Abstract
CONSPECTUS: The immune system has evolved over time to protect the host from foreign microorganisms. Activation of the immune system is predicated on a distinction between self and nonself. Unfortunately, cancer is characterized by genetic alterations in the host’s cells, leading to uncontrolled cellular proliferation and evasion of immune surveillance. Cancer immunotherapy aims to educate the host’s immune system to not only recognize but also attack and kill mutated cancer cells. While immune checkpoint blockers have been proven to be effective against multiple types of advanced cancer, the overall patient response rate still remains below 30%. Therefore, there is an urgent need to improve current cancer immunotherapies. In this Account, we present an overview of our recent progress on nanoparticle-based strategies for improving cancer vaccines and immunotherapies. We also present other complementary strategies to give a well-rounded snapshot of the field of combination cancer immunotherapy. The versatility and tunability of nanoparticles make them promising platforms for addressing individual challenges posed by various cancers. For example, nanoparticles can deliver cargo materials to specific cells, such as vaccines delivered to antigen-presenting cells for strong immune activation. Nanoparticles also allow for stimuli-responsive delivery of various therapeutics to cancer cells, thus forming the basis for combination cancer immunotherapy. Here, we focus on nanoparticle platforms engineered to deliver tumor antigens, whole tumor cells, and chemotherapeutic or phototherapeutic agents in a manner to effectively and safely trigger the host’s immune system against tumor cells. For each work, we discuss the nanoparticle platform developed, synthesis chemistry, and in vivo applications. Nanovaccines offer a unique platform for codelivery of personalized tumor neoantigens and adjuvants and elicitation of robust immune responses against aggressive tumors. Nanovaccines either delivering whole tumor cell lysate or formed from tumor cell lysate may increase the repertoire of tumor antigens as immune targets while exploiting immunogenic cell death to prime antitumor immune responses. We also discuss how antigen- and whole tumor cell-based approaches may open the door for personalized cancer vaccination and immunotherapy. On the other hand, chemotherapy, phototherapy, and radiotherapy are more standardized cancer therapies, and nanoparticle-based approaches may promote their ability to initiate T cell activation against tumor cells and improve antitumor efficacy with minimal toxicity. Finally, building on the recent progress in nanoparticle-based cancer immunotherapy, the field should set the ultimate goal to be clinical translation and clinical efficacy. We will discuss regulatory, analytical, and manufacturing hurdles that should be addressed to expedite the clinical translation of nanomedicine-based cancer immunotherapy.
Graphical Abstract
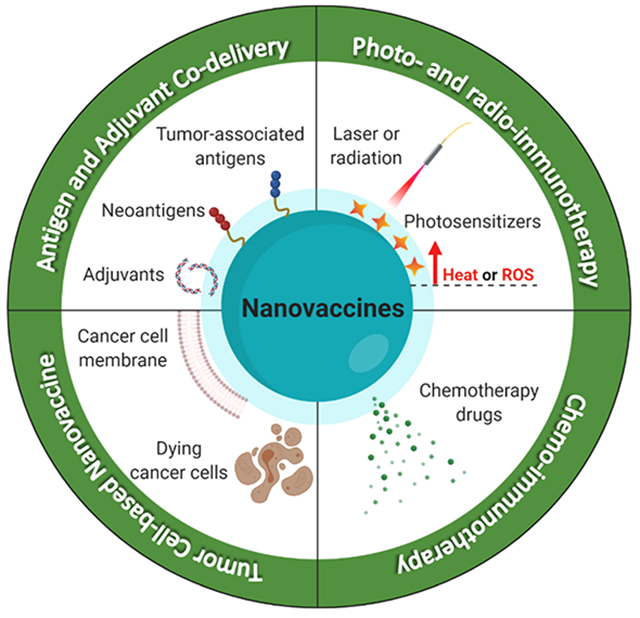
1. INTRODUCTION
Despite considerable improvement in our identification and understanding of cancer, standard treatment options have remained relatively unchanged. Surgical resection, chemotherapy, and radiation therapy are standard treatments, but unfortunately, there are numerous cases in which these treatments are unsuccessful and result in tumor recurrence or metastasis, thus necessitating a different treatment approach. Cancer immunotherapy has recently emerged as a major pillar of cancer treatment. To put it simply, cancer immunotherapy is a treatment that boosts the body’s natural immune response to cancer, establishing a population of highly active, tumor-specific T cells that can lyse tumor cells and eradicate malignancies.6 However, the most well-known cancer immunotherapy immune checkpoint blockers (ICBs) has overall patient response rates below 30%.2 Therefore, there is an urgent need to improve current cancer immunotherapies.
In this Account, we will introduce our recent efforts to develop and advance nanomaterial-based approaches for advancing cancer immunotherapy, and we also discuss relevant work from other research groups to put our work into context. We believe nanotechnology offers unique opportunities to improve cancer immunotherapy as nanoparticles can be designed for (1) targeted delivery of cargo materials to specific organs, tissues, and cells, (2) codelivery of antigens and adjuvants to antigen-presenting cells (APCs) for strong immune activation, and (3) noninvasive and stimuli-responsive delivery of therapeutics to cancer cells, while (4) providing a safe and biocompatible platform for combination immunotherapy. Here, we will first introduce our work on antigen-based nanovaccines focused on increasing the immunogenicity of cancer vaccines and amplifying antitumor T cell response. Next, we will discuss whole tumor cell-based nanovaccines, which present antigens in their native state. Lastly, we will present our strategy for combination cancer immunotherapy utilizing chemotherapeutics and photothermal therapies.
2. ANTIGEN- AND ADJUVANT-BASED NANOVACCINES
Antigens are proteins capable of eliciting a targeted immune response, specifically through activation of T cells and B cells. There are two major types of tumor antigens: tumor-associated antigens (TAAs) and tumor-specific antigens, also known as neoantigens. TAAs are overexpressed in tumor tissues but are still expressed at lower levels in normal tissues and assumed to elicit T cell responses against these self-proteins due to the following: (1) incomplete thymic depletion and/or peripheral tolerance of TAA-reactive T cells, (2) lower expression of TAA in the periphery, (3) low TCR binding affinity of TAA-reactive T cells, or (4) restricted TAA expression pattern during the development.7 Unfortunately, low immunogenicity of TAAs and the possible outcome of self-tolerance has led to limited therapeutic effects in clinical trials.7,8 Neoantigens, on the other hand, are mutated, nonself-proteins derived from tumor cells.9 Because neoantigens are selectively expressed by tumor cells alone, neoantigen-based vaccines may avoid central immune tolerance and induce tumor-specific T cells without safety concerns, thus providing key advantages over traditional TAA-based vaccines.9
Since their identification, neoantigens have been at the forefront of cancer immunotherapy research, and NPs designed to codeliver neoantigens and adjuvants may realize the therapeutic potential of neoantigen-based personalized immunotherapy.10 NP platforms intended for neoantigen-based cancer vaccination should exhibit good safety profiles, as well as ease of manufacturing and quality control. To achieve these design goals, we are developing a nanodisc platform based on synthetic high-density lipoproteins (sHDL) composed of phospholipids and apolipoprotein A1-mimetic peptides (Figure 1).1 Endogenous HDL plays a critical role in the transport and metabolism of lipids, such as cholesterol and triglycerides.11 As a platform for neoantigen-based vaccination, sHDL has ideal properties, including multiple cargo loading sites and a small size (10 nm) that mediates efficient codelivery of antigens and adjuvants to draining lymph nodes (LNs)—a crucial criterion for successful vaccines.12 Briefly, neoantigen peptides modified with a reduction-sensitive cysteine-serine-serine linker undergo a simple thiol reaction with a dioleoyl-sn-glycero-3-phosphoethanolamine-N-[3-(2-pyridyldithio) propionate] (PDP)-modified lipid to produce neoantigen-lipid conjugates. When mixed with sHDL, hydrophobic interactions allow incorporation of neoantigen-lipid conjugates and CpG (a Toll-like receptor-9 agonist) modified with cholesterol into nanodiscs. Nanodiscs are avidly taken up by DCs, leading to strong colocalization with endosomes/lysosomes, sustained epitope-MHC-I presentation, and cross-priming of CD8+ T cells. Nanodiscs carrying various neoantigens have been shown to induce robust antitumor responses in multiple types of cancer, a few of which will be highlighted below.
Figure 1.
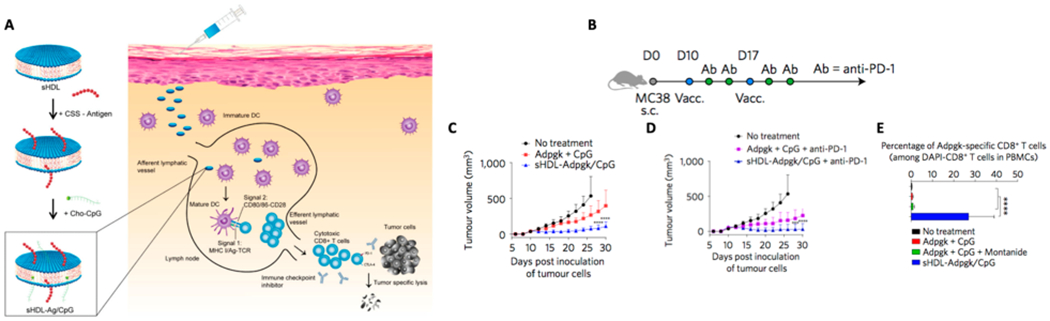
(A) sHDL nanodiscs, composed of phospholipids and apolipoprotein-1 mimetic peptides (22A), are coloaded with cysteine-modified tumor-specific mutated neoantigen peptides and CpG, an immunostimulatory adjuvant. (B) Immunization scheme of MC-38 study with nanodiscs. (C and D) Tumor growth curves of mice vaccinated with sHDL nanodiscs with or without anti-PD-1 IgG therapy. (E) Frequency of neoantigen-specific CD8+ T cells among PBMCs. Reproduced with permission from ref 1. Copyright 2017 Nature Publishing Group.
