Abstract
Objective
To evaluate the efficacy of rapid on-site cytological evaluation (ROSE) in determining specimen adequacy and diagnostic accuracy in the interventional diagnosis of lung lesions.
Methods
This retrospective study included 127 consecutive cases of lung lesions, which were sampled by bronchoscopy or transthoracic fine needle aspiration, and diagnosed on ROSE followed by histopathology. ROSE was performed by a trained pulmonologist and the diagnosis of ROSE was compared with the final diagnosis.
Results
The sensitivity of ROSE in determining adequacy of specimens was 97.5% and specificity in determining inadequacy was 85.7%. The diagnostic efficacy of ROSE for assessing malignancy (sensitivity of 94.5% and specificity of 100%) and non-malignancy (sensitivity of 97.8% and specificity of 100%) was excellent. The sensitivity of ROSE for diagnosing small cell carcinoma (100%) was highest, followed by adenocarcinoma (89.2%) and squamous cell carcinoma (75.0%). Performance of ROSE by a trained pulmonologist also determined tuberculosis with a high diagnostic sensitivity (83.3%) and specificity (100%).
Conclusions
A trained pulmonologist can reliably carry out ROSE to ensure the adequacy of the sample, distinguish between malignancy and non-malignancy, and make a preliminary diagnosis in a large number of cases.
Keywords: Cytology, lung lesion, rapid on-site evaluation, diagnostic accuracy, interventional pulmonology, malignancy
Introduction
Interventional pulmonology is defined as “the art and science of medicine as related to the performance of diagnostic and invasive therapeutic procedures that require additional training and expertise beyond that required in a standard pulmonary medicine training programme” by the European Respiratory Society/American Thoracic Society.1 Rapid development of diagnostic bronchoscopy and guidance technology, such as endobronchial ultrasound (EBUS), together with transthoracic fine needle aspiration (FNA) under computed tomographic (CT) guidance (CT-FNA) has raised diagnostic yield and reduced complications. This has led to minimally invasive interventional techniques being widely considered as the preferential diagnostic approaches for lung lesions. Moreover, 70% of lung cancer is unresectable in the advanced stages. Management of these patients includes personalized medicine where treatment decisions are made on the basis of specific histology and molecular characteristics of the tumor.2 Therefore, interventional diagnostic techniques play an important role in this situation.
The rapid on-site cytological evaluation (ROSE) system provides immediate feedback regarding adequacy of the specimens obtained during the examination and guides the operators to modify the sampling technique, and the site and depth sampled. Therefore, ROSE decreases the number of passes required for an adequate sample, improves the diagnostic yield, reduces the risk of complications, and reduces costs.3–5 Ideal ROSE reporting has two components of on-site adequacy and diagnostic category. There are various criteria for determining on-site adequacy, including a certain amount of lymphocytes, the presence of diagnostic smears, and abundant pigment-laden macrophages.6 The International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society have provided a standardized classification for diagnosis of lung cancer considering small biopsies and cytology.7 This classification cytomorphologically describes adenocarcinoma as a flat, cohesive sheet of relatively uniform-appearing glandular cells characterized by mild variability in nuclear sizes, a delicate cytoplasm, and a low level of disruption of polarity. Additionally, squamous cell carcinoma is described as a flat mosaic sheet of malignant epithelial cells characterized by a dense (or opaque) cyanophilic cytoplasm, and obviously hyperchromatic nuclei with small chromocenters and/or nucleoli.
The extent to which a pulmonary physician, who often performs ROSE, can master these cytological characteristics to aid interventional diagnosis of lung lesions is unclear. Therefore, we evaluated the efficacy of ROSE for determining specimen adequacy and diagnostic accuracy in lung lesions. We retrospectively compared the diagnosis of ROSE with the final diagnosis made on formalin-fixed and paraffin-embedded tissue obtained by biopsy during interventional sampling and/or on resected tissue from operable lesions.
