Abstract
BK virus (BKV)-related haemorrhagic cystitis (HC) is an important cause of morbidity following allogeneic haematopoietic stem cell transplantation (HSCT). The various risk factors include high-level BKV viruria and/or viremia, myeloablative conditioning, acute graft versus host disease (GVHD), cytomegalovirus viremia, and unrelated or HLA-mismatched donor. The presence of high plasma BK viral load and cytopenias have been implicated as important predictors for protracted disease course. These patients frequently require hospitalisation which may extend for several weeks. Supportive measures in the form of analgesics, intravenous hydration, bladder irrigation, and transfusion support remain the mainstay of management. Various drugs have been used with limited success in this setting. These include antiviral drugs, fluoroquinolones, leflunomide, growth factors, clotting factors, estrogens, and prostaglandins. The role of adoptive cellular immunotherapy has also been explored but lacks clinical validation. The strategies aimed at expediting urothelial repair like hyperbaric oxygen therapy (HBOT), intravesical fibrin glue and platelet-rich plasma (PRP) are emerging. Some patients with severe disease do require surgical intervention to relieve urinary obstruction. The frequent co-occurrence of acute GVHD and CMV disease further complicates the management in such patients. There is an unmet need for effective and evidence-based options for the prevention and management of this disease. Due to lack of robust data supported by randomised trials, the acceptability of the available guidelines to simplify the treatment is expected to be low. Despite the availability of various treatment options, the management of BKV-related HC in day-to-day practice continues to be a challenge. The aim of this article is to put forward an up-to-date review of the preventive and therapeutic strategies for BKV-related HC.
Keywords: BK Virus, cystitis
Introduction
BK virus (BKV)-related haemorrhagic cystitis (HC) is a well-recognised and significant complication that affects haematopoietic stem cell transplantation (HSCT) recipients.1–4 Apart from increasing the risk of death, BKV-HC increases the morbidity of HSCT recipients, prolongs their hospital stay, and increases healthcare costs.5 There is a paucity of definite preventive and therapeutic interventions for BKV-HC. The available therapeutic strategies for this clinical entity have yielded variable efficacy in different studies.
Considering the significant morbidity and mortality associated with BKV-related HC, there is a compelling need to explore novel drugs aimed at new therapeutic targets and optimise the available treatment options.
In this review, we attempt to provide an up-to-date review of the management strategies for BKV-related HC with a focus on novel strategies which have demonstrated encouraging results in recent years.
Epidemiology and risk factors for BKV-related HC
BK polyomavirus is a non-enveloped DNA virus that has a high seroprevalance in the general population.6 It is the predominant cause of HC and nephropathy in HSCT recipients and renal transplant recipients, respectively. Rarely, BKV can manifest as renal dysfunction in allogeneic HSCT recipients without causing HC.7
The reported incidence of post-HSCT BKV-related HC is highly variable among adults (7–54%) and children (8–25%).8–10 Although the frequency and pattern of BK viruria is comparable after autologous and allogeneic HSCT, BKV-related HC is specifically observed in allogeneic HSCT recipients. It has been suggested that rather than BK viruria per se, it is the allo-immunity that plays the most crucial role in the pathogenesis of BKV-related HC, especially in the setting of graft versus host disease (GVHD).11,12 Recent studies have reported a sharp rise in the incidence of BKV-related HC among allogeneic HSCT recipients with haploidentical donors. These patients generally receive post-transplant cyclophosphamide (PTCy) as part of GVHD prophylaxis.13,14 It has been postulated that PTCy use as GVHD prophylaxis contributes to BKV-related HC by two mechanisms. Firstly, it directly causes injury to bladder mucosa, regardless of the BKV involvement. Secondly, the delayed T-cell reconstitution observed after PTCy leads to paucity of BKV-specific T-cells in circulation, which favours BKV replication due to a lack of immune surveillance.15,16
The risk factors associated with BKV-related HC can be broadly classified into three main groups – patient-related, transplant-related and virus-related.17,18
patient-related: age, cytopenias
transplant-related: unrelated donor, stem cell source, myeloablative conditioning, GVHD, CMV seropositivity
virus-related: high level of BKV viruria and/or viremia.
