Abstract
Background:
Data on influenza burden in risk groups for severe influenza are important to guide targeted influenza immunization, especially in resource limited settings. However, this information is limited overall and in particular in low- and middle-income countries. We sought to assess the mean annual national burden of medically and non-medically attended influenza-associated mild, severe-non-fatal and fatal illness among potential target groups for influenza immunization in South Africa during 2013–2015.
Methods:
We used published mean national annual estimates of mild, severe-non-fatal, and fatal influenza-associated illness in South Africa during 2013–2015 and estimated the number of such illnesses occurring among the following risk groups: (i) children aged 6–59 months; (ii) individuals aged 5–64 years with HIV, and/or pulmonary tuberculosis (PTB), and/or selected underlying medical conditions (UMC); (iii) pregnant women; and (iv) individuals aged ≥65 years. We also estimated the number of individuals among the same risk groups in the population.
Results:
During 2013–2015, individuals in the selected risk groups accounted for 45.3% (24,569,328/54,086,144) of the population and 43.5% (4,614,763/10,598,138), 86.8% (111,245/128,173) and 94.5% (10,903/11,536) of the mean annual estimated number of influenza-associated mild, severe-non-fatal and fatal illness episodes, respectively. The rates of influenza-associated illness were highest in children aged 6–59 months (23,983 per 100,000 population) for mild illness, in pregnant women (930 per 100,000 population) for severe-non-fatal illness and in individuals aged ≥65 years (138 per 100,000 population) for fatal illness.
Conclusion:
Influenza immunization of the selected risk groups has the potential to prevent a substantial number of influenza-associated severe illness. Nonetheless, because of the high number of individuals at risk, South Africa, due to financial resources constrains, may need to further prioritize interventions among risk populations. Cost-burden and cost-effectiveness estimates may assist with further prioritization.
Keywords: Influenza, Disease burden, Risk groups, Immunization, South Africa
1. Introduction
Global studies have suggested a higher burden of influenza-associated severe illness, including death, in Africa compared to other regions [1,2]. This has been attributed, among other reasons, to the elevated number of individuals with predisposing factors for influenza-associated severe illness, such as a high number of children aged <5 years and a high prevalence of human immunodeficiency virus (HIV), tuberculosis, other underlying medical conditions, and pregnancy [3]. Nonetheless, the use of influenza vaccines remains limited in Africa [4], likely due to financial constraints and competing health priorities.
In resource-limited settings, governments may not have sufficient resources to provide vaccination to all individuals at increased risk of severe influenza and may need to prioritize between risk groups. In 2012, the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization highlighted that “country-specific information about risk groups, disease burden and cost-effectiveness are important to aid national policy makers […] in making informed decisions about target groups […] for vaccination” [5].
In South Africa, a middle-income country with a high burden of HIV and tuberculosis and a high pregnancy rate, approximately one million doses of influenza vaccine have been available annually in the public sector since 2010. Influenza vaccination is recommended for groups at increased risk of influenza-associated severe illness [6]; however, the available number of doses is insufficient to cover the recommended risk groups, estimated to be over 20 million individuals [7].
In South Africa, national estimates of the disease and economic burden of influenza-associated illness irrespective of risk groups are available [8,9] and individuals at increased risk of severe illness have been identified [10-20]. This information has been pivotal to the formulation of a national influenza policy [7]. A framework to guide the prioritization of risk groups for influenza immunization has also been developed [21], leading, in recent years, to prioritize influenza immunization for pregnant women and HIV-infected adults [6]. The risk groups selected in South Africa are among that recommended for influenza immunization by WHO [5]. Nonetheless, data on the cost-effectiveness of such interventions are lacking. This is mainly due to the lack of estimates of the burden and proportional contribution of influenza-associated illness among risk groups across levels of severity, whether the illness episode is medically or non-medically attended, and the cost of the illness episode to the health system and the society. Such data could assist in further refining the prioritization of risk groups and potentially assist in motivating for increased vaccination coverage in selected populations.
In this study, we sought to assess the mean annual national burden of medically and non-medically attended influenza-associated mild, severe-non-fatal and fatal illness among potential target groups for influenza immunization in South Africa during 2013–2015 to provide a basis for potential cost-burden and cost-benefit analysis of influenza immunization among different risk groups in the country.
2. Methods
2.1. Selection of potential target risk groups for influenza immunization
Following the WHO recommendations [5], we selected the potential target risk groups for influenza immunization based on increased risk for influenza-associated severe illness, including deaths, namely:
Children aged 6–59 months [10-15], including individuals with HIV, pulmonary tuberculosis (PTB) and certain underlying medical conditions associated with increased risk of influenza-associated severe illness and prevalent in South Africa [10,22] (UMC - including chronic lung, kidney, liver or heart diseases, diabetes mellitus, and asthma). Malnutrition and prematurity were considered as UMC only in this group [10].
Individuals aged 5–64 years with HIV [10-14,16], and/or PTB [11,18-20] and/or certain UMC as listed above [10,11,14] together, excluding pregnant women. In this groups we accounted for potential co-infections and co-morbidities in the same individual. We also considered any individual aged 5–64 years with HIV, PTB or UMC separately. The latter groups are not mutually exclusive as, for instance, individuals with HIV can also be co-infected with PTB or having other UMC.
Individuals aged ≥65 years [10-12,14,16], including individuals with HIV, PTB and other UMC as listed above.
Pregnant women [10,17], including individuals with HIV, PTB and other UMC.
In addition, we considered any individual aged ≥6 months with HIV and/or PTB and/or UMC together accounting for potential co-infections and co-morbidities in the same individual. This group is a subset of the groups described above. We also considered any individual aged ≥6 months with HIV, PTB or UMC separately. The latter groups are not mutually exclusive as commented above.
Furthermore, we considered infants aged <6 months separately because, although there are no influenza vaccines licensed for this group, they experience an elevated risk of influenza-associated severe illness [23] and can be protected through the vaccination of their mothers during pregnancy [24,25]. We considered individuals not in risk groups those aged 5–64 years without HIV, PTB and other UMC as defined above and not pregnant.
2.2. Definitions
For this study we defined the severity of influenza-associated illness in 3 levels as follows: (i) mild: not warranting hospitalization; (ii) severe-non-fatal: warranting hospitalization, excluding deaths; and (iii) deaths. We considered healthcare attendance of influenza-associated illness in two categories: (i) medically attended: attended by a registered medical care provider/institution excluding pharmacies; and (ii) non-medically attended: not attended by a registered medical care provider/institution, but including pharmacies and traditional healers.
