Abstract
Tendons have a uniaxially aligned structure with a hierarchical organization of collagen fibrils crucial for tendon function. Collagen XII is expressed in tendons and has been implicated in the regulation of fibrillogenesis. It is a non-fibrillar collagen belonging to the Fibril-Associated Collagens with Interrupted Triple Helices (FACIT) family. Mutations in COL12A1 cause myopathic Ehlers Danlos Syndrome with a clinical phenotype involving both joints and tendons supporting critical role(s) for collagen XII in tendon development and function. Here we demonstrate the molecular function of collagen XII during tendon development using a Col12a1 null mouse model. Col12a1 deficiency altered tenocyte shape, formation of interacting cell processes, and organization resulting in impaired cell-cell communication and disruption of hierarchal structure as well as decreased tissue stiffness. Immuno-localization revealed that collagen XII accumulated on the tenocyte surface and connected adjacent tenocytes by building matrix bridges between the cells, suggesting that collagen XII regulates intercellular communication. In addition, there was a decrease in fibrillar collagen I in collagen XII deficient tenocyte cultures compared with controls suggesting collagen XII signaling specifically alters tenocyte biosynthesis. This suggests that collagen XII provides feedback to tenocytes regulating extracellular collagen I. Together, the data indicate dual roles for collagen XII in determination of tendon structure and function. Through association with fibrils it functions in fibril packing, fiber assembly and stability. In addition, collagen XII influences tenocyte organization required for assembly of higher order structure; intercellular communication necessary to coordinate long range order and feedback on tenocytes influencing collagen synthesis. Integration of both regulatory roles is required for the acquisition of hierarchal structure and mechanical properties.
Keywords: Collagen XII, Tendon, Cell-cell communication, Collagen fibril assembly, Tendon extracellular matrix assembly, mechanical properties
INTRODUCTION
Tendons are responsible for transmission of mechanical force from muscle to bone, allowing for mobility and joint stability. Tendons have a uniaxially aligned collagenous extracellular matrix. This matrix is composed of hierarchically organized components: collagen molecules assemble into fibrils, fibrils assemble into fibers, and fibers together with tendon fibroblasts (tenocytes) are organized into fascicles [1–5]. This unique hierarchal structure is crucial for facilitating transmission of force and directly influences overall mechanical function of the tissue [1,5]. The hierarchical organization of collagen during development, growth and maturation determines connective tissue structure and function[5–7] and disruption of this assembly results in tissue dysfunction [8–11] that often underlies connective tissue diseases [12–17]. However, the mechanisms underlying development of the unique tendon hierarchical structure and, therefore, function have not been fully elucidated.
Tendon development is initiated by condensation of tenocytes, that become aligned into columns defining the tendon longitudinal axis [18–20]. As tendons develop, tenocytes secrete collagens and a series of sequential events leading to tissue-specific extracellular matrix assembly is initiated at the tenocyte surface [21–23]. These include: collagen nucleation initiating collagen fibril assembly, fibril assembly, and organization of fibrils into fibers [7]. Tenocyte alignment along the longitudinal axis of the developing tendon drives the longitudinal organization of the tendon extracellular matrix and structure [1]. On the other hand, in the transverse axis, tenocytes extend cell processes perpendicular to the tendon axis. These processes interact with adjacent tenocytes defining a series of specific domains where fibrils are organized into fibers and fiber assembly can be regulated [1,2,22]. These interacting aligned columns of tenocytes together with uniaxial collagen fibers constitute a primary tendon unit. Depolymerization of the tenocyte actin cytoskeleton results in disorganized tenocyte shape and disruption of cell process formation and organization as well as the loss of parallelism of the tendon extracellular matrix [24]. These lines of evidence indicate that tenocyte shape, cell process formation and organization are crucial for the development of tendon structure.
The tenocyte network responsible for tendon-specific assembly of the extracellular matrix has structural and communicating connections. The structural connections between tenocyte processes are via cadherin 11, which is the major adhesion molecule in tendon [25,26]. The inhibition of cadherin 11 expression decreases fibril deposition and distinct fiber-forming domains are lost [25]. This demonstrates the importance of intercellular interactions in formation of tendon hierarchal structure. In addition, tenocytes form a communicating network expressing two gap junction components; connexin 32 and 43 [27,28]. The major connexin is connexin 43 that localizes to the tips of tenocyte processes and to the cell body, whereas connexin 32 is localized only on the tenocyte cell body. In the response to mechanical stimuli, gap junctions have a role in mediating increased collagen I synthesis by tenocytes [28]. Therefore, the tenocyte gap junction mediated communicating network is critical not only for determining tendon structure, but also mediating the tendon response to mechanical stimuli.
We previously demonstrated that collagen XII is crucial in establishing/maintaining a communicating network via connexin 43 in osteoblasts during bone formation [29,30]. In addition, collagen XII expression is increased in response to the mechanical stimuli in vitro [31,32] and in vivo [33,34]. Furthermore collagen XII is highly expressed in tendons, compared to bones and muscles [30]. These lines of evidence indicate that collagen XII plays a critical role(s) in tendons.
Collagen XII is a member of the Fibril-Associated Collagens with Interrupted Triple Helices (FACIT) family, and is found associated with collagen I fibrils [35,36]. Its molecular structure and tissue localization suggest a role in regulation of collagen I fibrillogenesis [37–40]. Functionally, collagen XII has been implicated regulating intercellular communication during development [29,30] and tissue regeneration [41,42]. In addition, changes in collagen XII can result in altered growth factor availability with resulting changes in tenocyte behavior that would influence tissue function [15]. It has been shown that collagen XII is expressed in developing tendons and ligaments [38,40,43,44]. In chicken metatarsal tendon, the expression of collagen XII then becomes restricted to the endotenon, suggesting that collagen XII regulates the integration of developing tendon matrices and fascicles to functional units [38,43]. Clinically, patients with myopathic Ehlers-Danlos Syndrome have COL12A1 mutations with an overlapping phenotype combining clinical involvement from muscle and from connective tissue [16,17,45–48]. A number of COL12A1 dominant and recessive mutations have been identified. Both dominant and recessive mutations in the COL12A1 gene result in excess weakness at birth, strikingly hypermobile distal joints, and absence of deep tendon reflexes. These clinical manifestations indicate a critical role(s) of collagen XII in tendon and ligament development and function.
Here, we address the physiological roles of collagen XII in establishing tendon hierarchal structure and function. We define the developmental defects in our Col12a1−/− mouse model at molecular, cellular and tissue levels to probe the roles of collagen XII in the regulation of tendon extracellular matrix assembly and mechanical function.
RESULTS
Collagen XII content throughout tendon development and maturation
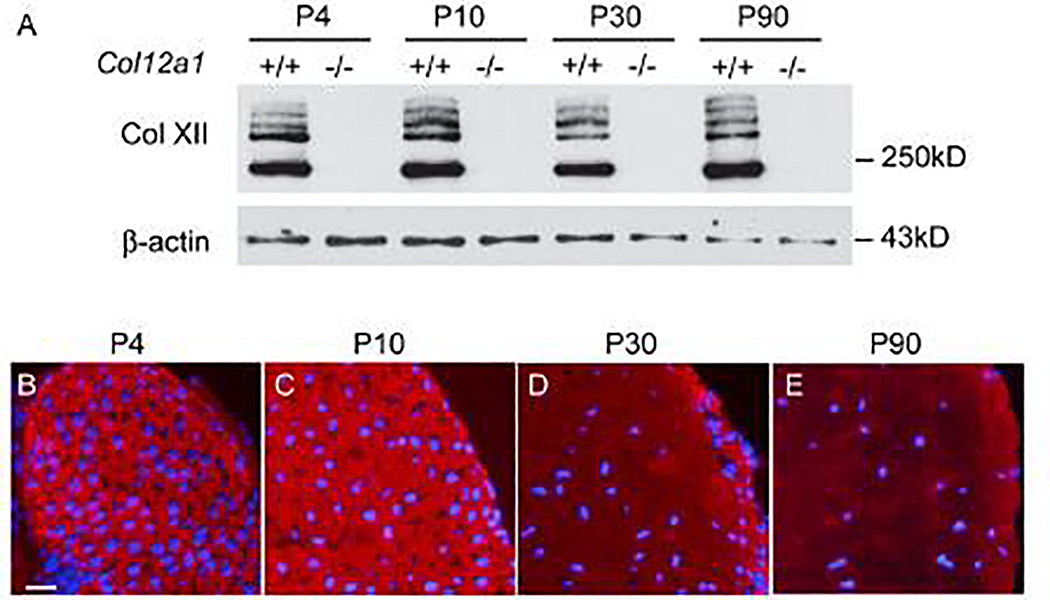
Collagen XII expression in tendon was analyzed in flexor digitorum longus (FDL) tendons by Western blotting. Collagen XII content was comparable in developing (P4, 10), maturing (P30) and mature (P90) wild type tendons. As expected, no collagen XII was present in Col12a1−/− tendons (Fig. 1A). The localization of collagen XII was analyzed using immunofluorescence. Collagen XII was expressed throughout the tendon extracellular matrix in transverse sections from P4, P10, P30 and P90 wild type mice (Fig. 1B–E). At all 4 stages pericellular localization is clear and the tendon proper demonstrated diffuse reactivity (Supplementary Fig. 1).
Fig 1. Expression of collagen XII during mouse tendon development and maturation.
(A) Quantitative analysis of collagen XII was performed by Western blotting from P4, P10, P30 and P90 Col12a1+/+ and Col12a1−/− mouse FDL tendons. The collagen XII expression was stable during tendon development. (B-E) Collagen XII localization was analyzed by immunofluoresence in P4, P10, P30 and P90 mouse FDLs. Collagen XII reactivity was present throughout the tendon with no change in distribution with age. At P30 and P90 reactivity was less than in the younger tendons, but with same distribution. DAPI, blue for nuclei. Bar = 50 μm.
