Abstract
Gravity determines shape of body tissue and affects the functions of life, both in plants and animals. The cellular response to gravity is an active process of mechanotransduction. Although plants and animals share some common mechanisms of gravity sensing in spite of their distant phylogenetic origin, each species has its own mechanism to sense and respond to gravity. In this review, we discuss current understanding regarding the mechanisms of cellular gravity sensing in plants and animals. Understanding gravisensing also contributes to life on Earth, e.g., understanding osteoporosis and muscle atrophy. Furthermore, in the current age of Mars exploration, understanding cellular responses to gravity will form the foundation of living in space.
Subject terms: Cell biology, Molecular biology
Introduction
Gravity defines the morphology of life on Earth. It affects the growth and development of plants and animals by regulating the proliferation of their constituent cells1. Gravity also plays crucial roles in cellular function. For example, plants grow leaves and roots in the correct direction by sensing gravity2. Animals regulate the densities of bones and muscles in response to gravitational load3,4. A response to gravity is an active activity inherent to the physiology of plants and animals.
Historically, elucidation of gravity sensing mechanisms in life originates from the study of plants. Abundant research in plant biology has laid the foundation for studying gravity sensing mechanisms in animals. In the first half of this article, we review the mechanisms of gravity sensing in plants from the viewpoint of cellular and molecular biology. Subsequently, the mechanisms of gravity sensing in animal cells will be discussed. Since both lunar base construction and manned Mars exploration plans are being discussed at present5,6, discussions regarding bone loss and muscle atrophy in microgravity environments are inevitable. This paper also summarizes the recent findings in this field. Through these discussions, we outline the common mechanisms of gravity sensing in plants and animals.
This paper expands on our understanding of gravity sensing in plant and animal cells and discusses the future direction of gravitational biology, with the ultimate purpose of contributing to the development of living in space.
Gravity sensing in plants
Auxin regulation of gravimorphogenesis in plants
The survival of sessile organisms, such as plants, depends upon their ability to avoid or mitigate various environmental stresses to which they are subjected. As one of such strategies, plants possess an ability to control directional growth by gravitropism (Fig. 1). Typically, coleoptiles and stems (shoots) grow upward in order to obtain light (negative gravitropism), whereas roots grow downward to acquire water and minerals (positive gravitropism). When plants in a vertical position are reoriented horizontally, the pattern of gravitropic response differs among plant species and organs. Other aspects of plant growth and development are also regulated by gravity. Accordingly, the terms “gravimorphism” or “gravimorphogenesis” can be used for gravity-regulated phenomena in plants.
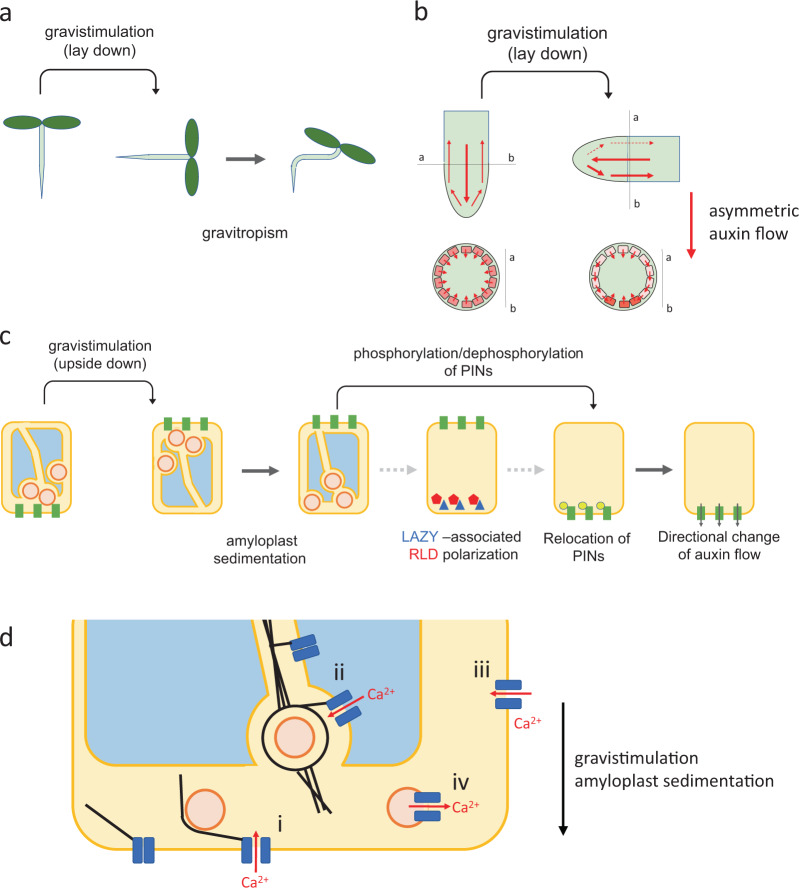
Fig. 1. Mechanism for directional growth in response to gravity in plants.
a Model of gravitropism. b Asymmetric auxin flow in horizontally reoriented plants, c cellular responses in gravisensing (endodermal) cells. At first, gravity causes sedimentation of amyloplasts. RLD proteins associated with LAZY proteins get polarized to the new bottom side. The LAZY proteins regulate the localization of PIN proteins, which are efflux carriers of plant hormone auxin. Finally, the change in direction of auxin flow causes asymmetric growth of plants. d Activation of mechanosensitive ion channels in plasma- and endomembrane upon amyloplast sedimentation (i, ii), deformation, compression and shear stress (iii), displacement of amyloplast (iv).
The plant hormone auxin plays an important role in plant gravimorphogenesis. A major endogenous auxin is indole-3-acetic acid (IAA) that regulates various aspects of plant growth and development. In auxin signaling pathway, Aux/IAA proteins inactivate the auxin response factor (ARF) when auxin level is low7. A high level of auxin results in forming a complex of the transcriptional repressor, Aux/IAA, and the AUXIN SIGNALING F-BOX PROTEIN co-repressor/auxin receptor, TIR/AFBs, which allows the degradation of Aux/IAA and the release of ARF repression for modulating the expressions of auxin-related genes7. Auxin concentration in the tissues is determined by regulating its biosynthesis, inactivation, and transport. A unique feature of auxin is the polar auxin transport that contributes to most of the directional auxin transport and local auxin concentration. This polar auxin transport is regulated by auxin efflux carriers PIN-FORMED (PIN) family and auxin influx carriers AUX/LAX family proteins. It is considered that auxin is directionally transported across the plasma membrane where PINs localize with a polarity8,9. ATP-binding cassette ABC transporters of the B class family (ABCB) also play a role in polar auxin transport10,11. TWISTED DWARF1 (TWD1) has been shown to interact with ABCB protein for auxin transport. ABCB could export auxin independently of PIN proteins, but the functional interaction of ABCB-PIN pairs for auxin transport is also considered. Abbreviations are listed in Table 1.
Table. 1.
