Abstract
Post-operative cognitive dysfunction (POCD) is the collection of cognitive impairments lasting days to months, experienced by individuals following a surgery. Persistent POCD is most commonly experienced by older individuals and is associated with a greater vulnerability to developing Alzheimer’s disease, but the underlying mechanisms are not known. It is known that laparotomy (exploratory abdominal surgery) in aged rats produces memory impairments for four days. Here we report that post-surgical treatment with morphine extends this deficit to at least two months while having no effects in the absence of surgery. Indeed, hippocampal-dependent long-term memory was impaired two, four, and eight weeks post-surgery only in aged, morphine-treated rats. Short-term memory remained intact. Morphine is known to have analgesic effects via μ-opioid receptor activation and neuroinflammatory effects through Toll-like receptor 4 activation. Here we demonstrate that persistent memory deficits were mediated independently of the μ-opioid receptor, suggesting that they were evoked through a neuroinflammatory mechanism and unrelated to pain modulation. In support of this, aging, laparotomized, and morphine-treated rats exhibited increased gene expression of various proinflammatory markers (IL-1β, IL-6, TNFα, NLRP3, HMGB1, TLR2, and TLR4) in the hippocampus at the two-week timepoint. Furthermore, central blockade of IL-1β signaling with the specific IL-1 receptor antagonist (IL-1RA), at the time of surgery, completely prevented the memory impairment. Finally, synaptophysin and PSD95 gene expression were significantly dysregulated in the hippocampus of aged, laparotomized, morphine-treated rats, suggesting that impaired synaptic structure and/or function may play a key role in this persistent deficit. This instance of long-term memory impairment following surgery closely mirrors the timeline of persistent POCD in humans and may be useful for future treatment discoveries.
Introduction
Post-operative cognitive dysfunction (POCD) is the constellation of cognitive symptoms, lasting anywhere from days to months, that many surgical patients experience immediately following a variety of surgical procedures (e.g., abdominal, orthopedic, and cardiac surgeries). These symptoms range from slight confusion to difficulties with executive functions, an inability to form long-term episodic memories, to Alzheimer’s disease (AD) and other dementias (Bedford, 1955; Ramaiah and Lam, 2009; Terrando et al., 2011; Rundshagen, 2014). Many POCD cases are transient but importantly, longer-lasting cases of POCD are more likely to develop into AD (McCusker et al., 2001; Wacker et al., 2006; Bickel et al., 2008) and thus are more devastating. Advanced age is known to be the strongest risk factor for this persistent form of POCD (Moller et al., 1998; Monk and Price, 2011), but preclinical research examining cognitive function following a surgical procedure, even in aged subjects, has failed to recapitulate this persistent form of POCD, typically finding only relatively brief deficits, on the order of days (Rosczyk et al., 2008; Wan et al., 2010; Barrientos et al., 2012; Wang et al., 2020).
In aging, microglial priming has been identified as a key mediator of exaggerated neuroinflammation, primarily in the hippocampus, following a variety of peripheral insults including surgery (Cunningham et al., 2005; Barrientos et al., 2015a; Norden et al., 2015). Exaggerated neuroinflammation can result in deteriorated cellular and molecular processes important for forming memories, which in turn causes precipitous long-term memory deficits (Hauss-Wegrzyniak et al., 2002; Cunningham et al., 2005; Godbout et al., 2005; Barrientos et al., 2006; Griffin et al., 2006; Abraham et al., 2008; Barrientos et al., 2009; Chapman et al., 2010; Frank et al., 2010b; Barrientos et al., 2012; Spencer et al., 2017; Tanaka et al., 2018). Thus, it is not surprising that previous preclinical POCD studies, including our own, have demonstrated a causal role for proinflammatory cytokines in surgery-induced cognitive impairments. However, as noted above, none of these works have found impairments lasting beyond one to seven days post-surgery (Rosczyk et al., 2008; Wan et al., 2010; Barrientos et al., 2012; Wang et al., 2020). Therefore, it is unknown whether similar mechanisms underlie persistent forms of POCD, and whether interventions shown to ameliorate these short-lived impairments would be effective in longer-lasting cases.
One potential risk factor surrounding the peri-operative setting that has been overlooked in rodent models of POCD thus far is the prevalent use of opioid analgesics among post-surgical patients. Indeed, about 90% of patients are prescribed morphine and other opioids for post-surgical pain management (Aubrun et al., 2012; Garimella and Cellini, 2013) owing to their potent analgesic effects mediated by activation of the μ-opioid receptor (Corder et al., 2018). Importantly, a growing preclinical literature has independently shown that morphine and other opioids are capable of triggering a robust neuroinflammatory response through activation of the pattern recognition receptor Toll-like receptor 4 (TLR4), which, paradoxical to its prescribed purpose, prolongs neuropathic and post-surgical pain (Hutchinson et al., 2007; Hutchinson et al., 2010a; Wang et al., 2012; Johnson et al., 2014; Grace et al., 2016; Zhang et al., 2018; Grace et al., 2019). Interestingly, aging rodents exhibit significantly increased TLR4 expression in the hippocampus compared to younger rats (Fonken et al., 2016), and activation of these receptors (with a peripheral E. coli infection) has resulted in exaggerated neuroinflammatory responses and memory impairments (Barrientos et al., 2009; Frank et al., 2010a; Barrientos et al., 2015b; Fonken et al., 2016). Therefore, we explored whether the combination of aging, surgery, and morphine treatment might cause a synergistic neuroinflammatory response strong enough to cause persistent POCD. If so, this would have implications for the use of opioid analgesic post-surgery in aging humans. We administered a seven-day regimen of morphine following laparotomy in young adult and aged rats and measured hippocampally-mediated memory at several time points to determine whether the combination of these factors would produce persistent memory impairments. Furthermore, we investigated mechanisms by which these factors might extend POCD symptoms in aging.
Materials and Methods
Experimental design
This study was comprised of 9 separate experiments which will be briefly summarized here for ease of reading. In experiment 1, young and old rats underwent either laparotomy or sham surgery. Immediately after surgery and for the next seven days, they received either saline or (−)morphine (i.p). Long-term contextual memory was assessed at two weeks post-surgery. In experiments 2 and 3, separate cohorts of aged rats underwent either laparotomy or sham surgery and received either saline or (−)morphine as in experiment 1, and long-term memory was assessed at four and eight weeks post-surgery, respectively. In experiment 4, a separate cohort of aged rats underwent either laparotomy or sham surgery and received either saline or the μ-opioid receptor-inactive isomer (+)morphine (same dose and regimen as (−)morphine experiments), and long-term memory was assessed at two weeks post-surgery. In experiment 5, a separate cohort of aged laparotomized rats were used to determine the effects of saline, (−) or (+)morphine on short-term memory at two weeks post-surgery. Experiments 6, 7, and 8 used separate cohorts of rats with the identical experimental designs as in experiments 1–3, respectively, but instead of assessing memory, hippocampi were extracted for measurements of gene expression at two, four, and eight weeks post-surgery. Experiment 9 used a separate cohort of aged rats to determine the contribution of neuroinflammation to the persistence of cognitive deficits. Rats received an intra-cisterna magna (icm) injection of either saline or IL-1RA immediately prior to laparotomy. They then received either saline, (−)morphine, or (+)morphine (i.p, for seven days) as in previous experiments, and long-term memory was assessed at two weeks post-surgery.
