Abstract
Objective:
Financial capacity is often one of the first instrumental activities of daily living to be affected in cognitively normal (CN) older adults who later progress to amnestic mild cognitive impairment (MCI) and Alzheimer’s disease (AD) dementia. The objective of this study was to investigate the association between financial capacity and regional cerebral tau.
Methods:
Cross-sectional financial capacity was assessed using the Financial Capacity Instrument – Short Form (FCI-SF) in 410 CN, 199 MCI, and 61 AD dementia participants who underwent flortaucipir tau positron emission tomography from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Linear regression models with backward elimination were used with FCI-SF total score as the dependent variable and regional tau and tau-amyloid interaction as predictors of interest in separate analyses. Education, age sex, Rey Auditory Verbal Learning Test Total Learning, and Trail Making Test B were used as covariates.
Results:
Significant associations were found between FCI-SF and tau regions (entorhinal: p<0.001; inferior temporal: p<0.001; dorsolateral prefrontal: p=0.01; posterior cingulate: p=0.03; precuneus: p<0.001; and supramarginal gyrus: p=0.005) across all participants. For the tau-amyloid interaction, significant associations were found in 4 regions (amyloid and dorsolateral prefrontal tau interaction: p=0.005; amyloid and posterior cingulate tau interaction: p=0.005; amyloid and precuneus tau interaction: p<0.001; and amyloid and supramarginal tau interaction: p=0.002).
Conclusions:
Greater regional tau burden was modestly associated with financial capacity impairment in early-stage AD. Extending this work with longitudinal analyses will further illustrate the utility of such assessments in detecting clinically meaningful decline, which may aid clinical trials of early-stage AD.
Keywords: Alzheimer’s disease, amyloid, financial capacity, instrumental activities of daily living, mild cognitive impairment, positron emission tomography, tau
Introduction
Alzheimer’s disease (AD) dementia is a neurodegenerative disorder consisting of cognitive, behavioral, and functional impairment that is estimated to affect about 14 million Americans over the age of 65 by the year 2050 [1]. Progression from a diagnosis of cognitively normal (CN) to amnestic mild cognitive impairment (MCI), followed by further decline and transition to AD dementia, is often accompanied by impairment in the ability to perform instrumental activities of daily living (IADL)[2, 3, 4]. In addition to negatively impacting the individual, IADL impairment increases caregiver mental and financial burden [5, 6, 7].
Financial capacity [8], performing household chores/repairs, and medication management are some of the many tasks that consist of IADL. Financial capacity has been defined as a complex IADL ability to manage one’s own finances in a consistent manner (e.g., making change, balancing the checkbook, and paying bills on time) [9]. Previous studies have shown that financial capacity often manifests as the first aspect of IADL decline in individuals who later progress to MCI and AD dementia [8, 10, 11] giving reason to further investigate them.
The association between a variety of neuroimaging modalities and IADL impairment across older adults has been an increasing topic of interest. Several studies have previously found that inferior temporal cortical thinning, frontal and parietal hypometabolism, frontal white matter hyperintensities, and bilateral prefrontal, striate, and anterior cingulate hypoperfusion have been associated with greater IADL impairment across the AD spectrum [12, 13, 14, 15]. More recently, Halawa and colleagues found that greater in vivo medial and inferior temporal tau burden and amyloid burden are associated with greater IADL impairment across the AD spectrum [16], while Marshall and colleagues found an association in MCI and AD dementia between IADL impairment and medial temporal and frontal tau burden in individuals with elevated amyloid [17]. These findings are in line with postmortem and cerebrospinal fluid (CSF) studies that have found that IADL impairment are associated with neurofibrillary tangles (tau burden) and neuritic plaques (amyloid burden) in medial temporal, frontal, and occipital regions in individuals with moderate to severe AD, as well as CSF lower amyloid-β 1–42 and greater total tau across the AD spectrum [18, 19].
As described above, previous studies have displayed an association between IADL and certain brain regions, but few studies have assessed the relationship between financial capacity and AD pathology. One such recent study found that higher cortical amyloid burden was significantly associated with higher impairment in financial capacity in participants with early-stage AD [20]. However, to our knowledge, no previous study has investigated the relationship between tau pathology and financial capacity.
Tau and amyloid accumulation are the hallmarks of AD pathology and correspond to cognitive decline [21, 22, 23]. Cohorts of older adults followed longitudinally with multi-modal imaging such as the Alzheimer’s Disease Neuroimaging Initiative (ADNI-3), utilize positron emission tomography (PET) tracers such as flortaucipir (FTP) (a.k.a AV-1451) and florbetapir (FBP) to measure tau and amyloid burden respectively [24, 25, 26, 27]. Cortical amyloid PET has also been used in clinical settings to assess biomarker burden in MCI and dementia patients [22]. Increases in amyloid and tau burden are associated with the progression of cognitive decline in older adults at risk for AD [28].