Absence of collagen XII altered both lateral tenocyte network formation and longitudinal columnar arrangement.
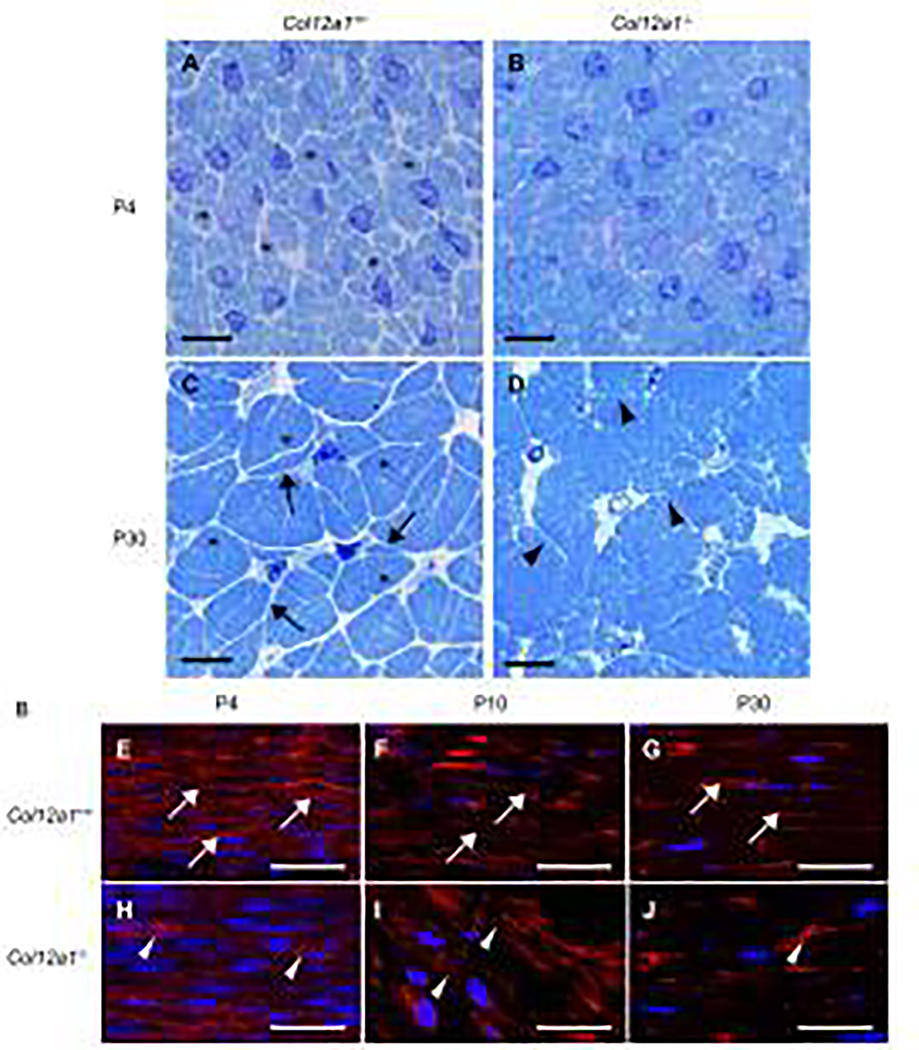
To determine the regulatory functions of collagen XII during tendon development, morphological analyses of Col12a1−/− and wild type control FDLs were performed. Transverse sections of FDLs were analyzed in P4 and P30 mice (Fig. 2A–D). At P4, wild type FDLs consisted of well-organized tenocytes with defined collagen fibers within distinct domains (Fig. 2A). In contrast, the tenocyte defined domains containing fibers were less distinct in Col12a1−/− FDLs than in wild type controls (Fig. 2B). At P30, wild type tenocytes extended attenuated cytoplasmic processes toward adjacent tenocytes establishing a well-organized interacting network (Fig. 2C). This well-defined tenocyte network compartmentalizes and clearly defines the maturing collagen fibers. In contrast, the Col12a1−/− tenocytes lacked organization and were characterized by ill-defined and shorter processes (Fig. 2D), resulting in a less defined tenocyte network compared to wild type controls. In addition, distinct fiber domains were not formed leading to a mass of disorganized collagen fibrils rather than distinct collagen fibers seen in the wild type controls. These data demonstrate that collagen XII regulates tenocyte shape and lateral network formation, compartmentalizing a fiber domain. Disruption of these domains in the absence of collagen XII results in dysfunctional fiber assembly.
Fig 2. Tenocytes and fiber domain structure in cross and logitudinal sections.
(A-D) Cross sections of FDLs were stained with Toluidine blue and (E-J) longitudinal sections were stained with phalloidin and DAPI. At P4, Col12a1+/+ FDLs consisted of tenocytes and collagen fibers (asterisks). The domains, defined by the tenocytes containing fibers, are clearly defined (A), whereas no clear fiber domains are detected in Col12a1−/− FDLs (B). At P30, Col12a1+/+ tenocyte processes (arrows) interact with processes from neighboring cells and clearly define the fiber domains (asterisks) (C). In contrast, tenocyte processes (arrow heads) and fiber domains are disorganized and poorly defined in Col12a1−/− FDLs (D). Phalloidin staining of longitudinal sections demonstrates that tenocytes (arrows) are parallel and orientated along with the longitudinal axis at P4 (E). At P10 and P30, the tenocytes become attenuated along the tendon axis (F, G). In Col12a1−/− FDLs at P4, the actin cytoskeleton is less developed, and tenocytes are poorly organized (arrowheads) (H). Tenocyte structure (cytoskeleton) is disrupted, and the tenocytes are disorganized with the tendon axis hard to define at P10 (I) and P30 (J). Scale bars 50 μm (A-D) and 25 μm (E-J).
In wild type tendons, tenocytes are longitudinally arranged in columns along the tendon axis. Longitudinal sections of FDLs were analyzed in P4, P10 and P30 mice (Fig. 2E–J). At P4 wild type, tenocytes defined by their cytoplasmic actin filaments were organized along with the longitudinal axis with a parallel alignment of tenocytes (Fig. 2E). At P10 and P30, the tenocytes became more attenuated with a retention of the parallel and axial alignment seen at P4 (Fig. 2F,G), consistent with maturation of tenocyte alignment. In contrast, in Col12a1−/− FDLs at P4, actin signal was reduced compared to controls suggesting a less developed cytoplasmic filamentous actin cytoskeleton. The tenocytes also were disorganized with respect to the tendon axis (Fig. 2H). At P10 and P30, the Col12a1−/− tenocytes did not attain the same parallel and axial alignment seen in the wild type tendons (Fig. 2I,J), suggesting that the columnar arrangement of tenocytes in longitudinal axis is disordered in Col12a1−/− FDLs. Therefore, the absence of collagen XII alters not only tenocyte lateral cell process formation, but also longitudinal columnar arrangement of tenocytes.
Collagen XII deficiency results in impaired tenocyte process formation and fiber organization.
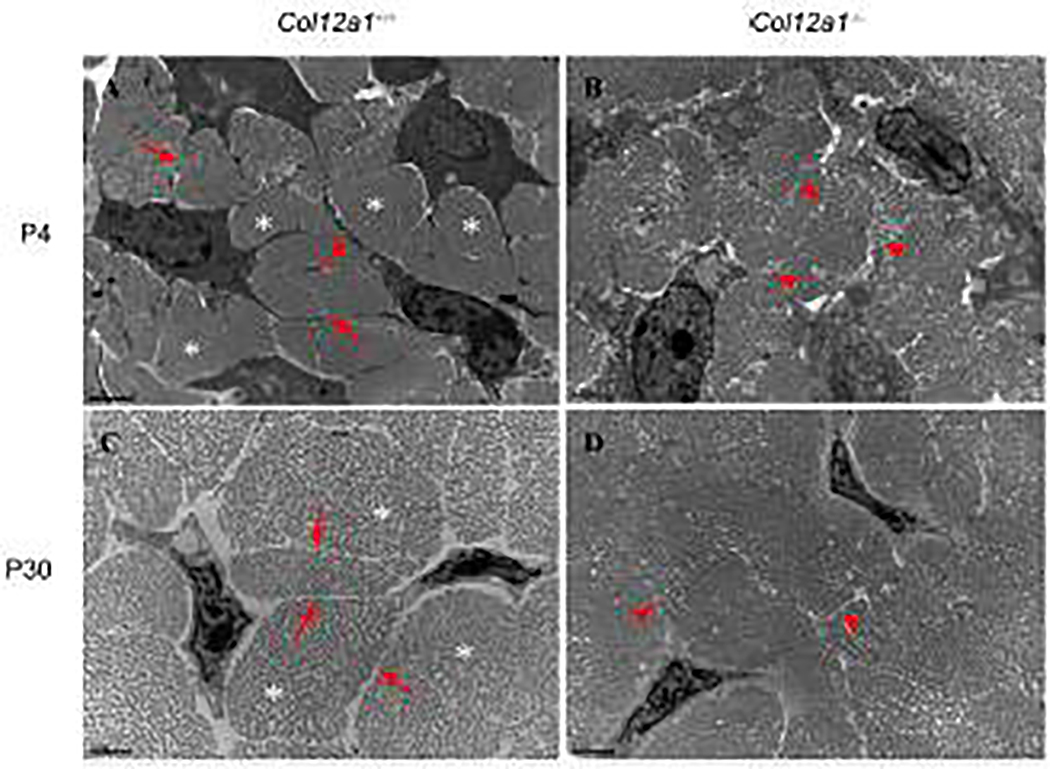
Ultrastructural analyses of the interactions involved in defining the tenocyte network and collagen fibers were done using transmission electron microscopy. Developing (P4) and maturing (P30) FDLs were analyzed in transverse sections from wild type and Col12a1−/− mice (Fig. 3). At P4, wild type tenocytes had extended processes that laterally interacted with adjacent cells to form clear domains containing well defined collagen fibers (Fig. 3A). In contrast, in Col12a1−/− FDLs the tenocyte processes were irregular and ill-defined (Fig. 3B). In addition, the interactions between adjacent cells were unclear, and fiber domains were not completely formed resulting in poorly defined and organized fibers. Similar to the developing FDLs, maturing wild type tenocytes had well-defined processes (Fig. 3C) and fiber-forming domains, resulting in well organized and defined tenocyte networks and well-organized fiber compartmentalization. In contrast, no clear tenocyte processes (Fig. 3D) and fiber-forming compartments were detected in mature Col12a1−/− FDLs, resulting in an ill-defined mass of collagen fibrils with tenocytes embedded in it. These data support a regulatory role for collagen XII in tenocyte process formation resulting in decreased tenocyte cell-cell connection and fiber domain formation.
