Abstract
Objective:
The association of renal dysfunction with tests of cognition in type 2 diabetes has been examined in individuals with moderate and advanced renal disease. Here we examine the association of renal dysfunction with tests of cognition in a cohort of middle-aged adults with short duration diabetes (mean 4.0±2.8 years).
Methods:
Baseline data were examined from the Glycemia Reduction Approaches in Diabetes (GRADE) study (n=4998). Renal dysfunction was defined by the presence of albumin in the urine or by estimated glomerular filtration rate (eGFR). Cognition was assessed with the Spanish English Verbal Learning Test, Letter and Animal fluency tests, and the Digit Symbol Substitution Test.
Results:
Participants with albuminuria or eGFR <60 ml/minute/1.73m2 had significantly lower test scores of information processing speed and perception, executive function and ability to categorize information, and of verbal learning and memory compared to participants without renal disease. Adjustment for hypertension, dyslipidemia, and waist circumference attenuated many of these findings but markers of impaired learning and executive function continued to remain lower in association with higher urine albumin levels.
Conclusion:
In type 2 diabetes of short duration, there are already subtle deficiencies in markers of cognition in association with renal disease in middle aged adults.
Keywords: albuminuria, reduced eGFR, cognitive impairment, diabetes, insulin resistance
INTRODUCTION
Individuals with type 2 diabetes are at an increased risk of having or developing cognitive impairment compared with people without diabetes (1–2). Depending on the duration of disease and the methods used to assess cognition, the risk of cognitive dysfunction may be increased several-fold (3). In addition to kidney disease (4), the causes of cognitive decline in patients with type 2 diabetes are manifold, including advanced age (5), hypertension (6), dyslipidemia (7), insulin resistance (8), adiposity (9), prevalent cardiovascular disease (CVD) (10), and hyperglycemia (11).
In the general population, an association between reduced estimated glomerular filtration rate (eGFR) and cognitive function has been reported (12, 13). Likewise, there appears to be an increased prevalence and risk of cognitive impairment with albuminuria (urine albumin/creatinine ratio [UACR] ≥30 mg/g) (14, 15). In middle-aged and older people with type 2 diabetes, the presence of kidney disease is especially relevant, since 25% of the diabetic population is estimated to have eGFR <60 mL/min/1.73m2 and 35% to have albuminuria by age ≥60 years (16).
To date, most studies of the association of reduced eGFR and albuminuria with cognitive impairment in type 2 diabetes have been carried out in individuals with longstanding disease (1–2) who are affected by other co-morbidities. This makes it difficult to ascertain the separate role of decreased eGFR and albuminuria in the pathogenesis of early cognitive impairment in type 2 diabetes. Since risk factors for cognitive impairment in type 2 diabetes may be more readily identified among individuals early in disease course, we examined the cross-sectional association between markers of renal dysfunction and cognitive function in the Glycemia Reduction Approaches in Diabetes (GRADE): A Comparative Effectiveness Study (17). This study examined the hypothesis that cognitive performance is lower in GRADE participants with increased UACR or lower eGFR.
METHODS
GRADE is a comparative effectiveness study examining the impact of 4 classes of glucose-lowering agents: insulin (glargine), a DPP4 inhibitor (sitagliptin), a sulfonylurea (glimepiride) and a GLP-1 agonist (liraglutide), in combination with metformin (17). Eligibility criteria included a diagnosis of type 2 diabetes <10 years duration; diabetes diagnosed at age ≥30 years in non-American Indian (AI)/Alaska Native (AN) patients, or age ≥20 for AI/AN; taking metformin monotherapy (at least 1,000 mg/day); HbA1c 6.8–8.5% at randomization; and willingness to take a second glucose-lowering medication, including daily injections of insulin. Key exclusion criteria included evidence of type 1 or secondary forms of diabetes; use of other diabetes medications within 6 months prior to study entry; intolerance or allergy to any of the proposed study medications or sulfa drugs; estimated glomerular filtration rate (eGFR) <30 mL/min/1.73m2; major cardiovascular event within the previous year; history of pancreatitis; heart failure (New York Heart Association Functional Classification ≥III); diagnosis or treatment of cancer (other than non-melanoma skin cancer) within 5 years; planned bariatric surgery; or planned pregnancy for women.
Study medications were randomly assigned and added to metformin (minimum 1000 mg to maximum 2000 mg per day). The primary metabolic outcome in the ongoing study is the time to primary treatment failure defined as an A1C>7.0%, subsequently confirmed, over an anticipated mean observation period of 4.8 (range 4–7) years. At baseline, participants underwent a 2-hour OGTT test with blood drawn every 30 minutes for plasma insulin and glucose levels. Baseline clinical examinations included medical and medications history, assessment of body weight and BMI, blood pressure, fasting serum lipids and ECG.
Tests of Cognition:
Four tests of cognitive function were performed at baseline. Quality of testing was ensured through training, certification, and monitoring of the staff administering the tests during the clinic visit. Test details appear in the Appendix, Table 1. The measure of memory and learning was the Spanish English Verbal Learning Test (SEVLT). The Digit Symbol Substitution Test (DSST) is a measure of processing speed, working memory, visuospatial processing, and attention. Two tests of Verbal Fluency were performed: a semantic test, which assesses the ability to concentrate and organize data, and a phonemic test which tests sounds from an endogenous mental glossary. Tests were conducted in English or Spanish.