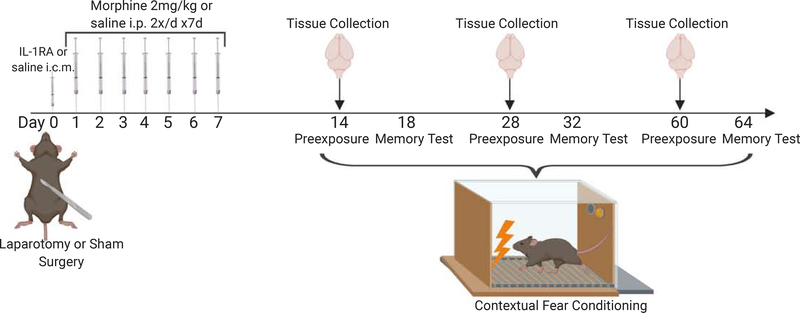
Separate cohorts were necessary for long-term and short-term memory experiments because exposure to the context during the memory test would initiate extinction of the fear memory and thus impact subsequent memory measurements. Also, separate groups of rats were used for the gene expression data because tissues were collected at the time that corresponded with memory consolidation rather than after memory test, and also to avoid the possible impact of behavioral testing on gene expression. A schematic depiction of these experiments is presented in Figure 1. The number of rats used per condition for each experiment is described in the Results section for each experiment.
Figure 1.
Schematic description of the study design. Generally, young adult or aged rats underwent either laparotomy or sham surgery. They subsequently received an i.p. injection of either saline or (−) or (+)morphine 2 mg/kg twice a day for seven days. Following this, either memory was assessed at two, four, or eight weeks post-surgery or hippocampi were collected for gene expression measurements. In one experiment, rats received an icm pre-operative treatment of either saline or IL-1RA.
Subjects
Subjects were male F344xBN F1 rats obtained from Charles River through the National Institute on Aging. This strain is particularly useful in the study of aging and aging-associated conditions as aged F344xBN F1 rats remain relatively healthy and show good cognitive function at baseline. Female rats of this strain were not available from this or any other vendor at the time these studies were completed. Therefore, they are not included here, but will be included in future studies as they become available. Upon arrival at our facility, rats were either 3 month old, weighing approximately 275 g, or 24 month old, weighing approximately 550 g. Importantly, the aged rats are not senescent at this age, and our previous work has shown that unchallenged animals at this age do not exhibit impaired function on contextual memory tasks compared to 3 month old controls (Barrientos et al., 2006; Frank et al., 2010b; Barrientos et al., 2012; Barrientos et al., 2015b; Spencer et al., 2017). Age- and condition-matched rats were housed 2 to a cage (52 L X 30 W X 21 H, cm). The animal colony was maintained at 22±1°C on a 12-h light/dark cycle (lights on at 07:00 h). All rats were allowed free access to food and water and were given at least 1 week to acclimate to colony conditions before experimentation began. All experiments were conducted in accordance with protocols approved by the University of Colorado and the Ohio State University Animal Care and Use Committees. All efforts were made to minimize the number of animals used and their suffering.
Surgery
Laparotomy (exploratory abdominal surgery) and sham surgeries were performed using aseptic procedures under isoflurane anesthesia according to a previously described method developed as a model of human abdominal exploratory surgery (Martin et al., 2005). The abdominal region was shaved and thoroughly cleaned with 70% ethanol and surgical scrub. Approximately 0.5 cm below the lower right rib, a 3 cm incision was made, penetrating the peritoneal cavity. Wearing sterile gloves, the surgeon vigorously manipulated the viscera and musculature. Approximately 10 cm of the small intestines were then exteriorized and vigorously rubbed between the surgeon’s thumb and index finger for 30 s. The intestines were then returned into the peritoneal cavity. Sterile chromic gut sutures (3–0, chromic gut, 27 in., PS-2; Ethicon) were used to suture the peritoneal lining and abdominal muscle in two layers. The skin was closed with surgical staples. To prevent infection, the wound was dressed with Polysporin (Pfizer, Morris Plains, NJ). Sham-operated rats were anesthetized, and abdominal area was shaved and cleaned as described above, but no incision was made. They remained on isoflurane for the same amount of time as their surgical counterpart (~25 min).
Drugs & Administration Procedures
We used the (−) and the (+) enantiomers of morphine in these studies. (−)Morphine, the natural enantiomer, is known to mediate analgesia through its actions at the μ-opioid receptor (Corder et al., 2018). More recently, it has also been shown to bind and activate TLR4, thus evoking an inflammatory response in the CNS (Hutchinson et al., 2007; Hutchinson et al., 2010a; Wang et al., 2012; Grace et al., 2016; Zhang et al., 2018; Grace et al., 2019). (+)Morphine, the unnatural enantiomer, is chromatographically and spectroscopically indistinguishable from the natural (−) enantiomer except for the sign of optical rotation. (+)Morphine has no μ-opioid receptor activity (Jacquet et al., 1977), but does activate TLR4 signaling (Hutchinson et al., 2010a; Hutchinson et al., 2011; Wang et al., 2012; Grace et al., 2015). Thus, (+)morphine is never used clinically as an analgesic, but rather serves as a useful tool for determining the relative contribution of the μ-opioid receptor vs TLR4 receptor in morphine-mediated effects. (−)Morphine, gifted by the NIDA drug repository, was injected i.p. at a dose of 2 mg/kg/ml, twice daily (~900 h and ~1700 h) for seven days, based on previous studies (Morgan et al., 2006; Hutchinson et al., 2009; Hutchinson et al., 2010b; Grace et al., 2019). (+)Morphine, gifted by Dr. Kenner Rice (NIDA/NIAAA), was also injected i.p. at a dose of 2 mg/kg/ml, twice daily for seven days. Both morphine compounds are reported as free base concentrations and were diluted in sterile saline (0.9%). The human morphine dose equivalency to the rat dose used in this study is 45 mg/day, falling within the recommended dose for managing post-operative pain for opioid-naïve patients of 30–60 mg/day (Reagan-Shaw et al., 2008; MD Anderson Cancer Center, 2018). Respective equivolumes of sterile saline were administered to controls.
For experiment 9, a single dose of IL-1 receptor antagonist (IL-1RA; Kineret) was injected intra-cisterna magna (icm) at a concentration of 112 μg/3 μl immediately prior to laparotomy. We opted to use icm injections, versus other routes of administration, for several reasons: First, peripherally administered IL-1RA is relatively short-lived (90–120 minutes) (Granowitz et al., 1993) with quick metabolism and excretion through the kidneys with little penetrance to the brain (Cawthorne et al., 2011). In contrast, a single icm administration of IL-1RA has been shown to protect the hippocampus against proinflammatory challenges for at least 4 days (Barrientos et al., 2012; Frank et al., 2012). Second, we wanted to directly target IL-1RA to the CNS in the least invasive way possible as to avoid causing additional inflammation, and icm injections do not require surgery or an indwelling cannula, and are completed in ~3 minutes. During these injections, rats were under isoflurane anesthesia. The dorsal aspect of the skull was first shaved and swabbed with 70% EtOH. A 27-gauge needle attached via PE50 tubing to a 25 μl Hamilton syringe was inserted into the cisterna magna. To verify entry into the cisterna magna, ~2 μl of clear cerebral spinal fluid was drawn and gently pushed back in and 3 μl total volume of IL-1RA was administered over 30 sec. An equal volume of sterile saline was injected (icm) into vehicle control animals.
