Abstract
Translation regulation in the context of aged-associated inflammation and behavioral impairments is not well characterized. Aged individuals experience lower life quality due to behavioral impairments. In this study, we used young and aged transgenic mice that are unable to activate the cap-binding protein, eukaryotic translation initiation factor 4E (eIF4E) to examine the role of protein translation control in aging, memory, depression, and anxiety. To determine how products of cap-dependent translation play a permissive role in aged-associated inflammation, we assessed levels of pro-inflammatory cytokines in various brain regions involved in the above-mentioned behaviors. We found that functional eIF4E is not necessary for age-related deficits in spatial and short-term memory but is important for depressive and anxiety-like behavior and this is correlated with pro-inflammatory cytokines in discrete brain regions. Thus, we have begun to elucidate a role for eIF4E phosphorylation in the context of aged-related behavioral impairments and chronic low-grade inflammation that may help identify novel immune modulators for therapeutic targets and decrease the burden of self-care among the geriatric population.
Keywords: Aging, eIF4E, inflammation, cognition, depression, anxiety
1. Introduction
Aging is a process that affects all living organisms and is characterized by changes in cellular processes at the molecular level that lead to detrimental activity of cells over time (Dilger and Johnson, 2008). Rapid growth of the elderly population has contributed to an increased financial burden to the medical care system that is associated with supporting health outcomes across the lifespan of individuals. The U.S. Census Bureau predicts a 150% increase of individuals 65 years or older by the year 2050, which will account for 20.5% of the population (Roberts, 2018). With aged individuals representing a large percentage of the population and the increased occurrence of neuropathological states associated with aging, such as mild cognitive impairment, depression, and anxiety; more studies on cognitive function, aging, and inflammation are critical.
An extensive literature has identified how age sensitizes, or “primes” the immune system causing a greater inflammatory cytokine release after various stimuli (Dilger and Johnson, 2008; Frank et al., 2010; Norden and Godbout, 2013; Perkins et al., 2018; Ye and Johnson, 1999). Many of these mediators sensitize microglia, the effective immune cell of the central nervous system (CNS) and potentiate neuropathological states (Dilger and Johnson, 2008; Rosczyk et al., 2008; Ye and Johnson, 1999). But how molecular biological processes like translation control interact with aging and the immune system in the brain to modulate behavioral outcomes are not well characterized.
Studies indicate that pro-inflammatory cytokines are excessively produced in aged individuals when compared to adults at baseline and after peripheral stimulus and this overproduction plays a critical role in inflammation-related changes in behavioral plasticity (Dilger and Johnson, 2008; Frank et al., 2010; Norden and Godbout, 2013; Sparkman et al., 2019; Ye and Johnson, 1999). However, there are no studies in the context of the role of subsets of mRNAs to understand how translation regulation is involved in inflammation and the onset of pathological states in aging.
Subsets of mRNAs to be translated are activated by binding of eukaryotic initiation factor 4F (eIF4F) to the 5’ 7-methylguanosine (m7G) cap and poly-A binding protein (PABP) to the 3’ end after the formation of the 43S pre-initiation complex (Merrick and Pavitt, 2018). The eIF4F complex comprises of eIF4G, a protein scaffold, eIF4E, which binds the 5’ cap, and eIF4A, which acts as an RNA helicase to unwind secondary structures near the 5’ cap. The binding of eIF4E to the 5’ m7G cap of mRNA is the rate-limiting step in translation initiation because it is directly responsible for mRNA recruitment to the 43S pre-initiation complex (Merrick and Pavitt, 2018). The activation of eIF4E is regulated by the mitogen-associated protein kinase (MAPK) pathway involving MAPK interacting proteins 1 and 2 (MNK1/2) (Joshi and Platanias, 2014). Both can phosphorylate eIF4E on serine 209 and cause cap-dependent translation. A loss of phosphorylation of eIF4E has been reported to reduce its affinity for the 5’ m7G mRNA cap, which reduces cap-dependent translation (Scheper et al., 2002). An additional layer of regulation comes from the mammalian target of Rapamycin complex (mTORC) pathway that is activated in response to hormones, nutrients, and growth factors. Signaling through mTORC phosphorylates inhibitory eIF4E binding proteins (4E-BPs) so that these cannot bind eIF4E and inhibit it (Joshi and Platanias, 2014; Scheper et al., 2002).
A general decrease of proteins has been reported during aging in C. elegans due to factors like decreased synthesis of translation machinery proteins, aberrant turnover of already synthesized proteins, and dysfunctional aggregation (Anisimova et al., 2018). As organisms age, the translational machinery, and controls, undergo changes. It has been shown that proteins of the MAPK pathway have a higher level of phosphorylation in older individuals and show a lesser degree of activation when stimulated (Williamson et al., 2003). Phosphorylated eIF4E has been shown to downregulate translation of a subset of mRNAs that affect antiviral responses and transcription of cytokines in immune cells (Herdy et al., 2012). Cap-dependent translation control is also a centralized pathway for regulating inflammation response and it has been shown that such regulation can occur on specific transcripts within UTR regions (Mazumder et al., 2010). Cap-dependent translation machinery is regulated by effectors downstream of the mTOR pathway and the MAPK pathway; signaling cascades that are responsive to stress, infections, or nutrition and also susceptible to age-related modulation of inflammation and behavior (Aguilar-Valles et al., 2018; Amorim et al., 2018b; Johnson et al., 2013; Shveygert et al., 2010; Thoreen et al., 2012). In the aged, tumor necrosis factor alpha (TNFα) mRNA abundance by the p38-MAPK-MNK pathway has been reported along with increased production of TNFα, interleukin 6 (IL-6) and interleukin 1 beta (IL-1β) (Pashenkov et al., 2017). With this observed increase of inflammation in aged individuals (Chung et al., 2019), it is not unreasonable to speculate that cap-dependent translation may be involved in modulating a pro-inflammatory profile during aging.
We hypothesized that eIF4E may play a causative role in age-related deficit of spatial memory, object recognition, depressive-like behavior, and anxiety-like behavior. This may be via the upregulation of pro-inflammatory cytokines such as TNFα and IL-1β and interferons like IFNα and γ in brain regions involved with these tasks and this regulation is tied to translational control. We are the first to assess the role of various pro-inflammatory cytokines in pertinent brain regions of aged eIF4E transgenic animals to understand the role of particular cytokines in cognitive and affective behaviors. We assessed behaviors in young and aged, wild-type (WT) and mice lacking eIF4E phosphorylation at serine residue 209 (eIF4ES209A) and found that there was no difference in spatial memory and recognition memory, due to genotype. However, both young and aged eIF4ES209A mice demonstrated more depressive-like behaviors than the corresponding age-matched WT mice, indicating that phosphorylation of eIF4E is important in the context of depression and age. We also found that aged eIF4E mutant mice were less prone to anxious behavior, despite exhibiting higher depressive-like behavior. Thus, eIF4E phosphorylation may play a role in modulating anxiety in the aged. There was a significant increase in the levels of TNFα and IL-1β in the striatum of WT and eIF4E aged animals. Interestingly, we observed a significant age and genotype difference in the level of IFN-α in the hippocampus of our animals.
The immune system is dynamic, and its modulation offers therapeutic opportunities for the subsequent modulation of neuronal activity in several conditions. During aging, behavioral impairments lead to a decrease in self-care and compliance, which are central tenets of medical concerns. We elucidate the correlation of cap-dependent translation control with aged-associated inflammation as well as behavioral deficits and thus hope to bridge key gaps regarding the contribution of eIF4E phosphorylation to inflammation leading to dysfunctions in depression and anxiety during aging.
2. Materials and Methods
2.1. Animals
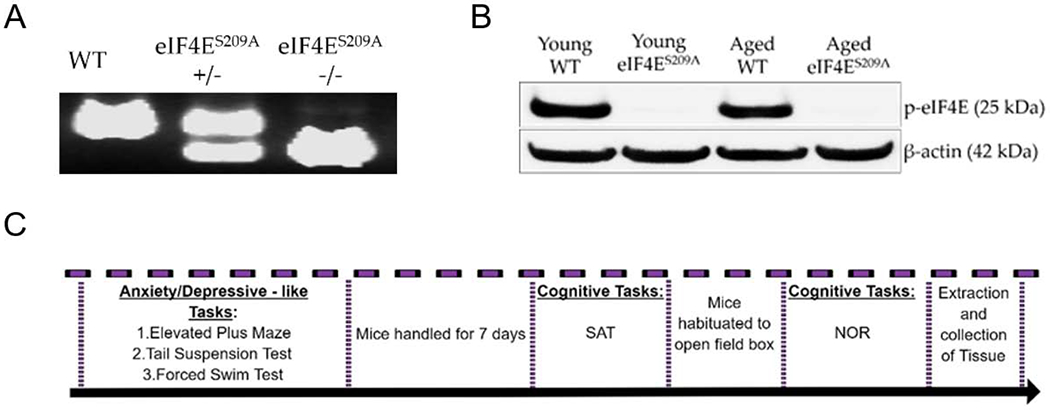
All mice used were from a C57BL/6 background. Wild-type (WT) animals were used as control; these mice were either bred in-house as littermates or purchased from Jackson Laboratory and allowed to age. Both male and female mice were used for the experiments; however, we did not find any sex-related differences, so the data was compiled before being presented here. Young (6-9 months old) and aged (22-26 months old) eIF4ES209A knock-in mice back-crossed to a C57B6/L background were generated in the Sonenberg laboratory at McGill University as previously described (Furic et al., 2010). These were a gift and were further bred to maintain genotypes at the University of Texas at Dallas vivarium to generate our experimental cohorts. In-house animals were weaned between 3 and 4 weeks of age and tail-clipped to verify genotypes. All young mice weighed between 25 g to 30 g and aged mice weighed between 30 g to 45 g at the time of experimental use. Animals were group housed in polypropylene cages with maximum five animals per cage. Cages were maintained at 21°C under a 12-h light dark cycle (lights on at 6:00 and off at 18:00) with ad libitum access to water and rodent chow. All procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Texas at Dallas Institutional Animal Care and Use Committee. A timeline of all behavioral tasks performed is depicted in Figure 1C.