Table 1:
Baseline characteristics of the GRADE cohort categorized by the presence or absence of albuminuria (≥ or < 30 mg albumin/gram creatinine) and eGFR ≥ or < 60 ml/minute/1.73 m2). Categorical variables are summarized as N (%). Continuous variables are summarized as mean ± SD or median [quartiles].
| ALBUMINURIA |
eGFR |
|||||
|---|---|---|---|---|---|---|
| <30 4210 (84.2%) |
≥30 788 (15.8%) |
P | ≥60 4873 (97.5%) |
<60 125 (2.5%) |
P | |
| Demographics | ||||||
| Sex (female, %) | 37.5 | 30.8 | <0.001 | 36.3 | 40.20 | 0.45 |
| Age (years) | 57.0 ± 9.9 | 57.6 ± 10.2 | 0.13 | 56.9 ± 9.9 | 67.6 ± 7.3 | <0.001 |
| Education (%) | 0.49 | 0.92 | ||||
| Less than high school | 7.0 | 8.4 | 7.2 | 6.4 | ||
| High school | 20.7 | 19.3 | 20.5 | 20.8 | ||
| College or more | 72.3 | 72.3 | 72.3 | 72.8 | ||
| Current smoking (%) | 12.9 | 18.4 | <0.001 | 13.9 | 11.2 | 0.47 |
| Race (%) | 0.64 | 0.01 | ||||
| African American | 20.0 | 18.1 | 19.9 | 12.0 | ||
| White | 65.8 | 65.4 | 65.5 | 76.0 | ||
| Other | 14.2 | 16.5 | 14.6 | 12.0 | ||
| Hispanic Ethnicity (%) | 761 (18.2) | 160 (20.4) | 0.17 | 909 (18.8) | 12 (9.7) | |
| Spanish used in Cognitive testing (%) | 8.6 | 10.5 | 0.10 | 9.1 | 3.2 | 0.03 |
| Diabetes | ||||||
| Duration (years), mean | 4.0 ± 2.8 | 4.1 ± 2.7 | 0.89 | 4.0 ± 2.8 | 5.0 ± 2.9 | <0.001 |
| Duration (years), median | 3.6 [1.7, 6.2] | 3.8 [1.8. 6.9] | 0.80 | 3.6 [1.7, 6.1] | 5.0 [2.5, 7.6] | <0.001 |
| HbA1c screening<8% (<64 mmol/mol) | 61.4 | 60.2 | 0.65 | 60.8 | 76.8 | 0.001 |
| HbA1c baseline <8% (<64 mmol/mol) | 79.7 | 77.4 | 0.29 | 79.1 | 86.4 | 0.06 |
| Anthropometrics | ||||||
| BMI (kg/m2) | 34.0 ± 6.6 | 35.7 ±7.5 | <0.001 | 34.3 ± 6.8 | 34.4 ± 7.0 | 0.85 |
| Waist (cm) | 111.6 ± 15.5 | 116.4 ± 16.9 | <0.001 | 112.3 ± 15.8 | 113.2 ± 13.5 | 0.48 |
| SBP (mmHg) | 127.6 ± 14.3 | 132.3 ± 16.2 | <0.001 | 128.3 ± 14.7 | 128.5 ± 16.1 | 0.89 |
| DBP (mmHg) | 77.0 ± 9.7 | 79.0 ± 10.7 | <0.001 | 77.4 ± 9.8 | 73.8 ± 11.2 | 0.001 |
| SBP>140 or HTN medication use (%) | 71.1 | 84.0 | <0.001 | 72.5 | 97.6 | <0.001 |
| Lipids (mg/dl) | ||||||
| Total cholesterol | 163.3 ± 37.4 | 166.3 ± 39.4 | 0.051 | 163.9 ± 37.8 | 158.0 ± 34.1 | 0.056 |
| HDL cholesterol | 43.7 ± 10.7 | 42.0 ± 9.7 | <0.001 | 43.4 ± 10.6 | 43.0 ± 10.0 | 0.63 |
| LDL cholesterol | 90.4 ± 31.5 | 91.2 ± 32.8 | 0.50 | 90.7 ± 31.7 | 83.3 ± 28.7 | 0.006 |
| Triglycerides | 150.3 ± 116.5 | 175.1 ± 145.3 | <0.001 | 154.2 ± 122.6 | 156.7 ± 81.7 | 0.74 |
| Cholesterol>240 or on medications (%) | 67.9 | 67.2 | 0.77 | 67.4 | 82.4 | <0.001 |
| Renal Testing | ||||||
| eGFR (ml/minute/1.73m2) | 95.0 ±16.6 | 94.1 ± 18.0 | 0.21 | 95.9 ± 15.7 | 54.6 ± 4.2 | <0.001 |
| eGFR (ml/minute/1.73m2) | 96.0 [84.0, 106.6] | 95.7 [82.3, 106.7] | 0.27 | 96.4 [85.0, 106.9] | 55.3 [53.1, 57.9] | <0.001 |
| eGFR < 60 ml/minute/1.73m2 (%) | 2.3 | 3.4 | 0.09 | 0.0 | 100.00 | <0.001 |
| ACR (mg/g) | 7.4 ± 6.6 | 120.6 ± 119.9 | <0.001 | 25.0 ± 62.6 | 36.8 ± 83.4 | 0.12 |
| ACR (mg/g) | 5.0[2.7, 9.7] | 71.3[42.4, 137.5] | <0.001 | 6.3[3.0, 16.7] | 8.4[3.7, 25.4] | 0.10 |
| ACR ≥30 mg/g (%) | 0.0 | 100.0 | <0.001 | 15.6 | 21.6 | 0.09 |
| Insulin Resistance or Sensitivity | ||||||
| Fasting glucose (mg/dl) | 150.7 ± 30.5 | 156.1 ± 32.6 | <0.001 | 151.7 ± 31.0 | 145.2 ± 27.1 | 0.008 |
