Abstract
The entorhinal cortex is subdivided into anterolateral (alERC) and posteromedial (pmERC) subregions, which are theorized to support distinct cognitive roles. This distinction is particularly important as the alERC is one of the earliest cortical regions affected by Alzheimer’s pathology and related neurodegeneration. The relative associations of alERC/pmERC with neuropsychological test performance have not been examined. We examined how alERC/pmERC volumes differentially relate to performance on 1) the ModRey, a verbal memory test designed to assess memory in normal/pre-clinical populations, 2) the Montreal Cognitive Assessment (MoCA), and 3) the National Alzheimer’s Coordinating Center (NACC) neuropsychological battery. We also examined whether alERC/pmERC volumes correlate with Alzheimer’s disease cerebrospinal fluid biomarkers. In 65 cognitively healthy (CDR = 0) older adults, alERC, but not pmERC volume was associated with ModRey memory retention. Only alERC volume differentiated between participants who scored above and below the MoCA cutoff score for impairment; evaluating the MoCA subdomains revealed that alERC was particularly associated with verbal recall. On the NACC battery, both alERC and pmERC volumes were associated with Craft story recall and Benson figure copy, but only alERC volume was associated with Craft story retention and semantic fluency. Neither alERC nor pmERC volume correlated with CSF levels of amyloid or tau, and regression analyses showed that alERC volume and CSF amyloid levels were independently associated with ModRey retention performance. Taken together, these results suggest that the alERC is important for memory performance, and that alERC volume differences are related to a pattern of neuropsychological test performance (i.e. impairments in episodic memory and semantic fluency) typically seen in clinical AD.
Keywords: entorhinal cortex, memory, neuropsychology, amyloid, aging
Introduction
The entorhinal cortex (ERC) is a critical brain region that connects the hippocampus to cortex (Duvernoy et al., 2013; Witter et al., 2000) and supports memory processes (Coutureau and Di Scala, 2009). Functional connectivity studies suggest the ERC can be subdivided into two subfields: the anterolateral entorhinal cortex (alERC), and the posteromedial entorhinal cortex (pmERC) (Maass et al., 2015; Navarro Schröder et al., 2015; however, see also Doan et al., 2019). How the ERC subfields differentially support ERC-dependent mnemonic processes has not been clearly addressed. This question is particularly important as Alzheimer’s disease (AD)-related tau pathology appears earliest in the alERC (Braak and Braak, 1991; Khan et al., 2014), and PET imaging studies show that tau deposition matches the pattern of neurodegeneration in AD (Ossenkoppele et al., 2016). In healthy older adults, ERC volume differences are associated with the combined presence of abnormal levels of AD cerebrospinal fluid (CSF) amyloid and tau biomarkers (Desikan et al., 2011), which in turn are related to differences in cognition (Desikan et al., 2012). Further, larger alERC, but not pmERC, volumes are related to abnormal CSF tau and amyloid levels in AD patients (Holbrook et al., 2019), as well as to better performance on the MoCA, a short assessment for cognitive decline (Olsen, Yeung et al., 2017).
The “posterior medial, anterior temporal” (PMAT) model suggests that the alERC is involved with learning about/representing concepts/items, whereas the pmERC is involved with constructing/applying contextual models (Ranganath and Ritchey, 2012; Ritchey et al., 2015; however, see also Nilssen et al., 2019 and Wang et al., 2020 for different interpretations of the cognitive functions of the ERC subfields). Experimental studies suggest that the alERC is involved in remembering and distinguishing similar visual objects (Berron et al., 2018; Reagh and Yassa, 2014; Schultz et al., 2012), processing spatial properties of objects (Tsao et al., 2013; Wilson et al., 2013; Yeung et al., 2019b, 2017), and encoding temporal information in episodic memory (Bellmund et al., 2019; Montchal et al., 2019; Tsao et al., 2018). In contrast, direct recording experiments suggest that the pmERC is important for spatial representation/navigation (Hafting et al., 2005; Jacobs et al., 2013; Killian et al., 2015, 2012; Meister and Buffalo, 2018; Sargolini et al., 2006; Solstad et al., 2008).
ERC volume loss is associated with verbal memory performance decline in both community samples (Gicas et al., 2019; Hays et al., 2019) and AD patients (Di Paola et al., 2007). A retrospective study of temporal lobe resection in epilepsy patients shows that the amount of ERC removed scales with decline in verbal memory (Liu et al., 2017), and smaller ERC volume is associated with worse verbal delayed recall in mild cognitive impairment (Guzman et al., 2013). Longitudinal ERC shrinkage is associated with decline in story and word recall (Stoub et al., 2010). Additionally, experimental work with tau PET tracers show that selective deposition of tau in the ERC/hippocampus and ERC atrophy are related to poorer episodic memory, including delayed verbal memory (Knopman et al., 2019; Maass et al., 2018).
Given the association between ERC volume and verbal memory, are alERC/pmERC volumes differentially related to aspects of verbal recall? The PMAT model would suggest that the alERC’s role in item memory underlies verbal memory, and the pmERC’s role in context memory also supports verbal recall. The Modified Rey Auditory Learning Test (“ModRey”, Hale et al., 2017) is a verbal memory test designed to be more sensitive to individual differences among preclinical/non-clinical populations than standard neuropsychological instruments. We previously showed that ModRey retention is specifically related to ERC cerebral blood volume (Brickman et al., 2014), which is related to subsequent conversion to AD (Khan et al., 2014), and is correlated with cerebrospinal fluid AD biomarkers in older adults without dementia (Yeung et al., 2019a).
This study addressed four questions. First, how do alERC and pmERC volumes in cognitively healthy older adults independently relate to verbal memory? Second, building on our previous work (Olsen, Yeung et al., 2017), how do alERC and pmERC volume relate to performance on the MoCA, a multi-domain screening instrument for cognitive impairment, particularly when accounting for differences in the hippocampal subfield volumes? Third, do the relationships of alERC and pmERC volumes with memory generalize to other cognitive domains, and do they match a prototypical AD cognitive profile? Fourth, how do alERC and pmERC volumes relate to AD biomarkers, and how do ERC subfield volumes and AD biomarkers interact in their effects on cognition? We hypothesized that 1) larger alERC volume is related to better ModRey verbal memory performance, 2) larger alERC volume is related to better MoCA performance, 3) larger alERC volume is related to better performance on standard clinical neuropsychological instruments that track with clinical AD progression, including episodic memory and semantic fluency, and 4) larger ERC subfield volumes are related to lower CSF tau biomarker levels.
Methods
Participants
Sixty-five older adults without cognitive impairment (CDR = 0) who were enrolled in the Columbia University Alzheimer’s Disease Research Center (ADRC) were included in the study. Participant demographic data are shown in Table 1. Participants were screened for neurological disease, as well as contraindications for MRI and gadolinium injection. All participants gave informed consent to participate in this study. The Columbia University Institutional Review Board approved all procedures used in this study. We recently reported data on a subset of these participants (Yeung et al., 2019a) with different analyses.
Table 1:
Participant Demographics
| Mean | Standard Deviation | Range | |
|---|---|---|---|
| Age | 70.5 | 7.1 | 56–93 |
| Education (Years) | 16.6 | 2.0 | 12–20 |
| Gender | 32 Women / 33 Men | ||
| Race/Ethnicity | 50 Non-Hispanic White (76.9%) 11 African American (16.9%) 2 Asians/Pacific Islander (3.1%) 2 Hispanic White (3.1%) |
||
| MoCA | 26.5 | 2.3 | 20–30 |
| Craft Story Immediate | 22.6 | 6.1 | 7–33 |
| Craft Story Delayed | 19.8 | 6.0 | 4–33 |
| Craft Story Retention | 0.878 | 0.164 | 0.571–1.500 |
| Digit Span Forwards | 7.0 | 1.3 | 4–9 |
| Digit Span Backwards | 5.3 | 1.5 | 2–8 |
| Trails A | 35.0s | 19.1s | 15s-148s |
| Trails B | 89.1s | 51.7s | 33s-300s |
| MINT | 30.1 | 1.9 | 22–32 |
| Semantic Fluency | 36.8 | 8.1 | 20–66 |
| Verbal Fluency | 30.7 | 7.4 | 17–46 |
| Benson Figure Copy | 16.1 | 1.0 | 14–17 |
| Benson Figure Delayed | 11.9 | 2.8 | 4–17 |
Structural Image Acquisition
Structural magnetic resonance images were acquired on a 3T GE MR750 scanner using a 32-channel head coil (56 subjects), and a 3T GE Signa Premier scanner using a 48-channel head coil (9 subjects) due to a scanner upgrade. There were no systematic differences in brain volume across the two scanners: total ERC volume (M = 1516.67 mm3, SD = 216.57 mm3 for the GE MR750, M = 1522.12 mm3, SD = 126.38 mm3 on the GE Signa Premier, t(63) = −0.073, p = 0.942), alERC volume (M = 1169.29 mm3, SD = 187.89 mm3 on the GE MR750, M = 1206.89 mm3, SD = 110.19 mm3 on the GE Signa Premier, t(63) = −0.582, p = 0.563), pmERC volume (M = 347.39 mm3, SD = 66.64 mm3 on the GE MR750, M = 315.24 mm3, SD = 57.77 mm3 on the GE Signa Premier, t(63) = 1.365, p = 0.177). Participants received a T1-weighted magnetization-prepared, rapid acquisition with gradient echo image (MPRAGE) whole-brain anatomical scan (TE/TR=2.63 ms/2000ms, 176 sagittal slices perpendicular to the AC-PC line, flip angle = 12°, voxel size=1×1×1 mm). The T1-weighted MPRAGE scan was used to obtain measures of brain and head size, and for slice placement during the acquisition of a subsequent high-resolution T2-weighted scan in an oblique-coronal plane, perpendicular to the hippocampal long axis (TE/TR = 68ms/3000ms, 40 slices, flip angle = 125°, voxel size = 0.43×0.43×2 mm). For the high-resolution T2-weighted scan, the first slice was placed anterior to the first appearance of the collateral sulcus, and the last slice placed posterior to the hippocampal tail, to ensure full coverage of the entire hippocampus and MTL cortices.