2.3. Data sources
2.3.1. Data source 1 (DS1): Population denominators for selected potential target risk groups for influenza immunization
a. Individuals aged <1, <5, 5–64 and ≥65 years
We obtained year-specific mid-year population denominators for individuals aged <1, <5, 5–64 and ≥65 years from projections of 2011 census data for South Africa [26-28]. South Africa had an estimated population of 54,860,530 individuals in 2015.
b. Pregnant women
We obtained year-specific mid-year estimates for number of pregnant women from the projection of the THEMBISA Model, an AIDS and demographic prediction model for South Africa [29].
c. HIV-infected individuals
We obtained year-specific mid-year estimates of the number of HIV-infected individuals among individuals aged <1, <5, 5–64 and ≥65 years and pregnant women from the projection of the THEMBISA Model [29].
d. Individuals with pulmonary tuberculosis
We obtained estimates of the number of individuals with PTB from published literature from South Africa [30].
e. Individuals with underlying medical conditions
We obtained estimates of the prevalence of the UMC considered among individuals aged <1, <5, 5–64 and ≥65 years from the 2012 South African National Health and Nutrition Examination Survey, which collects information on different UMC through community-based surveys [22].
2.3.2. Data source 2 (DS2): National estimates of influenza-associated illness by severity and medical attendance
We obtained national estimates of any influenza-associated illness (irrespective of risk groups) for South Africa during 2013–2015 from published literature [8]. These included estimates of any medically- and non-medically-attended influenza-associated mild, severe-non-fatal and fatal illness among individuals aged <1, <5, 5–64 and ≥65 years. The estimated mean annual number of influenza-associated illness episodes for all age groups was 10,737,847 (rate: 19,849.4 per 100,000 population). Of these episodes, 10,598,138 (98.7%; rate: 19,591.1 per 100,000 population), 128,173 (1.2%; rate: 236.9 per 100,000 population) and 11,536 (0.1%; rate: 21.3 per 100,000 population) were mild, severe-non-fatal, and fatal, respectively. We also obtained national estimates of medically- and non-medically-attended influenza-associated illness among individuals meeting the World Health Organization (WHO) case definition of influenza-like illness (ILI - for mild illness) and severe acute respiratory illness (SARI - for severe-non-fatal and fatal illness) only from the same source [8]. This constitutes a subset of any influenza-associated illness [8].
2.3.3. Data source 3 (DS3): Influenza surveillance among individuals hospitalized with severe acute respiratory illness
We obtained data on the prevalence of HIV, PTB and UMC co-infections and co-morbidities among patients aged <1, <5, 5–64 and ≥65 years hospitalized with influenza-associated severe acute respiratory illness (SARI) from laboratory-confirmed influenza surveillance conducted at seven hospitals situated in four provinces of South Africa (Gauteng, KwaZulu-Natal, North West, and Mpumalanga) during 2013–2015. The procedures of this surveillance program have been previously described [10-14,19,20]. Briefly, trained surveillance nurses completed case report forms that included demographic, clinical, and epidemiological information (including presence of UMC) for all enrolled cases. In-hospital deaths were also recorded. In addition, upper respiratory tract specimens were collected from all enrolled patients and tested for influenza viruses at the National Institute for Communicable Diseases (NICD), Johannesburg, South Africa using a real-time reverse transcription polymerase chain reaction assay [10-14,19,20]. HIV results were obtained from a combination of two sources: (i) patient clinical records when available and (ii) for consenting patients, a dried blood spot was tested at NICD. Sputa were systematically collected and tested for the detection of Mycobacterium tuberculosis [10,19,20]. A laboratory-confirmed tuberculosis case was defined as an individual with a positive result for M. tuberculosis on microscopy, culture, or PCR from the current hospital admission.
2.4. Estimation of the number of individuals in the general population within selected potential target groups for influenza immunization
We estimated the number of individuals within the selected potential target groups for influenza immunization in the general population using DS1.a to DS1.e. Because co-infections and co-morbidities with HIV, PTB, and UMC may occur in the same individual irrespective of age and pregnancy status we also estimated the number of individuals with such co-infections and co-morbidities. We considered the following categories: (i) HIV, PTB, or UMC only; (ii) HIV-PTB, HIV-UMC or PTB-UMC; and (iii) HIV-PTB-UMC. The detailed estimation approach for each risk group, including occurring co-infections and co-morbidities, is provided in Supplementary Material.
2.5. Estimation of the number of any influenza-associated mild illness episodes within selected potential target groups for influenza immunization
We obtained the total number of medically- and non-medically attended influenza-associated mild illness episodes by age group from available estimates from South Africa (DS2) [8]. For influenza-associated mild illness we first assumed the same prevalence of HIV, PTB, UMC (including co-infections and co-morbidities, referred as either HIV and PTB or HIV and UMC or PTB and UMC or HIV and PTB and UMC hereafter) and pregnancy as those estimated in the general population (as obtained above) and then discounted by the estimated number of cases that developed severe illness as previously described [31]. We also assumed the same proportion of individuals with influenza-associated mild illness and HIV, PTB, UMC or pregnancy among medically- and non-medically attended illness episodes.
2.6. Estimation of the number of any influenza-associated severe-non-fatal illness episodes within selected potential target groups for influenza immunization
We obtained the total number of medically- and non-medically attended influenza-associated severe-non-fatal illness episodes by age group from available estimates from South Africa (DS2) [8]. We estimated the number of individuals with influenza-associated severe illness and HIV, PTB or UMC using the following formula [32]:
| (1) |
Where:
Infli,j is the number of individuals with influenza-associated severe illness in age group i (i.e., <6 months, 6–59 months, 5–64 years and ≥65 years) and with condition j (i.e., HIV, PTB or UMC).
Popi,j is the number of individuals in the general population in age group i and with condition j.
Popi,n–j is the number of individuals in the general population in age group i and without condition j.
RRj is the relative risk for influenza-associated severe illness for condition j obtained from published literature from South Africa [10,11,12].
InflTotali is the number of influenza-associated severe illnesses in age group i obtained from DS2.
Thereafter, we estimated the number of individuals with HIV, PTB or UMC co-infections/co-morbidities using the prevalence of co-infections/co-morbidities observed among patients hospitalized with influenza-associated severe illness (DS3).
For pregnant women, we used the same approach described above among women of childbearing age (i.e., 15–49 years). The RR for influenza-associated severe illness among pregnant women was obtained from published literature [33].
We assumed the same proportion of individuals with influenza-associated severe illness and HIV, PTB, UMC, or pregnancy among medically- and non-medically-attended illness episodes.
2.7. Estimation of the number of any influenza-associated deaths within selected potential target groups for influenza immunization
We obtained the total number of in- and out-of-hospital influenza-associated deaths by age groups from available estimates from South Africa (DS2) [8]. We estimated the number of influenza-associated deaths among individuals with HIV or PTB using the same formula as for severe illness (Eq. (1)) with the following modifications: (i) we used the total number of influenza-associated deaths, instead of influenza-associated severe illness; and (ii) we used estimates of the RR for influenza-associated deaths, instead of severe illness from available literature from South Africa [15,16,18].
