Abstract
Long non-coding RNAs (lncRNAs) regulate transcription to control development and homeostasis in a variety of tissues and organs. However, their roles in the development of the cerebral cortex have not been well elucidated. Here, a bioinformatics pipeline was applied to delineate the dynamic expression and potential cis-regulating effects of mouse lncRNAs using transcriptome data from 8 embryonic time points and sub-regions of the developing cerebral cortex. We further characterized a sense lncRNA, SenZfp536, which is transcribed downstream of and partially overlaps with the protein-coding gene Zfp536. Both SenZfp536 and Zfp536 were predominantly expressed in the proliferative zone of the developing cortex. Zfp536 was cis-regulated by SenZfp536, which facilitates looping between the promoter of Zfp536 and the genomic region that transcribes SenZfp536. Surprisingly, knocking down or activating the expression of SenZfp536 increased or compromised the proliferation of cortical neural progenitor cells (NPCs), respectively. Finally, overexpressing Zfp536 in cortical NPCs reversed the enhanced proliferation of cortical NPCs caused by SenZfp536 knockdown. The study deepens our understanding of how lncRNAs regulate the propagation of cortical NPCs through cis-regulatory mechanisms.
Electronic supplementary material
The online version of this article (10.1007/s12264-020-00607-2) contains supplementary material, which is available to authorized users.
Keywords: Zfp536, Sense lncRNA, Self-renewal, Cortical development, Neural progenitor
Introduction
The mammalian cerebral cortex, also known as the neocortex, is a six-layer structure comprising a large cohort of neurons and glial cells, which is the basis for sophisticated cognitive functions. Cortical projection neurons (PNs) are functional units that make long-distance connections with other parts of the central nervous system. Cortical PNs in mouse and human are generated directly and indirectly from radial glial progenitor cells (RGs), including apical and basal RGs, lying in the dorsal part of the embryonic forebrain [1, 2]. Some cortical PNs are direct progeny of RGs, but most are the progeny of symmetrical cell divisions of basal neural progenitors, which are also derivatives of RGs and reside in the subventricular zone (SVZ) [3, 4]. With the exception of the first-born layer I neurons, deep layer PNs are born early and superficial layer PNs are born later. Newborn PNs migrate outward (radially), passing through deep layer PNs to settle in their final destination (an inside-out pattern, Fig. S1A) [5–7].
Extensive work has been focused on the molecular events underlying cell-fate specification of cortical neural progenitor cells (NPCs), including both RGs and basal neural progenitors, as well as the postmitotic maturation and migration of layer-specific PNs. Both extrinsic and intrinsic mechanisms balance the self-renewal and differentiation of NPCs during cortical development. Moreover, changes in the competency of NPCs over time during cortical development are likely due to temporal shifts of sets of transcription factors expressed in NPCs, but it is largely unknown how these changes occur [8–12]. Molecular dissection of these key properties will not only help to better understand how neural development proceeds, but also have implications for manipulating NPCs in vivo and in vitro to tackle neurological disorders such as neurodegenerative diseases and malignant brain tumors.
The transient expression, flexible structure, and dynamic localization of RNA molecules enable the fine-tuning of genome arrangement, scaffolding, and transcription functions, thus precisely regulating gene expression in a time- and site-specific manner. Recent work indeed points to critical roles of long noncoding RNAs (lncRNAs), transcripts longer than 200 nucleotides (nt) but without protein-coding potential, in controlling transcription by associating with epigenetic regulatory machinery and chromosomal architectural organization [13, 14]. Therefore, lncRNAs participate in numerous physiological and pathological processes including the maintenance of pluripotency, lineage specification, organogenesis, tumorigenesis, and metabolism [15–19]. Moreover, spatiotemporal co-expression of tissue-specific lncRNAs with nearby protein-coding genes is prevalent in mammals. LncRNAs can modulate neighboring transcription (cis-regulation) and participate in biological processes similar to those controlled by neighboring protein-coding genes [20]. Recent high-throughput studies have characterized spatiotemporally-regulated lncRNAs expressed in the developing brain of human and mouse [21, 22]. However, very few lncRNAs, particularly those with cis-regulatory roles, have been found to regulate the fate choices of cortical NPCs [23].
Here, we analyzed transcriptome data from developing mouse cerebral cortex to identify lncRNAs with spatiotemporal expression patterns and potential cis-regulatory functions. An example is SenZfp536, a sense lncRNA that partially overlaps with the 3′ end of Zfp536 and suppresses the self-renewal of cortical NPCs by cis-activating SenZfp536. The study advances the understanding of molecular mechanisms, particularly those mediated by lncRNAs, in controlling the self-renewal of cortical NPCs.
Materials and Methods
Mouse
All CD-1 mice were obtained from the Hunan SJA Laboratory Animal Co., Ltd (Changsha, China). The husbandry of CD-1 mice was carried out under specific pathogen-free (SPF) housing conditions and mouse experiments were approved by the Animal Care and Ethics Committee at Wuhan University. The day when a vaginal plug was detected was counted as embryonic (E) day 0.5.
Dissection and RNA-Sequencing of Dorsal Forebrain at E10.5 and E12.5
The dorsal forebrain (neocortex) was microdissected from E10.5 and E12.5 mouse embryos under a dissecting microscope and flash-frozen in liquid nitrogen. Total RNAs were extracted twice using TRIzol (Thermo Fisher Scientific, Waltham, United States) and treated with DNase I (NEB Biolabs, Ipswich, United States). The integrity of RNA was analyzed with an Agilent Bioanalyzer 2100 (Agilent, Santa Clara, United States). Removal of ribosomal RNAs and construction of libraries for strand-specific RNA-seq were performed in BGI Tech (Shenzhen, China). Totally ~410 million clean reads of putative polyadenylated and non-polyadenylated transcripts were generated from the cortices of the two developmental stages.
Processing of RNA-Seq Data
We used the Galaxy platform [24] to integrate our in-house RNA-seq data with the ENCODE RNA-seq data of mouse cortex at E10.5, E11.5, E12.5, E13.5, E14.5, E15.5, E16.5, and P0). Reads were mapped to the mouse genome (mm10) using HISAT2 [25] and then subjected to transcripts assembly using StringTie [26] with default parameters, followed by generating gene counts using the featureCount [27] software. Differentially-expressed genes of Tis21+ versus Tis21− apical radial glial cells were detected by edgeR [28]. Heatmaps and cluster analysis were performed with the R package ‘pheatmap’ [29]. See Table S1 for RNA-seq data used in this study.
LncRNA Catalog Assembly
The GTF file generated from StringTie was used as the input for GffCompare. The complete GENCODE gene annotation GTF file (release M22 GRCm38.p6) and NONCODE v5 mouse gene annotation GTF file were used as references. Completely matched protein-coding and non-coding genes were excluded. For transcripts not matched with the reference lncRNA database, another filtering was performed by Blastx in the kegg [30], nr [31], cog [32], and swissprot [33] protein databases. Transcripts mostly matched with known protein-coding genes (identity > 0.9 and coverage > 0.8) were removed from the final lncRNA list. For the remaining transcripts, intrinsic sequence feature-based software, CPC2 (Coding Potential Calculator) [34] and CPAT (Coding-Potential Assessment Tool) [35] were used to predict novel lncRNAs. The final lncRNA catalog contained known and novel lncRNAs (see Table S2).
Co-Expression Analysis
For co-expression analysis and module construction, WGCNA (Weighted Gene Co-Expression Network Analysis) [36], a topology distance-based R package, was employed as previously described [37]. The gene expression matrix was processed by a variance stabilizing transformation of the RNA-seq count data followed by the workflow of WGCNA to generate a gene co-expression network with the parameter ‘power = 5’. K-means clustering of RNA-seq data from E14.5 ventricular zone (VZ), SVZ/intermediate zone (IZ), and cortical plate (CP) was carried out by iDEP (integrated web application for differential expression and pathway analysis of RNA-seq data), a web-based RNA-seq data analysis tool [38]. See Tables S3 and S4 for WGCNA co-expression and K-means clustering results.
Gene Ontology Enrichment Analysis and Cis-Regulatory Position Analysis
Gene Ontology analysis was performed by DAVID [39, 40]. Further enrichment analysis was carried out on ShinyGO [41], which includes the Gene Signature Database (GeneSigDB) [42] and the L2L database [43]. GeneSigDB and L2L consist of lists of differentially-expressed genes, with their signatures extracted and manually curated from the literature and mammalian microarray studies respectively. Genomic Regions Enrichment of Annotations Tools (GREAT) [44] was employed for the cis-regulatory analysis.
Cell Cultures
The murine Neuro-2a neuroblastoma cell line was from the Cell Bank of the Chinese Academy of Sciences, and the human embryonic kidney (HEK) 293 cell line was a gift from Dr. Hongbing Shu at Wuhan University. Cells were cultured in the indicated culture media (Dulbecco’s modified Eagle’s medium or minimum essential medium) containing 10% fetal bovine serum and penicillin/streptomycin (Life Technologies, Carlsbad, United States). For neurosphere culture assays, cortical NPCs dissociated from E12.5 mouse cortex were cultured on ultra-low-attachment plates (Corning, New York, United States) and maintained in F12 medium (Life Technologies) with N2 and B27 supplements (1×, Life Technologies), 1 mmol/L Na-pyruvate, 1 mmol/L N-acetyl-l-cysteine (NAC), human recombinant FGF2, and EGF (20 ng/mL each; Life Technologies) [45].