Manual Entorhinal Cortex Subregion Segmentation
Manual segmentation of the ERC subfields was performed on the T2-weighed images (0.4×0.4mm in plane), in the participants’ native space, on the oblique coronal plane, perpendicular to long axis of the hippocampus. A single rater (L.-K.Y.) manually delineated the ERC and subdivided it into alERC and pmERC subregions in FSLView (FMRIB, Oxford, UK). The segmentation protocol used in this study was previously described in Olsen, Yeung et al. (2017).
The ERC was defined following the protocol described by Insausti et al. (1998) based on histological study. The subdivision of the entorhinal cortex into alERC and pmERC was adapted from the protocol of Maass et al. (2015), based on functional connectivity between the entorhinal cortex and the perirhinal and parahippocampal cortices respectively. The two protocols were combined by first defining the entire ERC based on the Insausti protocol, and subsequently subdividing the ERC into alERC and pmERC subfields using the Maass protocol. Moving anterior to posterior, the ERC appears at the level of the frontal-temporal junction. The pmERC appears at the superior tip of the ERC where the hippocampal head first appears, and increases in size moving posteriorly. The pmERC and alERC are equal in size approximately 2/3rds of the anterior/posterior extent of the hippocampal head, as described by Maass et al. (2015). The last slice of the ERC appears just posterior to the uncal apex. At this level, the pmERC covers the entire ERC.
As in our previous work (Olsen, Yeung et al., 2017), slight alterations were made to the Maass protocol to accommodate the thicker slices used in the current study. Further, as the Insausti protocol defines the lateral edge of the entorhinal cortex based on the depth of the collateral sulcus (as opposed to the Maass protocol, which places the lateral edge of the entorhinal cortex on the medial edge of the collateral sulcus) the entorhinal cortex subfields were extended laterally based on the Insausti protocol’s definition (see Figure 1).
Figure 1:
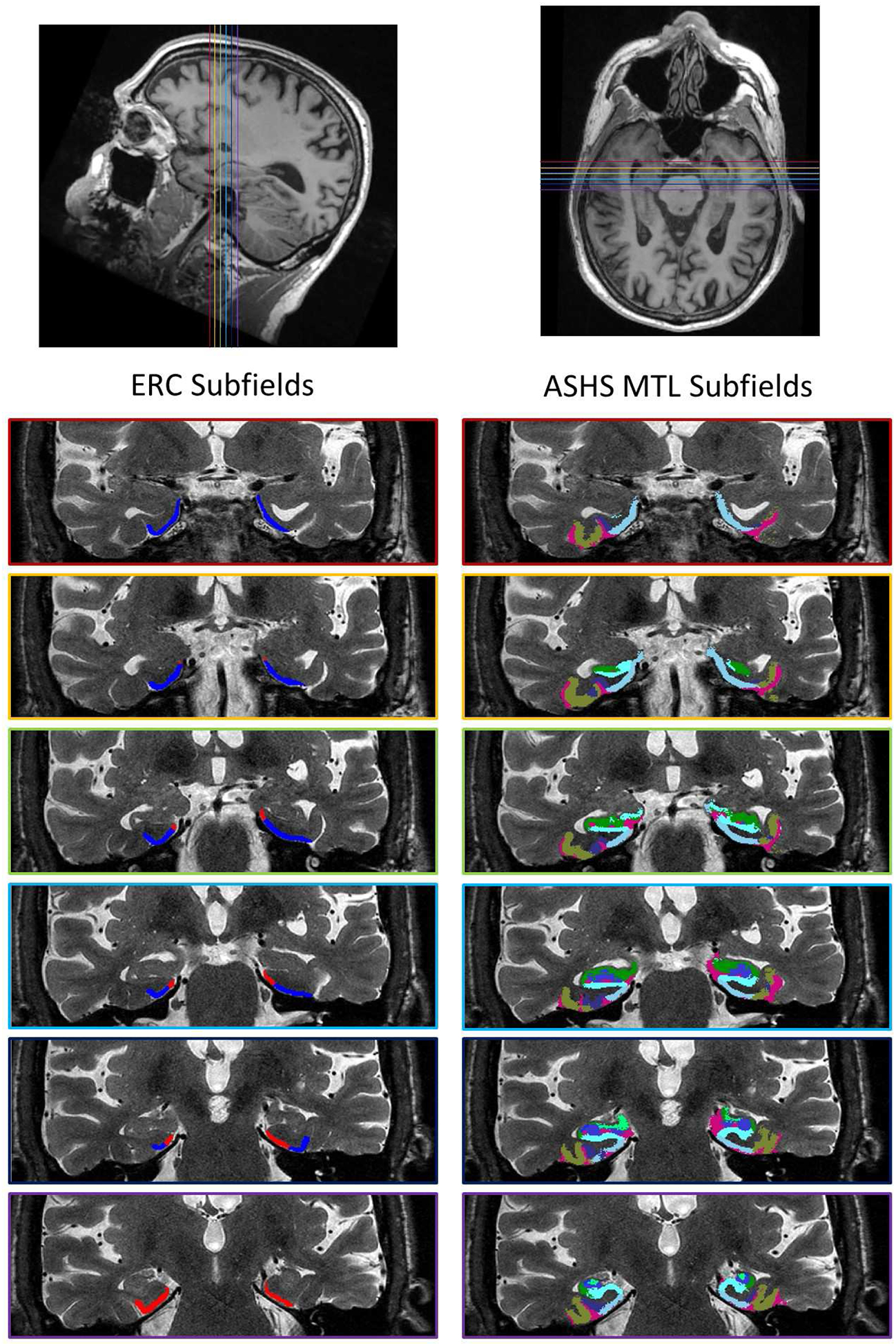
Example of manual segmentation of ERC subfields (left) and ASHS automated segmentation of hippocampal subfields/MTL regions (right), shown on coronal plane. Position of color-coded coronal slices on axial and sagittal views shown at top. In the ERC subfield segmentation, alERC indicated in blue, and pmERC indicated in red. In the ASHS hippocampal/MTL segmentation, CA1 indicated in green, dentate gyrus in blue, CA3 in light green, subiculum in light blue, BA35 indicated in violet, BA36 indicated in tan. CA2 and PHC were also measured, but are not visible on these slices. ERC (navy) and empty spaces (magenta) also shown in figure, but not used for analyses.
All entorhinal cortex volumes were corrected for head size using a regression-based method, to account for differences in brain size between participants. Estimated total intracranial volume was derived from the whole-brain T1-weighted scans using FreeSurfer (v6.0) (Buckner et al., 2004). A regression slope was obtained for each entorhinal cortex subfield in each hemisphere, by regressing the volume of that subfield with the total intracranial volume, and each individual participant’s subfield volume was corrected using that regression slope based on the difference between their intracranial volume compared to the mean intracranial volume. Corrected subfield volumes were subsequently summed across the two hemispheres, giving a single total volume for each region for each participant.
Intra-rater reliability was established by comparing the segmentation of ten randomly selected scans, after a delay of several months. Reliability was assessed using the intra-class correlation coefficient (ICC), which evaluates volume reliability (Shrout and Fleiss, 1979) and the Dice metric, which also takes spatial overlap into account (Dice, 1945), computed separately for each region in each hemisphere. Dice was derived using the formula 2*(intersecting region) / (original segmentation + repeat segmentation); a Dice overlap metric of 0 represents no overlap, while a metric of 1 represents perfect overlap. Intra-rater reliability results are shown in Table 2, and are similar to intra-rater reliability for ERC subfields in our previous work (Olsen, Yeung et al., 2017), and for manual segmentation of MTL regions more generally (Wisse et al., 2012; Yushkevich et al., 2015).
Table 2:
Intra-rater reliability for ERC subfield segmentations
| Intra-rater:Dice | Intra-rater ICC | |||
|---|---|---|---|---|
| Left | Right | Left | Right | |
| alERC | 0.82 | 0.81 | 0.86 | 0.93 |
| pmERC | 0.75 | 0.77 | 0.84 | 0.93 |
Automated Hippocampal Subfield Segmentation
Automated segmentation of the hippocampal subfields (CA1, CA2, CA3, dentate gyrus, subiculum) was performed using the Automatic Segmentation of Hippocampal Subfields (ASHS) package (Yushkevich et al., 2015) on both the T1-weighted MP-RAGE scans and the T2-weighted high resolution scans, using the Magdeburg atlas (Berron et al., 2017, see Figure 1). Note that subfields were partitioned in the hippocampal head and body sections, but not in the tail section, where the stratum radiatum lacunosum moleculare (SLRM), a strip of white matter that separates the subfields, is not visible at the 0.4×0.4mm in-plane resolution. Because it is unclear where the subfield boundaries lie in the hippocampal tail, it was excluded from the analyses in this study (see also Wisse et al., 2020 addressing concerns about segmenting hippocampal subfields where the SLRM is not visible).
ModRey
Participants were administered the ModRey (Hale et al., 2017), a test of list learning and declarative memory. Fifty-six participants were administered the ModRey on the day of MRI scanning. Of the remaining nine participants, the median delay between scanning and testing was 9 days (average delay = 43.8 days, SD = 48.1 days). Seven of the nine participants were tested within 1.5 months of scanning, and the remaining two were tested within 4 months of scanning. Cognitive performance did not differ on any measure tested in this study between participants who received the ModRey on the same day as scanning versus those who did not. The design of the ModRey is illustrated in Figure 2. In brief, participants first received three learning trials, where they were read 20 unique, semantically and phonetically unrelated words (List A), and were asked to recall these words after each trial. These trials were immediately followed by a 4th learning trial, where participants were read a distractor list of 20 additional semantically and phonetically unrelated words (List B). Subsequently, participants were asked to freely recall as many words as possible from List A (the “short delay free recall”). After an approximately 40-minute delay, during which unrelated cognitive testing occurred, participants were asked to freely recall as many words as possible from both List A and List B separately (“long delay free recall”). These trials were followed by a 66-item forced-choice recognition test where participants were asked to decide if a presented word belongs to List A; distractors came from both List B and a selection of phonetically/semantically related words. Finally, source memory was assessed by asking the participant to match words on a list to either List A or List B.