For pregnant women, we used the same approach described above among women of childbearing age (i.e., 15–49 years). The RR for influenza-associated death among pregnant women was obtained from published literature from South Africa [17].
We estimated the number of influenza-associated deaths among individuals with UMC using Eq. (1) with the following modifications: (i) we used the number of individuals with and without UMC among influenza-associated severe illness episodes (obtained as described above) instead of among the general population; and (ii) we used the RR of influenza-associated death for UMC among influenza-associated severe cases obtained from published literature from South Africa [14]. We used this approach because we did not have local estimates of the RR of influenza-associated deaths among individuals with UMC in the general population.
Thereafter, we estimated the number of individuals with HIV, PTB or UMC co-infections/co-morbidities using the prevalence of co-infections/co-morbidities observed among patients hospitalized with influenza-associated severe illness that died (DS3).
We assumed that the same proportions of influenza-associated deaths occurred among individuals with HIV, PTB, UMC or pregnant among deaths that occurred in or out of the hospital.
2.8. Estimation of the number of influenza-associated mild, severe-non-fatal and fatal illness episodes among individuals meeting the WHO ILI and SARI case definitions within selected potential target groups for influenza immunization
We obtained the total number of medically- and non-medically-attended influenza-associated illness episodes among individuals meeting the WHO ILI (mild illness) and SARI (severe-non-fatal and fatal illness) case definitions [34] by age groups from available estimates from South Africa (DS2) [8]. We used the same estimation approach among this group of individuals as for those with any influenza-associated illness as described above. This analysis was implemented to obtain estimates within syndromes (ILI or SARI) widely used for the estimation of influenza disease burden in Africa [35-41], including South Africa [12,13].
2.9. Estimation of rates and confidence intervals
We estimated rates of influenza-associated illness among the target risk groups by dividing the estimated number of illness episodes within each group by the population at risk. We expressed rates per 100,000 population. We used Monte Carlo simulations over 1,000 iterations to account for the variability associated with estimates of total influenza-associated mild, severe-non-fatal and fatal illness (DS2) [8], and RRs associated with severe illness or death for each risk group requiring RR adjustment (i.e. HIV, PTB, UMC, and pregnancy). All analyses were conducted using Stata version 14.2 (StataCorp, College Station, Texas, USA).
3. Results
3.1. Estimated mean annual number of individuals in the general population within selected potential target groups for influenza immunization
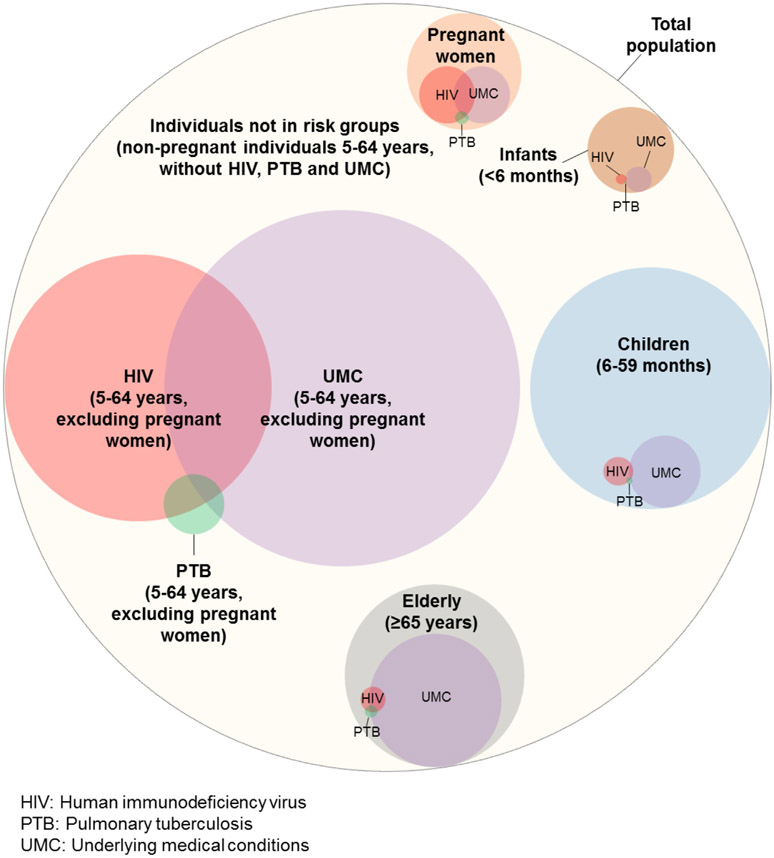
Individuals in the target risk groups accounted for 45.4% (24,569,328/54,086,144) of the population (Table 1 and Fig. 1). Within the selected risk groups, individuals aged 5–64 years with UMC accounted for the highest number of individuals (46.9%; 11,525,445/24,569,328) (Table 1 and Fig. 1). Among individuals aged ≥6 months with HIV, PTB or UMC, 16.2% (2,937,742/18,099,040) had more than one co-infection or co-morbidity (e.g. HIV and another UMC). The number and proportion of individuals with HIV/PTB/UMC co-infections and co-morbidities are provided in Fig. 1 and Table S13.
Table 1.
Estimated mean annual number of individuals within potential target risk groups for influenza immunization in South Africa, 2013–2015.
| Risk groups | Number | % of total individuals in risk groups |
% of total population |
|---|---|---|---|
| Children aged 6–59 monthsa | 5,203,824 | 21.2 | 9.6 |
| Individuals aged 5–64 years with HIV and/or PTB and/or UMC (excluding pregnant women)b | 15,425,229 | 62.8 | 28.5 |
| HIV | 6,429,939 | 26.2 | 11.9 |
| PTB | 332,656 | 1.4 | 0.6 |
| UMC | 11,525,445 | 46.9 | 21.3 |
| Individuals aged ≥65 yearsa | 3,014,877 | 12.3 | 5.6 |
| Pregnant womena | 925,398 | 3.8 | 1.7 |
| Individuals aged ≥6 months with HIV and/or PTB and/or UMC (including pregnant women)b |
18,099,040 | 73.7 | 33.5 |
| HIV | 6,766,575 | 27.5 | 12.5 |
| PTB | 357,348 | 1.5 | 0.7 |
| UMC | 13,997,956 | 57.0 | 25.9 |
| Totalc | 24,569,328 | 100.0 | 45.4 |
Abbreviations: HIV: human immunodeficiency virus; PTB: pulmonary tuberculosis; UMC: underlying medical conditions, including chronic lung, kidney, liver or cardiac disease, diabetes mellitus, and asthma as well as malnutrition and prematurity only among children aged <5 years.