Contextual Fear Conditioning
Memory function was measured using the contextual pre-exposure facilitation fear conditioning (CPF-FC) paradigm (detailed below), as it is widely accepted to be highly and specifically dependent on the hippocampus (Fanselow, 1990; Rudy et al., 2002), and because we have validated its utility in detecting memory impairments in young and aging rats following a variety of insults (Barrientos et al., 2006; Sobesky et al., 2014). This does not mean that no other brain areas participate (e.g., mPFC (Chakraborty et al., 2016), but that the hippocampus is required. The conditioning context consisted of one of two identical Igloo ice chests (54 L x 30 W x 27 H, cm) with white interiors. A speaker and an activated 24-V DC lightbulb were mounted on the ceiling of each chest. The conditioning chambers (26 L x 21 W x 24 H, cm), placed inside each chest, were made of clear plastic and had window screen tops. A foot shock could be delivered through a removable floor of stainless-steel rods 1.5 mm in diameter, spaced 1.2 cm center to center. Each rod was wired to a shock generator and scrambler (Coulbourn Instruments, Allentown, PA). Chambers were cleaned with water before each animal was conditioned or tested. Please refer to Figure 1 for timeline of when CPF-FC occurred relative to surgery.
The following CPF-FC paradigm procedures were used to measure long-term memory. For the short-term memory experiment, all phases of the paradigm were completed identically, with the sole exception that they were all conducted on a single day, with each phase occurring 1–2 h apart. Pre-exposure phase: On the first day, rats were taken two at a time from their home cage and transported in a black bucket to the conditioning context (one rat was placed in each context) where they were allowed to freely explore. This procedure was repeated six times (rats remained in the conditioning context for five min on the first exposure, and for 40 sec on each of the five subsequent exposures, with an approximate 40 sec interval in their home cage prior to each subsequent exposure). The purpose of these multiple exposures was to establish an association between the black bucket and activation of the conjunctive representation of the context (for further detail see (Rudy et al., 2002)). During the five-min pre-exposure, locomotion was scored to assess any confounding motor disturbances or generalized fear (described below). These responses were never observed. Immediate shock phase: Three days later, one animal at a time was taken from its home cage and transported in the same black bucket to the conditioning context where they immediately received one two-sec, 1.5 mA footshock. They were then quickly taken out of the context and transported back to their home cage. The rats’ time in the conditioning context in this phase never exceeded ten sec. Testing phase: Memory for the conditioning context was assessed 24 h after immediate shock (four days after pre-exposure) by placing the rat in the conditioning context and observing and scoring its fear (freezing) behavior. Freezing, the rat’s dominant defensive fear response, is a complete suppression of behavior that is accompanied by immobility, shallow breathing, and a variety of other autonomic changes including an increase in heart rate and pilo-erection (Fanselow and Lester, 1988). In these experiments, freezing was defined as the absence of all visible movement, except for respiration. Scoring began approximately ten sec after the rat was placed into the chamber, and continued every ten sec for a total of six min. Two hours later, locomotion and generalized fear were again assessed by placing the rat in an alternate (neutral/novel) context for another six min of observation for freezing behavior. Scoring was carried out by observers blind to experimental treatment, and inter-rater reliability exceeded 97% for all experiments.
Tissue dissection
Animals were given a lethal dose of sodium pentobarbital (Fatal Plus) and transcardially perfused with ice-cold saline (0.9%) for three min to remove peripheral immune leukocytes from the CNS vasculature. Brains were then rapidly extracted and placed on an ice-cold frosted glass plate and hippocampi were dissected. All tissues were quickly frozen in liquid nitrogen and stored at −70°C until the time of processing.
Quantitative PCR
Total RNA was isolated using a standard method of phenol:chloroform extraction (Chomczynski and Sacchi, 1987). cDNA amplification was performed using Quantitect SYBR Green PCR kit (Qiagen, Valenica, CA) in iCycler iQ 96-well PCR plates (Bio-Rad, Hercules, CA) on a MyiQ single Color Real-Time PCR Detection System (Bio-Rad). Primers were designed (Genbank, National Center for Biotechnology Information; www.ncbi.nlm.nih.gov) to span exon/exon boundaries and thus exclude amplification of genomic DNA, and were obtained from Invitrogen. Primer sequences are displayed in Table 1. Each sample was measured in duplicate using the MyiQ single Color Real-Time PCR Detection System (Bio-Rad). Primer specificity was verified by melt curve analysis. Threshold for detection of PCR product was set in the log-linear phase of amplification and the threshold cycle (CT) was determined for each reaction. The level of the target mRNA was quantified and expressed relative to the housekeeping gene β actin. β actin was not significantly different between groups.
Table 1.
PCR Primer Description and Sequences
| Gene | Primer Sequence: 5’ –> 3’ | Function |
|---|---|---|
| β-Actin | F: TTCCTTCCTGGGTATGGAAT R: GAGGAGCAATGATCTTGATC |
Cytoskeletal protein (housekeeping gene) |
| IL-1β | F: CCTTGTGCAAGTGTCTGAAG R: GGGCTTGGAAGCAATCCTTA |
Pro-inflammatory cytokine |
| IL-6 | F: AGAAAAGAGTTGTGCAATGGCA R: GGCAAATTTCCTGGTTATATCC |
Pro-inflammatory cytokine |
| TNFα | F: CAAGGAGGAGAAGTTCCCA R: TTGGTGGTTTGCTACGACG |
Pro-inflammatory cytokine |
| HMGB1 | F: GAGGTGGAAGACCATGTCTG R: AAGAAGAAGGCCGAAGGAGG |
Endogenous danger signal |
| NLRP3 | F: AGAAGCTGGGGTTGGTGAATT R: GTTGTCTAACTCCAGCATCTG |
IL-1 Inflammasome |
| TLR2 | F: TGGAGGTCTCCAGGTCAAATC R: ACAGAGATGCCTGGGCAGAAT |
PRR for motifs of gram-negative bacteria |
| TLR4 | F: TCCCTGCATAGAGGTACTTC R: CACACCTGGATAAATCCAGC |
PRR for motifs of gram-negative bacteria |
| Synaptophysin | F: ACCTCAGTGGTGTTTGGCTT R: CCCGTAATCGGGTTGATAAC |
Pre-synaptic density marker |
| PSD-95 | F: CAGATGGAAGTGCACTATGC R: CCGTCTATCTCATATTCCCG |
Post-synaptic density marker |
Table 1. Abbreviations: IL: interleukin, TNF (tumor necrosis factor alpha); HMGB1: high mobility group box 1, NLRP3: nod-like receptor protein 3, TLR (Toll-like receptor); PSD (post-synaptic density).
Statistical Analyses
Statistical analyses were conducted using StatView v.5 and Prism v.7 software. One-way, two-way and three-way ANOVAs were employed, as dictated by the experimental design. Following significant main effects or interactions, Tukey’s post-hoc tests were conducted to reveal pairwise differences between groups. Statistical significance for all tests was set at alpha = 0.05.