Pathogenesis of BKV-related HC
Primary infection with BKV, which generally occurs in early childhood, is either asymptomatic or may have symptoms indistinguishable from other acute viral illnesses.19 BKV remains in a latent form in the urothelial cells of immunocompetent individuals. Three distinct phases have been proposed in the development of BKV-related HC among HSCT recipients. Firstly, the direct injury to bladder mucosa is caused by various components of the conditioning regimen. Thereafter, the reactivation of latent BKV gets triggered in urothelial cells, which is also favoured by the ongoing immunosuppression. This is reflected by the enhanced shedding of BKV in urine. Finally, the immune reconstitution after engraftment initiates the immune-mediated urothelial injury that ultimately culminates in HC.20
Clinical manifestations, diagnosis and natural history of BKV-related HC
The timing of HC after HSCT is crucial in determining the underlying aetiology. ‘Early-onset’ HC observed in the initial 2 weeks is caused by the urothelial damage from constituents of conditioning regimens like alkylating agents (cyclophosphamide, busulfan) and total body irradiation.17 BKV-related HC usually manifests in the post-engraftment period and its onset may vary from 2 weeks to 6 months after HSCT. Rarely, adenovirus infection may give rise to late-onset HC that may have clinical features similar to BKV-related HC.21 It is essential to adopt a systematic approach towards evaluation of haematuria in allogeneic HSCT recipients as distinct underlying aetiologies can give rise to this serious complication (Figure 1).
Figure 1.
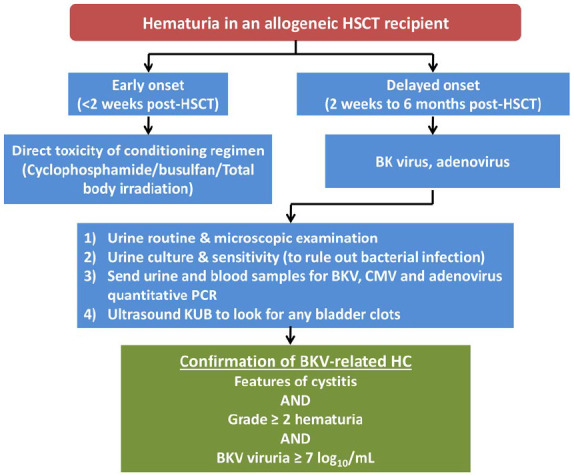
Approach to haematuria in an allogeneic HSCT recipient while considering BK virus-related cystitis.
The clinical features of BKV-related HC can be broadly divided into two groups – irritative syndrome and obstructive syndrome. The irritative syndrome is characterised by the lower urinary tract symptoms like burning micturition, painful micturition, dysuria, pelvic pain, etc. In contrast, the obstructive syndrome is characterised by urinary obstruction due to bladder involvement that ultimately leads to renal impairment by causing tubulo-interstitial nephritis.22
Bedi et al. described the grading for haematuria as following:
Grade 1 – microscopic haematuria
Grade 2 – macroscopic haematuria
Grade 3 – macroscopic haematuria with clots
Grade 4 – macroscopic haematuria with clots and renal dysfunction due to urinary obstruction.23
As per The European Conference on Infections in Leukemia guidelines, the ‘diagnostic triad’ of BKV-related HC is constituted by the presence of clinical features of cystitis, grade 2 or more haematuria, and BKV viruria ⩾7 log10/mL.17
The supportive measures and medical interventions often fail in patients with grades 3 and 4 BKV-related HC. Such patients require a more aggressive management, preferably with a combined modality approach, for attaining favourable outcomes.17
Role of BKV monitoring in blood and urine
A high prevalence of ‘asymptomatic’ BKV viruria in HSCT recipients has been reported by multiple studies but only a subset of these patients develop symptomatic HC.23,24 The patients exhibiting high-level BKV viruria, defined as urinary viral load >7 log10 genome equivalents (gEq)/mL, are considered more vulnerable for manifesting symptomatic BKV-related HC.25 Approximately two-thirds of patients have a high plasma viral load (>3–4 log10 copies/mL), which generally precedes the onset of clinical features. The decline in plasma viral load coincides with the clinical improvement in these patients.26,27
Cesaro et al. prospectively studied the association of urine and plasma BKV viral loads with clinical outcomes among paediatric HSCT recipients. The thresholds of BKV urine and blood viral loads with maximum sensitivity and specificity for HC were >107 gEq/mL (sensitivity 86% and specificity 60%) and >103 gEq/mL (sensitivity 100% and specificity 86%), respectively.28
A discordant relationship between clinical improvement and the trajectory of BK viral loads in urine and plasma has been reported among patients who receive various therapeutic interventions.29,30 The persistence of BK viral shedding in urine has been observed in up to 80% of patients despite clinical improvement.31 In a recent study, half of the patients continued to have BK viruria, despite attaining clinical benefit and a reduction in BK viremia with cidofovir.29
Laskin et al. studied the natural history of BKV in paediatric and adult HSCT recipients.18 The authors reported that asymptomatic high BK viremia (⩾104 copies/mL) in the initial 3 months was significantly associated with all-cause mortality (adjusted HR 2.2; 95% CI 1.1–4.2) and renal dysfunction at 2 years after HSCT. On the basis of these findings, the authors suggest that apart from symptomatic patients, there may be a role of screening for BKV viruria and viremia in asymptomatic HSCT recipients. In that study, instead of cidofovir exposure, BKV viral clearance was significantly associated with the immune reconstitution with recovery of endogenous BKV-specific T-lymphocytes. These findings support the potential role of cellular immunotherapy with BKV-specific T-lymphocytes to the treat BKV-related HC in future.