Figure 1. Information regarding behavior tests and genotype confirmation.
(A) A representative genotyping gel showing littermate genotypes for WT, heterozygous knock-in eIF4ES209A+/−, and homozygous knock-in eIF4ES209A−/− animals. (B) A representative western blot showing phosphorylated eIF4E protein in animals from the young WT and aged WT groups and none in eIF4ES209A knock-in young animals and eIF4ES209A knock-in aged animals. (C) A timeline showing when various behavior tasks were performed with the animal cohorts. SAT – spontaneous alternation task, NOR – novel object recognition.
2.2. Cognitive Behavior
All animals were handled 5 minutes a day once a day during their dark cycle for one week prior to any cognitive experiments. Habituation to testing apparatus and acclimation to the room started at the beginning of the dark cycle (18:00 hours) while start times of experiments were roughly one hour into the dark cycle (19:00 hours) unless otherwise noted. Animals were kept under red light during habituation and testing. All recording equipment was placed far enough to not create a shadow on the behavior apparatus. All experiments were recorded by a live 1080p webcam camera (Besteker) placed approximately four feet above of the behavior apparatus and data acquired using Anymaze software (Stoelting Co., IL).
2.2.1. Spontaneous Alternation Task (SAT)
A plus maze with the following dimensions: 35cm height, arm length 69cm, and arm width 4cm, was used. Curtains surrounding the entire plus maze were kept at least 10 cm away from maze to prevent animals holding onto them and held two extra maze spatial cues. Animals were pre-determinedly placed on the extreme end of one of the four arms of a plus maze. In this noreward spatial memory task, each animal was free to move to the end of any arm and continuously alternate arms for 12 minutes. The sequence of arm entries were recorded and counted only when half of the animal crossed half of the arm (from nose to abdomen). Repeated entries into the same arm were accounted for only if the whole body of the animal exited the previous arm to the maze center and/or beginning of another arm. Alternations were considered to be four different arm entries within five consecutive arm entries. For example, visiting arms ABCDA was counted as an alternation whereas visiting arms in the sequence ABCBA was not counted as an alternation. Alternations were normalized by total arm entries. Percent alternations were calculated by the following formula:
2.2.2. Familiar and Novel Object Recognition (FOR and NOR)
A wooden light gray box with dimensions 48.5 cm × 51 cm × 38cm was used. Curtains surrounded the entire box. Two spatial cues were placed on different walls of the box. Animals were introduced to the box facing one of the corners and allowed to explore the box 10 minutes for two days prior to testing. On test days, animals were subjected to a 10-minute habituation period in the box. After habituation, two familiar objects were taped to the bottom of the box, equidistant from opposite corners. Animals were allowed to explore the objects for 3 minutes followed by a 2-minute interval during which animals were returned to their home cage and one of the identical objects was replaced by a different object. Animals were then given another 3 minutes to explore both familiar and novel objects. Given that enough exploration of the familiar objects is required to access memory on the second trial, animals that had a total exploration time lower than 9 seconds were excluded from the analyses. The floor, walls, and objects were cleaned with 70% ethanol between every single trial to eliminate any odor cues. The novelty preference was calculated with the formula shown below where Tnov indicates time spent exploring novel object and Tfam indicates time spent exploring familiar object.
The discrimination index was calculated with the formula:
2.3. Depressive-like behavior – Forced Swim Test
Animals were habituated for one hour to the experimental room before each experiment. Tests were performed at beginning of the light cycle and animals were not handled before experiments. The plastic cylinder tanks with dimensions 22cm diameter and 25cm height used for the swim test were placed in the same spot relative to the table, for each test. Mice were placed in the tanks filled halfway with water (25°C ± 2°C) to prevent contact with the bottom of the apparatus. Testing was done for six minutes total, with a 2-minute adjustment period and a 4-minute recording period. Water was changed between cages and in between males and females. AnyMaze software was used to record the movements with the computer and camera placed 2 feet away from the animal being tested. Immobility time for each animal was noted as a measure of behavioral despair. Higher immobility times indicated a “learned helplessness,” signifying depressive-like behavior.
2.4. Anxiety-like behavior
An elevated plus maze with the dimensions 35cm height, 69cm arm length, and 4cm arm width, was used. Two arms of the four were enclosed. Black curtains were set up around the whole maze 10 cm from the maze extremities. A camera (Besteker) was held by a stand 122 cm from the floor. Animals were acclimated to the experimental room for 30 minutes before performing the test. Animal was placed in the center of the maze and the AnyMaze program was started. The test recording ran for six minutes total. The parameters noted were total distance traveled within maze, time spent in open and closed arms, and number of entries into open arms. Longer times spent in closed arms were indicators of anxiety-like behavior.
2.5. Western blots
Animals were deeply anesthetized with isoflurane and quickly decapitated. Neuronal tissue was rapidly dissected to isolate hippocampus, striatum, and pre-frontal cortex, and fresh frozen in liquid nitrogen. Tissue was thawed and ice cold protein extraction buffer (50mM Tris, 150mM NaCl, 1mM EDTA, 1% SDS, 0.5% Triton X-100, pH 7.4) was added. Protease inhibitor cocktail (Sigma P8340), phosphatase inhibitor cocktail 1 (Sigma P2850), and phosphatase inhibitor cocktail 2 (Sigma P5726) were added at 1% final concentration to extraction buffer immediately before addition of buffer to tissue samples. Tissue was homogenized by sonication at 30 Hz for 20-40 secs until no clumps remained followed by centrifugation at 13000 × g for 15 minutes at 4°C and clarified supernatant was used for western blotting. Total protein was estimated using the BCA protein Assay Reagent Kit (Pierce 23223) and 15μg protein loaded per lane of a 12% resolving, 4% stacking polyacrylamide gel. Proteins were transferred onto a 0.22μM nitrocellulose membrane and blocked with 5% nonfat dry milk. Membranes were probed with primary antibodies to IFNα (1:1000 Lifespan Biosciences LS-C192612), IFNγ (1:1000 Abcam ab9657), TNFα (1:500 Abcam ab1793), IL-1β (1:1000 Abcam ab9722), phosphorylated eIF4E (1:1000 Abcam ab76256), SK2 (1:500 Antibodies Inc. 75-403), CaMKIIα (1:1000 Sigma-Aldrich, 05-532 ), NR2B (1:1000 Sigma-Aldrich, 06-600), and β-actin (1:3000 Cell Signaling Technologies 8H10D10) at 4°C overnight followed by 3 washes with tris-buffered saline (50mM Tris, 150mM NaCl, 0.01% Tween-20 – TBST), and 1 hour incubation with horse radish peroxidase-conjugated secondary antibodies. Membranes were washed three more times using TBST, and target proteins detected via chemiluminescence. Levels were normalized according to β-actin signal. Normalized levels from eIF4ES209A samples were compared to WT samples for young and aged animal groups.
2.6. Statistical analyses
All statistical analyses were performed in GraphPad Prism version 8.4. Western blot analyses were performed in Image Lab software from BioRad. Area under the curve was collected as intensity and normalized according to intensity value changes for β-actin. All data are graphically represented by the mean and standard error of the mean. For behavior and western blots, to determine differences between genotypes (WT and eIF4ES209A), two-way ANOVA was used for each age (young and aged), followed by Bonferroni’s post-hoc analysis as well as for difference between age. A p-value ≤ 0.05 was considered significant.
3. Results
3.1. Timeline of behavioral tests and genotype confirmation
In this study, we used young and aged mice that were wild-type (WT) or transgenic mutants for eIF4E that substituted an alanine at serine position 209 (eIF4ES209A). This amino acid substitution does not allow MNK1/2 to phosphorylate eIF4E at position 209 (Ueda et al., 2004). In addition to confirming the mutation with PCR genotyping (Figure 1A), we conducted western blots for phosphorylated eIF4E (Figure 1B). Only the WT young and aged mice expressed phosphorylated eIF4E. To provide a clear timeline of when various behavior tasks were performed, we present the timeline shown in Figure 1C.