Results
Aging, surgery, and (−)morphine induced contextual memory impairments lasting two weeks
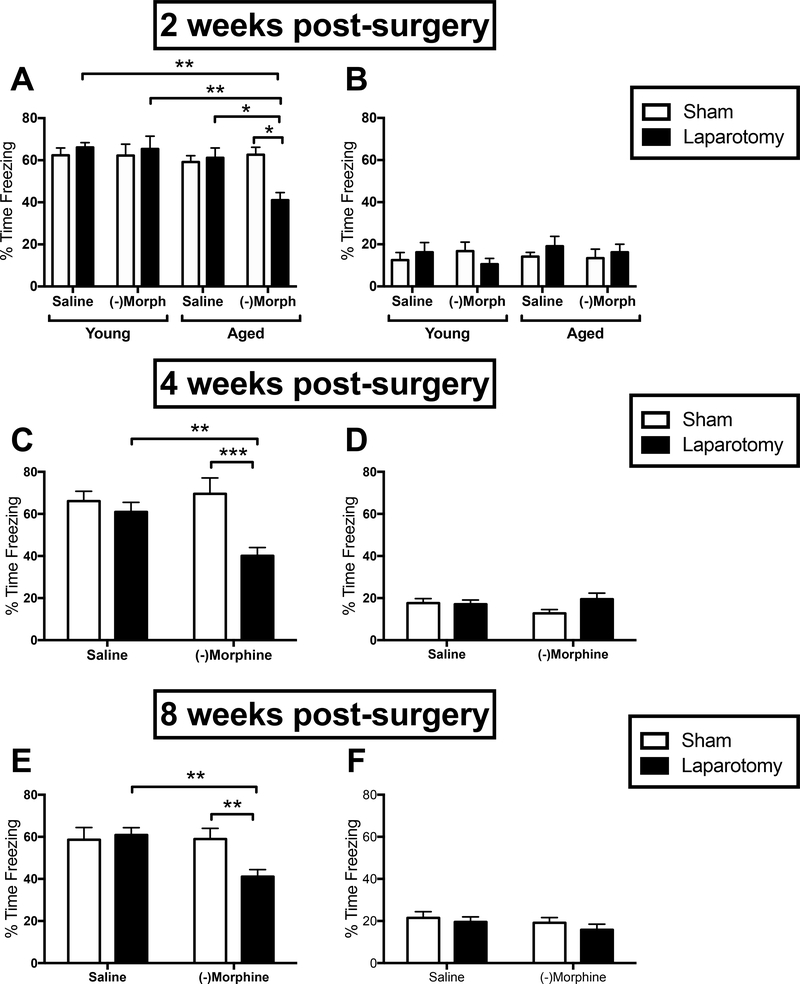
Contextual fear memory was measured two weeks post-surgery to determine whether the combination of aging, laparotomy, and (−)morphine would lead to hippocampal-dependent memory deficits beyond the four day-long impairment previously observed in laparotomized aged rats not administered morphine (Barrientos et al., 2012). A three-way ANOVA with age, surgery, and drug treatment (n = 12–14/group) revealed a main effect of age (F(1,97) = 6.889, p < 0.05), and significant age x surgery (F(1,97) = 4.73, p < 0.05) and drug x surgery (F(1,97) = 3.96, p < 0.05) interactions. Post-hoc analyses revealed that aged, laparotomized, (−)morphine-treated rats were significantly impaired on the memory test compared to age- and drug-matched sham controls (p < 0.05), age- and surgery-matched saline controls (p < 0.05), and drug- and surgery-matched young controls (p < 0.01; Fig. 2A). All other groups performed well and did not differ from one another. Freezing to a novel context was also measured to rule out the possibility of generalized fear or anxiety. All groups froze less than 20% of the time, indicating little to no fear, and there were no differences between any groups (p > 0.05; Fig. 2B).
Figure 2.
Hippocampal-dependent long-term memory as measured by % time freezing upon re-exposure to the conditioning context in young and aged (A) or just aged (C & E) rats that either had a laparotomy (black bars) or sham (white bars) surgery and were treated with either saline or (−)morphine. Generalized fear as measured by % time freezing upon exposure to a novel, control context in young and aged (B) or just aged (D & F) rats in the same conditions as above. Error bars represent S.E.M; *p < 0.05; **p < 0.01; ***p < 0.001.
Contextual memory at four and eight weeks post-surgery remained impaired
To measure the persistence of the (−)morphine plus surgery-induced memory impairment in aged rats, separate cohorts of rats were used to measure contextual memory four or eight weeks post-surgery. Because young rats were not impaired at the two-week time point, only aged rats were used for these experiments (four week: n = 8–10/group; eight week: n = 7–9/group). At four weeks, a drug x surgery interaction was observed (F(1,30) = 5.61, p < 0.05), and post-hoc analyses again revealed that aged, laparotomized, (−)morphine-treated rats drove this effect. They exhibited reduced levels of freezing (impaired memory) compared to (−)morphine-treated sham controls (p < 0.001) and surgery-matched saline controls (p < 0.01; Fig. 2C). Similar results were obtained at eight weeks. A drug x surgery interaction was observed (F(1,30) = 4.54, p < 0.05), and post-hoc analyses again demonstrated that aged, laparotomized, (−)morphine-treated rats had significant memory impairments compared to (−)morphine-treated sham controls (p < 0.01) and surgery-matched saline controls (p < 0.01; Fig. 2E). All other groups performed well and did not differ from one another. As observed in the two-week experiment, all groups froze less than 20% of the time in the novel context (in both four and eight week experiments), and did not differ across groups (p > 0.05; Figs. 2D & 2F) indicating little to no generalized fear.
(−)Morphine-induced persistent POCD in aged rats occurred independently of opioid receptors
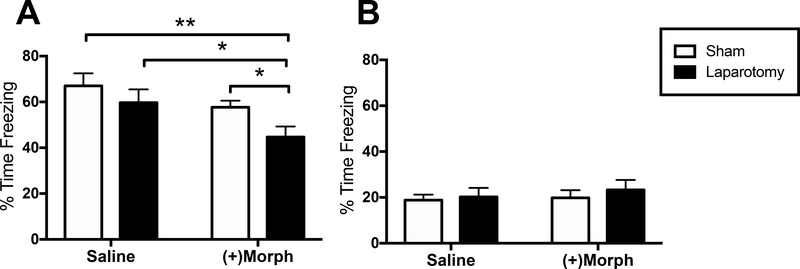
To determine the role of opioid receptors and analgesia in the observed (−)morphine-induced persistent memory impairment triggered in aged rats, a separate experiment was conducted wherein the unnatural stereoisomer (+)morphine, which activates TLR4 signaling (Hutchinson et al., 2010a) but has no μ-opioid receptor activity or analgesic properties (Jacquet et al., 1977), was used. All other experimental procedures were identical as in the previous experiments, and memory performance was measured two weeks post-surgery. Only aged rats were included here (n = 11–14/group), as young rats were not impaired in the original experiment. A two-way ANOVA indicated significant effects of drug (F(1,45) = 6.52, p < 0.05) and surgery (F(1,45) = 4.56, p < 0.05). Similar to the findings with (−)morphine, planned comparisons revealed a significant reduction in freezing (memory impairment) in laparotomized, (+)morphine-treated rats compared to (+)morphine-treated sham controls (p < 0.05), laparotomized saline-treated controls (p < 0.05) and sham saline-treated controls (p < 0.01; Fig. 3A). As before, freezing to a novel context indicated little to no fear (less than 20% of the time) across all groups, and none of the groups differed from one another (p > 0.05; Fig. 3B).
Figure 3.
(A) Hippocampal-dependent long-term memory as measured by % time freezing upon re-exposure to the conditioning context in aged rats that either had a laparotomy (black bars) or sham (white bars) surgery and were treated with either saline or the μ-opioid receptor inactive (+)morphine. (B) Generalized fear as measured by % time freezing upon exposure to a novel, control context in the same groups as (A). Error bars represent S.E.M; *p < 0.05; **p < 0.01.