Change in financial capacity, a complex, high-level aspect of IADL, may play an important role in the early clinical manifestation of AD. Therefore, if assessed with a sensitive enough test, deficits in financial capacity could be detected as early as the preclinical stage of AD. The objective of this study was to investigate the association between financial capacity and regional cerebral tau across CN, MCI, and AD dementia participants. The current study looked at six bilateral subcortical and cortical regions (entorhinal cortex, inferior temporal cortex, dorsolateral prefrontal cortex, posterior cingulate cortex, precuneus, and supramarginal gyrus), since these are regions that have been examined in the most recent study [16]. To assess financial capacity, we used the Financial Capacity Instrument – Short form (FCI-SF) [9], a performance-based IADL measure that evaluates one’s financial knowledge. We hypothesized that worse performance on the FCI-SF will be associated with greater tau burden primarily in temporal and frontal regions across CN and cognitively symptomatic participants. We further hypothesized that an interaction between greater tau and greater amyloid will be associated with worse financial capacity, given that Halawa and colleagues reported such results using a 10-item IADL questionnaire. Studying the cross-sectional relationship between regional tau deposition and financial capacity can serve as a basis for future longitudinal studies assessing the utility of IADL impairment in tracking and predicting disease progression in early-stage AD.
Materials and Methods
Participants
The study consisted of 410 CN, 199 amnestic MCI, and 61 AD dementia older adults ranging in age from 56–95 years old who were participants in the Alzheimer’s Disease Neuroimaging Initiative 3 (ADNI-3) study [29]. ADNI-3 was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD and involves 50 North American sites. Its primary goal has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessments can measure the progression from CN to MCI and mild AD dementia. Only participants who underwent flortaucipir (FTP) (a.k.a. AV-1451) tau PET imaging within a year of completing the Financial Capacity Instrument – Short Form (FCI-SF) were included in the sample. Participants were 48.1% female. Diagnoses were determined by the principal investigator or co-investigators at each site utilizing assessments of cognition, neuropsychiatric symptoms, and IADL (not including the FCI-SF). Participants with scores higher than 6 on the 15-item Geriatric Depression Scale (GDS) were excluded from the study. The study was approved by the local Institutional Review Board of each participating ADNI site. Participants and study partners reviewed and signed forms prior to completion of any study procedures.
Clinical Assessments
Financial capacity were assessed using the Financial Capacity Instrument – Short Form (FCI-SF) [9] that evaluates participants financial skills in the domain of monetary calculation, financial concepts, register usage, and bank statement usage. The FCI-SF consists of 37 items and five domain scores (Mental Calculation, Financial Conceptual Knowledge, Single Checkbook Register, Complex Checkbook Register, Bank Statement Management) that are summed into a Total Score (range of 0–74), with higher scores indicating better financial capacity. The total score is a summary of individual domains: Mental Calculation (0–4), Financial Conceptual Knowledge (0–8), Single Checkbook Register (0–20), Complex Checkbook Register (0–28), and Bank Statement Management (0–14).
Cognitive assessments were used as covariates in analyses. The Rey Auditory Verbal Learning Test (RAVLT) [30] total learning score assesses performance of episodic memory. In this assessment, participants are presented a list of 15 words over 5 trials and are asked to repeat them after a distractor list and a delay. The total learning score ranges between 0–75 and lower scores indicates poorer performance. The Trail Making Test B (TMT-B) [31] score was used to asses executive function (divided attention and task switching performance). It is recorded by the time it takes an individual to complete the task, where higher scores indicate greater impairment.
Tau and Amyloid PET Data
FTP and FBP PET imaging acquisition and processing was described in prior publications extensively [32, 33]. Briefly, FTP scans were co-registered to corresponding MRI scans that were segmented and parcellated with Freesurfer (version 5.3.0) in order to calculate the mean FTP uptake within each Freesurfer-defined region with cerebellar cortex as the reference region yielding a standardized uptake value ratio (SUVR) (UC Berkeley—AV1451 Analysis Methods, ADNI). Six bilateral subcortical and cortical regions of interest (ROI) were chosen based on prior studies assessing cerebral regional changes and IADL: entorhinal cortex, inferior temporal cortex, dorsolateral prefrontal cortex, posterior cingulate cortex, precuneus, and supramarginal gyrus [19, 34, 12, 35]. FBP scans were co-registered to corresponding MRI scans that were segmented and parcellated with Freesurfer (version 5.3.0) to define the amyloid cortical gray matter ROI (frontal, anterior cingulate, posterior cingulate, lateral parietal, and lateral temporal) that make up an aggregate cortical ROI with whole cerebellum as the reference region yielding an SUVR (UC Berkeley—AV45 Analysis Methods, ADNI). Amyloid status (positive or negative) was defined by a cutoff of FBP SUVR of 1.10 as previously described—amyloid-positive > 1.10 and amyloid-negative ≤ 1.10.
Statistical Analysis
All analyses for this study were carried out using SPSS version 26.0 (IBM). First, Pearson correlations between the FCI-SF total score and each tau region (entorhinal cortex, inferior temporal cortex, dorsolateral prefrontal cortex, posterior cingulate cortex, precuneus, and supramarginal gyrus) were performed to evaluate the unadjusted associations across all participants and then by diagnoses (CN and cognitively symptomatic consisting of MCI and AD dementia). The tau regions with associations of p < 0.05 were subsequently used in the primary analyses. Linear regression models with backward elimination (p < 0.10 for retention, p < 0.05 for significance) were used in the primary analyses to determine the association between the FCI-SF total score (dependent variable) and the significant tau regions identified from the unadjusted correlations. Each tau region was tested in its own model. We included the following covariates in the models: education, age, sex, RAVLT Total Learning Score, and TMT-B. RAVLT and TMT-B were included as covariates because memory impairment and executive dysfunction are associated with greater IADL impairments in individuals with MCI and AD dementia and have a wider range of performance across early-stage AD compared to a test of global cognition such as the Mini-Mental State Examination (MMSE) [36, 37].