List of abbreviations.
| ARHGAP | Rho GTPase Activating Protein |
| BRX | Brevis radix |
| CaN | Calcineurin |
| CBF/DREB1 | C-repeat binding factor / dehydration-responsive element-binding 1 |
| CCL | Conserved C terminus in the LAZY1 family |
| CICR | Ca2+-induced Ca2+-release |
| D6PK | Serine/threonine-protein kinase |
| DCC | Disposable cell culture |
| DEK1 | Defective Kernel 1 |
| GNOM | ARF guanine-nucleotide exchange factor |
| HEK | Human Embryonic Kidney |
| ISS | International Space Station |
| LAZY1 | Plant-specific genes with unknown molecular functions that are involved in gravitropism |
| MCA | Mid1 (Yeast mechanosensitive channel) complementing activity protein |
| MSC | Mesenchymal stem cells |
| MSL | MscS (bacterial mechanosensitive channel of small conductance) like protein |
| NFAT | Nuclear factor of activated T cells |
| NOX | NADPH oxidases |
| OSCA | reduced hyperosmolality- induced [Ca2+]i increase 1 |
| PDZ | Domains bind to a short region of the C-terminus of other specific proteins |
| PLC | Phospholipase C |
| RANKL | Receptor activator of nuclear factor-B ligand |
| RCC | Regulator of chromosome condensation |
| RCN | Reticulocalbin |
| RLD | Regulator of chromosome condensation-like domains |
| ROS | Reactive oxygen species |
| TRPC | Canonical transient receptor potential channel |
Gravisensing apparatuses reside in the endodermal cells of shoots and columella cells of roots, where amyloplast statoliths sediment upon plant reorientation12. However, the mechanisms of graviperception remain unsolved. The Cholodny–Went theory explains differential growth in tropisms through the redistribution of the plant hormone auxin in elongating organs13. In gravistimulated plants, more auxin accumulates in the lower side than the upper side of shoots and roots in a horizontal position, causing the upward bending of shoots and downward bending of roots. Auxin redistribution following gravistimulation has been verified. Indeed, molecular genetics with many gravitropic mutants has revealed the importance of the roles of auxin transport, redistribution, and response in gravitropism14. In particular, the identification of an auxin efflux carrier PIN-FORMED (PIN) was a significant breakthrough in our understanding of the mechanisms for asymmetric auxin transport and distribution in gravistimulated shoots and roots15,16. However, unlike some PINs, the pattern of ABCB expression and the phenotypes of ABCB mutants indicate that it is not directly involved in asymmetric redistribution of auxin during gravitropic response10,11. In roots, for example, PIN3 and PIN7 localize to the plasma membrane of gravisensing columella cells in the root cap. Within 10 min of gravistimulation by reorienting plant seedlings, PIN3 and PIN7 change their location to the new bottom of the plasma membrane, allowing auxin to move to the bottom side of the root cap. Thereafter, PIN2 localizing to the proximal side of the plasma membrane in the lateral root cap and the epidermis, plays a role in the basipetal transportation of auxin from the bottom side of the root cap to the elongation zone. In gravistimulated roots, auxin redistribution is thus established, and transcriptional regulation depending on the auxin level on the upper and lower sides leads to downward bending. Spaceflight experiments with cucumber seedlings in the Space Shuttle and the International Space Station (ISS) showed that the endodermal cells relocalize an auxin efflux carrier, CsPIN1, because of gravistimualtion in space and laterally transport auxin from the upper to lower flank17–19.
Some factors that could be involved in PIN polarization and thereby asymmetric auxin flow have been reported. That is, proteins such as RCN1, PINOID, and D6PK regulate PIN phosphorylation/dephosphorylation2,20,21. The phosphorylation status of PIN proteins, together with GNOM-dependent PIN recycling processes, is hypothesized to participate in polar localization of PIN proteins on the plasma membrane. Dynamics of microfilaments and microtubules (MTs) is an important factor involved in the regulation of trafficking of auxin transporters. It is reported that Sorting Nexin 1 (SNX1) plays a role in PIN2 recycling via interaction with MTs-associated protein CLASP22.
Recently, it was found that LAZY1 regulates PIN relocalization in gravisensing cells and determines negative gravitropism in shoots and positive gravitropism in roots23,24. Interestingly, the alteration of two amino acids in LAZY1 was found to successfully switch negative gravitropism to positive gravitropism in Arabidopsis shoots25. Furthermore, it was revealed that upon amyloplast sedimentation, LAZY1/LAZY1-like proteins get polarized to the plasma membrane of the bottom side of gravisensing cells26. The conserved C terminus in the LAZY1 family (CCL) domains interact with the Brevis radix (BRX) domains of the regulator of chromosome condensation (RCC)-like domains (RLD) proteins, thereby polarly recruiting RLD from the cytoplasm to the plasma membrane26. It was demonstrated that RLD1–4 localize in the root cap and modulate auxin transport through regulation of PIN localization, possibly via a GNOM-like function in PIN trafficking26. This process is required for controlling polarized auxin flow and gravitropic response. Thus, amyloplast position itself may play an important role in gravity sensing/signaling as discussed in next section “Gravity sensor in plants.” However, the mechanism underling the LAZY polarization upon amyloplast sedimentation in gravisensing cells still remains unknown.
Auxin biosynthesis and distribution in microgravity were also examined by spaceflight experiments in some plant species. Most of those results showed no differences between space- and ground-grown seedlings. Recently, transformed Arabidopsis lines with GFP reporter gene, pDR5r::GFP, pTAA1::TAA1-GFP, pSCR::SCR–GFP and pARR5::GFP, were used for spaceflight experiments on ISS27. The expressions of the auxin artificial AuxRE promoter construct (pDR5r::GFP), Tryptophan Aminotransferase of Arabidopsis fusion (pTAA1::TAA1-GFP) and Scarecrow fusion (pSCR::SCR–GFP) were used to monitor auxin level, auxin production and auxin-related signals, respectively. There were no differences in the expression patterns and levels of those genes in the primary root tips of seedlings grown under microgravity and 1 G ground conditions. These results implied that auxin gradient in plants is established independently of gravity. On the other hand, spaceflight experiments with pea and maize seedlings showed altered polar auxin transport in microgravity; polar auxin transport in microgravity was decreased in pea epicotyls and accelerated in maize coleoptiles and mesocotyls compared with the 1 G controls28,29. Recent spaceflight experiments immunohistochemically compared PsPIN1 localization in etiolated pea epicotyls grown under microgravity and 1 G conditions in space30. PsPIN1 proteins were detected in the lower side of the plasma membrane of 80–90% endodermal cells under artificial 1 G conditions, whereas number of those endodermal cells showing polarized PsPIN1 localization significantly decreased in microgravity. The authors consider the change in PsPIN1 localization pattern as a possible cause for the reduction of polar auxin transport in pea epicotyls under microgravity conditions. In maize seedlings, interestingly, the enhanced accumulation of ZmPIN1and the alteration of ZmPIN1a localization in parenchymatous cells of the coleoptiles were likely responsible for the enhanced polar auxin transport in microgravity31. However, species differences of polar auxin transport in microgravity are mysterious.
Thus, the PIN-mediated auxin transport and distribution are essential parts of plant gravimorphogenesis. It should be emphasized that some PIN proteins were verified to be gravity responsive in their relocalization on the plasma membrane of the gravisensing cells by spaceflight experiments. Polarization and function of LAZY1/LAZY1-like proteins appear to play a key role in the gravity-induced PIN relocalization and thereby asymmetric auxin flow. To understand the entire regulatory mechanism of plant gravimorphogenesis, it is important to clarify the graviperception mechanism that leads to the regulation of LAZY1/LAZY1-like proteins polarization and PIN relocalization in gravisensing cells.
Gravity sensor in plants
As discussed above, plants have a mechanism for gravity sensing using the sedimentation of organelles in order to establish the asymmetric transport of hormones. The most widely accepted model for plant gravity sensing is the starch-statolith hypothesis, in which intracellular sedimentation of the starch-filled organelle (amyloplast) plays a crucial role in the events triggering the initial phases of gravity sensing in plants32–34. Recent live-cell imaging technology has revealed, however, that the movement of the amyloplast is not static but saltatory because its dynamics are dependent on both the gravity vector and intracellular environments such as those of the cytoskeleton and vacuole35. In the shoot statocytes (endodermal cells) of Arabidopsis thaliana, the amyloplasts are tightly surrounded by the vacuolar membrane and are supposed to interact with actin filaments36. The abnormal behavior of the vacuolar membrane, however, pushes the amyloplasts to the periphery of the cell in the agravitropic mutant, shoot gravitropism (sgr) 2, which restricts the movement of the amyloplasts and renders them nonsedimentable37. Therefore, inflorescence stems in sgr2 mutants do not sense gravity and do not show a gravitropic response because of nonsedimentable amyloplasts37. distorted1 (dis1)/actin-related protein 3 (arp3) mutants possess irregular thick actin bundles surrounding amyloplasts in their root statocytes (columella cells), and consequently, the amyloplasts do not sediment fully from the actin filaments, resulting in a reduced gravitropic response in roots38. sgr9 mutants also have nonsedimentable, clustered amyloplasts entangled with actin filaments in the endodermal cells because of an excess of interaction between the amyloplasts and the actin filaments and exhibit a weak gravitropic response36. These abnormal phenotypes in both dis1 and sgr9 mutants were compensated by disrupting actin filaments, such as through the use of the actin filament-depolarizing drug latrunculin B36,38, suggesting that the actin filament is not an essential component for either gravity sensing or gravitropic responses, but rather acts as an intracellular component affecting amyloplast dynamics. Taken together, the dynamics of the vacuolar membrane and actin filaments could diffuse amyloplasts from the bottom of the cell, leading to the nonstatic, saltatory behavior of the plant statolith and amyloplast.