Plasmid Construction
Full-length mouse SenZfp536 and Zfp536 were amplified from cDNAs of E13.5 mouse cortex and then cloned into pCAGGS. The short hairpin RNA (shRNA) vectors were cloned by inserting annealed oligonucleotides targeting SenZfp536 or Zfp536 into pLKO.1-ZsGreen (LKO shRNA) or pCAG-mir30 (mir30 shRNA) vectors. For luciferase reporter assays, 5×UAS-TK-Luc, pcDNA3-Gal4-λN, and pcDNA3-BoxB-LacZ were gifts from Dr. Xiang Lv (Chinese Academy of Medical Sciences & Peking Union Medical College), with the LacZ cassette in pcDNA3-BoxB-LacZ replaced by the full-length SenZfp536 sequence. For SenZfp536 tethering assays, the sequences for SenZfp536, antisense SenZfp536, or Gfp were tagged with an sgRNA sequence to target the promoter of Zfp536. The fused cassettes were cloned into pcDNA3.1. For CRISPRa assays, sgRNA sequences targeting the SenZfp536 promoter were cloned into sgRNA (MS2) cloning vector (Addgene, #61424). See Table S5 for the oligonucleotide sequences used.
Lentivirus Packaging
HEK 293T cells were seeded at 3.8 × 106 cells per 10-cm plate and incubated at 37°C under 5% CO2 for ~20 h. Twelve micrograms of pLKO.1-ZsGreen, 6 μg psPAX2 and 6 μg pMD2.G vectors were transfected into 293T cells using calcium phosphate. The supernatant containing lentivirus particles was harvested at 48 h post-transfection and filtered through a Millex-GP filter unit (0.45 μm pore size, Merck, Darmstadt, Germany). The viral supernatant was aliquoted, snap-frozen in liquid nitrogen, and stored at −80°C.
Cell Proliferation Assay (CCK-8 Assay)
Vectors expressing shRNAs against SenZfp536 were transfected into 70%–80% confluent Neuro-2a cells. After two days, the cells were re-suspended and seeded into 96-well culture plates (1000 cells/well). For detection, 10 μL CCK-8 solution (C0039, Beyotime, Shanghai, China) was added to each well, followed by 37°C and 5% CO2 incubation for 1 h. The absorbance at 450 nm was measured with a microplate reader (ELx800, BioTek, Winooski, United States).
Immunofluorescence and Immunoblotting
Mouse brains were fixed overnight in 4% formaldehyde (Merck), then equilibrated in 20% sucrose-PBS and frozen-embedded in O.C.T. (Sakura, Torrance, United States). Cryosections (14 μm) were blocked in blocking buffer (3% heat-inactivated normal goat serum, 0.1% bovine serum albumin, and 0.1% Triton-X 100 in 10 mmol/L Tris-HCl, pH 7.4; 100 mmol/L NaCl) for 1 h at room temperature. The sections were incubated with diluted primary antibodies overnight at room temperature. After washing three times (5 min–10 min each) with 1×PBS, and incubation with secondary antibodies for 1 h at room temperature, the sections were mounted with anti-fade reagent with DAPI and stored at 4°C. BrdU immunoblotting assays were carried out according to a previous procedure [46]. Briefly, slides were treated with 20 μg/mL proteinase K (Merck) for 10 min followed by 2 mol/L HCl for 30 min prior to blocking. See Table S5 for antibodies used.
5′ and 3′ Rapid Amplification of cDNA Ends (5′ and 3′ RACE)
We designed nested primers to target regions of SenZfp536 according to previous studies [47] and our RNA-seq data. For 5′ RACE, two different approaches, a 5′-Full RACE Kit with TAP (Takara, Kusatsu, Japan) and a SMARTtm RACE Kit (Clontech, Mountain View, United States) were applied according to their manuals. 3′ RACE was carried out according to the manufacturer’s guide (Takara). PCR products were cloned into pGEM-T easy vectors (Promega, Madison, United States) and analyzed by Sanger sequencing to identify the 5′ and 3′ ends of SenZfp536.
Northern Blotting
Total RNAs were extracted using TRIzol (Thermo Fisher Scientific) from Neuro-2a cells. Twenty micrograms of total RNAs were subjected to formaldehyde denaturing agarose electrophoresis followed by transfer to a positively-charged nylon membrane (Beyotime) with 20×SSC buffer (3.0 mol/L NaCl and 0.3 mol/L sodium citrate, pH 7.0) through an ascending capillary transfer system. Wet membranes were cross-linked by UV irradiation (254 nm for 1 min 45s, 1.5 J/cm2) and treated with pre-warmed DIG Easy Hyb Hybridization solution (Roche, Carlsbad, United States) at 65°C for 1 h. DIG-labeled RNA probes (SenZfp536 and Gapdh) generated by in vitro transcription were denatured at 85°C for 5 min and chilled on ice. Hybridization was carried out at 65°C overnight for at least 10 h. Membranes were washed three times in wash buffer 1 (0.1×SSC and 0.1% SDS) for 15 min at 65°C, then rinsed in wash buffer 2 [0.1 mol/L maleic acid, 0.15 mol/L NaCl, 0.3% Tween 20 (pH 7.5)]. After incubation in blocking reagent (Roche) for 1 h at room temperature, the membrane was incubated with a 50,000-fold dilution of anti-DIG-AP Fab fragment (Roche) in blocking reagent for 30 min at room temperature, and washed three times in wash buffer 2 for 10 min at room temperature. After immersion in detection buffer [0.1 mol/L Tris-HCl, 0.1 mol/L NaCl (pH 9.5)] for 5 min, bands on the membrane were detected using the CDP-star chemiluminescent substrate for alkaline phosphatase (Roche) and X-ray film exposure. Primers used in generating probes for Northern blots are listed in Table S5.
Luciferase Reporter Assay
For BoxB-Gal4-λN luciferase reporter assays, Neuro-2a cells were seeded in 24-well plates for 24 h and transfected with mixed vectors of 50 ng 5×UAS-TK-Luc, 50 ng pcDNA3-Gal4-λN; and 50 ng pcDNA3-BoxB-LacZ/SenZfp536 along with 5 ng pTK-Ren vectors. Cells were harvested and delivered to the reporter activity testing assay by a Dual-Glo luciferase Assay System on the GloMax Luminometer System (Promega) at 24 h post-transfection. Data were gathered by calculating the Luc/Ren ratio of triplicate wells.
CRISPR/dCas9-Mediated Transcription Activation (CRISPRa) Assay
CRISPRa assays were performed according to published procedures [48, 49]. In short, sgRNA sequences targeting the promoter of SenZfp536 were cloned into the sgRNA (MS2) cloning vector (Addgene, #61424). Neuro-2a cells seeded for 24 h were transfected with mixed vectors of sgRNA, dCAS9-VP64-GFP, and MS2-P65-HSF1-GFP. Cells were harvested to extract RNAs for assessing SenZfp536 and Zfp536 RNA levels by RT-qPCR at 48 h post-transfection and for assessing ZFP536 protein levels by immunoblotting at 72 h post-transfection.
RNA Tethering
SgRNAs that activate Zfp536 transcription were identified by CRISPRa assays. The most efficient sgRNA was cloned into the pcDNA3.1 vector and tagged with Gfp, antisense SenZfp536 or SenZfp536 sequences. Neuro-2a cells were transfected with mixed vectors that expressed dCas9 along with sgRNA tagged with Gfp, antisense SenZfp536, or SenZfp536. Transfected cells were harvested to extract RNAs for assessing Zfp536 RNA levels by RT-qPCR at 48 h post-transfection.
Chromosome Conformation Capture (3C)
The 3C assay was performed according to the published protocol [50]. In short, Neuro-2a cells were harvested (1 × 107 cells) and crosslinked with 2% formaldehyde for 10 min at room temperature followed by quenching with 0.125 mol/L glycine for 5 min. Then extracted nuclei were digested by BamH I (NEB Biolabs) overnight at 37°C, followed by treatment with T4 ligase (Takara) for 30 min at room temperature.
DNAs were purified by phenol-chloroform extraction and analyzed by qPCR. The specificity and efficiency of all 3C primers were agarose-gel-verified by digestion and ligation of the BAC DNA (RP23-454K9) that contained the region of interest. For each primer pair (test primer to constant primer), we performed a triplicate quantification (Ct1, Ct2, and Ct3), and then calculated the mean Ct. The crosslinking value was calculated using parameters of the standard curve of BAC DNA: value = 10(Ct-b/a) (b: intercept; a: slope). These values were finally normalized to Gapdh to generate relative crosslinking frequency.
In Situ Hybridization
cDNA templates for antisense RNA probes were PCR amplified from mouse cDNA with primers specific to SenZfp536 and Zfp536. PCR products were gel-extracted and cloned into the pGEM-T Easy vector (Promega) that contains T7 and SP6 promoters. The vectors were linearized by restriction enzyme and then transcribed using a DIG-RNA labeling kit (Roche). The DIG-labeled probes were stored at −80°C. CD-1 mouse brains at E12.5, E14.5, and E16.5 were fixed overnight in RNase-free 4% formaldehyde (Sigma), then equilibrated in 20% sucrose-PBS and frozen-embedded in O.C.T. (Sakura). Cryosections (14 μm) were incubated with DIG-labeled RNA antisense probe (0.1 ng/μL–0.2 ng/μL in hybridization buffer) overnight at 65°C in humidified boxes after treatment with proteinase K (2 μg/mL, 10 min) and acetylation in 0.1 mol/L TEA (triethanolamine) for 10 min. Slides were washed four times (20 minutes each) in 0.1×SSC at 65°C followed by blocking with 10% heat-inactivated sheep serum in buffer B1 (0.1 mol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl) at room temperature for 2 h. Sections were incubated with the anti-DIG antibody (Roche; 1:5,000) overnight at 4°C and then washed three times with buffer B1. Slides were equilibrated with buffer B3 (0.1 mol/L Tris-HCl, 0.1 mol/L NaCl, 50 mmol/L MgCl2, 0.1% Tween-20, pH 9.5) twice for 10 min, followed by staining with NBT/BCIP (Roche; 4.5 μL/mL each in buffer B3) overnight in the dark. Slides were dehydrated using gradient ethanol and xylene sequentially, and mounted with neutral balsam.