Figure 2:
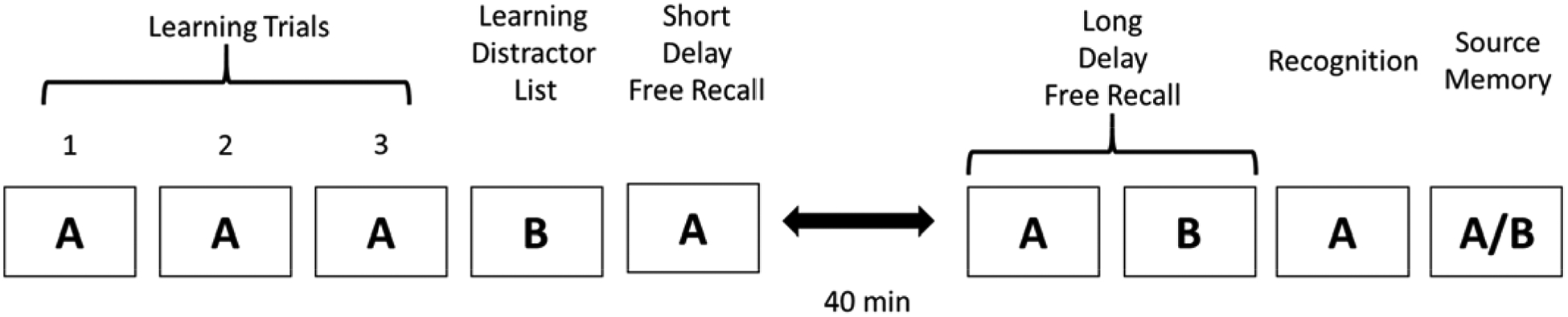
Experimental design of the ModRey verbal memory task
The primary ModRey outcome variable was the “short-delay retention” score: the ratio of the number of items from List A correctly recalled freely at the short delay, to the number of items correctly recalled freely during the last List A learning trial. This variable was chosen because it is tightly linked to function of the entorhinal cortex (Brickman et al., 2014, 2011; Fernández et al., 1999; Schon et al., 2004). Additional ModRey outcome variables analyzed in this study included total learning, short-delay free recall, long-delay free recall, recognition discrimination and source memory.
Two psychometrically similar (Hale et al., 2017) versions of the ModRey were used, each with its own unique Lists A and B. Half of the participants received one version of the ModRey, and the other half received the other. There were no differences in short-delay retention, or any of the other outcome variables between the two versions. Accordingly, all analyses collapsed across the two versions.
NACC-UDS3 Neuropsychological Battery
All of the participants received the National Alzheimer’s Coordinating Center (NACC) Uniform Data Set (UDS) neuropsychological battery, version 3 (NACC-UDS3, Weintraub et al., 2018). This is a standardized set of neuropsychological tests used by Alzheimer’s Disease Centers across the United States. The battery includes the MoCA (Nasreddine et al., 2005), Craft Story (Craft et al., 1996), Digit Span (Weschler, 1987), Semantic and Verbal Fluency (Morris et al., 1989), Trail Making Test Parts A and B (Reitan and Wolfson, 1985), Benson Complex Figure Test (Possin et al., 2011), and the Multilingual Naming Task (MINT, Gollan et al., 2012). The NACC-UDS3 is administered to all participants in the Columbia ADRC cohort annually.
Because of concerns about practice effects, the baseline performance on the entire NACC-UDS3 battery was used for each participant. Forty-two out of the 65 participants (64.6%) received the NACC-UDS3 battery for the first time within 1 year prior to scanning, and an additional 13 participants (20%) received the battery for the first time within 2 years prior scanning. No participant received the battery for the first time more than 3.5 years before the scan. There were no significant differences in performance between participants who were scanned within one year of taking the battery versus participants who were scanned before. The median delay between NACC-UDS3 testing and the scan was 103 days.
CSF Biomarkers
Cerebrospinal fluid (CSF) was obtained via lumbar puncture, performed with standard clinical research methods in aseptic fashion by a board-certified neurologist. CSF was obtained for 59 of the 65 participants in this study. CSF was always collected during a separate session from testing to avoid influencing performance on cognitive testing. Up to 15 cc of CSF was removed using a Sprotte 24G spinal needle and placed in two 12 cc polypropylene tubes. All samples were centrifuged briefly, aliquoted using polypropylene pipettes within 30 minutes, and stored for both biomarker analysis and CSF-banking at −80°C. Levels of three CSF biomarkers (Aβ42, phosphorylated tau, total tau) were analyzed in duplicate by a bead-based multiplex method using the Innogenetics Alz-Bio3 kits on a Luminex (LS-100) platform with 96-well plates. Coefficient of variation (CV) was generally less than 10% (samples with higher CV were repeat analyzed).
Statistical Analysis
ModRey performance & alERC/pmERC volumetric analyses
A multiple regression model was run to evaluate the relationship between alERC/pmERC volumes and ModRey performance. In this model, ModRey short-delay retention was the dependent variable, with alERC volume and pmERC volume as predictors, and age, years of education, gender and race/ethnicity as covariates.
These analyses were followed by additional exploratory multiple regression models that examined each of the other ModRey outcome measures (total learning, short-delay free recall, long-delay free recall, recognition discrimination and source memory) as the dependent variable, again with alERC and pmERC volume as predictors, and age, years of education, gender and race/ethnicity as covariates.
MoCA performance and alERC/pmERC volumetric analyses
To replicate analyses performed in our previous work in a separate sample (Olsen, Yeung et al., 2017), participants were divided into two groups based on their MoCA score (<26, ≥26; with 26 being the MoCA threshold score). The volumes of the alERC, pmERC, as well as the other segmented hippocampal subfields and MTL cortices were compared across these two groups using t-tests. Note that since these two groups did not differ in age, t(63) = 0.397, p = 0.693, 95% CI [−3.000, 4.488], years of education, t(63) = 1.169, p = 0.247, 95% CI [−0.433, 1.653], or gender, χ2(1, N=65) = 0.190, p = 0.663, structural volumes for these t-tests were not adjusted for age, gender or years of education.
Given that Olsen, Yeung et al. (2017) found that only alERC volume differed between the MoCA groups after correction for multiple comparisons, a multiple regression model was run with all the regional volumes as predictors, age, years of education, gender and race/ethnicity as covariates, and MoCA score as the dependent variable, with the goal of testing if alERC volume, or any other MTL/hippocampal subfield volume, was related to MoCA score after accounting for other subfield volume differences.
As a post-hoc analysis, performance on the MoCA was divided into subdomains (visuospatial/executive, naming, attention, language/abstraction, memory, and orientation). Pearson’s correlations were used to evaluate the association of each MoCA subdomain score with alERC/pmERC volumes, and age, years of education, gender and race/ethnicity as covariates.
NACC UDS-ERC subfield analyses
Exploratory multiple regression models were also used to evaluate the relationship of the alERC and pmERC (as regressors), with each of the NACC-UDS3 measures (as the dependent variable): Craft immediate recall, Craft delayed recall, Craft retention, digit span, semantic fluency, Trails A & B (and the ratio of Trails B to Trails A), Benson figure copy, Benson figure delayed recall, MINT, and verbal fluency. These multiple regression models included age, years of education, gender and race/ethnicity as covariates.
Pearson’s correlations were used to test if ERC subfield volumes correlated with three cerebrospinal fluid (CSF) AD biomarkers (Aβ−42, phospho-tau and total tau levels), or with ratios of the CSF tau biomarkers to the Aβ−42 biomarker, with age, years of education, gender and race/ethnicity as covariates. Post hoc analyses examined the correlations between hippocampal subfield volumes and CSF AD biomarker levels. Because our previous results in this sample showed that CSF amyloid was related to ModRey short-delay retention in cognitively healthy older adults (Yeung et al., 2019a), multiple regression analysis was also used to determine if CSF amyloid and alERC volume were independent predictors of ModRey performance, with age, years of education, gender and race/ethnicity as covariates.
Results
ModRey performance and alERC/pmERC volumetric analyses
The multiple regression model examining how ModRey short-delay retention performance was related to alERC and pmERC volumes showed that larger alERC volume was associated with better performance on the primary ModRey outcome measure, short-delay retention, t(58) = 2.009, p = 0.049, β = 0.258, 95% CI [0.001, 0.514], sr = 0.255 (Figure 3a). However pmERC volume was not significantly associated with ModRey performance, t(58) = 0.301, p = 0.764, β = 0.043, 95% CI [−0.243, 0.330], sr = 0.040.
