Abstract
Background:
Some individuals Parkinson’s Disease (PD) experience working memory and inhibitory difficulties, others learning and memory difficulties, while some only minimal to no cognitive deficits for many years.
Objective:
To statistically derive PD executive and memory phenotypes, and compare PD phenotypes on disease and demographic variables, vascular risk factors, and specific neuroimaging variables with known associations to executive and memory function relative to non-PD peers.
Methods:
Non-demented individuals with PD (n=116) and non-PD peers (n=62) were recruited to complete neuropsychology measures, blood draw, and structural magnetic resonance imaging. Tests representing the cognitive domains of interest (4 executive function, 3 memory) were included in a k-means cluster analysis comprised of the PD participants. Resulting clusters were compared demographic and disease-related variables, vascular risk markers, gray/white regions of interest, and white matter connectivity between known regions involved in executive and memory functions (dorsolateral prefrontal cortices to caudate nuclei; entorhinal cortices to hippocampi).
Results:
Clusters showed: 1) PD Executive, n=25; 2) PD Memory, n=35; 3) PD Cognitively Well; n=56. Even after disease variable corrections, PD Executive had less subcortical gray matter, white matter, and fewer bilateral dorsolateral-prefrontal cortex to caudate nucleus connections; PD Memory showed bilaterally reduced entorhinal-hippocampal connections. PD Cognitively Well group showed only reduced putamen volume and right entorhinal cortex to hippocampi connections relative to non-PD peers. Groups did not statistically differ on cortical integrity measures or cerebrovascular disease markers.
Conclusion:
PD cognitive phenotypes showed different structural gray and white matter patterns. We discuss data relative to phenotype demographics, cognitive patterns, and structural brain profiles.
Keywords: entorhinal cortex, white matter, hippocampus, prefrontal cortex, dementia, executive function, memory
Introduction
Non-demented individuals with idiopathic Parkinson’s Disease (PD) subjectively report attention and memory changes [1]. From a neuropsychological standpoint, these reports are consistent with recent research demonstrating that nondemented individuals with PD can present with reduced executive function and declarative memory relative to age matched peers[2–4]. Executive function weaknesses are often observed on measures of processing speed, working memory, and inhibitory functions (e.g., Digit Symbol Coding, Trail Making Test, Stroop Color Word Test) [3, 5, 6], while learning and memory difficulties are typically shown on unstructured list learning tasks (e.g., Hopkins Verbal Learning Test) [3, 5, 6] and, at times, story recall tests (e.g., Logical Memory Test) [6, 7]. The severity of executive and memory impairment varies as well; some individuals may show progressive decline in executive functions, others may show executive and memory difficulties, while others show minimal to no cognitive weaknesses for many years [8].
Researchers are attempting to differentiate the neuroanatomical and biomarker contributions to these executive and memory profiles within PD, but clear profile differences remain inconclusive. The most prominent theory, known as the dual syndrome hypothesis, proposes that working memory and executive dysfunction in PD are caused by dopaminergic denervation due to pathology in substantia nigra, while memory and accompanying visuospatial deficits are caused by cholinergic denervation due to pathology in basal forebrain [9]. However, there are other possible mechanisms underlying cognitive deficits in PD. Vascular disease and white matter disease changes associated with vascular insufficiency are potential contributors to the executive difficulties of PD [10], although some researchers show this pattern to be more prominent for non-PD than PD [11]. Reduced temporal cortex volume, particularly in the mesial structures, may also contribute to reduced encoding and retrieval in PD [4, 12]. For example, some hypothesize increased alpha-synuclein pathology in the anterior temporal cortex, which is typically detected as early as Braak Stage 4 [13]. Amyloid deposition in temporal lobes has also been associated with poor recall in PD, with selective vulnerability of the hippocampus in early PD patients without dementia [14]. There are also questions regarding differences in specific gray-white matter connectivity [6]. Building upon original circuitry research proposed by Alexander Delong and Strick [15], it is theorized that individuals with executive difficulties, specifically in working memory or processing speed, and difficulties on unstructured word-list learning tests may be experiencing disruptions to frontostriatal circuitry. The neural circuit between the caudate nucleus and the dorsolateral prefrontal cortex is particularly relevant to this dysfunction[7, 15]. By contrast, individuals with prominent episodic memory difficulties may have reduced connectivity or integrity within the perforant pathway or hippocampal-retrosplenial regions [4, 14, 16], as these pathways are well known to be involved in memory formation.
Collectively, these theories suggest that individuals with idiopathic PD with primary weaknesses in executive functioning should have distinct profiles from their peers with primary memory weaknesses. To date, however, we know of no study that has strategically examined demographics, vascular, gray-white matter, and a priori connectivity tractography neuroimaging profiles in PD executive and memory phenotypes. Towards this effort, our research team designed the current study.
Borrowing from recent statistical approaches examining cognitive phenotypes in prodromal profiles Alzheimer’s disease-vascular dementia spectrum disorders, (see [17–19]), we prospectively created empirically derived PD cognitive phenotypes among non-demented older adults with idiopathic PD. Clusters were based on neuropsychological measures assessing processing speed, working memory, inhibitory function, and declarative memory. We hypothesized that individuals would classify into three phenotypes: those with primary frontal-striatal (heretofore called ‘executive’) weakness, those with primary declarative memory weakness, and those with no cognitive weaknesses relative to non-PD peers. We then compared these statistically defined phenotypes in terms of demographic and cognitive behavioral features that have known significance to cognitive constructs (i.e., depression, apathy [20]), other cognitive domains (i.e., language, visuospatial) relevant to the dual-syndrome hypothesis [7], PD-related variables (disease severity, duration and dopaminergic medication dose), vascular risk profiles and white matter disease markers, gray and white matter neuroimaging parameters, and specific white-gray matter tracts associated with executive and memory function. Considering progression of the disease through Braak stages and considerations for the dual-syndrome hypothesis, we hypothesized individuals defined with an executive weakness would show the most reduction in cortical thickness in the dorsolateral prefrontal region, reduced white matter globally, and reduced connectivity between the dorsolateral prefrontal cortex (heretofore DLPFC) to caudate nucleus (CN). By contrast, individuals with memory weaknesses were hypothesized to show reduced cortical thickness within the entorhinal cortex (ERC) and reduced structural connectivity between hippocampus and entorhinal cortex (HIPP-ERC).
Methods
Participants with PD were recruited through movement disorder clinic referrals and advertisements to local MDC support groups affiliated with the movement disorder clinic. Idiopathic PD was confirmed by a fellowship-level movement disorder specialist, using UK Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria[21]. Individuals with early, mild to moderate PD with a Hoehn and Yahr scale[22] score between 1-3 were included. The predominant motor PD phenotype; i.e., tremor dominant vs. postural instability-gait difficulty was assessed clinically based on the first presenting symptom or predominant symptomatology on initial and subsequent exams (and also of primary concern to the patient). Non-PD participants were recruited through 1) mailings to demographically similar individuals in two counties, 2) community fliers, and 3) free community memory screenings. Exclusion criteria included other neurodegenerative disorders, significant disease that could limit lifespan, major psychiatric disorder, or dementia determined from structured telephone interview and medical record review. Depression and apathy were not exclusion criteria due to high prevalence in PD[23, 24]. This study was conducted through the Fixel Institute for Neurological Sciences, approved by the UF Institutional Review Board, required consent, and complied with the Declaration of Helsinki.
Procedures involved 1) a structured background interview for demographics (sex, age, years of education), comorbidity (Charlson Comorbidity Index[25]), handedness inventory [26], disease variables (PD duration, age at onset, first symptoms), medications (Magellan Anticholinergic Risk Scale[27] and levodopa dose[28]); 2) Mini-Mental Status Exam[29]; 3) Beck Depression Inventory, Second Edition (BDI-II[30]); Apathy Scale (AS[31, 32]); 4) a comprehensive research neuropsychological protocol; 5) Unified Parkinson’s Disease Rating Scale (UPDRS[33, 34]) completed on-medication at time of cognitive testing by trained staff and double scored by a trained rater blinded to diagnosis; 6) fasting blood draw for homocysteine and uric acid; and 7) a brain MRI conducted within 24 hours of the cognitive assessment. Individuals blinded to diagnosis double-scored and double-entered data. All procedures were completed by a trained movement disorder specialty neuropsychologist (CP, JT) and with review by a trained neurology movement disorder specialist (MF).