In Utero Electroporation (IUE)
In utero electroporation was carried out as published [51]. In brief, pregnant CD-1 mice with E13.5 embryos were anesthetized by injection of pentobarbital sodium (70 mg/kg), and the uterine horns were exposed for microinjection of mixed plasmids with 0.05% Fast Green (Sigma) into the lateral ventricles of brains. Five pulses (36 V; 50 ms on/950 ms off) were released across the heads of embryos by 5-mm platinum plate-electrodes connected to a CUY21VIVO-SQ electroporator (BEX, Tokyo, Japan). The uteri were replaced and the incisions were sutured. Mice were treated with analgesia (ibuprofen in drinking water) until sacrifice at E15.5 or E16.5.
Statistics
Statistical plots were generated and analyzed using Graphpad Prism 8.0 software. Results are presented as the mean ± SEM or ± SD. The two-tailed t-test was used to compare two groups. One-way ANOVA followed by Dunnett’s multiple comparison test was used for comparison of three or more groups with control groups. One-way ANOVA followed by Tukey’s multiple comparison test was used for comparison of three or more groups among each other. All animals and biochemical experiments were conducted with at least three replicates per group.
Data Access
Strand-specific RNA-seq of E10.5 and E12.5 CD-1 mouse forebrains have been deposited in the NCBI Gene Expression Omnibus under accession number GSE55600.
Results
Cataloging lncRNAs in Developing Mouse Cerebral Cortex
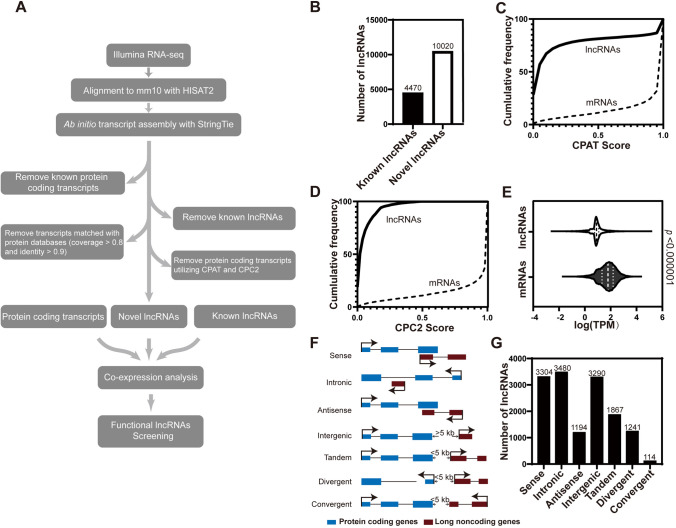
To systematically identify lncRNAs associated with cortical development, we used an ab initio approach (Fig. 1A). First, public RNA-seq data for E10.5, E11.5, E12.5, E13.5, E14.5, E15.5, E16.5, and P0 mouse forebrains (developing cerebral cortices, Fig. S1A) were obtained from ENCODE (Encyclopedia of DNA Elements) [52, 53]; and RNA-seq data for the VZ, SVZ/IZ, and CP at E14.5 [54] and the cerebral cortex at E17 [55] were downloaded. To supplement lncRNA without a 3′ polyadenylation (polyA) tail, strand-specific RNA-seq on ribosomal-depleted total RNA isolated from the dorsal forebrain at E10.5 and E12.5 was implemented at a sequencing depth of >200 million reads per sample. Moreover, RNA-seq data acquired from ESCs and ESC-derived NPCs [56] were incorporated into our transcriptome database for developing murine cerebral cortex (see also Table S1). Subsequently, we aligned >1,800 million RNA-seq clean reads to the reference genome (GRCm38/mm10) by HISAT2 [25], followed by ab initio transcript assembly using StringTie [26, 57]. In conformity with this pipeline (Fig. 1A), a total of 31,067 gene loci and 54,076 transcripts were eventually identified.
Fig. 1.
Comprehensive cataloging of lncRNAs in developing mouse cerebral cortex. A The bioinformatics pipeline for identification and annotation of lncRNAs expressed in developing mouse cerebral cortex. See also Table S1. B Numbers of known and novel lncRNAs identified in this study. Known lncRNAs have been annotated in GENCODE v25 and NONCODE v5. C Cumulative frequency of coding potential score for lncRNAs and mRNAs calculated by CPAT. D Cumulative frequency of coding potential scores for lncRNAs and mRNAs calculated by CPC2. E Violin plot showing average expression levels (transcripts per million, TPM) of mRNAs and lncRNAs. Statistical significance was determined using unpaired t-test with Welch’s correction. F Classification of lncRNAs based on their relationship with adjacent genes. G Numbers of lncRNAs classified according to their relationship with adjacent genes.
Using GffCompare [26] and Blastx [58], protein-coding genes and known lncRNAs were successfully characterized, which were also annotated in GENCODE M22 [59] and NONCODE v5 [60] respectively. After filtering with CPAT [35] and CPC2 [34], two protein-coding potential calculators, 4,470 known and 10,020 novel lncRNAs were authenticated (Fig. 1B). The re-use of CPC2 and CPAT validated the classification of lncRNA and protein-coding (mRNA) transcripts (Fig. 1C, D). Consistent with previous studies [56, 61, 62], lncRNAs associated with developing neocortex were expressed at significantly lower levels than mRNAs (Fig. 1E).
By cross-referencing with transcriptome data from apical radial glia (aRGs), basal intermediate progenitors (bIPs), basal radial glia (bRGs), and projection neurons [63] at E14.5, we identified numerous cell-type-specific mRNA and lncRNA transcripts. Interestingly, aRGs expressed the most cell-type-specific transcripts (Fig. S1B). Moreover, many mRNAs and lncRNAs were also differentially expressed between proliferative (Tis21−) and differentiative (Tis21+) mouse aRGs (Fig. S1C) [63]. Thus, the expression of lncRNAs is spatiotemporally regulated during cortical development and among cell types.
Cis-Regulatory Prediction of lncRNAs
Recent studies have shown that lncRNAs often cis-regulate their adjacent protein-coding genes [20], which would facilitate functional annotation of lncRNAs. To categorize lncRNAs and explore their potential biological roles, we described all lncRNAs according to their genomic distribution patterns relative to protein-coding loci. Among them, 22.8% (3,304) lncRNAs partially overlapped with adjacent protein-coding genes (PCGs) in the sense orientation, while the transcription of 8.2% (1,194) of lncRNAs were antisense and partially overlapped with adjacent PCGs. Twenty-four percent (3,480) of lncRNAs were transcribed from intronic regions of PCGs, and 22.7% (3,290) had no PCGs within their 5-kb flanking regions (intergenic). The rest, 22.2% (3,222), were tandem, divergent, and convergent lncRNAs that did not overlap with, but were transcribed within the 5-kb flanking regions of their PCG neighbors (Fig. 1F, G).
To predict the cis-regulatory roles of lncRNAs, the GREAT program [44] was applied to analyze the enrichment of molecular functions and pathways for their neighboring PCGs, excluding sense and intronic lncRNAs. Most enriched terms were associated with the cell cycle and regulation of mitosis, suggesting that lncRNAs have vigorous effects on cell proliferation during cortical development (Fig. S1D, E). In addition, the distances between lncRNA genes and transcription start sites (TSSs) of nearby PCGs ranged from 5 kb to 500 kb (Fig. S1F). Numerous HiC-Seq studies have shown that non-coding regions of the genome regulate the transcription of nearby PCGs in cis [64, 65]. Thus, Pearson correlation coefficients for expression levels between lncRNAs and nearby PCGs were calculated. As expected, the expression of > 20% of lncRNAs were strongly correlated (Pearson > 0.6) with their PCG neighbors during cortical development (Fig. S1G).
Co-Expression Analysis of Transcripts Expressed in Developing Cerebral Cortex
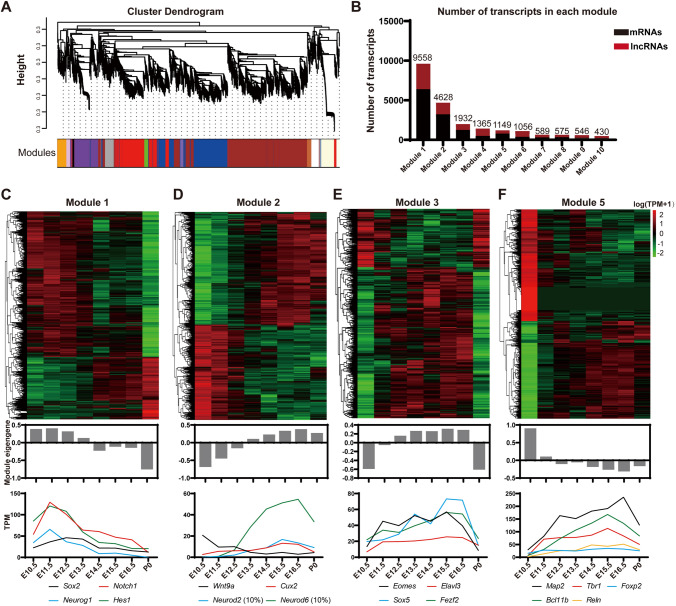
To associate expression patterns with developmental stages, WGCNA (Weighted Gene Co-Expression Network Analysis) [36, 66] was used to categorize groups of transcripts, or ‘Modules’. Transcripts in each module showed either positively or inversely correlated expression patterns, thus were probably involved in common biological processes or had regulatory relationships (Fig. 2A, B).
Fig. 2.
