Abstract
Diabetes is associated with cardiac metabolic disturbances and increased heart failure risk. Plasma fructose levels are elevated in diabetic patients. A direct role for fructose involvement in diabetic heart pathology has not been investigated. The goals of this study were to clinically evaluate links between myocardial fructose and sorbitol (a polyol pathway fructose precursor) levels with evidence of cardiac dysfunction, and to experimentally assess the cardiomyocyte mechanisms involved in mediating the metabolic effects of elevated fructose. Fructose and sorbitol levels were increased in right atrial appendage tissues of type 2 diabetic patients (2.8- and 1.5-fold increase respectively). Elevated cardiac fructose levels were confirmed in type 2 diabetic rats. Diastolic dysfunction (increased E/e’, echocardiography) was significantly correlated with cardiac sorbitol levels. Elevated myocardial mRNA expression of the fructose-specific transporter, Glut5 (43% increase), and the key fructose-metabolizing enzyme, Fructokinase-A (50% increase) was observed in type 2 diabetic rats (Zucker diabetic fatty rat). In neonatal rat ventricular myocytes, fructose increased glycolytic capacity and cytosolic lipid inclusions (28% increase in lipid droplets/cell). This study provides the first evidence that elevated myocardial fructose and sorbitol are associated with diastolic dysfunction in diabetic patients. Experimental evidence suggests that fructose promotes the formation of cardiomyocyte cytosolic lipid inclusions, and may contribute to lipotoxicity in the diabetic heart.
Subject terms: Cardiovascular diseases, Metabolism, Carbohydrates, Preclinical research
Introduction
Diabetes is associated with cardiomyocyte metabolic disturbances and cardiac dysfunction1. Population studies have associated diabetes and cardiovascular disease with escalating fructose consumption, but whether fructose plays a direct role remains controversial2,3. Experimentally, a high fructose diet induces cardiomyocyte metabolic and functional disturbances4–7. Although reported values of circulating fructose levels vary widely (5 µM–1.9 mM8,9), evidence suggests that plasma fructose is elevated in diabetic patients10. Myocardial tissue fructose levels in diabetic patients have not previously been investigated, and whether cardiomyocyte exposure to fructose contributes to cardiac pathology in diabetes is unknown. In type 1 diabetic rodent myocardium (streptozotocin-induced), upregulation of polyol pathway-mediated conversion of glucose to fructose has been demonstrated11. Thus, increased cardiomyocyte fructose levels in diabetes may derive from both circulating sources and endogenous production, but to date, rodent findings relating to polyol pathway disturbance have not been validated in the human context.
We have previously shown that the fructose-specific transporter GLUT5 is expressed in cardiomyocytes and provided evidence that fructose availability can modulate cardiomyocyte excitation-contraction coupling in vitro12. Additionally, fructose can directly impact structure and function of proteins via post-translational modifications which are both irreversible (advanced glycation end-products) and reversible (O-GlcNAcylation)4,13. The detrimental actions of fructose have been most well described in the liver, where increased fructose has been linked to lipid accumulation, ATP depletion, and insulin resistance14. Whilst pathologic impacts of fructose metabolism in the diabetic heart are yet to be characterized, some evidence of cardiac fructose involvement in heart failure progression in hypertrophic cardiomyopathy has been reported15.
The goals of this study were to characterize myocardial fructose levels in a clinical diabetic context, and to examine the cardiomyocyte metabolic consequences of fructose exposure using experimental approaches.
Methods
Fructose and sorbitol content was measured in right atrial appendage tissue from non-diabetic (ND) and type 2 diabetic (T2D) patients undergoing coronary artery bypass graft surgery, and cardiac fructose content and expression of fructose metabolic enzymes were assessed in left ventricle tissue of male Zucker diabetic fatty (ZDF) rats aged 20 weeks. All experiments were approved by the University of Auckland and the University of Otago Human and Animal Ethics Committees. Neonatal rat ventricular cardiomyocytes (NRVMs) were isolated from Sprague Dawley rats at days 1–2 and cultured in fructose (1 mM) or control (1 mM mannitol) conditions for 24 h prior to analysis for lipid inclusions (Oil Red O), protein expression (western blot) or metabolism (Seahorse XFp Bioanalyzer). Detailed methods are available in the online supplement.