A long-lasting question regarding gravity sensing in plants is how the physical process of amyloplast sedimentation is converted into intracellular signals. The most reasonable models, such as the inner ear (hair cell) system of vertebrates, suggest that amyloplast sedimentation activates mechanosensitive ion channels via actin, resulting in intracellular ionic signaling39–41. Changes in the gravity vector (inclining the specimens) elevate cytosolic calcium concentrations in Arabidopsis seedlings42,43. This Ca2+ response is attenuated with latrunculin B and mechanosensitive ion channel blockers such as Gd3+ 42, supporting a model involving actin filaments that function as a tether to activate mechanosensitive channels39. Actin filaments may have both a positive role in the activation of mechanosensitive channels upon gravity stimulation and a negative role in the sedimentary dynamics of amyloplasts as discussed above. To demonstrate this, direct observations of Ca2+ responses in both the shoot endodermal and root columella cells are needed. An alternative model is the position-sensing hypothesis in which the spatial distribution of amyloplasts upon gravity stimulation is detected as a signal for gravity sensing44. In this hypothesis, a putative machinery, rather than mechanosensitive channels sensing the gravitational force exerted on the amyloplasts, detects the position (state) of the amyloplasts in gravity sensing cells, consistent with data indicating that gravitropic responses in wheat coleoptile are dependent on the angle of inclination of the specimens but not on the amplitude of the gravitational force45. These data suggest a variety of gravity sensing mechanisms in diverse plant species such as monocots or dicots which are, however, quite different from those of animals.
Mechanosensitive channels in plants
As discussed above, gravity is the force that generates several effects such as the weight leading to the sedimentation of amyloplast statoliths, deformation and compression of the cells, and fluid shift in vasculature, all of which generate mechanical stresses in plasma and endomembranes. One of the earliest response to changes in gravity vector and magnitude is the Ca2+ response, which has been reported in many plant species46. Thus, it is plausible that mechanosensitive channels are the primary sensors of plant graviperception evoking the Ca2+ response.
MSL proteins share the C-terminal transmembrane (TM) segment corresponding to the pore-forming transmembrane segment of MscS (mechanosensitive channel of small conductance) in E. coli47. Among the 10 members of the MSL protein in Arabidopsis, MSL1 localizes to the inner mitochondrial membrane, whereas MSL2 and 3 are found at the inner plastid membrane, and MSL8, 9, and 10 localize to the plasma membrane48. MSL2 and 3 regulate the size and shape of the plastid, and MSL8 is required for the rehydration of the pollen grain, indicating that a major role of MSL proteins is osmotic regulation. Yeast two-hybrid assays demonstrated that MSL2 and 3 interact with each other, suggesting that some MSLs can form heteromeric channels49. MCA proteins have been identified as complements of the mid1 mutant of the yeast that is defective in Ca2+ influx50. MCA1 and MCA2 localize in the plasma membrane and mediate the cold-induced Ca2+ response that leads to cold tolerance according to the CBF/DREB1-independent pathway51. MCA1, but not MCA2, is required for the penetration of roots into harder agar and results in the retardation of the leafing and bolting of the mutant. The expression levels of MCA1 and MCA2 were increased under hypergravity conditions in the absence of light, and the hypocotyl elongation under these conditions was attenuated in the overexpressing seedlings. Therefore, MCA proteins might be responsible for resistance to gravity52. OSCA proteins, homologous to the TMEM63 family of proteins known throughout eukaryotes, have been identified from Arabidopsis mutants exhibiting a low hyperosmolality-induced Ca2+ response53. An ortholog of animal Piezo protein, the mechanosensitive cation channel for touch sensation and vascular development, is commonly conserved in monocots and suppresses the systemic movement of viruses54.
Mechanosensitive cation channel activity in MCAs and OSCAs has been recorded using patch-clamp techniques with heterologous expression in Xenopus oocytes and HEK cells55,56. Electrophysiological studies using Arabidopsis mutants and Xenopus oocytes have revealed that MSL proteins show a preference for anions over cations, leading to depolarization of the plasma membrane and the following Ca2+ response through the activation of voltage-dependent cation channels57. Most recently, a lack of rapidly activated mechanosensitive Ca2+-permeable channel activity (RMA) was reported in Arabidopsis DEK1 mutants58. Although there is no sequence homology, AtDEK1 has a high number of transmembrane helices as with mammalian Piezo proteins, and RMA shows low conductance and rapid inactivation. These electrophysiological studies have demonstrated that the thresholds of membrane stretch for the activation, conductance, and inactivation time constant of plant mechanosensitive channels are varied, even within the same family.
Although series of proteins and their physiological roles have been characterized in numerous aspects, the mechanosensitive channels responsible for gravitropism have not yet been identified. Overlapping tissue expression patterns suggest that mechanosensitive channels in the same tissue share physiological functions. Thus, most mutants, even those that lacking five MSLs (MSL4, 5, 6, 9, and 10), do not show a significant phenotype47. Interestingly, most mechanosensitive channels are expressed in the vasculature, where gravity-induced Ca2+ response is observed46. In root statocytes of Brassica grown in ISS, ten-minute onset (µg to 1 g) or removal (1 g to µg) of a gravity-induced Ca2+ response in absence of a significant statolith displacement59. Changes in gravity vector and magnitude promote a Ca2+ response with similar kinetics46. It suggests that multiple mechanosensitive channels in plasma- and endomembranes could be differentially activated by gravity, and promotes a small Ca2+ response that is amplified by common intracellular machineries. Thus, pharmacological studies suggest that gravity-induced Ca2+ response is greatly amplified by Ca2+-induced Ca2+-release (CICR) from organelles through signaling cascades, including PLC activation43. Subcellular and tissue-specific distributions of gravity-induced Ca2+ response and the underlying molecular mechanisms should be investigated more deeply in order to understand graviperception mechanisms in plants.
Gravity sensing in animals
YAP-mediated gravity response and 3D organ growth and maintenance
Although plants and animals share common mechanisms for gravity sensing, such as the homologous mechanosensitive ion channels discussed above, the transcriptional coactivator Yes-associated protein (YAP) is a mechanosensitive machinery specific to animals. The relationship between gravity and YAP was first revealed by the analysis of the medaka fish YAP mutant. The body of this mutant was flattened because of its inability to withstand gravity60. This demonstration that YAP is required for withstanding gravity in generating a 3D body/organ shape first suggested that YAP not only transduces gravity responses as a mechano-transducer61, but more strikingly acts as a mechano-effector for withstanding gravity, forming a mechanical negative-feedback60. Since YAP is the key regulator orchestrating organ growth62, this review will focus on the role of YAP in linking gravity response with organ growth and maintenance.
YAP and its paralog TAZ (transcriptional coactivator with PDZ-binding motif) act as transcriptional co-activators, mainly in the nucleus63,64. YAP nuclear localization is controlled mainly by the Hippo pathway and F-actin-mediated signaling responses to diverse signals, e.g., growth factors and mechanical stimuli65. YAP is able to expand the organ size when constitutively activated62 and is involved in such diseases as cancer and fibrosis65.