Materials and methods
Patients with pulmonary/mediastinal mass lesions that were sampled through minimally invasive techniques and diagnosed by ROSE followed by histopathology were included. Cases without ROSE or histopathological diagnosis were ruled out. Our retrospective study included 127 consecutive cases of pulmonary/mediastinal mass lesions during July 2018 to April 2019. For each patient, age and sex, techniques used for sampling, the site of sampling, the original ROSE report, and diagnosis made on histopathology were recorded. This retrospective study was approved by the Institutional Review Board of Hubei Public Health Clinical Center, the central Hospital of Wuhan (2020–196). All patients signed informed consent forms of sampling by interventional techniques and written informed consent for this study was waived.
The following techniques were used by pulmonologists on the basis of radiological characteristics of the lung lesions: transbronchial biopsy (TBB); endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) (22-gauge needle; Olympus Corporation, Tokyo, Japan and EBUS PENTAX; Miyagi Factory HOYA Corporation, Tokyo, Japan); transbronchial lung biopsy (TBLB) either directly or under radial probe endobronchial ultrasound guidance (UM-S20–20R; Olympus Corporation); and CT-FNA either alone or in combination. Surface anesthesia and/or general anesthesia with a laryngeal mask airway were freely decided by the operators.
ROSE was performed in the procedural room by a pulmonary physician who received training in cytopathology for 3 months. Every sample, brush, clamp, or needle aspiration was first evaluated by ROSE. A minimal amount of the specimen was smeared on slides, air dried, and stained with Diff-Quik (BA4150; Baso Diagnostics Corporation, Zhuhai, China,). The remaining specimen was kept for pathological tests and/or microbial tests. The staining process for ROSE was as follows. A drop of stain A (Diff-Quik, BA4150; Baso Diagnostics Corporation) was placed on freshly prepared air-dried smear for 30 s, washed with phosphate-buffered saline, stained with solution B for 40 s, and washed with phosphate-buffered saline. The smear was observed for specimen adequacy and diagnostic category. Adequacy of the smear for EBUS-TBNA was assessed6 as follows: (1) adequacy of lymphocytes where more than 41 lymphocytes were observed in the most cellular area of the slide on × 40 magnification; (2) abundant pigment-laden macrophages; and (3) diagnostic smears (malignancy or granulomas) were present. For other specimens, on-site adequacy was assessed by the presence of relatively abundant and well-visualized lesional material as proposed by Burlingame et al.8 The sampling stopped until ROSE reported an adequate specimen, and when inadequacy persisted, the biopsy was stopped on the basis of the operators’ and the patients’ tolerance.
ROSE also provided the diagnostic category, such as malignant, atypical, or negative for malignancy. Additionally, a specific diagnosis was further provided if possible because a pulmonologist can combine clinical features, radiological findings, and cytological characteristics together to make a diagnosis. Some cytological criteria of malignancy have been previously reported.4,7 Aggregates of epithelioid histiocytes associated with a necrotic background and a moderate amount of lymphocytes in cytological images, together with other features, such as symptoms of fever and cough, a positive result of interferon gamma release assay, and a tree-in-bud pattern, necrosis, or cavity in radiological images indicated the probable diagnosis of tuberculosis. Granulomatous inflammation without obvious necrosis in cytological images and symmetrical bilateral lymphadenopathy in radiological images highly indicated sarcoidosis.
The final diagnosis was made using histopathology and/or microbial tests, and polymerase chain reaction was used if necessary. For some cases, such as sarcoidosis, patients were followed up after treatment to confirm the diagnosis. Nonspecific inflammation was defined as inflammatory cells that were aggregated in tissues without definite microbial results. The diagnosis made by the pulmonologist who performed ROSE was compared with the final diagnosis.
In our study, the sampling method was decided by the operators and the proficiency of operators was different, which might be considered as investigator bias. Therefore, four experienced pulmonologists who participated in the study chose a sampling method and performed interventional sampling procedures independently to reduce investigator bias. The histopathological diagnosis was made by at least two pathologists who did not know the diagnosis of ROSE. However, the bias in performing ROSE could not be entirely eliminated because there was one pulmonologist who was not blinded to the patients and the diagnosis of ROSE depended on the experience of this pulmonologist.