Results
Increased cardiac fructose and sorbitol levels associated with diastolic dysfunction in diabetic patients
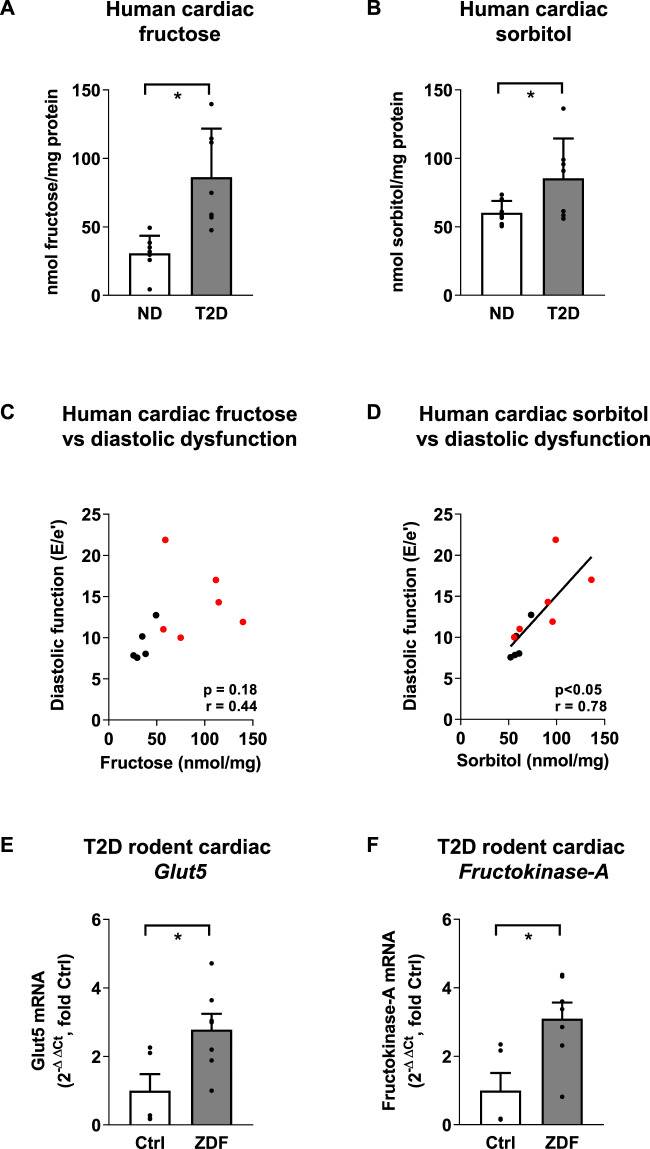
Non-diabetic and type 2 diabetic patients were clinically characterized, as shown in Table S1. Pre-surgical echocardiography revealed that left ventricular ejection fraction was preserved in both groups (T2D 56.0 ± 4.6% vs. ND 57.4 ± 5.8%). T2D patients exhibited diastolic dysfunction, evidenced by increased E/e’ (Table S1). Both cardiac fructose and sorbitol (polyol pathway intermediate) were markedly increased in right atrial appendage tissue from T2D patients (Fig. 1A, B, 2.8- and 1.5-fold increase respectively, T2D vs. ND, P < 0.05). Cardiac sorbitol levels and diastolic dysfunction were positively correlated (sorbitol vs. E/e’, Fig. 1D, r = 0.78, P < 0.05), suggesting involvement of endogenous cardiac fructose production in diastolic functional disturbance in T2D.
Fig. 1. Myocardial fructose metabolism in type 2 diabetes.