Co-expression modules categorize transcripts implicated in cortical development. A Hierarchical cluster dendrogram of co-expression modules for lncRNA genes and mRNA genes. A total of 46 modules are labeled with different color bands beneath corresponding tree branches. B Numbers of mRNAs and lncRNAs in Module 1–10. C–F Heatmaps and Eigengene bar plots of four modules. Upper panels: expression levels of lncRNAs and PCGs normalized to log10 (TPM+1) (red, higher expression; black, neutral expression; green, lower expression); middle panels: module Eigengene barplots representing expression levels of all transcripts, calculated by moduleEigengenes in the WGCNA package and in line with the first principal component obtained by singular value decomposition of each module: lower panels: broken line graphs exhibit expression patterns of representative protein-coding genes.
Through analyzing eight developmental time points of the forebrain (two samples for each time point from E10.5 to P0) from ENCODE, we successfully established 46 co-expression modules, each of which exhibited unique stage-specific characteristics. For instance, Modules 1 and 2 were enriched with transcripts whose expression levels gradually increased or decreased during cortical development. Genes essential for the stemness properties of neural progenitors such as Sox2, Notch1, and Hes1, and the proneural gene Neurog1 were assigned to Module1. Moreover, transcripts in Module 5 had the highest or lowest expression level at E10.5. Modules 2, 3, and 5 were enriched with genes involved in transit-amplifying and neuronal differentiation of the neocortex (Fig. 2C–F, S2A–D). To discover the functional significance of genes in Modules 1 and 2, enrichment analysis of PCGs in both modules was performed in L2L [43] and GeneSigDB [42], which consist of lists of differentially-expressed genes with their signatures extracted and manually curated from mammalian microarray studies and the literature, respectively. The results showed that genes in Modules 1 and 2 highly correlated with terms of stem cells and organ development (Fig. S2E–H).
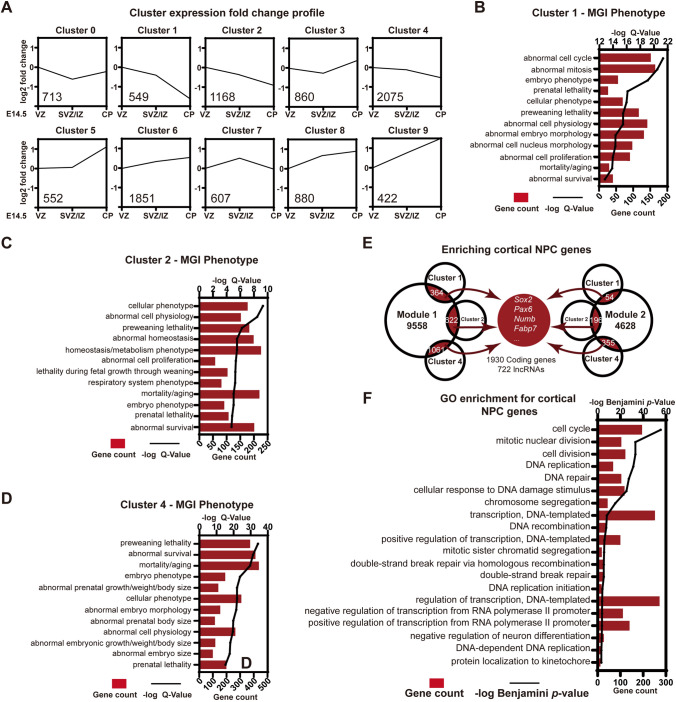
Clustering of Transcripts Based on Their Spatial Distribution to Identify Genes Associated with Self-Renewal of Cortical NPCs
We next analyzed spatial expression data from the dorsal forebrain at E14.5 [54]. RNA-seq data from the VZ, SVZ/IZ, and CP at E14.5 were analyzed and an unsupervised learning algorithm, K-means clustering, was performed to classify long noncoding and protein-coding transcripts based on their spatial distribution [67–69]. A total of 9,677 genes were categorized into 10 main clusters (Clusters 0–9), each exhibiting a spatially-specific pattern (Fig. 3A). Clusters 1, 2, and 4 contained the most NPC-related genes and shared similar expression trends, namely, highest expression levels in the VZ but reduced in the SVZ/IZ and CP at E14.5. Moreover, phenotype enrichment analysis based on the Gene List Analysis and Visualization (Vlad) [70] and MGI phenotypes [71] revealed that PCGs in Clusters 1, 2, and 4 were enriched for phenotypes related to abnormal cell cycle, mitosis, and cell lethality (Fig. 3B–D).
Fig. 3.
Spatial clustering of lncRNAs and characteristics of genes associated with cortical NPCs. A K-means clustering based on the spatial distribution of transcripts. Expression fold-changes are plotted to exhibit trends from E14.5 VZ, SVZ/IZ to the CP. Number of genes in each cluster is marked in the lower-left corner. B–D Top 12 MGI phenotype terms enriched in Clusters 1, 2, and 4. Bars display the gene count in each term and lines reflect −log Q values. E Venn diagrams showing intersections between Modules 1 and 2 and Clusters 1, 2, and 4. Intersected transcripts are identified as NPC-associated genes, with a few key genes listed. F Top 20 Gene Ontology (GO) Biological Process terms enriched in NPC-related PCGs, as determined by DAVID GO analysis. Bars display the gene count in each GO term and the line reflects −log Benjamin P values.
Aiming to identify lncRNAs with regulatory roles in controlling the self-renewal of cortical NPCs, we integrated temporal and spatial co-expression data. Specifically, 1,930 mRNAs and 722 lncRNAs were at intersections of Modules 1 and 2 with Clusters 1, 2, and 4, including PCGs implicated in stemness and self-renewal of cortical NPCs - Sox2, Pax6, Numb, and Fabp7 (Fig. 3E). Gene Ontology enrichment analysis indicated that PCGs at these intersections were associated with functions for cell cycle, DNA repair, and modulation of transcription (Fig. 3F). Collectively, we sorted out lncRNA candidates with similar spatiotemporal patterns and potential roles in regulating the self-renewal of NPCs during cortical development.
Characterization of SenZfp536
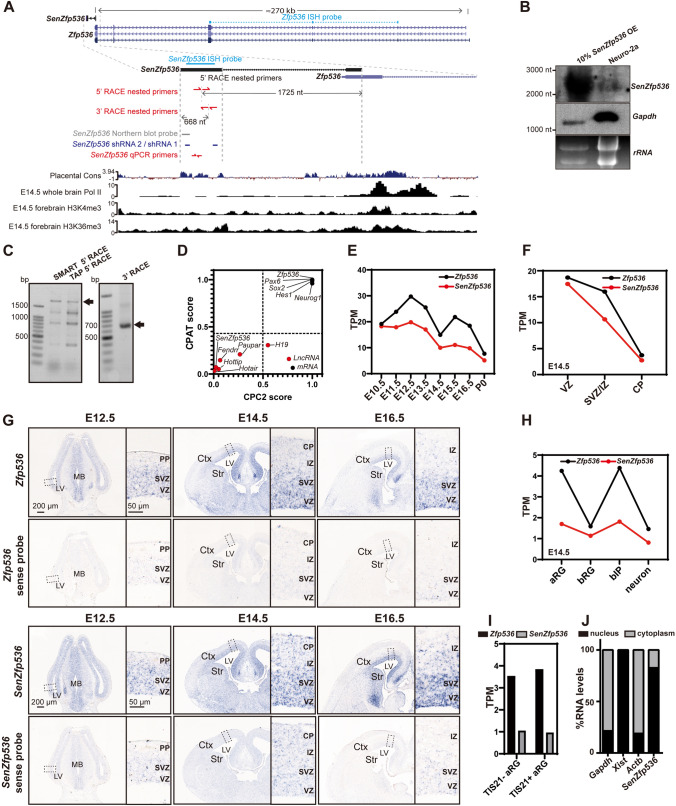
Among the 722 lncRNAs at intersections of Modules 1 and 2 with Clusters 1, 2, and 4 with potential roles in regulating NPC self-renewal, the 20 most VZ-enriched transcripts were screened using neurosphere culture assays. We identified a lncRNA that inhibits the proliferation of cortical NPCs. This lncRNA was previously annotated as AK163177 (NCBI) and subsequently termed SenZfp536 due to its partial overlap with the last exon of Zfp536 in the sense direction. The genomic region of SenZfp536 is evolutionarily conserved and is associated with RNA polymerase II (Pol II), H3K4me3, and H3K36me3 in mouse brain at E14.5 (Fig. 4A), suggesting its active transcription in the developing brain. Northern blot, 5′ and 3′ rapid amplification of cDNA end (RACE) revealed that SenZfp536 is a 2,542-base-long transcript, with its TSS and the first exon located inside the last exon of Zfp536 (Fig. 4A–C). Moreover, no protein-coding potential was detected in SenZfp536 using CPC2 and CPAT (Fig. 4D).
Fig. 4.