| Fasting insulin (mU/L)* | 21.0 ± 14.5 | 23.7 ± 16.0 | <0.001 | 21.4 ± 14.8 | 23.2 ± 15.5 | 0.28 |
| HOMA-IR* | 0.026 ± 0.02 | 0.029 ± 0.02 | 0.001 | 0.026±0.02 | 0.028 ± 0.02 | 0.34 |
| Matsuda Index* | 2.2 ± 1.5 | 2.0 ± 1.8 | 0.008 | 2.2 ± 1.5 | 1.8 ± 0.9 | <0.001 |
| Prevalent CVD | ||||||
| History of MI (%) | 4.6 | 6.7 | 0.02 | 4.9 | 10.4 | 0.01 |
| History of Stroke (%) | 1.8 | 3.1 | 0.02 | 2.0 | 0.8 | 0.53 |
| Alcohol (%) | 0.78 | 0.93 | ||||
| Never/Occasionally | 84.9 | 86.2 | 85.0 | 87.2 | ||
| Weekly | 11.3 | 10.5 | 11.2 | 9.6 | ||
| Daily | 3.8 | 3.3 | 3.7 | 3.2 | ||
| Depression (%) | 13.4 | 12.9 | 0.76 | 13.2 | 17.6 | 0.20 |
| General Health (%) | <0.001 | 0.04 | ||||
| Good, very good, excellent | 82.3 | 76.4 | 81.2 | 88.0 | ||
| Fair, poor | 17.7 | 23.6 | 18.8 | 12.0 | ||
| Peripheral Neuropathy | ||||||
| EDIC Instrument Score High | 15.3 | 19.4 | 0.005 | 15.8 | 22.0 | 0.08 |
| MNSI Instrument Score > 2 | 26.3 | 30.4 | 0.02 | 26.7 | 36.8 | 0.02 |
| MNSI Clinical score > 2 | 40.2 | 51.9 | <0.001 | 41.7 | 56.8 | 0.001 |
not measured in 1263 participants randomized to glargine insulin
Kidney Function Tests:
Participants provided a random urine sample for measurement of albumin and creatinine. Albumin was assayed using an immunoturbidimetric method (Roche Diagnostics, Indianapolis, IN) with a coefficient of variation (CV) of 2.1 – 4.0% and urine creatinine using an enzymatic method (Roche) with a CV of 2.6% – 4.5%, from which a urine albumin-to-creatinine ratio (UACR) was calculated. Values ≥30 mg albumin/g creatinine were classified as albuminuria. At the same visit, serum creatinine was measured using the enzymatic Roche assay; eGFR was calculated using the CKD-EPI equation (18). All assays were measured using a Roche cobas c501 analyzer (Roche Diagnostics, Indianapolis, IN) at the Central Biochemistry Laboratory at the University of Minnesota.
Covariates:
Information from the baseline year was collected on demographic factors (sex, age, race), smoking status, alcohol consumption, self-perceived health, and history of cardiovascular disease. Weight, height, waist circumference, and seated blood pressure were measured. Body mass index (kg/m2; BMI) was calculated. A quality of life questionnaire was administered. A positive response to the questions “are you depressed?” or “do you use of anti-depression medications?” were considered a history of depression. Insulin and C-peptide were measured in EDTA plasma on the Roche cobas e601 immunoassay analyzer using a sandwich immunoassay (Roche Diagnostics, Indianapolis, IN 46250). Insulin method CVs were 2.3 – 3.1%; and c-peptide method CVs were 2.6 – 3.4%. Insulin was not measured in the 1263 participants randomized to the glargine group. Measures of insulin resistance and sensitivity were derived from the OGTT tests: HOMA-IR and the Matsuda index, respectively (19). Prevalence of coronary artery disease (CAD) and stroke were tallied. Peripheral neuropathy was assessed by the modified Michigan Neuropathy Screening Instrument (MNSI) (20). Last, because tests of cognition were performed in Spanish and English among the 921 self-identified Hispanics (51.6% [n=475] took the tests of cognition in English and the rest in Spanish) we further examined the association of renal function and tests of cognition between Hispanics and non-Hispanics.