3.2. Loss of phosphorylation of eIF4E at Ser209 does not affect spatial memory in young or aged animals
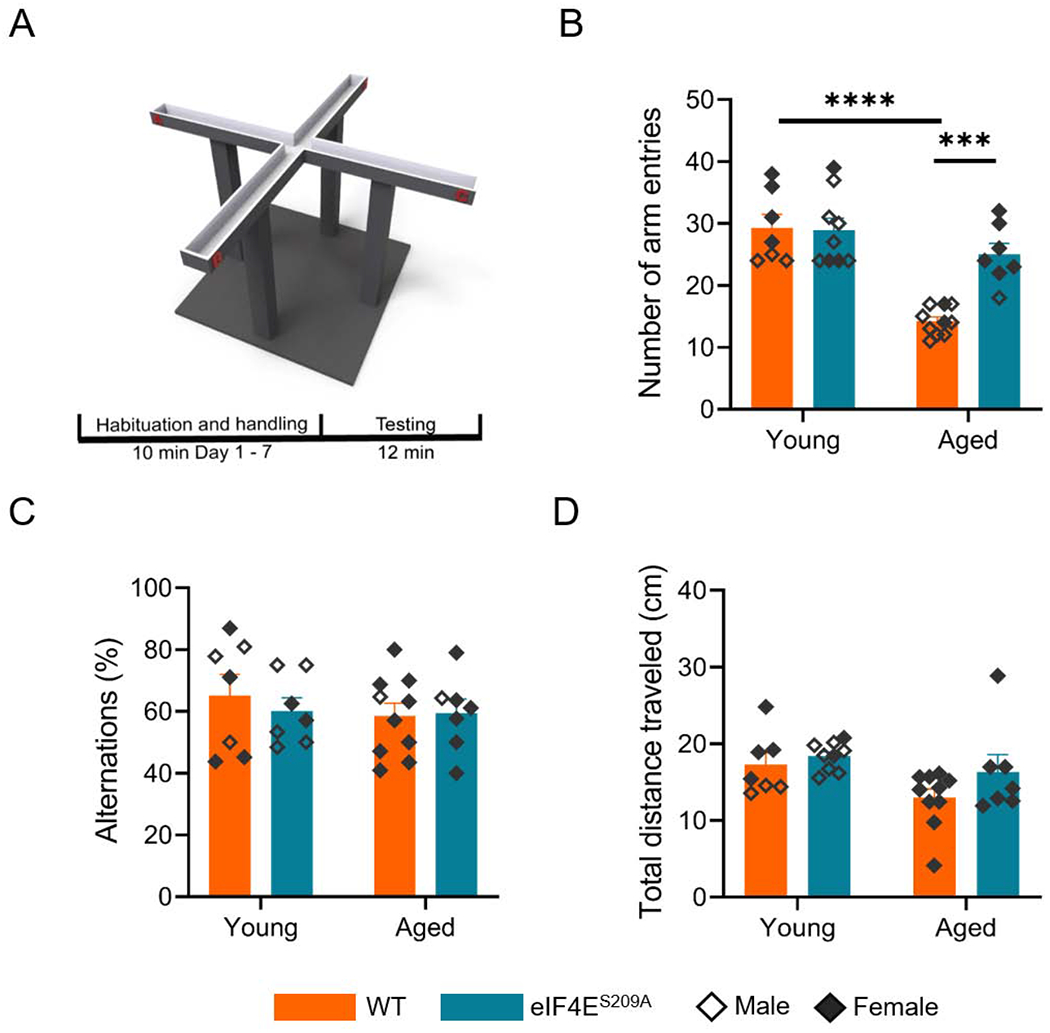
To determine if eIF4E activation is important for spatial memory processes in the aged, we subjected WT and eIF4ES209A mice to hippocampal-dependent behavior tasks. For examination of spatial memory, we performed the spontaneous alteration task using an elevated plus-maze that had four open arms. The animals were placed at the distal end of one of the four arms and allowed to explore the maze freely for 12 minutes while the number of arm entries, alternations, and total distance traveled were recorded. Rodents being naturally curious, tend to explore novel arms of the maze thus having high alterations and arm entries (Ukai et al., 1995). We observed that aged WT mice had a significantly lower number of arm entries compared to the young WT animals, while aged mice with eIF4ES209A mutation had a similar number of arm entries as the young animals (Figure 2A; F (1, 29) = 11.09, p 0.0024). All four groups showed similar alternations (Figure 2B). The aged mice traveled significantly less total distance in the maze (F (1, 29) = 5.172, p 0.0305), as was expected (Figure 2C). From this data, we suggest that although the aged animals prefer to move and explore less, there is no impairment of spatial or short-term memory due to their age or loss of eIF4E function. It also appears that absence of active eIF4E may partially restore natural exploratory behavior in the aged (Figure 2B).
Figure 2. Spatial memory is not impaired by lack of eIF4E phosphorylation.
(A) A schematic diagram showing the structure of the 4-arm maze used for the spontaneous alternation tests. (B) Number of arm entries during spontaneous alternation task by various cohorts. Individual values are depicted, separated by sex - males empty diamonds and females filled diamonds. Data are represented as means with SEM (n = 7-10 per group). Two-way ANOVA with Bonferroni’s post-hoc. ***p 0.0004, ****p < 0.0001 (C) Percent alternations by the animals shown in B. (D) Total distance traveled within the elevated plus maze.
3.3. Phosphorylation of eIF4E at S209 is unimportant for recognition or short-term memory
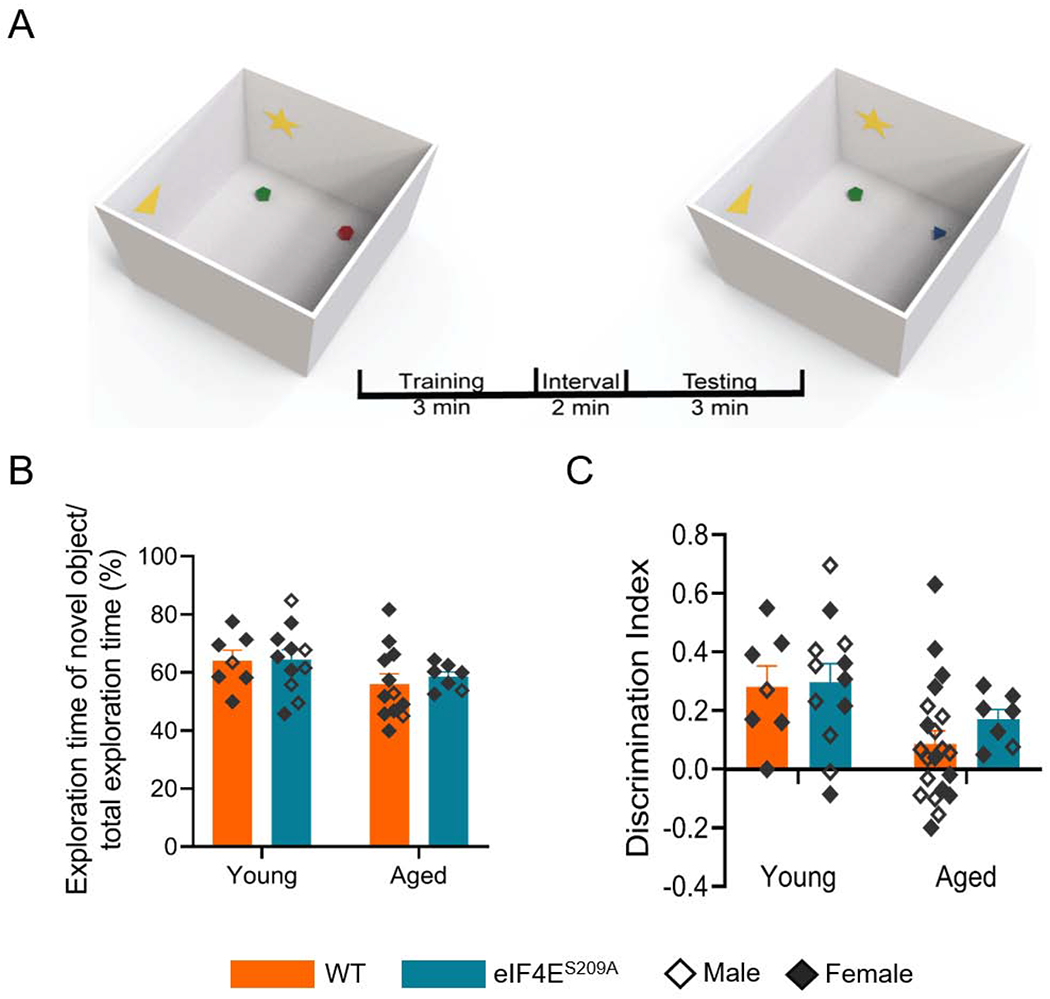
Rodents have an inherent tendency to explore new objects (Blaser and Heyser, 2015). This is represented as novelty preference defined by how much time rodents spend on exploration of novel object within total experiment time, and discrimination index defined by how well the mice recognize an object as novel as opposed to familiar. We observed a reduction in novelty preference (F (1,33) = 3.832, p 0.0588) and discrimination index (F (1,43) = 6.591, p 0.0138) in aged mice compared to the young (Figures 3B and 3C). This indicated that recognition memory declined with increasing age. There was no difference due to genotype between the aged groups (see Table 1 for exact statistical values) and this suggests that loss of phosphorylation of eIF4E does not affect recognition memory. Thus, cap-dependent translation may have an enhancing effect on exploratory behavior but does not play a role in spatial or recognition memory, within the context of aging.
Figure 3. Loss of phosphorylation of eIF4E does not impact short-term recognition memory.
(A) A graphic showing the timeline of the object recognition experiment. The yellow star and triangle indicate representative spatial cues provided in the box. The red and green objects were familiar to the animals. During the interval, the red five-sided object was replaced with the blue three-sided object.(B) Graph showing preference of novel object. Two-way ANOVA was performed followed by Bonferroni’s post-hoc analysis for multiple comparisons between ages and genotypes. (C) Graph showing how well the cohorts can distinguish familiar from novel objects. Statistical analyses were done as for B, except multiple comparisons were made between age groups and not based on genotype. Individual values are separated by males (empty diamonds) and females (filled diamonds) and data are represented as means with SEMs (n = 7-12 per group).