Figure 3:
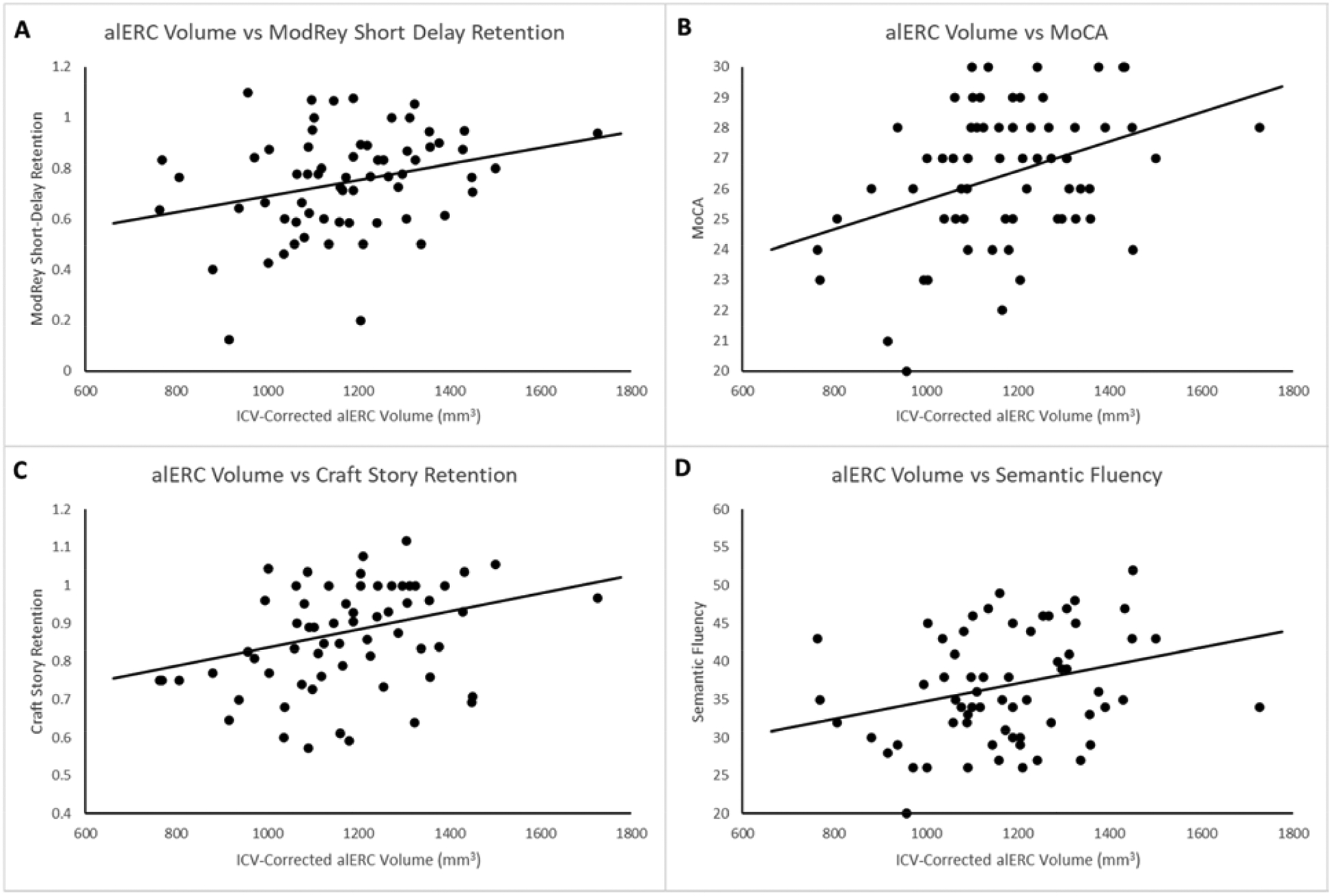
Correlation of alERC volume with a) ModRey short-delay retention, b) MoCA, c) Craft story retention, and d) semantic fluency
Exploratory multiple regression analyses examining the effects of alERC and pmERC volume on other ModRey measures showed that larger alERC volume was also significantly associated with better performance on total learning, short-delay accuracy, and number of false positive errors (Table 3), while pmERC volume was only significantly associated with the number of intrusions.
Table 3 –
alERC/pmERC volumes as predictors in multiple regression models for additional ModRey outcome variables. Each row represents an individual multiple regression model, with the specified ModRey outcome measure as the dependent variable, and alERC volume, pmERC volume, age, years of education, gender, and race/ethnicity as predictors. p < 0.05 indicated in bold
| alERC | pmERC | |||||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | t | p | β | 95% CI | t | p | |
| Total Learning | 0.276 | [0.032, 0.520] | 2.268 | 0.027 | 0.144 | [−0.129, 0.416] | 1.055 | 0.296 |
| Total Perseverations | 0.086 | [−0.157, 0.330] | 0.708 | 0.482 | −0.045 | [−0.317, 0.227] | −0.329 | 0.743 |
| Total Intrusions | 0.020 | [−0.237, 0.278] | 0.159 | 0.874 | −0.288 | [−0.576, −0.001] | −2.006 | 0.049 |
| Short-Delay Accuracy | 0.341 | [0.099, 0.583] | 2.818 | 0.007 | 0.092 | [−0.179, 0.363] | 0.680 | 0.499 |
| Long-Delay Accuracy | 0.195 | [−0.054, 0.444] | 1.567 | 0.123 | 0.119 | [−0.159, 0.397] | 0.856 | 0.396 |
| Long-Delay Retention | 0.046 | [−0.211, 0.304] | 0.360 | 0.720 | 0.085 | [−0.203, 0.373] | 0.590 | 0.557 |
| Recognition (Hits) | 0.006 | −0.257, 0.269] | 0.045 | 0.964 | 0.122 | [−0.172, 0.416] | 0.828 | 0.411 |
| Recognition (False Positives) | −0.259 | [−0.510, −0.008] | −2.064 | 0.044 | −0.071 | [−0.352, 0.209] | −0.510 | 0.612 |
| Source Memory | 0.159 | [−0.103, 0.421] | 1.215 | 0.229 | 0.122 | [−0.171, 0.415] | 0.835 | 0.407 |
MoCA performance and alERC/pmERC volumetric analyses
The mean MoCA score across all participants was 26.5 (SD = 2.3, range = 20–30). Independent samples t-tests showed that participants with MoCA scores of 26 or above (i.e. above the MoCA threshold score) had larger alERC volumes compared to those with MoCA scores below 26 (see Table 4). In contrast, the volumes of pmERC and the other measured hippocampal subfields or MTL cortical areas did not differ between the two groups.
Table 4 –
Average volumes of entorhinal cortex subfields, hippocampal subfields, and MTL cortices in participant groups divided by MoCA threshold score, and t-tests comparing volume differences between those groups, df = 63, p < 0.05 indicated in bold
| pmERC | 345.66 ± 65.34 | 337.61 ± 68.56 | [−26.75, 42.85] | 0.454 | 0.651 |
| alERC | 1211.14 ± 164.56 | 1102.86 ± 187.94 | [17.82, 198.75] | 2.254 | 0.028 |
| CA1 | 1310.35 ± 189.26 | 1271.32 ± 220.41 | [−65.83, 143.88] | 0.705 | 0.484 |
| CA2 | 60.90 ± 23.37 | 59.94 ± 21.83 | [−11.03, 12.93] | 0.162 | 0.872 |
| DG | 693.38 ± 158.88 | 694.39 ± 148.21 | [−82.41, 80.40] | 0.025 | 0.980 |
| CA3 | 244.76 ± 84.30 | 230.41 ± 60.70 | [−26.11, 54.81] | 0.786 | 0.435 |
| Subiculum | 2156.78 ± 276.51 | 2148.36 ± 240.61 | [−130.43, 147.27] | 0.127 | 0.900 |
| BA 35 | 1065.91 ± 194.73 | 1071.42 ± 253.12 | [−118.62, 107.62] | 0.089 | 0.929 |
| BA 36 | 4410.12 ± 742.56 | 4349.05 ± 831.62 | [−344.05, 466.18] | 0.289 | 0.773 |
| PHC | 735.20 ± 161.92 | 760.07 ± 173.78 | [−111.81, 62.07] | 0.556 | 0.580 |
Because the volume of MTL regions and hippocampal subfields are necessarily highly correlated (see Table 5), multiple regression analysis was performed to evaluate the specific associations of each regional volume (alERC, pmERC, BA35, BA36, CA1, CA2, CA3, dentate gyrus, subiculum) with MoCA score. Among all the regions evaluated, only alERC volume uniquely related to MoCA score positively, t(50) = 3.532, p = 0.001, β = 0.518, 95% CI [0.224, 0.813], sr = 0.417 (Figure 3b).
Table 5 –
Pearson’s correlations between the volumes of all segmented regions. df = 65 for all comparisons. p < 0.05 indicated in bold, p < 0.10 indicated in italics
| alERC | pmERC | CA1 | CA2 | DG | CA3 | Sub. | BA35 | BA36 | PHC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| alERC | r | 1 | 0.251 | 0.093 | 0.361 | 0.312 | 0.235 | 0.044 | .331 | 0.061 | 0.216 |
| p | 0.044 | 0.463 | 0.003 | 0.011 | 0.059 | 0.730 | 0.007 | 0.627 | 0.084 | ||
| pmERC | r | 0.251 | 1 | 0.376 | 0.054 | 0.106 | 0.369 | 0.445 | 0.299 | 0.042 | 0.276 |
| p | 0.044 | 0.002 | 0.669 | 0.399 | 0.002 | 0.000 | 0.016 | 0.741 | 0.026 | ||
| CA1 | r | 0.093 | 0.376 | 1 | 0.409 | 0.646 | 0.391 | 0.516 | 0.348 | 0.230 | 0.351 |
| p | 0.463 | 0.002 | 0.001 | 0.000 | 0.001 | 0.000 | 0.004 | 0.066 | 0.004 | ||
| CA2 | r | .0361 | 0.054 | 0.409 | 1 | 0.736 | 0.203 | 0.122 | 0.388 | 0.443 | 0.402 |
| p | 0.003 | 0.669 | 0.001 | 0.000 | 0.104 | 0.331 | 0.001 | 0.000 | 0.001 | ||
| DG | r | 0.312 | 0.106 | 0.646 | 0.736 | 1 | 0.173 | 0.215 | 0.368 | 0.321 | 0.401 |
| p | 0.011 | 0.399 | 0.000 | 0.000 | 0.167 | 0.085 | 0.003 | 0.009 | 0.001 | ||
| CA3 | r | 0.235 | 0.369 | 0.391 | 0.203 | 0.173 | 1 | 0.328 | 0.530 | 0.194 | 0.344 |
| p | 0.059 | 0.002 | 0.001 | 0.104 | 0.167 | 0.008 | 0.000 | 0.121 | 0.005 | ||
| Sub. | r | 0.044 | 0.445 | 0.516 | 0.122 | 0.215 | .328 | 1 | 0.487 | 0.323 | 0.185 |
| p | 0.730 | 0.000 | 0.000 | 0.331 | 0.085 | 0.008 | 0.000 | 0.009 | 0.140 | ||
| BA35 | r | 0.331 | 0.299 | 0.348 | 0.388 | 0.368 | 0.530 | .487 | 1 | 0.591 | 0.411 |
| p | 0.007 | 0.016 | 0.004 | 0.001 | 0.003 | 0.000 | 0.000 | 0.000 | 0.001 | ||
| BA36 | r | 0.061 | 0.042 | 0.230 | 0.443 | 0.321 | 0.194 | 0.323 | 0.591 | 1 | 0.156 |
| p | 0.627 | 0.741 | 0.066 | 0.000 | 0.009 | 0.121 | 0.009 | 0.000 | 0.216 | ||
| PHC | r | 0.216 | 0.276 | 0.351 | 0.402 | 0.401 | 0.344 | 0.185 | 0.411 | 0.156 | 1 |
| p | 0.084 | 0.026 | 0.004 | 0.001 | 0.001 | 0.005 | 0.140 | 0.001 | 0.216 |
As a post-hoc analysis, the association between MoCA subdomain scores (visuospatial/executive, naming, attention, language, abstraction, memory, orientation) and alERC volumes was tested using Pearson’s correlations. These analyses showed that only the memory subdomain score, which is a measure of delayed recall, was significantly positively associated with alERC volume, r(59) = 0.367, 95% CI [0.123, 0.569], p = 0.004.We note that alERC volume was also marginally correlated with the visuospatial/executive subdomain, r(59) = 0.248, 95% CI [−0.008, 0.473], p = 0.054, and the orientation subdomain, r(59) = 0.241, 95% CI [−0.016, 0.468], p = 0.061.