Neuropsychological Protocol
Participants were assessed in a private room, and all PD participants were on prescribed PD medication(s) at the time of testing in order to be comfortable and provide best performance. The comprehensive research assessment targeted processing speed, working memory, inhibitory function, language, visuospatial function, declarative memory, reasoning, and fine motor function. Seven neuropsychology outcome variables of interest were a priori selected for cluster analysis: executive function [Wechsler Adult Intelligence Scale-III Digit Symbol (total score)[35, 36] and Letter Number Sequencing (total score)[37]; Trail Making Test Part B (total time)[38–40]; Stroop Color-Word Test (total correct in 45)[41]] and declarative memory [Wechsler Adult Intelligence Scale-III (WMS-III) Logical Memory Total Recall [42], Hopkins Verbal Learning Test, Revised (HVLT-R) delay free recall [43], HVLT-R recognition discriminability]. We selected executive function measures known for the assessment of processing speed, working memory, and cognitive flexibility. Individuals with idiopathic PD (no dementia) show reduced performance on these measures [6, 44], and these measures associate with frontal-striatal regions [45] altered in earlier (no dementia) disease stages of PD [6]. We created composites for both domains (executive and memory, respectively) by averaging the standardized scores. This allowed us to compare the cognitive phenotypes for performance differences after cluster analysis. We assess the cognitive phenotypes and non-PD peers on additional external measures of reasoning [Wechsler Abbreviated Scale of Intelligence Matrix Reasoning (total score)[46], Delis-Kaplan Executive Function System - Tower Test (total achievement score)[48]], and learning/memory (Philadelphia Repeatable Verbal Learning Test; delay score controlling for initial recall[4, 49, 50]). Additionally, we provide scores on measure of language (Animal Fluency[51], Boston Naming Test[39, 52], total scores) and visuospatial function(Judgement of Line Orientation, total correct[53]) to guide future research and assist those interested in a more comprehensive cognitive profile for the PD cognitive phenotypes in the current study. For missing values on the Stroop Color-Word test due to color blindness, regression imputation was used to replace the missing variable[54]. Neuropsychology outcome variables were standardized using z-scores based on published normative age based references. Normative references for the Trail Making Test Part B corrected for age and years of education [55].
Neuroimaging Protocol
Participants were scanned on a Siemens 3T Verio scanner and 8-channel head coil with 1) one T1-weighted scan (TR: 2500 ms; TE: 3.77 ms; 176 sagittal 1 mm slices; 1 mm isotropic resolution; 256x256x176 matrix) optimized for gray/white matter segmentation, 2) two separate single-shot EPI diffusion scans, with gradients applied in 6 directions (b =100s/mm2) and 64 directions (b =1000s/mm2) (TR: 17300 ms; TE: 81 ms; 73 transversal 2mm slices; 2 mm isotropic resolution; 256x256x146 matrix); and 3) a Fluid Attenuated Inversion Recovery (FLAIR) scan (TR: 6000 ms; TE: 395 ms; TI: 2100ms;176 sagittal 1mm slices, 1 mm isotropic resolution; 256x256x176 matrix).
Gray/White Matter Volume/ Thickness:
A trained rater (JJT) applied FreeSurfer (Version 6.0; http://surfer.nmr.mgh.harvard.edu/)[56–59] for T1-weighted cortical reconstruction and volumetric segmentation of total gray matter volume, total cortical gray matter volume, total subcortical gray matter volume, and total white matter volume, hippocampus volume, putamen volume, caudate nucleus volume, and thalamus volume. This program also estimated ERC thickness and DLPFC thickness. Total intracranial volume (TICV)[60] served as a control variable for all structural metrics. Leukoaraiosis (LA)[61] was quantified as a marker of vascular disease.
White Matter Tract Connectivity:
Two a priori regions of interest (ROIs) were examined: 1) DLPFC-CN with DLPFC quantified by a reliable rater (DSC intra-rater range = 0.80–0.95; mean±SD=0.89±0.05; Inter-rater range=0.83–0.99; mean±SD=0.97 ± 0.04) in ITK-SNAP (www.itksnap.org)[62] following published guidelines[63]; 2) ERC-HIPP connectivity with regions extracted from FreeSurfer. ROI tractography was acquired through edge weight connectivity by first interpolating the diffusion data to an isotropic resolution of 1 x 1 x 1 mm3. Diffusion tensor imaging (DTI)[64] was performed on each data set to extract a fractional anisotropy (FA) image. This FA image was used to create a seed mask of each brain where voxels with an FA larger than .05 were seeded with 125 seed points. To inform tractography, the data were modeled to calculate the water probability density function (PDF) in each voxel using the Mixtures of Wishart tensor distribution model[65]. Up to 5 maxima of the PDF were identified, and peak directions were used to lead the tractography algorithm. Deterministic tractography was performed using a fiber step-size of half a voxel and step-to-step track deviation less than 50°. The tractogram was filtered by keeping only tracts that connected the ROI pairs.
To analyze the strength of connectivity between the ROI pairs, a normalized edge weight analysis was performed to bypass biases from standard tract counting techniques[66]. The normalized edge weight representing the connection strength between two ROIs is defined as:
Vvoxel is the volume of a voxel, Pvoxel is the number of seed points per voxel, A is the surface area of the connected nodes, ni and nj, and l(f) is the length of the fibers, f, connecting the nodes.
Vascular Marker Considerations
To consider the relative contribution of vascular factors to the white matter and cognitive profile differences in the PD phenotypes we acquired the following: 1) Fasting Blood Draw–for cardiovascular risk markers (homocysteine, uric acid); 2) Leukoaraiosis (LA[61])– a marker of small vessel vascular disease linked to executive function in non-demented older adults.[67] White matter abnormalities were quantified by a reliable rater (DSC intra-rater range=0.84–0.93; mean±SD= 0.84 ± 0.12; Inter-rater range = 0.80–0.83) using an in-house macro within ImageJ[68, 69] on FLAIR scans and associating highly with segmentation via FLAIR and T1 images using a k-nearest neighbor algorithm with high reliability manual segmentations[70]. Dependent variables = LA mm3 and LA relative to TICV.
Statistical Analyses
Analyses completed with SPSS v25. Using only individuals with PD, a principal component analysis (PCA) was used on neuropsychological outcome variables with both orthogonal and oblique rotations to confirm memory and executive cognitive constructs. As per Kasier (1960) [71], components of eigenvalue greater than 1 were retained. The regression scores derived from the factor loadings for the retained components were used in subsequent cluster analyses. A hierarchical cluster analysis with Ward’s method determined the optimum number of clusters, followed by a k-means analysis for final cluster determination. To test the reliability of the PD cluster solution, we applied a cross-validation approach in which a k-means procedure was completed on a random sample of 50% of the participants five times. These new classifications were then compared to the original k-means analysis solution. Generally, 90% or greater agreement is considered very stable, 80-90% agreement is considered stable, and 75-80% is considered somewhat stable[72]. One-way analyses of variance (ANOVAs) compared group differences on cognitive composite scores. Individual z-scores from the seven tests used within the cluster analyses were also compared between PD phenotypes and non-PD peers. We report how covarying for Wechsler Adult Intelligence Scale-III Digit Symbol (total score) changed group comparisons on the inhibition and set-switching measures (Stroop Color Word Test and Trail Making Test, Part B). ANOVAs compared the groups on the external validation measures of reasoning, memory, language, and visuospatial function.
Non-PD peers were used as a reference group in all comparative analyses, as it is clinically useful and assists with rigor and reliability to compare deviation from age matched non-PD peer group. One-way analyses of variance (ANOVAs) compared PD cognitive phenotypes and non-PD peers on age, education, disease duration, and mood scores (BDI-II, AS) and were followed up with pair-wise independent samples t-tests. Kruskal-Wallis analyses compared UPDRS-III, CCI, Magellan score, and MMSE, as these variables were not normally distributed. A MANCOVA controlling for age, education, and TICV compared MRI volumes of interest (total brain volume, cortical gray matter volume, subcortical gray matter volume, and total white matter volume) between groups. This MANCOVA was repeated controlling for disease duration. ANCOVAs controlling for age, education and TICV compared volume of caudate nucleus, putamen, and thalamus and thickness of DLPFC and ERC between groups. ANCOVAs compared groups in edge weight strength controlling for age and sex. The DLFPC-CN edge weight analysis controlled for TICV, as this edge weight was significantly correlated with TICV. DLPFC-CN edge weights were log-transformed to achieve normality. Alpha level set at 0.05 for all comparisons. Partial Eta squared (ηp2) provides an estimate of effect size with small (ηp=0.01), medium (ηp2=0.06), and large (ηp2=0.14).
Results
Of 211 individuals screened, 181 completed cognitive testing (116 PD, 65 non-PD peers; mental Table 1 for demographic information), and 174 of these completed the full neuroimaging protocol (2 missing diffusion scans; 5 did not complete due to claustrophobia). Six participants had to have regression imputation for the Stroop Color-Word test due to color blindness. Cluster analyses were conducted on the 116 PD participants. The PCA of the seven cognitive outcome measures showed 2 factors with eigenvalues greater than 1 (eTable 2). Hierarchical cluster analysis showed the greatest reduction in squared Euclidian distance between two and three clusters (Supplemental Figure 1). K-means cluster analysis with three clusters yielded three PD cognitive phenotype clusters: 1) PD Executive (n=25); 2) PD Memory (n=35), and PD Cognitively Well (n=56). Cross-validation agreement with the full PD sample ranged from 86.7% to 96.7%, with an average agreement of 92.3%. (eTable 3).