Neither (−) nor (+)morphine impaired short-term contextual memory
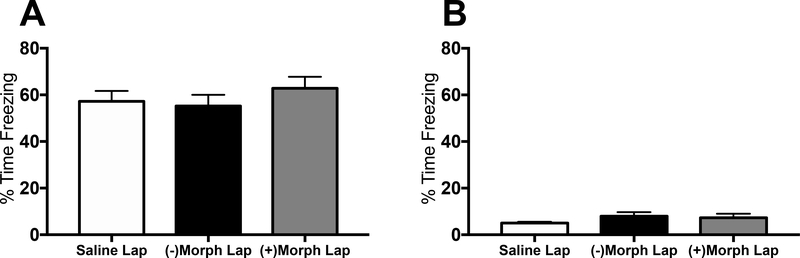
To investigate whether the memory deficits observed in the previous experiments were specific to long-term memory, the effects of (−) and (+)morphine on short-term memory were examined two weeks post-surgery in a separate cohort of rats (n = 5–8/group). To minimize the number of animals used, only aged, laparotomized rats were used. A one-way ANOVA showed no significant effects between saline-, (−)morphine- or (+)morphine-treated groups (p > 0.05), indicating no impairments in short-term contextual fear memory (Fig. 4A). As expected, no significant differences in freezing behavior during exposure to a novel context were observed between any of the groups (p > 0.05; Fig. 4B).
Figure 4.
(A) Hippocampal-dependent short-term memory as measured by % time freezing upon re-exposure to the conditioning context in aged rats that had a laparotomy surgery and were treated with either saline, (−)morphine or (+)morphine. (B) Generalized fear as measured by % time freezing upon exposure to a novel, control context in the same groups as (A). Error bars represent S.E.M.
(−)Morphine-induced persistent POCD in aged rats was associated with increased gene expression of pro-inflammatory markers in hippocampus
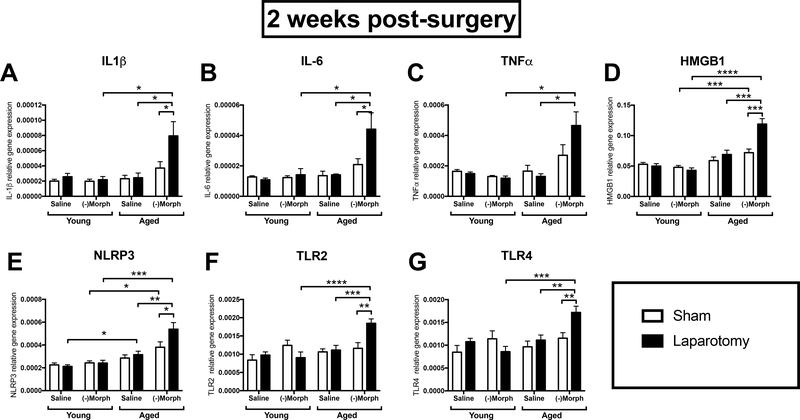
Using qPCR, we measured mRNA expression levels of several key inflammation-related genes (IL-1β, IL-6, TNFα, NLRP3, HMGB1, TLR2, and TLR4) in the hippocampus. A three-way ANOVA with age, surgery, and drug treatment (n = 8–10/group) as the three factors run at the two-week time point revealed a similar trend across all genes. That is, interaction effects were observed with each gene: (IL-1β (F(1,62) = 9.34, p < 0.01), IL-6 (F(1,40) = 7.36, p < 0.01), TNFα (F(1,40) = 16.60, p < 0.001), HMGB1 (F(1,61) = 19.07, p < 0.0001), NLRP3 (F(1,63) = 7.04, p < 0.01), TLR2 (F(1,61) = 7.99, p < 0.01), and TLR4 (F(1,62) = 6.11, p < 0.05)). Post-hoc analyses revealed that aged, laparotomized, (−)morphine-treated rats had significantly increased mRNA levels of each gene compared to age- and drug-matched sham controls, age- and surgery-matched saline controls, and drug- and surgery-matched young controls (p < 0.05–0.0001; Fig. 5A–G). At four weeks post-surgery, there were no significant interactions between the factors for any of the genes measured. However, there were main effects of surgery elevating gene expression of IL-6 (F(1,24) = 5.66, p < 0.05), HMGB1 (F(1,24) = 5.69, p < 0.05), and TLR4 (F(1,24) = 6.28, p < 0.05; Fig. S1 (n = 5–8)). At eight weeks post-surgery, there were no significant main effects or interactions for any gene (S2 (n = 7–10).
Figure 5.
Hippocampal gene expression of IL-1β (A), IL-6 (B), TNFα (C), HMGB1 (D), NLRP3 (E), TLR2 (F), and TLR4 (G) in young and aged, laparotomized, (−)morphine-treated rats compared to controls two weeks post-surgery. Error bars represent S.E.M.*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
IL-1RA pre-treatment prevents persistent morphine-induced POCD
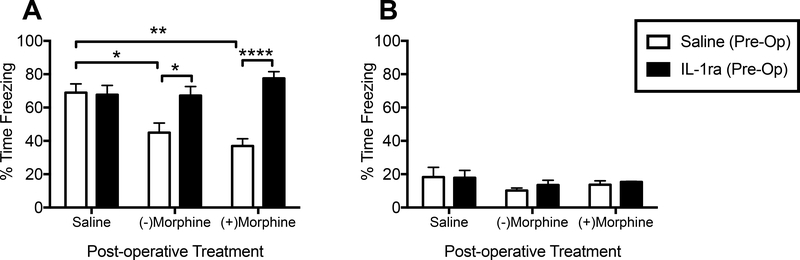
To further determine whether neuroinflammation played a role in both (−) and (+)morphine-induced memory impairments at the 2-week time point, we examined the extent to which a single IL-1RA injection at the time of surgery would prevent the memory impairments caused by both morphine compounds in laparotomized aged rats (n = 7–8/group). Young rats were not included since they showed no impairments at any time with either compound. Rats were given a single pre-operative icm injection of either saline or IL-1RA. Post-operatively, rats were treated with either saline, (−)morphine or (+)morphine as in all previous experiments. A two-way ANOVA indicated a significant interaction (F(2,41) = 8.21, p < 0.001) between the pre-operative and post-operative treatment factors. Post-hoc analyses revealed that aged, laparotomized rats treated pre-operatively with saline and post-operatively with (−)morphine (p < 0.05) or (+)morphine (p < 0.01) were significantly impaired compared to control rats treated post-operatively with saline. Furthermore, pre-operative treatment with IL-1RA completely prevented both the (−)morphine-induced (p < 0.05) and the (+)morphine-induced (p < 0.0001) memory impairments (Fig. 6A). As before, all groups froze less than 20% of the time in the novel context, and did not differ across groups (p > 0.05; Figs. 6B) indicating little to no generalized fear.
Figure 6.