We have coloaded CpG and Adpgk, a neoantigen derived from MC-38 colon carcinoma, into nanodiscs (sHDL-Adpgk/CpG), which significantly increased antigen delivery to draining LNs upon subcutaneous (SC) administration.1 When administered in MC-38 tumor-bearing mice, nanodiscs induced a 31fold greater frequency of neoantigen-specific IFN-γ+ and TNF-α+ cytotoxic CD8+ T-lymphocytes (CTLs), compared with soluble Adpgk peptide admixed with CpG. Although nanodisc vaccination alone slowed tumor growth, this did not lead to tumor elimination. When nanodisc vaccination was combined with anti-PD-1 ICB, ~88% mice had complete tumor regression, and of that fraction, 100% mice survived rechallenge, indicating immunological memory.1 Similar results were obtained in a highly aggressive, poorly immunogenic B16F10 melanoma model, where multiple MHC I- and MHC II-restricted neoantigens (M27 and M30, respectively) were loaded into nanodiscs to create a cocktail. In this model, nanodisc vaccination combined with anti-PD-1 and anti-CTLA-4 led to ~90% tumor elimination.1 Notably, compared with the traditional route of intramuscular vaccination, SC route of nanodisc vaccination increased antigen delivery to draining LNs by 3.3-fold and generated a 7-fold higher frequency of antigen-specific CD8+ T cells,13 suggesting increased lymphatic accessibility of the SC administration contributed to improved T cell priming.
Having established nanodiscs as a platform for neoantigen-based vaccination, we have recently shown that (1) nanodiscs offer advantages over other leading cancer vaccines, (2) nanodiscs deliver clinically relevant human HLA-restricted antigens, and (3) nanodiscs exert antitumor efficacy against tumors that are difficult to treat using conventional immunotherapies. We have loaded CpG-nanodiscs with HPV16 E7 antigen found in human papillomavirus (HPV), a prevalent virus that contributes to the pathogenesis of head and neck cancer as well as cervical cancer. Both cancers originate in mucosal tissues, which have low induction and infiltration of T cells.14 Three rounds of sHDL-E7/CpG vaccination generated ~32% E7-specific CD8+ T cells among peripheral blood mononuclear cells (PBMCs), representing a 29-fold increase, compared with the soluble vaccine or E7-CpG-Montanide control.15 Currently, one of the leading cancer vaccines is live-attenuated vectors encoding antigens (e.g., Listeria).16 Notably, the Listeria vaccine is a late-stage cancer vaccine platform, which unfortunately failed to show antitumor efficacy and safety in a phase III clinical trial.17 We directly compared our sHDL with a Listeria vector vaccine; MC-38 tumor-bearing mice were vaccinated with either sHDL-Adpgk/CpG (SC) or Listeria-Adpgk (IV) in combination with anti-PD-1.15 Both nanodisc- and Listeria-based treatment groups induced similar levels of neoantigen-specific CD8+ T cells and regression of established MC-38 tumors. On the basis of the ease of manufacturing and SC administration, as well as well-documented safety of nanodiscs,11 these results highlight the promising potential of nanodiscs for cancer vaccination.
We have also successfully demonstrated that nanodiscs elicit T cell responses against human HLA-restricted antigens, specifically neoantigen HLA-A02 from a melanoma patient. HLA-A02 transgenic mice that received prime-boost-boost immunizations of HLA-A02 neoantigen peptide in Complete Freund’s Adjuvant (a potent yet toxic adjuvant system) generated only basal levels of IFNγ+ T cell responses, whereas switching the last boost immunization with sHDL-HLA-A02 neoantigen/CpG achieved 200-fold greater IFNγ+ T cell responses.15
Finally, to determine if nanodiscs were able to elicit antitumor immunity against disseminated tumors, we have recently evaluated nanodisc vaccination against highly aggressive gliomas.18 Glioma patients respond poorly to conventional treatments, including ICBs, and a dismal clinical prognosis of less than 15 months underscores the urgent need for new treatment options.19,20 In particular, traditional glioma treatments have been ineffective because of tumor heterogeneity, immunosuppressive tumor microenvironments, and the blood–brain barrier (BBB), which severely hampers the transport of therapeutics to the central nervous system. Importantly, when orthotopic GL261 tumor-bearing mice were treated SC with nanodiscs carrying three neoantigens21 in combination with anti-PD-L1, we observed elicitation of up to 100-fold higher IFNγ+ T cell responses, compared with soluble vaccine + anti-PD-L1 treatment.18 Furthermore, whereas the soluble vaccine + anti-PD-L1 group had no survivors, nanodisc vaccination + anti-PD-L1 therapy eradicated gliomas from 30% of mice in two different orthotopic models of GL261 and mutant IDH1 gliomas. There were no signs of recurrence through day 90, and surviving mice rechallenged in the contralateral hemisphere on day 90 did not show any signs of neurological deficit. These exciting results have demonstrated immunological memory and the ability of glioma-specific T cells to traverse the BBB and exert cytotoxic potential against gliomas. Together, these examples show that nanodiscs offer a promising platform that can be readily plugged in with various tumor antigens, including neoantigens, for inducing robust T cell responses against aggressive and disseminated tumors.
While neoantigens provide specific and effective targets for cancer vaccination, many types of cancer do not have a high mutational load.22 In addition, timely and efficient neoantigen identification and production of personalized cancer vaccines are major hurdles to overcome. This leaves TAAs as the remaining option to use for peptide-based therapeutic cancer vaccination. We have recently shown that nanodiscs can also be used for TAA-based immunotherapy.23 In particular, cancer stem cells (CSCs) are a subset of cancer cells that can self-renew and drive tumor pathogenesis, recurrence, metastasis, and chemo-resistance.24 We hypothesized that SC nanodisc vaccination against well-studied CSC markers like aldehyde dehydrogenase (ALDH) would elicit T cells against ALDHHigh CSCs with potent antitumor efficacy.23 We first identified two ALDH epitopes and tested nanodiscs delivering them in murine models of D5 melanoma and 4T1 breast cancer known to harbor CSCs. D5-bearing mice immunized with sHDL-ALDH/CpG in combination with anti-PD-L1 generated >44-fold higher frequencies of ALDH-specific CD8+ T cells, compared with the soluble vaccine + anti-PD-L1 group, leading to a significant reduction in the frequency of ALDHHigh CSCs, tumor growth inhibition, and extended animal survival.23 We also confirmed these responses in 4T1 tumor-bearing mice, thus showing the potential of off-the-shelf cancer vaccine targeted against CSCs.
We have also examined whether adjuvant molecules loaded in nanodiscs can further amplify DC activation and T cell responses against TAAs.25 We loaded sHDL with CpG and monophosphoryl lipid A (MPLA, a TLR-4 agonist) to activate DCs through two distinct pathways. Each adjuvant molecule is currently used in FDA-approved vaccines, and the combination of CpG and MPLA has shown synergy in prior studies.26 Compared with the single adjuvant-loaded nanodiscs, sHDL-MPLA/CpG induced the upregulation of CD80 and CD86 on DCs and Th1 immune responses. Treatment with sHDL-MPLA/CPG in B16F10-OVA tumor-bearing mice generated strong antigen-specific CD8+ T cells, decreasing the tumor growth rate. These findings were also confirmed in a second tumor model of HPV16 E7+ TC-1; sHDL-MPLA/CpG admixed with E7 antigen elicited robust E7-specific T cell responses that eliminated established HPV16 E7+ TC-1 tumors.25
In addition to nanodiscs, we have recently reported the development of PEGylated reduced graphene oxide nanosheets (RGO-PEG) as a multifunctional nanovaccine platform for efficient delivery of neoantigen peptides and adjuvant CpG to draining LNs (Figure 2).2 After SC administration, RGO-PEG exhibited rapid, efficient, and sustained (up to 72 h) accumulation in LNs, achieving >100-fold improvement in LN-targeted delivery, compared with soluble vaccines.2 Interestingly, RGO-PEG induced intracellular reactive oxygen species (ROS) in DCs, promoting antigen processing and presentation to T cells. A single injection of RGO-PEG vaccine elicited neoantigen-specific T cell responses lasting up to 30 days and eliminated established MC-38 colon carcinoma. For the treatment of poorly immunogenic B16F10 melanoma, we combined RGO-PEG vaccination with anti-PD-1 therapy, leading to potent therapeutic efficacy. These results suggest that LN-targeting nanomaterials with immune-activating properties may serve as a powerful delivery platform for cancer vaccination.
Figure 2.