The discovery that YAP could act as a mechanoeffector uncovered a negative feedback control of YAP activity: F-actin polymerization activates YAP66 and its target gene ARHGAP18 and then negatively regulates F-actin polymerization, suppressing YAP activity60 (Fig. 2a). This is a mechanical negative feedback since the negative regulation of F-actin polymerization by YAP optimizes F-actin turnover and maximizes actomyosin contractility, i.e., cell/tissue tension. Cell/tissue tension then controls 3D tissue formation and tissue alignment necessary for generating a 3D organ consisting of multiple tissues, e.g., an eye consisting of the lens and eye cup60 (Fig. 2b). It is hypothesized that a YAP-mediated response to gravity is involved in the maintenance of bones and skeletal muscles, since YAP is known to control the organ size through Hippo signaling and is expressed in the stem cells of many organs, including skeletal muscles.
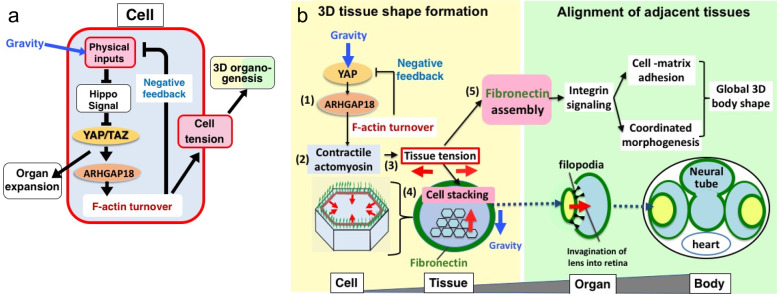
Fig. 2. YAP-mediated 3D organ/tissue formation withstanding gravity.
a Mechanical negative feedback maintaining YAP activity in a cell. b YAP-mediated 3D organ/tissue formation withstanding gravity. In (a), YAP/TAZ acts as a mechanotransducer and mechanoeffector. As a mechanotransducer, it provides physical inputs, including gravity activation of YAP/TAZ that leads to an expansion of organ size. As a mechanoeffector, it activates YAP, which, in turn, controls F-actin turnover, leading to the suppression of YAP as part of a negative feedback mechanism. F-actin turnover controls the cell/tissue tension that mediates 3D organogenesis. b YAP is essential for the formation of complex 3D organs by coordinating 3D tissue shape (left) and tissue alignment (right). In response to external forces, including gravity, YAP activates (1) ARHGAP18 expression, which mediates (2) contractile actomyosin formation controlling (3) tissue tension. Tissue tension is required for both (4) cell stacking to form a 3D tissue shape and (5) fibronectin assembly required for adjacent tissue alignment, e.g., the alignment of the lens and eye-cup.
YAP orchestrates the response to gravity by controlling actomyosin contractility by negatively regulating F-actin polymerization through its target gene ARHGAP18. Since actomyosin both generates mechanical forces and acts as a mechanical sensor67, actomyosin is a putative gravity sensor. Gravity could promote F-actin polymerization, activating YAP in order to maintain a 3D organ size. This is consistent with reports that simulated microgravity inhibits the osteogenic differentiation of mesenchymal stem cells via the de-polymerization of F-actin that inhibits TAZ nuclear translocation68. Further detailed studies are necessary to elucidate the mechanisms by which the YAP-mediated gravity response is linked with organ growth and maintenance. These studies will be useful in alleviating compromises to health, such as the loss of bones and skeletal muscles that arises from periods of “life in space.”
Gravity sensing in bones
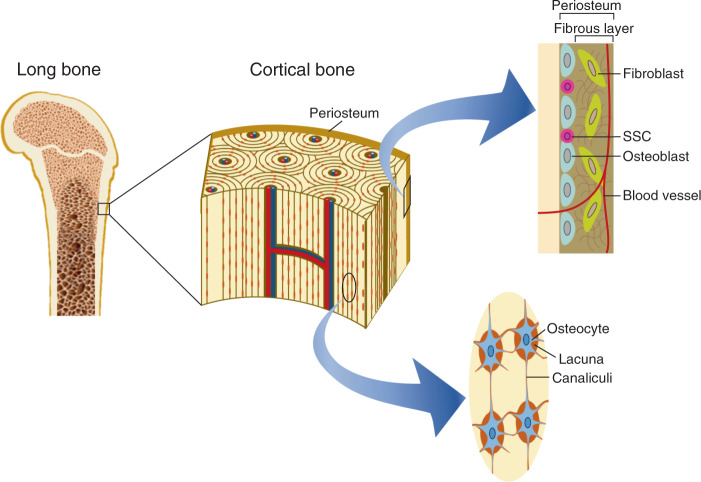
Bone loss is one of the major health problems facing organisms that experience life in space. The structure of bones is shown in Fig. 3. Here we discuss the sensing mechanisms of gravitational loads in the trabecular bone and cortical bone.
Fig. 3. The structure of the long bone, cortical bone, and periosteum.
Osteocytes are embedded in the lacuna of the bone matrix and are connected with each other through dendrites surrounded by canaliculi. At the periphery of the bone, SSCs, and fibroblasts form the periosteum together with osteoblasts. Osteocytes and the periosteum are mechanical sensors in the bone tissue.
Mechanical sensing by osteocytes, a commander for osteoclasts and osteoblasts
Bone homeostasis is maintained as a result of the balanced action of osteoblasts for bone formation and osteoclasts for bone resorption69. In bone destructive diseases, such as osteoporosis, bone resorption is favored over bone formation, leading to bone loss. In particular, bone loss in unloading conditions, such as microgravity in space, is caused by enhanced bone resorption by osteoclasts and suppressed bone formation by osteoblasts.
Osteocytes, another type of bone cell, are differentiated from osteoblasts and embedded in the bone matrix have many dendrites formed through osteocytogenesis that communicate either with each other or with the osteoblasts and osteoclasts at the bone surface70. Osteocytes also have a critical role in bone homeostasis, functioning as a commander for osteoclasts and osteoblasts by regulating the expression of genes involved in the receptor activator of nuclear factor-B ligand (RANKL), an osteoclast differentiation factor, and as a negative regulator of osteoblast differentiation by sclerostin (Sost)71. Importantly, expressions of these genes vary in response to mechanical loading or unloading to osteocytes in the bone. Osteocyte cell body and dendrites reside in the lacunae and canaliculi, respectively, and mechanical loading induces minute changes in the structure of the bone that generate interstitial fluid flow in the lacuno-canalicular system. This flow acts as a mechanical loading, similar as pressure and shear stress, and affects osteocytes directly72.
Such mechanical loadings to osteocytes as described above can activate mechanotransduction mediators such as ion channels, connexins, integrins, and cytoskeleton-related molecules73. In addition, the cytosolic signaling adapter protein p130Cas, a cellular mechanosensing molecule74, is involved in the regulation of bone homeostasis in response to mechanical loading in osteocytes75. Interestingly, p130Cas translocates into the nucleus and negatively regulates NF-κB activity to suppress bone resorption by downregulating the expression of RANKL. These findings suggest that the p130Cas- NF-κB axis in osteocytes is a potential target for treatment against disuse osteoporosis.
Although the critical significance of mechanical loading to the bone has been clearly elucidated, a large portion of the molecular mechanisms underlying the mechanical regulation of bone homeostasis is not understood. Efforts to clarify these mechanisms will be a promising strategy to prevent bone loss during future space missions.
Periosteum might sense physical loading
The deterioration of the bone microarchitecture during spaceflight occurs not only in the trabecular bone but also in the cortical bone76. It is thought to be triggered by enhanced osteoclast-mediated bone resorption at the endocortical surface and suppressed bone-forming activity in the periosteum. The periosteum, the highly vascularized outer membrane that covers all bones except for joints, generates the cortical bone in physiological and pathological situations through the provision of osteoblasts77. The periosteum contains two layers: an outer layer of fibroblasts and an inner layer composed of bone-forming osteoblasts. Although the periosteum is not highly sensitive to mechanical loading compared to the endocortical surface, it nonetheless responds to loading and gives rise to bones in a variety of animal models. The unloading model of the hind limb reduces bone formation in the cortical bone as well as in the trabecular bone78. Conversely, periosteal bone formation is stimulated by enhanced loading using in vivo models of axial loading and three-point bending79,80. The alteration of gene expression patterns and cell morphologies within the periosteum after loading provides evidence that periosteal cells sense loading stimuli80,81.