Table 1.
Demographic and General Cognition Characteristics Between PD Cognitive Phenotypes and Non-PD Peers
| Variable | PD Executive (n=25) | PD Memory (n=35) | PD Cognitively Well (n=56) | Non-PD (n=65) | p-value |
|---|---|---|---|---|---|
| Gender (M:F) | 19:6 | 27:8 | 37:19 | 48:17 | .63 |
| Age (yrs) | 69.08±6.30, 55/81 | 66.48±6.19, 55/84 | 68.84±6.01, 56/82 | 68.23±5.4, 55/80 | .25 |
| Education (yrs) | 15.58±2.37, 12/21 | 16.06±2.37, 10/20 | 17.11±2.71, 11/23 | 17.19±2.23, 12/22 | .02* |
| Handedness | 20.20±4.53 | 21.46±3.18 | 19.75±5.45 | 20.88±4.84 | .35 |
| Disease Duration (yrs) | 8.92±6.89, 1/26 | 5.74±3.22, 1/12 | 6.64±4.33, 1/23 | -- | .04 |
| UPDRS III | 22.84±9.31, 5/44 | 22.36±11.26, 4/57 | 18.45±10.70, 2/46 | 4.13±4.62, 0/18 | .08** |
| l-Dopa Equiv. Score | 665.95±371.29, 0/1232 | 648.83±416.57, 0/1800 | 661.55±277.12, 100/1450 | -- | .98 |
| Magellan Score | 0.81±1.39, 0/6 | 0.76±1.15, 0/3 | 0.89±1.15, 0/4 | 0.25±0.82, 0/5 | <.01 |
| Charlson Comorbidity Index | 0.33±0.57, 0/2 | 0.17±0.38, 0/1 | 0.44±0.76, 0/4 | 0.33±0.66, 0/2 | .34 |
| MMSE Total | 28.16±1.57, 24/30 | 28.20±1.49, 24/30 | 28.95±1.00, 26/30 | 29.20±0.98, 25/30 | <.01 |
| BDI-II* | 11.00±5.64, 3/23 | 8.11±5.64, 0/28 | 7.46±5.75, 0/24 | 3.12±4.63, 0/27 | <.01* |
| Apathy Scale | 13.09±5.94, 4/26 | 9.43±5.42, 2/26 | 9.80±5.80, 0/24 | 9.35±4.87, 1/30 | .03* |
This sample represents Caucasian non-Hispanic individuals within Florida, United States, at time of study. All values presented as Mean±SD, Min/Max. p-value represents overall between group (PD Executive, PD Memory, PD Well, Non-PD) comparison difference;
indicates significant difference between PD phenotypes.
p-value when comparing PD phenotypes only. Apathy scale[32]; BDI-II = Beck Depression Inventory-2[30]; Handedness = Modification of Annette (1976) inventory with range −24 to 24 (higher positive = right-side dominance; [26]; l-Dopa Equiv. Score = Levodopa Equivalent Sore = Total Daily levodopa dosage intake in milligrams[28]; MMSE = Mini-Mental State Exam[29]; UPDRS III = United Parkinson’s Disease Rating Scale III [33].
External Validation of PD Cognitive Phenotypes (eTable 2,4,5)
PD phenotypes and non-PD peers differed on the neuropsychological composites for executive function (F[3,177]=65.35, p<.01, ηp2=.526) and memory (F[3,177]=60.65, p<.01, ηp2=.507). PD Executive scored lowest on the executive composite, while the PD Memory scored lowest on the memory composite. This pattern was present for each individual test even when correcting for Wechsler Adult Intelligence Scale-III Digit Symbol (total score) performance [Stroop (F[3, 174]=5.962, p<.01, ηp2=.097) and Trail Making Test Part B (F[3, 174]=19.968, p<.01, ηp2=.160). Groups also differed on the standardized external reasoning measures (Matrix Reasoning; F[3,177]=13.38, p<.01, ηp2=.185; PD Executive<PD Cognitively Well, non-PD; Tower Test F[3,177]=4.431, p<.01, ηp2=.070, PD Executive < non-PD), and an external memory measure (PrVLT; F[3,173]=5.98, ηp2=.094, p’s<.01; PD Memory<all other groups).
Other cognitive domains: Groups differed on a language test composite (F[3,177]=10.585, p<.01, ηp2=.106) such that PD Executive and PD Memory had significantly lower scores than PD Cognitively Well and non-PD peers (p<.01 in all cases). Groups differed in visuospatial function (F[3,177]=7.098, p<.01, ηp2=.107) such that PD Executive had significantly lower scores than PD Cognitively Well and non-PD peers (p<.01 in both cases).
Demographic and Clinical Comparisons (Table 1)
Demographics:
Demographic analyses included all participants regardless of their completion of the neuroimaging protocol. Groups were significantly different in years of education (F[3,177]=4.84, p=.02, ηp2=.056) with PD Executive having two years less than PD Cognitively Well (p<.02) and non-PD peers (p<.01). Age and sex ratio were not different between groups. All participants were Caucasian. A second set of demographic analyses were completed with only individuals containing a full neuroimaging protocol (eTable 6). Results show the same pattern of findings.
Disease and Medication:
PD phenotypes differed in disease duration (F[2,113]=3.41, p=0.04, ηp2=.057; PD Executive>PD Memory, p=.01; PD Executive>PD Cognitively Well, p<.05). PD phenotypes did not differ in UPDRS-III or levodopa equivalency dose. Anticholinergic load was higher for PD relative to non-PD (p<.01), but not within PD phenotypes (p=.56). Comorbidity severity was not significantly different between PD phenotypes or to non-PD peers.
Mood/General Cognition:
Groups differed in depression (F[3,177]=16.30, p<.01, ηp2=.216) and apathy (F[3,177]=2.95, p=.03, ηp2=.048) with PD Executive highest in both (Depression: PD Executive>PD Memory, PD Cognitively Well; p’s<.04; Apathy: PD Executive>all other groups, p’s<.01). MMSE was significantly lower than non-PD peers for both low cognition phenotypes (PD Executive and PD Memory<non-PD; p’s<.01; no difference within PD phenotypes).
Vascular Marker Considerations (eTable 7):
There were no significant differences between phenotypes in homocysteine (F[3,175]=1.84, p=.14, ηp2=.031) or uric acid (F[3,176]=0.06, p=.98, ηp2=.001). There was no significant difference in overall LA after controlling for TICV (F[3,169]=1.48, p=.22, ηp2=.026).
General Brain Volumetrics (Figure 1; Table 2)
Figure 1.
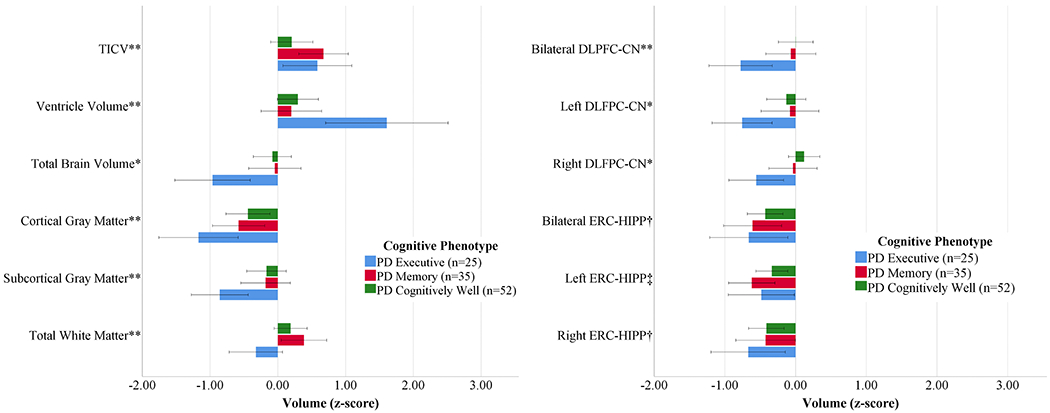
Volumetric and Structural Connectivity Measures by PD Cognitive Phenotype Referenced to Non-PD Peers
Left: Brain Volume Measures; Right: ROI to ROI Structural Connectivity (Edge Weight) Measures normalized by Non-PD peers (n=62). Bars demonstrate the raw z-scores after normalizing for non-PD peers, and are uncorrected for age or total intracranial volume. Error bars display unadjusted standard error. TICV=Total Intracranial Volume. *PD Executive significantly different from PD Cognitively Well and Non-PD (p<.05). **PD Executive significantly different from PD Memory and PD Cognitively Well (p<.05) †Non-PD significantly different from all phenotypes. ‡Non-PD significantly different from PD Memory. All volume analyses controlled for age, education, and TICV. DLPFC/Caudate controlled for age, education and TICV. ERC/Hippocampus controlled for age and education.