The efficacy of ROSE in assessing specimen adequacy was shown by the sensitivity, specificity, positive predictive value, and negative predictive value. The efficacy of ROSE for sub-classifying the morphological type of lung lesions was shown by diagnostic sensitivity. SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA) was used for analysis.
Results
The mean (±standard deviation) age of the patients was 54.9 ± 11.7 years and there were 72 men and 55 women. As shown in Table 1, six (4.7%) cases were undiagnosed because these patients refused surgery or other techniques for sampling after failing to obtain an adequate specimen by bronchoscopy. Three (2.4%) cases were diagnosed as atypical cells. A total of 94.5% (69 cases) of the 73 malignant cases were pulmonary source tumors and 5.5% (4 cases) were non-pulmonary source tumors. Adenocarcinoma accounted for the majority of lung cancer, followed by squamous cell carcinoma and small cell carcinoma. One patient received CT-FNA immediately after failing to obtain adequate tissue by TBLB, and the diagnosis of adenocarcinoma was finally made. In the non-malignant group, the diagnosis was mostly nonspecific inflammation and tuberculosis. The majority of specimens were obtained by TBB (53 cases) and EBUS-TBNA (30 cases), followed by TBLB (24 cases) and CT-FNA (21 cases). The distribution of the lesions sampled is shown in Table 2. The right lower lobe location was slightly preferred (26 cases). The locations of EBUS-TBNA were 4R and 7 lymph node stations, and both 4R and 7 lymph nodes were sampled in two patients.
Table 1.
Distribution of specimen types that were sampled by interventional diagnostic techniques on the basis of the final diagnosis.
| Categories | Percentage (number of cases/total number of cases) | Diagnosis | Percentage (number of cases/total number of cases) | Interventional diagnostic techniques used (n) |
|||
|---|---|---|---|---|---|---|---|
| TBB | TBLB | EBUS-TBNA | CT-FNA | ||||
| Malignancy | 57.5 (73/127) | ||||||
| Pulmonary source tumors | 94.5 (69/73) | Adenocarcinoma | 53.6 (37/69) | 16 | 6 | 4 | 12 |
| Squamous cell carcinoma | 29.0 (20/69) | 14 | 2 | 4 | |||
| Small cell carcinoma | 14.5 (10/69) | 4 | 1 | 4 | 1 | ||
| Sarcomatoid carcinoma | 2.9 (2/69) | 2 | |||||
| Non-pulmonary source tumors | 5.5 (4/73) | Lymphoma | 25 (1/4) | 1 | |||
| Adenoid cystic carcinoma | 25 (1/4) | 1 | |||||
| Metastatic malignancy | 50 (2/4) | 1 | 1 | ||||
| Non-malignancy | 35.4 (45/127) | Sarcoidosis | 6.7 (3/45) | 3 | |||
| Tuberculosis | 26.7 (12/45) | 5 | 3 | 3 | 1 | ||
| Nonspecific inflammation | 62.2 (28/45) | 6 | 12 | 8 | 2 | ||
| Aspergillosis | 2.2 (1/45) | 1 | |||||
| Leiomyoma | 2.2 (1/45) | 1 | |||||
| Other | 7.1 (9/127) | Atypical cells | 2.4 (3/127) | 2 | 1 | ||
| Undiagnosed | 4.7 (6/127) | 4 | 2 | ||||
TBB, transbronchial biopsy; TBLB, transbronchial lung biopsy; EBUS-TBNA, endobronchial ultrasound-transbronchial needle aspiration; CT-FNA, fine needle aspiration under computed tomographic guidance.
Table 2.
Sampling locations of different interventional diagnostic techniques.
| Sampling locations | Interventional diagnostic techniques used (n) |
|||
|---|---|---|---|---|
| TBB | TBLB | CT-FNA | EBUS-TBNA | |
| LUL | 6 | 6 | 5 | |
| LLL | 13 | 4 | 3 | |
| RUL | 9 | 7 | 6 | |
| RML | 7 | 3 | 2 | |
| RLL | 17 | 4 | 5 | |
| Trachea | 1 | |||
| 4R lymph nodes | 11 | |||
| 4L lymph nodes | 6 | |||
| 7 lymph nodes | 15 | |||
TBB, transbronchial biopsy; TBLB, transbronchial lung biopsy; CT-FNA, fine needle aspiration under computed tomographic guidance; EBUS-TBNA, endobronchial ultrasound-transbronchial needle aspiration; LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe.