Mechanical loading is possibly translated into bone formation through the periosteal skeletal stem cell
Among the cells responsible for sensing physical loading is skeletal stem cell (SSC) because loading-induced bone formation requires activation of the periosteal SSC to give rise to osteoblasts. The periosteal SSC displays a unique gene expression pattern and exhibits high regenerative capacity in response to bone injury when compared to bone marrow skeletal stem cells (BMSC)82. A recent study has revealed that Cathepsin K (CTSK)-lineage populations within the periosteum contain postnatal self-renewing and multipotent stem cells83. The deletion of Osterix, encoded by the Sp7 gene, in CTSK-lineage cells results in impaired bone formation and fracture healing83. Furthermore, Prx1-lineage mesenchymal cells that contain SSC sense loading stimuli through their primary cilia indicate loading-dependent bone formation78,82. More recently, Nestin+ and Leptin+ cells have been shown to generate osteoblasts for periosteal bone formation84. On the other hand, some studies have shown that loading alters gene expression patterns, including extracellular molecules in osteoblasts85. Thus, the osteoblast may function as a mechanotransducer that induces osteogenic differentiation of SSCs in the periosteum. Accordingly, direct or indirect loading can activate several types of SSC to induce cortical bone formation.
Gravity sensing in muscles
Muscle atrophy is another major health problem in life in space and involves the decrease of muscle mass in response to the reduction of hemodynamic loads. It is known to be caused by microgravity, long term bed rest, and cancer cachexia86. Unloading-induced muscle wasting is mediated by a decrease of protein synthesis in the homeostasis of muscle cells and an increase of catabolism. Consequently, there must be a molecule that senses and transduces the signals originating from mechanical loading. One of the candidates for such a load transducer is a nonselective cation channel, the canonical transient receptor potential channel (TRPC). Members of the TRPC channels, namely, TRPC1, TRPC3, and TRPC6, are reportedly activated downstream of mechanical signals in addition to phospholipase C-coupled cell surface receptor activation87. TRPC channels play important roles in the activation of protein phosphatase calcineurin (CaN). CaN regulates the Ca2+-dependent transcription factor, the nuclear factor of activated T cells (NFAT), and the peroxisome proliferator-activated receptor γ88. Both proteins are important for myogenesis. Exposure of C2C12 skeletal myoblasts to microgravity induces the reduction of TRPC1 expression, which arrests the cell cycle at the G2/M phase, thereby inhibiting myoblast proliferation89. The importance of TRPC1 has also been demonstrated in muscle regrowth after unloading-induced atrophy. Hind limb unloading induces the reduction of TRPC1 expression, which persists even after reloading90. The expression of the TRPC3 channel is also suppressed at complete atrophy and in the early recovered phase90. These changes in the expression of the TRPC1 and TRPC3 channels are consistent with the muscle mass, suggesting that these channels play important roles in load-dependent muscle growth.
It is widely accepted that oxidative stresses caused by the aberrant production of reactive oxygen species (ROS) or reactive nitrogen species are key regulators for catabolic muscle wasting91. ROS are produced as a byproduct of the mitochondrial respiratory chain or are produced enzymatically by NADPH oxidases (NOX) within the cell. In cardiac muscles, ROS production by the NOX2 protein is physiologically important for Ca2+ homeostasis and is activated mechanically during diastole92. However, in pathological conditions, NOX2-mediated ROS production causes cardiac remodeling in response to various stresses. It has also been noted that pathological situations in muscle tissue can engender abnormal Ca2+ signaling. Since some NOX isoforms require Ca2+ for activation, it is plausible that there exists a crosstalk between pathological NOX activation and abnormal Ca2+ signaling. TRPC3 and NOX2 proteins exist at this crossroads of signaling pathways. Additionally, it has been demonstrated that the TRPC3 channels play an important role in NOX2 protein stabilization by protecting them from proteasomal degradation93. ROS production mediated by TRPC3 and NOX2 coupling causes cardiac muscle atrophy in stressed hearts, in which the hemodynamic load is reduced94,95. Therefore, TRPC channels might have dual roles in unloading-induced muscle atrophy: the first is the regulation of myoblast proliferation via CaN activation, and the second is the production of ROS, which induces catabolic remodeling of muscle tissue.
Gravity sensing in mesenchymal stem cells
Mesenchymal stem cells (MSCs) are crucial in the field of regenerative medicine by virtue of their self-renewal and multi-differentiation potentials96. MSC self-renewal and differentiation are known to be controlled by a diverse set of soluble factors, including growth factors or cytokines. In addition, the fate of MSCs has been shown to be influenced by mechanical stresses or surrounding physical microenvironments, such as substrate stiffness97, or changes in gravity. Many space experiments and ground-based studies have demonstrated that MSCs are very sensitive to the modulation of gravitational stimuli and exhibit various responses against such effects98. The exposure of MSCs to microgravity or simulated microgravity induces characteristic physiological responses, including remodeling of the cytoskeleton and the disruption of the stress fiber99,100, reduced activity in transcriptional coactivator YAP/TAZ68, suppression of osteoblastic differentiation, and the promotion of adipogenesis101,102, some of which were also observed in other nonspecialized animal cells.
How can MSCs sense a microgravity environment? Ordinary mechanical forces, including stretch or shear stress, can be sensed by animal cells through cell mechanosensors that convert mechanical stimuli into electrical or chemical signals. To date, mechanosensitive channels, focal adhesion proteins (p130Cas and Talin), and actin fibers have been established to function as mechanosensors for various types of cells. It has been postulated that MSCs also utilize these common sensors to detect changes in gravity, since MSCs have no specific gravity sensors, as is the case for organs such as the animal gravity sensor statocyst. Recent studies have proposed that the cytoskeleton may function as an initial sensor for microgravity103. In the early phase (30 min to 6 h) of exposure to microgravity, environmental changes experienced by the cytoskeleton have been observed, including a reduced amount or thinning of stress fibers103 (unpublished data in Fig. 4) and the redistribution of microtubules. In addition, genetic restoration of the arrangement of actin fibers or the pharmacological stabilization of actin cytoskeleton could maintain the osteogenic differentiation of MSCs under modeled microgravity68,99. This indicates that changes in the actin cytoskeleton in the cells transferred under microgravity conditions could have a crucial role in cellular responses against changed gravity. However, it remains unclear if the cytoskeleton acts as an initial and primary mechanosensor for gravity sensing103. It has been proposed that the loss of gravitational forces acting on heavy organelles, including the nucleus and mitochondria, could affect the cytoskeleton. Further studies will provide deeper insight regarding gravity sensing and transduction.
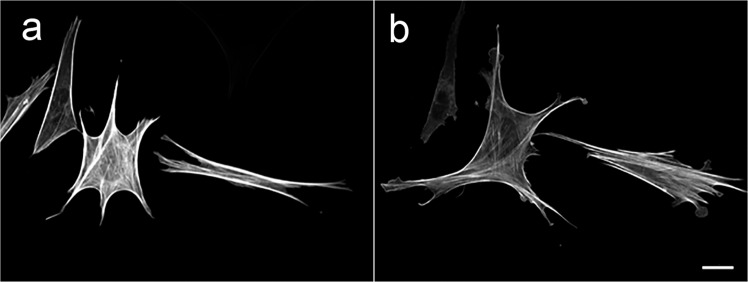
Fig. 4. Stress fiber remodeling in MSCs exposed to simulated microgravity as analyzed by confocal fluorescence microscopy.
MSCs expressing Lifeact-TagGFP2 contained thick stress fibers under 1 G conditions (a), whereas an exposure to simulated microgravity for 6 h led to the appearance of thinner stress fibers (b). The images in (a) and (b) showing the same field of view, were recorded, processed and presented in an identical condition (Kobayashi, unpublished). Scale bar: 20 μm.