Includes individuals with HIV and/or PTB and/or UMC.
The HIV, PTB and UMC categories are not mutually exclusive (e.g. the HIV category includes any individual with HIV within the specified age group, including individuals with tuberculosis co-infection and/or underlying medical conditions).
The total number of individuals in the selected risk groups accounts for coinfection and co-morbidities. It is calculated as the sum of the following: (i) children aged 6–59 months; (ii) individuals aged 5–64 years (excluding pregnant women) with HIV and/or PTB and/or UMC; (iii) individuals aged ≥65 years; and (iv) pregnant women.
Fig. 1.
Estimated mean annual number of individuals within potential target risk groups for influenza immunization (including coinfections with HIV and PTB and co-morbidities) in South Africa, 2013–2015. Each circle’s size is proportional to the number of individuals it represent. Inner circles represent a subpopulation of the outer circle. Overlapping circles represent co-infection and co-morbidities (e.g. HIV and another condition such as PTB and/or other UMC).
3.2. Estimated mean annual number of influenza-associated mild illness episodes within selected potential target groups for influenza immunization
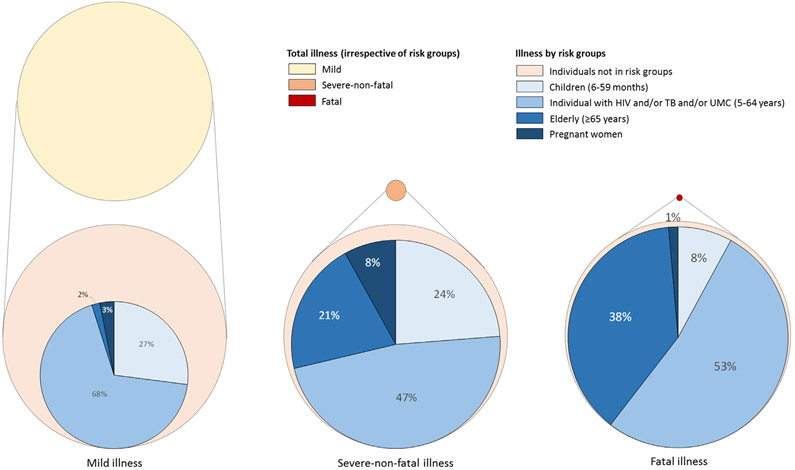
Individuals in the target risk groups accounted for 43.5% (4,614,763/10,598,138) of any influenza-associated mild illness episodes (Table 2 and Fig. 2). Within the selected risk groups, individuals aged 5–64 years with UMC accounted for the highest number of influenza-associated mild illness episodes (50.9%; 2,347,300/4,614,763) (Table 2), whereas children aged 6–59 months experienced the highest rate (23,983 per 100,000 population). Among individuals with influenza-associated mild illness aged ≥6 months with HIV, PTB or UMC, 17.3% (583,899/3,383,536) had more than one co-infection/co-morbidity of the above conditions. The number and proportion of any influenza-associated mild illness episodes among individuals with HIV/PTB/UMC co-infections and co-morbidities are provided in Table S14. The number and rates of any medically- and non-medically-attended influenza-associated mild illness episodes in the selected risk groups are provided in Table S1; whereas those among individuals meeting the WHO ILI case definition are provided in Tables S5 and S6.
Table 2.
Estimated mean annual rates and number of total (medically and non-medically-attended) influenza-associated mild illness (any) among potential target risk groups for influenza immunization in South Africa, 2013–2015.
| Risk group | Rate (95% CI) | Number (95% CI) | % of total illness in risk groups |
% of total illnessa |
|---|---|---|---|---|
| Children aged 6–59 monthsb | 23,983 (17,268–30,698) | 1,248,027 (898,579–1,597,475) | 27.0 | 11.8 |
| Individuals aged 5–64 years with HIV and/or PTB and/or UMC (excluding pregnant women)c | 20,366 (16,089–24,643) | 3,141,540 (2,481,817–3,801,263) | 68.1 | 29.6 |
| HIV | 20,366 (15,275–25,458) | 1,309,537 (982,153–1,636,921) | 28.4 | 12.4 |
| PTB | 20,366 (12,627–28,106) | 67,750 (42,005–93,495) | 1.5 | 0.6 |
| UMC | 20,366 (15,071–25,661) | 2,347,300 (1,737,002–2,957,598) | 50.9 | 22.1 |
| Individuals aged ≥65 yearsb | 2,709 (1,108–4,291) | 81,667 (33,398–129,381) | 1.8 | 0.8 |
| Pregnant womenb | 15,510 (11,322–19,698) | 143,529 (104,776–182,282) | 3.1 | 1.4 |
| Individuals aged ≥6 months with HIV and/or PTB and/or UMC (including pregnant women)c | 18,695 (15,143–22,247) | 3,383,536 (2,740,664–4,026,408) | 73.3 | 31.9 |
| HIV | 20,104 (15,882–24,326) | 1,360,334 (1,074,664–1,646,004) | 29.5 | 12.8 |
| PTB | 19,627 (13,346–25,907) | 70,135 (47,692–92,578) | 1.5 | 0.7 |
| UMC | 18,246 (13,867–22,625) | 2,554,114 (1,941,127–3,167,101) | 55.3 | 24.1 |
| Totald | 18,783 (14,321–23,242) | 4,614,763 (3,518,570–5,710,401) | 100.0 | 43.5 |
Abbreviations: HIV: human immunodeficiency virus; PTB: pulmonary tuberculosis; UMC: underlying medical conditions, including chronic lung, kidney, liver or cardiac disease, diabetes mellitus, and asthma as well as malnutrition and prematurity only among children aged <5 years; CI: confidence intervals.
Rates reported per 100,000 person year.
Total influenza-associated mild illness (10,598,138) obtained from Tempia et al. [8].
Includes individuals with HIV and/or PTB and/or UMC.
The HIV, PTB and UMC categories are not mutually exclusive (e.g. the HIV category includes any individual with HIV within the specified age group, including individuals with tuberculosis co-infection and/or underlying medical conditions).
The total number of individuals in the selected risk groups accounts for co-infection and co-morbidities. It is calculated as the sum of the following: (i) children aged 6–59 months; (ii) individuals aged 5–64 years (excluding pregnant women) with HIV and/or PTB and/or UMC; (iii) individuals aged ≥65 years; and (iv) pregnant women.
Fig. 2.
Estimated mean annual number of total influenza-associated mild, severe-non-fatal and fatal illness (irrespective of risk groups) and illness among potential target risk groups for influenza immunization in South Africa, 2013–2015. Total illness estimates obtained from Tempia et al. [8]. The circles’ size is proportional to the number of influenza-associated illness cases.