A, B Cardiac fructose and sorbitol levels are increased in human type 2 diabetic (T2D, n = 7) patient right atrial appendage samples relative to non-diabetic (ND, n = 8) patients. Mean ± SD. C Correlation of cardiac fructose levels and diastolic dysfunction (E/e’ ratio) in ND (black, n = 5) and T2D (red, n = 6) patients (r, Pearson correlation coefficient). D A significant positive correlation is evident between cardiac sorbitol levels and diastolic dysfunction (E/e’ ratio) for ND (black, n = 5) and diabetic (red, n = 6) patients (r, Pearson correlation coefficient). E, F Glut5 and Fructokinase-A mRNA expression is increased in T2D rat hearts (Zucker diabetic fatty rats (ZDF), n = 7; Controls, n = 5). Non-parametric Mann Whitney U test A, E, F, unpaired Students t-test (B), *p-value < 0.05, mean ± SEM.
Diabetic upregulation of expression of fructose-related genes in rat myocardium
Gene expression of the fructose-specific transporter, Glut5, and fructose metabolizing enzyme, Fructokinase-A (also termed ketohexokinase-A, phosphorylates fructose to fructose-1-phosphate), were evaluated in T2D rat hearts (Zucker Diabetic Fatty (ZDF) rat). Increased cardiac fructose levels were confirmed in ZDF rats (Fig. S1). Increases in mRNA expression levels of both Glut5 and Fructokinase-A were detected (Fig. 1E, F, 2.7- and 3.1-fold increase respectively, ZDF vs. control, P < 0.05). These findings suggest that cardiac fructose transport and metabolism are increased in diabetes.
Experimental evidence of fructose-derived cardiomyocyte glycolytic flux in vitro
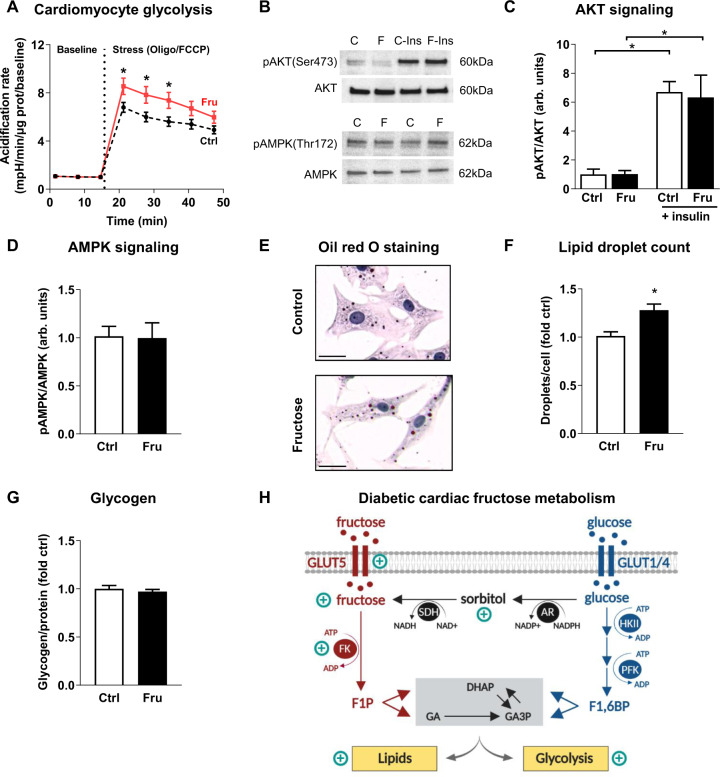
To obtain information regarding the involvement of glycolytic and mitochondrial metabolism in a more controlled setting, responses of NRVMs cultured in elevated fructose or mannitol (osmotic control) were investigated. In early development, cardiomyocyte glycolysis demand is accentuated, and NRVMs provide an optimal model system for evaluating hexose sugar metabolic impacts15. No difference in glycolysis between fructose and control incubated cardiomyocytes (extracellular acidification rate (ECAR)) was observed at baseline (Fig. 2A, fructose 1.0 ± 0.1 vs. control 1.1 ± 0.1 mpH/min/µg protein). In response to FCCP-Oligomycin induced depletion of mitochondrial-derived ATP, glycolysis (ECAR) increased to a greater extent in cardiomyocytes exposed to fructose than control (Fig. 2A, fructose: 8.5-fold increase, control: 6.8-fold increase, P < 0.05). No change in mitochondrial respiration was observed (Fig. S2A). Cardiomyocyte metabolic signaling via the canonical energy sensor pathways was unaffected by fructose exposure, indicated by no change in AKT and AMPK phosphorylation (Fig. 2B–D, Fig. S3). Collectively, these data suggest that fructose influence on glycolytic metabolism is consistent with direct fructose utilization as a glycolytic substrate, rather than secondary to altered energy signaling.