Fig 3. Altered tenocyte process formation and fibril spacing in the absence of collagen XII.
FDL cross sections from P4 and P30 Col12a1+/+ and Col12a1−/− mice were analyzed by transmission electron microscopy (TEM). At P4, Col12a1+/+ tenocytes extend their processes (arrows) and interact with adjacent cells. Fibers (asterisks) are surrounded by tenocyte processes (A). In contrast, tenocyte processes (arrowheads) are unclear and no obvious, well defined fibers are observed in Col12a1−/− FDLs (B). At P30, similar to P4, Col12a1+/+ tenocytes have fine processes (arrows) and clear fiber domains (asterisks) (C), whereas no clear tenocyte processes (arrowheads) and fiber domains are found in the Col12a1−/− FDL (D). Scale bars 2 μm.
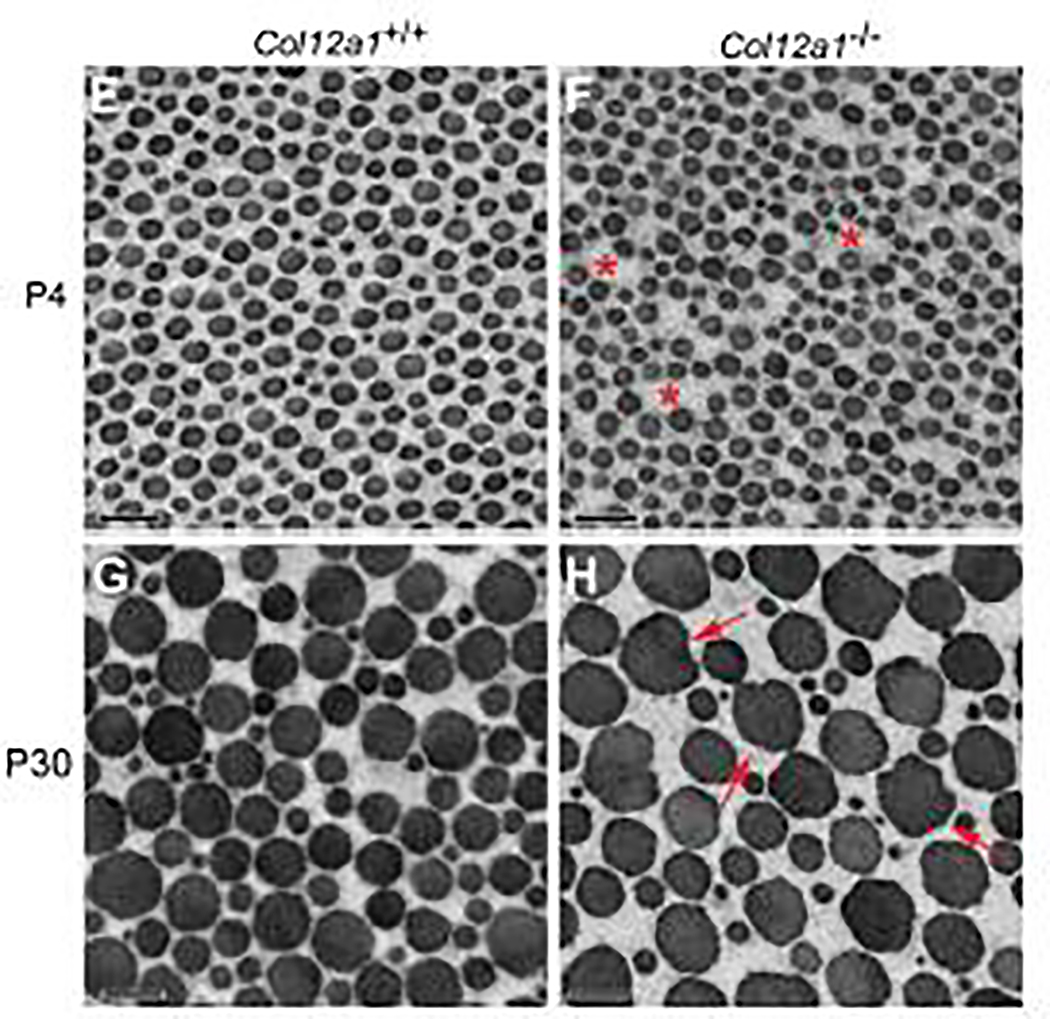
Next, collagen fibril assembly as a function of collagen XII content was analyzed. Transverse sections of FDLs at P4 and P30 from wild type and Col12a1−/− mice were analyzed (Fig. 4). At P4, wild type fibrils were regularly packed, and the fibrils were uniform with normal circular cross-sectional profiles (Fig. 4A). Fibril packing was less regular with increased interfibrillar spacing in Col12a1−/− FDLs compared to wild type controls (Fig. 4B). In mature wild type FDLs, fibrils were well packed with circular cross-sectional profiles similar to the developing tendon (Fig. 4C). However, collagen XII deficiency increased interfibrillar spacing and impaired fibril packing consistent with P4. At P4, collagen fibril structures were comparable with fibrils from both genotypes having circular profiles. The mean fibril diameter was similar in both genotypes, 55.0±4.9nm and 50.1±3.1nm for wild type and Col12a1−/− respectively (Supplementary Fig. 2). At P30, a similar situation was observed with mean diameters being 115.5±16.5 nm versus 117.4±14.7 nm respectively (Supplementary Fig. 2). However, fibrils in P30 Col12a1−/− FDLs demonstrated some irregularity in fibril profile compared to controls. Specifically, many of the larger fibrils show non-circular transverse fibril profiles. The collagen XII deficient FDL fibrils show ‘bumps’ in the contours consistent with unregulated lateral fusion of fibrils. The data suggest a role for collagen XII in mediating fibril packing consistent with a functional role in fiber assembly. The irregularity in transverse fibril profiles suggest collagen XII may stabilize the fibril surface with its absence resulting in abnormal lateral interactions.
Fig. 4. Abnormal tendon collagen fibril packing in the absence of collagen XII.
Cross sections of fibrils from FDLs were analyzed using transmission electron microscopy. (A-B) At P4, fibril diameters are uniform with normal circular cross-sectional profiles in Col12a1+/+ FDLs. In Col12a1−/− FDLs, fibril spacing is increased (asterisks) and fibril packing is irregular. (C-D) At P30, Col12a1+/+ fibrils are well packed with circular crosssectional profiles (C), whereas in Col12a1−/− FDLs aberrant fibril packing is observed. In addition, abnormal fibrils are seen with altered fibril growth resulting in irregular cross-sectional profiles. Arrows indicate irregular fibril profiles (D.) Scale bars 200nm.
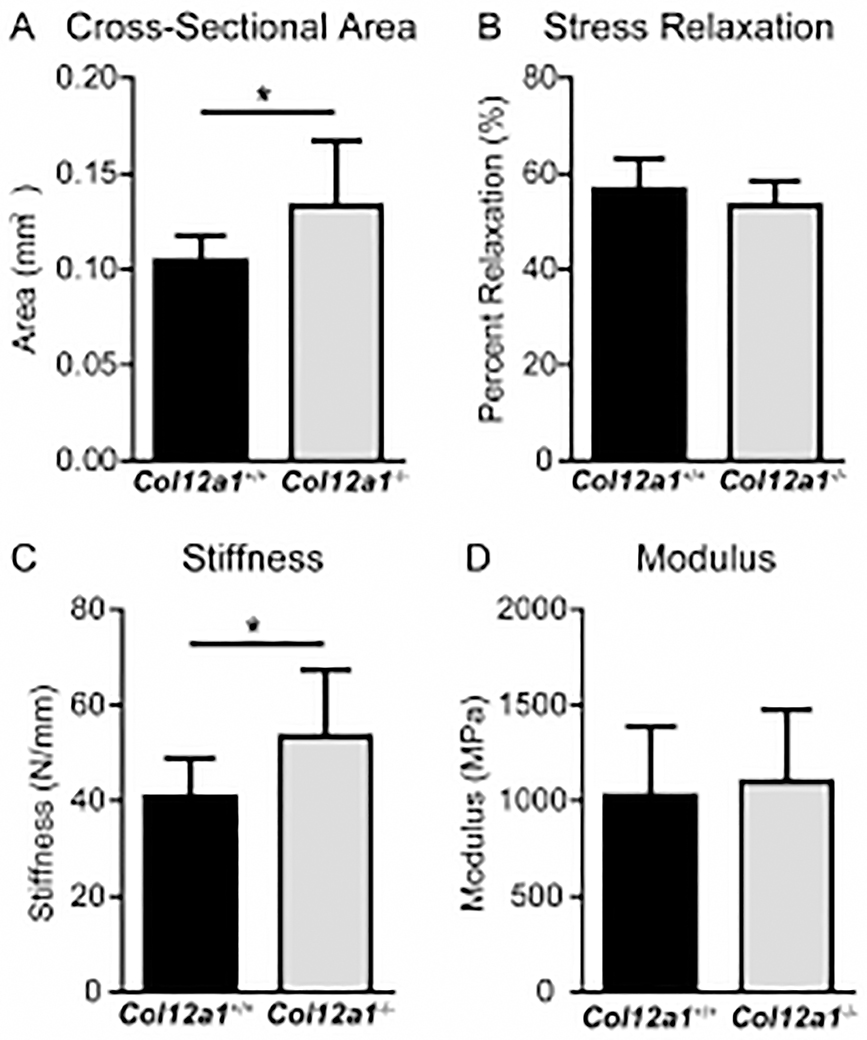
Collagen XII deficiency alters FDL stiffness
Cellular arrangement and fibril packing are important determinants of biomechanical properties in mature tendon [49–51]. Therefore, an assessment of the biomechanical properties in mature wild type controls and Col12a1−/− FDLs was done (Fig. 5). Collagen XII deficient FDLs were significantly larger in cross-sectional area than the wild type FDLs. Col12a1−/− FDLs exhibited ~1.2 fold increase in stiffness compared to controls. However, no difference in modulus was observed when compared to wild type. Also, there was no difference in percent relaxation, a measure of viscoelasticity, between genotypes.