NACC UDS-ERC subfield analyses
Multiple regression analyses were run to examine the associations of alERC and pmERC volume with performance on each measure from the NACC-UDS3 battery. Table 6 lists the association of the ERC subfield predictors in these models. Notably, pmERC volume was significantly associated with performance on Craft story immediate recall and delayed recall. alERC volume was significantly associated with both Craft story delayed recall and retention (Figure 3c). Both subfield volumes were associated with Benson figure copy performance, while alERC volume, but not pmERC volume was significantly related to performance on a test of semantic fluency (Figure 3d).
Table 6 –
alERC & pmERC volumes as predictors in multiple regression models for performance on NACC battery tasks (with age, gender, race/ethnicity and years of education as covariates). Each row represents an individual multiple regression model, with the specified NACC-UDS3 outcome measure as the dependent variable, and alERC volume, pmERC volume, age, years of education, gender, and race/ethnicity as predictors. p < 0.05 indicated in bold, p < 0.10 indicated in italics
| alERC | pmERC | |||||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | t | p | β | 95% CI | t | p | |
| Craft Story Immediate | 0.148 | [−0.100. 0.396] | 1.194 | 0.237 | 0.369 | [0.091, 0.646] | 2.662 | 0.010 |
| Craft Story Delayed | 0.274 | [0.028, 0.519] | 2.230 | 0.030 | 0.296 | [0.021, 0.571] | 2.161 | 0.035 |
| Craft Story Retention | 0.246 | [−0.013, 0.504] | 1.899 | 0.062 | −0.009 | [−0.292, 0.274] | −0.063 | 0.950 |
| Digit Span Forwards | −0.009 | [−0.259, 0.241] | −0.071 | 0.943 | −0.111 | [−0.389, 0.166] | −0.802 | 0.426 |
| Digit Span Backwards | −0.058 | [−0.303, 0.187] | −0.477 | 0.635 | 0.116 | [−0.157, 0.390] | 0.852 | 0.398 |
| Trails A | −0.178 | [−0.428, 0.073] | −1.421 | 0.161 | 0.225 | [−0.055, 0.504] | 1.608 | 0.113 |
| Trails B | −0.065 | [−0.311, 0.182] | −0.525 | 0.601 | −0.066 | [−0.342, 0.209] | −0.481 | 0.632 |
| Trails B / Trails A | 0.041 | [−0.211, 0.292] | 0.324 | 0.747 | −0.246 | [−0.527, 0.035] | −1.756 | 0.084 |
| MINT | 0.123 | [−0.128, 0.374] | 0.980 | 0.331 | 0.007 | [−0.274, 0.289] | 0.051 | 0.959 |
| Semantic Fluency | 0.277 | [0.023, 0.530] | 2.187 | 0.033 | −0.062 | [−0.346, 0.221] | −0.441 | 0.661 |
| Verbal Fluency | 0.084 | [−0.173, 0.342] | 0.657 | 0.514 | 0.144 | [−0.143, 0.431] | 1.003 | 0.320 |
| Benson Figure Copy | 0.323 | [0.086, 0.561] | 2.721 | 0.009 | 0.257 | [−0.009, 0.523] | 1.935 | 0.058 |
| Benson Figure Delayed | 0.152 | [−0.109, 0.413] | 1.167 | 0.248 | −0.028 | [−0.320, 0.264] | −0.193 | 0.847 |
CSF biomarker analyses
Pearson’s correlations were run to explore the associations between CSF biomarkers in this participant cohort. Aβ42 levels were not correlated with phosphorylated tau levels, r(59) = 0.159, 95% CI [−0.101, 0.398], p = 0.228, but Aβ42 levels were inversely correlated with total tau, r(59) = 0.307, 95% CI [0.056, 0.522], p = 0.018. Phosphorylated tau and total tau levels were strongly correlated, r(59) = 0.656, 95% CI [0.481, 0.780], p < 0.001.
Pearson’s correlations were run to examine the relationship between ERC subfield volume and CSF biomarkers of AD. Neither alERC volume, nor pmERC volume correlated with CSF levels of Aβ−42, phospho-tau or total tau, or the ratios of the tau measures with Aβ−42 (see Table 7). Exploratory post hoc analyses using Pearson’s correlations to examine the relationship between MTL region/hippocampal subfield volumes and CSF biomarkers showed that among all the segmented regions, only the dentate gyrus volume was significantly correlated with CSF Aβ−42.
Table 7 –
Pearson’s correlations of CSF biomarker levels with alERC/pmERC volumes, controlling for age, years of education, gender, and race/ethnicity, df = 53. p < 0.05 indicated in bold, p < 0.10 indicated in italics
| Aβ42 | Phospho-Tau | Total Tau | Phospho-Tau / Aβ42 | Total Tau / Aβ42 | ||
|---|---|---|---|---|---|---|
| alERC | r | 0.055 | −0.082 | −0.145 | −0.119 | −0.171 |
| p | 0.688 | 0.553 | 0.292 | 0.387 | 0.213 | |
| 95% CI | [−0.218, 0.320] | [−0.344, 0.192] | [−0.399, 0.130] | [−0.377, 0.156] | [−0.421, 0.104] | |
| pmERC | r | 0.001 | −0.105 | −0.099 | −0.003 | −0.039 |
| P | 0.996 | 0.447 | 0.473 | 0.984 | 0.777 | |
| 95% CI | [−0.269, 0.271] | [−0.364, 0.170] | [−0.359, 0.176] | [−0.273, 0.267] | [−0.306, 0.233] | |
| CA1 | r | 0.201 | 0.120 | 0.162 | −0.056 | −0.039 |
| p | 0.141 | 0.385 | 0.237 | 0.686 | 0.778 | |
| 95% CI | [−0.073, 0.447] | [−0.155, 0.378] | [−0.113, 0.414] | [−0.321, 0.217] | [−0.306, 0.233] | |
| CA2 | r | 0.186 | 0.216 | 0.107 | −0.026 | −0.098 |
| p | 0.174 | 0.114 | 0.435 | 0.853 | 0.479 | |
| 95% CI | [−0.088, 0.434] | [−0.057, 0.459] | [−0.168, 0.366] | [−0.294, 0.246] | [−0.358, 0.176] | |
| DG | r | 0.268 | 0.181 | 0.226 | −0.202 | −0.167 |
| p | 0.048 | 0.185 | 0.097 | 0.140 | 0.224 | |
| 95% CI | [0.000, 0.502] | [−0.093, 0.430] | [−0.047, 0.467] | [−0.447, 0.072] | [−0.418, 0.108] | |
| CA3 | r | 0.055 | 0.128 | 0.167 | 0.211 | 0.185 |
| p | 0.688 | 0.351 | 0.223 | 0.122 | 0.177 | |
| 95% CI | [−0.218, 0.320] | [−0.147, 0.384] | [−0.108, 0.418] | [−0.062, 0.455] | [−0.089, 0.433] | |
| Sub. | r | −0.144 | 0.076 | −0.125 | 0.229 | 0.064 |
| p | 0.295 | 0.582 | 0.362 | 0.092 | 0.641 | |
| 95% CI | [−0.398, 0.131] | [−0.198, 0.339] | [−0.382, 0.150] | [−0.044, 0.470] | [−0.209, 0.328] | |
| BA35 | r | 0.186 | 0.128 | 0.132 | 0.009 | −0.053 |
| p | 0.174 | 0.351 | 0.336 | 0.945 | 0.699 | |
| 95% CI | [−0.088, 0.434] | [−0.147, 0.384] | [−0.143, 0.388] | [−0.261, 0.278] | [−0.318, 0.220] | |
| BA36 | r | 0.175 | 0.191 | 0.028 | −0.060 | −0.178 |
| p | 0.201 | 0.163 | 0.840 | 0.666 | 0.192 | |
| 95% CI | [−0.100, 0.425] | [−0.083, 0.438] | [−0.244, 0.296] | [−0.325, 0.213] | [−0.427, 0.096] | |
| PHC | r | −0.068 | −0.005 | −0.066 | −0.054 | −0.085 |
| p | 0.622 | 0.970 | 0.632 | 0.696 | 0.537 | |
| 95% CI | [−0.332, 0.206] | [−0.274, 0.265] | [−0.330, 0.208] | [−0.319, 0.219] | [−0.347, 0.189] |
As both alERC volume and CSF Aβ−42 (Yeung et al., 2019a) have been shown to relate to ModRey short-delay retention in this cohort, multiple regression analysis was performed to examine the independence of their contributions to ModRey short-delay retention. alERC volume and CSF Aβ−42 levels were entered as predictors for ModRey short-delay retention performance. This model showed that alERC volume, t(52) = 2.043, p = 0.046, β = 0.257, 95% CI [0.004, 0.510], sr = 0.254, and CSF Aβ−42 levels, t(52) = 2.186, p = 0.033, β = 0.345, 95% CI [0.029, 0.662], sr = 0.271 were independently associated with ModRey short delay retention, accounting for age, education, gender and race/ethnicity as covariates. Post-hoc analyses revealed no significant interaction between alERC volume and CSF Aβ−42 (i.e. no moderation), and neither variable mediated the relationship between the other and ModRey short-delay retention.