Statistical Methods:
Baseline characteristics of the cohort are presented by the presence or absence of albuminuria (≥30 mg albumin/g creatinine) and by eGFR below or ≥ 60 ml/minute/1.73m2. We considered four measures of renal dysfunction: UACR as a continuous variable; UACR dichotomized as above/equal to or below 30 ug/g; eGFR as a continuous variable; and eGFR dichotomized as above/equal to or below 60 ml/minute/1.73m2. To mitigate the effect of extreme outliers in UACR, values above the 99th percentile (460.7 ug/g) were set to 460.7, i.e. were winsorized at the 99th percentile. Analyses are presented as dichotomous variables for ease of presentation, but continuous variables are considered the primary outcomes owing to their increased power compared to dichotomous variables.
We fit separate linear regression models with each of the measures of cognition as the dependent variable and each of the four measures of renal dysfunction as the independent variable of primary interest. In the regression models with UACR as a continuous variable, the log2 of UACR was used so that its regression coefficient represents the effect of doubling in UACR. In the regression models with eGFR as a continuous variable, eGFR was rescaled so that its regression coefficient represents the effect of a decrease of 5 ml/minute/1.73m2.
For each combination of cognitive and renal dysfunction measures, we considered three regression models: Model 1 (M1) is adjusted for age, sex, race, and Hispanic ethnicity, and the interaction of sex x age; Model 2 (M2) additionally adjusted for the highest level of attained education, waist circumference, current smoking, current use of alcohol, history of stroke, elevated SBP (≥140 mmHg) or treatment for hypertension, elevated total cholesterol (>240 mg/dl) or use of anti-cholesterol medications, and depression; Model 3 (M3) additionally adjusted for HbA1c at the screening visit, plus either UACR in models with eGFR or eGFR in models with UACR. All these factors were significantly related to cognition in preliminary analysis in GRADE.
Finally, interactions between the renal dysfunction variable and sex (M vs F), age (<55 vs ≥55 years), total cholesterol >240 mg/dl or use of anti-lipid treatment (Y vs N), and age x sex categories (M vs F, age <55 vs ≥55 years) were tested one at a time in the full model (M3). If an interaction was found to be significant at the nominal 0.01 level, separate renal dysfunction effects were estimated for each of the subgroups implied by the interaction. For example, if a renal dysfunction by age and sex was statistically significant, separate estimates were obtained for men <55, men ≥55, women <55, and women ≥55 years.
The Matsuda index of insulin sensitivity was considered but not included in models since it uses fasting insulin levels, which were not available in participants randomized to the glargine group (~25% of the GRADE cohort). Exploratory analyses in the subset with complete data showed, however, little difference in model outcomes whether Matsuda index was or was not included.
To better comprehend significant differences in tests of cognition between participants with and without an impaired renal measure, we calculated differences as the number of years of chronological age between them (“years of equivalent aging”) by dividing the coefficient of the value in model 3 by the coefficient of age in the same model. An asymptotic standard error for this ratio was calculated using the delta method.
In addition to fitting the individual regression models, we performed a pooled analysis using the Wei-Lachin test (21) to assess the overall effect of renal dysfunction on cognition. In this analysis, the regression effects from all M3 models are standardized and combined into an overall 1-degree freedom test of the null hypothesis of no effect in any regression model versus the alternative hypothesis of effects in at least some of the regression models, all of them in the same direction. For M3 models with significant interactions, we obtained separate effects for each subgroup as described above and included these effects in the pooled analysis with a weight proportional to the fraction of participants in each subgroup.
Analyses were done using the R statistical package, version 3.6.0 (22).
RESULTS
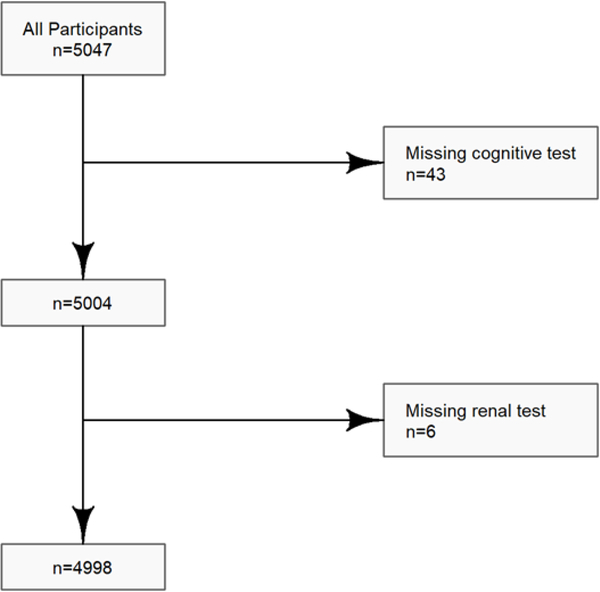
More than 99% of the GRADE cohort was included in this analysis; only 43 of the 5047 participants did not have cognitive testing and only 6 did not have renal testing (Figure 1).
Figure 1:
Analytic subset of the GRADE cohort used for this study.