Table 2.
Brain Volume/Thickness Characteristics by PD Cognitive Phenotype and Non-PD peers
| Variable | PD Executive (n=25) | PD Memory (n=35) | PD Cognitively Well (n=56) | Non-PD (n=62) | p-value |
|---|---|---|---|---|---|
|
Brain Volumes (cm3) | |||||
| Total Intracranial | 1699 ± 161 1395/2047 | 1711 ± 140 1435/2112 | 1650 ± 147 1263/1928 | 1623 ± 131 1343/1887 | .02* |
| Ventricle Volume | 54.45 ± 33.22 7.93/147.01 | 34.08 ± 18.08, 11.31/87.49 | 34.54 ± 16.14, 9.35/78.39 | 29.78 ± 14.12, 6.74/65.52 | <.01* |
| Brain Volume | 1101 ± 83.0 973/1283 | 1150±113.3 946/1526 | 1106 ± 99.87 805/1307; 1145 | 1091 ± 96.65 906/1306 | <.01* |
| Total Gray Matter Volume | 620.08 ± 50.76 514.26/745.92 | 635.48 ± 57.82 522.22/826.68 | 619.62 ± 50.25 459.54/714.02 | 619.06 ± 48.24 523.85/734.14 | <.01 |
| Cortex Volume | 458.29 ± 40.87 373.42/555.65 | 473.90 ± 46.22 387.58/627.46 | 458.73 ± 39.87 343.91/551.97 | 459.48 ± 38.89 388.29/558.97 | <.01 |
| Subcortical Grey Volume | 53.96 ± 3.88 47.83/61.31 | 56.85 ± 5.69 45.90/71.68 | 54.81 ± 5.26 41.18/64.54 | 54.40 ± 4.80 46.03/66.11 | .02* |
| White Matter Volume | 454.83±37.51 375.51/534.85 | 484.07±59.45 401.51/667.24 | 460.09±53.91 329.04/612.48 | 446.70±53.02 340.65/581.36 | <.01* |
| Hippocampus Volume | 3.68±0.33 3.17/4.31 | 3.93±0.44 2.97/5.07 | 3.86±0.38 3.12/4.86 | 3.87±0.43 2.87/4.94 | <.01* |
| Caudate Volume | 3.55±0.64 2.61/5.60 | 3.54±0.64, 2.41/6.03 | 3.43±0.52 2.39/4.64 | 3.44±0.53 2.43/5.41 | .67 |
| Putamen Volume | 4.42±0.35 3.76/5.10 | 4.66±0.73 3.58/7.28 | 4.43±0.67 2.70/5.84 | 4.64±0.53 3.50/5.94 | .02 |
| Thalamus Volume | 6.75±0.64 5.51/8.05 | 7.07±0.76 5.38/8.79 | 6.91±0.79 4.73/9.59 | 6.61±0.67 5.21/8.32 | .04 |
| Cortical Thickness (mm) | |||||
| ERC Thickness | 3.30±0.27 2.53/3.75 | 3.32±0.24 2.86/3.74 | 3.32±0.23 2.77/3.86 | 3.36±0.4 2.80/3.84 | .64 |
| DLPFC Thickness | 2.34±0.10 2.11/2.47 | 2.36±0.09 2.16/2.54 | 2.35±0.09 2.17/2.56 | 2.36±0.09 2.11/2.5 | .83 |
All values reported as Mean±SD, Min/Max. All volumes reported in cm3; thickness is presented in mm. ERC=Entorhinal Cortex. DLPFC=Dorsolateral Prefrontal Cortex.
indicates significant difference between PD phenotypes after controlling for disease duration.
In the MANCOVA controlling for age, education, and TICV, the groups differed in the multivariate analysis (F[15,495]=2.92, p<.01, ηp2=.081). The follow-up univariate analyses showed group differences for total brain (F[3,167]=7.97, p<.01, ηp2=.125), ventricle (F[3, 167]=11.20, p<.01, ηp2=.167), cortical gray matter (F[3,167]=5.10, p<.01, ηp2=.084), subcortical gray matter (F[3,167]=3.51, p=.02, ηp2=.059), and total white matter (F[3, 167]=3.99, p=.09, ηp2=.067) volumes. Post-hoc pairwise analyses showed that PD Executive had lower volumes in total brain, subcortical gray matter, white matter, and higher ventricular volume than all others (p<.01 in all cases) with less cortical gray matter volume than PD Cognitively Well and Non-PD peers (p<.01 in both cases). The differences in total brain, ventricular, subcortical gray, and white matter volumes remained significant after controlling for disease duration. Cortical gray matter was no longer significant after controlling for disease duration. There were no significant group differences in ERC thickness or DLPFC thickness.
Region of Interest Volumetrics (Figure 1; Table 2)
After controlling for age, years of education and TICV, groups differed significantly in putamen volume (F[3,167]=3.795, p=.01, ηp2=.064) such that PD Executive and PD Cognitively Well had significantly smaller volume than non-PD peers (p=.03 and p<.05, respectively). Groups differed significantly in thalamus volume (F[3,167]=2.849, p=.04, ηp2=.049) such that PD Executive had significantly smaller volume than PD Cognitively Well (p=.01). Groups did not differ in caudate volume, DLFPC thickness or ERC thickness.
A Priori Tractography (Figures 1&2; eTable 8)
Figure 2.
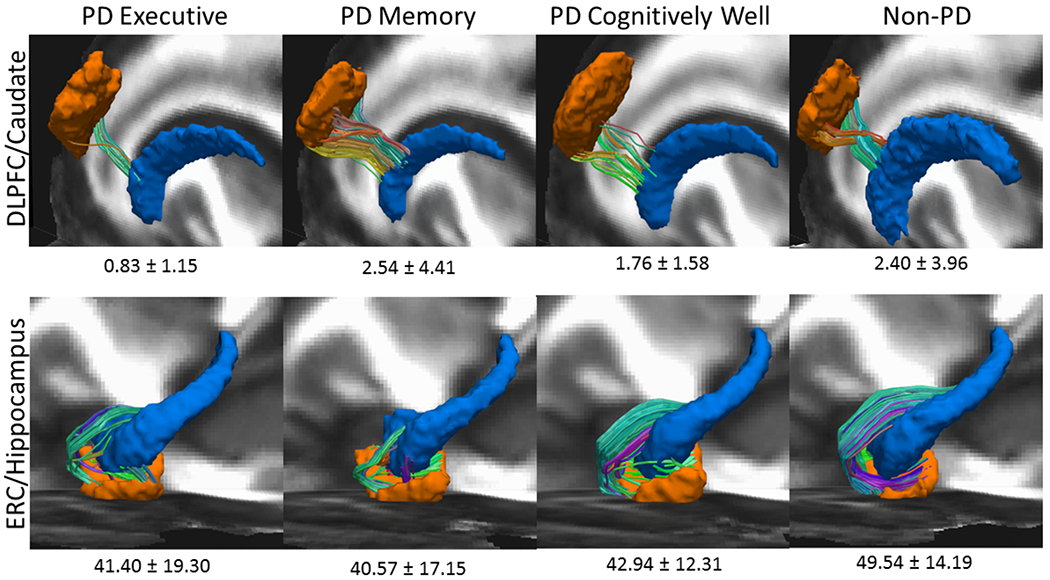
Region of interest Raw Edge Weights by PD Cognitive Phenotype (Executive = 25; Memory = 35; Well n=56) and Non-PD Peers (n=62)
Left hemisphere shown only. To create this image, FA images from all datasets were first aligned to the FMRIB58_FA template using nonlinear registration. This nonlinear registration was applied each participants’ diffusion data and region of interest masks. Voxel-wise diffusion data averaging was then performed across subjects for each subgroup to create a group-wise averaged diffusion dataset. Deterministic tractography was performed using the parameters and methodology listed above to create a group-wise average tractogram. Registered ROI masks of the dorsolateral prefrontal cortex (DLPFC), caudate nucleus (CN) Hippocampus, (HIPP) and entorhinal cortex (ERC) for each phenotype group were superimposed and thresholded to create group-wise averaged ROI masks. The tractogram of each group was then filtered using the corresponding ROI masks to obtain the tracts connecting the DLPFC to CN, and the ERC to HIPP.TOP ROW: DLFPC in orange, CN in blue. Bilaterally, PD Executive < all groups after controlling for age, sex, and TICV. BOTTOM ROW: ERC in orange, HIPP in blue. Non-PD > all cognitive PD phenotypes in bilaterally and in right hemisphere; PD < Non-PD the left hemisphere.