3.3. Estimated mean annual number of influenza-associated severe-non-fatal illness episodes within selected potential target groups for influenza immunization
Individuals in the target risk groups accounted for 86.8% (111,245/128,173) of any influenza-associated severe-non-fatal illness episodes (Table 3 and Fig. 2). Within the selected risk groups, individuals aged 5–64 years with HIV accounted for the highest number of influenza-associated severe-non-fatal illness episodes (39.8%; 44,305/111,245) (Table 3), whereas pregnant women experienced the highest rate (970 per 100,000 population). Among individuals with influenza-associated severe-non-fatal illness aged ≥6 months with HIV, PTB or UMC, 33.5% (28,464/84,973) had more than one co-infection/co-morbidity of the above conditions. The number and proportion of any influenza-associated severe-non-fatal illness episodes among individuals with HIV/PTB/UMC co-infections and co-morbidities are provided in Table S15. The number and rates of any medically- and non-medically-attended influenza-associated severe-non-fatal illness episodes in the selected risk groups are provided in Table S2; whereas those among individuals meeting the WHO SARI case definition are provided in Tables S7 and S8.
Table 3.
Estimated mean annual rates and number of total (medically and non-medically-attended) influenza-associated severe-non-fatal illness (any) among potential target risk groups for influenza immunization in South Africa, 2013–2015.
| Risk group | Rate (95% CI) | Number (95% CI) | % of total illness in risk groups |
% of total illnessa |
|---|---|---|---|---|
| Children aged 6–59 monthsb | 508 (366–650) | 26,429 (19,029–33,829) | 23.8 | 20.6 |
| Individuals aged 5–64 years with HIV and/or PTB and/or UMC (excluding pregnant women)c | 343 (271–415) | 52,875 (41,771–63,979) | 47.5 | 41.3 |
| HIV | 689 (517–861) | 44,305 (33,229–55,381) | 39.8 | 34.6 |
| PTB | 825 (511–1,138) | 2,743 (1,701–3,785) | 2.5 | 2.1 |
| UMC | 261 (193–329) | 30,126 (22,293–37,959) | 27.1 | 23.5 |
| Individuals aged ≥65 yearsb | 762 (324–1,356) | 22,966 (9,773–40,887) | 20.6 | 17.9 |
| Pregnant womenb | 970 (708–1,232) | 8,975 (6,552–11,398) | 8.1 | 7.0 |
| Individuals aged ≥6 months with HIV and/or PTB and/or UMC (including pregnant women)c | 469 (380–559) | 84,972 (68,827–101,117) | 76.4 | 66.3 |
| HIV | 784 (619–949) | 53,043 (41,904–64,182) | 47.7 | 41.4 |
| PTB | 1,117 (759–1,474) | 3,990 (2,713–5,267) | 3.6 | 3.1 |
| UMC | 412 (313–511) | 57,702 (43,854–71,550) | 51.9 | 45.0 |
| Totald | 453 (314–611) | 111,245 (77,125–150,093) | 100.0 | 86.8 |
Abbreviations: HIV: human immunodeficiency virus; PTB: pulmonary tuberculosis; UMC: underlying medical conditions, including chronic lung, kidney, liver or cardiac disease, diabetes mellitus, and asthma as well as malnutrition and prematurity only among children aged <5 years; CI: confidence intervals.
Rates reported per 100,000 person year.
Total influenza-associated severe-non-fatal illness (128,173) obtained from Tempia et al. [8].
Includes individuals with HIV and/or PTB and/or UMC.
The HIV, PTB and UMC categories are not mutually exclusive (e.g. the HIV category includes any individual with HIV within the specified age group, including individuals with tuberculosis co-infection and/or underlying medical conditions).
The total number of individuals in the selected risk groups accounts for co-infection and co-morbidities. It is calculated as the sum of the following: (i) children aged 6–59 months; (ii) individuals aged 5–64 years (excluding pregnant women) with HIV and/or PTB and/or UMC; (iii) individuals aged ≥65 years; and (iv) pregnant women.
3.4. Estimated mean annual number of influenza-associated deaths within selected potential target groups for influenza immunization
Individuals in the target risk groups accounted for 94.5% (10,903/11,266) of any influenza-associated deaths (Table 4 and Fig. 2). Within the selected risk groups, individuals aged 5–64 years with HIV accounted for the highest number of influenza-associated deaths (47.5%; 5,184/10,903) (Table 4); whereas individuals aged ≥65 years experienced the highest rate (138 per 100,000 population). Among individuals with influenza-associated fatal illness aged ≥6 months with HIV or PTB or UMC, 46.0% (4,076/8,864) had more than one co-infection/co-morbidity of the above conditions. The number and proportion of any influenza-associated deaths among individuals with HIV/PTB/UMC co-infections and co-morbidities are provided in Table S16. The number and rates of any medically- and non-medically-attended influenza-associated deaths in the selected risk groups are provided in Table S3; whereas those among individuals meeting the WHO SARI case definitions are provided in Tables S9 and S10.
Table 4.
Estimated mean annual rates and number of total (in- and out-of-hospital) influenza-associated deaths (any) among potential target risk groups for influenza immunization in South Africa, 2013–2015.
| Risk group | Rate (95% CI) | Number (95% CI) | % of total deaths in risk groups | % of total deathsa |
|---|---|---|---|---|
| Children aged 6–59 monthsb | 17 (12–21) | 868 (625–1,111) | 8.0 | 7.5 |
| Individuals aged 5–64 years with HIV and/or PTB and/or UMC (excluding pregnant women)c | 37 (29–45) | 5,727 (4,524–6,930) | 52.5 | 49.6 |
| HIV | 81 (60–101) | 5,184 (3,888–6,480) | 47.5 | 44.9 |
| PTB | 136 (84–188) | 454 (281–627) | 4.2 | 3.9 |
| UMC | 28 (21–36) | 3,252 (2,406–4,098) | 29.8 | 28.2 |
| Individuals aged ≥65 yearsb | 138 (57–249) | 4,159 (1,709–7,498) | 38.1 | 36.1 |
| Pregnant womenb | 16 (12–20) | 149 (109–189) | 1.4 | 1.3 |
| Individuals aged ≥6 months with HIV and/or PTB and/or UMC (including pregnant women)c | 49 (40–58) | 8,864 (7180–10548) | 81.3 | 76.8 |
| HIV | 96 (76–116) | 6,474 (5,114–7,834) | 59.4 | 56.1 |
| PTB | 150 (102–198) | 535 (364–706) | 4.9 | 4.6 |
| UMC | 44 (33–54) | 6,143 (4,669–7617) | 56.3 | 53.3 |
| Totald | 44 (28–64) | 10,903 (6,967–15,728) | 100.0 | 94.5 |
Abbreviations: HIV: human immunodeficiency virus; PTB: pulmonary tuberculosis; UMC: underlying medical conditions, including chronic lung, kidney, liver or cardiac disease, diabetes mellitus, and asthma as well as malnutrition and prematurity only among children aged <5 years; CI: confidence intervals.