(A) Hippocampal-dependent long-term memory as measured by % time freezing upon re-exposure to the conditioning context in aged laparotomized rats that received pre-operatively either saline or IL-1RA (icm) and were post-operatively treated with either saline, (−)morphine or (+)morphine. (B) Generalized fear as measured by % time freezing upon exposure to a novel, control context in the same groups as (A). Error bars represent S.E.M; *p < 0.05. *p < 0.05; **p < 0.01; ****p < 0.0001
(−) Morphine induced abnormal synaptic marker expression in hippocampus
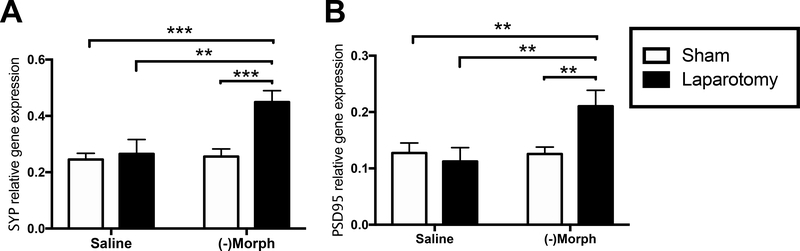
To determine if mRNA transcripts of synaptic structures important for forming long-term memories may have been altered with the combination of aging, laparotomy, and (−)morphine, we examined mRNA expression of the pre- and post-synaptic markers synaptophysin and PSD95, respectively, at the two-week time point in aged rats (n = 7–8/group). Two-way ANOVAs indicated significant interactions between treatment and surgery for synaptophysin (F(1,27) = 5.84, p < 0.05) and PSD95 (F(1,27) = 5.46, p < 0.05). Post-hoc analyses showed that laparotomy and (−)morphine treatment caused a significant increase in gene expression of synaptophysin (p < 0.01; Fig. 7A) and PSD95 (p < 0.01; Fig. 7B) compared to all other groups.
Figure 7.
Synaptophysin (A) and PSD-95 (B) gene expression two weeks post-surgery in the hippocampus of aged laparotomized rats that received (−)morphine. Error bars represent S.E.M; **p < 0.01; ***p < 0.001.
Discussion
We found that a seven-day treatment regimen with (−)morphine following surgery produced a long-lasting detrimental effect on hippocampal memory in aged, but not young rats. Rats showed impaired ability to form a long-term contextual memory two, four, and eight weeks post-surgery (we did not measure beyond eight weeks). This impairment robustly outlasted the impairment reported in our previous study in which the combination of aging and surgery, without morphine, produced a memory impairment lasting 4, but not 12 days post-surgery (Barrientos et al., 2012). Importantly, short-term memory was spared, indicating that the rats’ ability to explore, sample, and encode the contextual representation, or to express fear for what was learned was not disrupted. Together, these findings indicate that this combination of factors interfered with memory consolidation and not learning. It is worth noting that in all behavioral experiments, all groups demonstrated little to no freezing to the novel context, indicating that fear to the conditioning context was specific for what was learned and not due to disturbances in locomotion, generalized fear or anxiety. It is also important to note that neither aging, surgery, or morphine alone was sufficient to produce a long-lasting memory deficit, nor were any two factors combined sufficient. Indeed, all 3 factors combined (i.e., aging, laparotomy, and morphine) were necessary to result in this persistent memory deficit.
The persistent memory effect was associated with significantly increased gene expression of various proinflammatory markers (IL-1β, IL-6, TNFα, NLRP3, HMGB1, TLR2, and TLR4) in the hippocampus of aged, laparotomized, (−)morphine-treated rats at the two-week time point. HMGB1, an early danger-associated molecular pattern which alerts the CNS microenvironment of impending threat (Bianchi, 2007), can initiate a neuroinflammatory response through activation of TLR2 and TLR4 (Yang and Tracey, 2010). The activation of TLR2 and TLR4, both of which were also found to be significantly elevated here, can initiate a proinflammatory cascade (Trotta et al., 2014). This cascade begins with NFκB signaling which promotes assembly of the NLRP3 inflammasome; activation of the NLRP3 inflammasome then leads to cleavage of caspase-1 and pro-IL-1 to trigger mature IL-1β release (Martinon et al., 2002; Khare et al., 2010). IL-1β release and signaling, in turn, initiates the release of other proinflammatory cytokines such as IL-6 and TNFα (Morris et al., 2014). Thus, these data strongly suggest that an intense and long-lasting (at least two weeks) neuroinflammatory response was triggered with these three combined factors. Expression levels of these markers were no longer significantly different across key groups at later time points, but memory deficits persisted suggesting that downstream processes may have been altered (discussed below).
To further characterize the processes that may underlie these effects, we used the (+)morphine enantiomer to determine the role of the μ-opioid receptor. This enantiomer shares the exact molecular structure of (−)morphine, but because it is the mirror image of the (−) compound does not bind to the stereoselective μ-opioid receptor (Jacquet et al., 1977), but does bind to the non-stereoselective TLR4 (Hutchinson et al., 2011; Wang et al., 2012; Grace et al., 2015). Using this pharmacological tool, we replicated the long-lasting memory deficits achieved with (−)morphine, suggesting that these persistent memory deficits occurred independently of μ-opioid receptors. These findings favor a TLR4-mediated mechanism, though experiments to determine its specific role are the subject of a future study.
To determine whether and to what extent neuroinflammation contributes to this morphine-induced persistent memory impairment, we blocked the IL-1 receptor with a central injection of IL-1RA at the time of surgery and measured memory performance at the two-week time point. It should be noted that we have previously shown that a single icm injection of IL-1RA confers protection against neuroinflammation for four days (Barrientos et al., 2012; Frank et al., 2012). Here, this intervention completely prevented both the (−) and (+)morphine-induced memory deficits, strongly suggesting that early neuroinflammation is necessary for morphine to exert a persistent deteriorating effect on cognitive function. That is, blocking the initial neuroinflammatory response triggered by surgery and the first several days of morphine treatment was sufficient to preserve long-term memory function in aged rats. These findings suggest that pre-operative interventions aimed at reducing neuroinflammation may hold promise as a neuroprotective approach prior to a planned surgery. For example, behavioral interventions such as physical exercise or intermittent fasting are known to reduce neuroinflammation in aged animals and humans (Barrientos, 2011; Barrientos et al., 2011; Kohman et al., 2012; Woods et al., 2012; Kohman et al., 2013; Vasconcelos et al., 2014; Hu et al., 2019). Likewise, administration of anti-neuroinflammatory drugs before surgery may also provide some neuroprotective benefit during surgery.
It is well-known that long-term memory formation depends on synaptic integrity and plasticity (Kandel, 2001). We have previously reported that synaptic plasticity is severely impaired in the aged hippocampus following a bacterial infection-evoked exaggerated neuroinflammatory response (Chapman et al., 2010; Tanaka et al., 2018). Furthermore, systemic inflammation has been linked to synapse loss and cognitive decline (Cunningham et al., 2009). Therefore, to explore a possible disruption to memory-forming processes downstream of neuroinflammation in our model, we measured the gene expression of the presynaptic marker synaptophysin, and the post-synaptic marker post-synaptic density (PSD)-95, in the hippocampus of aged laparotomized rats treated with (−)morphine two weeks post-surgery. We found that (−)morphine caused a robust dysregulation in both synaptophysin and PSD-95 compared to controls. Surprisingly, expression levels of these markers were robustly elevated. Although these data were initially unexpected, evidence from several studies indicates this may be a common finding following various neurological insults. For example, studies examining the detrimental effects of high-fat diet consumption on memory consolidation have shown that short-term consumption in aged rats (Spencer et al., 2017; Spencer et al., 2019) and chronic consumption in young adult mice (Denver et al., 2018) resulted in hippocampal long-term memory impairments, elevated brain inflammation, and subsequent upregulation of synaptophysin in various regions of the hippocampus. In a controlled cortical impact model of traumatic brain injury, rats exhibited an initial robust decrease in PSD-95 expression following injury, but two and four weeks later these levels were potently elevated (Svirsky et al., 2020). These findings, in conjunction with those of the current study, suggest the possibility of a compensatory rebound response following injury or insult. Given that tissues in the current study were not collected earlier than two weeks post-surgery, additional work needs to be done to determine if synaptic structures were indeed damaged and the extent of that damage.