Fig 5. Biomechanics in Col12a1+/+ and Col12a1−/− FDLs.
Biomechanics were analyzed in P60 Col12a1+/+ and Col12a1−/− mouse FDL tendons. (A) Col12a1−/− FDLs are significantly larger in cross-sectional area than Col12a1+/+ FDLs. (B) There was no difference in percent relaxation, a measure of viscoelasticity, between the two groups. (C) Col12a1−/− FDLs exhibits increased stiffness compared to wild type controls. (D) No significant change is detected in modulus in Col12a1−/−, when compared to the wild type controls.
Altered tenocyte intercellular communication in the absence of collagen XII
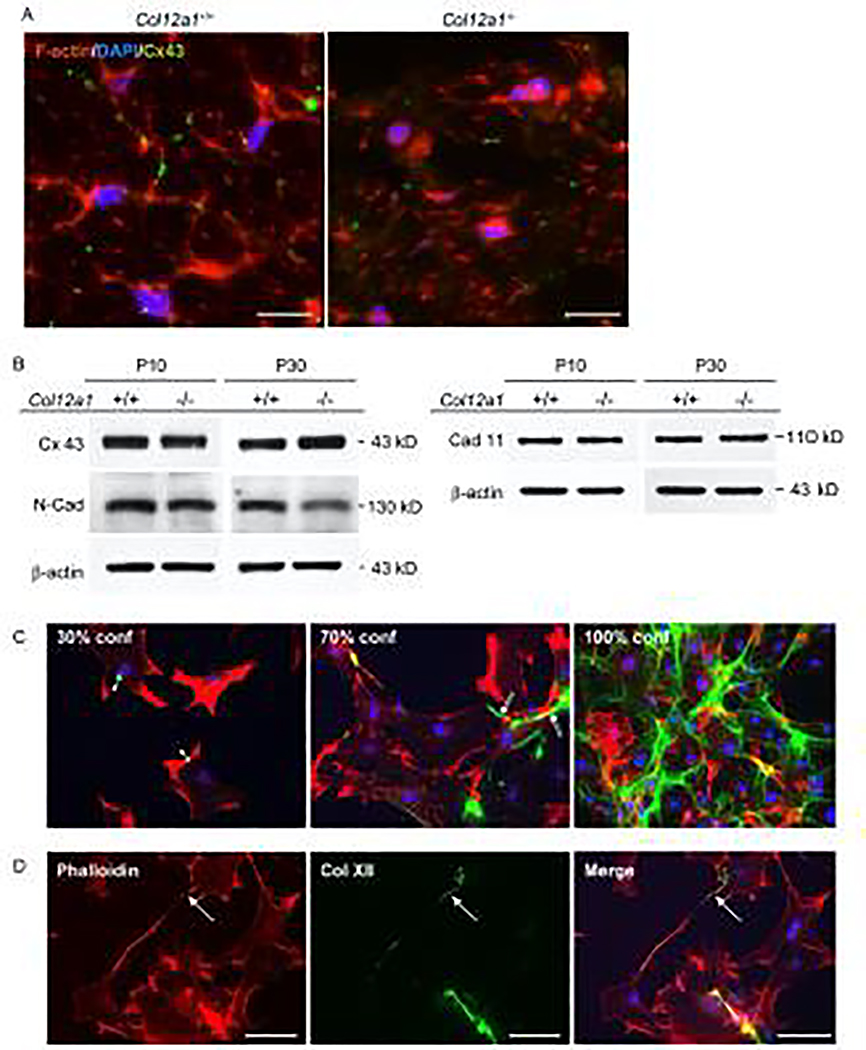
We found that collagen XII deficiency alters tenocyte cell shape, arrangement and interactions leading to dysfunctional fiber assembly resulting in increased tendon stiffness. Tenocyte junctional interactions were analyzed. Tenocytes communicate via gap junctions [20,27,28] and are connected by various adhesion molecules [25]. The gap junction protein, connexin 43 was localized in wild type and Col12a1−/− FDLs using immunofluorescence microscopy of transverse sections (Fig. 6A). Wild type tenocytes demonstrated a dendritic morphology with cellular processes extending laterally toward adjacent cells. Connexin 43 was localized to where adjacent tenocyte processes interact, as expected. In contrast, the dendritic morphology was markedly disrupted/absent in Col12a1−/− FDLs. Connexin 43 immunoreactivity was found in Col12a1−/− tenocytes, however it was poorly localized and difficult to characterize. No interacting tenocyte processes reactive for connexin 43 were observed. Western blots (Fig. 6B) demonstrated that connexin 43 expression was comparable in wild type and Col12a1−/− FDLs from both developing (P10) and maturing (P30) mice. Together, these data indicate that collagen XII is required for appropriate localization in tenocytes, but does not affect quantity of connexin. Cadherin 11, a major cell adhesion molecule in tenocytes, and N-cadherin were analyzed (Fig. 6B). No substantial changes were observed in the absence of collagen XII, cadherin 11 was equivalently expressed between wild type and Col12a1−/− FDLs at P10 and P30, whereas N-cadherin expression slightly declined in Col12a1−/− FDLs at P30. These data indicate that pericellular collagen XII mediates tenocyte interaction and communication.
Fig 6. Collagen XII regulates establishment of a communicating tenocyte network.
(A) Connexin 43 immunolocalization was performed in primary tenocytes obtained from FDLs of Col12a1+/+ and Col12a1−/− neonatal mice. Tenocyte structure was visualized after staining of the cytoplasmic filamentous actin network with Alexa 594 Phalloidin (red) and nuclei were stained with DAPI (blue). Col12a1+/+ tenocytes are dendritic and connexin 43 (green) is localized where tenocyte processes interacted with other processes from adjacent tenocytes. In contrast, Col12a1− /− tenocytes have altered shapes with no clear processes, and no specific localization of connexin 43 is observed. Scale bars 25 μm. (B) Connexin 43 and cell adhesion molecules were analyzed by Western blotting in P10 and P30 FDLs from Col12a1+/+ and Col12a1−/− mice. Connexin 43 and cadherin 11 expression is equivalent between Col12a1+/+ and Col12a1−/− at both P10 and P30. N-cadherin expression is similar between genotypes at P4, but slightly decreased in P30 Col12a1−/− FDLs when compared to Col12a1+/+. b-actin was used as an internal control. (C,D) Collagen XII localization was analyzed in primary tenocytes obtained from Col12a1+/+ FDLs. (C) Immunostaining for collagen XII (green), phalloidin (red) and DAPI (blue) was analyzed in 30%, 70% and 100% confluent cell cultures. At 30% confluence, few intercellular connections are formed. Collagen XII is detected as punctate structures on the tenocyte surface (arrowheads). At 70% confluence, intercellular connections begin to form. Collagen XII is localized along the processes connecting neighboring cells (double arrows). At 100% confluence, collagen XII forms a network connecting neighboring cells. (D) Collagen XII forms bridges between neighboring cells before they establish physical connection. Scale bars 100 μm.
To investigate whether pericellular collagen XII is involved in establishing functional interactions between tenocytes, collagen XII localization at the cellular level was analyzed using primary tenocytes obtained from wild type mouse FDLs (Fig. 6C,D). Collagen XII immunoreactivity was seen as puncta on the tenocyte cell surface in sub-confluent (under 30% confluent) conditions (Fig. 6C). However, as cell density increased, collagen XII reactivity became less focal and was spread along the cell surface (70% confluent) enveloping the confluent tenocytes. Interestingly, we found that collagen XII was localized along tenocyte process and bridged adjacent cells prior to cell-cell contact being established (Fig. 6D). These data suggest that pericellular collagen XII provides a physical connection between neighboring cells thereby stabilizing cell shape and regulating cell-cell connection/communication.
Pericellular collagen XII influences collagen I synthesis.