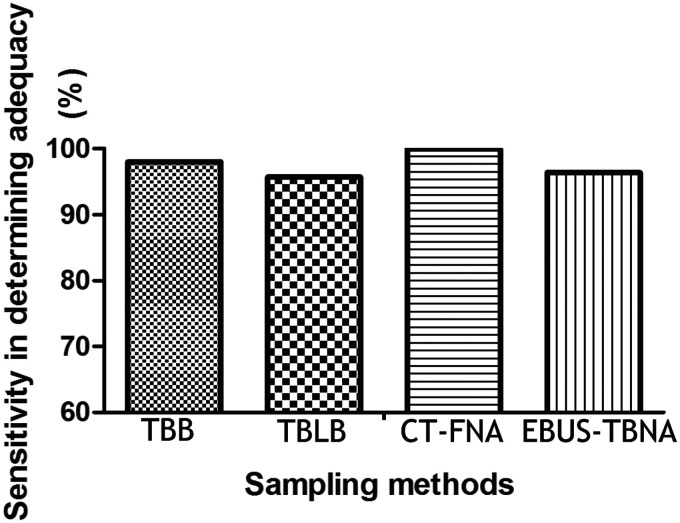
The efficacy of ROSE for determining adequacy is shown in Table 3. ROSE successfully confirmed adequacy in most cases. However, three cases were falsely determined as inadequate by ROSE, while they were diagnosed as adenocarcinoma, primary tracheal leiomyoma, and atypical cells on histopathology. Notably, there was one case in our study in which ROSE was adequate and the case was diagnosed as adenocarcinoma, but histopathology suggested an inadequate specimen. Although ROSE failed in determining adequacy in this case, it resulted in the correct diagnosis because the patient was confirmed as having adenocarcinoma by histopathology after surgery in another hospital. The sensitivity of ROSE in determining adequacy was 97.5% and specificity in determining inadequacy was 85.7%. The positive predictive value was 99.2% and the negative predictive value was 66.7%. The sensitivity of ROSE in determining adequacy was highest in the CT-FNA group, followed by the TBB group and the EBUS-TBNA group (Figure 1). ROSE was more sensitive when the lesion was more precisely located and a larger specimen was obtained.
Table 3.
Efficacy of ROSE in assessing specimen adequacy.
| Adequacy by ROSE | Adequacy by histopathology |
|
|---|---|---|
| Positive (n) | Negative (n) | |
| Positive | 117 | 1 |
| Negative | 3 | 6 |
| Total | 120 | 7 |
ROSE, rapid on-site cytological evaluation.
Figure 1.
Sensitivity of rapid on-site cytological evaluation in determining adequacy of specimens for TBB, TBLB, CT-FNA, and EBUS-TBNA.
TBB, transbronchial biopsy; TBLB, transbronchial lung biopsy; CT-FNA, fine needle aspiration under computed tomographic guidance; EBUS-TBNA, endobronchial ultrasound-transbronchial needle aspiration.
The association between ROSE and histopathology, as well as the diagnostic sensitivity and specificity of ROSE in various lung lesions, are shown in Table 4. The diagnostic efficacy of ROSE for determining malignancy (sensitivity of 94.5% and specificity of 100%) and non-malignancy (sensitivity of 97.8% and specificity of 100%) was excellent. ROSE was used to further make a specific diagnosis when possible. The sensitivity of ROSE for diagnosing small cell carcinoma was highest, followed by adenocarcinoma and squamous cell carcinoma. In the non-malignant group, ROSE also determined tuberculosis with a high diagnostic sensitivity (83.3%) and specificity (100%).
Table 4.