Table 1.
Statistical values for analyses performed on behavioral data. All datasets were analyzed with ordinary two-way ANOVA followed by Bonferroni’s post hoc. WT – wildtype, eIF4ES209A – knock-in animals for non-activatable eIF4E, OR – object recognition, SAR – spontaneous alternation, FST – forced swim test, EPM – elevated plus maze.
| Dataset | Main Effect | Interactions | Multiple Comparisons | ||||
|---|---|---|---|---|---|---|---|
| F(DFn,DFd) | p-value | F(DFn,DFd) | p-value | effect | groups | p-value | |
| SAR number of arm entries | age: F(1,29)=31.85 | p<0.0001 | F(1,29)=11.09 | p=0.0024 | age | WT | p<0.0001 |
| genotype: F(1,29)=9.573 | p=0.0043 | eIF4ES209A | p=0.3848 | ||||
| genotype | young | p=0.9984 | |||||
| aged | p=0.0004 | ||||||
| SAR Percent Alternations | age: F(1,27)=0.5413 | p=0.4683 | F(1,27)=0.3295 | p=0.5707 | |||
| genotype: F(1,27)=0.1618 | p=0.6907 | ||||||
| SAR total distance traveled | age: F(1,29)=5.172 | p=0.0305 | F(1,29)=0.6582 | p=0.4238 | age | WT | p=0.0696 |
| genotype: F(1,29)=2.572 | p=0.1196 | eIF4ES209A | P=0.5304 | ||||
| genotype | young | p>0.9999 | |||||
| aged | p=0.5688 | ||||||
| OR Novelty Preference | age: F(1,33)=3.832 | p=0.0588 | F(1,33)=0.01552 | p=0.9015 | |||
| genotype: F(1.33)=0.1638 | p=0.6883 | ||||||
| OR Discrimination Index | age: F(1,43)=6.591 | p=0.0138 | F(1,43)=0.2938 | p=0.5906 | age | WT | p=0.3821 |
| genotype: F(1,43)=0.6208 | p=0.4351 | eIF4ES209A | p=0.4517 | ||||
| FST immobile time | age: F(1,50)=0.4947 | p=0.4851 | F(1,50)=0.4030 | p=0.5284 | genotype | young | p=0.0213 |
| genotype: F(1,50)=17.49 | p<0.0001 | aged | p=0.0035 | ||||
| EPM distance traveled | age: F(1,61)=0.3345 | p=0.5652 | F(1,61)=5.387 | p=0.0236 | age | WT | p=0.5331 |
| genotype: F(1,61)=1.348 | p=0.2502 | eIF4ES209A | p=0.2411 | ||||
| genotype | young | p=0.8202 | |||||
| aged | p=0.1012 | ||||||
| EPM open arm entries | age: F(1,58)=0.8684 | p=0.3553 | F(1,58)=0.00888 | p=0.9252 | n/a | ||
| genotype: F(1,58)=0.1405 | p=0.7091 | ||||||
| EPM time spent in open arms | age: F(1,58)=4.727 | p=0.0338 | F(1.58)=3.867 | p=0.0540 | age | WT | p>0.9811 |
| genotype F(1,58)=0.8561 | p=0.3587 | eIF4ES209A | p=0.0246 | ||||
3.4. Role of eIF4E phosphorylation in depressive-like behavior in the context of aging
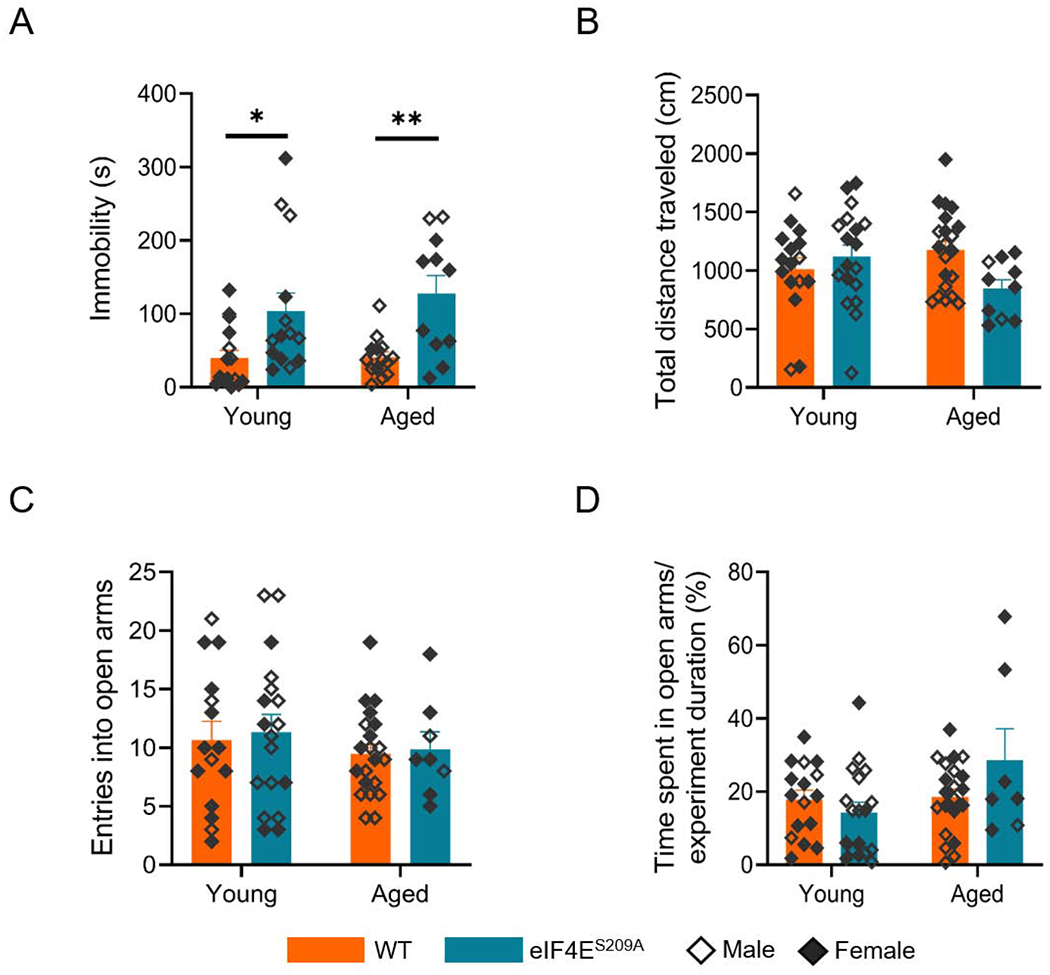
The forced swim test (FST) measures behavioral despair after subjecting the animal to a container filled with water and allowing it time to make escape efforts (Yankelevitch-Yahav et al., 2015). After initial tries for escape, the animal exhibits immobility that indicates despair. Animals exhibiting higher immobile time were considered as exhibiting more depressive-like behavior. As presented in Figure 4A, both young and aged WT animals exhibited similar levels of immobility. The eIF4ES209A mutants, however, were significantly different compared to the respective young or aged WT counterparts (F(1,50) = 17.89, p < 0.0001). This suggests a role for eIF4E phosphorylation in mediating depressive-like behavior. Thus, our data suggests that absence of phosphorylated eIF4E is linked with more depressive-like behavior, with age.
Figure 4. eIF4E phosphorylation mediates depressive-like and anxiety-like behavior in young and aged mice.
(A) Immobile time (seconds) during the forced swim test. *p 0.0213, **p 0.0035. (B) Total distance traveled by the four groups (C) Number of entries made by each group into the open arms of the maze. No significant differences were found. (D) Percentage of time spent in the open arms of the maze relative to entire experiment duration. *p 0.0246. Individual values are separated by males (empty diamonds) and females (filled diamonds) and data are represented as means with SEM (n=10-21 per group). Two-way ANOVA with Bonferroni’s post-hoc was performed.
3.5. A lack of eIF4E phosphorylation mediates decreased anxiety-like behavior in the aged
To evaluate anxiety-like behavior in all of our cohorts, we used an elevated plus maze test with two covered arms and two open arms. This test is based on the rodents’ exploratory nature and also natural apprehension of new, open surroundings (Rodgers and Dalvi, 1997). Rodents usually prefer dark, enclosed spaces so are expected to spend more time in the closed arms of the maze. They are also naturally quite active and travel a good distance while exploring the maze. We found that aged eIF4ES209A mice traveled significantly less distances (F (1,61) = 5.387, p 0.0236) compared to the young eIF4ES209A mice and WT mice (Figure 4B). Aged WT mice were no different than their young WT group. These differences were apparent even though all groups had similar number of entries into the maze’s open arms (Figure 4C). These differences are also reflected in more time spent in the open arms relative to experiment duration, by the aged eIF4ES209A animals (Figure 4D; F(1,58) = 4.727, p 0.0338). Therefore, we suggest based on this data, that a lack of phosphorylation of eIF4E, especially in old age, may be linked with less anxiety.