Rates reported per 100,000 person year.
Total influenza-associated deaths (11,536) obtained from Tempia et al. [8].
Includes individuals with HIV and/or PTB and/or UMC.
The HIV, PTB and UMC categories are not mutually exclusive (e.g. the HIV category includes any individual with HIV within the specified age group, including individuals with tuberculosis co-infection and/or underlying medical conditions).
The total number of individuals in the selected risk groups accounts for co-infection and co-morbidities. It is calculated as the sum of the following: (i) children aged 6–59 months; (ii) individuals aged 5–64 years (excluding pregnant women) with HIV and/or PTB and/or UMC; (iii) individuals aged ≥65 years; and (iv) pregnant women.
3.5. Estimated mean annual number of influenza-associated illnesses among infants aged <6 months and non-pregnant individuals aged 5–64 year without HIV, PTB or UMC (non-risk group)
During 2013–2015, infants aged <6 months accounted for 1.5% (155,484/10,598,138), 5.4% (6,923/128,173) and 3.1% (362/11,266) of influenza-associated mild, severe-non-fatal and fatal illness episodes, respectively (Table 5). Individuals not in risk groups accounted for 55.0% (5,827,891/10,598,138), 7.8% (10,004/128,173) and 2.3% (270/11,266) of influenza-associated mild, severe-non-fatal and fatal illness episodes, respectively (Table 5). The number and rates of any medically- and non-medically-attended influenza-associated illness in these groups are provided in Table S4; whereas those among individuals meeting the WHO ILI or SARI case definitions are provided in Tables S11 and S12. The number and proportion of any influenza-associated illness episodes among infants with HIV/PTB/UMC co-infections and co-morbidities are provided in Table S17.
Table 5.
Estimated mean annual rate and number of any influenza-associated illness among infants aged <6 months and individuals aged 5–64 years without HIV, pulmonary tuberculosis (PTB) or other underlying medical conditions in South Africa, 2013–2015.
| Category | Rate (95% CI) | Number (95% CI) | % of total |
|---|---|---|---|
| Infants aged <6 months | |||
| Population | N/A | 592,660 | 1.5 |
| Influenza-associated mild illness | 26,235 (17,840–34,630) | 155,484 (105,729–205,239) | 1.5 |
| Influenza-associated severe-non-fatal illness | 1,168 (794–1,542) | 6,923 (4708–9138) | 5.4 |
| Influenza-associated death | 61 (42–81) | 362 (246–478) | 3.1 |
| Individuals aged 5–64 years without HIV, PTB or UMC (excluding pregnant women) | |||
| Population | N/A | 28,924,156 | 53.1 |
| Influenza-associated mild illness | 20,149 (14,709–25,589) | 5,827,891 (4,254,360–7,401,422) | 55.0 |
| Influenza-associated severe-non-fatal illness | 35 (25–44) | 10,004 (7,303–12,705) | 7.8 |
| Influenza-associated death | 0.9 (0.7–1.2) | 270 (197–343) | 2.3 |
Abbreviations: CI: confidence intervals.
Rates reported per 100,000 person year.
4. Discussion
During 2013–2015 an estimated 45.4% (24.6 million people) of the South African population were in the target risk groups we defined for influenza immunization. This proportion was similar (43.5%) among individuals with influenza-associated mild illness. In comparison, individuals in risk groups accounted for the majority of influenza-associated severe-non-fatal (86.8%) and fatal (94.5%) illness. In a study conducted in the USA, the prevalence of individuals at increased risk for influenza-associated severe illness also increased across levels of severity, but was lower than in our study: 23.5%, 49.7%, and 92.7% among individuals with influenza-associated mild, severe-non-fatal and fatal illness, respectively [31]. However, in the US study individuals at risk were considered those with Advisory Committee on Immunization Practices-identified high-risk conditions, which excluded young children and older adults without such conditions as at-risk individuals. In addition, a higher burden of HIV and PTB is experienced in South Africa compared to the USA. These two factors could potentially explain the observed differences.
An increased risk of influenza-associated severe illness among the risk groups considered in this study has previously been reported in South Africa [10-20]. However, the proportional contribution of such individuals to total influenza-associated illness had not been described in the country prior to this study. Compared to the other risk groups considered in this study, individuals aged 5–64 years with UMC accounted for the highest proportion of individuals in the general population (21.3%) and among those with influenza-associated mild illness (22.1%). Individuals aged 5–64 years with HIV accounted for the highest proportion of individuals with severe-non-fatal (34.6%) and fatal (44.9%) illness, respectively. The proportional contribution of a risk group on influenza-associated severe illness is related to the prevalence of the specific risk groups in the population and its relative risk/rate associated with severe illness.
For prioritization of interventions, differences in the relative risk/rate associated with severe disease or the proportional contribution to total illness can be considered. This would depend on the aim of the intervention. If the goal of an interventions, such as vaccination, is to maximize the number of deaths prevented per dose of vaccine utilized, the program would target groups with the highest mortality rates; whereas if the goal is to prevent the highest number of deaths irrespective of the number of doses utilized, the program would target groups with the highest proportional contribution to total deaths. For instance, in our study the highest rates of influenza-associated deaths were among individuals aged ≥65 years (138 per 100,000 population and 36.1% of total deaths); whereas the highest proportion of deaths (44.9% and rate 81 per 100,000 population) were among individuals aged 5–64 years with HIV. This would identify different target groups based on the aim of the intervention.
Thus far, an approach that targets risk groups with the highest rates of influenza-associated severe illness (i.e. HIV-infected adults and pregnant women) has been undertaken in South Africa [6,21] as it would maximize the health benefits per dose of vaccine used. The estimates of influenza-associated severe illness provided in this study among these two risk groups support this decision. Nonetheless, other factors such as the feasibility of vaccinating the selected risk groups as well as the influenza vaccine effectiveness in the target populations should be considered in the selection process [21]. In addition, in South Africa no considerations were made on the effect of vaccination on mild illness in the selected risk groups. The influenza virus attack rate and associated mild illness is known to vary across age groups [12,40,41] and this was also observed in our study. Such information can further assist with prioritization of risk groups within a cost-effectiveness framework.