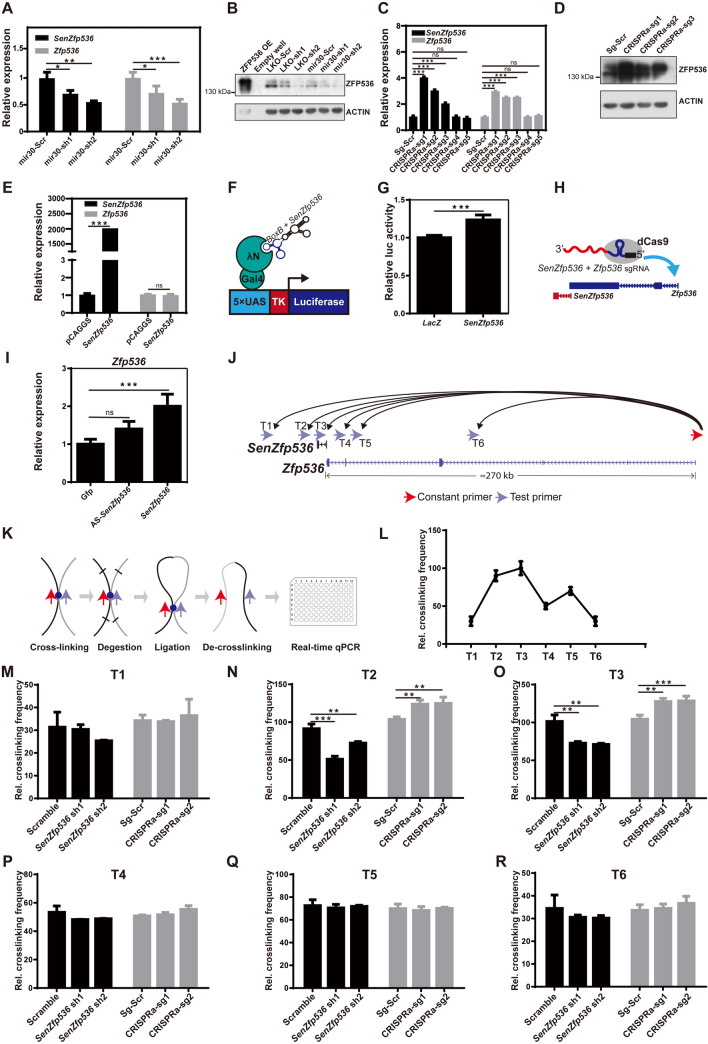
The lncRNA SenZfp536 is a sense lncRNA overlapping with Zfp536. A Schematic of the murine SenZfp536 locus (UCSC mm10). Upper track: the sequence conservation (Cons) among placental animals; lower tracks: ChIP-seq signals for Pol II, H3K4me3, and H3K36me3 in whole brain or forebrain at E14.5. Locations of the ISH probes, Northern probe, shRNA targets, qPCR primers and nested primers for 5′ and 3′ RACE are also shown. B Northern blot analysis of RNA extracted from Neuro-2a cells expressing exogenous SenZfp536 (left lane, 10% input) and wild-type Neuro-2a cells (right lane). C Agarose gels showing products of 5′ and 3′ RACE nested PCR for SenZfp536. Two 5′ RACE approaches, SMART and TAP, were applied. All visible bands were collected for DNA sequencing. Arrows indicate specific SenZfp536 products. D Coding potential scores for SenZfp536 and other known long non-coding and protein-coding transcripts. Dotted lines indicate cutoffs for coding and non-coding values. E Line plots for the temporal expression of SenZfp536 and Zfp536 in developing cortex. F Line plots for the spatial expression of SenZfp536 and Zfp536, using RNA-seq data from the VZ, SVZ/IZ, and CP at E14.5. G In situ hybridization of SenZfp536 and Zfp536 in coronal sections of embryonic mouse brains using anti-sense (upper panels) and sense probes (lower panels). Magnified images are displayed in the right panels. Ctx, cortex; Str, striatum; LV, lateral ventricle; PP, preplate; CP, cortical plate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone. H Line plots for expression of SenZfp536 and Zfp536 in apical radial glia (aRG), basal radial glia (bRG), basal intermediate progenitors (bIP), and neurons at E14.5. I Expression of SenZfp536 and Zfp536 in proliferative (Tis21-GFP-) and differentiative (Tis21-GFP+) aRG of cortex at E14.5. J Subcellular distribution of SenZfp536 in Neuro-2a cells. Most SenZfp536 RNAs reside in the nucleus. Gapdh, Xist, and Actb are reference RNAs.
Quantitative analysis of RNA-seq data confirmed the correlated expression trends between Zfp536 and SenZfp536 in the developing cerebral cortex, with SenZfp536 expressed at 1/2 to 2/3 the levels of Zfp536 (Fig. 4E). Consistent with their relatively high expressions in the VZ and SVZ/IZ (Fig. 4F), in situ hybridization demonstrated that both SenZfp536 and Zfp536 were dominantly expressed in the VZ/SVZ of the developing cerebral cortex, whereas sense probes did not elicit specific signals (Fig. 4G). Quantitative analysis of high-throughput data from aRGs, bIPs, bRGs, and neurons at E14.5 [63] revealed that both Zfp536 and SenZfp536 were expressed at higher levels in aRGs and bIPs than bRGs and neurons (Fig. 4H). Moreover, Zfp536 and SenZfp536 were expressed at comparable levels in proliferative (Tis21−) and differentiative (Tis21+) mouse aRGs (Fig. 4I). Subcellular fractionation followed by quantitative PCR showed that ~80% of SenZfp536’s transcripts were located in the nucleus, indicating related roles (Fig. 4J).
SenZfp536 Positively Regulates the Expression of Zfp536
Since SenZfp536 is transcribed from the last exon of Zfp536, we then explored whether SenZfp536 regulates the transcription of Zfp536. Knocking down SenZfp536 by shRNAs significantly downregulated the expression of Zfp536 in Neuro-2a cells (Fig. 5A). Accordingly, knocking down SenZfp536 using two types of shRNA-expressing vectors, pCAG-mir30 and pLKO.1, attenuated ZFP536 at the protein level (Fig. 5B).
Fig. 5.
SenZfp536 maintains the expression of Zfp536 in cis. A RNA levels of SenZfp536 and Zfp536 in Neuro-2a cells transfected with vectors expressing shRNAs against SenZfp536 or scrambled control for two days. For SenZfp536 expression, F = 15.56, P = 0.0078; for Zfp536 expression, F = 24.86, P = 0.0056 (one-way ANOVA). B Immunoblots of ZFP536 and ACTIN of Neuro-2a cells transfected with vectors expressing shRNA against SenZfp536 for three days. C RNA levels of SenZfp536 and Zfp536 in Neuro-2a cells transfected with indicated CRISPRa vectors for two days. For SenZfp536 expression, F = 102.4, P < 0.0001; for Zfp536 expression, F = 61.54, P < 0.0001 (one-way ANOVA). D Immunoblots of ZFP536 and ACTIN of Neuro-2a cells transfected with vectors that cis-activate SenZfp536 expression for three days. E RNA levels of SenZfp536 and Zfp536 in Neuro-2a cells transfected with indicated vectors for two days. For SenZfp536 expression, P < 0.0001; for Zfp536 expression, P = 0.83 (two-tailed unpaired Student’s t-test). F Schematic of the Gal4-λN/BoxB RNA tethering system. G Relative luciferase activity of Neuro-2a cells transfected with plasmids expressing BoxB-tagged LacZ or SenZfp536 along with Gal4-λN and 5×UAS-TK-Luciferase for 24 h. Two-tailed unpaired student’s t-test was used. H Schematic of dCas9-mediated RNA tethering assay. I Relative expression of Zfp536 in Neuro-2a cells transfected with plasmids expressing Zfp536-TSS-targeted sgRNA tagged with Gfp, SenZfp536, or antisense-SenZfp536 along with MS2-P65-HSF1-GFP and dCAS9-VP64-GFP for 48 h. F = 16.64, P = 0.0076 (one-way ANOVA). J Design of the chromosome conformation capture (3C) assay. Red arrow, constant primer at the Zfp536 promoter; blue arrows; test primers; curved arrows, potential interactions between test loci and the Zfp536 promoter. K Schematic of 3C-qPCR. L Relative crosslinking values of loci T1-T6 with the Zfp536 promoter in Neuro-2a cells. M–R Relative crosslinking values of loci T1-T6 in Neuro-2a cells with depletion or enhanced expression (CRISPRa) of SenZfp536 for two days. was performed. No significant differences were detected in T1, T4, T5, and T6. For short hairpin RNAs in (N), F = 155.1, P < 0.0001; for CRISPRa in (N), F = 35.05, P = 0.0005; for short hairpin RNAs in (O), F = 45.35, P = 0.0003; for CRIPSPa in (O), F = 120.2, P < 0.0001. Replicates in each experiment, n = 3. *P < 0.05; **P <0.01; ***P < 0.001; ns, not significant (one-way ANOVA). Results are presented as mean ± SD.
Next, we evaluated whether activation of SenZfp536’s transcription up-regulates the expression of Zfp536 using the CRISPR/dCas9-mediated transcription activation system (CRISPRa) [48, 49]. Activation of SenZfp536 also elevated the levels of transcripts and proteins for Zpf536 in Neuro-2a cells (Fig. 5C–D). Notably, the extent of transcriptional repression and activation of Zfp536 correlated with that of SenZfp536, ruling out off-target effects by shRNAs or CRISPRa. However, episomal expression of SenZfp536 had no effect on Zfp536 expression (Fig. 5E), implying that SenZfp536 is a positive cis-regulator of Zfp536 in neural cells.
SenZfp536 is a Cis Transcriptional Activator of Zfp536
To determine whether SenZfp536 has an intrinsic ability to promote gene expression, we first used the Gal4-λN/BoxB system to tether SenZfp536 to the promoter of a heterologous reporter (Fig. 5F). Luciferase activity was measured in Neuro-2a cells 24 h after transfection. SenZfp536 enhanced the luciferase activity by ~25% in comparison with the LacZ control (Fig. 5G). Next, transcripts for SenZfp536, antisense SenZfp536, and Gfp were tagged with Zfp536-TSS-targeted sgRNA for the RNA tethering assay. The data showed that tethering SenZfp536, but not antisense SenZfp536, to the TSS of Zfp536 markedly induced the expression of Zfp536 (Fig. 5H, I).
Since SenZfp536 activates transcription of Zfp536 in cis but not in trans, we went on to investigate if the locus that transcribes SenZfp536 can physically associate with the promoter of Zfp536. We thus performed chromosome conformation capture (3C) assays using one constant primer on the promoter of Zfp536 along with six testing primers around SenZfp536 (Fig. 5J, K). qPCR showed that T2 and T3, the loci nearest SenZfp536, were significantly crosslinked with the promoter of Zfp536 (Fig. 5L). Furthermore, depletion or activation of SenZfp536 significantly attenuated or enhanced, respectively, the association between T2/T3 and the Zfp536 promoter, suggesting that transcribed SenZfp536 maintains a chromatin loop to bring close the cis elements around SenZfp536 with the promoter of Zfp536 (Fig. 5M–R).