Fig. 2. Effect of fructose on cardiomyocyte glycolysis, energy signaling, lipid droplets, and glycogen content in vitro.
Neonatal rat ventricular myocytes were cultured for 24 h with 1 mM fructose (Fru) vs. control (Ctrl, 1 mM mannitol). A Increased glycolytic capacity with fructose was evident during the stress test (extracellular acidification rate normalized to protein & baseline). Oligo, oligomycin; FCCP, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone. n = 9 wells/group, 3 independent cultures. B Representative immunoblots for C, D (C, control; F, fructose; Ins, insulin). Images have been cropped for concise presentation. C Ratio of phosphorylated (Ser473) to total AKT was unchanged with fructose in baseline and insulin-stimulated conditions (1 µm insulin, 30 min). D Ratio of phosphorylated (Thr172) to total AMPK was unchanged with fructose. E Representative images of control and fructose cultured cardiomyocytes, oil red O-stained for assessment of lipids (high-intensity red lipid droplets show as near black in some instances, scale bar 20 µm). F Cardiomyocyte lipid droplet count normalized to cell number (estimated by nuclei count), is increased with fructose (n = 5–6 wells/group, 10–15 images per well, 2 independent cultures). G Glycogen content was similar in cardiomyocytes cultured in fructose and control media (n = 7–11 wells/group, 3 independent cultures). H Schematic representation of the cardiomyocyte consequences of high fructose levels in the diabetic heart. FK, fructokinase (also termed ketohexohinase); F1P, fructose-1-phosphate; DHAP, dihydroxyacetone phosphate; GA, glyceraldehyde; GA3P, glyceraldehyde-3-phosphate; HKII, hexokinase II; PFK, phosphofructokinase; F1,6BP, fructose-1,6-biphosphate; AR, aldose reductase; NADPH, reduced form of nicotinamide adenine dinucleotide phosphate (NADP + ); SDH, sorbitol dehydrogenase; NADH, reduced form of nicotinamide adenine dinucleotide (NAD). Schematic created with biorender.com. Repeated measures ANOVA with Bonferroni post-hoc tests (A), 2-way ANOVA with Bonferroni post-hoc tests (C), Mann Whitney U non-parametric test (F), Unpaired Students t-test (D, G), *p-value < 0.05, mean ± SEM.
Fructose increased cardiomyocyte lipid content but not glycogen content in vitro
Lipid accumulation is a key feature of diabetic cardiomyopathy and studies in non-cardiac cells have demonstrated that both lipids and glycogen can be derived from fructose16,17. NRVM histology showed that fructose exposure significantly increased cardiomyocyte cytosolic lipid droplet count (Fig. 2E, F, 28% increase, fructose vs. control, P < 0.05). This finding was confirmed in H9c2 rat cardiomyoblast cell line using spectrophotometric quantification of Nile Red lipid droplet staining (Fig. S2B). In contrast, fructose exposure did not influence glycogen stores in NRVMs (Fig. 2G).
Discussion
This study is the first to demonstrate a clinical link between elevated myocardial fructose and sorbitol levels, and cardiac dysfunction in diabetes. Experimental exploration of the mechanisms involved revealed that myocardial tissues of a T2D rodent model exhibited upregulated gene expression of the Glut5 fructose transporter and the fructose-phosphorylating enzyme, Fructokinase-A. In vitro analyses showed that fructose exposure exerts direct cardiomyocyte metabolic influence, conferring increased glycolytic capacity associated with cardiomyocyte cytosolic lipid droplet accumulation. Collectively these findings indicate a pathogenic role for elevated fructose and sorbitol in diabetic heart disease.