In secondary analyses, models were repeated for the FCI-SF total score but with the addition of an interaction term between cortical regional tau and cortical amyloid. Additional analyses looked at FCI-SF total score and cortical tau burden associations, separately, in cognitively asymptomatic (CN) and symptomatic (combined MCI and AD dementia) participants. Lastly, models were repeated using the five FCI-SF domain scores to assess regional tau association with each FCI-SF domain (see Supplementary Materials).
Unstandardized coefficients (β) with 95% confidence intervals (CI), Partial regression correlations (pr), and significance test results (p values) were reported. We did not adjust for multiple comparisons in any of the above analyses.
Results
Participant demographics and characteristics, including cognitive, tau, and amyloid values by diagnostic group can be found in table 1. FCI-SF total scores and domain scores (Mental Calculation, Financial Conceptual Knowledge, Single Checkbook Register, Complex Checkbook register, and Bank Statement Management) can be found in table 2.
Table 1-.
Participant Demographics and Characteristics
| Diagnosis | All | CN | MCI | AD Dementia |
|---|---|---|---|---|
| 670 | 410 | 199 | ||
| 74.5 ± 7.8†† | 73.6 ± 7.3 | 75.4 ± 8.4 | ||
| 48.1 | 41.2 | 58.3 | ||
| 16.6 ± 2.5†† | 16.8 ± 2.3 | 16.3 ± 2.6 | ||
| 90.7 | 88.8 | 93.5 | ||
| 28.1 ± 2.6* | 29.1 ± 1.2 | 27.8 ± 2.0 | ||
| 0.9 ± 1.9* | 0.1 ± 0.2 | 1.5 ± 0.9 | ||
| 91.7 ± 58.4* | 72.9 ± 33.9 | 104.2 ± 56.7 | ||
| 41.3 ± 12.8* | 47.1 ± 10.4 | 34.8 ± 10.0 | ||
| 1.17 ± 0.23* | 1.10 ± 0.13 | 1.22 ± 0.26 | ||
| 1.25 ± 0.28* | 1.18 ± 0.15 | 1.27 ± 0.27 | ||
| 1.04 ± 0.18** | 1.01 ± 0.09 | 1.04 ± 0.16 | ||
| 1.11 ± 0.18** | 1.07 ± 0.10 | 1.12 ± 0.16 | ||
| 1.13 ± 0.22** | 1.08 ± 0.09 | 1.13 ± 0.16 | ||
| 1.09 ± 0.20‡ | 1.06 ± 0.10 | 1.09 ± 0.17 | ||
| 1.15 ± 0.23* | 1.10 ± 0.15 | 1.21 ± 0.28 | ||
| 40.3 | 30.0 | 49.5 |
MMSE, Mini-Mental State Examination; CDR, Clinical Dementia Rating; TMT-B, Trail Making Test B; RAVLT, Rey Auditory Verbal Learning Test. All values (except n, sex, and race) represent mean ± standard deviation (range).
p<0.05 for CN versus MCI and CN versus AD dementia.
p<0.001 for CN versus MCI, CN versus AD dementia and MCI versus AD dementia.
p<0.05 for CN versus MCI, and p<0.001 for CN versus AD and MCI versus AD dementia.
p<0.001 for CN versus AD dementia and MCI versus AD dementia.
Table 2-.
Financial Capacity Instrument – Short Form Performance Scores
| Diagnosis | All | CN | MCI | AD Dementia |
|---|---|---|---|---|
| 64.01 ± 12.30* | 68.59 ± 5.77 | 61.47 ± 10.57 | ||
| 3.42 ± 1.20** | 3.65 ± 0.87 | 3.39 ± 1.29 | ||
| 7.18 ± 1.29‡ | 7.54 ± 0.84 | 7.04 ± 1.28 | ||
| 17.56 ± 3.56* | 18.71 ± 1.85 | 17.32 ± 2.98 | ||
| 23.58 ± 6.32* | 25.70 ± 3.50 | 22.45 ± 6.01 | ||
| 12.03 ± 2.68* | 12.91 ± 1.74 | 11. 41 ± 2.74 |
p<0.001 for CN versus MCI, CN versus AD dementia and MCI versus AD dementia.
p<0.05 for CN versus MCI, and p<0.001 for CN versus AD dementia and MCI versus AD dementia.
p<0.001 for CN versus AD dementia and MCI versus AD dementia.
Unadjusted correlations across all participants showed significant associations between the FCI-SF total score and all 6 tau regions (entorhinal cortex: r=−0.40, p<0.001; inferior temporal cortex: r=−0.48, p<0.001; dorsolateral prefrontal cortex: r=−0.39, p<0.001; posterior cingulate cortex: r=−0.36, p<0.001; precuneus: r=−0.44, p<0.001; and supramarginal gyrus: r=−0.41, p<0.001). Unadjusted correlations performed within CN participants showed significant associations between the FCI-SF total score and 2 tau regions (entorhinal cortex: r=−0.15, p=0.003; and inferior temporal cortex: r=−0.13, p=0.008) (see Figure 1). Lastly, for the symptomatic participants (combined MCI and AD dementia), unadjusted correlation showed significant associations between the FCI-SF total score and all 6 tau regions (entorhinal cortex: r=−0.30, p<0.001; inferior temporal cortex: r=−0.46, p<0.001; dorsolateral prefrontal cortex: r=−0.39, p<0.001; posterior cingulate cortex: r=−0.36, p<0.001; precuneus: r=−0.42, p<0.001; and supramarginal gyrus: r=−0.41, p<0.001) (see Figure 1).