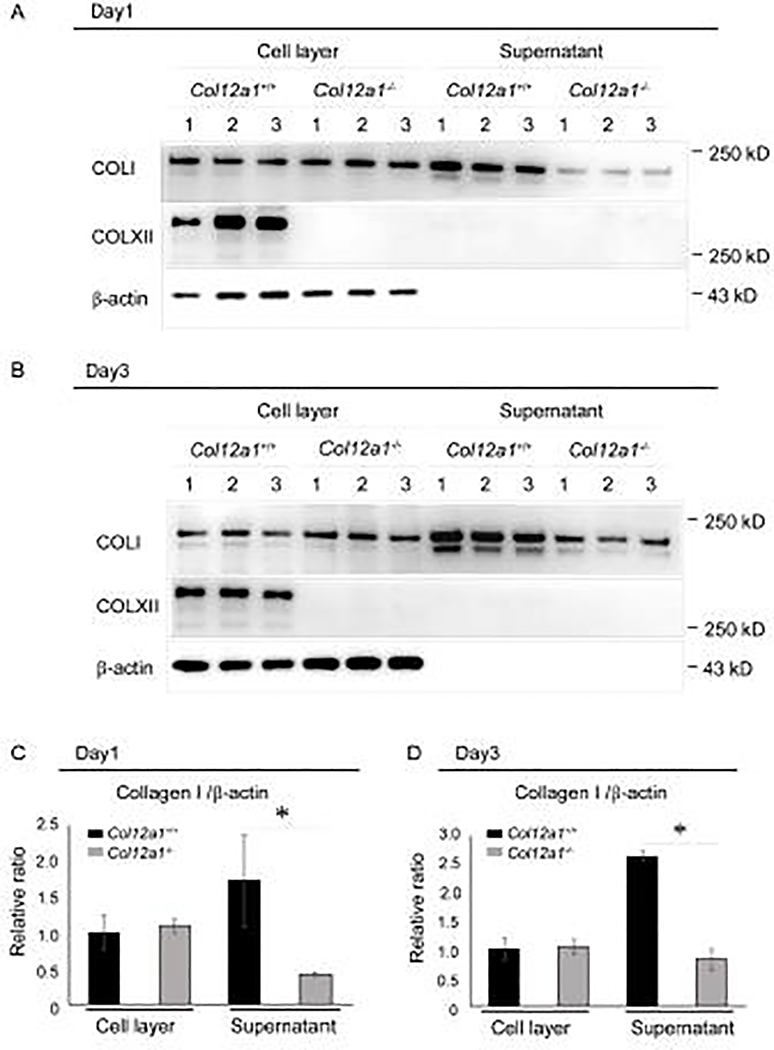
To further define the role of collagen XII in tendons, its localization was defined using cultured primary tenocytes from wild type and Col12a1−/− FDLs. Culture supernatant and cell layers were analyzed using Western blots (Fig. 7). Collagen XII was restricted to the cell layer in wild type tenocytes at both day 1 and 3 post confluence. Collagen XII was absent from both cell layer and supernatant of Col12a1−/− tenocyte cultures, as expected. This data supports a pericellular phenotype at tissue level. Since collagen I fibril assembly is initiated at the tenocyte surface, the role of cell-associated collagen XII in regulation of collagen I fibril assembly was examined. Collagen I was present in the cell layer and supernatant in both wild type and Col12a1−/− cultures. Quantitative analysis of collagen I normalized to β-actin demonstrated that the amount of collagen I in cell layer was comparable between wild type and Col12a1−/− cultures at both day 1 and 3 post confluence. However, in the supernatant, collagen I was decreased at both day 1 and 3 in Col12a1−/− compared to wild type cultures. This is consistent with decreased collagen I in the absence of collagen XII suggesting that collagen XII signaling may increase collagen I synthesis.
Fig 7. Collagen XII is associated with the pericellular matrix and influences collagen I secretion.
Collagen I secretion was analyzed in primary tenocytes obtained from Col12a1+/+ and Col12a1−/− FDLs. Cell layer and culture supernatant were harvested at 1 (A) and 3 (B) days after confluence and analyzed for collagens I and XII content by Western blotting. b-actin is used as an internal control. Collagen XII was found in cell layer at day1 and 3, but not in the supernatant (A, B). Collagen I was found in both the cell layer and culture supernatant as expected. In the cell layer, the collagen I content is comparable between genotypes on both culture days. However, collagen I in the supernatant is decreased in Col12a1−/− cultures at both time points when compared to wild type. (C,D) Quantitative analysis of collagen I at day 1 (C) and day 3 (D) was performed. In wild type controls, more collagen I is present in the supernatant than in the cell layer at both time points, whereas there is less in the Col12a1−/−cell layer. Compared to wild type controls, a significant reduction of collagen I in Col12a1−/− supernatants is found in both culture days.
In summary, the absence of collagen XII alters tenocyte structure, interactions and intercellular communication. The loss of regulatory domains resulting from disruption in tenocyte organization and interaction results in abnormal tendon extracellular matrix assembly, specifically fiber assembly. The fibril associated roles of collagen XII influence fibril packing and fiber stability contributing to the abnormal matrix assembly. The data suggest structural changes in tenocytes and tendon fibrillar matrix in the absence of collagen XII as causative of the altered mechanical properties. In addition, disruption the tenocyte communicating network in the absence of collagen XII would be expected to have effects on the coordination of regulatory steps necessary to establish long range order.
DISCUSSION
Both cell- and extracellular matrix mediated collagen XII regulation of tendon structure and function is supported by this work. We demonstrated that collagen XII regulates tendon structure and mechanical properties through the regulation of tenocyte structure and organization, cell-cell communication, and via interaction with tendon collagen fibrils. All of these regulatory foci coordinately regulate assembly of the tendon hierarchal structure and therefore function. The dual regulatory roles for collagen XII are critical in determination of the hierarchal structure necessary for tendon function. The absence of collagen XII resulted in disruption of fibril, fiber and tenocyte structure as well as organization with the overall result being a structurally abnormal and mechanically deficient tendon.
Extracellularly, collagen XII interacts with collagen fibrils and mediates fibril packing both during fiber assembly and in mature fibers. In addition, collagen XII-fibril interactions may act to stabilize fibrils within fibers. Our analysis demonstrated that collagen XII regulates fibril packing with an increase in interfibrillar space in the absence of collagen XII. This phenotype also is seen in Col14a1 null mouse model, but only through perinatal stages when collagen XIV is expressed [51]. Collagen XIV is a FACIT and structurally very similar to collagen XII [51–53], fibril spacing regulation may be a general extracellular function of FACITs due to their large non-collagenous domains in the interfibrillar space during matrix assembly. There is no indication that a knockout of collagen XII influences expression of collagen XIV (data not shown).
Collagen I is the major protein in tendons and its extracellular matrix assembly into fibrils begins at the tenocyte surface [7] and collagen XII has been implicated in the regulation of fibrillogenesis [36,38]. Our data also shows that in the absence of collagen XII there are mild fibril structure changes consistent with abnormal lateral fibril growth [6,54]. Therefore, fibril associated collagen XII may also stabilize fibrils. However, other less direct mechanisms may be in effect. Small leucine rich proteoglycans (SLRP) are known regulators of lateral fibril growth in a variety of tissues including tendons [55–57]. Collagen XII is known to bind to decorin, a SLRP [58] that is a crucial regulator of lateral fibril growth [56]. Decorin expression also is reduced in human patients with a COL12A1 mutation [47], alteration of fibril fusion may be caused by the decreased decorin signaling. Since FACITs and SLRPs are fibril associated molecules and collagen XII can be a proteoglycan [7], there may be some overlap in function that requires further investigations. Collagen V has a critical role of collagen fibril nucleation at the cell surface [6], including in tendon [59]. Since the targeted deletion of collagen V in tendon alters fiber organization [30], collagens V and XII may interact pericellularly in the regulation of fiber organization and fibrillogenesis. However, further studies are required. In addition to the morphological findings, our cell culture study revealed that total secreted collagen I was decreased in Col12a1−/− tenocytes while pericellular collagen I was not changed. This suggest that collagen XII has a role in regulating signaling in collagen I secretion that may influence on fibril assembly and growth. Taken together, pericellular collagen XII may regulate collagen I fibril assembly as well as signaling that influences collagen I secretion, potentially together with other matrix molecules. Overall, extracellularly, collagen XII regulates fibril stability and has a role is assembly of fibrils into fibers.
Collagen XII also interacts with tenocytes. This works demonstrates that the lack of pericellular collagen XII alters tenocyte structure and organization. In the presence of collagen XII, wild type tenocytes extend of cellular processes in the transverse plane that interact with adjacent tenocyte processes. This compartmentalization of the developing tendon extracellular matrix is critical in regulation of tissue-specific fibrillar matrix assembly [1,2,60]. Specifically, these tenocyte-defined domains are the sites of fiber assembly. In the absence of collagen XII impaired lateral cytoplasmic process formation and compartmentalization was observed. This dysfunctional compartmentalization resulted in poorly defined fiber-forming domains with abnormal fiber that were poorly defined and just masses of collagen fibrils with no structure. Therefore, the alteration in tenocyte compartmentalization of the extracellular matrix resulted in a significant disruption of tendon hierarchal structure. In addition, collagen XII deficiency altered the columnar arrangement of tenocytes in longitudinal axis. This columnar organization precedes organization of the tendon fibrillar matrix during development and is important in the determination of tendon structure [25,61]. Our data indicate key roles for pericellular collagen XII in the regulation of the lateral and longitudinal tenocyte organization. Longitudinal and lateral tenocyte interactions are critical is establishing the tenocyte network necessary to coordinate long range assembly in the tendon.
Cell-cell interaction is regulated not only collagen XII but also collagen VI, and both collagens are required for establishing matrix bridges [29]. Collagen VI is a network forming collagen and often enriched in pericellular region[59]. Collagen VI related myopathies have have an overlap of symptoms with myopathic EDS, including joint hyperlaxity. In addition, recent studies demonstrated that collagen XII localization is altered in tenocytes from the patients with COL6A1 mutations [62], suggesting their coordinate regulation. In tendon, the absence of collagen VI alters tenocyte shape and hierarchical structure, similar to collagen XII deficient tendon[63]. However, in contrast to Col12a1 deficiency, fibrils are packed with less interfibrillar space, and decreased stiffness in Col6a1 deficient FDLs. Thus, collagens VI and XII coordinately regulate cell-cell communication in pericellular region, but these could be functionally different.