Baseline characteristics of the cohort are shown in Table 1. 15.8% of participants had albuminuria. As compared with participants without albuminuria, participants with albuminuria were more likely to be male, smokers, and have higher HbA1c levels; to be heavier with greater waist circumferences, and to have higher blood pressure and lower HDL cholesterol levels. Participants with albuminuria also had higher fasting insulin and glucose levels; increased HOMA-IR levels; more history of myocardial infarction and stroke; lower perceived general health; and more peripheral neuropathy than participants without albuminuria. eGFR, duration of type 2 diabetes, and HbA1c levels at screening and baseline were the same in participants with and without albuminuria. Only ~3% of participants with albuminuria had eGFR <60 ml/minute/1.73m2 (or 0.5% of the total cohort).
Approximately 2.5% of all participants had eGFR <60 ml/minute/1.73m2. This group was characterized by older age, longer duration of type 2 diabetes, higher HbA1c levels at screening, more hypertension, lower LDL-cholesterol levels and a higher proportion with cholesterol level >240 mg/dl or using lipid lowering medications, higher fasting glucose levels, lower insulin sensitivity as measured by the Matsuda formula, and higher prevalence of myocardial infarction compared to participants with preserved eGFR levels. Interestingly, participants with diminished eGFR had better self-reported general health than participants with intact eGFR. On the other hand, participants with reduced eGFR had more peripheral neuropathy. Of the 125 participants with reduced eGFR, 27 (21.6%) had albuminuria.
Further testing (Supplementary Table 2) between Hispanics and non-Hispanics from baseline data showed statistically significant differences between groups in terms of race, education, diabetes duration, and renal testing.
In un-adjusted analyses (Table 2), participants with albuminuria and participants with eGFR <60 ml/minute/1.73m2 had statistically significant lower SEVLT and DSST values than their respective counterparts without renal impairments. There were no differences in the Word Fluency Letters test between participants with and without albuminuria or with and without decreased eGFR. Participants with reduced eGFR had significantly lower Word Fluency Animal values than those with preserved eGFR.
Table 2:
Tests of Cognition (raw values) of the GRADE cohort categorized by the presence or absence of albuminuria (≥30 or < 30 mg albumin/gram creatinine) and diminished eGFR (≥ 60 or < 60 ml/minute/1.73 m2). Significant differences are highlighted. Results are unadjusted.
| ALBUMINURIA | eGFR | |||||
|---|---|---|---|---|---|---|
| <30 4210 (84.2%) |
≥30 788 (15.8%) |
P | ≥60 4873 (97.5%) |
<60 125 (2.5%) |
P | |
| SEVLT | 34.7 + 8.1 | 34.1 ± 8.1 | 0.048 | 34.7 ± 8.1 | 32.4 ± 8.0 | 0.002 |
| DSST | 46.3 ± 13.8 | 44.9 ± 13.8 | 0.01 | 46.2 ± 13.8 | 41.3 ± 13.0 | <0.001 |
| Word Fluency – Letters | 12.5 ± 4.4 | 12.2 ± 4.4 | 0.14 | 12.4 ± 4.4 | 11.7 ± 4.7 | 0.10 |
| Word Fluency – Animals | 19.3 ± 5.4 | 19.0 ± 5.4 | 0.15 | 19.3 ± 5.4 | 18.2 ± 5.3 | 0.03 |
There were significant differences in unadjusted tests of cognition between Hispanics and non-Hispanics (Supplementary Table 2). Because of these differences as well as the renal differences, Hispanic ethnicity was included as a confounder for regression analyses.
The linear regressions of albuminuria with SEVLT and DSST after adjustment for age, sex, race, and Hispanic ethnic group (Table 3, Model 1) were statistically non-significant. Further adjustment for education, waist, current smoking, alcohol status, prevalent stroke, hypertension, hyperlipidemia and depression (Table 3, Model 2), and for screening HbA1c and eGFR (Table 3, Model 3), changed regression values very little and estimates remained statistically non-significant. All models showed albuminuria to be associated with lower cognitive values. When UACR was examined (Supplemental Table 3), a doubling of UACR was associated with a significantly lower Word Fluency Animal test (p=0.049), equivalent to 0.84 (0.00, 1.68) years of chronological aging. There was a borderline significantly lower DSST score (p=0.06), equivalent to 0.33 (−0.02, 0.69) years of aging. There were no significant interaction terms between final model outcomes (either as a dichotomous or continuous variable) with age, sex, diabetes duration, BMI, or cholesterol status.