Efficacy of ROSE in diagnostic accuracy.
| Diagnosis | ROSE (number of cases) | Histopathology (number of cases) | Diagnostic efficacy of ROSE (%) |
|
|---|---|---|---|---|
| Sensitivity | Specificity | |||
| Malignancy | 69 | 73 | 94.5 | 100 |
| Adenocarcinoma | 33 | 37 | 89.2 | 100 |
| Squamous cell carcinoma | 15 | 20 | 75.0 | 100 |
| Small cell carcinoma | 10 | 10 | 100 | 100 |
| Sarcomatoid carcinoma | 0 | 2 | ||
| Lymphoma | 1 | 1 | ||
| Adenoid cystic carcinoma | 0 | 1 | ||
| Metastatic malignancy | 0 | 2 | ||
| Unclassified | 10 | 0 | ||
| Non-malignancy | 44 | 45 | 97.8 | 100 |
| Sarcoidosis | 1 | 3 | ||
| Tuberculosis | 10 | 12 | 83.3 | 100 |
| Nonspecific inflammation | 32 | 28 | 100 | 96.0 |
| Aspergillosis | 1 | 1 | ||
| Leiomyoma | 0 | 1 | ||
| Atypical cells | 5 | 3 | ||
| Undiagnosed | 9 | 6 | ||
ROSE, rapid on-site cytological evaluation.
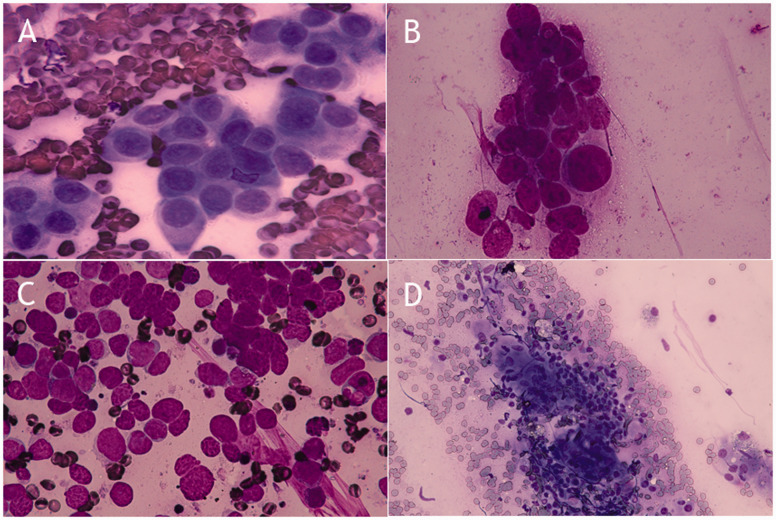
We summarized several cytomorphological characteristics in relation to the histotype of the tumor and tuberculosis (Figure 2). Adenocarcinoma showed small clusters of uniform-appearing glandular cells with a delicate cytoplasm (Figure 2a). For squamous cell carcinoma, tumor cells with large nuclei, macronucleoli, and varying nuclear/cytoplasmic ratios were observed in a dirty, necrotic background, and keratinization was occasionally observed (Figure 2b). Small cell carcinoma cells had a scant cytoplasm, and their nuclei were round, oval, or spindle-shaped. Additionally, these cells showed nuclear molding with a dispersed granular chromatin pattern (Figure 2c). For tuberculosis, aggregates of epithelioid histiocytes associated with a necrotic background with a moderate amount of lymphocytes were observed (Figure 2d).
Figure 2.
Cytomorphological characteristics with rapid on-site cytological evaluation (stained with Diff-Quik). In adenocarcinoma, small clusters of uniform-appearing glandular cells with a delicate cytoplasm can be seen (100× magnification) (a). In squamous cell carcinoma, tumor cells with large nuclei, macronucleoli, and varying nuclear/cytoplasmic ratios in a dirty necrotic background can be seen (100× magnification) (b). In small cell carcinoma, tumor cells with a scant cytoplasm, nuclear molding, and dispersed granular chromatin can be seen (100× magnification) (c). In tuberculosis, aggregates of epithelioid histiocytes associated with a necrotic background and a moderate amount of lymphocytes can be seen (40× magnification) (d).