3.6. Levels of pro-inflammatory mediators in different brain regions
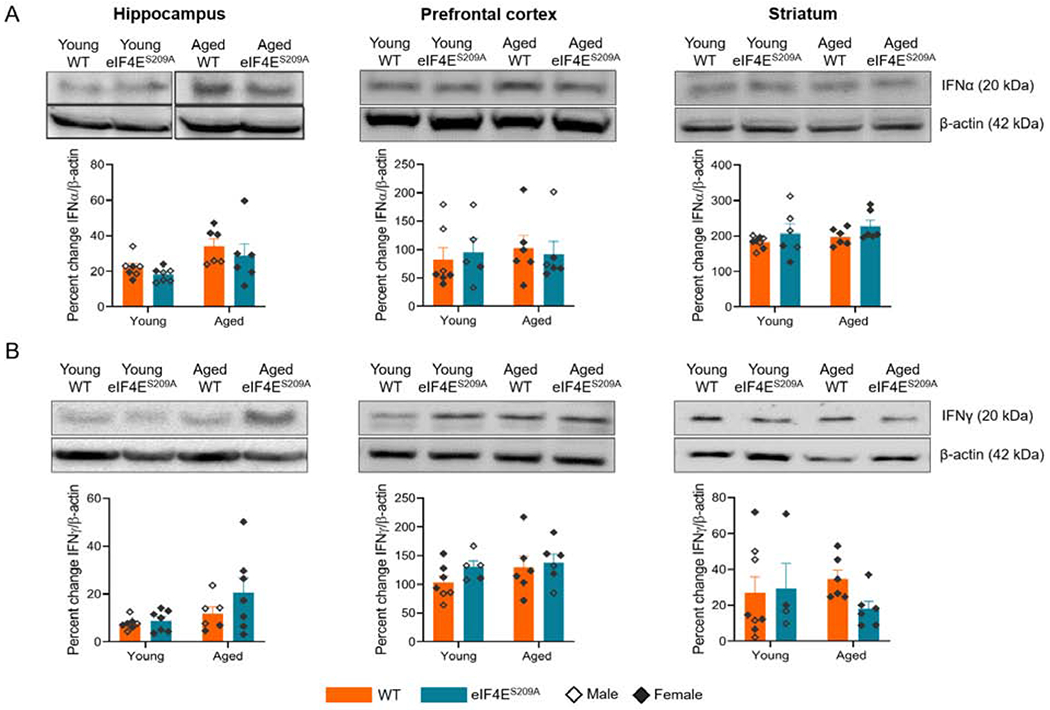
As we identified effects of non-phosphorylatable eIF4E on various behaviors, we next assessed whether the levels of inflammatory mediators namely interferon α (IFNα), interferon γ (IFNγ), TNFα, and IL-1β were affected in the hippocampus, pre-frontal cortex and striatum (Figures 5 and 6), which could be participating in changing affective behavior. We found a significant age effect for levels of IFNα (F (1,22) = 8.168, p 0.0091) and IFNγ (F (1,23) = 4.863, p 0.0377) in the hippocampus (Figure 5A left panel). However, no genotype differences were identified, suggesting that interferons are unaltered in response to loss of phosphorylation of eIF4E There were no statistically significant differences in levels of IFNα and IFNγ for other tissues (Figure 5A and 5B), indicating that interferons may not be involved in mediating behavior changes due to age or loss of eIF4E phosphorylation.
Figure 5. Levels of IFNα are increased with loss of eIF4E phosphorylation in aged animals.
(A) Representative western blots for IFNα with their densitometric analyses in hippocampus, pre-frontal cortex, and striatum from various cohorts. (B) Representative western blots for IFNγ in hippocampus, pre-frontal cortex, and striatum from various cohorts with densitometry. Individual values are separated by males (empty diamonds) and females (filled diamonds) and data are represented as means with SEM (n=4-11 per group). Two-way ANOVA with Bonferroni’s post-hoc was performed.
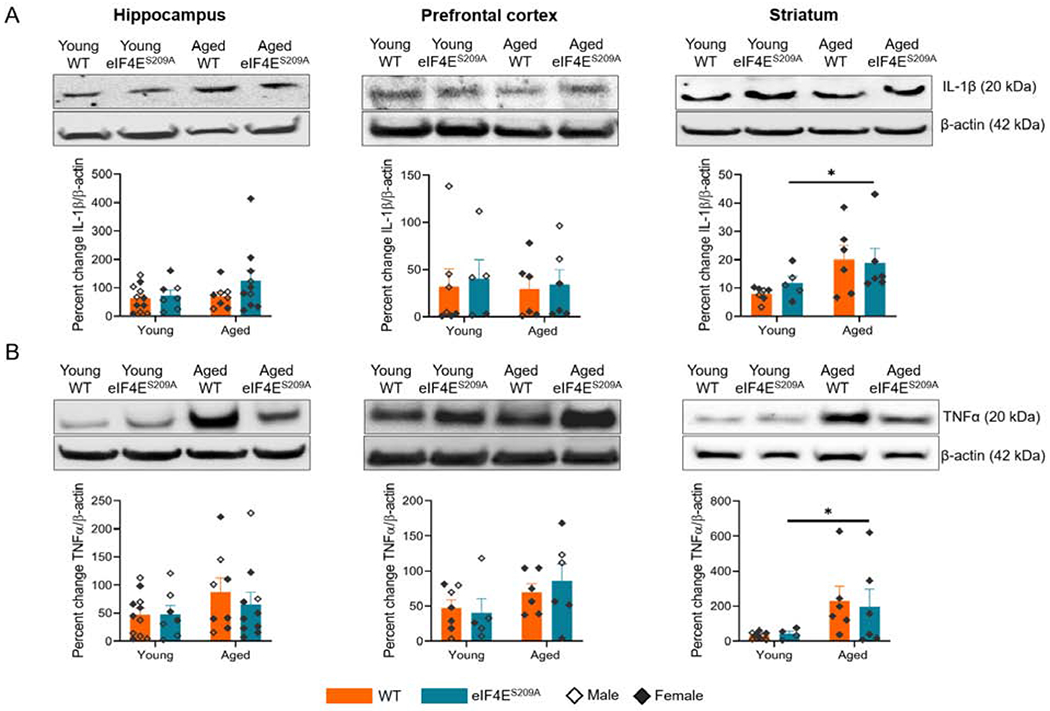
Figure 6. Levels of IL-1β and TNFα are increased in striatum of aged animals.
(A) Representative western blots for IL-1β with their densitometric analyses in hippocampus, pre-frontal cortex, and striatum from various cohorts. (B) Representative western blots for TNFα in hippocampus, pre-frontal cortex, and striatum from various cohorts with densitometry. Individual values are separated by males (empty diamonds) and females (filled diamonds) and data are represented as means with SEM (n=4-11 per group). Two-way ANOVA with Bonferroni’s post-hoc was performed.
We found that the aged animals had significantly higher levels for IL-1β (F (1, 20) = 6.773, p 0.0170) and TNFα (F (1, 20) = 6.756, p 0.0172) in the striatum compared to young animals, irrespective of whether phosphorylatable eIF4E was present or not (Figure 6A and B right panels). This suggests that eIF4E phosphorylation is not necessary for the effects on IL-1β and TNFα production, during aging. It also confirmed that a low level of inflammation is present in aged animals with upregulated levels of pro-inflammatory cytokines.
3.7. Proteins involved in synaptic plasticity do not change with genotype
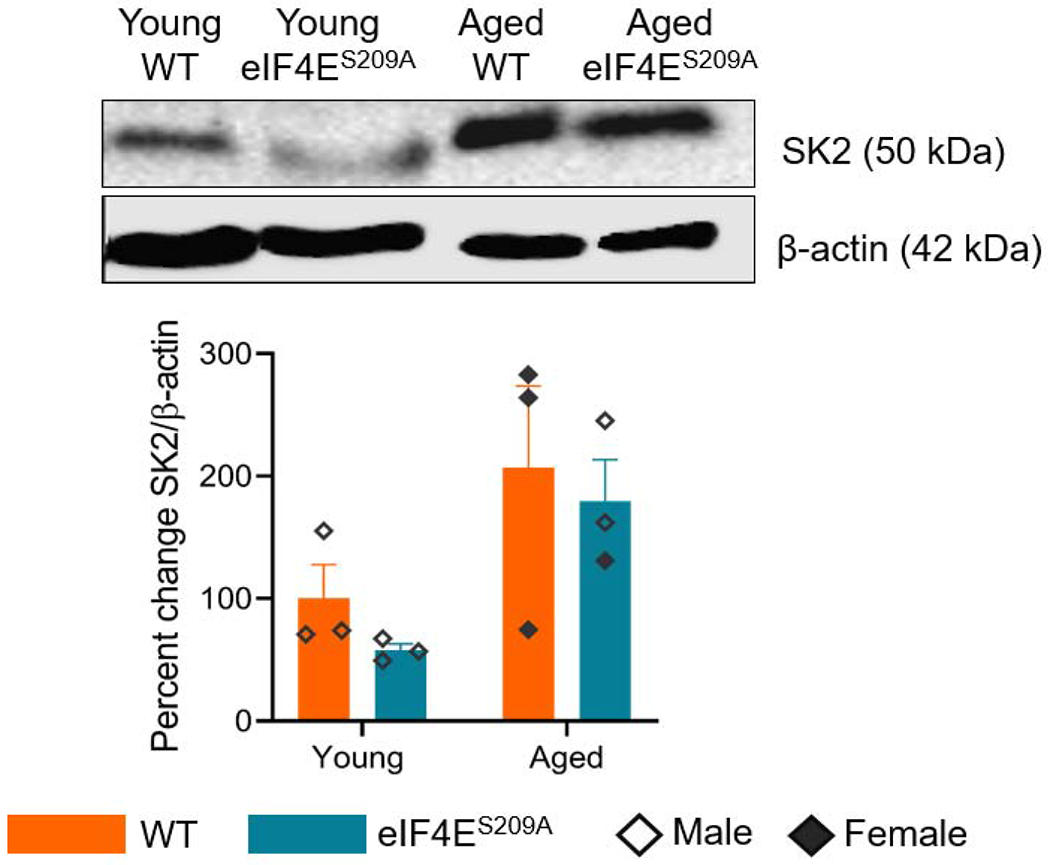
We performed western blots to test levels of SK2, CaMKIIα, and NR2B as all of these proteins are involved in synaptic plasticity correlated with learning, memory, and long-term potentiation (Buffington et al., 2014). We did not find differences for eIF4ES209A mice compared to WT for any of these three proteins. A representative western blot for SK2 is shown in figure 7. There was a statistically significant increase in SK2 levels in the prefrontal cortex (F (1,8) = 8.205, p 0.0210) for aged animals, compared to young.