Gravity sensitivity of the cell cycle
Regulation of the cell cycle is crucial for the maintenance of organs, such as bones and muscles. Technological innovation in bio-imaging has recently used fluorescent proteins, and we advocate real-time, single-cell imaging techniques to accurately and comprehensively dissect molecular and cellular principles and explain natural and sample-originated heterogeneity in biology104. For example, our fluorescent, ubiquitination-based cell cycle indicator (Fucci) technology harnesses the cell-cycle-dependent proteolysis of Cdt1 and Geminin fused to fluorescent proteins of different colors105,106. Although considerable progress has been made toward understanding the mechanisms of cell cycle progression on Earth107, much less is known about how cell proliferation is affected by microgravity in outer space, where humans will live in the future.
As discussed above, regulation of the cell cycle is crucial for growth and maintenance of organs. To directly understand the precise molecular mechanisms of how individual cells in organs respond to microgravity, several simulated microgravity experiments have been performed on the ground using cultured cells. The relationship between gravity force and cell cycle progression has been reviewed108, but remains controversial. Because individual studies use different experimental setups and different types of cells, discrepancies in results might arise inevitably. The 3D-clinorotation system provides time-averaged simulated microgravity as an alternative to real microgravity conditions109, and the effects of microgravity on living cells have been studied using this device. For example, cultured cells were exposed to microgravity for several days. Cells were seeded in 25 cm2 flasks or on a DCC (disposable cell culture) plate110, which were then fully filled with a CO2-equilibrated medium and accommodated on the 3D-clinorotation device. After exposure, the number of cells was quantified to determine whether microgravity inhibits the proliferation of cells.
To visualize the progression of the cell cycle of cultured cells in real time at the single-cell level during exposure to microgravity, we are currently developing a 3D-clinorotation 2D-microscopy system. The system accommodates a portable fluorescence microscope that we invented on the basis of a smartphone and a DCC-G (glass-based DCC110) plate for fluorescence observation. This system can be used in the cell biology experiment facility of the ISS. We have already generated a variety of human cell lines that constitutively express Fucci probes. Hopefully, this will enable us to better understand how cells behave in outer space.
Conclusion
Response to gravity is a cellular process of mechanotransduction in both plants and animals. Interestingly, although plants and animals seem to be very genetically distant, they share common mechanisms for gravity sensing, e.g., an actin cytoskeleton and mechanosensitive ion channels combined to this skeleton. On the other hand, animals evolved unique systems for gravisensing as exemplified by the transcriptional coactivator YAP/TAZ, which affects the cell fate of bones, muscles, and stem cells. Knowledge derived from extensive studies of gravisensing will contribute to medicine on Earth, e.g., understanding osteoporosis, muscle atrophy, and cancer biology. However, in the present age of preparation for human space exploration and colonization, or example through the Moon base and Mars exploration, understanding cellular responses to gravity will form the foundations of living in space.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research on Innovative Areas [No. 15H05935, 15H05936].
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Data availability
All data are available in the main text.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ruden DM, et al. Effects of gravity, microgravity or microgravity simulation on early mammalian development. Stem Cells Dev. 2018;27:1230–1236. doi: 10.1089/scd.2018.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrasek J, Friml J. Auxin transport routes in plant development. Development. 2009;136:2675–2688. doi: 10.1242/dev.030353. [DOI] [PubMed] [Google Scholar]
- 3.Vico L, et al. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355:1607–1611. doi: 10.1016/S0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 4.Vandenburgh H, Chromiak J, Shansky J, Del Tatto M, Lemaire J. Space travel directly induces skeletal muscle atrophy. FASEB J. 1999;13:1031–1038. doi: 10.1096/fasebj.13.9.1031. [DOI] [PubMed] [Google Scholar]
- 5.Baisden DL, et al. Human health and performance for long-duration spaceflight. Aviat. Space Environ. Med. 2008;79:629–635. doi: 10.3357/ASEM.2314.2008. [DOI] [PubMed] [Google Scholar]
- 6.Sehlke A, et al. Requirements for portable instrument suites during human scientific exploration of Mars. Astrobiology. 2019;19:401–425. doi: 10.1089/ast.2018.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavy M, Estelle M. Mechanisms of auxin signaling. Development. 2016;143:3226–3229. doi: 10.1242/dev.131870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paponov IA, Teale WD, Trebar M, Blilou I, Palme K. The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends Plant Sci. 2005;10:170–177. doi: 10.1016/j.tplants.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 9.van Berkel K, de Boer RJ, Scheres B, ten Tusscher K. Polar auxin transport: models and mechanisms. Development. 2013;140:2253–2268. doi: 10.1242/dev.079111. [DOI] [PubMed] [Google Scholar]
- 10.Spalding EP. Diverting the downhill flow of auxin to steer growth during tropisms. Am. J. Bot. 2013;100:203–214. doi: 10.3732/ajb.1200420. [DOI] [PubMed] [Google Scholar]
- 11.Geisler M, Aryal B, di Donato M, Hao P. A critical view on ABC transporters and their interacting partners in auxin transport. Plant Cell Physiol. 2017;58:1601–1614. doi: 10.1093/pcp/pcx104. [DOI] [PubMed] [Google Scholar]
- 12.Kiss JZ. Mechanisms of the early phases of plant gravitropism. CRC Crit. Rev. Plant Sci. 2000;19:551–573. doi: 10.1080/07352680091139295. [DOI] [PubMed] [Google Scholar]
- 13.Went, F. W. & Thimann, K. V. Phytohormones. (The Macmillan Company, 1937).
- 14.Muday GK. Auxins and tropisms. J. Plant Growth Regul. 2001;20:226–243. doi: 10.1007/s003440010027. [DOI] [PubMed] [Google Scholar]
- 15.Muller A, et al. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 17.Kamada M, et al. Control of gravimorphogenesis by auxin: accumulation pattern of CS-IAA1 mRNA in cucumber seedlings grown in space and on the ground. Planta. 2000;211:493–501. doi: 10.1007/s004250000321. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe C, et al. Gravistimulation changes the accumulation pattern of the CsPIN1 auxin efflux facilitator in the endodermis of the transition zone in cucumber seedlings. Plant Physiol. 2012;158:239–251. doi: 10.1104/pp.111.188615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamazaki C, et al. The gravity-induced re-localization of auxin efflux carrier CsPIN1 in cucumber seedlings: spaceflight experiments for immunohistochemical microscopy. NPJ Microgravity. 2016;2:16030. doi: 10.1038/npjmgrav.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleine-Vehn J, et al. PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis. Plant Cell. 2009;21:3839–3849. doi: 10.1105/tpc.109.071639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zourelidou, M. et al. Auxin efflux by PIN-FORMED proteins is activated by two different protein kinases, D6 PROTEIN KINASE and PINOID. Elife10.7554/eLife.02860 (2014). [DOI] [PMC free article] [PubMed]
- 22.Ambrose C, et al. CLASP interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana. Dev. Cell. 2013;24:649–659. doi: 10.1016/j.devcel.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Yoshihara T, Spalding EP. LAZY genes mediate the effects of gravity on auxin gradients and plant architecture. Plant Physiol. 2017;175:959–969. doi: 10.1104/pp.17.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taniguchi M, et al. The Arabidopsis LAZY1 family plays a key role in gravity signaling within statocytes and in branch angle control of roots and shoots. Plant Cell. 2017;29:1984–1999. doi: 10.1105/tpc.16.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshihara, T. & Spalding, E. P. Switching the direction of stem gravitropism by altering two amino acids in AtLAZY1. Plant Physiol. 10.1104/pp.19.01144 (2019). [DOI] [PMC free article] [PubMed]