To address concerns that the presence of biomarker-positive participants might drive the observed structure-cognition associations, the multiple regression models predicting ModRey retention were repeated, excluding participants who were biomarker positive, based on previously-established CSF thresholds (Aβ−42 < 325 pg/mL, t-tau > 72 pg/mL, p-tau > 31 pg/mL, p-tau/Aβ−42 ratio < 0.1, see Yeung et al., 2019a). As shown in Table 8, excluding participants who were biomarker positive did not greatly affect the observed effect of the alERC predictor on ModRey retention.
Table 8 –
The effect of the alERC predictor in multiple regression models predicting ModRey short-delay retention in biomarker-negative only subgroups, with pmERC, age, education, gender and race/ethnicity as additional predictors. p < 0.05 indicated in bold, p < 0.10 indicated in italics
| Group | N | β | sr | t | P |
|---|---|---|---|---|---|
| All participants | 65 | 0.258 | 0.249 | 2.009 | 0.049 |
| Aβ-42 negative | 51 | 0.313 | 0.303 | 2.178 | 0.035 |
| P-tau negative | 52 | 0.244 | 0.235 | 1.685 | 0.099 |
| T-tau negative | 52 | 0.290 | 0.276 | 2.040 | 0.047 |
| P-Tau/Amyloid < 0.1 | 54 | 0.304 | 0.293 | 2.194 | 0.033 |
| Negative for all 3 biomarkers | 41 | 0.354 | 0.350 | 2.177 | 0.037 |
Discussion
In a group of cognitively-unimpaired older adults, we demonstrated that larger alERC volume is related to better performance short-delay retention on the ModRey (Hale et al., 2017), a memory test designed for preclinical and cognitively-healthy older adults. Among hippocampal and ERC subfields, alERC volume is also selectively positively associated with performance on the MoCA, a short assessment that tracks cognitive decline, and differs between participants who score above/below the MoCA threshold score; this observation replicates the findings of Olsen, Yeung et al. (2017) in a separate participant sample. Extending this finding, we showed that the positive relationship between alERC and MoCA is largely based on the delayed recall component of the MoCA. Further, we demonstrated that on the NACC-UDS3 neuropsychological battery, the volume of both alERC and pmERC is positively related to clinical measures of memory and visuospatial processing, while alERC volume, but not pmERC volume, is also positively related to memory retention and semantic fluency. Finally, we showed that higher alERC volume and CSF amyloid levels (i.e. healthier levels of CSF amyloid) have independent positive associations with ModRey retention.
Taken together, our results suggest that the alERC broadly supports memory processing, including recall, retention, and rejecting false positives, across multiple neuropsychological instruments, while the pmERC may be necessary only when contextual information is needed for memory. Interpreting our results within the “posterior medial, anterior temporal” (PMAT) framework (Ritchey et al., 2015), alERC volume is positively related to memory measures across numerous different neuropsychological instruments, and to semantic fluency scores, because these require memory for single items and their associations, without necessarily requiring additional contextual information. In turn, pmERC volumes are positively related to memory only on the Craft story task because the narrative structure of the stimuli makes contextual information more useful for recall. Likewise, the positive relationship of both alERC and pmERC volume to better Benson copy performance might be explained by the importance of spatial relations between the different line segments in that task. Interestingly, alERC volume was positively associated with immediate recall on both the Benson complex figure task and the ModRey, but not with delayed recall. However, on the Craft story, alERC volume was positively associated with performance on both immediate and delayed recall trials. This pattern of alERC-memory relationships does not seem to be related to the length of the delay, as the delay for the ModRey (40min) was greater than the Benson complex figure task (15 min) or the Craft story (20 min). Rather, our results might reflect the importance of the alERC (as the main hippocampal input) for narrative events (Rosenbaum et al., 2008) versus single items, or the greater impact of alERC dysfunction on more interconnected stimuli. The lack of association between alERC volumes and ModRey long-delay recall in our study is not necessarily discrepant with previously-reported association between delayed recall and functional activity in the ERC (Brickman et al., 2011). A recent study highlighted how performance on spatial navigation tasks within a reasonable range of normal performance - which have previously been shown to elicit functional activity in the hippocampus - do not relate to hippocampal volume in healthy participants (Clark et al., 2020). This model suggests that within a certain range, functional activation and cognitive performance might be linked independent of brain structure volume. Relationships between brain volume and cognitive function may emerge only brain volume limits the amount of functional activation possible, e.g. when task performance is sufficiently taxing, or when participants are undergoing volumetric decline. In the context of aging, this model would predict that it is plausible that structure-cognition relationships become more significant as atrophy progresses. This idea aligns with the prediction that arises from the PMAT model that we should expect to see pmERC volume differences have a larger effect on memory performance in more cognitively impaired populations, because contextual information would become more helpful for recall as memory for individual items weakens. Additionally, the alERC results are broadly in line with the revised models (Doan et al., 2019; Nilssen et al., 2019), which argue that the alERC is a critical convergence zone for sensory information representing the content of episodic memory. We note however, that the associations between pmERC volume and cognition we report do not necessarily align with the allocentric spatial coding proposed by this model. This discrepancy may arise through our functional connectivity-based definition of the ERC subfields, which could attribute some portion of the alERC as being pmERC instead, in contrast to the histological definition used in the revised model.
It is well established that ERC atrophy is commonly observed in AD patients (Bobinski et al., 1999; Du et al., 2001; Herukka et al., 2008; Juottonen et al., 1998; Killiany et al., 2000; Pennanen et al., 2004) and correlates with clinical severity (Du et al., 2003; Fennema-Notestine et al., 2009; McDonald et al., 2009). Based on the lack of associations between alERC volume and CSF biomarkers in our sample (in contrast to the findings of Holbrook et al., 2019 in AD patients), and the independent effects of CSF amyloid and alERC volume on ModRey retention, one interpretation is that our results represent either a range of normal variation in alERC volume relating to cognitive performance in healthy older adults, or a non-AD related neurodegeneration effect. On the other hand, alERC volume in our data is related specifically to a canonically AD-like pattern of cognitive test performance on the NACC-UDS3 neuropsychological battery (i.e. specific deficits in episodic memory and semantic fluency), with relatively spared performance in other cognitive domains (Salmon et al., 2002, 1999). Speculatively, when our findings are viewed in context with those of Holbrook et al (2019), it is possible that our results reflect some antecedent cause of alERC neurodegeneration, that combined with the presence of amyloid/tau biomarkers, may subsequently be related to development of AD. Longitudinal follow-up testing on these participants will help untangle which of these two interpretations is more accurate.
There are important limitations that need to be considered in interpreting these findings. It is important to note that our participant group are all cognitively unimpaired (CDR = 0), and it is unknown whether they will go on to develop AD. Thus, any interpretation of our data in the context of AD is necessarily speculative. Because our sample is drawn from an ongoing study involving both lumbar punctures to collect cerebrospinal fluid and gadolinium-contrast MRI, the sample size is not particularly large. The sample was mostly white and highly educated, and the findings may not generalize to a more diverse or less educated population. Further, there is an underlying assumption that entorhinal cortex volume loss and synaptic/neuronal loss are linearly related, which may not actually be the case. It is possible that neuronal loss might not initially cause detectable volume loss using MRI, which would suggest our data are understating the strength of the alERC-cognition association. As we examined grey matter volumes, our method does not account for changes in white matter structure (e.g. the integrity of the perforant pathway from the ERC into the hippocampus). Our study has a cross-sectional design, and we can only speak to the association of ERC subfield volume differences to cognition. While it is possible to extrapolate that our results reflect individuals at different points along a trajectory of neurodegeneration, longitudinal data are necessary to confirm this hypothesis.
An interesting implication of the results is that the ModRey might be a particularly sensitive instrument for detecting cognitive changes in otherwise asymptomatic older adults, which may be related to AD. We previously showed in the same group of participants that ModRey short-delay retention is related to CSF levels of amyloid and tau (Yeung et al., 2019a), which are biomarkers for AD. This study shows ModRey performance in cognitively healthy older adults is related to alERC volume differences, a neurodegenerative biomarker for AD. Strikingly, we find that alERC volumes are related to a classical neuropsychological presentation of AD: relative deficits in delayed recall and semantic fluency. Combining these two results suggests that ModRey performance might reflect subtle preclinical cognitive changes that are not currently regarded as pathological, but may predict subsequent decline. If that is true, then the ModRey may be a useful instrument for the early detection of AD, and for tracking progression in very early clinical phases.
The anterolateral entorhinal cortex (alERC) is affected by AD pathology early
In unimpaired older adults, alERC volume correlates with ModRey, corrected for age
alERC volume also correlates with other neuropsych tasks impaired early in AD
alERC volume and CSF amyloid independently related to ModRey memory retention
alERC volume differences might underlie preclinical AD-related cognitive changes
Acknowledgements:
This work was supported by the NIH/NIA (grant P50 AG008702 to A.M.B; center PI: S.A.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: A.M.B., S.A.S. & L.-K.Y. have filed an invention report on the ModRey task with Columbia University.