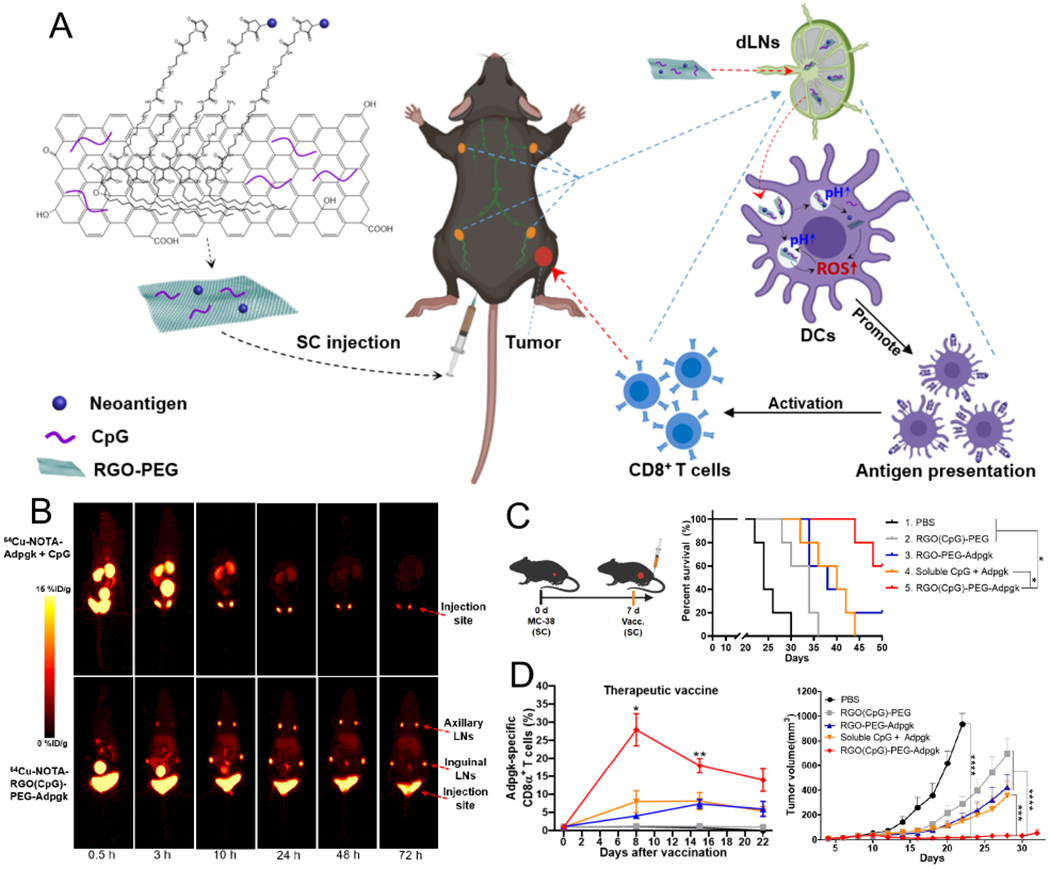
(A) Schematic illustration of RGO-based cancer nanovaccine. Shown are the structure of RGO(CpG)-PEG-neoantigen and its proposed mechanism of LN draining and ROS-enhanced antigen presentation. (B) PET imaging of 64Cu-NOTA-Adpgk + CpG and 64Cu-NOTA-RGO(CpG)-PEG-Adpgk after SC injection. (C) Treatment regimen and overall survival curve for MC-38 tumor therapeutic vaccine study. (D) Frequencies of Adpgk-specific CD8α+ T cells and the average tumor growth curves in the MC-38 tumor model. Reproduced with permission from ref 2. Copyright 2020 American Chemical Society.
Another example using the self-assembly method has been reported by Lynn et al., who have developed charge-modified neoantigen-TLR7/8a conjugates for self-assembly into stable, uniformly sized 20 nm micelles (SNP-7/8a).27 When evaluated in murine models of B16F10 and TC-1 tumors, as well as tumor-free nonhuman primates, SNP-7/8a induced a breadth of CD4+ and CD8+ T cell responses and increased immunogenicity of otherwise nonimmunogenic neoantigens. In particular, IV route of SNP-7/8a administration inhibited tumor growth more effectively than the SC route. When used in combination with anti-PD-L1 therapy, SNP-7/8a nanovaccine administered IV exerted stronger antitumor efficacy against B16F10 and TC1 tumors, compared with long peptide + polyICLC (administered SC) combined with anti-PD-L1 therapy.27
Taken together, neoantigen- and TAA-based nanovaccines could be a staple of cancer immunotherapy given the current regulatory, analytical, and manufacturing landscape. Not only do they provide the host immune system with a confirmed immunogenic target, but the NP design and adjuvant addition can further aid the activation of APCs through costimulatory molecule upregulation and subsequent antigen presentation. However, the rate-limiting factor of this technology is the identification and validation of immunogenic neoantigens in a patient-specific manner.10 Given this limitation, it is critical to explore other immunotherapy strategies that complement personalized neoantigen vaccination, as we discuss below.
3. WHOLE TUMOR CELL-BASED NANOVACCINES
The cancer nanovaccines discussed above have utilized defined tumor antigens, but this strategy requires extensive antigen discovery, synthesis, and optimization methods. Additionally, these vaccines utilize only a small, defined number of antigens. Alternatively, cancer vaccines may employ the whole tumor cell or a subset of whole tumor cells (e.g., tumor cell membrane) as the source of antigens, and these may obviate the need to identify immunogenic antigens while providing a plethora of diverse TAAs as immune targets. However, clinical trials with traditional tumor lysate (TL) vaccines have shown limited efficacy.28 For instance, clinical trials for Melacine, an allogenic melanoma TL vaccine administered with DETOX adjuvant, resulted in only 6–7% objective clinical responses.29 A few reasons for these disappointing results include poor delivery and uptake of TL by APCs and inefficient T cell priming.30 To overcome these challenges, NP platforms are being explored for TL-based personalized vaccination.
Our group developed a method to form PEG-coated NPs from TL.31 When tumor cells undergo freeze–thaw lysis, probe-tip sonication, and centrifugation, this results in nanovesicles composed of tumor cell plasma membranes; however, they are prone to aggregation. We have sought to address this by adding a PEG layer to stabilize the nanovesicles, which also increased trafficking to LNs, compared with freeze–thaw lysate (FT lysate) and unPEGylated nanovesicles.31 Subsequently, PEGylated NPs containing transmembrane TAAs and CpG were taken up by DCs, significantly increasing and sustaining CD8+ T cell expansion. B16F10-OVA tumor-bearing mice vaccinated SC with PEG-NPs increased the frequency of OVA-specific CD8+ T cells among PBMCs by 3.7-fold, compared with FT lysate-vaccinated mice.31 PEG-NP treatment group had the median survival of 55 days, compared with 27 days for the FT lysate group. Co-administration of the PEG-NP TL vaccine with anti-PD-1 yielded complete tumor regression in 63% of mice and 100% protection against tumor rechallenge among the survivors.31
Jin et al. recently advanced this approach one step further.32 Using cancer cell membrane fractions (CCMF) of TLs, they coated PLGA NPs to serve as “artificial cancer cells”, which increased NP trafficking to LNs and also maintained surface proteins to disrupt cancer cell–stromal cell interaction and prevent tumor cell proliferation.32 The presence of CCMF-PLGA NPs inhibited the migration of tumor cells to human mammary fibroblasts by 30% in vitro, which translated to tumor protection in a murine lung metastasis model. Mice vaccinated SC with CCMP-PLGA-NP also reduced lung metastasis by day 21 postinoculation. This was likely due to targeted activation of CTLs because CCMF-PLGA NPs were found to accumulate in LNs where significantly more IFN-γ-producing CD4+ and CD8+ T cells were present.32
In addition to the TL-based approach, tumor cells undergoing immunogenic cell death (ICD) can also serve as a rich source of tumor antigens and danger signals.34 During ICD, tumor cells are subject to stressors (e.g., chemotherapeutics, phototherapies, radiation therapies), which cause up-regulation of “eat me” and “danger” signals (e.g., calreticulin and HMGB1) and trigger tumor-specific immune activation (Figure 3).34–37 We have reported a NP-based approach to amplify the potency of ICD through surface-modification of tumor cells with adjuvant-loaded nanodepots.38 Briefly, we prepared unilamellar liposomes using maleimide-functionalized lipid DOBAQ-MAL, DOTAP, and DOPC lipids, followed by incubation with thiolated hyaluronic acid and CpG to cross-link the unilamellar vesicles (Figure 4).38 This led to the formation of homogeneous multilamellar liposomes with diameters of ~250 nm and increased NP stability in vivo. To promote ICD, B16F10-OVA tumor cells were treated with mitoxantrone, a potent ICD-inducing anthracenedione agent. Maleimide-displaying CpG nanodepots were then tethered to the surface of dying tumor cells via free sulfhydryls on endogenous cell-membrane proteins (Mit-B 16F10-OVA-CpG-NPs). Mit-B16F10-OVA-CpG-NPs significantly upregulated costimulatory molecules CD40 and CD86 as well as inflammatory cytokines (IL-12p70, TNF-α, IFN-β) necessary for optimal antigen presentation and T cell activation.38 Mice vaccinated SC with Mit-B16F10-OVA-CpG-NPs exhibited a 2.4-fold increase in antigen-specific CD8+ T cell responses, compared with dying tumor cells.38 When mice were challenged with B16F10-OVA cells 8 days postvaccination, 100% of mice treated with Mit-B16F10-OVA-CpG-NP rejected tumor cells, compared with 20% rate in the Mit-B16F10-OVA group. Moreover, Mit-CT-26-CpG-NP combined with anti-PD-1 ICB regressed CT-26 carcinoma in ~78% mice, and 100% of survivors were protected against tumor rechallenge on day 70, demonstrating long-term immunity.38
Figure 3.