The unique tendon hierarchal structure is crucial for facilitating force transmission and mechanical function [1,5,64]. Our data show that the structural changes resulting from collagen XII deficiency alters tendon mechanics suggesting that collagen XII is necessary for normal tendon function. Specifically, collagen XII deficiency resulted in increased cross-sectional area and increased tendon stiffness without affecting other material parameters, such as stress relaxation and modulus. Collagen fiber/fibril organization, size, and connectivity all influence macroscale tissue function through dynamic interactions during mechanical loading (collagen re-alignment, uncrimping, and sliding). While these were not within the scope of the present work, they could be more sensitive to the changes reported and result in functional differences in dynamic or repetitive tissue function. In addition, changes in other compositional parameters such as crosslinking or in the non-collagenous matrix, could compensate for fibrillar deficiencies thus leading to unchanged tissue function. These remain open avenues to fully understand functional deficits in collagen XII knockout tendons and should be pursued in future studies.
Other collagens also regulate tendon biomechanics such as collagen V, VI, XIV. However, in contrast to collagen XII, deficiency of Col5a1[65], Col6a1[63] and Col14a1[51] demonstrate decreased cross sectional area and/or decreased tendon stiffness. Similar to collagen XII, these collagens also are enriched in the pericellular compartment, and some common features in their clinical manifestation, and have been suggested to have coordinated functions in the regulation of tendon matrix assembly and therefore function. Fiber compartmentalization allows for tendon deformation and movements and disruption at this level would be expected to impact this function. In addition, the evidence is also supported by the in vivo mouse data and clinical phenotype in myopathic EDS that results from mutations in COL12A1. Both the mouse model and affected patients show walking abnormality, hind limb expansion and/or contraction abnormality, and decreased grip strength in mice [30,45], and joint hypermobility and absence of deep tendon reflexes in myopathic EDS patients [16,17,45–47]. Because immobility is one of the causes of increased stiffness, lack of collagen XII may exacerbate the effects of immobilization.
We show that tenocyte network formation required collagen XII for proper formation. Therefore, we addressed the role of collagen XII in intercellular communication and adhesion. Our previous work in bone indicated that collagen XII regulates gap junction formation and intercellular communication [30]. In addition, collagen XII forms matrix bridges that provide a connection between adjacent osteoblasts during mouse bone development [29] and axon outgrowth after spinal cord injury in zebrafish [66]. Here, we identify collagen XII bridges connecting adjacent tenocyte processes. In addition, collagen XII deficiency disrupted connexin 43 localization and decreased cadherin consistent with a disruption in communication and cell adhesion. Therefore, in tendons, collagen XII in matrix bridges may provide a physical connection that stabilizes cell surface molecules for intercellular communication and adhesion. In addition, collagen XII is a mechanosensitive molecule [32–34,67,68], and mechanical stress caused by intercellular adhesion or tissue development [69] may be an inducer of collagen XII. Our culture studies demonstrated a decrease in collagen I in collagen XII deficient tenocytes. These data suggest that collagen XII provides a signal to tenocytes that up-regulates collagen I and, therefore, increases tendon matrix assembly. These lines of evidence suggest that increased mechanical stress on tenocytes during development induces collagen XII bridge formation, regulating the formation of stable functional communicating tenocyte networks that may coordinate signals necessary for long range coordination of tendon assembly.
In our tenocyte culture assays, we demonstrated the pericellular function of collagen XII. We found collagen XII on tenocyte surfaces, and as cell density is increased surface-associated collagen XII becomes more prominent and is found along processes and connecting neighboring tenocytes. This localization is consistent with collagen XII deficient mice demonstrating tenocyte disorganization, suggesting pericellular regulation. In addition, the presence on tenocyte processes suggests roles in regulating process interaction and matrix bridge formation. These findings suggest that pericellular collagen XII is a key regulator of tenocytes. In conclusion, our results indicate that collagen XII mediated cellular and extracellular mechanisms are critical in establishment of tendon structure and function.
Experimental procedures
Animals
The production of Col12a1 null mouse model used in this study has been previously described [30]. Male mice were used at P30, P60 and P90. At P14 or less gender was not determined. All animal studies were performed in compliance with IACUC approved animal protocols.
Primary tenocyte culture
Primary tenocytes were isolated after enzymatic digestion of excised tendons from neonatal mouse flexor digitorum longus (FDL) tendons using Collagenase B (Roche) as described previously [59]. Tenocytes were cultured in DMEM supplemented with 10% FBS (Invitrogen), 1mM 2-phospho-L-ascorbic acid (Sigma), and 1% antibiotics (Invitrogen). The medium was changed every 2 days. The collected cells were used for immuno-fluorescence and Western blotting analyses.
Immuno-localization analysis
FDL tendons were dissected from wild type and Col12a1−/− mice at P4, P10, P30 and P90. Tissues were fixed with 4% paraformaldehyde, embedded in OCT medium, and stored at −80 °C. Transverse and longitudinal sections were cut and used for immuno-localization studies. Primary tenocytes isolated from neonatal mouse FDLs were cultured on glass coverslips and collected at 30%, 70% and 100% confluence. The coverslips were fixed with 4% paraformaldehyde for 15 min at room temperature and used for immuno-localization studies. Immunofluorescence localization was performed as previously described[30]. Rabbit anti-type XII collagen (1:1000 dilution; KR33), rabbit anti-Cx43 (1:10000 dilution, Sigma-Aldrich), and Alexa Flour 594 Phalloidin (Invitrogen) were used. Images were captured using an Olympus BX61 fluorescence microscope and Zeiss LSM980 with Airyscan confocal microscopy. Identical conditions and set integration times were used to facilitate comparisons.
Western blotting analysis
FDL tendons dissected from P4 and P30 wild type and Col12a1−/− mice, and primary tenocytes from neonatal mouse FDL described above were used for Western blotting analysis. Tendon preparation and immuno-blots were performed as previously described [51,59]. Cultured primary tenocytes were seeded at 1X105 cells/6cm dish and collected on day 1 and day 3 after confluence. The medium was replaced with serum-free medium 24 hrs before collection, and the conditioned medium was collected. The tenocytes were washed twice with PBS and extracted with 20 mM Tris-HCL pH 7.4 and 0.3% Brij O10, Sigma-Aldrich. The total protein concentration was determined using a BCA protein assay kit (Pierce, Rockford, IL). Constant protein amounts were loaded for each sample; FDL tissue (10 μg), cell culture (15 μg) and 20 μl of cell culture medium and used for SDS-PAGE using NuPAGE Protein gel system (Invitrogen). Westerns blots were done using rabbit anti-type XII collagen (1:1000 dilution; KR33), rabbit anti-type I collagen (1:1000 dilution; Millipore), rabbit anti-Cadherin11 (1:500 dilution; Invitrogen), and rabbit anti-N-cadherin (1:50 dilution; Cell Signaling), and β-actin (1:2000 dilution; Millipore) were used as loading controls at 1:1000 dilution. The signals were detected with an ECL system (Amersham Biosciences). The films were scanned using Amersham imager system and semi-quantitative analysis was performed by measuring the individual band densities in Quantity One software. Statistics were performed using Student’s t tests. The numeral data were presented as means ± SD.
Transmission Electron Microscopy
FDL tendons from P4 and P30 wild type and Col12a1−/− mice were analyzed by transmission electron microscopy (TEM). FDL tendons were dissected and fixed in 4% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M sodium cacodylate, pH 7.4, with 8.0 mM CaCl2, post-fixed with 1% osmium tetroxide [1,65]. After dehydration in an ethanol series, followed by propylene oxide, the tissue samples were infiltrated and embedded in a mixture of EMbed 812, nadic methyl anhydride, dodecenyl succinic anhydride, and DMP-30 (Electron Microscopy Sciences, Hatfield, PA). Ultra-thin sections (90 nm) were cut using a Leica UCT ultramicrotome and post-stained with 2% aqueous uranyl acetate. Cross sections from the mid-plantar regions of FDL tendons were examined at 80 kV using a JEOL 1400 transmission electron microscope equipped with a Gatan Ultrascan US1000 2K digital camera. Semi thin sections (~1um) were cut and stained with toluidine blue and the images were captured with an Olympus BX61 light microscope.
Fibril morphological analysis
For each genotype, 4–5 tendons from 4 different animals at P4 and 3 tendons from 3 different animals at P60 were analyzed. Fibril diameters were measured with a RM Biometrics-Bioquant Image Analysis System (Nashville, TN) using randomly chosen, masked digital images analyzed at a final magnification of 118,790X. For measurements at P4, a constant area of 0.47 μm2 per image was utilized that contained cross-sectional fibril profiles free of cell processes. The count per image was 104–110 fibrils measured, with 24 images measured for wild-type (total fibril count of 2643) and 30 images for the null genotype (total fibril count of 3118). For measurements at P60, a constant area of 1.46 μm2 per image was utilized that contained cross-sectional profiles free of cell processes. The count per image was 62–84 fibrils measured, with 15 images measured for wild-type (total fibril count of 1266) and 15 images for the Col12a1−/− genotype (total fibril count of 926).
Biomechanical Analysis
Biomechanics were analyzed in P60 wild type and Col12a1−/− mouse FDLs as previously described [14,51,65]. Briefly, FDLs were dissected and cleaned of surrounding soft tissue. Cross-sectional area of the FDL was measured using a custom-built laser-based device as previously described [70]. Each end of the tendon was glued to sandpaper 5mm apart and stain lines were placed 2.5mm apart in the midsubstance to allow for optical strain tracking [51]. Tendons were then immersed in a 37°C phosphate-buffered saline bath and tested in an Instron 5543 mechanical testing machine (Instron Corp.). Samples were clamped in custom test fixtures and subjected to a standard testing protocol consisting of preconditioning, a stress relaxation test and a ramp to failure as described previously [71]. All mechanical parameters were calculated from optical tracking data using custom software. Statistical comparisons of mechanical parameters, specifically cross-sectional area, percent relaxation, stiffness, and modulus, were performed using Student t-tests (significance at p ≤ 0.05).
Supplementary Material
Highlights.