Data Verification: A subset of the data (the ModRey results and the CSF biomarkers) in this manuscript was previously published in Neurobiology of Aging (Yeung et al., 2019). However, none of the analyses presented in this manuscript have previously been published, and this manuscript does not include any duplicate analyses from our earlier work (which this manuscript builds upon). This manuscript has not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
Ethics Approval: The Institutional Review Board of Columbia University approved all procedures used in this study. All participants gave informed consent before participating in this study.
Author Approval: All authors have reviewed the contents of the manuscript being submitted, approve of its contents and validate the accuracy of the data.
References
- Bellmund JL, Deuker L, Doeller CF, 2019. Mapping sequence structure in the human lateral entorhinal cortex. Elife 8, 1–20. 10.7554/eLife.45333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berron D, Neumann K, Maass A, Schütze H, Fliessbach K, Kiven V, Jessen F, Sauvage M, Kumaran D, Düzel E, 2018. Age-related functional changes in domain-specific medial temporal lobe pathways. Neurobiol. Aging 65, 86–97. 10.1016/j.neurobiolaging.2017.12.030 [DOI] [PubMed] [Google Scholar]
- Berron D, Vieweg P, Hochkeppler A, Pluta JB, Maass A, Luther A, Das SR, Wolk DA, Wolbers T, Yushkevich PA, Wisse LEM, 2017. A protocol for manual segmentation of medial temporal lobe subregions in 7 Tesla MRI. NeuroImage Clin. 15, 466–482. 10.1016/j.nicl.2017.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobinski M, de Leon MJ, Convit A, De Santi S, Wegiel J, Tarshish CY, Saint Louis LA, Wisniewski HM, 1999. MRI of entorhinal cortex in mild Alzheimer’s disease. Lancet 353, 38–40. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259. 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- Brickman AM, Khan UA, Provenzano FA, Yeung L-K, Suzuki WA, Schroeter H, Wall M, Sloan RP, Small SA, 2014. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci 17, 1798–1803. 10.1038/nn.3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Stern Y, Small SA, 2011. Hippocampal subregions differentially associate with standardized memory tests. Hippocampus 21, 923–928. 10.1002/hipo.20840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IA, Monk AM, Hotchin V, Pizzamiglio G, Liefgreen A, Callaghan MF, Maguire EA, 2020. Does hippocampal volume explain performance differences on hippocampal-dependant tasks? Neuroimage 221, 117211 10.1016/j.neuroimage.2020.117211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutureau E, Di Scala G, 2009. Entorhinal cortex and cognition. Prog. Neuro-Psychopharmacology Biol. Psychiatry 33, 753–761. 10.1016/j.pnpbp.2009.03.038 [DOI] [PubMed] [Google Scholar]
- Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A, 1996. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol. Aging 17, 123–130. 10.1016/0197-4580(95)02002-0 [DOI] [PubMed] [Google Scholar]
- Desikan RS, McEvoy LK, Thompson WK, Holland D, Brewer JB, Aisen PS, Sperling RA, Dale AM, Initiative ADN, 2012. Amyloid-β-Associated Clinical Decline Occurs Only in the Presence of Elevated P-tau. Arch. Neurol 69 10.1001/archneurol.2011.3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, McEvoy LK, Thompson WK, Holland D, Rddey JC, Blennow K, Aisen PS, Brewer JB, Hyman BT, Dale AM, 2011. Amyloid-β associated volume loss occurs only in the presence of phospho-tau. Ann. Neurol 70, 657–661. 10.1002/ana.22509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola M, Macaluso E, Carlesimo GA, Tomaiuolo F, Worsley KJ, Fadda L, Caltagirone C, 2007. Episodic memory impairment in patients with Alzheimer’s disease is correlated with entorhinal cortex atrophy: A voxel-based morphometry study. J. Neurol 254, 774–781. 10.1007/s00415-006-0435-1 [DOI] [PubMed] [Google Scholar]
- Dice L, 1945. Measures of the amount of ecologic association between species. Ecology 26. [Google Scholar]
- Doan TP, Lagartos-Donate MJ, Nilssen ES, Ohara S, Witter MP, 2019. Convergent Projections from Perirhinal and Postrhinal Cortices Suggest a Multisensory Nature of Lateral, but Not Medial, Entorhinal Cortex. Cell Rep. 29, 617–627.e7. 10.1016/j.celrep.2019.09.005 [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Zhu XP, Jagust WJ, Miller BL, Reed B, Kramer JH, Mungas D, Yaffe K, Chui HC, Weiner MW, 2003. Atrophy rates of entorhinal cortex in AD and normal aging. Neurology 60, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuv N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yave K, Kramer JH, Reed B, Norman D, Chui HC, Weiner MW, Street C, 2001. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J. Neurol 71, 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM, Cattin F, Risold P-Y, 2013. The human hippocampus: functional anatomy, vascularization and serial sections with MRI, 4th ed Springer, New York, NY. [Google Scholar]
- Fennema-Notestine C, Hagler DJ, McEvoy LK, Fleisher AS, Wu EH, Karow DS, Dale AM, 2009. Structural MRI biomarkers for preclinical and mild Alzheimer’s disease. Hum. Brain Mapp 30, 3238–53. 10.1002/hbm.20744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández G, Brewer JB, Zhao Z, Glover GH, Gabrieli JDE, 1999. Level of sustained entorhinal activity at study correlates with subsequent cued-recall performance: A functional magnetic resonance imaging study with high acquisition rate. Hippocampus 9, 35–44. [DOI] [PubMed] [Google Scholar]
- Gicas KM, Thornton AE, Waclawik K, Wang N, Jones AA, Panenka WJ, Lang DJ, Smith GN, Vila-Rodriguez F, Leonova O, Barr AM, Procyshyn RM, Buchanan T, Su W, Vertinsky AT, Rauscher A, MacEwan GW, Honer WG, 2019. Volumes of the Hippocampal Formation Differentiate Component Processes of Memory in a Community Sample of Homeless and Marginally Housed Persons. Arch. Clin. Neuropsychol 34, 548–562. 10.1093/arclin/acy066 [DOI] [PubMed] [Google Scholar]
- Gollan TH, Weissberger GH, Runnqvist E, Montoya RI, Cera CM, 2012. Self-ratings of spoken language dominance: A Multilingual Naming Test (MINT) and preliminary norms for young and aging Spanish-English bilinguals. Bilingualism 15, 594–615. 10.1017/S1366728911000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman VA, Carmichael OT, Schwarz C, Tosto G, Zimmerman ME, Brickman AM, 2013. White matter hyperintensities and amyloid are independently associated with entorhinal cortex volume among individuals with mild cognitive impairment. Alzheimer’s Dement. 9, S124–S131. 10.1016/j.jalz.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI, 2005. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806. 10.1038/nature03721 [DOI] [PubMed] [Google Scholar]
- Hale C, Last BS, Meier IB, Yeung L-K, Budge M, Sloan RP, Small SA, Brickman AM, 2017. The ModRey: An Episodic Memory Test for Nonclinical and Preclinical Populations. Assessment 107319111772311. 10.1177/1073191117723113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays CC, Zlatar ZZ, Meloy MJ, Bondi MW, Gilbert PE, Liu TT, Helm JL, Wierenga CE, 2019. APOE modifies the interaction of entorhinal cerebral blood flow and cortical thickness on memory function in cognitively normal older adults. Neuroimage 202, 116162 10.1016/j.neuroimage.2019.116162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herukka S-K, Pennanen C, Soininen H, Pirttilä T, 2008. CSF Aβ42, tau and phosphorylated tau correlate with medial temporal lobe atrophy. J. Alzheimer’s Dis 14, 51–57. 10.3233/JAD-2008-14105 [DOI] [PubMed] [Google Scholar]
- Holbrook A, Tustison N, Marquez F, Roberts J, Yassa MA, Gillen D, 2019. Anterolateral entorhinal cortex thickness as a new biomarker for early detection of Alzheimer’s disease. medRxiv. 10.1101/19011825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A, Pitka A, 1998. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am. J. Neuroradiol 19, 659–71. [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Weidemann CT, Miller JF, Solway A, Burke JF, Wei X-X, Suthana NA, Sperling MR, Sharan AD, Fried I, Kahana MJ, 2013. Direct recordings of grid-like neuronal activity in human spatial navigation. Nat. Neurosci 16, 1188–1190. 10.1038/nn.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juottonen K, Laakso MP, Insausti R, Lehtovirta M, Pitkänen A, Partanen K, Soininen H, 1998. Volumes of the entorhinal and perirhinal cortices in Alzheimer’s disease. Neurobiol. Aging 19, 15–22. 10.1016/S0197-4580(98)00007-4 [DOI] [PubMed] [Google Scholar]
- Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan RP, Mayeux R, Duff KE, Small SA, 2014. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat. Neurosci 17, 304–11. 10.1038/nn.3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian NJ, Jutras MJ, Buffalo EA, 2012. A map of visual space in the primate entorhinal cortex. Nature 491, 761–764. 10.1038/nature11587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian NJ, Potter SM, Buffalo EA, 2015. Saccade direction encoding in the primate entorhinal cortex during visual exploration. Proc. Natl. Acad. Sci. 201417059 10.1073/pnas.1417059112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, Albert MS, 2000. The use of structural MRI to predict who will get Alzheimer’s disease. Ann. Neurol 47, 430–439. [PubMed] [Google Scholar]
- Knopman DS, Lundt ES, Therneau TM, Vemuri P, Lowe VJ, Kantarci K, Gunter JL, Senjem ML, Mielke MM, Machulda MM, Boeve BF, Jones DT, Graff-Radford J, Albertson SM, Schwarz CG, Petersen RC, Jack CR, 2019. Entorhinal cortex tau, amyloid-β, cortical thickness and memory performance in non-demented subjects. Brain 142, 1148–1160. 10.1093/brain/awz025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Thesen T, Barr W, Morrison C, Dugan P, Wang X, Meager M, Doyle W, Kuzniecky R, Devinsky O, Blackmon K, 2017. Parahippocampal and Entorhinal Resection Extent Predicts Verbal Memory Decline in an Epilepsy Surgery Cohort. J. Cogn. Neurosci 29, 869–880. 10.1162/jocn_a_01089 [DOI] [PubMed] [Google Scholar]