Pathophysiology of endogenous fructose and sorbitol elevation in the diabetic heart
The extent of myocardial fructose elevation in the T2D diabetic patient cohort reported here is substantial, and demonstrates the clinical relevance of deranged fructose handling. In this heterogenous cohort of patients with limited sample size and variable medication status, a dramatic 2.8 fold increase in cardiac fructose content was observed. Previous reports of elevated myocardial fructose have been limited to rodent studies of pharmacologically induced or genetically produced diabetic conditions11,18. Our data show that cardiac fructose and sorbitol elevation occur in parallel, indicative of altered polyol pathway flux (i.e., conversion of glucose to sorbitol to fructose, Fig. 2H). Increased production of sorbitol via aldose reductase has been linked to cellular oxidative stress19 and advanced glycation end-product formation (methylglyoxyl)20. Clinical findings show that pharmacologic inhibition of the polyol pathway improves cardiac systolic function in diabetes21. Interestingly, our study found a significant correlation between cardiac sorbitol and diastolic dysfunction (increased E/e’) suggesting that upregulation of the polyol pathway intermediate (sorbitol) may have a specific pathophysiologic link relatively early in the progression of diabetic cardiomyopathy, even while systolic function is maintained. Diastolic dysfunction in diabetes is highly prevalent and the underlying mechanisms are not well understood. Potential contributors include increased cell death with fibrotic infiltration22,23, increased cardiomyocyte stiffness, and metabolic disturbance1.
Further work is required to understand the metabolic drivers of sorbitol elevation in the diabetic myocardium. Investigation into the influence of anti-diabetic medications on cardiac fructose uptake and/or production would be informative, and with large datasets it may be possible to systematically evaluate these possibilities. In a setting where cardiomyocyte glucose influx is restricted (by insulin insufficiency or resistance), sorbitol production directly from incoming glucose would be expected to be limited. A more complex metabolic derangement such as disruption in the localization of enzymatic complexes involved in glycolysis may favor sorbitol production in the diabetic cardiomyocyte.
Diabetes-induced upregulation of cardiac fructose-related genes
Sorbitol increase in the diabetic cardiomyocyte may also be a kinetic consequence of elevated trans-sarcolemmal GLUT5-mediated fructose influx suppressing sorbitol dehydrogenase activity. In the present study, increased cardiac mRNA expression of the fructose-specific transporter, Glut5, was evident in a setting of elevated cardiac fructose levels in T2D rat hearts. GLUT5 exhibits high specificity for fructose, with negligible capacity to transport glucose24. Thus GLUT5-mediated fructose transport is realistic even in a context of elevated blood glucose (e.g., diabetes). Given that GLUT5 expression can be positively regulated by its substrate24, it is feasible that increased cardiac fructose in diabetes may trigger upregulation of GLUT5 expression, and the extent of cardiomyocyte fructose influx or efflux may be determined by the (as yet undefined) sarcolemmal fructose concentration gradient. Fructokinase-A mRNA expression, the enzyme that catalyzes the conversion of fructose to fructose-1-phosphate, was also increased in rodent T2D hearts, consistent with an increased capacity for cardiomyocyte fructose metabolism in diabetes. Future studies using isotopic labeling of fructose may be informative for understanding the metabolic fate of fructose in the heart.
Metabolic consequences of elevated cardiomyocyte fructose
Previously, using adult cardiomyocytes in vitro, we demonstrated that fructose can be utilized as a fuel source, supporting contractility and Ca2+ handling12. In the present study, we have shown that cardiomyocyte fructose exposure increases glycolytic capacity without affecting key insulin and AMPK regulatory signals. This indicates that increased glycolytic capacity is related to the direct utilization of fructose as a glycolytic substrate. The effect of fructose on glycolysis was only evident under conditions of mitochondrial ATP inhibition (oligomycin), suggesting that fructose could be a potential glycolytic substrate in settings where aerobic metabolism is limited (e.g., hypoxia/ischemia), but is unlikely to contribute to glycolytic flux in ATP replete states. As fructose bypasses the glycolytic rate-limiting enzyme, phosphofructokinase, its metabolism can proceed in a relatively unregulated manner with the potential to increase lactate production and induce cellular acidosis. Whether fructose-derived glycolysis affects cellular ATP production, and can confer benefit or detriment in diabetic cardiomyocytes is yet to be determined.