Together, these results underscored that SenZfp536 cis-activates Zfp536, probably by facilitating the chromatin loop between the SenZfp536 locus and the Zfp536 promoter.
SenZfp536 Negatively Regulates the Self-renewal of Cortical NPCs
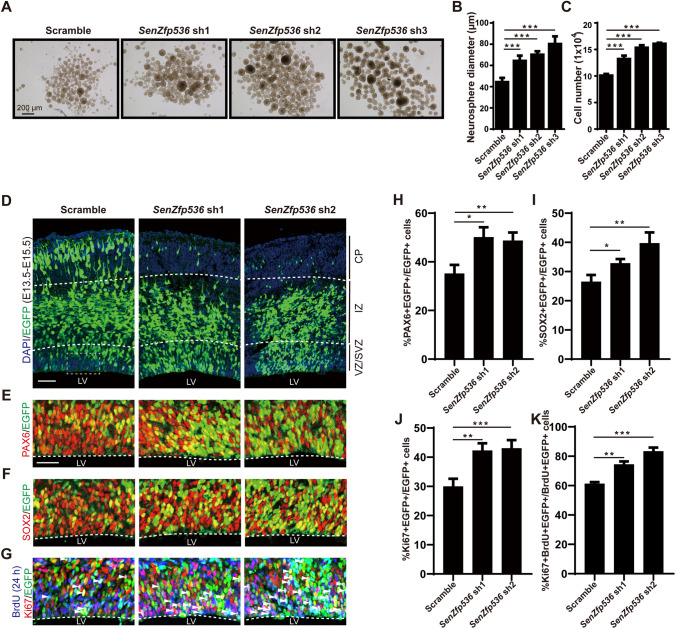
SenZfp536 is highly expressed in the VZ/SVZ, the proliferative zone, of the developing neocortex. To determine if SenZfp536 controls the self-renewal of cortical NPCs, suspension-cultured cortical NPCs at E12.5 were transduced with lentiviruses expressing shRNAs against SenZfp536 or scrambled control. The results showed that depletion of SenZfp536 greatly increased the sphere sizes and cell numbers of cortical NPCs (Fig. 6A–C). Interestingly, knockdown of SenZfp536 in Neuro-2a cells did not enhance their proliferation, suggesting a cell type-specific function of SenZfp536 (Fig. S3A).
Fig. 6.
SenZfp536 regulates the proliferation of cortical NPCs. A Representative images showing E12.5 cortices cultured under sphere conditions and transduced with the indicated shRNAs for 7 days. B Bar plots of the average diameter of neurospheres as in A. F = 43.86, P < 0.0001 (one-way ANOVA followed by Dunnett’s multiple comparison test). C Bar plots of the cell numbers of neurospheres as in A. F = 112.4, P < 0.0001 (one-way ANOVA followed by Dunnett’s multiple comparison test). D–K E13.5 mouse cortices were electroporated with a mix of shRNA-expressing and EGFP-expressing vectors; embryos were sacrificed at E15.5 for immunofluorescent analyses. Spatial distributions of transduced cells (D). VZ/SVZ immunofluorescent images and quantification of PAX6+ (E, H), SOX2+ (F, I), and Ki67+ (G, J) transduced cells (EGFP+) are displayed. Co-immunostaining and quantification for 24-h BrdU (E14.5-E15.5), Ki67, and EGFP reveal that SenZfp536 knockdown leads to more cells staying in the cell cycle as measured by the percentages of BrdU+/Ki67+/EGFP+ among BrdU+/EGFP+ cells (G, K). For H, F = 30.30, P < 0.001; for (I, F = 34.05, P < 0.001; for J, F = 103.9, P < 0.0001; for K, F = 123.6, P < 0.0001 (one-way ANOVA followed by Dunnett’s multiple comparison test). Embryos in each experiment: scrambled, n = 4; SenZfp536 sh1, n = 4; SenZfp536 sh2, n = 3. Scale bars, 50 μm. *P < 0.05; **P <0.01; ***P < 0.001; ns, not significant. Results are presented as mean ± SEM.
We next address whether SenZfp536 suppresses the self-renewal of cortical NPCs in vivo. E13.5 cortices were electroporated with plasmids expressing shRNAs against SenZfp536 or scrambled control for phenotypic analysis at E15.5, with transduced cells labeled with co-expressed EGFP (Fig. 6D). BrdU was administered at E14.5, 24 h prior to sacrifice. Significantly more SenZfp536-shRNA-transduced cortical cells expressed PAX6 and SOX2, markers for RGCs (Fig. 6E, F, H, and I). Moreover, significantly more SenZfp536-shRNA-transduced cortical cells expressed Ki67, with more cells double positive for Ki67 and 24-h BrdU, suggesting that SenZfp536-depleted cells tend to remain as proliferative neural progenitors (Fig. 6G, J, and K).
We next extended the phenotypic analyses to E16.5. More SenZfp536-depleted cortical cells resided in the VZ/SVZ and IZ and fewer in the CP than in scrambled-shRNA-transduced cells. Moreover, SenZfp536-depleted cells were more likely to co-localize with PAX6, SOX2, and BrdU (30 min), further supporting the idea that loss of SenZfp536 promotes the self-renewal of cortical NPCs (Fig. S3B–I). We next determined whether loss of SenZfp536 affects cortical layer specification. Although more SenZfp536-depleted cells resided in the IZ, the percentages of SenZfp536-depleted cells that expressed SATB2 or CTIP2, markers for superficial and deep cortical layers, respectively, were the same as for scrambled controls, indicating unaltered cortical layer fate upon loss of SenZfp536 (Fig. S3J–M).
We further used CRISPRa to enhance the transcription of SenZfp536 in the developing cortex [48, 49], and this led to decreased co-localization of SenZfp536-activated cells with PAX6 and SOX2 (Fig. S4A–D). In contrast, episomal expression of SenZfp536 did not have an effect on the self-renewal of cortical NPCs (Fig. S4E–J). These data support the idea that SenZfp536 controls cortical NPC self-renewal in cis but not in trans.
Zfp536 Expression Rescues SenZfp536 Knockdown-Associated Phenotypes
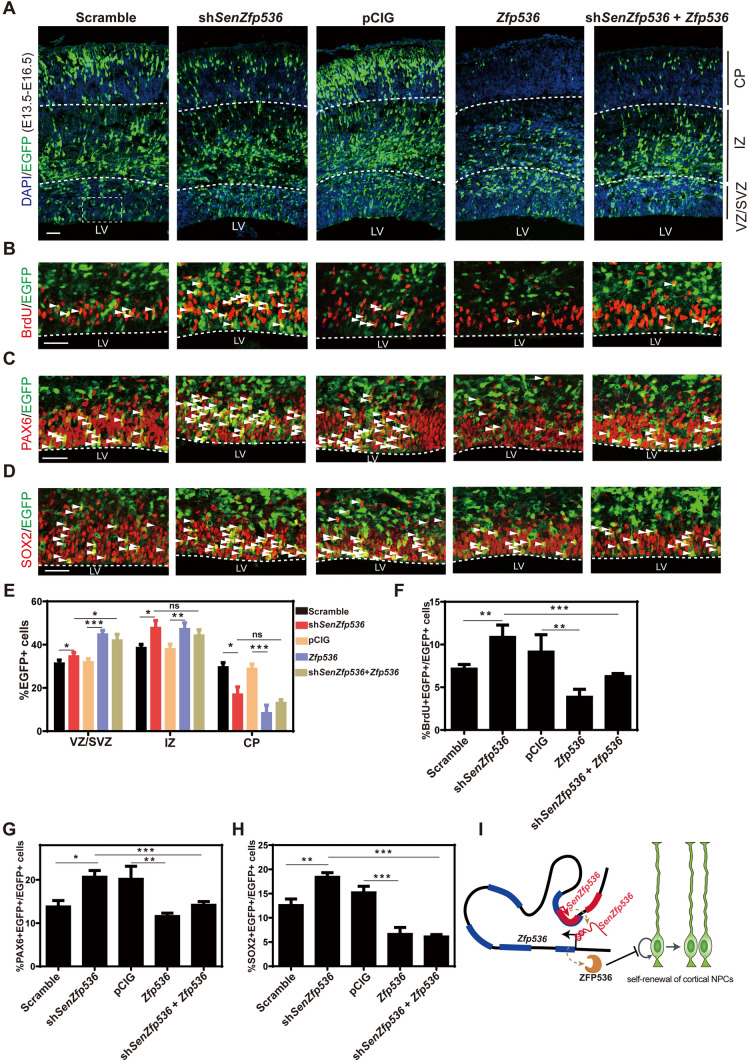
Similar to the phenotypes found in SenZfp536 knockdown, depletion of Zfp536 in cortical NPCs also led to more transduced cells expressing PAX6 and SOX2, indicating enhanced self-renewal (Fig. S4K–N). In contrast, overexpression of Zfp536 caused compromised self-renewal of transduced cortical NPCs, as significantly fewer Zfp536-expressing cells were labeled with BrdU (30 min), PAX6, and SOX2 without causing more cell death (Figs 7A–H and S4O). Notably, Zfp536 overexpression also hampered the radial migration of cortical projection neurons, evidenced by fewer transduced cells located in the CP, indicating that proper expression levels of Zfp536 are essential for neuronal differentiation. Last, overexpressing Zfp536 reversed the enhanced self-renewal of cortical NPCs caused by SenZfp536 depletion, supporting the idea that SenZfp536 inhibits the propagation of cortical NPCs by cis-activating Zfp536 (Fig. 7A–H).