Figure 1.
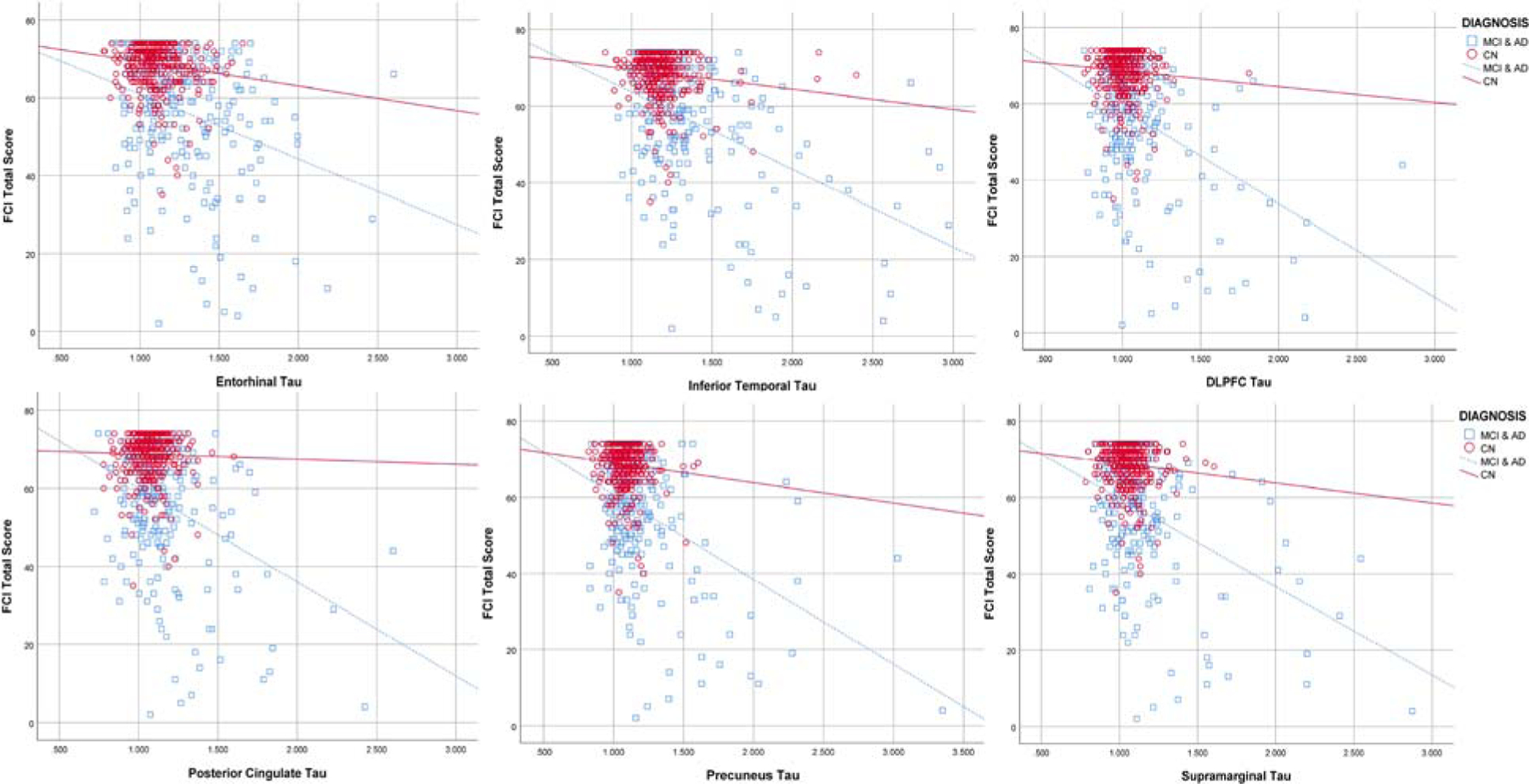
Scatter plot of entorhinal cortex, inferior temporal cortex, Dorsolateral prefrontal cortex (DLPFC), posterior cingulate, precuneus, and supramarginal FTP tau vs. FCI-SF total score, stratified by diagnosis (CN vs. symptomatic participants—combined MCI and AD dementia).
Primary Analyses
Linear regression models with backward elimination were run for tau regions that had significant associations with unadjusted correlations across all participants utilizing the FCI-SF total score with education, age, sex, RAVLT Total Learning Score, and TMT-B as covariates. FCI-SF total scores were significantly associated with greater tau burden in all 6 regions (see Table 3).
Table 3 –
Results of separate linear regression models across all participants with tau region as the predictor of interest, the FCI-SF total score as the dependent variable, and education, age, sex, RAVLT Total Learning Score, and TMT-B as covariates.
| Tau Region | β | 95% CI for β | pr | P-value | |
|---|---|---|---|---|---|
| −6.75 | −9.74, −3.75 | −0.18 | |||
| −6.07 | −8.74, −3.39 | −0.18 | |||
| −5.11 | −9.14, −1.07 | −0.10 | |||
| −4.61 | −8.67, −0.55 | −0.09 | |||
| −7.26 | −11.00, −3.53 | −0.15 | |||
| −5.54 | −9.39, −1.68 | −0.11 | |||
β, unstandardized coefficients; CI, confidence interval; pr, partial correlation coefficient.