Figure 7. Lack of eIF4E phosphorylation does not affect synaptic plasticity.
A representative western blot of SK2 in the mouse prefrontal cortex for all groups is shown with its corresponding quantification. Data are represented as mean and standard error of the mean (n=3 per group). Two-way ANOVA was performed with Bonferroni’s post-hoc.
4. Discussion
The regulation of cap-dependent translation is known to mediate depressive-like behaviors (Almorim et al., 2018; Aguilar-Valles et al., 2018) and the expression of a subset of pro-inflammatory cytokines (Herdy et al., 2012; Mazumder et al., 2010). In young eIF4E transgenic animals exhibiting increased depressive-like behavior and pro-inflammatory markers. Interestingly, aged individuals exhibit a continuous baseline low-grade inflammation and stimulated secretion of pro-inflammatory cytokines that may be cap-dependent (Chung et al., 2019). Thus, we investigated whether the inhibition of eIF4E phosphorylation and subsequent loss of cap-dependent translation led to amelioration of age-related cognitive decline, depressive-like, and anxiety-like behaviors. We also examined levels of pro-inflammatory cytokines and a subset of proteins involved in synaptic plasticity in pertinent brain regions to determine if these were altered in our eIF4ES209A mutant animals, in the context of aging.
Recent evidence has shown that a loss of eIF4E has no effect on spatial memory (Amorim et al., 2018a), and leads to depressive-like behaviors in young animals, and this is mediated by translational control of the inhibitor of kappa B protein (IκBα) (Aguilar-Valles et al., 2018). Another group reported similar behavioral results, but through gamma interferon activated inhibitor of translation (GAIT) complex (Amorim et al., 2018a). Importantly, these studies were performed in young adult rodent models, so how these mechanisms influence age-related, chronic low-grade inflammation and behavioral deficits, remained to be elucidated. Our data from aged eIF4ES209A mice suggest that a lack of phosphorylation of eIF4E does not further impair spatial or working memory, similar to the published report in young adult mice (Amorim et al., 2018a). In addition, upregulation of IFNα and IFNγ in the aged mice hippocampus (Figure 6A) suggested that cognition may be affected without any contribution from eIF4e phosphorylation. Higher expression of type I interferons can be engendered by a reduction in expression of NFKB, which is a consequence of inhibited eIF4E phosphorylation (Herdy et al., 2012). Other groups have shown that interferons can affect the p70 S6 kinase downstream in the PI3K pathway, which phosphorylates 4E binding proteins leading to their inhibition and subsequent activation of eIF4E (Lekmine et al., 2003), which is contrary to our finding. There is ample evidence of IFNα-induced sickness behaviors across many animal models (Capuron and Miller, 2011; Felger et al., 2007) as well as clinical studies in humans (Lotrich et al., 2007; Raison et al., 2010).
Our study is the first to highlight that there is a correlation between cap dependent translation and age, which can mediate anxiety or depression. Between our young and aged groups, we report a decrease in mobility of the aged animals (Figure 2D). As reviewed by Toth, and published by Shoji et.al, aging mice demonstrate decreased locomotor activity, higher depressive-like behaviors, and anxiety (Shoji and Miyakawa, 2019; Toth, 2018). Interleukin signaling in the aged mouse brain is involved in contextual fear conditioning and can also facilitate recovery from inflammation sickness behavior (Burton and Johnson, 2012; Burton et al., 2011). In our dataset, all aged animals, independent of genotype, demonstrated a lower ability to distinguish between novel and familiar objects (Figure 4C). It has been published that aged WT mice and rats tend to treat novel objects as familiar and this is correlated with decreased hippocampal function (Baxter, 2010). However, ours is the first study to show that translation control machinery is not involved in the decrease in novel object recognition.
The aged eIF4ES209A animals showed considerably more exploratory behavior (Figure 2C). This is a novel finding suggesting that decreased cap-dependent translation of a subset of mRNAs, may engender phenotypes more reflective of younger animals. Taking these findings along with the increased levels of Il-1β and TNFα in striatum (Figure 7), we suggest that cap-dependent translation participates in age-related inflammation and it affects a subset of behaviors depending upon tissue and potential cell types involved. We suggest that microglia are the main culprits in mediating these behavioral phenotypes via age-related increases in inflammation. However, it is also possible that neuronal activity via eIF4E or mTORC signaling could explain differences observed in behavior. While this was not our focus, T-cells also secrete cytokines such as TNFα, IL-1β, and interferons, which may also be responsible for regulating behavioral changes. Recent literature shows an increased presence of T-cells in the lymphatic system that drains the brain as well as leptomeninges (Weller et al., 2019).
In our studies, the young and aged eIF4ES209A mice exhibited clear depressive-like behavior compared to their age-matched WT groups in the forced swim test (Figure 4A). It is known that young eIF4ES209A mice exhibit depressive-like behavior (Amorim et al., 2018a) and aged mice demonstrate depressive-like behaviors due to activation of the peripheral innate immune system (Godbout et al., 2008). However, our finding regarding aged eIF4ES209A mice is novel in the context of age-related depressive-like behavior and cap-dependent translation. Studies have also shown that inhibition of MNK 1/2, kinases that phosphorylate eIF4E, led to depression and anxiety-like behavior in young adult mice, similar to absence of phosphorylatable eIF4E (Aguilar-Valles et al., 2018). It has been published that eIF4E phosphorylation engenders depressive-like and anxiety-like behavior through an increase in TNFα levels in young adult mice (Amorim et al., 2018a). We have presented data that aged animals demonstrate more depressive-like behavior along with increased levels of TNFα and an absence of phosphorylatable eIF4E does not affect this (Figures 4 and 7B). While changes in nociception could contribute to changes in depressive-like behaviors (Mitsi and Zachariou, 2016), the knock-in mice have no changes in baseline nociception (Mody et al., 2020; Moy et al., 2017), so we do not expect a baseline influence of nociception on affective behaviors. On the other hand, the same aged mice show considerably less anxiety-like behavior (Figure 4) despite increased interferon in the hippocampus (Figure 5). This suggests that anxiety and depression may be regulated by different pathways but may involve the same pro-inflammatory cytokines or it may be that specific cell types are affected differently by the loss of phosphorylatable eIF4E.
Another consideration for changes in behavior due to genotype is the connection between eIF4E phosphorylation and circadian rhythm. The MAPK/MNK pathway is responsive to light and circadian rhythm, which upregulates eIF4E phosphorylation during the day (Cao et al., 2015; Saraf et al., 2014). For animals lacking eIF4E phosphorylation, this could have contributed to behavioral changes seen in eIF4E mice compared to WT.
In adult neurons, the major post-translational modification for the eIF4E binding proteins (4E-BPs) is deamidation, which inhibits their activity and leads to increased cap-dependent translation (Bidinosti et al., 2010). In non-neuronal cells, however, 4E-BPs are inhibited by phosphorylation via the mTORC pathway (Thoreen et al., 2012). Neuronal activity, synaptic plasticity, and translation modulation are linked with impaired cognition, depression, anxiety (Buffington et al., 2014), But we did not find any differences in levels of a subset of proteins important for synaptic plasticity in animals lacking eIF4E phosphorylation compared to WT. We know that overall neurotransmission and synaptic plasticity are downregulated with normal aging. We believe that the aged-associated inflammation drives this downregulation. There is evidence that TNFα modulates synaptic plasticity and long-term potentiation during aging via the MAPK pathway (Maggio and Vlachos, 2018, Moynagh et al., 2004). It is apparent from our dataset that cap-dependent translation can effect heightened pro-inflammatory cytokine levels during aging (Figure 8). Previous work identified a serotonin specific contribution of eIF4E phosphorylation to regulate depressive like behavior (Amorim et al., 2018). However, there is a lot to be elucidated in the context of synaptic plasticity. Detailed future studies to investigate synaptic markers and neurotransmission in context of cap-dependent translation and age-related inflammation would fill in these exciting new questions.
Figure 8. Graphical abstract of our aging and transgenic model.