Collagen XII is localized both in the tendon matrix and associated with tenocytes in developing, maturing and mature tendons.
Collagen XII regulates collagen I fibril packing and fiber assembly.
Col12a1 deficiency alters tenocyte organization and disrupts cell-cell connections, resulting in deformation of tendon hierarchal structure and reducing mechanical properties.
Collagen XII regulates intercellular communication by building matrix bridges in between adjacent tenocytes facilitating gap junction formation.
Acknowledgement
We thank Dr. Mei Sun (University of South Florida) and Mr. Thomas H. Adams for support all the experiments.
Funding
This study was supported by NIH/NIAMS grant AR044745 (DEB), and NIH/NIAMS grant AR050950, supporting the Penn Center for Musculoskeletal Disorders (LJS), and by Takeda Science Foundation, the Naito Foundation, JSPS KAKENHI Grant Number JP18K09042, Imabari city and Ehime prefecture (YI).
Footnotes
Competing interests
The author(s) declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Birk DE, Trelstad RL, Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation., J Cell Biol. 103 (1986) 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Birk DE, Zycband E, Assembly of the tendon extracellular matrix during development., J. Anat. 184 (1994) 457–63. [PMC free article] [PubMed] [Google Scholar]
- [3].Graham HK, Holmes DF, Watson RB, Kadler KE, Identification of collagen fibril fusion during vertebrate tendon morphogenesis. The process relies on unipolar fibrils and is regulated by collagen-proteoglycan interaction, J. Mol. Biol. 295 (2000) 891–902. 10.1006/jmbi.1999.3384. [DOI] [PubMed] [Google Scholar]
- [4].Canty EG, Kadler KE, Procollagen trafficking, processing and fibrillogenesi, J Cell Sci. 118 (2005) 1341–1353. 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- [5].Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE, Development of tendon structure and function: Regulation of collagen fibrillogenesis, J. Musculoskelet. Neuronal Interact. 5 (2005) 5–21. [PubMed] [Google Scholar]
- [6].Birk DE, Bruckner P, Collagens, suprastructures and collagen fibril assembly., Springer, Berlin, Heidelberg, New York, 2011. . [Google Scholar]
- [7].Mienaltowski MJ, Birk DE, Structure, physiology, and biochemistry of collagens., Adv. Exp. Med. Biol. 802 (2014) 5–29. [DOI] [PubMed] [Google Scholar]
- [8].Walraven M, Hinz B, Therapeutic approaches to control tissue repair and fibrosis: Extracellular matrix as a game changer, Matrix Biol. 71–72 (2018) 205–224. . [DOI] [PubMed] [Google Scholar]
- [9].Wang C, Brisson BK, Terajima M, Li Q, Hoxha K, Han B, Goldberg AM, Sherry Liu X, Marcolongo MS, Enomoto-Iwamoto M, Yamauchi M, Volk SW, Han L, Type III collagen is a key regulator of the collagen fibrillar structure and biomechanics of articular cartilage and meniscus, Matrix Biol. 85–86 (2020) 47–67. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Delbaere S, Van Damme T, Syx D, Symoens S, Coucke P, Willaert A, Malfait F, Hypomorphic zebrafish models mimic the musculoskeletal phenotype of β4GalT7-deficient Ehlers-Danlos syndrome, Matrix Biol. 89 (2020) 59–75. . [DOI] [PubMed] [Google Scholar]
- [11].Cabral WA, Fratzl-Zelman N, Weis M, Perosky JE, Alimasa A, Harris R, Kang H, Makareeva E, Barnes AM, Roschger P, Leikin S, Klaushofer K, Forlino A, Backlund PS, Eyre DR, Kozloff KM, Marini JC, Substitution of murine type I collagen A1 3-hydroxylation site alters matrix structure but does not recapitulate osteogenesis imperfecta bone dysplasia, Matrix Biol. 90 (2020) 20–39. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Karamanos NK, Theocharis AD, Neill T, V Iozzo R, Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases, Matrix Biol. 75–76 (2019) 1–11. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].V Iozzo R, Gubbiotti MA, Extracellular matrix: The driving force of mammalian diseases, Matrix Biol. 71–72 (2018) 1–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sun M, Luo EY, Adams SM, Adams T, Ye Y, Shetye SS, Soslowsky LJ, Birk DE, Collagen XI Regulates the Acquisition of Collagen Fibril Structure, Organization and Functional Properties in Tendon, Matrix Biol. (2020). . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schönborn K, Willenborg S, Schulz J-N, Imhof T, Eming SA, Quondamatteo F, Brinckmann J, Niehoff A, Paulsson M, Koch M, Eckes B, Krieg T, Role of collagen XII in skin homeostasis and repair, Matrix Biol. (2020). In press . [DOI] [PubMed] [Google Scholar]
- [16].Mohassel P, Liewluck T, Hu Y, Ezzo D, Ogata T, Saade D, Neuhaus S, Bolduc V, Zou Y, Donkervoort S, Medne L, Sumner CJ, Dyck PJB, Wierenga KJ, Tennekoon G, Finkel RS, Chen J, Winder TL, Staff NP, Foley AR, Koch M, Bönnemann CG, Dominant collagen XII mutations cause a distal myopathy, Ann. Clin. Transl. Neurol. 6 (2019) 1980–1988. 10.1002/acn3.50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Punetha J, Kesari A, Hoffman EP, Gos M, Kamińska A, Kostera-Pruszczyk A, Novel Col12A1 variant expands the clinical picture of congenital myopathies with extracellular matrix defects, Muscle Nerve. 55 (2017) 277–281. 10.1002/mus.25232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Squier CA, Bausch WH, Three-dimensional organization of fibroblasts and collagen fibrils in rat tail tendon, Cell Tissue Res. 238 (1984) 3193–27. 10.1007/BF00217304. [DOI] [PubMed] [Google Scholar]
- [19].Birk DE, Southern JF, Zycband EI, Fallon JT, Trelstad RL, Collagen fibril bundles: A branching assembly unit in tendon morphogenesis, Development. 107 (1989) 437–443. [DOI] [PubMed] [Google Scholar]
- [20].McNeilly CM, Banes AJ, Benjamin M, Ralphs JR, Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions, J Anat. 189 (1996) 593–600. [PMC free article] [PubMed] [Google Scholar]
- [21].Smith SM, Thomas CE, Birk DE, Pericellular proteins of the developing mouse tendon: A proteomic analysis, Connect. Tissue Res. 53 (2012) 2–13. 10.3109/03008207.2011.602766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Canty EG, Lu Y, Meadows RS, Shaw MK, Holmes DF, Kadler KE, Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon Elizabeth, J Cell Biol. 65 (2004) 553–563. 10.1083/jcb.200312071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kalson NS, Starborg T, Lu Y, Mironov A, Humphries SM, Holmes DF, Kadler KE, Nonmuscle myosin II powered transport of newly formed collagen fibrils at the plasma membrane, Proc. Natl. Acad. Sci. U. S. A. 110 (2013). 10.1073/pnas.1314348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Canty EG, Starborg T, Lu Y, Humphries SM, Holmes DF, Meadows RS, Huffman A, O’Toole ET, Kadler KE, Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon, J. Biol. Chem. 281 (2006) 38592–38598. [DOI] [PubMed] [Google Scholar]
- [25].Richardson SH, Starborg T, Lu Y, Humphries SM, Meadows RS, Kadler KE, Tendon development requires regulation of cell condensation and cell shape via cadherin-11-mediated cell-cell junctions, Mol. Cell. Biol. 27 (2007) 6218–6228. 10.1128/MCB.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Theodossiou SK, Murray JB, Schiele NR, Cell-cell junctions in developing and adult tendons, Tissue Barriers. 8 (2019) 1695491 10.1080/21688370.2019.1695491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ralphs JR, Benjamin M, Waggett AD, Russell DC, Messner K, Gao J, Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation., J. Anat. 193 (1998) 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Waggett AD, Benjamin M, Ralphs JR, Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load, Eur. J. Cell Biol. 85 (2006) 1145–1154. 10.1016/j.ejcb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- [29].Izu Y, Ezura Y, Koch M, Birk DE, Noda M, Collagens VI and XII form complexes mediating osteoblast interactions during osteogenesis, Cell Tissue Res. 364 (2016) 677–679. 10.1007/s00441-015-2345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Izu Y, Sun M, Zwolanek D, Veit G, Williams V, Cha B, Jepsen KJ, Koch M, Birk DE, Type XII collagen regulates osteoblast polarity and communication during bone formation, J. Cell Biol. 193 (2011) 1115–1130. 10.1083/jcb.201010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Arai K, Nagashima Y, Takemoto T, Nishiyama T, Mechanical strain increases expression of type XII collagen in murine osteoblastic MC3T3-E1 cells., Cell Struct. Funct. 33 (2008) 203–210. [DOI] [PubMed] [Google Scholar]
- [32].Trächslin J, Koch M, Chiquet M, Rapid and reversible regulation of collagen XII expression by changes in tensile stress, Exp. Cell Res. 247 (1999) 320–328. 10.1006/excr.1998.4363. [DOI] [PubMed] [Google Scholar]
- [33].Fluck M, Tunc-Civelek V, Chiquet M, Rapid and reciprocal regulation of tenascin-C and tenascin-Y expression by laoading of skeletal muscle, J. Cell Sci. 113 (2000) 3583–3591. [DOI] [PubMed] [Google Scholar]
- [34].Karimbux NY, Nishimura I, Temporal and spatial expressions of type XII collagen in the remodeling periodontal ligament during experimental tooth movement, J. Dent. Res. 74 (1995) 313–318. 10.1177/00220345950740010501. [DOI] [PubMed] [Google Scholar]
