Sir,
The need for broad and accessible testing coverage has been recognised as one of the key elements to control the coronavirus disease 2019 (Covid-19) pandemic. The reverse transcriptase-polymerase chain reaction (RT-PCR) technique has been the gold standard for the diagnosis of Covid-19. However, it also represents a bottleneck for broadening testing policies, particularly in low-resource settings, because it depends on specialised laboratories, requires trained personnel and has relatively high turn-around times. In this regard, Chow et al. [1] proposed the pooling strategy to increase mass screening capacity, which is particularly necessary in low-income countries. Herein, we aim to present the importance of evaluating RT-PCR-sparing strategies to overcome testing shortages by deploying available point-of-care (PoC) tests.
In countries such as Brazil, RT-PCR testing for patients reliant on the public health system is concentrated in a few public laboratories and a network of additional support laboratories requisitioned in response to the ongoing crisis. Given the enormous demand for testing, results are often reported 1 week after sample collection or even later. This delay hinders adherence to self-isolation practices and the implementation of public-health measures to avoid transmission to close contacts [2].
Recently, PoC tests based on either viral antigen or RNA detection have been developed and evaluated, showing high specificity and sensitivity (although the latter has been more variable) [3–8]. Nonetheless, the sensitivity of these tests increases as Ct values decrease, (indicating higher viral loads), which is the situation most often seen in symptomatic patients. Additionally, these tests usually have a lower cost than RT-PCR, are easy to use and are scalable [4–8].
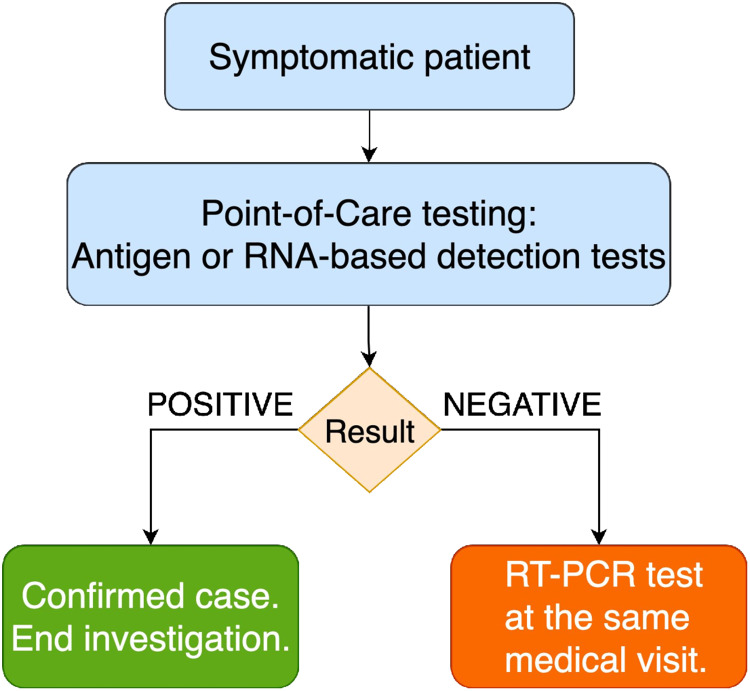
We consider that widespread use of available PoC tests (Fig. 1) should be urgently evaluated as an RT-PCR sparing strategy, with potential individual and public-health benefits, particularly in settings with high RT-PCR positivity rates. The benefits include possibility of diagnosis on initial presentation, which may facilitate adherence to self-isolation; faster notification of close contacts, allowing more easy and effective tracing; and, as patients receive appropriate medical guidance in a single visit, prevention of unnecessary mobility of symptomatic patients. Furthermore, public-health laboratories would face a lower demand for RT-PCR processing, which would likely decrease turnaround time. The disadvantage is the need for two swab collections at the same visit in patients with negative PoC tests.
Fig. 1.
Proposed diagnostic algorithm for symptomatic patients with suspected Covid-19 using an RT-PCR-sparing strategy based on point-of-care testing.
The use of PoC tests for detection of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) in symptomatic patients before RT-PCR may be a valuable strategy to overcome limited RT-PCR availability and mitigate its consequences. Studies modelling such strategies and assessing their implementation are urgently needed.
Conflict of interest
I declare no competing interests.
References
- 1.Chow WK and Chow CL (2021) A discussion on implementing pooling detection tests of novel coronavirus (SARS-CoV-2) for large population. Epidemiology & Infection 149, e17, 1–6. doi: 10.1017/S0950268820003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kretzschmar ME et al. (2020) Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. The Lancet Public Health 5, e452–e459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinnes J et al. (2020) Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database of Systematic Reviews 8, Cd013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler VL et al. (2021) A highly effective reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay for the rapid detection of SARS-CoV-2 infection. Journal of Infection 82, 117–125. doi: 10.1016/j.jinf.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howson ELA et al. (2021) Preliminary optimisation of a simplified sample preparation method to permit direct detection of SARS-CoV-2 within saliva samples using reverse-transcription loop-mediated isothermal amplification (RT-LAMP). Journal of Virological Methods 289, 114048. doi: 10.1016/j.jviromet.2020.114048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilarowski G et al. (2020) Field performance and public health response using the BinaxNOW TM rapid SARS-CoV-2 antigen detection assay during community-based testing. Clinical Infecttious Diseases, ciaa1890. doi: 10.1093/cid/ciaa1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strömer A et al. (2020) Performance of a point-of-care test for the rapid detection of SARS-CoV-2 antigen. Microorganisms 9, E58.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toptan T, et al. (2020) Evaluation of a SARS-CoV-2 rapid antigen test: potential to help reduce community spread? Journal of Clinical Virology 135, 104713. [DOI] [PMC free article] [PubMed] [Google Scholar]