Whereas the disease burden among risk groups accounts for the majority of influenza-associated severe-non-fatal (86.8%) and fatal (94.5%) illness, there is a considerable number of individuals in the risk groups that are only mildly affected. The estimated mean annual cost of medically attended influenza-associated mild illness irrespective of risk groups in South Africa was $107 million; whereas those of medically attended influenza-associated severe illness (i.e. hospitalization) was $47 million [9]. This highlights the importance of medically attended mild illness in our setting.
In our study, we estimated that among individuals with influenza-associated illness aged ≥6 months with HIV or PTB or UMC the proportion that had more than one co-infection/comorbidity increased with the level of severity: 17.3%, 33.5%, and 46.0% among individuals with influenza-associated mild, severe-non-fatal, and fatal illness, respectively. This suggests that individuals with more than one co-infection/co-morbidity may have an increased risk of influenza-associated severe illness compared to individuals having HIV or PTB or UMC only.
Our study has limitations that warrant discussion. First, we did not have estimates of differential likelihood of developing influenza-associated mild illness following influenza virus infection among individuals with HIV, PTB or UMC compared to individuals without such conditions. Therefore, we parametrized the rates of influenza-associated mild illness in these groups to be the same as those in the general population within the selected age groups. Second, we did not have estimates of differential propensity to seek care among individuals with influenza-associated mild or severe illness with HIV or PTB or UMC compared to individuals without such conditions. If individuals with such conditions would be more likely to seek care compared to individuals without the condition, then we would have underestimated the proportion of medically-attended illness in these groups. From a health system perspective non-medically attended illness would have no impact within a cost-effectiveness framework; however, such illnesses could be of importance from a societal perspective. It is estimated that approximately 74% and 56% of influenza-associated mild and severe illness, respectively is non-medically attended in South Africa [8], and the mean annual cost for such illnesses was $115 million [9]. This highlights the importance of non-medically attended illness in our setting. Underestimating medically attended and overestimating non-medically attended illness in certain populations would result in minimum estimates of such risk groups within a cost-effectiveness framework. Lastly, we were not powered to estimate the influenza-associated burden among person with different underlaying medical conditions such as chronic lung, heart and kidney diseases or diabetes, separately. This may further improve the prioritization of risk groups for influenza immunization if differences on the burden of influenza-associated illness are identified among individuals with different underlaying medical conditions.
5. Conclusions
In conclusion, individuals in the selected risk groups accounted for the majority of influenza-associated severe-non-fatal and fatal illness, with individuals aged 5–64 years with HIV accounting for the largest proportion of such illnesses. However, a considerable number of influenza-associated mild illness was also estimated among the selected risk groups. Annual influenza immunization of the selected risk groups has the potential to prevent a substantial number of influenza-associated severe illness. Nonetheless, due to competing health priorities and the high number of individuals in the risk groups (24.5 million, 45.4% of the general population), it is unlikely that the South African Government will be able to provide annual influenza immunization coverage for all risk groups in the near future.
This study supports the WHO recommendations pertaining vaccination of groups at increased risk of influenza-associated severe illness [5]. In addition, this study provides a framework and a reference for further prioritization among risk groups based on disease burden and the basis for future cost-burden and cost-effectiveness analyses of influenza immunization among different risk groups in South Africa. These future studies will assist in further prioritizing influenza immunization among risk groups and potentially lead to increased vaccination coverage, should influenza immunization be found cost-effective in selected populations.
Supplementary Material
Acknowledgments
The authors would like to gratefully acknowledge the contribution of Professor Demetre Labadarios and team at the Human Sciences Research Council for access to SANHANES data, all members involved in SRI and ILI surveillance for the collection and testing of specimens and the data management team at the National Institute for Communicable Diseases for data quality control and assurance.
Financial disclosure
This work was supported by the National Institute for Communicable Diseases, of the National Health Laboratory Service and the US Centers for Disease Control and Prevention (co-operative agreement number: 5U51IP000155).
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute for Communicable Diseases.
Footnotes
Ethics
The SARI and SRI protocol was approved by the University of the Witwatersrand Human Research Ethics Committee (HREC) and the University of KwaZulu-Natal Human Biomedical Research Ethics Committee (BREC) protocol numbers M081042 and BF157/08, respectively. All other data were publicly available.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.04.045.
References
- [1].Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018;391 (10127):1285–300. 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lafond KE, Nair H, Rassoly MH, et al. Global role and burden of influenza in pediatric respiratory hospitalizations, 1982–2012: a systematic analysis. PLoS Med 2016;13(3):. 10.1371/journal.pmed.1001977e1001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Katz MA, Schoub BD, Heraud JM, et al. Influenza in Africa: uncovering the epidemiology of a long-overlooked disease. J Infect Dis 2012;206(Suppl 1): S1–4. 10.1093/infdis/jis548. [DOI] [PubMed] [Google Scholar]
- [4].Duque J, McMorrow ML, Cohen AL. Influenza vaccines and influenza antiviral drugs in Africa: are they available and do guidelines for their use exist?. BMC Public Health 2014;14:41 10.1186/1471-2458-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].World Health Organization (WHO). Vaccines against influenza - WHO position paper – November 2012. Wkly Epidemiol Rec. 2012;47(87):461–76. [PubMed] [Google Scholar]
- [6].Blumberg L, Cohen C, Dawood H, et al. Influenza NICD Recommendations for the Diagnosis, Prevention, Management and Public Health Response. Available at: http://www.nicd.ac.za/wp-content/uploads/2017/03/Influenza-guidelines-rev_-23-April-2018.pdf [accessed on 29 May 2019].
- [7].South Africa Department of Health. National Influenza Policy and Strategic Plan – 2017 to 2021. Available at: http://www.health.gov.za/index.php/component/phocadownload/category/339 [accessed on 29 May 2019].