- [35].Keene DR, Lunstrum GP, Morris NP, Stoddard DW, Burgeson RE, Two type XII-like collagens localize to the surface of banded collagen fibrils., J. Cell Biol. 113 (1991) 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Koch M, Bohrmann B, Matthison M, Hagios C, Trueb B, Chiquet M, Large and small splice variants of collagen XII : Differential expression and ligand binding preparation of polyclonal antiserum, J. Cell Biol. 130 (1995) 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Agarwal P, Zwolanek D, Keene DR, Schulz JN, Blumbach K, Heinegard D, Zaucke F, Paulsson M, Krieg T, Koch M, Eckes B, Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure., J. Biol. Chem. 287 (2012) 22549–22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang G, Young BB, Birk DE, Differential expression of type XII collagen in developing chicken metatarsal tendons., J. Anat. 202 (2003) 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shaw LM, Olsen BR, FACIT collagens: Diverse molecular bridges in extracellular matrices, Trends Biochem. Sci. 16 (1991) 191–194. [DOI] [PubMed] [Google Scholar]
- [40].Oh SP, Griffith CM, Hay ED, Olsen BR, Tissue-specific expression of type XII collagen during mouse embryonic development., Dev. Dyn. 196 (1993) 37–46. [DOI] [PubMed] [Google Scholar]
- [41].Wehner D, Tsarouchas TM, Michael A, Haase C, Weidinger G, Reimer MM, Becker T, Becker CG, Wnt signaling controls pro-regenerative Collagen XII in functional spinal cord regeneration in zebrafish, Nat. Commun. 8 (2017) 126 10.1038/s41467-017-00143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mcneill EP, Zeitouni S, Pan S, Haskell A, Cesarek M, Clough BH, Krause U, Dobson LK, Garcia M, Kung C, Saunders WB, Liu F, Kaunas R, Gregory CA, Tahan D, Zhao Q, Characterization of a pluripotent stem cell-derived matrix with powerful osteoregenerative capabilities, Nat. Commun. 11 (2020) 3025 10.1038/s41467-020-16646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wälchli C, Koch M, Chiquet M, Odermatt BF, Trueb B, Tissue-specific Expression of the Fibril-Associated Collagens XII and XIV, J. Cell Sci. 107 (1994) 669–681. [DOI] [PubMed] [Google Scholar]
- [44].Bohme K, Li Y, Paul S, Olsen BR, Primary structure of the long and short splice variants of mouse collagen XII and their tissue-specific expression during embryonic development, Dev. Dyn. 204 (1995) 432–445. [DOI] [PubMed] [Google Scholar]
- [45].Zou Y, Zwolanek D, Izu Y, Gandhy S, Schreiber G, Brockmann K, Devoto M, Tian Z, Hu Y, Veit G, Meier M, Stetefeld J, Hicks D, Straub V, Voermans NC, Birk DV, Barton ER, Koch M, Bönnemann CG, Recessive and dominant mutations in COL12A1 cause a novel EDS/myopathy overlap syndrome in humans and mice, Hum. Mol. Genet. 23 (2014) 2339–2352. 10.1093/hmg/ddt627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hicks D, Farsani GT, Laval S, Collins J, Sarkozy A, Martoni E, Shah A, Zou Y, Koch M, Bönnemann CG, Roberts M, Lochmüller H, Bushby K, Straub V, Mutations in the collagen XII gene define a new form of extracellular matrix-related myopathy, Hum. Mol. Genet. 23 (2014) 2353–2363. 10.1093/hmg/ddt637. [DOI] [PubMed] [Google Scholar]
- [47].Delbaere S, Dhooge T, Syx D, Petit F, Goemans N, Destrée A, Vanakker O, De Rycke R, Symoens S, Malfait F, Novel defects in collagen XII and VI expand the mixed myopathy/Ehlers–Danlos syndrome spectrum and lead to variant-specific alterations in the extracellular matrix, Genet. Med. 22 (2019) 112–123. 10.1038/s41436-019-0599-6. [DOI] [PubMed] [Google Scholar]
- [48].Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, Bloom L, Bowen JM, Brady AF, Burrows NP, Castori M, Cohen H, Colombi M, Demirdas S, Backer JDE, Paepe ADE, Fournel-gigleux S, Frank M, Ghali N, Giunta C, Grahame R, Hakim A, Jeunemaitre X, Johnson D, Juul-kristensen B, Kapferer-seebacher I, Kazkaz H, Kosho T, Lavallee ME, Levy H, Mendoza-londono R, Pepin M, Pope FM, Reinstein E, Robert L, Rohrbach M, Sanders L, Sobey GJ, Damme TIMVAN, Vandersteen A, Mourik CVAN, Voermans N, Wheeldon N, Zschocke J, The 2017 International Classification of the Ehlers–Danlos Syndromes, Am. J. Med. Genet. Part C. 26 (2017) 8–26. 10.1002/ajmg.c.31552. [DOI] [PubMed] [Google Scholar]
- [49].Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HRC, Specialization of tendon mechanical properties results from interfascicular differences, J. R. Soc. Interface. (2012) 3108–3117. 10.1098/rsif.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ, Decorin expression is important for age-related changes in tendon structure and mechanical properties, Matrix Biol. 32 (2013) 3–13. 10.1016/j.matbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ansorge HL, Meng X, Zhang G, Veit G, Sun M, Klement JF, Beason DP, Soslowsky LJ, Koch M, Birk DE, Type XIV collagen regulates fibrillogenesis premature collagen fibril growth and tissue dysfunction in null mice, J. Biol. Chem. 284 (2009) 8427–8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chiquet M, Birk DE, Bönnemann CG, Koch M, Collagen XII: Protecting bone and muscle integrity by organizing collagen fibrils, Int. J. Biochem. Cell Biol. 53 (2014) 51–54. 10.1016/j.biocel.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gerecke DR, Meng X, Liu B, Birk DE, Complete primary structure and genomic organization of the mouse Col14a1 gene, Matrix Biol. 22 (2004) 595–601. 10.1016/S0945-053X. [DOI] [PubMed] [Google Scholar]
- [54].Banos CC, Thomas AH, Kuo CK, Collagen fibrillogenesis in tendon development: Current models and regulation of fibril assembly, Birth Defects Res. Part C - Embryo Today Rev. 84 (2008) 228–244. 10.1002/bdrc.20130. [DOI] [PubMed] [Google Scholar]
- [55].Chen S, Birk DE, The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly, FEBS J. 280 (2013) 2120–2137. 10.1111/febs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE, Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development, J. Cell. Biochem. 98 (2006) 1436–1449. 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- [57].Ezura Y, Chakravarti S, Oldberg Å, Chervoneva I, Birk DE, Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons, J Cell Biol. 151 (2000) 779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Font B, Eichenberger D, Rosenberg LM, van der Rest M, Characterization of the interactions of type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin, Matrix Biol. 15 (1996) 341–348. [DOI] [PubMed] [Google Scholar]
- [59].Smith SM, Zhang G, Birk DE, Collagen V localizes to pericellular sites during tendon collagen fibrillogenesis, Matrix Biol. 33 (2013) 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Birk DE, Trelstad RL, Extraceilular compartments in matrix morphogenesis : collagen fibril, bundle, and Llmellar formation by corneal fibroblasts, J Cell Biol. 99 (1984) 2024–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Landis WJ, Silver FH, The structure and function of normally mineralizing avian tendons, Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 133 (2002) 1135–1157. 10.1016/S1095-6433(02)00248-9. [DOI] [PubMed] [Google Scholar]
- [62].Antoniel M, Traina F, Merlini L, Andrenacci D, Tigani D, Santi S, Cenni V, Sabatelli P, Faldini C, Squarzoni S, Tendon Extracellular Matrix Remodeling and Collagen VI Mutations, (2020). [DOI] [PMC free article] [PubMed]
- [63].Izu Y, Ansorge HL, Zhang G, Soslowsky LJ, Bonaldo P, Chu M-L, Birk DE, Dysfunctional tendon collagen fibrillogenesis in collagen VI null mice, Matrix Biol. 30 (2011) 53–61. 10.1016/j.matbio.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Edom-Vovard F, Schuler B, Bonnin MA, Teillet MA, Duprez D, Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons, Dev. Biol. 247 (2002) 351–66. 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- [65].Sun M, Connizzo BK, Adams SM, Freedman BR, Wenstrup RJ, Soslowsky LJ, Birk DE, Targeted deletion of collagen V in tendons and ligaments results in a classic Ehlers-Danlos syndrome joint phenotype, Am. J. Pathol. 185 (2015) 1436–1447. 10.1016/j.ajpath.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wehner D, Becker T, Becker CG, Restoration of anatomical continuity after spinal cord transection depends on Wnt/β-catenin signaling in larval zebrafish, Data Br. (2018). 10.1016/j.dib.2017.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chiquet M, Mumenthaler U, Wittwer M, Jin W, Koch M, The chick and human collagen alpha1(XII) gene promoter--activity of highly conserved regions around the first exon and in the first intron., Eur. J. Biochem. 257 (1998) 362–371. [DOI] [PubMed] [Google Scholar]
- [68].Arai K, Nagashima Y, Takemoto T, Nishiyama T, Mechanical strain increases expression of type XII collagen in murine osteoblastic MC3T3-E1 cells, CELL Struct. Funct. 33 (2008) 203–210. [DOI] [PubMed] [Google Scholar]
- [69].Vining KH, Mooney DJ, Mechanical forces direct stem cell behaviour in development and regeneration, Nat. Rev. Mol. Cell Biol. 18 (2017) 728–742. 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Favata M, Beredjiklian PK, Zgonis MH, Beason DP, Crombleholme TM, Jawad AF, Soslowsky LJ, Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment, J. Orthop. Res. 24 (2006) 2124–2132. 10.1002/jor.20271. [DOI] [PubMed] [Google Scholar]
- [71].Robinson PS, Huang TF, Kazam E, Iozzo RV, Birk DE, Soslowsky LJ, Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice, J. Biomech. Eng. 127 (2005) 181–185. 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.