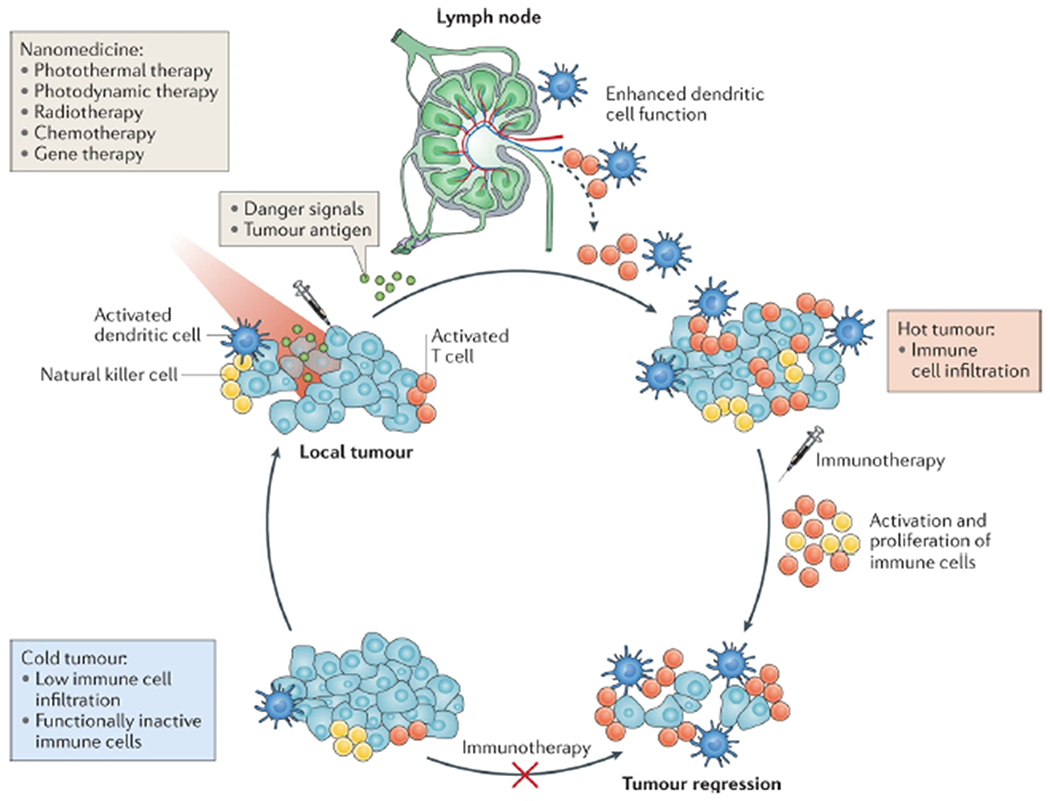
Nanomedicine for combination cancer immunotherapy. Nonimmunogenic, “cold” tumors are resistant to immunotherapies as they have various immune evasion mechanisms, including poor T cell infiltration and immunosuppressive pathways. Nanomedicines designed for photothermal therapy, photodynamic therapy, radiotherapy, chemotherapy, or gene therapy can be used to convert “cold” tumors into immunogenic, “hot” tumors. Nanomedicines can exert cytotoxic effects against tumor cells in the immunosuppressive tumor microenvironment, leading to debulking of the tumor mass, releasing of tumor antigens and danger signals, and dendritic cell-mediated antitumor immunity. Reproduced with permission from ref 33. Copyright 2019 Nature Publishing Group.
Figure 4.
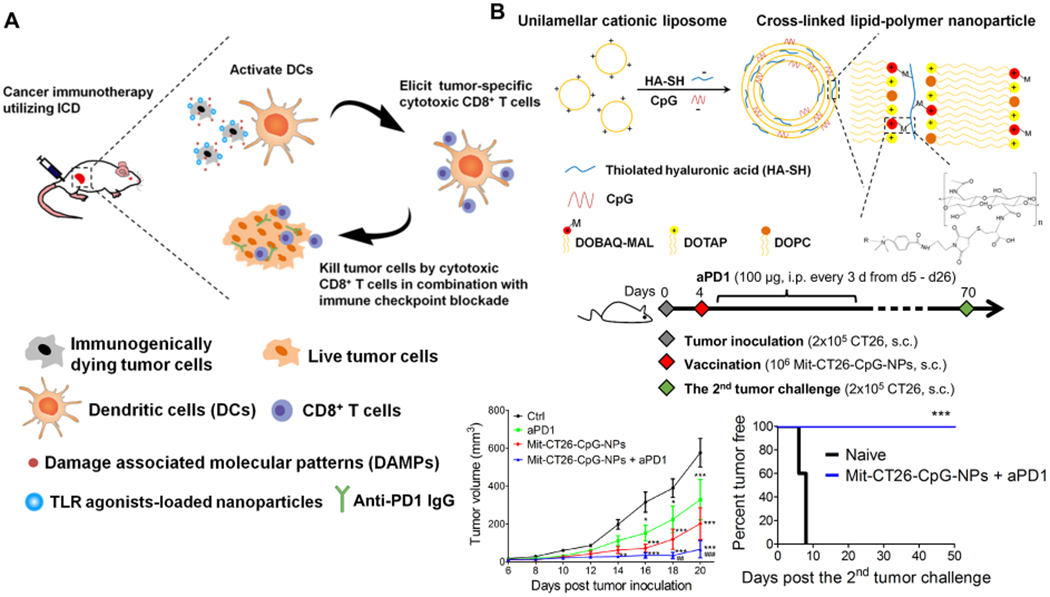
(A) Immunogenically dying tumor cells surface-decorated with TLR agonist-loaded NPs release tumor antigens and damage-associated molecular patterns, triggering activation of DCs, and induction of tumor-specific CD8α+ T cells that can kill tumor cells. (B) We synthesized the lipid-polymer hybrid NP encapsulating CpG by complexing cationic liposomes with thiolated HA-SH, an anionic biopolymer, followed by cross-link-mediated stabilization. (C) CT26 tumor study: tumor growth and survival curves. Reproduced with permission from ref 38. Copyright 2017 American Chemical Society.
Taken together, whole tumor cell and tumor membrane nanovaccines offer a promising platform for personalized immunotherapy. These strategies eliminate the laborious task of identifying immunogenic antigens, and the large array of antigens may provide multiple targets for the immune system.
4. NANOPARTICLES FOR CHEMO-IMMUNOTHERAPY
Chemotherapy is one of the standard cancer treatments because of its versatility and ease of use; however, chemotherapy is limited by systemic toxicity and chemo-resistance. Nanotechnology may address some of these limitations through reduction of both the dose and the frequency of administration, while providing a new and safe pathway for combination chemo-immunotherapy.39 For example, we have employed sHDL nanodiscs for tumor-targeted delivery of doxorubicin (DOX), a potent ICD-inducer (Figure 5).5 The main objectives were to utilize the long-circulating property of sHDL to enhance intratumoral delivery of DOX and promote pH-responsive release of DOX in endolysosomes of cancer cells for induction of ICD and antitumor immunity. Briefly, DOX was tethered to sHDL via a hydrophobic anchor with a hydrazone linker, allowing for stable drug incorporation into sHDL at pH 7.4 but rapid DOX release at pH 5. Tumor cells incubated with sHDL-DOX initiated the ICD cascade. CT-26 tumor-bearing mice treated with sHDL-DOX + anti-PD-1 generated 8-fold and 4-fold greater antigen-specific CD8+ T cell expansion among PBMCs, compared with anti-PD-1 and free DOX + anti-PD-1, respectively.5 This led to robust antitumor efficacy with 88% survival rate in an orthotopic colon carcinoma metastasis model, whereas the free DOX + anti-PD-1 group had only to 13% response rate, demonstrating the potential of nanotechnology for combination chemo-immunotherapy.5
Figure 5.
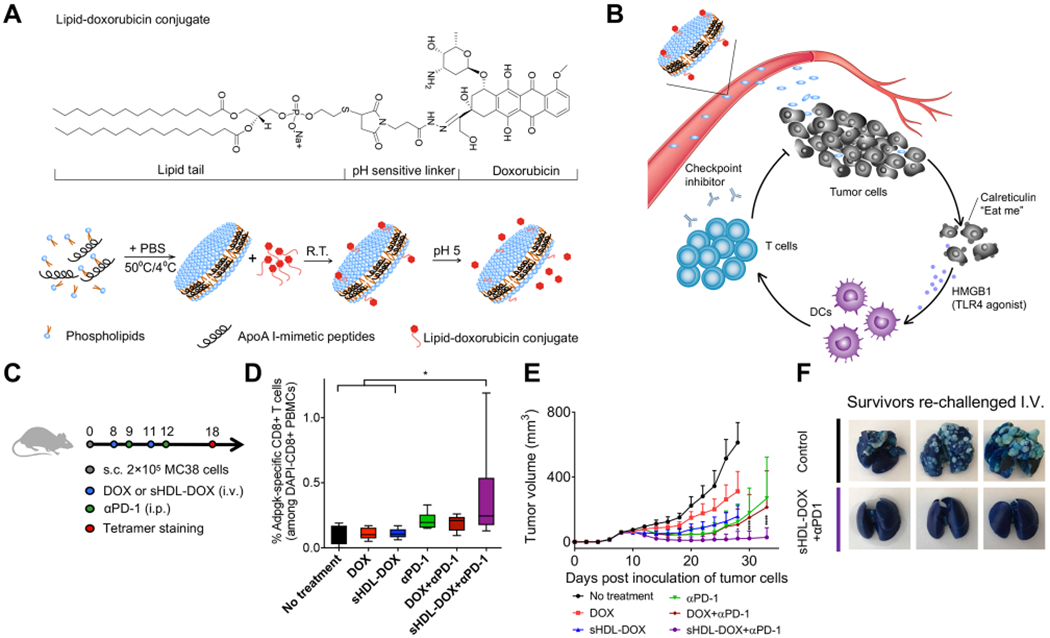
(A) sHDL-DOX is formed by incubation of lipid-DOX with preformed sHDL. (B) Ultrasmall size and prolonged circulation of sHDL enable intratumoral delivery of DOX. Released DOX kills tumor cells and triggers ICD, promoting the recruitment of DCs and antigen-specific T cells. Antitumor immunity primed with sHDL-DOX synergizes with immune checkpoint blockade, leading to efficient elimination of established tumors and prevention of tumor relapse. (C) Immunization scheme of MC38 study with sHDL-DOX. (D) Frequency of neoantigen-specific CD8+ T cells among PBMCs. (E) Tumor growth curves of mice treated with indicated formulations. (F) Lung metastasis of MC38 cells after IV tumor rechallenge. Reproduced with permission from ref 5. Copyright 2018 American Association for the Advancement of Science.