- 26.Furutani M, et al. Polar recruitment of RLD by LAZY1-like protein during gravity signaling in root branch angle control. Nat. Commun. 2020;11:76. doi: 10.1038/s41467-019-13729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferl RJ, Paul AL. The effect of spaceflight on the gravity-sensing auxin gradient of roots: GFP reporter gene microscopy on orbit. NPJ Microgravity. 2016;2:15023. doi: 10.1038/npjmgrav.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda J, et al. Growth and development, and auxin polar transport in higher plants under microgravity conditions in space: BRIC-AUX on STS-95 space experiment. J. Plant Res. 1999;112:487–492. doi: 10.1007/PL00013904. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto K, et al. Polar auxin transport is essential to maintain growth and development of etiolated pea and maize seedlings grown under 1g conditions: Relevance to the international space station experiment. Life Sci. Space Res. (Amst.) 2019;20:1–11. doi: 10.1016/j.lssr.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Kamada M, et al. Gravity-regulated localization of PsPIN1 is important for polar auxin transport in etiolated pea seedlings: Relevance to the International Space Station experiment. Life Sci. Space Res. (Amst.) 2019;22:29–37. doi: 10.1016/j.lssr.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Oka, M. et al. Altered localisation of ZmPIN1a proteins in plasma membranes responsible for enhanced-polar auxin transport in etiolated maize seedlings under microgravity conditions in space. Funct. Plant Biol. 10.1071/FP20133 (2020). [DOI] [PubMed]
- 32.Morita MT. Directional gravity sensing in gravitropism. Annu. Rev. Plant Biol. 2010;61:705–720. doi: 10.1146/annurev.arplant.043008.092042. [DOI] [PubMed] [Google Scholar]
- 33.Toyota M, Gilroy S. Gravitropism and mechanical signaling in plants. Am. J. Bot. 2013;100:111–125. doi: 10.3732/ajb.1200408. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura M, Nishimura T, Morita MT. Gravity sensing and signal conversion in plant gravitropism. J. Exp. Bot. 2019;70:3495–3506. doi: 10.1093/jxb/erz158. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura M, Toyota M, Tasaka M, Morita MT. Live cell imaging of cytoskeletal and organelle dynamics in gravity-sensing cells in plant gravitropism. Methods Mol. Biol. 2015;1309:57–69. doi: 10.1007/978-1-4939-2697-8_6. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura M, Toyota M, Tasaka M, Morita MT. An Arabidopsis E3 ligase, SHOOT GRAVITROPISM9, modulates the interaction between statoliths and F-actin in gravity sensing. Plant Cell. 2011;23:1830–1848. doi: 10.1105/tpc.110.079442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toyota M, et al. Amyloplast displacement is necessary for gravisensing in Arabidopsis shoots as revealed by a centrifuge microscope. Plant J. 2013;76:648–660. doi: 10.1111/tpj.12324. [DOI] [PubMed] [Google Scholar]
- 38.Zou JJ, et al. The role of Arabidopsis Actin-Related Protein 3 in amyloplast sedimentation and polar auxin transport in root gravitropism. J. Exp. Bot. 2016;67:5325–5337. doi: 10.1093/jxb/erw294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perbal G, Driss-Ecole D. Mechanotransduction in gravisensing cells. Trends Plant Sci. 2003;8:498–504. doi: 10.1016/j.tplants.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Tatsumi H, et al. Mechanosensitive channels are activated by stress in the actin stress fibres, and could be involved in gravity sensing in plants. Plant Biol. 2014;16(Suppl 1):18–22. doi: 10.1111/plb.12095. [DOI] [PubMed] [Google Scholar]
- 41.Toyota, M., Furuichi, T. & Iida, H. Molecular mechanisms of mechanosensing and mechanotransduction. In: Plant Biomechanics. (eds. Geitmann A., Gril J.) Springer International Publishing AG, 375–397 (2018).
- 42.Toyota M, Furuichi T, Tatsumi H, Sokabe M. Cytoplasmic calcium increases in response to changes in the gravity vector in hypocotyls and petioles of Arabidopsis seedlings. Plant Physiol. 2008;146:505–514. doi: 10.1104/pp.107.106450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toyota M, Furuichi T, Sokabe M, Tatsumi H. Analyses of a gravistimulation-specific Ca2+ signature in Arabidopsis using parabolic flights. Plant Physiol. 2013;163:543–554. doi: 10.1104/pp.113.223313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pouliquen O, et al. A new scenario for gravity detection in plants: the position sensor hypothesis. Phys. Biol. 2017;14:035005. doi: 10.1088/1478-3975/aa6876. [DOI] [PubMed] [Google Scholar]
- 45.Chauvet H, Pouliquen O, Forterre Y, Legue V, Moulia B. Inclination not force is sensed by plants during shoot gravitropism. Sci. Rep. 2016;6:35431. doi: 10.1038/srep35431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toyota M, Furuichi T, Tatsumi H, Sokabe M. Hypergravity stimulation induces changes in intracellular calcium concentration in Arabidopsis seedlings. Adv. Space Res. 2007;39:1190–1197. doi: 10.1016/j.asr.2006.12.012. [DOI] [Google Scholar]
- 47.Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr. Biol. 2008;18:730–734. doi: 10.1016/j.cub.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 48.Basu D, Haswell ES. Plant mechanosensitive ion channels: an ocean of possibilities. Curr. Opin. Plant Biol. 2017;40:43–48. doi: 10.1016/j.pbi.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JS, Wilson ME, Richardson RA, Haswell ES. Genetic and physical interactions between the organellar mechanosensitive ion channel homologs MSL1, MSL2, and MSL3 reveal a role for inter-organellar communication in plant development. Plant Direct. 2019;3:e00124. doi: 10.1002/pld3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakagawa Y, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl Acad. Sci. USA. 2007;104:3639–3644. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mori K, et al. Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Sci. Rep. 2018;8:550. doi: 10.1038/s41598-017-17483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hattori, T. et al. MCA1 and MCA2 Are Involved in the Response to Hypergravity in Arabidopsis Hypocotyls. Plants (Basel)10.3390/plants9050590 (2020). [DOI] [PMC free article] [PubMed]
- 53.Yuan F, et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature. 2014;514:367–371. doi: 10.1038/nature13593. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z, et al. Genetic analysis of a Piezo-like protein suppressing systemic movement of plant viruses in Arabidopsis thaliana. Sci. Rep. 2019;9:3187. doi: 10.1038/s41598-019-39436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furuichi T, Iida H, Sokabe M, Tatsumi H. Expression of Arabidopsis MCA1 enhanced mechanosensitive channel activity in the Xenopus laevis oocyte plasma membrane. Plant Signal Behav. 2012;7:1022–1026. doi: 10.4161/psb.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murthy, S. E. et al. OSCA/TMEM63 are an evolutionarily conserved family of mechanically activated ion channels. Elife10.7554/eLife.41844 (2018). [DOI] [PMC free article] [PubMed]
- 57.Maksaev G, Haswell ES. MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc. Natl Acad. Sci. USA. 2012;109:19015–19020. doi: 10.1073/pnas.1213931109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tran D, et al. A mechanosensitive Ca2+ channel activity is dependent on the developmental regulator DEK1. Nat. Commun. 2017;8:1009. doi: 10.1038/s41467-017-00878-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bizet F, et al. Both gravistimulation onset and removal trigger an increase of cytoplasmic free calcium in statocytes of roots grown in microgravity. Sci. Rep. 2018;8:11442. doi: 10.1038/s41598-018-29788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Porazinski S, et al. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature. 2015;521:217–221. doi: 10.1038/nature14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 62.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sudol M, et al. Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J. Biol. Chem. 1995;270:14733–14741. doi: 10.1074/jbc.270.24.14733. [DOI] [PubMed] [Google Scholar]