Taken together, these studies demonstrated that the combination of aging, surgery, and morphine treatment produced very long-lasting memory deficits that closely resemble the post-operative cognitive deficits exhibited by human surgical patients who subsequently develop Alzheimer’s disease and other dementias (McCusker et al., 2001; Wacker et al., 2006; Bickel et al., 2008). Furthermore, findings indicated that an initial intense neuroinflammatory response elicited by these three factors was followed by dysregulated gene expression of markers found in hippocampal pre and post-synaptic structures, possibly reflecting dysfunction in the hippocampus and rendering it unable to support long-term memory formation. Future studies should investigate the possible involvement of the TLR4, and the subsequent mechanisms that might lead to synaptic dysfunction.
The present data have potential implications for the development and amelioration of POCD in humans as 90% of all surgical patients are prescribed morphine for post-surgical pain (Aubrun et al., 2012; Garimella and Cellini, 2013), and limited clinical research suggests that opioids increase the risk of delirium in elderly patients (Swart et al., 2017). These findings suggest either the use of non-opioid analgesics after surgery or anti-neuroinflammatory interventions prior to surgery may ameliorate cognitive decline after surgery in older individuals.
Supplementary Material
Figure S1. Hippocampal gene expression of IL-1β (A), IL-6 (B), TNFα (C), HMGB1 (D), NLRP3 (E), TLR2 (F), and TLR4 (G) in aged, laparotomized, (−)morphine-treated rats compared to controls 4 weeks post-surgery. Error bars represent S.E.M.
Figure S2. Hippocampal gene expression of IL-1β (A), IL-6 (B), TNFα (C), HMGB1 (D), NLRP3 (E), TLR2 (F), and TLR4 (G) in aged, laparotomized, (−)morphine-treated rats compared to controls 8 weeks post-surgery. Error bars represent S.E.M.
Highlights.
Surgery & morphine induced memory deficits lasting 8 wk in aged, but not young rats
Morphine-induced POCD in aged rats occurred independently of opioid receptors
Morphine-induced POCD was associated with increased hippocampal proinflammatory markers
IL-1RA pre-treatment prevented morphine-induced POCD in aged rats
Morphine-induced POCD was associated with dysregulated hippocampal synaptic markers
Acknowledgements:
This work was supported, in part, by a grant from the National Institute on Aging RF1AG028271 to R.M.B. & S.F.M. A portion of this work was supported by the Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and National Institute of Alcohol Abuse and Alcoholism (NIAAA).
The data contained in the manuscript being submitted have not been previously published, have not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
All experiments were conducted in accordance with protocols approved by the University of Colorado and the Ohio State University Animal Care and Use Committees.
All authors have reviewed the contents of the manuscript being submitted, approve of its contents, and validate the accuracy of the data.
Footnotes
All authors declare no actual or potential conflicts of interests.
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW (2008) Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging 29:614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubrun F, Mazoit JX, Riou B (2012) Postoperative intravenous morphine titration. British journal of anaesthesia 108:193–201. [DOI] [PubMed] [Google Scholar]
- Barrientos RM (2011) Voluntary exercise as an anti-neuroinflammatory therapeutic. Brain Behav Immun 25:1061–1062. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Kitt MM, Watkins LR, Maier SF (2015a) Neuroinflammation in the normal aging hippocampus. Neuroscience 309:84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF (2012) Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci 32:14641–14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF (2009) Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun 23:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF (2006) Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging 27:723–732. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF (2011) Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci 31:11578–11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Thompson VM, Kitt MM, Amat J, Hale MW, Frank MG, Crysdale NY, Stamper CE, Hennessey PA, Watkins LR, Spencer RL, Lowry CA, Maier SF (2015b) Greater glucocorticoid receptor activation in hippocampus of aged rats sensitizes microglia. Neurobiol Aging 36:1483–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford PD (1955) Adverse cerebral effects of anaesthesia on old people. Lancet 269:259–263. [DOI] [PubMed] [Google Scholar]
- Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5. [DOI] [PubMed] [Google Scholar]
- Bickel H, Gradinger R, Kochs E, Forstl H (2008) High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dement Geriatr Cogn Disord 26:26–31. [DOI] [PubMed] [Google Scholar]
- Cawthorne C, Prenant C, Smigova A, Julyan P, Maroy R, Herholz K, Rothwell N, Boutin H (2011) Biodistribution, pharmacokinetics and metabolism of interleukin-1 receptor antagonist (IL-1RA) using [(1)(8)F]-IL1RA and PET imaging in rats. Br J Pharmacol 162:659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T, Asok A, Stanton ME, Rosen JB (2016) Variants of contextual fear conditioning induce differential patterns of Egr-1 activity within the young adult prefrontal cortex. Behav Brain Res 302:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman TR, Barrientos RM, Ahrendsen JT, Maier SF, Patterson SL (2010) Synaptic correlates of increased cognitive vulnerability with aging: peripheral immune challenge and aging interact to disrupt theta-burst late-phase long-term potentiation in hippocampal area CA1. J Neurosci 30:7598–7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159. [DOI] [PubMed] [Google Scholar]
- Corder G, Castro DC, Bruchas MR, Scherrer G (2018) Endogenous and Exogenous Opioids in Pain. Annu Rev Neurosci 41:453–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH (2005) Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci 25:9275–9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH (2009) Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry 65:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denver P, Gault VA, McClean PL (2018) Sustained high-fat diet modulates inflammation, insulin signalling and cognition in mice and a modified xenin peptide ameliorates neuropathology in a chronic high-fat model. Diabetes Obes Metab 20:1166–1175. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (1990) Factors governing one trial contextual conditioning. Anim Learn Behav 18:264–270. [Google Scholar]
- Fanselow MS, Lester LS (1988) A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior In: Evolution and Learning (Bolles RC, Beecher MD, eds), pp 185–211. Hillside, NJ: Erlbaum. [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, D’Angelo HM, Norden DM, Weber MD, Barrientos RM, Godbout JP, Watkins LR, Maier SF (2016) The Alarmin HMGB1 Mediates Age-Induced Neuroinflammatory Priming. J Neurosci 36:7946–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF (2010a) Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol 226:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF (2010b) IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun 24:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Thompson BM, Weber MD, Watkins LR, Maier SF (2012) IL-1RA injected intra-cisterna magna confers extended prophylaxis against lipopolysaccharide-induced neuroinflammatory and sickness responses. J Neuroimmunol 252:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garimella V, Cellini C (2013) Postoperative pain control. Clin Colon Rectal Surg 26:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW (2005) Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J 19:1329–1331. [DOI] [PubMed] [Google Scholar]