DLPFC-CN:
Groups differed in bilateral DLFPC-CN connectivity (F[3,165]=3.00, p=.03, ηp2=.052; PD Executive<all groups, p’s≤.02). Exploratory hemispheric follow-up analyses found group differences in right DLPFC-CN connectivity (F[3,165]=2.70, p<.05, ηp2=.047; PD Executive < PD Cognitively Well, Non-PD, p’s <.05), but not left DLPFC-CN (F[3,165]=1.69, p=.17, ηp2=.030). These patterns were unchanged after correcting for disease duration.
ERC-HIPP:
Bilateral ERC-HIPP connectivity differed between groups (F[3,165]=4.09, p<.01, ηp2=.069; non-PD>all PD phenotypes, p’s<.01). Exploration of hemisphere showed a group difference was present for the left hemisphere (F[3,165]=4.24, p<.01, ηp2=.071) but only PD Memory was lower than non-PD peers (p<.01), while all three PD phenotypes differed from non-PD peers in the right hemisphere (p’s ≤.03). These findings were unchanged after correcting for disease duration.
Discussion
The present study reliably separated individuals with idiopathic PD into three cognitive phenotypes, which differ in a manner consistent with prior literature relating cognition, disease duration, and mood variables. Even after correcting for disease duration, the cognitive phenotypes differed in gray and white matter volume and showed different structural white matter connectivity, with particularly pronounced and lower connectivity between the dorsolateral prefrontal cortex and the caudate nucleus only for PD individuals with executive dysfunction. Each PD group had reduced bilateral white matter connectivity between the entorhinal cortices and hippocampi relative to non-PD peers. Exploratory hemispheric analyses suggests this reduction for PD is largely based on right HIPP-ERC weaknesses; only the PD Memory phenotype had lower connectivity in both the left and right hemisphere. Collectively, these findings provide convincing evidence that individuals with PD can be differentiated into cognitive phenotypes based on performance on executive and memory measures, and that these phenotypes warrant additional neuroscientific investigation. In the paragraphs below we discuss the data relative to phenotype demographics and cognitive profile patterns, brain volume and vascular disease findings, and white matter connectivity results.
Cognitive and Behavioral Profile Differences by PD Phenotype:
The PD phenotype cluster shows that approximately 48% of our participants with PD were classified as cognitively well, with remaining classified as PD Executive (~22%), and PD Memory (~30%). These two cognitive phenotypes performed almost 1.5 standard deviations below their peers in respective executive or memory domains. Although the PD Executive and Memory phenotypes also showed significantly lower language and visuospatial composites relative to the non-PD peers, we note the scores are in the average range suggesting these cognitive functions were not a dominant area of weakness at the time of testing. The PD Cognitively Well group did not statistically differ from the non-PD peers in terms of cognition and performed average or higher in all domains.
Cognitive phenotypes differed in disease duration and mood symptoms, with findings consistent with expectations. PD Executive averaged nine years of disease duration – approximately three years longer than PD Memory and two years longer than PD Cognitively Well. Longer disease duration is associated with increases in a number of motor and nonmotor symptoms, including worsening bradykinesia and rigidity, more frequent freezing of gait, declining balance, increased sleep disturbances, and reduced heart rate variability [73]. This association between longer disease duration and motor symptoms is reflected in our data; the PD Executive phenotype averaged 4 points higher on the on-medication UPDRS motor subscore than the PD Cognitively Well phenotype, reflecting a 23.8% difference in on-medication motor symptom severity. This difference was not significant (p=.08), but nonetheless suggests a possible association between disease duration and motor symptom severity for our PD Executive phenotype sample. Furthermore, executive function also declines with longer disease duration [74]. Therefore, the PD Executive phenotype’s disease-related variables are largely consistent with the existing literature. The PD Memory, however, had relatively similar disease duration to the PD-Well with an average of 6 and 7 years, respectively. Despite this, the PD Memory phenotype has dominant memory weaknesses (~ 1.5 standard deviations below the PD Cognitively Well and non-PD peers). Research to date has limited information on individuals with PD and a primary memory deficit, but has noted that memory deficits in PD associate with abnormalities in temporal regions [4, 75].
In contrast to the PD Executive and Memory cognitive phenotypes, the PD Cognitively Well phenotype is notable for cognitive performance on par with non-PD peers despite similar disease duration to PD Memory peers and comparable levodopa dosage to other phenotypes. They showed milder motor symptoms than the other phenotypes, with the on-medication UPDRS III four points lower than both other phenotypes. Some of this may be due to their higher level of education, as PD Cognitively Well averaged almost one additional year of education than the other two phenotypes. This suggests higher cognitive reserve [76, 77]. Education is a major component of cognitive reserve, and higher education in PD associates with better global cognition, attention, visuospatial functioning, executive functioning and memory [78], as well as less severe PD motor symptoms [79], which may explain the lower symptom severity within this phenotype. This underscores the importance of considering degree of education when assessing current cognitive function and prognosis of individuals presenting with PD.
In addition to varying disease duration, the phenotypes differed in mood symptom severity. This is important to mention given the relevance of this symptom to cognition and brain profiles. The PD Executive phenotype had significantly more severe depressive and apathy symptoms than other phenotypes, though all three phenotypes had significantly more severe depressive symptoms than non-PD peers. These findings are consistent with the extant literature, which consistently finds associations between apathy and executive function, as well as associations between depression and both executive function and memory [80]. In addition, given that the PD Executive phenotype also had the highest disease duration, this finding is consistent with prior literature reporting depressive and apathy symptoms increase over the disease course [81]. Thus, the findings in both disease duration and mood variables are consistent with prior research, lending further validity to the derived cognitive phenotypes.
Phenotype Structural Neuroimaging Profiles
As expected, phenotypes showed distinct subcortical white matter, subcortical gray matter volume, and gray-white matter connectivity differences even after correcting for disease duration. PD Executive had less overall subcortical gray than non-PD peers and the lowest putamen, thalamus, and white matter volumes as well as the largest ventricles of the three phenotypes. This pattern was present without significant group differences in vascular disease risk markers, dopaminergic medication or anticholinergic medication dosages. These findings, combined with the cortical gray matter volume differences, suggests that the PD Executive phenotype has a unique subcortical gray and white matter profile differentiating them from their PD Memory and PD Cognitively Well peers. This is consistent with prior research, which has demonstrated an association between white matter integrity and executive function performance in PD [82, 83].
It is important to emphasize that while PD Executive had lower executive function than the other phenotypes, their cognitive impairments are generally mild; some individuals within the group might not be classified as impaired within clinical settings. However, given that they display significant subcortical and white matter volume reduction, this group may be more likely to convert to PD dementia than other phenotypes. Prior research has shown that PD pathology in subcortical regions and white matter generally predates cortical atrophy [84]; cortical atrophy is more typical of individuals with PDD [85]. Therefore, the Executive participants may be at the most risk for further cognitive decline. Follow-up analyses should determine whether those in this phenotype convert to PDD at a higher rate than other phenotypes. In addition to widespread volumetric differences, PD Executive had significantly lower DLPFC-CN white matter structural connectivity than other phenotypes. This is consistent with established theory suggesting structural abnormalities in corticostriatal circuits influence executive dysfunction in PD [15, 82].
In contrast to the widespread brain abnormalities in PD Executive, the PD Memory phenotype showed lower left ERC-HIPP connectivity relative to non-PD peers, with no evidence of significantly reduced cortical gray, subcortical gray, or white matter volumes. This suggests focal white matter neurodegeneration in the area of the perforant pathway without broader apparent atrophy in adjacent gray matter – a finding consistent with previous reports of lower temporal lobe white matter connectivity for non-demented PD with memory weaknesses (e.g., retrosplenial to entorhinal cortex [4]). Previous studies have shown ERC atrophy largely in those with PD dementia [12], although one group showed reduced right entorhinal thickness over time in PD MCI [86]. This reduced connectivity may be secondary atrophy caused by cholinergic denervation, as proposed by the dual syndrome hypothesis [9], or the result of direct synuclein pathology in anterior temporal lobe, which is typically the first region of cortex to show Lewy bodies [13]. If this is the case, our findings suggest that individuals with PD presenting with primary memory deficits might have more temporal synuclein pathology than individuals of other phenotypes. It is also important to note that the nature of the memory deficits in this phenotype might vary between participants, as some individuals with PD show deficits in memory encoding, while others show deficits in memory retrieval [4, 43]. Further investigations of memory deficits in PD should focus on comparisons in temporal region and differentiate deficits in retrieval verses recall.
The PD Cognitively Well group had lower putamen volume and lower connectivity between the right entorhinal cortex and hippocampus than non-PD peers. We previously reported on extensive putamen and hippocampal morphometric differences in a subgroup of this sample [87]. Thus, the differences from non-PD peers in putamen and hippocampal regions for the PD Cognitively Well group are not surprising. It is possible these regions serve as sensitive markers of Parkinson’s disease without cognitive phenotype specificity.