- [8].Tempia S, Walaza S, Moyes J, et al. Quantifying how different clinical presentations, levels of severity and healthcare attendance shape the burden of influenza-associated illness: a modelling study from South Africa. Clin Infect Dis. 2018. doi: 10.1093/cid/ciy1017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tempia S, Moyes J, Cohen AL, et al. Health and Economic Burden of Influenza-Associated Illness in South Africa, 2013–2015. Influenza Other Respir Viruses 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tempia S, Walaza S, Moyes J, et al. Risk factors for influenza-associated severe acute respiratory illness hospitalization in South Africa, 2012–2015. Open Forum Infectious Diseases. 2017;4(1):ofw262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Abadom TR, Smith AD, Tempia S, et al. Risk factors associated with hospitalisation for influenza-associated severe acute respiratory illness in South Africa: a case-population study. Vaccine 2016;34(46):5649–55. 10.1016/j.vaccine.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tempia S, Walaza S, Moyes J, et al. The effects of the attributable fraction and the duration of symptoms on burden estimates of influenza-associated respiratory illnesses in a High HIV-prevalence setting, South Africa, 2013–2015. Influenza Other Respir Viruses 2018;12(3):360–73. 10.1111/irv.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cohen C, Moyes J, Tempia S, et al. Severe influenza-associated lower respiratory tract infection in a high HIV-Prevalence setting – South Africa, 2009–2011. Emerg Infec Dis 2013;19(11):1766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cohen C, Moyes J, Tempia S, et al. Mortality amongst patients with influenza-associated severe acute respiratory illness, South Africa, 2009–2013. PLoS ONE 2015;10(3):e0118884 10.1371/journal.pone.0118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tempia S, Walaza S, Viboud C, et al. Mortality associated with seasonal and pandemic influenza and respiratory syncytial virus among children <5 years of age in a high HIV prevalence setting—South Africa, 1998–2009. Clin Infect Dis 2014;58(9):1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tempia S, Walaza S, Viboud C, et al. Deaths associated with respiratory syncytial and influenza viruses among persons ≥5 years of age in HIV-prevalent area, South Africa, 1998–2009. Emerg Infect Dis 2015;21(4):600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tempia S, Walaza S, Cohen AL, et al. Mortality associated with seasonal and pandemic influenza among pregnant and nonpregnant women of childbearing age in a high-HIV-prevalence setting-South Africa, 1999–2009. Clin Infect Dis 2015;61(7):1063–70. 10.1093/cid/civ448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Walaza S, Cohen C, Nanoo A, et al. Excess mortality associated with influenza among tuberculosis deaths in South Africa, 1999–2009. PLoS ONE 2015;10(6):. 10.1371/journal.pone.0129173.eCollectione0129173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Walaza S, Tempia S, Dawood H, et al. Influenza virus infection is associated with increased risk of death amongst patients hospitalized with confirmed pulmonary tuberculosis in South Africa, 2010–2011. BMC Infect Dis 2015;15:26 10.1186/s12879-015-0746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Walaza S, Tempia S, Dawood H, et al. The impact of influenza and tuberculosis interaction on mortality among individuals aged ≥15 years hospitalized with severe respiratory illness in South Africa, 2010–2016. Open Forum. Infect Dis 2019;6(3):ofz020 10.1093/ofid/ofz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McMorrow ML, Tempia S, Walaza S, et al. Prioritization of risk groups for influenza vaccination in resource limited settings - a case study from South Africa. Vaccine 2019;37(1):25–33. 10.1016/j.vaccine.2018.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shisana O, Labadarios D, Rehle T, et al. The South African National Health and Nutrition Examination Survey, 2012 (SANHANES-1). Available at: http://www.hsrc.ac.za/en/research-outputs/view/6493 [accessed on 18 Jan 2019]. [Google Scholar]
- [23].McMorrow ML, Tempia S, Walaza S, et al. The Role of Human Immunodeficiency Virus in Influenza- and Respiratory Syncytial Virus-associated Hospitalizations in South African Children, 2011–2016. Clin Infect Dis 2019;68(5):773–80. 10.1093/cid/ciy532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Madhi SA, Cutland CL, Kuwanda L, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med 2014;371(10):918–31. [DOI] [PubMed] [Google Scholar]
- [25].Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008;359(15):1555–64. [DOI] [PubMed] [Google Scholar]
- [26].Statistics South Africa. Mid-year population estimates: 2013. Available at: https://www.statssa.gov.za/publications/P0302/P03022013.pdf [accessed on 18 Jan 2019].
- [27].Statistics South Africa. Mid-year population estimates: 2014. Available at: https://www.statssa.gov.za/publications/P0302/P03022014.pdf [accessed on 18 Jan 2019].
- [28].Statistics South Africa. Mid-year population estimates: 2015. Available at: https://www.statssa.gov.za/publications/P0302/P03022015.pdf [accessed on 18 Jan 2019].
- [29].THEMBISA Model. Available at: http://www.thembisa.org/ [accessed on 18 Jan 2019].
- [30].Nanoo A, Izu A, Ismail NA, et al. Nationwide and regional incidence of microbiologically confirmed pulmonary tuberculosis in South Africa, 2004–12: a time series analysis. Lancet Infect Dis 2015;15(9):1066–76. 10.1016/S1473-3099(15)00147-4. [DOI] [PubMed] [Google Scholar]
- [31].Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007;25(27):5086–96. [DOI] [PubMed] [Google Scholar]
- [32].von Gottberg, de Gouveia L, Tempia S, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med 2014;371(20):1889–99. 10.1056/NEJMoa1401914. [DOI] [PubMed] [Google Scholar]
- [33].Mertz D, Geraci J, Winkup J, et al. Pregnancy as a risk factor for severe outcomes from influenza virus infection: a systematic review and metaanalysis of observational studies. Vaccine 2017;35(4):521–8. 10.1016/j.vaccine.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].WHO. Interim global epidemiological surveillance standards for influenza. WHO,2012. Available at: http://www.who.int/influenza/resources/documents/influenza_surveillance_manual/en/ [accessed on 18 December 2019]. [Google Scholar]
- [35].Ntiri M, McMorrow ML, Frimpong JA, et al. Incidence of medically attended influenza among residents of Shai-Osudoku and Ningo-Prampram Districts, Ghana, May 2013-April 2015. BMC Infect Dis 2016;16(1):757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dawa JA, Chaves SS, Nyawanda B, et al. National burden of hospitalized and non-hospitalized influenza-assocaited severe acute respiratory illness in Kenya, 2012–2014. Influenza Other Respir Viruses 2018;12(1):30–7. 10.1111/irv.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nyamusore J, Rukelibuga J, Mutagoma M, et al. The national burden of influenza-associated severe acute respiratory illness hospitalization in Rwanda, 2012–2014. Influenza Other Respir Viruses 2018;12(1):38–45. 10.1111/irv.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Theo A, Tempia S, Cohen AL, et al. The national burden of influenza-associated severe acute respiratory illness hospitalization in Zambia, 2011–2014. Influenza Other Respir Viruses 2018;12(1):46–53. 10.1111/irv.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rabarison JH, Tempia S, Harimanana A, et al. Burden and epidemiology of influenza- and respiratory syncytial virus-associated severe acute respiratory illness hospitalization in Madagascar, 2011–2016. Influenza Other Respir Viruses 2018. 10.1111/irv.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Emukule GO, Khagayi S, McMorrow ML, et al. The burden of influenza and RSV among inpatients and outpatients in rural western Kenya, 2009–2012. PLoS ONE 2014;9(8):. 10.1371/journal.pone.0105543e105543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Babakazo P, Lubula L, Disasuani W, et al. The national and provincial burden of medically attended influenza-associated influenza-like illness and severe acute respiratory illness in the Democratic Republic of Congo, 2013–2015. Influenza Other Respir Viruses 2018;12(6):695–705. 10.1111/irv.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.