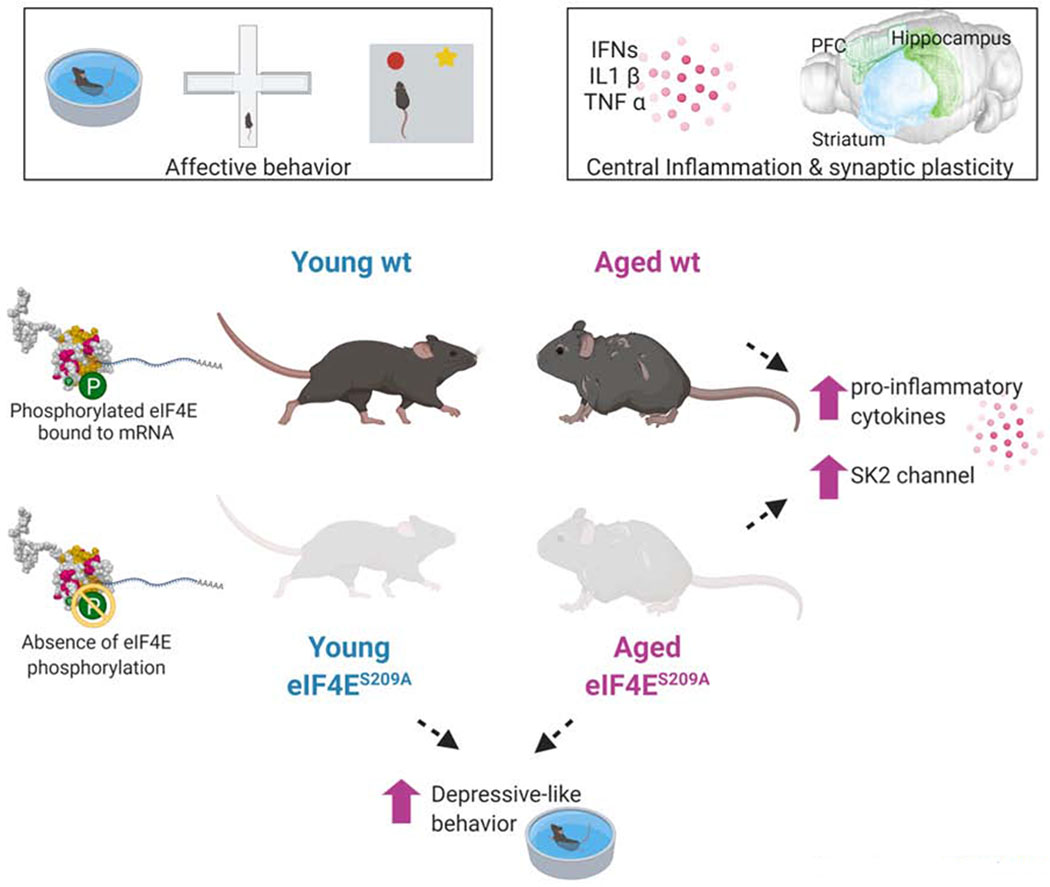
A representative image of pathways involved in aging, inflammation, cap-dependent translation, and behavioral deficits. Created with BioRender.
An important component of the age-related cognitive deficits is the underlying low-grade inflammation (Rea et al., 2018). Our data suggests that cap-dependent translation modulation is linked to this increased inflammation and overproduction of pro-inflammatory cytokines. Microglia have been implicated as culprits for this low-grade inflammation and dysfunctional social behaviors, in aged rodents (Burton et al., 2016; Garner et al., 2018; Perkins et al., 2018). It can be speculated that cap-dependent translation in microglia in aged animals is responsible for the over production of pro-inflammatory cytokines. In conclusion, how cap-dependent eIF4E-mediated translation of a key subset of mRNAs change with age, represents a central mechanistic explanation as to how translational control is causal for discrepancies in pro-inflammatory pathways, exploratory behavior, depression, and anxiety.
Table 2.
Statistical values for analyses performed on western blot data. All datasets were analyzed with ordinary two-way ANOVA followed by Bonferroni’s post hoc. WT – wildtype, eIF4ES209A – knock-in animals for non-activatable eIF4E, IFNα – interferon alpha, IFNγ – interferon gamma, IL-1β – interleukin 1 beta, TNFα – tumor necrosis factor alpha, PFC – prefrontal cortex, SK2 – Small conductance calcium-activated potassium channel protein 2.
| Protein | Region | Main effects | Interactions | Multiple comparisons | ||||
|---|---|---|---|---|---|---|---|---|
| F(DFn,DFd) | p values | F(DFn,DFd) | p values | effect | groups | p-value | ||
| IFNα | hippocampus | age: F(1,22)=8.168 | 0.0091 | F(1,22)=02769 | 0.8694 | age | WT | 0.0877 |
| genotype: F(1,22)=1.479 | 0.2368 | eIF4ES209A | 0.1404 | |||||
| genotype | young | >0.9999 | ||||||
| aged | 0.0756 | |||||||
| PFC | age: F (1, 20) = 0.1238 | 0.7287 | F (1, 20) = 0.2526 | 0.6208 | n/a | |||
| genotype: F (1, 20) = 0.03744 | 0.9536 | |||||||
| striatum | age: F (1, 22) = 1.231 | 0.2793 | F (1, 22) = 0.02607 | 0.8732 | n/a | |||
| genotype: F (1, 22) = 2.953 | 0.0997 | |||||||
| IFNγ | hippocampus | age:F (1, 23) = 4.863 | 0.0377 | F (1, 23) = 1.130 | 0.2989 | age | WT | >0.9999 |
| genotype:F (1, 23) = 1.861 | 0.1857 | eIF4ES209A | 0.1633 | |||||
| genotype | young | >0.9999 | ||||||
| aged | 0.6354 | |||||||
| PFC | age:F (1, 20) = 1.318 | 0.2645 | F (1, 20) = 0.3790 | 0.5451 | n/a | |||
| genotype: F (1, 20) = 1.481 | 0.2379 | |||||||
| striatum | age: F (1, 20) = 0.3904 | 0.8454 | F (1, 20) = 1.301 | 0.2675 | n/a | |||
| genotype: F (1, 20) = 0.7035 | 0.4115 | |||||||
| IL-1β | hippocampus | age:F (1, 32) = 1.444 | 0.2382 | F (1, 32) = 0.8404 | 0.3661 | n/a | ||
| genotype: F (1, 32) = 1.696 | 0.2021 | |||||||
| PFC | age: F (1, 20) = 0.06551 | 0.8006 | F (1, 20) = 0.007876 | 0.9302 | n/a | |||
| genotype: F (1, 20) = 0.1471 | 0.7054 | |||||||
| striatum | age: F (1, 20) = 6.773 | 0.0170 | F (1, 20) = 0.4306 | 0.5192 | age | WT | 0.1548 | |
| genotype: F (1, 20) = 0.1288 | 0.7233 | eIF4ES209A | >0.9999 | |||||
| genotype | young | >0.9999 | ||||||
| aged | >0.9999 | |||||||
| TNFα | hippocampus | age: F (1, 32) = 2.327 | 0.1369 | F (1, 32) = 0.3358 | 0.5663 | |||
| genotype: F (1, 32) = 0.3057 | 0.5842 | |||||||
| PFC | age:F (1, 20) = 3.780 | 0.0661 | F (1, 20) = 0.4390 | 0.5152 | ||||
| genotype: F (1, 20) = 0.08185 | 0.7777 | |||||||
| striatum | age: F (1, 20) = 6.756 | 0.0172 | F (1, 20) = 0.09033 | 0.7669 | age | WT | 0.2110 | |
| genotype: F (1, 20) = 0.1299 | 0.8804 | eIF4ES209A | 0.8976 | |||||
| genotype | young | >0.9999 | ||||||
| aged | >0.9999 | |||||||
| SK2 | PFC | Age: F(1,8)=8.205 | 0.0210 | F(1,8)=03279 | 0.8608 | Age | WT | 0.1798 |
| Genotype: F(1,8)=0.7691 | 0.4601 | eIF4ES209A | 0.1228 | |||||
Highlights.
Spatial/working memory is unaffected by impaired cap dependent translation in aging
Loss of eIF4E-mediated translation alleviates anxiety in the aged
Age-related inflammation in specific brain regions affects a subset of behaviors
Acknowledgments:
We thank Vivien Lai and Aspen Samuel for their technical assistance. We also thank Leticia Mariane dos Santos for the architectural renderings of the apparatus used for behavioral tests.
Funding: This research was funded by the NIH/NINDS, grant number K22NS096030 (M.D.B.), the University of Texas System Rising STARS program research support grant (M.D.B.), the American Pain Society Future Leaders Grant (M.D.B.), and the Rita Allen Foundation Grant (M.D.B.).