- Grace PM, Maier SF, Watkins LR (2015) Opioid-induced central immune signaling: implications for opioid analgesia. Headache 55:475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Galer EL, Strand KA, Corrigan K, Berkelhammer D, Maier SF, Watkins LR (2019) Repeated Morphine Prolongs Postoperative Pain in Male Rats. Anesth Analg 128:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR (2016) Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A 113:E3441–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granowitz EV, Porat R, Mier JW, Orencole SF, Callahan MV, Cannon JG, Lynch EA, Ye K, Poutsiaka DD, Vannier E, et al. (1993) Hematologic and immunomodulatory effects of an interleukin-1 receptor antagonist coinfusion during low-dose endotoxemia in healthy humans. Blood 82:2985–2990. [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA (2006) The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem 99:1263–1272. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Lynch MA, Vraniak PD, Wenk GL (2002) Chronic brain inflammation results in cell loss in the entorhinal cortex and impaired LTP in perforant path-granule cell synapses. Exp Neurol 176:336–341. [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhang M, Chen Y, Yang Y, Zhang JJ (2019) Postoperative intermittent fasting prevents hippocampal oxidative stress and memory deficits in a rat model of chronic cerebral hypoperfusion. Eur J Nutr 58:423–432. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR (2007) Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal 7:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR (2011) Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev 63:772–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, Somogyi AA, Yin H, Maier SF, Rice KC, Watkins LR (2010a) Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience 167:880–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW (2009) Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav Immun 23:240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR et al. (2010b) Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun 24:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet YF, Klee WA, Rice KC, Iijima I, Minamikawa J (1977) Stereospecific and nonstereospecific effects of (+)- and (−)-morphine: evidence for a new class of receptors? Science 198:842–845. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Rolan PE, Johnson ME, Bobrovskaya L, Williams DB, Johnson K, Tuke J, Hutchinson MR (2014) Codeine-induced hyperalgesia and allodynia: investigating the role of glial activation. Transl Psychiatry 4:e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294:1030–1038. [DOI] [PubMed] [Google Scholar]
- Khare S, Luc N, Dorfleutner A, Stehlik C (2010) Inflammasomes and their activation. Crit Rev Immunol 30:463–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Bhattacharya TK, Wojcik E, Rhodes JS (2013) Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J Neuroinflammation 10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, DeYoung EK, Bhattacharya TK, Peterson LN, Rhodes JS (2012) Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun 26:803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Kahn WR, Eisenach JC (2005) Abdominal surgery decreases food-reinforced operant responding in rats: relevance of incisional pain. Anesthesiology 103:629–637. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular cell 10:417–426. [DOI] [PubMed] [Google Scholar]
- McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F (2001) Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ 165:575–583. [PMC free article] [PubMed] [Google Scholar]
- MD Anderson Cancer Center (2018) Post-Operative Pain Management. In: (The University of Texas MD Anderson Cancer Center, ed). [Google Scholar]
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS (1998) Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 351:857–861. [DOI] [PubMed] [Google Scholar]
- Monk TG, Price CC (2011) Postoperative cognitive disorders. Current opinion in critical care 17:376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Fossum EN, Stalding BM, King MM (2006) Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J Pain 7:358–366. [DOI] [PubMed] [Google Scholar]
- Morris MC, Gilliam EA, Li L (2014) Innate immune programing by endotoxin and its pathological consequences. Front Immunol 5:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Muccigrosso MM, Godbout JP (2015) Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 96:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiah R, Lam AM (2009) Postoperative cognitive dysfunction in the elderly. Anesthesiology clinics 27:485–496. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661. [DOI] [PubMed] [Google Scholar]
- Rosczyk HA, Sparkman NL, Johnson RW (2008) Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol 43:840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O’Reilly RC (2002) Hippocampal formation supports conditioning to memory of a context. Behav Neurosci 116:530–538. [DOI] [PubMed] [Google Scholar]
- Rundshagen I (2014) Postoperative cognitive dysfunction. Dtsch Arztebl Int 111:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobesky JL, Barrientos RM, De May HS, Thompson BM, Weber MD, Watkins LR, Maier SF (2014) High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1beta, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav Immun 42:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, D’Angelo H, Soch A, Watkins LR, Maier SF, Barrientos RM (2017) High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol Aging 58:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Basri B, Sominsky L, Soch A, Ayala MT, Reineck P, Gibson BC, Barrientos RM (2019) High-fat diet worsens the impact of aging on microglial function and morphology in a region-specific manner. Neurobiol Aging 74:121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svirsky S, Henchir J, Li Y, Ma X, Carlson S, Dixon CE (2020) Neurogranin Protein Expression Is Reduced after Controlled Cortical Impact in Rats. J Neurotrauma 37:939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart LM, van der Zanden V, Spies PE, de Rooij SE, van Munster BC (2017) The Comparative Risk of Delirium with Different Opioids: A Systematic Review. Drugs Aging 34:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Cortese GP, Barrientos RM, Maier SF, Patterson SL (2018) Aging and an Immune Challenge Interact to Produce Prolonged, but Not Permanent, Reductions in Hippocampal L-LTP and mBDNF in a Rodent Model with Features of Delirium. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N, Brzezinski M, Degos V, Eriksson LI, Kramer JH, Leung JM, Miller BL, Seeley WW, Vacas S, Weiner MW, Yaffe K, Young WL, Xie Z, Maze M (2011) Perioperative cognitive decline in the aging population. Mayo Clin Proc 86:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta T, Porro C, Calvello R, Panaro MA (2014) Biological role of Toll-like receptor-4 in the brain. J Neuroimmunol 268:1–12. [DOI] [PubMed] [Google Scholar]
- Vasconcelos AR, Yshii LM, Viel TA, Buck HS, Mattson MP, Scavone C, Kawamoto EM (2014) Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J Neuroinflammation 11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker P, Nunes PV, Cabrita H, Forlenza OV (2006) Post-operative delirium is associated with poor cognitive outcome and dementia. Dement Geriatr Cogn Disord 21:221–227. [DOI] [PubMed] [Google Scholar]
- Wan Y, Xu J, Meng F, Bao Y, Ge Y, Lobo N, Vizcaychipi MP, Zhang D, Gentleman SM, Maze M, Ma D (2010) Cognitive decline following major surgery is associated with gliosis, beta-amyloid accumulation, and tau phosphorylation in old mice. Crit Care Med 38:2190–2198. [DOI] [PubMed] [Google Scholar]
- Wang P, Velagapudi R, Kong C, Rodriguiz RM, Wetsel WC, Yang T, Berger M, Gelbard HA, Colton CA, Terrando N (2020) Neurovascular and immune mechanisms that regulate postoperative delirium superimposed on dementia. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H (2012) Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A 109:6325–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JA, Wilund KR, Martin SA, Kistler BM (2012) Exercise, inflammation and aging. Aging Dis 3:130–140. [PMC free article] [PubMed] [Google Scholar]
- Yang H, Tracey KJ (2010) Targeting HMGB1 in inflammation. Biochim Biophys Acta 1799:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cui F, Chen H, Zhang T, Yang K, Wang Y, Jiang Z, Rice KC, Watkins LR, Hutchinson MR, Li Y, Peng Y, Wang X (2018) Dissecting the Innate Immune Recognition of Opioid Inactive Isomer (+)-Naltrexone Derived Toll-like Receptor 4 (TLR4) Antagonists. J Chem Inf Model 58:816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Hippocampal gene expression of IL-1β (A), IL-6 (B), TNFα (C), HMGB1 (D), NLRP3 (E), TLR2 (F), and TLR4 (G) in aged, laparotomized, (−)morphine-treated rats compared to controls 4 weeks post-surgery. Error bars represent S.E.M.
Figure S2. Hippocampal gene expression of IL-1β (A), IL-6 (B), TNFα (C), HMGB1 (D), NLRP3 (E), TLR2 (F), and TLR4 (G) in aged, laparotomized, (−)morphine-treated rats compared to controls 8 weeks post-surgery. Error bars represent S.E.M.