Other Considerations
It is useful to compare our group findings relative to the dual syndrome hypothesis. This hypothesis proposes that dopaminergic denervation contributes to working memory and inhibitory difficulties in PD, while cholinergic denervation contributes to learning/memory and accompanying visuospatial difficulties. While we do report two distinct executive and memory phenotypes in PD, there is no compelling difference between these two groups on measures of language or visuospatial function. Relative to the non-PD and PD Cognitively Well, only the Executive phenotype performed significantly worse on a measures of visuospatial function. Perhaps participants in the PD Executive group are experiencing some of the cholinergic denervation proposed to drive the visuospatial and semantic fluency deficits suggested in the dual syndrome hypothesis [9]. Prefrontal cholinergic activity is associated with executive function [88], and cortical cholinergic denervation is associated with executive dysfunction in PD [89]. The Memory phenotype, by contrast, may reflect temporal dysfunction not directly driven by the cholinergic system, different levels of amyloid-β and tau [90], or altered levels of alpha-synuclein [91–93]. These are areas for future research.
It is also useful to consider the possible relationship between motor and cognitive phenotypes in our sample, as some researchers show relationships between motor profiles and cognitive weaknesses e.g.,[94] and cognitive change over time e.g.,[95]. To address topic, we report here on a post-hoc analysis of motor phenotype classification using our on-dopaminergic medication UPDRS cluster scores for tremor dominant (TD) and postural instability/ gait difficulty (PIGD) [34, 96]. Our participant sample classified as 31.6%TD, 28.9% intermediate, and 39.5% PIGD classification. Motor groups did not significantly differ in memory or executive profile composites (all p’s > .80) or by edge weight structural measurements (all p values > .21). These post-hoc findings suggest on-medication motor type does not predict the cognitive phenotypes defined in this investigation.
While we do recognize limitations for assessing cognition in PD while on dopaminergic medication, we planned this approach so that participants would be more comfortable and able to provide best effort. Being on-medication, however, may have stabilized executive function subdomains (processing speed, working memory, inhibitory function) without improving memory function [97]. This might explain why the PD Executive phenotype was the smallest of the three, despite executive dysfunction being the most typical deficit in PD [6]. It might also explain our higher percentage of participants in the Cognitively Well phenotype. For these reasons, we encourage future research to consider if cognitive phenotypes differ on versus off dopaminergic medication and if these profiles relate to motor type presentation and disease progression.
Other considerations include the homogeneous demographics, standardization approach, focus on structural imaging only, and need for replication. Our sample is highly educated and Caucasian and so the sample findings cannot be extended to other demographic samples. Regarding our cognitive metrics, our memory measures were verbally biased, limiting our appreciation for memory for more visual based information. This may be particularly important to the right ERC-HIPP connectivity role in our PD phenotypes. In addition, the cluster analysis used z-scores from published normative test sources which may not reflect sociodemographics of the current study sample. Due to our concern for normative referenced potential bias on our cluster analyses, we replicated (post-hoc) the cluster analyses using age and education corrected z-scores based on our local non-PD sample. These post-hoc analyses show adequate agreement to our original normative reference clusters (102/116 clustered in the same domains; 87.9% agreement). We encourage future researchers to consider the value of published versus local normative reference groups for their sample of cognitive phenotypes[98]. For imaging methodology, we exclusively examined structural brain variables. There is extensive literature, however, demonstrated resting state network dysfunction in PD [99]. Future analyses should examine differences in resting state function between these phenotypes, particularly the interaction between established resting state networks such as the default mode network and salience network. Finally, although we used an a priori hypothesis approach and report results with effect sizes, we recognize the findings need replication.
Despite limitations and the need for follow-up research, the current study has many design strengths. These include the prospective and hypothesis driven nature of the investigation, recruitment of individuals who received the same cognitive and neuroimaging protocols to strategically examine gray-white matter connectivity regions of interest using tractography methods, and the comprehensive neuropsychological test protocol providing opportunity to assess cognitive phenotypes even among individuals with largely average cognition. Cognitive phenotypes were also considered relative to demographic and clinical characteristics, vascular markers, dopaminergic medication dose, anticholinergic medications, and depressive and apathy symptoms. The results provide convincing evidence that individuals with idiopathic non-dementia PD can be differentiated into cognitive phenotypes based on performance on executive and memory measures, and that these phenotypes present with unique neuroimaging profiles at least from a structural standpoint.
Supplementary Material
Acknowledgements:
We wish to sincerely thank the participants who provided time and effort towards this investigation. Without them, this study and research would certainly not have been possible. Also thank you to the research coordinators Donna Webe and Kristy Ayers for their valuable time in study coordination and assistance. Additional thanks to Michael Okun, M.D., UF, for his study encouragement.
Financial Disclosures
Sources of funding include NINDS R01 NS082386 (CP), K23 NS060660 (CP), and T32-NS082128 (DB, SC), as well as UL1TR001427 (UF CTSI).
Funding Sources: This work was conducted at the University of Florida. Sources of funding include NINDS R01 NS082386 (CP), K23 NS060660 (CP), and T32-NS082128 (DB; SC on training grant). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Footnotes
Conflicts of Interest/Financial Disclosures: None
References
- [1].Raein KL, Ortiz-Hernandez S, Benge JF (2019) Cognitive Problems in Parkinson Disease: Perspectives and Priorities of Patients and Care Partners. Cogn Behav Neurol 32, 16–24. [DOI] [PubMed] [Google Scholar]
- [2].Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, Burn D, Barone P, Pagonabarraga J, Allcock L, Santangelo G, Foltynie T, Janvin C, Larsen JP, Barker RA, Emre M (2010) Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 75, 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hoogland J, van Wanrooij LL, Boel JA, Goldman JG, Stebbins GT, Dalrymple-Alford JC, Marras C, Adler CH, Junque C, Pedersen KF, Mollenhauer B, Zabetian CP, Eslinger PJ, Lewis SJG, Wu RM, Klein M, Rodriguez-Oroz MC, Cammisuli DM, Barone P, Biundo R, de Bie RMA, Schmand BA, Troster AI, Burn DJ, Litvan I, Filoteo JV, Geurtsen GJ, Weintraub D, Disease ISGVoMCIiP (2018) Detecting Mild Cognitive Deficits in Parkinson’s Disease: Comparison of Neuropsychological Tests. Mov Disord 33, 1750–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tanner JJ, Mareci TH, Okun MS, Bowers D, Libon DJ, Price CC (2015) Temporal Lobe and Frontal-Subcortical Dissociations in Non-Demented Parkinson’s Disease with Verbal Memory Impairment. PLoS One 10, e0133792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aarsland D, Kurz MW (2010) The epidemiology of dementia associated with Parkinson disease. J Neurol Sci 289, 18–22. [DOI] [PubMed] [Google Scholar]
- [6].Price CC, Tanner J, Nguyen PT, Schwab NA, Mitchell S, Slonena E, Brumback B, Okun MS, Mareci TH, Bowers D (2016) Gray and White Matter Contributions to Cognitive Frontostriatal Deficits in Non-Demented Parkinson’s Disease. PLoS One 11, e0147332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lafo JA, Jones JD, Okun MS, Bauer RM, Price CC, Bowers D (2015) Memory Similarities Between Essential Tremor and Parkinson’s Disease: A Final Common Pathway? Clin Neuropsychol 29, 985–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weintraub D, Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Siderowf A, Aarsland D, Barone P, Burn D, Chahine LM, Eberling J, Espay AJ, Foster ED, Leverenz JB, Litvan I, Richard I, Troyer MD, Hawkins KA, Parkinson’s Progression Markers I (2015) Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson’s disease. Mov Disord 30, 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kehagia AA, Barker RA, Robbins TW (2013) Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neurodegener Dis 11, 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chahine LM, Dos Santos C, Fullard M, Scordia C, Weintraub D, Erus G, Rosenthal L, Davatzikos C, McMillan CT (2019) Modifiable vascular risk factors, white matter disease and cognition in early Parkinson’s disease. Eur J Neurol 26, 246–e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jones JD, Tanner JJ, Okun M, Price CC, Bowers D (2017) Are Parkinson’s Patients More Vulnerable to the Effects of Cardiovascular Risk: A Neuroimaging and Neuropsychological Study. J Int Neuropsychol Soc 23, 322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goldman JG, Stebbins GT, Bernard B, Stoub TR, Goetz CG, deToledo-Morrell L (2012) Entorhinal cortex atrophy differentiates Parkinson’s disease patients with and without dementia. Mov Disord 27, 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Del Tredici K, Braak H (2016) Review: Sporadic Parkinson’s disease: development and distribution of alpha-synuclein pathology. Neuropathol Appl Neurobiol 42, 33–50. [DOI] [PubMed] [Google Scholar]