- 64.Kanai F, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sansores-Garcia L, et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30:2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirata H, et al. Actomyosin bundles serve as a tension sensor and a platform for ERK activation. EMBO Rep. 2015;16:250–257. doi: 10.15252/embr.201439140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Z, Luo Q, Lin C, Kuang D, Song G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells via depolymerizing F-actin to impede TAZ nuclear translocation. Sci. Rep. 2016;6:30322. doi: 10.1038/srep30322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seeman E, Martin TJ. Antiresorptive and anabolic agents in the prevention and reversal of bone fragility. Nat. Rev. Rheumatol. 2019;15:225–236. doi: 10.1038/s41584-019-0172-3. [DOI] [PubMed] [Google Scholar]
- 70.Bonewald LF. The amazing osteocyte. J. Bone Min. Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atkins GJ, Findlay DM. Osteocyte regulation of bone mineral: a little give and take. Osteoporos. Int. 2012;23:2067–2079. doi: 10.1007/s00198-012-1915-z. [DOI] [PubMed] [Google Scholar]
- 72.Yavropoulou MP, Yovos JG. The molecular basis of bone mechanotransduction. J. Musculoskelet. Neuronal Interact. 2016;16:221–236. [PMC free article] [PubMed] [Google Scholar]
- 73.Wittkowske C, Reilly GC, Lacroix D, Perrault CM. In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front Bioeng. Biotechnol. 2016;4:87. doi: 10.3389/fbioe.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sawada Y, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miyazaki T, et al. Mechanical regulation of bone homeostasis through p130Cas-mediated alleviation of NF-kappaB activity. Sci. Adv. 2019;5:eaau7802. doi: 10.1126/sciadv.aau7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sibonga JD, Spector ER, Johnston SL, Tarver WJ. Evaluating Bone Loss in ISS Astronauts. Aerosp. Med. Hum. Perform. 2015;86:A38–A44. doi: 10.3357/AMHP.EC06.2015. [DOI] [PubMed] [Google Scholar]
- 77.Rauch F. Bone growth in length and width: the Yin and Yang of bone stability. J. Musculoskelet. Neuronal Interact. 2005;5:194–201. [PubMed] [Google Scholar]
- 78.Moore ER, Zhu YX, Ryu HS, Jacobs CR. Periosteal progenitors contribute to load-induced bone formation in adult mice and require primary cilia to sense mechanical stimulation. Stem Cell Res. Ther. 2018;9:190. doi: 10.1186/s13287-018-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugiyama T, et al. Bones’ adaptive response to mechanical loading is essentially linear between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition. J. Bone Min. Res. 2012;27:1784–1793. doi: 10.1002/jbmr.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakai D, et al. Remodeling of actin cytoskeleton in mouse periosteal cells under mechanical loading induces periosteal cell proliferation during bone formation. PLoS ONE. 2011;6:e24847. doi: 10.1371/journal.pone.0024847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raab-Cullen DM, Thiede MA, Petersen DN, Kimmel DB, Recker RR. Mechanical loading stimulates rapid changes in periosteal gene expression. Calcif. Tissue Int. 1994;55:473–478. doi: 10.1007/BF00298562. [DOI] [PubMed] [Google Scholar]
- 82.Duchamp de Lageneste O, et al. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat. Commun. 2018;9:773. doi: 10.1038/s41467-018-03124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Debnath S, et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562:133–139. doi: 10.1038/s41586-018-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao B, et al. Macrophage-lineage TRAP+ cells recruit periosteum-derived cells for periosteal osteogenesis and regeneration. J. Clin. Invest. 2019;129:2578–2594. doi: 10.1172/JCI98857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen X, et al. Cyclic compression stimulates osteoblast differentiation via activation of the Wnt/beta-catenin signaling pathway. Mol. Med. Rep. 2017;15:2890–2896. doi: 10.3892/mmr.2017.6327. [DOI] [PubMed] [Google Scholar]
- 86.Bajotto G, Shimomura Y. Determinants of disuse-induced skeletal muscle atrophy: exercise and nutrition countermeasures to prevent protein loss. J. Nutr. Sci. Vitaminol. (Tokyo) 2006;52:233–247. doi: 10.3177/jnsv.52.233. [DOI] [PubMed] [Google Scholar]
- 87.Numaga-Tomita T, et al. TRPC channels in exercise-mimetic therapy. Pflug. Arch. 2019;471:507–517. doi: 10.1007/s00424-018-2211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang KC. Key signalling factors and pathways in the molecular determination of skeletal muscle phenotype. Animal. 2007;1:681–698. doi: 10.1017/S1751731107702070. [DOI] [PubMed] [Google Scholar]
- 89.Benavides Damm T, et al. Calcium-dependent deceleration of the cell cycle in muscle cells by simulated microgravity. FASEB J. 2013;27:2045–2054. doi: 10.1096/fj.12-218693. [DOI] [PubMed] [Google Scholar]
- 90.Zhang BT, Yeung SS, Cheung KK, Chai ZY, Yeung EW. Adaptive responses of TRPC1 and TRPC3 during skeletal muscle atrophy and regrowth. Muscle Nerve. 2014;49:691–699. doi: 10.1002/mus.23952. [DOI] [PubMed] [Google Scholar]
- 91.Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35:411–429. doi: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- 92.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 93.Kitajima N, et al. TRPC3 positively regulates reactive oxygen species driving maladaptive cardiac remodeling. Sci. Rep. 2016;6:37001. doi: 10.1038/srep37001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sudi SB, et al. TRPC3-Nox2 axis mediates nutritional deficiency-induced cardiomyocyte atrophy. Sci. Rep. 2019;9:9785. doi: 10.1038/s41598-019-46252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shimauchi, T. et al. TRPC3-Nox2 complex mediates doxorubicin-induced myocardial atrophy. JCI Insight.10.1172/jci.insight.93358 (2017). [DOI] [PMC free article] [PubMed]
- 96.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 97.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 98.Ulbrich C, et al. The impact of simulated and real microgravity on bone cells and mesenchymal stem cells. Biomed. Res. Int. 2014;2014:928507. doi: 10.1155/2014/928507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meyers VE, Zayzafoon M, Douglas JT, McDonald JM. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J. Bone Min. Res. 2005;20:1858–1866. doi: 10.1359/JBMR.050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gershovich PM, Gershovich JG, Buravkova LB. Cytoskeleton structure and adhesion properties of human stromal precursors under conditions of simulated microgravity. Cell Tissue Biol. 2009;3:423. doi: 10.1134/S1990519X09050046. [DOI] [PubMed] [Google Scholar]
- 101.Nishikawa M, et al. The effect of simulated microgravity by three-dimensional clinostat on bone tissue engineering. Cell Transpl. 2005;14:829–835. doi: 10.3727/000000005783982477. [DOI] [PubMed] [Google Scholar]
- 102.Zayzafoon M, Gathings WE, McDonald JM. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology. 2004;145:2421–2432. doi: 10.1210/en.2003-1156. [DOI] [PubMed] [Google Scholar]
- 103.Vorselen D, Roos WH, MacKintosh FC, Wuite GJ, van Loon JJ. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J. 2014;28:536–547. doi: 10.1096/fj.13-236356. [DOI] [PubMed] [Google Scholar]
- 104.Miyawaki A, Niino Y. Molecular spies for bioimaging–fluorescent protein-based probes. Mol. Cell. 2015;58:632–643. doi: 10.1016/j.molcel.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 105.Sakaue-Sawano A, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 106.Sakaue-Sawano A, et al. Genetically Encoded Tools for Optical Dissection of the Mammalian Cell Cycle. Mol. Cell. 2017;68:626–640.e625. doi: 10.1016/j.molcel.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 107.Nurse P. A long twentieth century of the cell cycle and beyond. Cell. 2000;100:71–78. doi: 10.1016/S0092-8674(00)81684-0. [DOI] [PubMed] [Google Scholar]
- 108.Luna C, Yew AG, Hsieh AH. Effects of angular frequency during clinorotation on mesenchymal stem cell morphology and migration. NPJ Microgravity. 2015;1:15007. doi: 10.1038/npjmgrav.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Becker JL, Souza GR. Using space-based investigations to inform cancer research on Earth. Nat. Rev. Cancer. 2013;13:315–327. doi: 10.1038/nrc3507. [DOI] [PubMed] [Google Scholar]
- 110.Harada-Sukeno A, et al. “Myo Lab”: A JAXA Cell Biology Experiment in “Kibo (JEM)” of the International Space Station. Biol. Sci. Space. 2009;23:189–193. doi: 10.2187/bss.23.189. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the main text.