Fig. 7.
Zfp536 expression rescues the enhanced proliferation of cortical NPCs caused by SenZfp536 knockdown. A–H E13.5 mouse cortices were electroporated with indicated vectors and transduced cells were labeled with EGFP; embryos were sacrificed at E16.5 for immunofluorescent analyses. A, E Spatial distribution of transduced cells. VZ/SVZ immunofluorescent images and quantification for BrdU (B, F), PAX6 (C, G), and SOX2 (D, H) are displayed. White arrows, co-labeled cells. For VZ/SVZ in E, F = 36.22, P < 0.0001; for IZ in E, F = 30.23, P < 0.0001; for CP in E, F = 46.32, P < 0.0001. For F, F = 33.35, P < 0.0001; for G, F = 38.23, P < 0.0001. for H, F = 44.24, P < 0.0001 (one-way ANOVA followed by Tukey’s multiple comparison test). Samples per experiment: Scrambled, n = 6; shSenZfp536 n = 4; pCIG, n = 7; Zfp536, n = 3; shSenZfp536 + Zfp536, n = 5. Scale bars, 50 μm. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant. Results are presented as mean ± SEM. I Model showing that SenZfp536 negatively regulates the self-renewal of cortical NPCs by cis-activating Zfp536.
To sum up, we characterized lncRNAs with spatiotemporal expression patterns in developing cerebral cortex. Among them, SenZfp536 restricts the proliferation of cortical NPCs by cis-activating the expression of Zfp536. Mechanistically, SenZfp536 promotes a local chromatin conformation to bring close the SenZfp536 locus and the Zfp536 promoter (Fig. 7I).
Discussion
Although many lncRNAs are expressed during neural development, very few have been reported to play regulatory roles. In general, the expression levels of lncRNAs are much lower than mRNAs, which argues that lncRNAs could be a result of transcriptional noise. However, lncRNAs with relatively high expression and/or displaying spatiotemporal distributions are more likely to have a functional capacity. Moreover, current knowledge of epigenetic mechanisms, including lncRNA-mediated transcriptional regulation, in regulating cell-fate choices during cortical development is limited. In the present study, we integrated in-house and public transcriptome data of developing mouse neocortex to characterize lncRNAs with potential functional roles in regulating the self-renewal of cortical NPCs. In fact, aRGs, the predominant cortical NPCs in developing mouse neocortex, specifically express the most lncRNAs. We further found that SenZfp536, a lncRNA overlapping the 3′ of Zfp536 in the sense direction, positively regulates the expression of Zfp536 and inhibits the self-renewal of cortical NPCs both in vitro and in vivo. SenZfp536 facilitates DNA looping and the association between the Zfp536 promoter and the genomic region that transcribes SenZfp536.
Recent progress in single-cell transcriptome studies identified lncRNAs involved in brain development and homeostasis [72, 73]. However, due to the current technical bottleneck, low-expression transcripts cannot be efficiently resolved. Thus, by integrating temporal and spatial transcriptome data from the developing mouse cortex, our approach managed to characterize both coding and non-coding transcripts implicated in the self-renewal of cortical NPCs. Moreover, ribosomal-depleted and strand-specific sequencing of the dorsal forebrain at E10.5 and E12.5 greatly supplemented the lncRNA annotation, as many non-polyadenylated transcripts were also identified.
Although many lncRNAs overlap with mRNAs in the sense direction, very few have been functionally dissected. Sense lncRNAs are commonly regarded as non-coding transcripts transcribed from alternative TSSs of PCGs, and are functionally related to their host PCGs, but the molecular mechanisms underlying the actions of lncRNAs are largely unknown. SenZfp536 is a sense lncRNA that is transcribed from an independent TSS; this is supported by Northern blotting, 5′ RACE experiments, and enriched Pol II and H3K4me3 around the SenZfp536 TSS. SenZfp536 is polyadenylated and largely resides in the nucleus.
Ablation of SenZfp536 downregulated the expression of Zfp536, whereas transcriptional activation of SenZfp536 elevated the Zfp536 level, and the expression levels of SenZfp536 and Zfp563 were correlated in the developing cortex and in manipulated Neuro-2a cells. However, episomal expression of SenZfp536 had no effect on Zfp536 expression. In addition, both reporter assay and RNA tethering experiments indicated that the transcript of SenZfp536 has transactivating activity. All these data support the conclusion that SenZfp536 maintains Zfp536 expression in cis but not in trans and rule out possible off-target effects by shRNAs or CRIPSRa. Using 3C assays, we further revealed that the genomic region that transcribes SenZfp536 interacts with the promoter of Zfp536 to form an enhancer-promoter loop structure, which is facilitated by SenZfp536. CTCF and cohesion might work with SenZfp536 to support the loop formation [74]. Moreover, the locus that transcribes SenZfp536 could be an enhancer for Zfp536, a scenario similar to the role of the lncRNA CCAT1-L in controlling MYC expression [75].
Both SenZfp536 and Zfp536 are highly expressed in VZ/SVZ, the germinal zone of the developing cerebral cortex, with almost identical patterns. Surprisingly, they suppressed but did not promote the self-renewal of cortical NPCs both in vitro and in vivo, probably affecting NPC cell-cycle length. A previous study demonstrated that ZFP536 negatively modulates the neural differentiation of P19 cells by participating in retinoic acid receptor-mediated gene regulation [76]. In the current study, however, we largely used primary cortical NPCs and developing cortex rather than the P19 carcinoma cells in Qin’s study [76]; they more faithfully reflect the physiological function of ZFP536, namely, limiting cortical NPC self-renewal without affecting cortical layer specification.
Together, we found large amounts of lncRNAs are expressed during cortical development. Furthermore, we illustrated the cis-regulatory role of the sense lncRNA SenZfp536 to control self-renewal of cortical NPCs. Given that numerous sense lncRNAs are expressed in radial glial NPCs, this could be a general theme for balancing the self-renewal and differentiation of cortical NPCs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1. Summary of RNA-seq data used in this study (PDF 70 kb)
Table S2. Identified LncRNAs in the GTF format (TXT 7554 kb)
Table S3. Gene List in WGCNA Co-expression Network Analysis (TXT 16342 kb)
Table S4. K-means cluster analysis (TXT 226 kb)
Table S5. Oligo sequences and antibodies (PDF 341 kb)
Acknowledgements
We thank members of the Zhou lab for critical reading of the manuscript. This work was supported by grants from the National Key R&D Program of China (2018YFA0800700), the National Natural Science Foundation of China (31970770, 31970676, and 31671418), the Natural Science Foundation of Hubei Province, China (2018CFA016), Fundamental Research Funds for the Central Universities, the Medical Science Advancement Program (Basic Medical Sciences) of Wuhan University (TFJC2018005) and State Key Laboratory Special Fund 2060204.
Conflict of interest
All authors claim that there are no conflicts of interest.
Footnotes
Kuan Tian and Andi Wang have contributed equally to this work.
Contributor Information
Ying Liu, Email: y.liu@whu.edu.cn.
Yan Zhou, Email: yan.zhou@whu.edu.cn.
References
- 1.Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci. 2013;14:755. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 4.Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caviness VS, Jr, Sidman RL. Time of origin or corresponding cell classes in the cerebral cortex of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol. 1973;148:141–151. doi: 10.1002/cne.901480202. [DOI] [PubMed] [Google Scholar]
- 6.Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Zheng J, Qi S, Shen Q. NONO regulates cortical neuronal migration and postnatal neuronal maturation. Neuroscience Bulletin. 2019;35:1097–1101. doi: 10.1007/s12264-019-00428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–348. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Guillemot F. Spatial and temporal specification of neural fates by transcription factor codes. Development. 2007;134:3771–3780. doi: 10.1242/dev.006379. [DOI] [PubMed] [Google Scholar]
- 10.Imayoshi I, Kageyama R. bHLH factors in self-renewal, multipotency, and fate choice of neural progenitor cells. Neuron. 2014;82:9–23. doi: 10.1016/j.neuron.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Cadwell CR, Bhaduri A, Mostajo-Radji MA, Keefe MG, Nowakowski TJ. Development and Arealization of the Cerebral Cortex. Neuron. 2019;103:980–1004. doi: 10.1016/j.neuron.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzortzopoulos A, Thomaidou D, Gaitanou M, Matsas R, Skoulakis E. Expression of Mammalian BM88/CEND1 in Drosophila Affects Nervous System Development by Interfering with Precursor Cell Formation. Neuroscience Bulletin. 2019;35:979–995. doi: 10.1007/s12264-019-00386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nature Reviews Genetics. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 14.Rinn JL, Chang HY. Genome Regulation by Long Noncoding RNAs. Annual Review of Biochemistry. 2012;81(81):145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang YW, Flynn RA, Chen Y, Qu K, Wan BB, Wang KC, et al. Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. Elife 2014, 3. [DOI] [PMC free article] [PubMed]
- 16.Ramos AD, Diaz A, Nellore A, Delgado RN, Park KY, Gonzales-Roybal G, et al. Integration of Genome-wide Approaches Identifies lncRNAs of Adult Neural Stem Cells and Their Progeny In Vivo. Cell Stem Cell. 2013;12:616–628. doi: 10.1016/j.stem.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Chang HY. Physiological roles of long noncoding RNAs: insight from knockout mice. Trends Cell Biol. 2014;24:594–602. doi: 10.1016/j.tcb.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu T, Chen C, Yang L, Zhang M, Zhang X, Jia J, et al. Distinct lncRNA expression profiles in the prefrontal cortex of SD rats after exposure to methylphenidate. Biomed Pharmacother. 2015;70:239–247. doi: 10.1016/j.biopha.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Yan P, Luo S, Lu JY, Shen X. Cis- and trans-acting lncRNAs in pluripotency and reprogramming. Curr Opin Genet Dev. 2017;46:170–178. doi: 10.1016/j.gde.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Lipovich L, Tarca AL, Cai J, Jia H, Chugani HT, Sterner KN, et al. Developmental Changes in the Transcriptome of Human Cerebral Cortex Tissue: Long Noncoding RNA Transcripts. Cerebral Cortex. 2013;24:1451–1459. doi: 10.1093/cercor/bhs414. [DOI] [PubMed] [Google Scholar]
- 22.Wang A, Wang J, Liu Y, Zhou Y. Mechanisms of Long Non-Coding RNAs in the Assembly and Plasticity of Neural Circuitry. Front Neural Circuits. 2017;11:76. doi: 10.3389/fncir.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Shen W, Zhang B, Tian K, Li Y, Mu L, et al. Long non-coding RNA LncKdm2b regulates cortical neuronal differentiation by cis-activating Kdm2b. Protein Cell. 2020;11:161–186. doi: 10.1007/s13238-019-0650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Afgan E, Baker D, Batut B, Van Den Beek M, Bouvier D, Čech M, et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic acids research. 2018;46:W537–W544. doi: 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nature methods. 2015;12:357. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nature biotechnology. 2015;33:290. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2013;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 28.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolde R. pheatmap: Pretty Heatmaps. 2015.