Secondary Analyses
As shown on Table 4, models were repeated including an interaction term between cortical amyloid and regional tau across all participants and revealed associations between FCI-SF total score and greater tau burden within participants with greater cortical amyloid in 4 regions (cortical amyloid and dorsolateral prefrontal cortex tau interaction, cortical amyloid and posterior cingulate tau interaction, cortical amyloid and precuneus tau interaction, and cortical amyloid and supramarginal tau interaction (see Figure 2).
Table 4 –
Results of separate linear regression models across all participants with the interaction of cortical amyloid and regional tau as the predictor of interest, the FCI-SF total score as the dependent variable, and education, age, sex, RAVLT Total Learning Score, and TMT-B as covariates.
| Amyloid × Tau Interaction | β | 95% CI for β | pr | P-value |
|---|---|---|---|---|
| −5.28 | −8.95 ± −1.60 | −0.15 | ||
| −2.95 | −4.97 ± −0.92 | −0.16 | ||
| −3.61 | −5.58 ± −1.63 | −0.19 | ||
| −3.12 | −5.08 ± −1.16 | −0.17 |
β, unstandardized coefficients; CI, confidence interval; pr, partial correlation coefficient.
Figure 2.
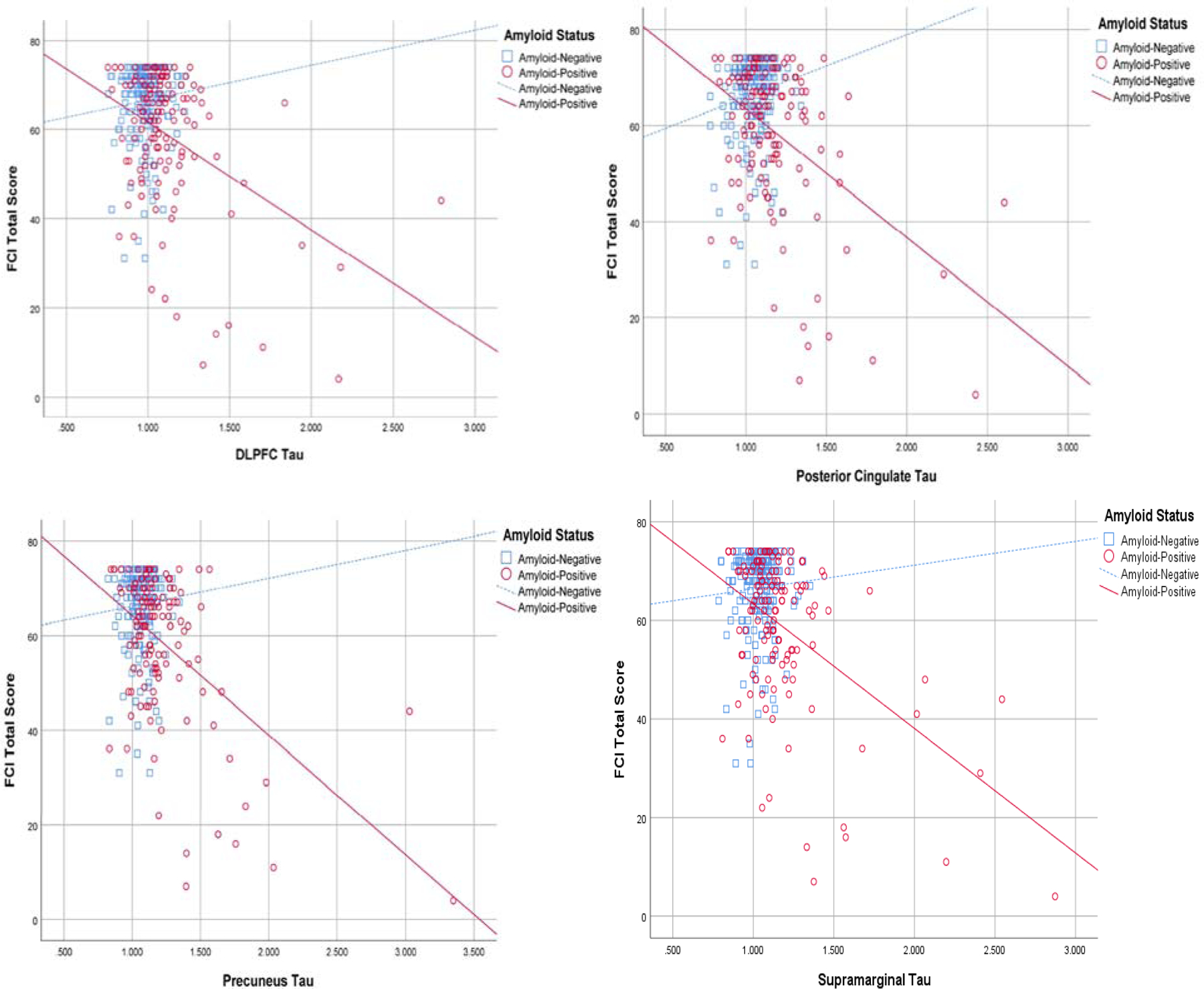
Scatter plot of Dorsolateral prefrontal cortex (DLPFC), posterior cingulate, precuneus, and supramarginal FTP tau vs. FCI-SF total score in all participants, stratified by Amyloid status (Amyloid-Positive=florbetapir SUVR>1.10; Amyloid-Negative=florbetapir SUVR≤1.10).