For some types of cancer, other chemotherapeutic agents are more effective but require a delivery system to reach and release the agents in the tumor microenvironment. Glioblastoma multiforme (GBM), an aggressive primary brain tumor that is protected by the BBB, is an example of this type of cancer.40 To overcome GBM’s physical resistance to chemotherapeutic treatment, we have coloaded sHDL with docetaxel (DTX) and CpG (sHDL-DTX-CpG).40 GL26 glioma-bearing mice treated by intrathecal injections of sHDL-DTX-CpG had a ~2-fold increase in survival, compared with DTX, DTX-CpG, or DTX-sHDL treatment. CTL activity is normally suppressed in GBM, but sHDL-DTX-CpG triggered ICD, as evidenced by high expression of calreticulin and HMGB1 on the surface of tumor cells in an antigen-specific manner while CpG helped to recruit APCs. CD8+ T cells were required for the efficacy of DTX-sHDL-CpG, as the same treatment was not efficacious in CD8-KO mice. Since the standard therapy for GBM is the combination of radiation therapy and chemotherapy, GBM-bearing mice were treated by sHDL-DTX-CpG combined with radiation therapy, resulting in 80% tumor regression rate with no evidence of tumor recurrence after tumor rechallenge.40 As exemplified here, it is crucial for the future of chemo-immunotherapy to amplify ICD, and nanoparticle platforms designed for codelivery of ICD-inducers and adjuvants can activate DAMP production while enhancing APC recruitment.33,36 The addition of ICBs can further potentiate this immune response as well.
To increase the tumor-targeting capacity of NPs, Yong et al. developed DOX-loaded exosome-sheathed porous silicon NPs (DOX@E-PSiNPs).41 Exosome membrane proteins facilitate cellular uptake and target-homing capabilities, and PsiNPs have a high loading capacity for DOX.41 DOX@E-PSiNPs’ small size (~260 nm) allowed accumulation in and diffusion throughout the tumor, leading to increased animal survival by 40 days in H22 hepatocellular carcinoma-bearing mice.41 Mice treated IV with DOX@E-PSiNPs exhibited efficient cross-reactive killing of cancer cells and CSCs, limited metastasis, and reduced tumor volume in murine models of 4T1 breast cancer and B16F10 melanoma.
Taken together, chemotherapy-loaded NPs may be employed to target tumors and initiate ICD, promoting innate immune activation and antitumor T cell expansion.
5. EMERGING COMBINATION IMMUNOTHERAPIES
Phototherapies, including photodynamic therapy (PDT) and photothermal therapy (PTT), are noninvasive and effective ablation approaches for the treatment of local tumors. Phototherapeutic agents or photosensitizers can be activated by a specific wavelength of laser irradiation at the tumor site, resulting in selective killing of cancer cells with reduced systemic toxicity.42 However, it remains challenging to completely eradicate large sold tumors with conventional phototherapy due to tumor relapse from residual tumor cells, especially at the treatment margin. Furthermore, phototherapies, which require direct access to the source of irradiation, are powerless in treating disseminated, metastatic tumor cells and in controlling tumor recurrence.43 Recent studies have shown that such limitations could be addressed by combining phototherapy with immunotherapy.44 Here, we highlight recent studies that achieved synergistic antitumor effects by combining antigen-based cancer vaccines with phototherapies.
PDT employs photosensitizer molecules to generate cytotoxic reactive oxygen species (ROS, e.g., singlet oxygen), which damage plasma membranes and subcellular organelles to induce tumor cell apoptosis and necrosis.45 Dying tumor cells release tumor-associated antigens and cytosolic components that can cause inflammation and stimulate potent immune responses.46 However, in the immunosuppressive tumor microenvironment, PDT alone is unable to elicit sufficient antitumor immune responses.47 Strategies combining cancer vaccines or ICBs with PDT have been widely reported to improve overall therapeutic efficacy.47,48 We have recently developed biodegradable mesoporous silica NPs (bMSN) carrying photosensitizer chlorin e6, CpG, and neoantigen peptides and have demonstrated their antitumor efficacy in bilateral tumor models, mimicking local and metastatic tumors (Figure 6).4 NPs were injected IV, and PDT was only applied to the primary tumor. Treatment with neoantigen, CpG, and PDT elicited a very potent systemic, neoantigen-specific CD8+ T cell response, compared with PDT alone. In local tumors, PDT + neoantigen + CpG treatment increased tumor infiltration of T cells and activated DCs. As a result, strong antitumor efficacy was achieved not only in locally PDT-treated tumors but also in distant, untreated tumors after the combination PDT-immunotherapy.4 Similar synergistic therapeutic effects and robust antitumor immunity have been reported by the combination of PDT and tumor cell fragments.49
Figure 6.
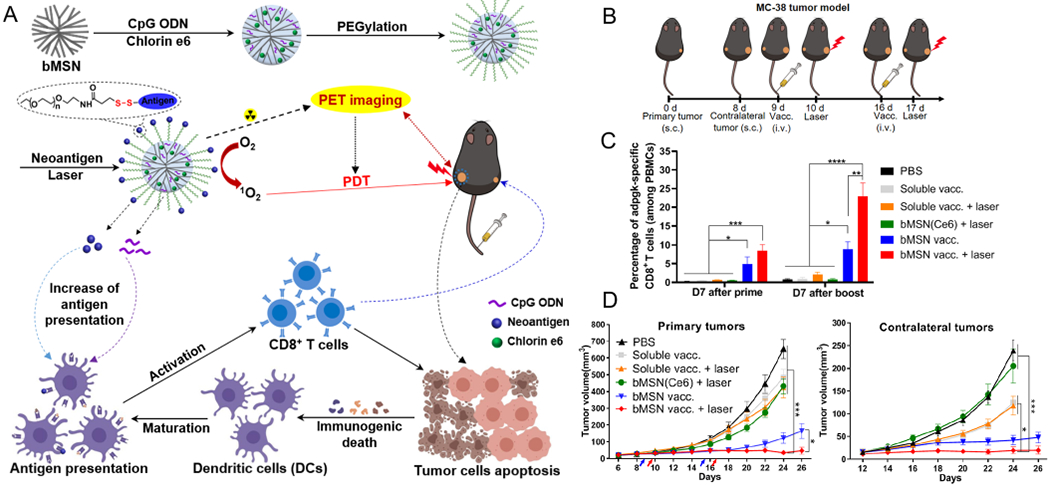
(A) Schematic illustration of neoantigen-based cancer nanovaccine: bMSN(CpG/Ce6)-neoantigen and its proposed mechanism of action for the combination PDT-immunotherapy. CpG and Ce6 were loaded into bMSN by electrostatic and hydrophobic interactions, respectively. Neoantigen peptides were conjugated to bMSN via the formation of disulfide bonds. PDT with laser irradiation (660 nm) generated cytotoxic ROS and eliminated tumor cells while triggering local immune activation for antitumor immunity. (B) Antitumor therapy study in MC-38 tumor-bearing mice. (C) Percentage neoantigen-specific CD8α+ T-cells in PBMCs. (D) Average tumor growth curves for the treated, primary tumors and untreated contralateral tumors. Reproduced with permission from ref 4. Copyright 2019 American Chemical Society.
Thermal ablation of local tumor cells with PTT is another promising and minimally invasive approach.50 Conventional PTT treatments employ the temperature in the range of 42–45 °C, which induces tumor cell death and releases TAAs.51,52 With near-infrared (NIR) radiation and NP-based phototherapeutic agents, PTT is able to penetrate deeper into solid tumors than PDT.53 We have recently reported that PTT mediated by polydopamine-coated spiky gold NPs (SGNP@PDA) could effectively treat ~100 mm3 CT-26 primary tumors and elicit moderate antitumor T-cell immunity simultaneously through the release of TAAs and immuno-stimulatory molecules, such as HSP70 and MULT-1.3 Our results also pointed out that hyperthermia might induce the accumulation of immunosuppressive myeloid-derived suppressor cells and regulatory T cells, particularly with nonlethal thermal doses, and that PTT monotherapy failed to show significant therapeutic efficacy in advanced and metastatic tumor models.33 Therefore, it is necessary to combine PTT with supplementary treatment methods, such as chemotherapy, cancer vaccines, or ICBs to enhance the antitumor immune response post PTT treatment.33,54,55 In our studies, we sought to amplify the immune activation triggered by PTT by inducing ICD of tumor cells with DOX chemotherapy. The combination of PTT and DOX chemotherapy elicited robust systemic immune responses and promoted the activation of CD8+ T cells and NK cells, resulting in significantly improved therapeutic efficacy against distant, untreated tumors.