- [14].Stav AL, Johansen KK, Auning E, Kalheim LF, Selnes P, Bjornerud A, Hessen E, Aarsland D, Fladby T (2016) Hippocampal subfield atrophy in relation to cerebrospinal fluid biomarkers and cognition in early Parkinson’s disease: a cross-sectional study. NPJ Parkinsons Dis 2, 15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9, 357–381. [DOI] [PubMed] [Google Scholar]
- [16].La C, Linortner P, Bernstein JD, Ua Cruadhlaoich MAI, Fenesy M, Deutsch GK, Rutt BK, Tian L, Wagner AD, Zeineh M, Kerchner GA, Poston KL (2019) Hippocampal CA1 subfield predicts episodic memory impairment in Parkinson’s disease. Neuroimage Clin 23, 101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Price CC, Tanner JJ, Schmalfuss IM, Brumback B, Heilman KM, Libon DJ (2015) Dissociating Statistically-Determined Alzheimer’s Disease/Vascular Dementia Neuropsychological Syndromes Using White and Gray Neuroradiological Parameters. J Alzheimers Dis 48, 833–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Libon DJ, Drabick DA, Giovannetti T, Price CC, Bondi MW, Eppig J, Devlin K, Nieves C, Lamar M, Delano-Wood L, Nation DA, Brennan L, Au R, Swenson R (2014) Neuropsychological syndromes associated with Alzheimer’s/vascular dementia: a latent class analysis. J Alzheimers Dis 42, 999–1014. [DOI] [PubMed] [Google Scholar]
- [19].Clark LR, Delano-Wood L, Libon DJ, McDonald CR, Nation DA, Bangen KJ, Jak AJ, Au R, Salmon DP, Bondi MW (2013) Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Int Neuropsychol Soc 19, 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Szymkowicz SM, Dotson VM, Jones JD, Okun MS, Bowers D (2018) Symptom Dimensions of Depression and Apathy and Their Relationship With Cognition in Parkinson’s Disease. J Int Neuropsychol Soc 24, 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ (1992) What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology 42, 1142–1146. [DOI] [PubMed] [Google Scholar]
- [22].Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17, 427–442. [DOI] [PubMed] [Google Scholar]
- [23].Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF (2008) A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord 23, 183–189; quiz 313. [DOI] [PubMed] [Google Scholar]
- [24].den Brok MG, van Dalen JW, van Gool WA, Moll van Charante EP, de Bie RM, Richard E (2015) Apathy in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 30, 759–769. [DOI] [PubMed] [Google Scholar]
- [25].Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40, 373–383. [DOI] [PubMed] [Google Scholar]
- [26].Briggs GG, Nebes RD (1975) Patterns of hand preference in a student population. Cortex 11, 230–238. [DOI] [PubMed] [Google Scholar]
- [27].Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE (2008) The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 168, 508–513. [DOI] [PubMed] [Google Scholar]
- [28].Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25, 2649–2653. [DOI] [PubMed] [Google Scholar]
- [29].Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [30].Beck AT (1999), ed. Steer RA The Psychological Corporation, San Antonio, TX. [Google Scholar]
- [31].Marin RS, Biedrzycki RC, Firinciogullari S (1991) Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 38, 143–162. [DOI] [PubMed] [Google Scholar]
- [32].Kirsch-Darrow L, Zahodne LB, Marsiske M, Okun MS, Foote KD, Bowers D The trajectory of apathy after deep brain stimulation: from pre-surgery to 6 months post-surgery in Parkinson’s disease. Parkinsonism Relat Disord 17, 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fahn S, Elton RL et al. (1987) Unified Parkinson’s Disease Rating Scale In Recent Developments in Parkinson’s Disease, Fahn S, Marsden CD, Goldstein M, Calne DB, ed. Macmillan Publishing Company, New York, pp. 153–164. [Google Scholar]
- [34].Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I, et al. (1990) Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 40, 1529–1534. [DOI] [PubMed] [Google Scholar]
- [35].Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Tabert MH, de Leon MJ (2007) Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology 68, 828–836. [DOI] [PubMed] [Google Scholar]
- [36].Goldman JG, Holden S, Ouyang B, Bernard B, Goetz CG, Stebbins GT (2015) Diagnosing PD-MCI by MDS Task Force criteria: how many and which neuropsychological tests? Mov Disord 30, 402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pfeiffer HC, Lokkegaard A, Zoetmulder M, Friberg L, Werdelin L (2014) Cognitive impairment in early-stage non-demented Parkinson’s disease patients. Acta Neurol Scand 129, 307–318. [DOI] [PubMed] [Google Scholar]
- [38].Botwinick J, Storandt M, Berg L, Boland S (1988) Senile dementia of the Alzheimer type. Subject attrition and testability in research. Arch Neurol 45, 493–496. [DOI] [PubMed] [Google Scholar]
- [39].Heaton RK, Psychological Assessment Resources Inc. (2004) Revised comprehensive norms for an expanded Halstead-Reitan battery : demographically adjusted neuropsychological norms for African American and Caucasian adults, professional manual, Psychological Assessment Resources, Lutz, Fla. [Google Scholar]
- [40].Goldman WP, Baty JD, Buckles VD, Sahrmann S, Morris JC (1998) Cognitive and motor functioning in Parkinson disease: subjects with and without questionable dementia. Arch Neurol 55, 674–680. [DOI] [PubMed] [Google Scholar]
- [41].Janvin CC, Aarsland D, Larsen JP (2005) Cognitive predictors of dementia in Parkinson’s disease: a community-based, 4-year longitudinal study. J Geriatr Psychiatry Neurol 18, 149–154. [DOI] [PubMed] [Google Scholar]
- [42].Carlson MC, Helms MJ, Steffens DC, Burke JR, Potter GG, Plassman BL (2008) Midlife activity predicts risk of dementia in older male twin pairs. Alzheimers Dement 4, 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Weintraub D, Moberg PJ, Culbertson WC, Duda JE, Stern MB (2004) Evidence for impaired encoding and retrieval memory profiles in Parkinson disease. Cogn Behav Neurol 17, 195–200. [PubMed] [Google Scholar]
- [44].Muslimovic D, Post B, Speelman JD, Schmand B (2005) Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65, 1239–1245. [DOI] [PubMed] [Google Scholar]
- [45].Alexander GE, Delong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9, 357–381. [DOI] [PubMed] [Google Scholar]
- [46].Wechsler D (1999) Wechsler Abbreviated Scale of Intelligence, The Psychological Corporation, San Antonio, Tx. [Google Scholar]
- [47].McKinlay A, Grace RC, Dalrymple-Alford JC, Roger D (2010) Characteristics of executive function impairment in Parkinson’s disease patients without dementia. J Int Neuropsychol Soc 16, 268–277. [DOI] [PubMed] [Google Scholar]
- [48].Delis D, Kaplan E, Kramer JH (2001) Delis-Kaplan Executive Functional System: Technical Manual, Harcourt Assessment Company, San Antonio, Tx. [Google Scholar]
- [49].Davis Garrett K, Price CC, Lamar M, Giovannetti T, Delano-Wood L, Penney DL, Swenson R, Bondi MW, Delis DC, Libon DJ (2013) Assessing Verbal List Learning: The California Verbal Learning Test and the Philadelphia (Repeatable) Verbal Learning Test In The Boston Process Approach to Neuropsychological Assessment: A Practitioner's Guide OUP USA, New York City, pp. 148–169. [Google Scholar]
- [50].Libon DJ, Bondi MW, Price CC, Lamar M, Eppig J, Wambach DM, Nieves C, Delano-Wood L, Giovannetti T, Lippa C, Kabasakalian A, Cosentino S, Swenson R, Penney DL (2011) Verbal serial list learning in mild cognitive impairment: a profile analysis of interference, forgetting, and errors. J Int Neuropsychol Soc 17, 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tombaugh TN, Kozak J, Rees L (1999) Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 14, 167–177. [PubMed] [Google Scholar]
- [52].Tombaugh TN, Hubley AM (1997) The 60-item Boston Naming Test: norms for cognitively intact adults aged 25 to 88 years. J Clin Exp Neuropsychol 19, 922–932. [DOI] [PubMed] [Google Scholar]
- [53].Steinberg BA, Bieliauskas LA, Smith GE, Langellotti C, Ivnik RJ (2005) Mayo’s Older Americans Normative Studies: Age- and IQ-Adjusted Norms for the Boston Naming Test, the MAE Token Test, and the Judgment of Line Orientation Test. Clin Neuropsychol 19, 280–328. [DOI] [PubMed] [Google Scholar]