Discussion
With the development of interventional pulmonology, an increasing amount of lung lesions can be sampled by minimally invasive techniques.1 ROSE is recommended in the sampling process to improve diagnostic accuracy, reduce the risk of complications, and enable appropriate specimen triage.9–11 With ROSE, the yield of conventional TBNA is similar to that of EBUS-TBNA and this is especially relevant in the case of sarcoidosis.12 Even at centers where an EBUS facility is available, conventional TBNA with ROSE can provide a diagnostic yield similar to EBUS. This is useful in settings where EBUS-TBNA is not available. With ROSE, the diagnostic yield may be optimized.13 Because ROSE is just beginning to be used in China, the experience of this technique should be summarized and many questions need to be clarified. Unfortunately, few studies have focused on the diagnostic accuracy of ROSE by comparing it with histopathology.
Consistent with a study by Anila et al.,14 we found that ROSE had excellent efficacy in determining adequacy of specimens. In three cases, ROSE resulted in a false negative result of inadequacy and these cases were diagnosed as adenocarcinoma, primary tracheal leiomyoma, and atypical cells by histopathology. In our study, Minnesota Criteria6 and criteria proposed by Burlingame et al.8 were used to determine on-site adequacy. Currently, there are no uniform criteria for on-site adequacy of ROSE. Moreover, a small amount of specimens, mass red cell contamination, and a lack of experience in cytopathology by a pulmonologist might account for these false negative results. Additionally, in one patient, there was a false positive result in determining adenocarcinoma by ROSE and inadequacy with histopathology. This patient was finally diagnosed with adenocarcinoma after surgery. A similar situation was previously reported,14 and there was speculation that a difficult biopsy or intolerance of the patient caused few specimens to be obtained. Additionally, fragmentation of specimens into pieces and diagnostic material with a different distribution might have caused this situation. However, falsely determined adequacy appeared to be an advantage, instead of a limitation, of ROSE in our study.
ROSE was useful in determining malignancy and in preliminarily identifying subtypes of lung tumors in our study, which would help to define the number of samples to be taken. In cases of squamous cell or small cell carcinoma, a single sample may be sufficient, whereas multiple samples are required for adenocarcinoma to permit biomolecular characterization. Additionally, tuberculosis can also be preliminarily diagnosed by ROSE when symptoms, imaging features, and cytological characteristics are considered together. Several studies4,7,15 have reported cytological characteristics of common lung diseases. These studies showed the presence of nucleoli and small/medium cell clusters in adenocarcinoma and the presence of large cluster-forming cells and extensive necrosis in squamous cell carcinoma. Additionally, single cells with molding and moderate necrosis were characteristic of small cell carcinoma, and granulomatous inflammation with a large amount of necrosis was indicative of tuberculosis.
Training a pulmonologist to perform ROSE when a cytopathologist is not available regularly is an alternative. A trained pulmonologist can reliably assess adequacy and malignancy for endosonography-derived samples.16 Furthermore, an 81% overall agreement was reported between a pulmonologist and cytopathologist in evaluating ROSE when determining adequacy of specimens.17 Some “user-friendly” criteria of ROSE have been reported to histologically classify cytological specimens.4,15 A pulmonologist can better combine clinical features and cytological characteristics together to make a diagnosis compared with a cytopathologist. Therefore, the pulmonologist who performed ROSE in our study not only assessed adequacy, but also provided a preliminary diagnosis of the most frequent lung diseases, and the diagnostic accuracy was high.