Table 3:
Linear regression of cognition tests with albuminuria (≥ 30 mg/gram creatinine) and eGFR <60 ml/minute/1.73m2; β with 95% CI.
| SEVLT | DSST | Word Fluency Letters | Word Fluency Animal | |
|---|---|---|---|---|
| Model 1 | ||||
| ALB ≥30 | −0.06 (−0.62, 0.50) | −0.66 (−1.58, 0.26) | −0.14 (−0.47, 0.19) | −0.17 (−0.56, 0.21) |
| eGFR <60 | −1.04 (−2.35, 0.28) | −1.72 (−3.89, 0.46) | −0.46 (−1.24, 0.33) | −0.64 (−1.55, 0.27) |
| Model 2 | ||||
| ALB ≥30 | −0.19 (−0.73, 0.36 | −0.70 (−1.58, 0.16) | −0.12 (−0.45, 0.20) | −0.31 (−0.68, 0.07) |
| eGFR <60 | −1.09 (−2.37, 0.18) | −1.60 (−3.62, 0.42) | −0.40 (−1.16, 0.37) | −0.60 (−1.48, 0.28) |
| Model 3 | ||||
| ALB ≥ 30* | −0.17 (−0.72, 0.37) | −0.67 (−1.54, 0.19) | −0.12 (−0.45, 0.21) | −0.30 (−0.68, 0.72) |
| eGFR <60† | −1.03 (−2.31, 0.24) | −1.59 (−3.61, 0.43) | −0.38 (−1.15, 0.38) | −0.55 (−1.43, 0.33) |
Model 1: Adjusted for age, sex, race, Hispanic ethnicity, and an interaction term for age × sex.
Model 2: Model 1 plus education level, waist circumference, current smoking, alcohol status, history of stroke, HTN, hyperlipidemia and depression.
Model 3: Model 2 plus screening HbA1c, and either eGFR or UACR. In model 3 interaction terms were included for: sex (M vs F), age (<55 vs ≥55 years), lipid treatment and/or TC >240 (Y vs N), and by age × sex categories.
There were no significant interactions for < vs ≥ 30 mg/g creatinine.
There were no significant interactions for eGFR < vs ≥60 ml/minute/1.73m2.
An eGFR value <60ml/minute/1.73m2 was not significantly associated with any test of cognitive function (Table 3). When examined as a continuous variable (per 5 ml/minute/1.73m2 decrements) (Supplementary Table 3), eGFR decrements were also not associated with tests of cognition. In all cases lower eGFR values were not associated with significantly lower levels of tests of cognition. There were, however, two significant interactions by age and sex for SEVLT and Word Fluency Animals. In both cases, men >55 years had larger declines compared to younger men and to women of either age group.
The pooled analysis is summarized in the Supplementary Figure. Since the sex x age x eGFR interaction was nominally significant for models of SEVLT and Word Fluency Animals, separate eGFR effects are reported for the sub-groups: women <55 years, men <55 years, women ≥55 years, and men ≥55 years, for a total of 22 regression effects. Most effects are negative, i.e. corresponding to a decrease in cognitive function for an increase in renal dysfunction. Three of these effects are nominally significant at the 0.05 level, and several approach statistical significance. The pooled Wei-Lachin estimate is negative as well and statistically significant (p=0.004).
DISCUSSION
In this cross-sectional analysis of predominantly middle-aged participants, with a mean duration of type 2 diabetes of <5 years and a low burden of prevalent CVD and advanced renal disease, we found statistically significant unadjusted differences in tests of frontal lobe executive function, memory, and learning between participants with the presence of albuminuria or increasing UACR and with eGFR <60 ml/minute/1.73m2 or with decrements of 5 ml/minute/1.73m2 in eGFR versus their counterparts without these disorders.
Adjustment for demographic factors attenuated the associations of diminished eGFR on learning and memory (SEVLT), visuospatial processing and attention (DSST), and language skill (Word fluency animals), suggesting that non-vascular risk factors mediate the association of eGFR with these forms of cognition. These findings are not surprising given the relatively young age of the cohort, the mild degree of renal dysfunction (per study design), and the short duration of diabetes. Also, memory impairment is mostly affected by neurodegenerative changes (such as Alzheimer’s disease) which occurs mostly after age 65 years. In addition, diminished eGFR is caused by factors such as fibrosis (23) which may not be relevant to cognitive dysfunction. Last, low eGFR leads to cognitive impairment through metabolic abnormalities which occur only with more advanced disease than present in the current cohort. Nonetheless, despite these caveats, all associations between low eGFR in our cohort (as a dichotomous or continuous variable) were negative.
Adjustment of albuminuria for demographic factors also attenuated significant associations with tests of cognition. On the other hand, UACR (as a continuous variable) had a statistically significant negative association with Word Fluency–Animals test results, and a borderline association with DSST scores. Other tests of urine albumin with cognition were not statistically significant but remained negative. The mechanisms by which UACR could be associated with cognitive impairment were not sought here. One could hypothesize that because UACR is part of a systemic disorder of the microvasculature and is a marker of endothelial dysfunction (24), that it may be related to cerebral microvascular disease and impaired blood brain barrier structural and functional properties (25–27). The current results contrast with a prior study in which there was a strong negative association of albuminuria with DSST results (28). That finding was in an older and sicker cohort with longer duration of diabetes. Hence it is possible that UACR may initiate cognitive decline, but factors associated with increased diabetes duration enhance its effects.