Abbreviations
- 4E-BP
eIF4E binding protein
- CaMKIIα
Ca++/calmodulin dependent protein kinase 2 alpha
- CNS
Central nervous system
- eIF4E
Eukaryotic initiation factor 4e
- IFNα
Interferon alpha
- IFNγ
Interferon gamma
- IL-1β
Interleukin 1 beta
- m7G
7-methylguanosine cap
- MAPK
Mitogen activated protein kinase
- MNK 1/2
MAPK interacting kinase 1/2
- mTORC
Mammalian target of Rapamycin complex
- NR2B
N-methyl D-aspartate receptor subtype 2B
- PABP
Poly A binding protein
- PNS
Peripheral nervous system
- SK2
small conductance Ca++ activated potassium channel 2
- TNFα
Tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
Conflicts of Interest: The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Aguilar-Valles A, Haji N, De Gregorio D, Matta-Camacho E, Eslamizade MJ, Popic J, Sharma V, Cao R, Rummel C, Tanti A, Wiebe S, Nuñez N, Comai S, Nadon R, Luheshi G, Mechawar N, Turecki G, Lacaille J-C, Gobbi G, Sonenberg N, 2018. Translational control of depression-like behavior via phosphorylation of eukaryotic translation initiation factor 4E. Nature Communications 9(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim IS, Kedia S, Kouloulia S, Simbriger K, Gantois I, Jafarnejad SM, Li Y, Kampaite A, Pooters T, Romano N, Gkogkas CG, 2018a. Loss of eIF4E Phosphorylation Engenders Depression-like Behaviors via Selective mRNA Translation. J Neurosci 38(8), 2118–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim IS, Kedia S, Kouloulia S, Simbriger K, Gantois I, Jafarnejad SM, Li Y, Kampaite A, Pooters T, Romanò N, Gkogkas CG, 2018b. Loss of eIF4E Phosphorylation Engenders Depression-like Behaviors via Selective mRNA Translation. J Neurosci 38(8), 2118–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimova AS, Alexandrov AI, Makarova NE, Gladyshev VN, Dmitriev SE, 2018. Protein synthesis and quality control in aging. Aging (Albany NY) 10(12), 4269–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, 2010. “I’ve seen it all before”: explaining age-related impairments in object recognition. Theoretical comment on Burke et al. (2010). Behav Neurosci 124(5), 706–709. [DOI] [PubMed] [Google Scholar]
- Bidinosti M, Ran I, Sanchez-Carbente MR, Martineau Y, Gingras AC, Gkogkas C, Raught B, Bramham CR, Sossin WS, Costa-Mattioli M, DesGroseillers L, Lacaille JC, Sonenberg N, 2010. Postnatal deamidation of 4E-BP2 in brain enhances its association with raptor and alters kinetics of excitatory synaptic transmission. Mol Cell 37(6), 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser R, Heyser C, 2015. Spontaneous object recognition: a promising approach to the comparative study of memory. Front Behav Neurosci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington SA, Huang W, Costa-Mattioli M, 2014. Translational control in synaptic plasticity and cognitive dysfunction. Annu Rev Neurosci 37, 17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton M, Rytych J, R A, Johnson R, 2016. Dietary Luteolin Reduces Proinflammatory Microglia in the Brain of Senescent Mice. Rejuvenation research 19(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton MD, Johnson RW, 2012. Interleukin-6 trans-signaling in the senescent mouse brain is involved in infection-related deficits in contextual fear conditioning. Brain Behav Immun 26(5), 732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton MD, Sparkman NL, Johnson RW, 2011. Inhibition of interleukin-6 trans-signaling in the brain facilitates recovery from lipopolysaccharide-induced sickness behavior. J Neuroinflammation 8, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Gkogkas CG, de Zavalia N, Blum ID, Yanagiya A, Tsukumo Y, Xu H, Lee C, Storch KF, Liu AC, Amir S, Sonenberg N, 2015. Light-regulated translational control of circadian behavior by eIF4E phosphorylation. Nat Neurosci 18(6), 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Miller A, 2011. Immune System to Brain Signaling: Neuropsychopharmacological Implications. Pharmacology & therapeutics 130(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Kim DH, Lee EK, Chung KW, Chung S, Lee B, Seo AY, Chung JH, Jung YS, Im E, Lee J, Kim ND, Choi YJ, Im DS, Yu BP, 2019. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis 10(2), 367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW, 2008. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol 84(4), 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH, 2007. Effects of Interferon-Alpha on Rhesus Monkeys: A Nonhuman Primate Model of Cytokine-Induced Depression. Biological psychiatry 62(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF, 2010. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol 226(1-2), 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furic L, Rong L, Larsson O, Koumakpayi IH, Yoshida K, Brueschke A, Petroulakis E, Robichaud N, Pollak M, Gaboury LA, Pandolfi PP, Saad F, Sonenberg N, 2010. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci U S A 107(32), 14134–14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner KM, Amin R, Johnson RW, Scarlett EJ, Burton MD, 2018. Microglia priming by interleukin-6 signaling is enhanced in aged mice. J Neuroimmunol 324, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, O’Connor J, Castanon N, Kelley KW, Dantzer R, Johnson RW, 2008. Aging Exacerbates Depressive-like Behavior in Mice in Response to Activation of the Peripheral Innate Immune System. Neuropsychopharmacology 33(10), 2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdy B, Jaramillo M, Svitkin YV, Rosenfeld AB, Kobayashi M, Walsh D, Alain T, Sean P, Robichaud N, Topisirovic I, Furic L, Dowling RJO, Sylvestre A, Rong L, Colina R, Costa-Mattioli M, Fritz JH, Olivier M, Brown E, Mohr I, Sonenberg N, 2012. Translational control of the activation of transcription factor NF-κB and production of type I interferon by phosphorylation of the translation factor eIF4E. Nat Immunol 13(6), 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M, 2013. mTOR is a key modulator of ageing and age-related disease. Nature 493(7432), 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Platanias LC, 2014. Mnk kinase pathway: Cellular functions and biological outcomes. World J Biol Chem 5(3), 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekmine F, Uddin S, Sassano A, Parmar S, Brachmann SM, Majchrzak B, Sonenberg N, Hay N, Fish EN, Platanias LC, 2003. Activation of the p70 S6 Kinase and Phosphorylation of the 4E-BP1 Repressor of mRNA Translation by Type I Interferons. The Journal of biological chemistry 278(30). [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Rabinovitz M, Gironda P, Pollock BG, 2007. Depression Following Pegylated Interferon-Alpha: Characteristics and Vulnerability. Journal of psychosomatic research 63(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder B, Li X, Barik S, 2010. Translation Control: A Multifaceted Regulator of Inflammatory Response. J Immunol 184(7), 3311–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick WC, Pavitt GD, 2018. Protein Synthesis Initiation in Eukaryotic Cells. Cold Spring Harb Perspect Biol 10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsi V, Zachariou V, 2016. Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience 338, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody PH, Dos Santos NL, Barron LR, Price TJ, Burton MD, 2020. eIF4E phosphorylation modulates pain and neuroinflammation in the aged. GeroScience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy JK, Khoutorsky A, Asiedu MN, Black BJ, Kuhn JL, Barragán-Iglesias P, Megat S, Burton MD, Burgos-Vega CC, Melemedjian OK, Boitano S, Vagner J, Gkogkas CG, Pancrazio JJ, Mogil JS, Dussor G, Sonenberg N, Price TJ, 2017. The MNK–eIF4E Signaling Axis Contributes to Injury-Induced Nociceptive Plasticity and the Development of Chronic Pain. J Neurosci 37(31), 7481–7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP, 2013. Microglia of the Aged Brain: Primed to be Activated and Resistant to Regulation. Neuropathol Appl Neurobiol 39(1), 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins AE, Piazza MK, Deak T, 2018. Stereological analysis of microglia in aged male and female Fischer 344 rats in socially-relevant brain regions. Neuroscience 377, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH, 2010. CSF Concentrations of Brain Tryptophan and Kynurenines During Immune Stimulation With IFN-alpha: Relationship to CNS Immune Responses and Depression. Molecular psychiatry 15(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA, 2018. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front Immunol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA, 2018. The Population 65 Years and Older in the United States. United States Census Bureau, https://www.census.gov. [Google Scholar]
- Rodgers RJ, Dalvi A, 1997. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev 21(6), 801–810. [DOI] [PubMed] [Google Scholar]
- Rosczyk H, Sparkman NL, Johnson R, 2008. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol 43(9), 840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraf A, Luo J, Morris DR, Storm DR, 2014. Phosphorylation of Eukaryotic Translation Initiation Factor 4E and Eukaryotic Translation Initiation Factor 4E-binding Protein (4EBP) and Their Upstream Signaling Components Undergo Diurnal Oscillation in the Mouse Hippocampus: Implications for Memory Persistence. The Journal of biological chemistry 289(29). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper GC, van Kollenburg B, Hu J, Luo Y, Goss DJ, Proud CG, 2002. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J Biol Chem 277(5), 3303–3309. [DOI] [PubMed] [Google Scholar]
- Shoji H, Miyakawa T, 2019. Age-related behavioral changes from young to old age in male mice of a C57BL/6J strain maintained under a genetic stability program. Neuropsychopharmacol Rep 39(2), 100–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shveygert M, Kaiser C, Bradrick SS, Gromeier M, 2010. Regulation of Eukaryotic Initiation Factor 4E (eIF4E) Phosphorylation by Mitogen-Activated Protein Kinase Occurs through Modulation of Mnk1-eIF4G Interaction⍰. Mol Cell Biol 30(21), 5160–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Dos Santos NL, Johnson RW, Burton MD, 2019. Aging sensitizes male mice to cognitive dysfunction induced by central HIV-1 gp120. Exp Gerontol 126, 110694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM, 2012. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485(7396), 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth LA, 2018. Identifying and Implementing Endpoints for Geriatric Mice. Comp Med 68(6), 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R, 2004. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol 24(15), 6539–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukai M, Miura M, Kameyama T, 1995. Effects of galanin on passive avoidance response, elevated plus-maze learning, and spontaneous alternation performance in mice. Peptides 16(7), 1283–1286. [DOI] [PubMed] [Google Scholar]
- Weller RO, Sharp MM, Christodoulides M, Carare RO, Møllgård K, 2019. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS | SpringerLink. Acta Neuropathologica 135(3), 363–385. [DOI] [PubMed] [Google Scholar]
- Williamson D, Gallagher P, Harber M, Hollon C, Trappe S, 2003. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol 547(Pt 3), 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankelevitch-Yahav R, Franko M, Huly A, Doron R, 2015. The forced swim test as a model of depressive-like behavior. J Vis Exp (97). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SM, Johnson RW, 1999. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol 93(1-2), 139–148. [DOI] [PubMed] [Google Scholar]