In conclusion, ROSE is comparable with histology in ensuring the adequacy of samples and sub-classifying the morphological type of lung lesions in many cases. This would be beneficial in clinical practice. A trained pulmonologist can reliably carry out ROSE.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Mingli Yuan https://orcid.org/0000-0003-0960-5156
References
- 1.Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J 2002; 19: 356–373. [DOI] [PubMed] [Google Scholar]
- 2.Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc 2009; 15: 201–205. [DOI] [PubMed] [Google Scholar]
- 3.Chen CH, Cheng WC, Wu BR, et al. Improved diagnostic yield of bronchoscopy in peripheral pulmonary lesions: combination of radial probe endobronchial ultrasound and rapid on-site evaluation. J Thorac Dis 2015; 7: S418–S425. DOI: 10.3978/j.issn.2072-1439.2015.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandra S, Chandra H, Sindhwani G. Role of rapid on-site evaluation with cyto-histopathological correlation in diagnosis of lung lesion. J Cytol 2014; 31: 189–193. DOI: 10.4103/0970-9371.151128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sehgal IS, Dhooria S, Aggarwal AN, et al. Impact of Rapid On-Site Cytological Evaluation (ROSE) on the Diagnostic Yield of Transbronchial Needle Aspiration During Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis. Chest 2018; 153: 929–938. [DOI] [PubMed] [Google Scholar]
- 6.Jeffus SK, Joiner AK, Siegel ER, et al. Rapid on-site evaluation of EBUS-TBNA specimens of lymph nodes: Comparative analysis and recommendations for standardization. Cancer Cytopathol 2015; 123: 362–372. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 2013; 137: 668–684. DOI: 10.5858/arpa.2012-0263-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burlingame OO, Kesse KO, Silverman SG, et al. On-site adequacy evaluations performed by cytotechnologists: correlation with final interpretations of 5241 image-guided fine-needle aspiration biopsies. Cancer Cytopathol 2012; 120: 177–184. DOI: 10.1002/cncy.20184. [DOI] [PubMed] [Google Scholar]
- 9.Jain D, Allen TC, Aisner DL, et al. Rapid On-Site Evaluation of Endobronchial Ultrasound-Guided Transbronchial Needle Aspirations for the Diagnosis of Lung Cancer: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med 2018; 142: 253–262. DOI: 10.5858/arpa.2017-0114-SA. [DOI] [PubMed] [Google Scholar]
- 10.Peng TF, Ren T, Wang HS, et al. Diagnostic value of rapid on-site evaluation for CT-guided percutaneous fine needle aspiration in the diagnosis of pulmonary occupying lesions. Biomed Res Int 2020. DOI: 10.1155/2020/9842768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo H, Liu S, Guo J, et al. Rapid on-site evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of hilar and mediastinal lymphadenopathy in patients with lung cancer. Cancer Lett 2016; 371: 182–186. DOI: 10.1016/j.canlet.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 12.Madan K, Dhungana A, Mohan A, et al. Conventional Transbronchial Needle Aspiration Versus Endobronchial Ultrasound-guided Transbronchial Needle Aspiration, With or Without Rapid On-Site Evaluation, for the Diagnosis of Sarcoidosis: A Randomized Controlled Trial. J Bronchology Interv Pulmonol 2017; 24: 48–58. doi:10.1097/LBR.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 13.Madan NK, Madan K, Jain D, et al. Utility of conventional transbronchial needle aspiration with rapid on-site evaluation (c-TBNA-ROSE) at a tertiary care center with endobronchial ultrasound (EBUS) facility. J Cytol 2016; 33: 22–26. doi:10.4103/0970-9371.175493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anila KR, Nayak N, Venugopal M, et al. Role of Rapid On-site Evaluation in CT-guided Fine Needle Aspiration Cytology of Lung Nodules. J Cytol 2018; 35: 229–232. DOI: 10.4103/JOC.JOC_134_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravaioli S, Bravaccini S, Tumedei MM, et al. Easily detectable cytomorphological features to evaluate during ROSE for rapid lung cancer diagnosis: from cytology to histology. Oncotarget 2017; 8: 11199–11205. DOI: 10.18632/oncotarget.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natali F, Cancellieri A, Tinelli C, et al. A Trained Pulmonologist Can Reliably Assess Endosonography-Derived Lymph Node Samples during Rapid On-Site Evaluation. Respiration 2019; 97: 540–547. [DOI] [PubMed] [Google Scholar]
- 17.Gasparini S, Bonifazi M. Rapid on-site cytological evaluation of transbronchial needle aspiration: Why not? Lung India 2014; 31: 203–204. DOI: 10.4103/0970-2113.135751. [DOI] [PMC free article] [PubMed] [Google Scholar]