Other examples of combination PTT-immunotherapy include the work from Chen et al.54 who designed a hybrid eukaryotic and prokaryotic nanoplatform composed of fused melanoma cytomembrane and bacterial outer membrane (as immune adjuvant). They used the nanoplatform as a cancer vaccine in combination with PTT, achieving enhanced antitumor efficacy with durable suppression of tumor cell growth. Zhang et al. generated gold NPs (AuNPs) intracellularly in B16F10 cells and then extracted the AuNPs retaining tumor antigens.55 This technology has shown the potential benefits of AuNP-mediated PTT combined with antigen-based immunotherapy, which led to efficient eradication of primary tumors and inhibition of tumor metastasis and relapse. A similar strategy has been previously reported based on polymeric NPs capturing tumor antigens for improving radio-immunotherapy.56
Similar to PDT, radiotherapy (RT) generates ROS, leading to DNA or organelle damage and cell death. While RT alone is often insufficient to treat metastatic tumors,57 radiotherapy combined with immunotherapy is emerging as a promising therapeutic strategy.58,59 Poly(lactic-co-glycolic) acid (PLGA)-based NPs carrying catalase (cat) and imiquimod (R837, a Toll-like-receptor-7 agonist) have been shown to enhance the radiotherapy efficiency by modulating the immune-suppressive tumor microenvironment.58 Furthermore, the combination therapy triggered the release of TAAs, leading to the inhibition of tumor metastases with a strong abscopal effect.58 Compared with phototherapy, radiation reaches deeper tissue, and this property can be used to excite the photosensitizer for ROS generation in a process known as radiodynamic therapy (RDT). Lu et al. developed a strategy that combines metal–organic framework (MOF) mediated RDT with ICBs for the effective elimination of both local and distant tumors.59 Intratumoral injection of MOF-carrying an indoleamine 2,3-dioxygenase (IDO) inhibitor combined with low doses of X-ray radiation eradicated local tumors and rejected distal tumors.
Chemodynamic therapy (CDT) is a new form of cancer therapy that can convert the intratumoral hydrogen peroxide (H2O2) to one of the most harmful ROS, hydroxyl radical (•OH), through a Fenton/Fenton-like reactions.60 CDT could elevate the level of tumor oxidative stress, leading to ICD of tumor cells and alleviation of immunosuppression in the tumor microenvironment.60 Notably, a recent study reported a combination cancer immunotherapy based on CDT, PDT, and starvation therapy.61 Hydroxyl radicals generated from glucose oxidase-loaded Cu2MoS4 (GOx-CMS) NPs induced ICD of tumor cells. In addition, the catalase-like property of CMS decomposed H2O2 to O2, thus enhancing the PDT and starvation therapy. When combined with anti-CTLA4, the combination therapy induced a strong antitumor immunity and effectively eradicated both primary and metastatic tumors.
Overall, antigen- and adjuvant-based cancer vaccines combined with other therapeutic strategies can effectively reverse the immunosuppressive tumor microenvironment, promote antitumor immunity, and inhibit tumor recurrence. In addition, the efficacy of cancer vaccines can be further amplified when tumors are debulked using either phototherapies, RT, or CDT. Therefore, combination therapy, such as photoimmunotherapy, radio-immunotherapy, or chemodynamic-immunotherapy, capable of ablating large local tumors while simultaneously eliciting a systemic antitumor immune response, offers a promising approach for the treatment of advanced cancers.
6. SUMMARY AND FUTURE OUTLOOK
In this Account, we have presented our recent progress on NP-based cancer immunotherapies. The commonality of these immunotherapies is nanotechnology, which offers various advantages. Nanoparticles can be designed for (1) targeted delivery of cargo materials to specific organs, tissues, and cells, (2) codelivery of antigens and adjuvants to APCs for strong immune activation, and (3) noninvasive and stimuli-responsive delivery of therapeutics to cancer cells, while (4) providing a safe and biocompatible platform for combination immunotherapy. Antigen-based nanovaccines increase the immunogenicity of tumor antigens and amplify antitumor T cells responses, while whole tumor cell-based nanovaccines present antigens in their native state. Nanomaterials designed for tumor-targeting could be employed to deliver chemotherapeutics for inducing ICB, while nanomedicine for phototherapies could be combined with immunotherapies to enhance their antitumor efficacy using minimally invasive methods. Each nanomedicine discussed in this Account is different from one another, and as they offer tunable physicochemical properties, an array of platforms can be designed to address individual challenges posed by different types of cancers.
However, key challenges remain in the clinical translation of NP-based cancer immunotherapy.62 Nanovaccines are shown to be efficacious in the research setting, but their clinical translation has been rather lackluster. Part of the reason for this is a focus on efficacy and a lack of understanding in correlations between nanovaccine behavior and both patient biology and disease heterogeneity.62 Therefore, studying mechanisms of action for NP-based therapies encompassing therapeutic efficacy, safety, biodistribution, and pharmacokinetics in animal models relevant to human disease is crucial.63 Furthermore, regulatory, analytical, safety, and manufacturing challenges remain for clinical translation of nanovaccines, which are generally more complex to produce than traditional small molecule and biologic cancer drugs. NPs, in general, require laborious, multistep synthesis processes that are difficult to manufacture at a large-scale during good manufacturing (cGMP) production.64–66 As discussed, nanovaccines have many properties (or parameters) that need to be evaluated to ensure quality control. In addition to a large number of parameters, the analytical instrumentation used to evaluate those that affect in vivo performance needs to be standardized to eliminate redundant, costly optimization efforts by multiple parties.65–67 A uniformly accepted definition of nanomedicine does not exist, partially due to this variability, making it difficult to not only classify nanomedicines, but establish specific regulatory guidelines for development and characterization at the biophysical level.68 In addition, biosafety of nanovaccines should be thoroughly evaluated in clinical trials because although they have the potential to be efficacious, synthetic base materials in nanovaccines could be reactive, and they are not subjected to the gastrointestinal absorption process after SC, IV, or IM injection, potentially leading to long-term accumulation in tissues or negative interactions with the host tissues.69
In summary, engineered nanomaterials offer promising platforms for improving cancer immunotherapy. Improvements in synthesis, manufacturing, and analysis of nanoparticles will be one aspect that dictates the speed at which nanomedicine for cancer immunotherapy becomes clinical drug products. Another aspect is the design of the nanomaterial itself. The goal should be set on translation, commercialization, and making treatments available to patients. Rather than solely focusing on novelty, which certainly has its place in the field, it is crucial that we continue to develop and improve on existing NP platforms, as well as design new NP-based cancer immunotherapies with these hurdles in mind.
KEY REFERENCES.
Kuai, R.; Ochyl, L. J.; Bahjat, K. S.; Schwendeman, A.; Moon, J. J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496.1 This is the first demonstration of nanovaccines personalized with neoantigens for induction of tumor-specific T cells with potent antitumor efficacy.
Xu, C.; Hong, H.; Lee, Y.; Park, K. S.; Sun, M.; Wang, T.; Aikins, M. E.; Xu, Y.; Moon, J. J. Efficient Lymph Node-Targeted Delivery of Personalized Cancer Vaccines with Reactive Oxygen Species-Inducing Reduced Graphene Oxide Nanosheets. ACS Nano 2020, 10.1021/acsnano.0c05062.2 Reactive oxygen species-inducing nanovaccine promotes antigen presentation by dendritic cells and delivers neoantigens to lymph nodes, generating strong antitumor T cell immunity.
Nam, J.; Son, S.; Ochyl, L. J.; Kuai, R.; Schwendeman, A.; Moon, J. J. Chemo-photothermal therapy combination elicits antitumor immunity against advanced metastatic cancer. Nat. Commun. 2018, 9, 1074.3 Photothermal therapy combined with chemotherapy triggers immunogenic cell death and antitumor immune responses, resulting in elimination of local, as well as untreated, distant tumors.
Xu, C.; Nam, J.; Hong, H.; Xu, Y.; Moon, J. J. Positron Emission Tomography-Guided Photodynamic Therapy with Biodegradable Mesoporous Silica Nanoparticles for Personalized Cancer Immunotherapy. ACS Nano 2019, 13, 12148–12161.4 Biodegradable mesoporous silica nanoparticles is a multifunctional platform for personalized cancer vaccination combined with imaging-guided photodynamic therapy.
ACKNOWLEDGMENTS
This work was supported in part by NIH (R01AI127070, R01CA210273, R01DK125087, U01CA210152). J.J.M. is supported by NSF CAREER Award (1553831).
Biographies
Biographies
Marisa Aikins is a Ph.D. student in the laboratory of Prof. James Moon. She received her bachelor’s degree from Oberlin College in 2015 and her M.S. in Biomedical Engineering from University of Michigan, Ann Arbor, in 2017. Her research interests focus on the cost-effective design and application of nanomaterials for cancer immunotherapy.