Models were repeated separately in CN and symptomatic participants. For CN participants, no significant associations were found in any tau regions. When an interaction term between cortical amyloid and regional tau was added for CN participants, FCI-SF total score was significantly associated with 2 regions (cortical amyloid and inferior temporal cortex tau interaction: β=−31.00, 95% CI=−55.37, −6.62, pr=−0.17, p=0.01; cortical amyloid and precuneus tau interaction: β=−34.69, 95% CI=−69.32, −0.05, pr=−0.13, p=0.05). In symptomatic participants, FCI-SF total score was significantly associated with 2 tau regions (inferior temporal cortex: β=−4.83, 95% CI=−9.04, −0.61, pr=−0.15, p=0.03; and precuneus: β=−6.51, 95% CI=−12.27, −0.76, pr=−0.15, p=0.03). However, no significant associations were found in any regions when an interaction term between cortical amyloid and regional tau was added in symptomatic participants. Models were repeated using the five FCI-SF domain scores (see Supplementary Materials).
Discussion
This study explored the cross-sectional association between the regional cerebral accumulation of tau and financial capacity across CN, MCI, and AD dementia participants. Our findings suggest that greater regional tau in the entorhinal, inferior temporal, dorsolateral prefrontal, posterior cingulate, precuneus, and supramarginal gyrus are modestly associated with greater financial capacity impairment across these diagnostics groups. The current study, which employed a performance-based IADL test, the FCI-SF, confirms results of previous studies, which employed an IADL questionnaire, the Functional Activities Questionnaire (FAQ), and showed an association between IADL impairment and greater tau burden in temporal and frontal regions in MCI and AD dementia [16, 17].
Our study also examined the associations between financial capacity and the interaction of tau and amyloid. Our results showed that dorsolateral prefrontal cortical tau, posterior cingulate tau, precuneus tau, and supramarginal tau were associated with greater financial capacity impairment in individuals with elevated cortical amyloid. Halawa et al., previously found an association between IADL and the interaction of entorhinal and inferior temporal cortex tau with cortical amyloid [16], while Marshall et al. found an association between IADL and the interaction of entorhinal and orbitofrontal cortex tau and cortical amyloid [17]. The differences in regional associations could be due to a difference in the test used to assess IADL—both of the previous studies used a study partner-reported questionnaire targeting a variety of IADL, the FAQ, while the current study used a performance-based test targeting financial capacity, the FCI-SF.
Additional analyses explored tau accumulation separately within diagnostic groups. When looking only at the asymptomatic (CN) group, a modest association between greater IADL impairment and inferior temporal and precuneus tau within individuals with elevated cortical amyloid was seen. In the symptomatic (combined MCI and AD dementia) group, an association between greater IADL impairment and inferior temporal and precuneus tau was observed. However, the interactions of regional tau and cortical amyloid were not significant in the symptomatic group. This finding was contrary to Halawa et al., as they found an association within the symptomatic group but not within the asymptomatic group. While association were modest in strength, this suggests that the FCI-SF, used in the current study, may be more sensitive to detecting the earliest IADL changes in the preclinical stage of AD, while the FAQ, used in the previous study, may be more geared toward detecting IADL changes at the stage of MCI and mild AD dementia. A few prior studies have shown an association between IADL changes and cortical amyloid within CN older adults [20, 38]. However, to our knowledge, this is the first study to show an association between regional tau burden and IADL within CN older adults.
When assessing the regional tau association with each of the FCI-SF five domains (see Supplementary Materials), similar results were obtained, suggesting that within this sample there was not a particular domain of financial capacity that drove the findings.
A strength of the current study was that we utilized the ADNI dataset, which represents a multicenter study of clinical and multi-modal imaging in a well-characterized cohort across early-stage AD. The FCI-SF was added to the third iteration of ADNI (ADNI-3) and currently few participants had repeat assessments. Since longitudinal tau and clinical data are collected in ADNI-3, when the data becomes available, we plan to extend our analyses to determine the longitudinal relationship between financial capacity and regional tau burden.
Limitations to our study consisted of a lack of diversity within the ADNI-3 participants. Most participants (about 91%) reported being White and this contributed to the data being less generalizable to the older population, since we know that Blacks and African Americans are twice as likely to develop AD [39]. Moreover, the sample was highly educated with over 16 years of education on average, again making it less generalizable. Another limitation is that the same size of the AD dementia group was much smaller than that of the other two diagnostic groups (CN and MCI). For that reason, we combined the symptomatic groups together for the analyses that were divided by diagnostic group. An additional limitation is that while the FCI-SF serves as a promising tool for the baby boomer generation, it may no longer be relevant to future generations since the usage of monetary tools and transfers have been more and more transitioned to electronic interfaces. The current study did not correct for multiple comparisons; therefore, the results should be interpreted with caution. Future studies need to replicate these findings. Another limitation is that the current study focused on cross-sectional PET and FCI-SF total scores. As mentioned above, we plan on analyzing the longitudinal data as it becomes available. Lastly, the current study focused on the regional cerebral tau burden associations with financial capacity. In future studies using ADNI and other datasets, we would like to determine the association between CSF and plasma tau markers, financial capacity, and other sensitive IADL tests.
In summary, our study showed that greater regional tau burden was modestly associated with financial capacity impairment in early-stage AD. These associations were independent of age, education, sex, memory, and executive function. We also showed that the interaction of regional tau and cortical amyloid was associated with financial capacity changes, even within CN older adults. Future longitudinal analyses will help determine how well AD pathology predicts changes financial capacity at the earliest stages of AD. This in turn will help guide the use of clinically meaningful assessments of IADL, such as the FCI-SF, in upcoming clinical trials in early-stage AD aiming to find better treatments and slowing disease progression.