In the present study, primary rat cardiomyocytes cultured in fructose exhibited an increase in the occurrence of cytosolic lipid inclusions, with no change in glycogen content. It can be speculated that cardiomyocyte fructose exposure may elicit lipid accumulation via direct production of lipids using fructose as a substrate (de novo lipogenesis or glyceroneogenesis) and/or via a fructose-induced signaling response promoting lipid production or inhibiting lipid breakdown. Given that myocardial lipid content has been shown to be an independent predictor of diastolic dysfunction in type 2 diabetic patients, fructose-induced lipids may have important implications for diabetic cardiomyopathy25. Further characterization of the cardiomyocyte metabolic fate of fructose is now required to probe the detailed mechanisms of fructose-associated cardiomyocyte lipid storage increase, potentially inducing lipotoxicity.
Conclusion
In conclusion, this is the first study to identify that myocardial fructose and sorbitol are elevated in T2D patients, and to demonstrate that elevated intracellular fructose production (polyol pathway) may be linked to clinically-detected diastolic dysfunction. In vitro mechanistic evidence suggests that fructose promotes increased cardiomyocyte cytosolic lipid inclusions. Taken together these in vivo and in vitro studies provide a compelling case for cardiac fructose involvement in cellular lipotoxicity and metabolic dysregulation in diabetic cardiomyopathy. These findings provide the impetus for more extensive interrogation of cardiac fructose and sorbitol metabolism in the etiology of diabetic cardiomyopathy.
Supplementary information
Acknowledgements
We acknowledge expert technical assistance from the Biomedical Imaging Research Unit at the University of Auckland, and Dr Praju Vikas Anekal for assistance with developing a custom ImageJ macro for lipid quantitation. The research was supported by funding from the Health Research Council of New Zealand (#16/300).
Author contributions
L.J.D., M.A., L.M.D.D., and K.M.M. conceived and designed the research. L.J.D., M.A., P.K., and X.L. performed the cell cultures, biochemical assays, histology, and molecular experiments. C.T.B. and R.R.L. provided the diabetic rodent samples. R.K., R.W.B, P.D., and I.V.H. provided the clinical samples and patient data. S.C. performed echocardiography analysis. L.J.D., M.A., P.K., X.L., L.M.D.D., and K.M.M. interpreted results of experiments. L.J.D., M.A., L.M.D.D., and K.M.M. drafted the manuscript. All authors approved the final version.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lorna J. Daniels, Marco Annandale
Supplementary information
The online version contains supplementary material available at 10.1038/s41387-021-00150-7.
References
- 1.Glatz JFC, Dyck JRB, Des Rosiers C. Cardiac adaptations to obesity, diabetes and insulin resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:1905–1907. doi: 10.1016/j.bbadis.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Stanhope KL. Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit. Rev. Clin. Lab. Sci. 2015;53:52–67. doi: 10.3109/10408363.2015.1084990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tappy L. Fructose-induced alterations of glucose and lipid homeostasis: progressive organ dysfunction leading to metabolic diseases or mere adaptive changes? Am. J. Clin. Nutr. 2020;111:244–245. doi: 10.1093/ajcn/nqz323. [DOI] [PubMed] [Google Scholar]
- 4.Delbridge LM, Benson VL, Ritchie RH, Mellor KM. Diabetic cardiomyopathy: the case for a role of fructose in disease etiology. Diabetes. 2016;65:3521–3528. doi: 10.2337/db16-0682. [DOI] [PubMed] [Google Scholar]
- 5.Mellor KM, Bell JR, Young MJ, Ritchie RH, Delbridge LM. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J. Mol. Cell Cardiol. 2011;50:1035–1043. doi: 10.1016/j.yjmcc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Mellor KM, Wendt IR, Ritchie RH, Delbridge LM. Fructose diet treatment in mice induces fundamental disturbance of cardiomyocyte Ca2+ handling and myofilament responsiveness. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H964–H972. doi: 10.1152/ajpheart.00797.2011. [DOI] [PubMed] [Google Scholar]