- 30.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic acids research. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benson DA, Boguski MS, Lipman DJ, Ostell J, Ouellette BF. GenBank. Nucleic acids research. 1998;26:1–7. doi: 10.1093/nar/26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tatusov RL, Fedorova ND, Jackson JD, Jacobs AR, Kiryutin B, Koonin EV, et al. The COG database: an updated version includes eukaryotes. BMC bioinformatics. 2003;4:41. doi: 10.1186/1471-2105-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boeckmann B, Bairoch A, Apweiler R, Blatter MC, Estreicher A, Gasteiger E, et al. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic acids research. 2003;31:365–370. doi: 10.1093/nar/gkg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang YJ, Yang DC, Kong L, Hou M, Meng YQ, Wei L, et al. CPC2: a fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic acids research. 2017;45:W12–W16. doi: 10.1093/nar/gkx428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Park HJ, Dasari S, Wang S, Kocher JP, Li W. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic acids research. 2013;41:e74–e74. doi: 10.1093/nar/gkt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langfelder P, Horvath S, Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 2009;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heng X, Guo Q, Leung AW, Li JYH. Analogous mechanism regulating formation of neocortical basal radial glia and cerebellar Bergmann glia. eLife. 2017;6:e23253. doi: 10.7554/eLife.23253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ge SX, Son EW, Yao R. iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinformatics. 2018;19:534. doi: 10.1186/s12859-018-2486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 40.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic acids research. 2008;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ge S, Jung D. ShinyGO: a graphical enrichment tool for animals and plants. bioRxiv 2018: 315150. [DOI] [PMC free article] [PubMed]
- 42.Culhane AC, Schröder MS, Sultana R, Picard SC, Martinelli EN, Kelly C, et al. GeneSigDB: a manually curated database and resource for analysis of gene expression signatures. Nucleic Acids Research. 2011;40:D1060–D1066. doi: 10.1093/nar/gkr901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newman JC, Weiner AM. L2L: a simple tool for discovering the hidden significance in microarray expression data. Genome biology. 2005;6:R81–R81. doi: 10.1186/gb-2005-6-9-r81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, et al. GREAT improves functional interpretation of cis-regulatory regions. Nature biotechnology. 2010;28:495. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo Z, Mu L, Zheng Y, Shen W, Li J, Xu L, et al. NUMB enhances Notch signaling by repressing ubiquitination of NOTCH1 intracellular domain. J Mol Cell Biol. 2020;12:345–358. doi: 10.1093/jmcb/mjz088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallagher S, Chakavarti D. Immunoblot Analysis. JoVE 2008: e759. [DOI] [PMC free article] [PubMed]
- 47.Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, Kondo S, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–573. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- 48.Luo S, Lu JY, Liu L, Yin Y, Chen C, Han X, et al. Divergent lncRNAs regulate gene expression and lineage differentiation in pluripotent cells. Cell Stem Cell. 2016;18:637–652. doi: 10.1016/j.stem.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 49.Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hagège H, Klous P, Braem C, Splinter E, Dekker J, Cathala G, et al. Quantitative analysis of chromosome conformation capture assays (3C-qPCR) Nature Protocols. 2007;2:1722. doi: 10.1038/nprot.2007.243. [DOI] [PubMed] [Google Scholar]
- 51.Xie Z, Moy LY, Sanada K, Zhou Y, Buchman JJ, Tsai LH. Cep120 and TACCs control interkinetic nuclear migration and the neural progenitor pool. Neuron. 2007;56:79–93. doi: 10.1016/j.neuron.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DunhamI K, AldredSF C, Consortium EP An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis CA, Hitz BC, Sloan CA, Chan ET, Davidson JM, Gabdank I, et al. The Encyclopedia of DNA elements (ENCODE): data portal update. Nucleic Acids research. 2017;46:D794–D801. doi: 10.1093/nar/gkx1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayoub AE, Oh S, Xie Y, Leng J, Cotney J, Dominguez MH, et al. Transcriptional programs in transient embryonic zones of the cerebral cortex defined by high-resolution mRNA sequencing. Proceedings of the National Academy of Sciences. 2011;108:14950–14955. doi: 10.1073/pnas.1112213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dillman AA, Hauser DN, Gibbs JR, Nalls MA, McCoy MK, Rudenko IN, et al. mRNA expression, splicing and editing in the embryonic and adult mouse cerebral cortex. Nat Neurosci. 2013;16:499–506. doi: 10.1038/nn.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guttman M, Garber M, Levin JZ, Donaghey J, Robinson J, Adiconis X, et al. Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat Biotech. 2010;28:503–510. doi: 10.1038/nbt.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protocols. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of molecular biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 59.Frankish A, Diekhans M, Ferreira AM, Johnson R, Jungreis I, Loveland J, et al. GENCODE reference annotation for the human and mouse genomes. Nucleic acids research. 2018;47:D766–D773. doi: 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang S, Zhang L, Guo J, Niu Y, Wu Y, Li H, et al. NONCODEV5: a comprehensive annotation database for long non-coding RNAs. Nucleic Acids Research. 2017;46:D308–D314. doi: 10.1093/nar/gkx1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hudson WH, Prokhnevska N, Gensheimer J, Akondy R, McGuire DJ, Ahmed R, et al. Expression of novel long noncoding RNAs defines virus-specific effector and memory CD8+ T cells. Nature Communications. 2019;10:196. doi: 10.1038/s41467-018-07956-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marta F, Mareike A, Elena T, Takashi N, Holger B, Eric L, et al. Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science. 2015;347:1465–1470. doi: 10.1126/science.aaa1975. [DOI] [PubMed] [Google Scholar]
- 64.de la Torre-Ubieta L, Stein JL, Won H, Opland CK, Liang D, Lu D, et al. The Dynamic Landscape of Open Chromatin during Human Cortical Neurogenesis. Cell. 2018;172(289–304):e218. doi: 10.1016/j.cell.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, et al. The accessible chromatin landscape of the human genome. Nature. 2012;489:75–82. doi: 10.1038/nature11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol2005, 4: Article17. [DOI] [PubMed]
- 67.Forgy EW. Cluster analysis of multivariate data: efficiency versus interpretability of classifications. Biometrics. 1965;21:768–769. [Google Scholar]
- 68.Le Cam L, Neyman J, Scott EL. Proceedings of the Sixth Berkeley Symposium on Mathematical Statistics and Probability: Held at the Statistical Laboratory, University of California, June 21-July 18, 1970. Univ of California Press, 1972.
- 69.Lloyd S. Least squares quantization in PCM. IEEE transactions on information theory. 1982;28:129–137. [Google Scholar]
- 70.Richardson JE, Bult CJ. Visual annotation display (VLAD): a tool for finding functional themes in lists of genes. Mammalian genome : official journal of the International Mammalian Genome Society. 2015;26:567–573. doi: 10.1007/s00335-015-9570-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bult CJ, Eppig JT, Kadin JA, Richardson JE, Blake JA, Group MGD The Mouse Genome Database (MGD): mouse biology and model systems. Nucleic acids research. 2008;36:D724–D728. doi: 10.1093/nar/gkm961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu SJ, Nowakowski TJ, Pollen AA, Lui JH, Horlbeck MA, Attenello FJ, et al. Single-cell analysis of long non-coding RNAs in the developing human neocortex. Genome Biol. 2016;17:67. doi: 10.1186/s13059-016-0932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luo YP, Coskun V, Liang AB, Yu JH, Cheng LM, Ge WH, et al. Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell. 2015;161:1175–1186. doi: 10.1016/j.cell.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ong CT, Corces VG. CTCF: an architectural protein bridging genome topology and function. Nature Reviews Genetics. 2014;15:234. doi: 10.1038/nrg3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiang JF, Yin QF, Chen T, Zhang Y, Zhang XO, Wu Z, et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Research. 2014;24:513–531. doi: 10.1038/cr.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qin Z, Ren F, Xu X, Ren Y, Li H, Wang Y, et al. ZNF536, a Novel Zinc Finger Protein Specifically Expressed in the Brain, Negatively Regulates Neuron Differentiation by Repressing Retinoic Acid-Induced Gene Transcription. Molecular and Cellular Biology. 2009;29:3633–3643. doi: 10.1128/MCB.00362-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of RNA-seq data used in this study (PDF 70 kb)
Table S2. Identified LncRNAs in the GTF format (TXT 7554 kb)
Table S3. Gene List in WGCNA Co-expression Network Analysis (TXT 16342 kb)
Table S4. K-means cluster analysis (TXT 226 kb)
Table S5. Oligo sequences and antibodies (PDF 341 kb)