Dr. Cheng Xu is a postdoctoral fellow in the laboratory of Prof. James Moon. He received his Ph.D. in 2016 from Hunan University, China. His research interests focus on the biomedical application of inorganic nanomaterials.
Dr. James Moon is John Gideon Searle Associate Professor in the Department of Pharmaceutical Sciences and Biomedical Engineering at University of Michigan, Ann Arbor. His interdisciplinary research program aims to develop novel biomaterials-based strategies to improve vaccines and immunotherapies. Dr. Moon received his bachelor’s degree from University of California at Berkeley and his Ph.D. in Bioengineering from Rice University. He completed his postdoctoral training at MIT in the laboratory of Prof. Darrell Irvine.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.accounts.0c00456
The authors declare the following competing financial interest(s): Patent applications for the nanodisc technology have been filed. J.J.M. is a co-founder of EVOQ Therapeutics, LLC., that developed the nanodisc technology for applications in vaccines and immunotherapies.
Contributor Information
Marisa E. Aikins, Department of Pharmaceutical Sciences and Biointerfaces Institute, University of Michigan, Ann Arbor, Michigan 48109, United States
Cheng Xu, Department of Pharmaceutical Sciences and Biointerfaces Institute, University of Michigan, Ann Arbor, Michigan 48109, United States.
James J. Moon, Department of Pharmaceutical Sciences, Biointerfaces Institute, and Department of Biomedical Engineering, University of Michigan, Ann Arbor, Michigan 48109, United States.
REFERENCES
- (1).Kuai R; Ochyl LJ; Bahjat KS; Schwendeman A; Moon JJ Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater 2017, 16, 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Xu C; Hong H; Lee Y; Park KS; Sun M; Wang T; Aikins ME; Xu Y; Moon JJ Efficient Lymph Node-Targeted Delivery of Personalized Cancer Vaccines with Reactive Oxygen Species-Inducing Reduced Graphene Oxide Nanosheets. ACS Nano 2020, DOI: 10.1021/acsnano.0c05062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Nam J; Son S; Ochyl LJ; Kuai R; Schwendeman A; Moon JJ Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun 2018, 9, 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Xu C; Nam J; Hong H; Xu Y; Moon JJ Positron Emission Tomography-Guided Photodynamic Therapy with Biodegradable Mesoporous Silica Nanoparticles for Personalized Cancer Immunotherapy. ACS Nano 2019, 13, 12148–12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kuai R; Yuan W; Son S; Nam J; Xu Y; Fan Y; Schwendeman A; Moon JJ Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci. Adv 2018, 4, eaao1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Emens LA; Middleton G The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol. Res 2015, 3, 436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Li L; Goedegebuure SP; Gillanders WE Preclinical and clinical development of neoantigen vaccines. Ann. Oncol 2017, 28, xii11–xii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Melero I; Gaudernack G; Gerritsen W; Huber C; Parmiani G; Scholl S; Thatcher N; Wagstaff J; Zielinski C; Faulkner I; Mellstedt H Therapeutic vaccines for cancer: an overview of clinical trials. Nat. Rev. Clin. Oncol 2014, 11, 509–24. [DOI] [PubMed] [Google Scholar]
- (9).Schumacher TN; Schreiber RD Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [DOI] [PubMed] [Google Scholar]
- (10).Scheetz L; Park KS; Li Q; Lowenstein PR; Castro MG; Schwendeman A; Moon JJ Engineering patient-specific cancer immunotherapies. Nat. Biomed Eng 2019, 3, 768–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Kuai R; Li D; Chen YE; Moon JJ; Schwendeman A High-Density Lipoproteins: Nature’s Multifunctional Nanoparticles. ACS Nano 2016, 10, 3015–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Irvine DJ; Aung A; Silva M Controlling timing and location in vaccines. Adv. Drug Delivery Rev 2020, DOI: 10.1016/j.addr.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Kuai R; Sun X; Yuan W; Xu Y; Schwendeman A; Moon JJ Subcutaneous Nanodisc Vaccination with Neoantigens for Combination Cancer Immunotherapy. Bioconjugate Chem. 2018, 29, 771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Sandoval F; Terme M; Nizard M; Badoual C; Bureau MF; Freyburger L; Clement O; Marcheteau E; Gey A; Fraisse G; Bouguin C; Merillon N; Dransart E; Tran T; Quintin-Colonna F; Autret G; Thiebaud M; Suleman M; Riffault S; Wu TC; Launay O; Danel C; Taieb J; Richardson J; Zitvogel L; Fridman WH; Johannes L; Tartour E Mucosal imprinting of vaccine-induced CD8(+) T cells is crucial to inhibit the growth of mucosal tumors. Sci. Transl Med 2013, 5, 172ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Kuai R; Singh PB; Sun X; Xu C; Hassani Najafabadi A; Scheetz L; Yuan W; Xu Y; Hong H; Keskin DB; Jain R; Schwendeman A; Moon JJ Robust Anti-Tumor T Cell Response with Efficient Intratumoral Infiltration by Nanodisc Cancer Immunotherapy. Advanced Therapeutics 2020, 3, 2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Flickinger JC; Rodeck U; Snook AE Listeria monocytogenes as a Vector for Cancer Immunotherapy: Current Understanding and Progress. Vaccines (Basel, Switz.) 2018, 6, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Medina E; Guzman CA Use of live bacterial vaccine vectors for antigen delivery: potential and limitations. Vaccine 2001, 19, 1573–80. [DOI] [PubMed] [Google Scholar]
- (18).Scheetz L; Kadiyala P; Sun X; Son S; Hassani Najafabadi A; Aikins M; Lowenstein PR; Schwendeman A; Castro MG; Moon JJ Synthetic high-density lipoprotein nanodiscs for personalized immunotherapy against gliomas. Clin. Cancer Res 2020, 26, 4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hanif F; Muzaffar K; Perveen K; Malhi SM; Simjee Sh U Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac J. Cancer Prev 2017, 18, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Filley AC; Henriquez M; Dey M Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget 2017, 8, 91779–91794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Johanns TM; Ward JP; Miller CA; Wilson C; Kobayashi DK; Bender D; Fu Y; Alexandrov A; Mardis ER; Artyomov MN; Schreiber RD; Dunn GP Endogenous Neoantigen-Specific CD8 T Cells Identified in Two Glioblastoma Models Using a Cancer Immunogenomics Approach. Cancer Immunol. Res 2016, 4, 1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Vogelstein B; Papadopoulos N; Velculescu VE; Zhou S; Diaz LA Jr.; Kinzler KW Cancer genome landscapes. Science 2013, 339, 1546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hassani Najafabadi A; Zhang J; Aikins ME; Najaf Abadi ZI; Liao F; Qin Y; Okeke EB; Scheetz LM; Nam J; Xu Y; Adams D; Lester P; Hetrick T; Schwendeman A; Wicha MS; Chang AE; Li Q; Moon JJ Cancer Immunotherapy via Targeting Cancer Stem Cells Using Vaccine Nanodiscs. Nano Lett. 2020, DOI: 10.1021/acs.nanolett.0c03414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Jordan CT; Guzman ML; Noble M Cancer stem cells. N. Engl. J. Med 2006, 355, 1253–61. [DOI] [PubMed] [Google Scholar]
- (25).Kuai R; Sun X; Yuan W; Ochyl LJ; Xu Y; Hassani Najafabadi A; Scheetz L; Yu MZ; Balwani I; Schwendeman A; Moon JJ Dual TLR agonist nanodiscs as a strong adjuvant system for vaccines and immunotherapy. J. Controlled Release 2018, 282, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Madan-Lala R; Pradhan P; Roy K Combinatorial Delivery of Dual and Triple TLR Agonists via Polymeric Pathogen-like Particles Synergistically Enhances Innate and Adaptive Immune Responses. Sci. Rep 2017, 7, 2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Lynn GM; Sedlik C; Baharom F; Zhu Y; Ramirez-Valdez RA; Coble VL; Tobin K; Nichols SR; Itzkowitz Y; Zaidi N; Gammon JM; Blobel NJ; Denizeau J; de la Rochere P; Francica BJ; Decker B; Maciejewski M; Cheung J; Yamane H; Smelkinson MG; Francica JR; Laga R; Bernstock JD; Seymour LW; Drake CG; Jewell CM; Lantz O; Piaggio E; Ishizuka AS; Seder RA Peptide-TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat. Biotechnol 2020, 38, 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Sondak VK; Sosman JA Results of clinical trials with an allogenic melanoma tumor cell lysate vaccine: Melacine. Semin. Cancer Biol 2003, 13, 409–15. [DOI] [PubMed] [Google Scholar]