- 7.Szucs G, et al. Prediabetes induced by fructose-enriched diet influences cardiac lipidome and proteome and leads to deterioration of cardiac function prior to the development of excessive oxidative stress and cell damage. Oxid. Med. Cell Longev. 2019;2019:3218275. doi: 10.1155/2019/3218275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le MT, et al. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism. 2012;61:641–651. doi: 10.1016/j.metabol.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui H, et al. Direct spectrophotometric determination of serum fructose in pancreatic cancer patients. Pancreas. 2009;38:706–712. doi: 10.1097/MPA.0b013e3181a7c6e5. [DOI] [PubMed] [Google Scholar]
- 10.Kawasaki T, Akanuma H, Yamanouchi T. Increased fructose concentrations in blood and urine in patients with diabetes. Diabetes Care. 2002;25:353–357. doi: 10.2337/diacare.25.2.353. [DOI] [PubMed] [Google Scholar]
- 11.Kashiwagi A, et al. Increase in cardiac muscle fructose content in streptozotocin-induced diabetic rats. Metabolism. 1992;41:1041–1046. doi: 10.1016/0026-0495(92)90283-G. [DOI] [PubMed] [Google Scholar]
- 12.Mellor KM, et al. Fructose modulates cardiomyocyte excitation-contraction coupling and Ca2+ handling in vitro. PLoS ONE. 2011;6:e25204. doi: 10.1371/journal.pone.0025204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssens JV, et al. Cardiac troponins may be irreversibly modified by glycation: novel potential mechanisms of cardiac performance modulation. Sci. Rep. 2018;8:16084. doi: 10.1038/s41598-018-33886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masson S, Henriksen O, Stengaard A, Thomsen C, Quistorff B. Hepatic metabolism during constant infusion of fructose; comparative studies with 31P-magnetic resonance spectroscopy in man and rats. Biochim. Biophys. Acta. 1994;1199:166–174. doi: 10.1016/0304-4165(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 15.Mirtschink P, et al. HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease. Nature. 2015;522:444–449. doi: 10.1038/nature14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varma V, et al. Metabolic fate of fructose in human adipocytes: a targeted (13)C tracer fate association study. Metabolomics. 2015;11:529–544. doi: 10.1007/s11306-014-0716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciudad CJ, Carabaza A, Guinovart JJ. Glycogen synthesis from glucose and fructose in hepatocytes from diabetic rats. Arch. Biochem Biophys. 1988;267:437–447. doi: 10.1016/0003-9861(88)90049-5. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, et al. Polyol pathway and modulation of ischemia-reperfusion injury in Type 2 diabetic BBZ rat hearts. Cardiovasc. Diabetol. 2008;7:33. doi: 10.1186/1475-2840-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang WH, et al. Cardiac contractile dysfunction during acute hyperglycemia due to impairment of SERCA by polyol pathway-mediated oxidative stress. Am. J. Physiol. Cell Physiol. 2010;299:C643–C653. doi: 10.1152/ajpcell.00137.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Wang R, Desai K, Wu L. Upregulation of aldolase B and overproduction of methylglyoxal in vascular tissues from rats with metabolic syndrome. Cardiovasc. Res. 2011;92:494–503. doi: 10.1093/cvr/cvr239. [DOI] [PubMed] [Google Scholar]
- 21.Johnson BF, et al. Cardiac abnormalities in diabetic patients with neuropathy: effects of aldose reductase inhibitor administration. Diabetes Care. 2004;27:448–454. doi: 10.2337/diacare.27.2.448. [DOI] [PubMed] [Google Scholar]
- 22.Lamberts RR, et al. Impaired relaxation despite upregulated calcium-handling protein atrial myocardium from type 2 diabetic patients with preserved ejection fraction. Cardiovasc. Diabetol. 2014;13:72. doi: 10.1186/1475-2840-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rawal S, et al. Down-regulation of miR-15a/b accelerates fibrotic remodelling in the Type 2 diabetic human and mouse heart. Clin. Sci. (Lond.) 2017;131:847–863. doi: 10.1042/CS20160916. [DOI] [PubMed] [Google Scholar]
- 24.Douard V, Ferraris RP. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 2008;295:E227–E237. doi: 10.1152/ajpendo.90245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rijzewijk LJ, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2008;52:1793–1799. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.