Supplementary Material
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Disclosures
CG, NST, DB, MJP, and REA have nothing to disclose. GAM has received research salary support from Eisai Inc., Eli Lilly and Company, Janssen Alzheimer Immunotherapy, Novartis, and Genentech, and consulting fees from Grifols Shared Services North America, Inc., Eisai Inc., and Pfizer.
References
- 1. 2019 Alzheimer’s Disease Facts and Figures Alzheimer’s Dementia. 2019 https://alz.org/media/Documents/alzheimers-facts-and-figures-2019-r.pdf.
- 2.Tabira T, Hotta M, Murata M, Yoshiura K, Han G, Ishikawa T, Koyama A, Ogawa N, Maruta M, Ikeda Y, Mori T, Yoshida T, Hashimoto M, Ikeda M. Age-Related Changes in Instrumental and Basic Activities of Daily Living Impairment in Older Adults with Very Mild Alzheimer’s Disease. Dement Geriatr Cogn Dis Extra. 2020;10(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jekel K, Damian M, Wattmo C, Hausner L, Bullock R, Connelly PJ, Dubois B, Eriksdotter M, Ewers M, Graessel E, Kramberger MG, Law E, Mecocci P, Molinuevo JL, Nygard L, Olde-Rikkert MG, Orgogozo JM, Pasquier F, Peres K, Salmon E, Sikkes SA, Sobow T, Spiegel R, Tsolaki M, Winblad B, Frolich L. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimers Res Ther. 2015;7(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn IS, Kim JH, Kim S, Chung JW, Kim H, Kang HS, Kim DK. Impairment of instrumental activities of daily living in patients with mild cognitive impairment. Psychiatry Investig. 2009;6(3):180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dauphinot V, Delphin-Combe F, Mouchoux C, Dorey A, Bathsavanis A, Makaroff Z, Rouch I, Krolak-Salmon P. Risk factors of caregiver burden among patients with Alzheimer’s disease or related disorders: a cross-sectional study. J Alzheimers Dis. 2015;44(3):907–916. [DOI] [PubMed] [Google Scholar]
- 6.Marshall GA, Amariglio RE, Sperling RA, Rentz DM. Activities of daily living: where do they fit in the diagnosis of Alzheimer’s disease? Neurodegener Dis Manag. 2012;2(5):483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raccichini A, Castellani S, Civerchia P, Fioravanti P, Scarpino O. The caregiver’s burden of Alzheimer patients: differences between live-in and non-live-in. Am J Alzheimers Dis Other Demen. 2009;24(5):377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marson DC, Triebel K, Knight A. Financial capacity In: Civil capacities in clinical neuropsychology: Research findings and practical applications. New York, NY, US: Oxford University Press; 2012:39–68. [Google Scholar]
- 9.Gerstenecker A, Eakin A, Triebel K, Martin R, Swenson-Dravis D, Petersen RC, Marson D. Age and education corrected older adult normative data for a short form version of the Financial Capacity Instrument. Psychol Assess. 2016;28(6):737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Triebel KL, Martin R, Griffith HR, Marceaux J, Okonkwo OC, Harrell L, Clark D, Brockington J, Bartolucci A, Marson DC. Declining financial capacity in mild cognitive impairment: A 1-year longitudinal study. Neurology. 2009;73(12):928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherod MG, Griffith HR, Copeland J, Belue K, Krzywanski S, Zamrini EY, Harrell LE, Clark DG, Brockington JC, Powers RE, Marson DC. Neurocognitive predictors of financial capacity across the dementia spectrum: Normal aging, mild cognitive impairment, and Alzheimer’s disease. J Int Neuropsychol Soc. 2009;15(2):258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy K, Pepin LC, Philiossaint M, Lorius N, Becker JA, Locascio JJ, Rentz DM, Sperling RA, Johnson KA, Marshall GA. Regional fluorodeoxyglucose metabolism and instrumental activities of daily living across the Alzheimer’s disease spectrum. J Alzheimers Dis. 2014;42(1):291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogama N, Sakurai T, Nakai T, Niida S, Saji N, Toba K, Umegaki H, Kuzuya M. Impact of frontal white matter hyperintensity on instrumental activities of daily living in elderly women with Alzheimer disease and amnestic mild cognitive impairment. PLoS One. 2017;12(3):e0172484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melrose RJ, Ettenhofer ML, Harwood D, Achamallah N, Campa O, Mandelkern M, Sultzer DL. Cerebral metabolism, cognition, and functional abilities in Alzheimer disease. J Geriatr Psychiatry Neurol. 2011;24(3):127–134. [DOI] [PubMed] [Google Scholar]
- 15.Salmon E, Lespagnard S, Marique P, Peeters F, Herholz K, Perani D, Holthoff V, Kalbe E, Anchisi D, Adam S, Collette F, Garraux G. Cerebral metabolic correlates of four dementia scales in Alzheimer’s disease. J Neurol. 2005;252(3):283–290. [DOI] [PubMed] [Google Scholar]
- 16.Halawa OA, Gatchel JR, Amariglio RE, Rentz DM, Sperling RA, Johnson KA, Marshall GA, Alzheimer’s Disease Neuroimaging I. Inferior and medial temporal tau and cortical amyloid are associated with daily functional impairment in Alzheimer’s disease. Alzheimers Res Ther. 2019;11(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall GA, Gatchel JR, Donovan NJ, Muniz MC, Schultz AP, Becker JA, Chhatwal JP, Hanseeuw BJ, Papp KV, Amariglio RE, Rentz DM, Sperling RA, Johnson KA. Regional Tau Correlates of Instrumental Activities of Daily Living and Apathy in Mild Cognitive Impairment and Alzheimer’s Disease Dementia. J Alzheimers Dis. 2019;67(2):757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Neuropathologic correlates of activities of daily living in Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(1):56–59. [DOI] [PubMed] [Google Scholar]