- Maass A, Berron D, Libby LA, Ranganath C, Duzel E, 2015. Functional subregions of the human entorhinal cortex. Elife 4, 1–20. 10.7554/eLife.06426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Lockhart SN, Harrison TM, Bell RK, Mellinger T, Swinnerton K, Baker SL, Rabinovici GD, Jagust WJ, 2018. Entorhinal Tau Pathology, Episodic Memory Decline, and Neurodegeneration in Aging. J. Neurosci 38, 530–543. 10.1523/JNEUROSCI.2028-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, McEvoy LK, Gharapetian L, Fennema-Notestine C, Hagler DJ, Holland D, Koyama A, Brewer JB, Dale AM, 2009. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology 73, 457–465. 10.1212/WNL.0b013e3181b16431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister MLR, Buffalo EA, 2018. Neurons in primate entorhinal cortex represent gaze position in multiple spatial reference frames. J. Neurosci 2432–17. 10.1523/JNEUROSCI.2432-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montchal ME, Reagh ZM, Yassa MA, 2019. Precise temporal memories are supported by the lateral entorhinal cortex in humans. Nat. Neurosci 22, 284–288. 10.1038/s41593-018-0303-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C, 1989. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assesment of Alzheimer’s disease. Neurology 39, 1159–1159. 10.1212/WNL.39.9.1159 [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H, 2005. The Montreal Cognitive Assessment, MoCA : A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- Navarro Schröder T, Haak KV, Zaragoza Jimenez NI, Beckmann CF, Doeller CF, 2015. Functional topography of the human entorhinal cortex. Elife 4, 1–17. 10.7554/eLife.06738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilssen ES, Doan TP, Nigro MJ, Ohara S, Witter MP, 2019. Neurons and networks in the entorhinal cortex: A reappraisal of the lateral and medial entorhinal subdivisions mediating parallel cortical pathways. Hippocampus 1–17. 10.1002/hipo.23145 [DOI] [PubMed] [Google Scholar]
- Olsen RK, Yeung L-K, Noly-Gandon A, D’Angelo MC, Kacollja A, Smith VM, Ryan JD, Barense MD, 2017. Human anterolateral entorhinal cortex volumes are associated with cognitive decline in aging prior to clinical diagnosis. Neurobiol. Aging 57, 195–205. 10.1016/j.neurobioaging.2017.04.025 [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, O’Neil JP, Janabi M, Lazaris A, Cantwell A, Vogel JW, Santos M, Miller ZA, Bettcher BM, Vossel KA, Kramer JH, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD, 2016. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139, 1551–1567. 10.1093/brain/aww027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hänninen T, Laakso MP, Hallikainen M, Vanhanen M, Nissinen A, Helkala E-L, Vainio P, Vanninen R, Partanen K, Soininen H, 2004. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol. Aging 25, 303–310. 10.1016/S0197-4580(03)00084-8 [DOI] [PubMed] [Google Scholar]
- Possin KL, Laluz VR, Alcantar OZ, Miller BL, Kramer JH, 2011. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer’s disease and behavioral variant frontotemporal dementia. Neuropsychologia 49, 43–48. 10.1016/j.neuropsychologia.2010.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M, 2012. Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci 13, 713–726. 10.1038/nrn3338 [DOI] [PubMed] [Google Scholar]
- Reagh ZM, Yassa MA, 2014. Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proc. Natl. Acad. Sci. U. S. A 111, E4264–73. 10.1073/pnas.1411250111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D, 1985. The Halstead-Reitan Neuropsychological Test Battery: Therapy and Clinical Interpretation. Neuropsychological Press, Tucson, AZ. [Google Scholar]
- Ritchey M, Libby LA, Ranganath C, 2015. Cortico-hippocampal systems involved in memory and cognition: The PMAT framework, in: Progress in Brain Research. Elsevier, pp. 45–64. 10.1016/bs.pbr.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Moscovitch M, Foster JK, Schnyer DM, Gao F, Kovacevic N, Verfaellie M, Black SE, Levine B, 2008. Patterns of autobiographical memory loss in medial-temporal lobe amnesic patients. J. Cogn. Neurosci 20, 1490–506. 10.1162/jocn.2008.20105 [DOI] [PubMed] [Google Scholar]
- Salmon DP, Heindel WC, Lange KL, 1999. Differential decline in word generation from phonemic and semantic categories during the course of Alzheimer’s disease: Implications for the integrity of semantic memory. J. Int. Neuropsychol. Soc 5, 692–703. 10.1017/S1355617799577126 [DOI] [PubMed] [Google Scholar]
- Salmon DP, Thomas RG, Pay MM, Booth A, Hofstetter CR, Thal LJ, Katzman R, 2002. Alzheimer’s disease can be accurately diagnosed in very mildly impaired individuals. Neurology 59, 1022–1028. 10.1212/WNL.59.7.1022 [DOI] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, NcNaughton BL, Witter MP, Moser M-B, Moser EI, 2006. Conjunctive representation of position, direction, and velocity in entorhinal Cortex. Science (80-.) 312, 758–762. 10.1126/science.1125572 [DOI] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, LoPresti ML, Tricarico MD, Stern CE, 2004. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: A functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J. Neurosci 24, 11088–11097. 10.1523/JNEUROSCI.3807-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz H, Sommer T, Peters J, 2012. Direct Evidence for Domain-Sensitive Functional Subregions in Human Entorhinal Cortex. J. Neurosci 32, 4716–4723. 10.1523/JNEUROSCI.5126-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL, 1979. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull 10.1037/0033-2909.86.2.420 [DOI] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser M-B, Moser EI, 2008. Representation of geometric borders in the entorhinal cortex. Science (80-.) 322, 1865–8. 10.1126/science.1166466 [DOI] [PubMed] [Google Scholar]
- Stoub TR, Rogalski EJ, Leurgans S, Bennett DA, deToledo-Morrell L, 2010. Rate of entorhinal and hippocampal atrophy in incipient and mild AD: Relation to memory function. Neurobiol. Aging 31, 1089–1098. 10.1016/j.neurobiolaging.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao A, Moser M-B, Moser EI, 2013. Traces of experience in the lateral entorhinal cortex. Curr. Biol 23, 399–405. 10.1016/j.cub.2013.01.036 [DOI] [PubMed] [Google Scholar]
- Tsao A, Sugar J, Lu L, Wang C, Knierim JJ, Moser M-B, Moser EI, 2018. Integrating time from experience in the lateral entorhinal cortex. Nature 561, 57–62. 10.1038/s41586-018-0459-6 [DOI] [PubMed] [Google Scholar]
- Wang C, Chen X, Knierim JJ, 2020. Egocentric and allocentric representations of space in the rodent brain. Curr. Opin. Neurobiol 60, 12–20. 10.1016/j.conb.2019.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FC, Giordani B, Kramer J, Loewenstein D, Marson D, Mungas D, Salmon D, Welsh-Bohmer K, Zhou XH, Shirk SD, Atri A, Kukull WA, Phelps C, Morris JC, 2018. Version 3 of the Alzheimer Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Dis. Assoc. Disord 32, 10–17. 10.1097/WAD.0000000000000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D, 1987. Wechsler Memory Scale - Revised Manual. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wilson DIG, Langston RF, Schlesiger MI, Wagner M, Watanabe S, Ainge JA, 2013. Lateral entorhinal cortex is critical for novel object-context recognition. Hippocampus 23, 352–66. 10.1002/hipo.22095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse LEM, Chételat G, Daugherty AM, Flores R, Joie R, Mueller SG, Stark CEL, Wang L, Yushkevich PA, Berron D, Raz N, Bakker A, Olsen RK, Carr VA, 2020. Hippocampal subfield volumetry from structural isotropic 1 mm 3 MRI scans: A note of caution. Hum. Brain Mapp. hbm.25234 10.1002/hbm.25234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse LEM, Gerritsen L, Zwanenburg JJM, Kuijf HJ, Luijten PR, Biessels GJ, Geerlings MI, 2012. Subfields of the hippocampal formation at 7 T MRI: in vivo volumetric assessment. Neuroimage 61, 1043–9. 10.1016/j.neuroimage.2012.03.023 [DOI] [PubMed] [Google Scholar]
- Witter MP, Naber PA, Van Haeften T, Machielsen WCM, Rombouts SARB, Barkhof F, Scheltens P, Lopes Da Silva FH, 2000. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus 10, 398–410. [DOI] [PubMed] [Google Scholar]
- Yeung L-K, Hale C, Last BS, Andrews H, Sloan RP, Honig LS, Small SA, Brickman AM, 2019a. Cerebrospinal fluid amyloid levels are associated with delayed memory retention in cognitively normal biomarker-negative older adults. Neurobiol. Aging 84, 90–97. 10.1016/j.neurobiolaging.2019.08.010 [DOI] [PubMed] [Google Scholar]
- Yeung L-K, Olsen RK, Bild-Enkin HEP, D’Angelo MC, Kacollja A, McQuiggan DA, Keshabyan A, Ryan JD, Barense MD, 2017. Anterolateral Entorhinal Cortex Volume Predicted by Altered Intra-Item Configural Processing. J. Neurosci 37, 5527–5538. 10.1523/JNEUROSCI.3664-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung L-K, Olsen RK, Hong B, Mihajlovic V, D’Angelo MC, Kacollja A, Ryan JD, Barense MD, 2019b. Object-in-place memory predicted by anterolateral entorhinal cortex and parahippocampal cortex volume in older adults. J. Cogn. Neurosci 31, 711–729. 10.1162/jocn_a_01385 [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Pluta JB, Wang H, Xie L, Ding S-L, Gertje EC, Mancuso L, Kliot D, Das SR, Wolk DA, 2015. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum. Brain Mapp 36, 258–287. 10.1002/hbm.22627 [DOI] [PMC free article] [PubMed] [Google Scholar]