- [54].Enders CK (2010) Applied missing data analysis, Guilford Press, New York. [Google Scholar]
- [55].Heaton RK, Miller WS, Taylor MJ, Grant I (2004) Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults, Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- [56].Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. [DOI] [PubMed] [Google Scholar]
- [57].Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM (2004) Sequence-independent segmentation of magnetic resonance images. Neuroimage 23 Suppl 1, S69–84. [DOI] [PubMed] [Google Scholar]
- [58].Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B (2004) A hybrid approach to the skull stripping problem in MRI. Neuroimage 22, 1060–1075. [DOI] [PubMed] [Google Scholar]
- [59].Fischl B (2012) FreeSurfer. Neuroimage 62, 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Crowley SJ, Tanner JJ, Ramon D, Schwab NA, Hizel LP, Price CC (2018) Reliability and Utility of Manual and Automated Estimates of Total Intracranial Volume. J Int Neuropsychol Soc 24, 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hachinski VC, Potter P, Merskey H (1987) Leuko-araiosis. Arch Neurol 44, 21–23. [DOI] [PubMed] [Google Scholar]
- [62].Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116–1128. [DOI] [PubMed] [Google Scholar]
- [63].Al-Hakim R, Fallon J, Nain D, Melonakos J, Tannenbaum A (2006) in Medical Imaging 2006: Image Processing International Society of Optics and Photonics, p. 61440J. [Google Scholar]
- [64].Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jian B, Vemuri BC, Ozarslan E, Carney PR, Mareci TH (2007) A novel tensor distribution model for the diffusion-weighted MR signal. Neuroimage 37, 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Colon-Perez LM, Spindler C, Goicochea S, Triplett W, Parekh M, Montie E, Carney PR, Price C, Mareci TH (2015) Dimensionless, Scale Invariant, Edge Weight Metric for the Study of Complex Structural Networks. PLoS One 10, e0131493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wiggins ME, Tanner J, Schwab N, Crowley SJ, Schmalfuss I, Brumback B, Libon DJ, Heilman K, Price CC (2018) Regional leukoaraiosis and cognition in non-demented older adults. Brain Imaging Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Abràmoff MD, Magalhāes PJ, Ram SJ (2004) Image Processing with ImageJ. Biophotonics International 11, 36–42. [Google Scholar]
- [69].Price CC, Mitchell SM, Brumback B, Tanner JJ, Schmalfuss I, Lamar M, Giovannetti T, Heilman KM, Libon DJ (2012) MRI-leukoaraiosis thresholds and the phenotypic expression of dementia. Neurology 79, 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jiang J, Liu T, Zhu W, Koncz R, Liu H, Lee T, Sachdev PS, Wen W (2018) UBO Detector - A cluster-based, fully automated pipeline for extracting white matter hyperintensities. Neuroimage 174, 539–549. [DOI] [PubMed] [Google Scholar]
- [71].Kaiser HF (1960) The application of electronic computers to factor analysis. Educational and Psychological Measurement 20, 141–151. [Google Scholar]
- [72].Hair JF (2010) Multivariate data analysis, Prentice Hall, Upper Saddle River, NJ. [Google Scholar]
- [73].Maetzler W, Liepelt I, Berg D (2009) Progression of Parkinson’s disease in the clinical phase: potential markers. Lancet Neurol 8, 1158–1171. [DOI] [PubMed] [Google Scholar]
- [74].Muslimovic D, Schmand B, Speelman JD, de Haan RJ (2007) Course of cognitive decline in Parkinson’s disease: a meta-analysis. J Int Neuropsychol Soc 13, 920–932. [DOI] [PubMed] [Google Scholar]
- [75].Carlesimo GA, Piras F, Assogna F, Pontieri FE, Caltagirone C, Spalletta G (2012) Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology 78, 1939–1945. [DOI] [PubMed] [Google Scholar]
- [76].Stern Y (2002) What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 8, 448–460. [PubMed] [Google Scholar]
- [77].Satz P (1993) Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology 7, 273–295. [Google Scholar]
- [78].Hindle JV, Martyr A, Clare L (2014) Cognitive reserve in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 20, 1–7. [DOI] [PubMed] [Google Scholar]
- [79].Lee PC, Artaud F, Cormier-Dequaire F, Rascol O, Durif F, Derkinderen P, Marques AR, Bourdain F, Brandel JP, Pico F, Lacomblez L, Bonnet C, Brefel-Courbon C, Ory-Magne F, Grabli D, Klebe S, Mangone G, You H, Mesnage V, Brice A, Vidailhet M, Corvol JC, Elbaz A, Group DS (2019) Examining the Reserve Hypothesis in Parkinson’s Disease: A Longitudinal Study. Mov Disord 34, 1663–1671. [DOI] [PubMed] [Google Scholar]
- [80].Alzahrani H, Venneri A (2015) Cognitive and neuroanatomical correlates of neuropsychiatric symptoms in Parkinson’s disease: A systematic review. J Neurol Sci 356, 32–44. [DOI] [PubMed] [Google Scholar]
- [81].Wen MC, Chan LL, Tan LC, Tan EK (2016) Depression, anxiety, and apathy in Parkinson’s disease: insights from neuroimaging studies. Eur J Neurol 23, 1001–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gallagher C, Bell B, Bendlin B, Palotti M, Okonkwo O, Sodhi A, Wong R, Buyan-Dent L, Johnson S, Willette A, Harding S, Ninman N, Kastman E, Alexander A (2013) White matter microstructural integrity and executive function in Parkinson’s disease. J Int Neuropsychol Soc 19, 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Foo H, Mak E, Yong TT, Wen MC, Chander RJ, Au WL, Sitoh YY, Tan LC, Kandiah N (2017) Progression of subcortical atrophy in mild Parkinson’s disease and its impact on cognition. Eur J Neurol 24, 341–348. [DOI] [PubMed] [Google Scholar]
- [84].Zeighami Y, Ulla M, Iturria-Medina Y, Dadar M, Zhang Y, Larcher KM, Fonov V, Evans AC, Collins DL, Dagher A (2015) Network structure of brain atrophy in de novo Parkinson’s disease. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Rektor I, Svatkova A, Vojtisek L, Zikmundova I, Vanicek J, Kiraly A, Szabo N (2018) White matter alterations in Parkinson’s disease with normal cognition precede grey matter atrophy. PLoS One 13, e0187939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Jia X, Wang Z, Yang T, Li Y, Gao S, Wu G, Jiang T, Liang P (2019) Entorhinal Cortex Atrophy in Early, Drug-naive Parkinson’s Disease with Mild Cognitive Impairment. Aging Dis 10, 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tanner JJ, McFarland NR, Price CC (2017) Striatal and Hippocampal Atrophy in Idiopathic Parkinson’s Disease Patients without Dementia: A Morphometric Analysis. Front Neurol 8, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Howe WM, Berry AS, Francois J, Gilmour G, Carp JM, Tricklebank M, Lustig C, Sarter M (2013) Prefrontal cholinergic mechanisms instigating shifts from monitoring for cues to cue-guided performance: converging electrochemical and fMRI evidence from rats and humans. J Neurosci 33, 8742–8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bohnen NI, Albin RL, Muller ML, Petrou M, Kotagal V, Koeppe RA, Scott PJ, Frey KA (2015) Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of Parkinson disease and evidence of interaction effects. JAMA Neurol 72, 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lim EW, Aarsland D, Ffytche D, Taddei RN, van Wamelen DJ, Wan YM, Tan EK, Ray Chaudhuri K, Kings Parcog group MDSNsg (2019) Amyloid-beta and Parkinson’s disease. J Neurol 266, 2605–2619. [DOI] [PubMed] [Google Scholar]
- [91].Quinn MT, Gauss KA (2004) Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol 76, 760–781. [DOI] [PubMed] [Google Scholar]
- [92].Rocha EM, De Miranda B, Sanders LH (2018) Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol Dis 109, 249–257. [DOI] [PubMed] [Google Scholar]
- [93].Kim C, Lee HJ, Masliah E, Lee SJ (2016) Non-cell-autonomous Neurotoxicity of alpha-synuclein Through Microglial Toll-like Receptor 2. Exp Neurobiol 25, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].D’Iorio A, Maggi G, Vitale C, Amboni M, Di Meglio D, Trojano L, Santangelo G (2019) Prospective memory in Parkinson’s disease: the role of the motor subtypes. J Neurol 266, 2505–2511. [DOI] [PubMed] [Google Scholar]
- [95].Johnson AR, Bucks RS, Kane RT, Thomas MG, Gasson N, Loftus AM (2016) Motor Subtype as a Predictor of Future Working Memory Performance in Idiopathic Parkinson’s Disease. PLoS One 11, e0152534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC (2013) How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord 28, 668–670. [DOI] [PubMed] [Google Scholar]
- [97].Lange KW, Robbins TW, Marsden CD, James M, Owen AM, Paul GM (1992) L-dopa withdrawal in Parkinson’s disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology (Berl) 107, 394–404. [DOI] [PubMed] [Google Scholar]
- [98].Wyman-Chick KA, Martin PK, Weintraub D, Sperling SA, Erickson LO, Manning CA, Barrett MJ (2018) Selection of Normative Group Affects Rates of Mild Cognitive Impairment in Parkinson’s Disease. Mov Disord 33, 839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Baggio HC, Segura B, Junque C (2015) Resting-state functional brain networks in Parkinson’s disease. CNS Neurosci Ther 21, 793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