- 19.Marshall GA, Lorius N, Locascio JJ, Hyman BT, Rentz DM, Johnson KA, Sperling RA. Regional cortical thinning and cerebrospinal biomarkers predict worsening daily functioning across the Alzheimer’s disease spectrum. J Alzheimers Dis. 2014;41(3):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tolbert S, Liu Y, Hellegers C, Petrella JR, Weiner MW, Wong TZ, Murali Doraiswamy P. Financial Management Skills in Aging, MCI and Dementia: Cross Sectional Relationship to 18F-Florbetapir PET Cortical beta-amyloid Deposition. J Prev Alzheimers Dis. 2019;6(4):274–282. [DOI] [PubMed] [Google Scholar]
- 21.Lee JC, Kim SJ, Hong S, Kim Y. Diagnosis of Alzheimer’s disease utilizing amyloid and tau as fluid biomarkers. Exp Mol Med. 2019;51(5):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suppiah S, Didier MA, Vinjamuri S. The Who, When, Why, and How of PET Amyloid Imaging in Management of Alzheimer’s Disease-Review of Literature and Interesting Images. Diagnostics (Basel). 2019;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7(8):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franzmeier N, Neitzel J, Rubinski A, Smith R, Strandberg O, Ossenkoppele R, Hansson O, Ewers M, Alzheimer’s Disease Neuroimaging I. Functional brain architecture is associated with the rate of tau accumulation in Alzheimer’s disease. Nat Commun. 2020;11(1):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K, Marshall G, Albers M, Mauro S, Pepin L, Alverio J, Judge K, Philiossaint M, Shoup T, Yokell D, Dickerson B, Gomez-Isla T, Hyman B, Vasdev N, Sperling R. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Yao Z, Yu Y, Zou Y, Fu Y, Hu B, Alzheimer’s Disease Neuroimaging I. Brain network alterations in individuals with and without mild cognitive impairment: parallel independent component analysis of AV1451 and AV45 positron emission tomography. BMC Psychiatry. 2019;19(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie L, Das SR, Wisse LEM, Ittyerah R, Yushkevich PA, Wolk DA, Alzheimer’s Disease Neuroimaging I. Early Tau Burden Correlates with Higher Rate of Atrophy in Transentorhinal Cortex. J Alzheimers Dis. 2018;62(1):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sperling RA, Mormino EC, Schultz AP, Betensky RA, Papp KV, Amariglio RE, Hanseeuw BJ, Buckley R, Chhatwal J, Hedden T, Marshall GA, Quiroz YT, Donovan NJ, Jackson J, Gatchel JR, Rabin JS, Jacobs H, Yang HS, Properzi M, Kirn DR, Rentz DM, Johnson KA. The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann Neurol. 2019;85(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR, Jagust W, Liu E, Morris JC, Petersen RC, Saykin AJ, Schmidt ME, Shaw L, Siuciak JA, Soares H, Toga AW, Trojanowski JQ, Alzheimer’s Disease Neuroimaging I. The Alzheimer’s Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012;8(1 Suppl):S1–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rey A. L’examen clinique en psychologie [The clinical examination in psychology]. Presses Universitaries De France; 1964. [Google Scholar]
- 31.Reitan R Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- 32.Landau SM, Breault C, Joshi AD, Pontecorvo M, Mathis CA, Jagust WJ, Mintun MA, Alzheimer’s Disease Neuroimaging I. Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med. 2013;54(1):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tosun D, Landau S, Aisen PS, Petersen RC, Mintun M, Jagust W, Weiner MW, Alzheimer’s Disease Neuroimaging I. Association between tau deposition and antecedent amyloid-beta accumulation rates in normal and early symptomatic individuals. Brain. 2017;140(5):1499–1512. [DOI] [PubMed] [Google Scholar]
- 34.Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ, Alzheimer’s Disease Neuroimaging I. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32(7):1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidoni ED, Honea RA, Burns JM. Neural correlates of impaired functional independence in early Alzheimer’s disease. J Alzheimers Dis. 2010;19(2):517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitter-Edgecombe M, Woo E, Greeley DR. Characterizing multiple memory deficits and their relation to everyday functioning in individuals with mild cognitive impairment. Neuropsychology. 2009;23(2):168–177. [DOI] [PubMed] [Google Scholar]
- 37.Marshall GA, Rentz DM, Frey MT, Locascio JJ, Johnson KA, Sperling RA, Alzheimer’s Disease Neuroimaging I. Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2011;7(3):300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lilamand M, Cesari M, Cantet C, Payoux P, Andrieu S, Vellas B, the MDSAsg. Relationship Between Brain Amyloid Deposition and Instrumental Activities of Daily Living in Older Adults: A Longitudinal Study from the Multidomain Alzheimer Prevention Trial. J Am Geriatr Soc. 2018;66(10):1940–1947. [DOI] [PubMed] [Google Scholar]
- 39.Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, Andrews H, Feng L, Tycko B, Mayeux R. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279(10):751–755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