Strengths of this study include a large and well-phenotyped cohort. Adjustment for candidate pathways for cognitive dysfunction were performed. Analyses were performed as dichotomous and as continuous variables. Several cognitive domains were tested. There was a low burden of prevalent CVD allowing us to analyze findings without its confounding effects. While we included participants with albuminuria in the eGFR groups, and vice versa, there was little overlap between albuminuria and diminished eGFR so that the two renal markers appeared to represent separate disease processes in this cohort. Studies of older adults with type 2 diabetes have shown that these two renal phenotypes can arise independently of one another (29). Finally, we performed pooled analyses to test whether our statistically significant outcomes occurred by chance (they did not). Limitations of this study include a cross-sectional design which does not allow for causal inference. The tests of cognition used here are exemplars of cognitive functions but are not comprehensive markers of such domains. Last, variables hypothesized to play a role in cognitive decline – e.g., oxidative stress - were not available for testing.
In conclusion, in middle aged people with early type 2 diabetes, there are subtle changes in cognitive function in association with renal dysfunction. Risk factors that associate with type 2 diabetes appear to mediate most of the associations of reduced eGFR with cognition. On the other hand, urine albumin appears to have an independent negative association with several markers of cognitive function.
Supplementary Material
BLURB FOR JDC.
The impact of renal dysfunction on cognition was examined in early diabetes.
The effect of reduced eGFR on cognition was explained by hypertension and obesity.
Albuminuria was independently associated with impaired executive function.
ACKNOWLEDGEMENTS
Support: The GRADE Study is supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health under Award Number U01-DK-098246. The planning of GRADE was supported by a U34 planning grant from the NIDDK (U34-DK-088043). The American Diabetes Association supported the initial planning meeting for the U34 proposal. The National Heart, Lung, and Blood Institute and the Centers for Disease Control and Prevention also provided funding support. The Department of Veterans Affairs provided resources and facilities. Additional support was provided by grant numbers P30 DK017047, P30 DK020541-44, P30 DK020572, P30 DK072476, P30 DK079626, P30 DK092926, U54 GM104940, UL1 TR000439, UL1 TR000445, UL1 TR001108, UL1 TR001409, UL1 TR001449, UL1 TR002243, UL1 TR002345, UL1 TR002378, UL1 TR002489, UL1 TR002489, UL1 TR002529, UL1 TR002535, UL1 TR002537, and UL1 TR002548. Educational materials have been provided by the National Diabetes Education Program. Material support in the form of donated medications and supplies has been provided by Becton, Dickinson and Company, Bristol-Myers Squibb, Merck, NovoNordisk, Roche Diagnostics, and Sanofi. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Guarantor: JIB and NY are the guarantors of this work and as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of Interest: RPB reports grants from National Institute of Diabetes and Digestive and Kidney Diseases, NIH (U34 DK088043 and U01 DK098246) during the conduct of the study; grants from Astra Zeneca, personal fees from Novo Nordisk, Bayer, and Boehringer Ingelheim outside the submitted work. ES reports other support from MannKind, Zucara, ABIM, Web MD, Sanofi, and grants and other support from Lilly, outside the submitted work. JAL reports personal fees from vTV Therapeutics, and other support from Wolters Kluwer, outside the submitted work. JIB, HF, NY, and CFY have nothing to disclose.
Footnotes
The authors of this study have no conflicts of interest regarding its contents
Prior presentation: There has been no prior presentation of this material.
REFERENCES
- 1.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia. 2005; 48 (12):2460–2469. [DOI] [PubMed] [Google Scholar]
- 2.Moheet A, Mangia S, Seaquist ER. Impact of diabetes on cognitive function and brain structure. Ann N Y Acad Sci. 2015; 1353: 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biessels GJ, Staekenborg S, Brunner E, Scheltens P. Risk of dementia in diabetes mellitus: a systemic review. Lancet Neurol. 2006; 5 (2): 64–74 [DOI] [PubMed] [Google Scholar]
- 4.Drew DA, Weiner DE, Sarnak MJ. Cognitive Impairment in CKD: Pathophysiology, Management, and Prevention. Am J Kidney Dis. 2019. December; 74(6):782–790. doi: 10.1053/j.ajkd.2019.05.017. Epub 2019 Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009; 30(4): 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker KA, Power MC, Gottesman RF. Defining the Relationship Between Hypertension, Cognitive Decline, and Dementia: a Review. Curr Hypertens Rep. 2017. March; 19 (3):24 Doi: 10.1007/s11906-017-0724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panza F, D’Introno A, Colacicco AM, Capurso C, Pichichero G, Capurso SA, Capurso A, Solfrizzi V. Lipid metabolism in cognitive decline and dementia. Brain Res Rev. 2006; 51(2):275–292 [DOI] [PubMed] [Google Scholar]