- (29).Joshi VB; Geary SM; Gross BP; Wongrakpanich A; Norian LA; Salem AK Tumor lysate-loaded biodegradable microparticles as cancer vaccines. Expert Rev. Vaccines 2014, 13, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Chesson CB; Zloza A Nanoparticles: augmenting tumor antigen presentation for vaccine and immunotherapy treatments of cancer. Nanomedicine (London, U. K.) 2017, 12, 2693–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Ochyl LJ; Bazzill JD; Park C; Xu Y; Kuai R; Moon JJ PEGylated tumor cell membrane vesicles as a new vaccine platform for cancer immunotherapy. Biomaterials 2018, 182, 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Jin J; Krishnamachary B; Barnett JD; Chatterjee S; Chang D; Mironchik Y; Wildes F; Jaffee EM; Nimmagadda S; Bhujwalla ZM Human Cancer Cell Membrane-Coated Biomimetic Nanoparticles Reduce Fibroblast-Mediated Invasion and Metastasis and Induce T-Cells. ACS Appl. Mater. Interfaces 2019, 11, 7850–7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Nam J; Son S; Park KS; Zou W; Shea LD; Moon JJ Cancer nanomedicine for combination cancer immunotherapy. Nature Reviews Materials 2019, 4, 398–414. [Google Scholar]
- (34).Montico B; Nigro A; Casolaro V; Dal Col J Immunogenic Apoptosis as a Novel Tool for Anticancer Vaccine Development. Int. J. Mol. Sci 2018, 19, 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kroemer G; Galluzzi L; Kepp O; Zitvogel L Immunogenic cell death in cancer therapy. Annu. Rev. Immunol 2013, 31, 51–72. [DOI] [PubMed] [Google Scholar]
- (36).Chao Y; Liang C; Tao H; Du Y; Wu D; Dong Z; Jin Q; Chen G; Xu J; Xiao Z; Chen Q; Wang C; Chen J; Liu Z Localized cocktail chemoimmunotherapy after in situ gelation to trigger robust systemic antitumor immune responses. Sci. Adv 2020, 6, eaaz4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Chen Q; Chen M; Liu Z Local biomaterials-assisted cancer immunotherapy to trigger systemic antitumor responses. Chem. Soc. Rev 2019, 48, 5506–5526. [DOI] [PubMed] [Google Scholar]
- (38).Fan Y; Kuai R; Xu Y; Ochyl LJ; Irvine DJ; Moon JJ Immunogenic Cell Death Amplified by Co-localized Adjuvant Delivery for Cancer Immunotherapy. Nano Lett. 2017, 17, 7387–7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Wu J; Waxman DJ Immunogenic chemotherapy: Dose and schedule dependence and combination with immunotherapy. Cancer Lett. 2018, 419, 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Kadiyala P; Li D; Nunez FM; Altshuler D; Doherty R; Kuai R; Yu M; Kamran N; Edwards M; Moon JJ; Lowenstein PR; Castro MG; Schwendeman A High-Density Lipoprotein-Mimicking Nanodiscs for Chemo-immunotherapy against Glioblastoma Multiforme. ACS Nano 2019, 13, 1365–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Yong T; Zhang X; Bie N; Zhang H; Zhang X; Li F; Hakeem A; Hu J; Gan L; Santos HA; Yang X Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun 2019, 10, 3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Gao D; Guo X; Zhang X; Chen S; Wang Y; Chen T; Huang G; Gao Y; Tian Z; Yang Z Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Mater. Today Bio 2020, 5, 100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Hou X; Tao Y; Pang Y; Li X; Jiang G; Liu Y Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int. J. Cancer 2018, 143, 3050–3060. [DOI] [PubMed] [Google Scholar]
- (44).Chen Q; Xu L; Liang C; Wang C; Peng R; Liu Z Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun 2016, 7, 13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Dolmans DE; Fukumura D; Jain RK Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–7. [DOI] [PubMed] [Google Scholar]
- (46).Sun X; Cao Z; Mao K; Wu C; Chen H; Wang J; Wang X; Cong X; Li Y; Meng X; Yang X; Yang YG; Sun T Photodynamic therapy produces enhanced efficacy of antitumor immunotherapy by simultaneously inducing intratumoral release of sorafenib. Biomaterials 2020, 240, 119845. [DOI] [PubMed] [Google Scholar]
- (47).He C; Duan X; Guo N; Chan C; Poon C; Weichselbaum RR; Lin W Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun 2016, 7, 12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Shao Y; Liu B; Di Z; Zhang G; Sun LD; Li L; Yan CH Engineering of Upconverted Metal-Organic Frameworks for Near-Infrared Light-Triggered Combinational Photodynamic/Chemo-/Immunotherapy against Hypoxic Tumors. J. Am. Chem. Soc 2020, 142, 3939–3946. [DOI] [PubMed] [Google Scholar]
- (49).Ding B; Shao S; Yu C; Teng B; Wang M; Cheng Z; Wong KL; Ma P; Lin J Large-Pore Mesoporous-Silica-Coated Upconversion Nanoparticles as Multifunctional Immunoadjuvants with Ultrahigh Photosensitizer and Antigen Loading Efficiency for Improved Cancer Photodynamic Immunotherapy. Adv. Mater 2018, 30, e1802479. [DOI] [PubMed] [Google Scholar]
- (50).Xu C; Yang D; Mei L; Li Q; Zhu H; Wang T Targeting chemophotothermal therapy of hepatoma by gold nanorods/graphene oxide core/shell nanocomposites. ACS Appl. Mater. Interfaces 2013, 5, 12911–20. [DOI] [PubMed] [Google Scholar]
- (51).Zhu X; Feng W; Chang J; Tan YW; Li J; Chen M; Sun Y; Li F Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat. Commun 2016, 7, 10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Tian Q; Hu J; Zhu Y; Zou R; Chen Z; Yang S; Li R; Su Q; Han Y; Liu X Sub-10 nm Fe3O4@Cu(2-x)S core-shell nanoparticles for dual-modal imaging and photothermal therapy. J. Am. Chem. Soc 2013, 135, 8571–7. [DOI] [PubMed] [Google Scholar]
- (53).Liu Y; Bhattarai P; Dai Z; Chen X Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev 2019, 48, 2053–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Chen Q; Huang G; Wu W; Wang J; Hu J; Mao J; Chu PK; Bai H; Tang G A Hybrid Eukaryotic-Prokaryotic Nanoplatform with Photothermal Modality for Enhanced Antitumor Vaccination. Adv. Mater 2020, 32, e1908185. [DOI] [PubMed] [Google Scholar]
- (55).Zhang D; Wu T; Qin X; Qiao Q; Shang L; Song Q; Yang C; Zhang Z Intracellularly Generated Immunological Gold Nanoparticles for Combinatorial Photothermal Therapy and Immunotherapy against Tumor. Nano Lett. 2019, 19, 6635–6646. [DOI] [PubMed] [Google Scholar]
- (56).Min Y; Roche KC; Tian S; Eblan MJ; McKinnon KP; Caster JM; Chai S; Herring LE; Zhang L; Zhang T; DeSimone JM; Tepper JE; Vincent BG; Serody JS; Wang AZ Antigencapturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol 2017, 12, 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Ko EC; Formenti SC Radiation therapy to enhance tumor immunotherapy: a novel application for an established modality. Int. J. Radiat. Biol 2019, 95, 936–939. [DOI] [PubMed] [Google Scholar]
- (58).Chen Q; Chen J; Yang Z; Xu J; Xu L; Liang C; Han X; Liu Z Nanoparticle-Enhanced Radiotherapy to Trigger Robust Cancer Immunotherapy. Adv. Mater 2019, 31, e1802228. [DOI] [PubMed] [Google Scholar]
- (59).Lu K; He C; Guo N; Chan C; Ni K; Lan G; Tang H; Pelizzari C; Fu YX; Spiotto MT; Weichselbaum RR; Lin W Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed Eng 2018, 2, 600–610. [DOI] [PubMed] [Google Scholar]
- (60).Wang X; Zhong X; Liu Z; Cheng L Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today 2020, 35, 100946. [Google Scholar]
- (61).Chang M; Wang M; Wang M; Shu M; Ding B; Li C; Pang M; Cui S; Hou Z; Lin J A Multifunctional Cascade Bioreactor Based on Hollow-Structured Cu2MoS4 for Synergetic Cancer Chemo-Dynamic Therapy/Starvation Therapy/Phototherapy/Immunotherapy with Remarkably Enhanced Efficacy. Adv. Mater 2019, 31, e1905271. [DOI] [PubMed] [Google Scholar]
- (62).Hare JI; Lammers T; Ashford MB; Puri S; Storm G; Barry ST Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Delivery Rev 2017, 108, 25–38. [DOI] [PubMed] [Google Scholar]
- (63).Hua S; de Matos MBC; Metselaar JM; Storm G Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol 2018, 9, 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Kumar Teli M; Mutalik S; Rajanikant GK Nanotechnology and nanomedicine: going small means aiming big. Curr. Pharm. Des 2010, 16, 1882–92. [DOI] [PubMed] [Google Scholar]
- (65).Tinkle S; McNeil SE; Muhlebach S; Bawa R; Borchard G; Barenholz YC; Tamarkin L; Desai N Nanomedicines: addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci 2014, 1313, 35–56. [DOI] [PubMed] [Google Scholar]
- (66).Sainz V; Conniot J; Matos AI; Peres C; Zupanǒiǒ E; Moura L; Silva LC; Florindo HF; Gaspar RS Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun 2015, 468, 504–510. [DOI] [PubMed] [Google Scholar]
- (67).Gaspar R Regulatory issues surrounding nanomedicines: setting the scene for the next generation of nanopharmaceuticals. Nanomedicine (London, U. K.) 2007, 2, 143–7. [DOI] [PubMed] [Google Scholar]
- (68).Agrahari V; Agrahari V Facilitating the translation of nanomedicines to a clinical product: challenges and opportunities. Drug Discovery Today 2018, 23, 974–991. [DOI] [PubMed] [Google Scholar]
- (69).Su H; Wang Y; Gu Y; Bowman L; Zhao J; Ding M Potential applications and human biosafety of nanomaterials used in nanomedicine. J. Appl Toxicol 2018, 38, 3–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