- 8.Ekblad LL, Rinne JO, Puukka P, Laine H, Ahtiluoto S, Sulkava R, Viitanen M, Jula A. Insulin resistance predicts cognitive decline: an 11-year follow-up of a nationally representative adult population sample. Diabetes Care. 2017; 40 (6):751–758. [DOI] [PubMed] [Google Scholar]
- 9.Luchsinger JA, Biggs ML, Kizer JR, Barzilay J, Fitzpatrick A, Newman A, Longstreth WT, Lopez O, Siscovick D, Kuller L. Adiposity and cognitive decline in the cardiovascular health study. Neuroepidemiology. 2013; 40 (4):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feinkohl I, Keller M, Robertson CM, Morling JR, Williamson RM, Nee LD, McLachlan S, Sattar N, Welsh P, Reynolds RM, Russ TC, Deary IJ, Strachan MW, Price JF; Edinburgh Type 2 Diabetes Study (ET2DS) Investigators. Clinical and subclinical macrovascular disease as predictors of cognitive decline in older patients with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2013; 36(9): 2779–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feinkohl I, Keller M, Robertson CM, Morling JR, McLachlan S, Frier BM, Deary IJ, Strachan MW, Price JF. Cardiovascular risk factors and cognitive decline in older people with type 2 diabetes. Diabetologia. 2015; 8(7): 1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaffe K, Falvey C, Hamilton N, Schwartz AV, Simonsick EM, Satterfield S, Cauley JA, Rosano C, Launer LJ, Strotmeyer ES, Harris TB. Diabetes, Glucose Control, and 9-Year Cognitive Decline Among Older Adults Without Dementia. Arch Neurol. 2012; 69 (9):1170–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, Satterfield S, Ayonayon H, Yaffe K. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005; 16 (7):2127–2133. [DOI] [PubMed] [Google Scholar]
- 14.Barzilay JI, Fitzpatrick AL, Luchsinger J, Yasar S, Bernick C, Jenny NS, Kuller LH. Albuminuria and dementia: a community study. Am J Kid Dis. 2008; 52 (2): 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barzilay J, Gao P, O’Donnell M, Mann JF, Anderson C, Fagard R, Probstfield J, Dagenais GR, Teo K, Yusuf S; ONTARGET and TRANSCEND Investigators. Albuminuria and decline in cognitive function: The ONTARGET / TRANSCEND studies. Arch Intern Med. 2011; 171 (2): 142–150. [DOI] [PubMed] [Google Scholar]
- 16.Garg AX, Kiberd BA, Clark WF, Haynes RB, Clase CM. Albuminuria and renal insufficiency prevalence guides population screening: results from the NHANES III. Kidney Int. 2002; 61(6):2165–2175. [DOI] [PubMed] [Google Scholar]
- 17.Nathan DM, Buse JB, Kahn SE, Krause-Steinrauf H, Larkin ME, Staten M, Wexler D, Lachin JM; GRADE Study Research Group. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013; 36 (8):2254–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, Warnock DG, Wen CP, Coresh J, Gansevoort RT, Hemmelgarn BR, Levey AS; Chronic Kidney Disease Prognosis Consortium. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012; 307(18): 1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furugen M, Saitoh S, Ohnishi H, Akasaka H, Mitsumata K, Chiba M, Furukawa T, Miyazaki Y, Shimamoto K, Miura T. Matsuda-DeFronzo insulin sensitivity index is a better predictor than HOMA-IR of hypertension in Japanese: the Tanno-Sobetsu study. J Hum Hypertens. 2012; 26(5):325–333. [DOI] [PubMed] [Google Scholar]
- 20.Herman WH, Pop-Busui R, Braffett BH, Martin CL, Cleary PA, Albers JW, Feldman EL; DCCT/EDIC Research Group. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012; 29(7): 937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lachin JM. Applications of the Wei-Lachin multivariate one-sided test for multiple outcomes on possibly different scales. PLoS One. 2014. October 17;9(10): e108784. doi: 10.1371/journal.pone.0108784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- 23.Humphreys BD. Mechanisms of Renal Fibrosis. Annu Rev Physiol. 2018; 80: 309–326. [DOI] [PubMed] [Google Scholar]
- 24.Stehouwer CDA. Microvascular Dysfunction and Hyperglycemia: A Vicious Cycle With Widespread Consequences. Diabetes 2018: 67(9):1729–1741. [DOI] [PubMed] [Google Scholar]
- 25.Christensen PK, Hommel EE, Clausen P, Feldt-Rasmussen B, Parvin HH. Impaired autoregulation of the glomerular filtration rate in patients with nephropathies. Kid Int. 1999; 56 (4):1517–1523. [DOI] [PubMed] [Google Scholar]
- 26.Ding J, Strachan MW, Fowkes FG, Wong TY, Macgillivray TJ, Patton N, Gardiner TA, Deary IJ, Price JF. Association of retinal arteriolar dilatation with lower verbal memory: the Edinburgh Type 2 Diabetes Study. Diabetologia. 2011; 54(7):1653–1662. [DOI] [PubMed] [Google Scholar]
- 27.Bůžková P, Barzilay JI, Fink HA, Robbins JA, Cauley JA, Fitzpatrick AL. Ratio of urine albumin to creatinine attenuates the association of dementia with hip fracture risk. J Clin Endocrinol Metab. 2014; 99(11):4116–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barzilay JI, Lovato JF, Murray AM, Williamson J, Ismail-Beigi F, Karl D, Papademetriou V, Launer LJ. Albuminuria and cognitive decline in people with diabetes and normal renal function. Clin J Am Soc Nephrol. 2013; 8(11):1907–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klimontov VV, Korbut AI. Albuminuric and Non-Albuminuric Patterns of Chronic Kidney Disease in Type 2 Diabetes. Diabetes Metab Syndr 2019; 13 (1): 474–479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.