Overall, the use of BKV viremia or viruria as a screening tool for HC has yielded variable results among adults as well as paediatric patients, and therefore their clinical utility is uncertain.28,32,33 As per the ECIL-6 guidelines, the routine monitoring with plasma BKV viral load among allogeneic HSCT recipients is not recommended.17
Preventive measures
Hyperhydration with or without mesna
Intravenous hyperhydration with or without mesna minimises the exposure of the bladder mucosa to the alkylating agents and their toxic metabolites. Although the role of mesna has been mainly described for the prevention of conditioning regimen-related early HC, recent studies have suggested its potential preventive role in patients receiving PTCy-based haploidentical HSCT. Jaiswal et al. prospectively evaluated patients receiving PTCy-based haploidentical HSCT who were given continuous mesna infusion as HC prophylaxis.34 None of their patients developed early HC and the incidence of high BK viruria (⩾104 copies/mL) with HC was 5.4% at 30 days. The lesser incidence of BKV-related HC in this study was attributed to enhanced urothelial protection with continuous mesna infusion which probably reduced the magnitude of the first hit in the pathogenesis of BKV-related HC.34 There is no clear consensus on the optimal dosing schedule for mesna administration in HSCT recipients receiving PTCy-based GVHD prophylaxis. Intermittent mesna dosing schedules have not proved to be beneficial in reducing the incidence of HC among allogeneic HSCT recipients.23 In a recent study by Arango and Cardona the continuous infusion of mesna was found to be more useful than recurrent bolus administration in preventing HC among haploidentical HSCT recipients receiving PTCy as GVHD prophylaxis.35 It is expected that the use of PTCy as GVHD prophylaxis will increase in future for both haploidentical and full-matched donor HSCTs. Strategies focussed at minimising urothelial damage from PTCy may have a potential role in reducing the subsequent development of BKV-related HC in these patients.
Fluoroquinolones
There is limited evidence in favour of ciprofloxacin as a prophylaxis against BKV-related HC. Despite in vitro studies supporting its inhibitory action on BKV replication, then use of ciprofloxacin as a prophylactic intervention against BKV has yielded conflicting results. Its prolonged use in the setting of allogeneic HSCT can possibly give rise to antimicrobial resistance.36 Alteration in gut microbiota due to ciprofloxacin may also potentially affect transplant outcomes. The increased risk of Clostridium difficile infection is another drawback with fluoroquinolone use.
General supportive measures
Supportive measures for the management of BKV-related HC have been the standard of care for the past few years (Figure 2). BKV-related HC is often associated with intense pain. Antispasmodic agents such as tolterodine and oxybutynin relieve pain by reducing bladder spasms. Urinary analgesics such as phenazopyridine (pyridium) have some role in relieving the symptoms arising from irritation of lower urinary tract mucosa. Patients with high-grade HC require parenteral opioid analgesics (morphine, fentanyl, tramadol, etc.) for pain relief.37 The application of transdermal patches containing opioid analgesics (e.g. fentanyl, buprenorphine) may also be considered. Aggressive intravenous hydration with normal saline is essential for the clearance of haematuria. Forced diuresis with furosemide is indicated for patients with low urine output. Continuous bladder irrigation (CBI) with cold normal saline after placing a three-way catheter is often required for patients with high-grade HC. Simultaneous placement of a suprapubic catheter may be required when CBI is not possible due to frequent obstruction of the urinary catheter.38 The achievement and maintenance of a platelet count ⩾50,000/µL has been shown to be beneficial in decreasing the severity of haematuria in BKV-related HC patients. The transfusion of packed RBCs to maintain haemoglobin ⩾8 g/dL is essential.39 The reduction or cessation of immunosuppression will be considered as an attempt to augment anti-BKV immunity. However, the decision regarding the same may not be straightforward. There is always a substantial risk of precipitation or aggravation of ongoing GVHD when immunosuppression is tapered in allogeneic HSCT recipients.17 The use of fibrinolytic drugs is contraindicated in these patients to prevent the formation of clots which may precipitate urinary obstruction.
Figure 2.

Approach to a confirmed BKV-related HC in an allogeneic HSCT recipient.
Treatment
Systemic therapy
Multiple therapeutic approaches have been used for the management of BKV-related HC with variable results across the studies. There are noteworthy caveats that should be kept in mind while interpreting the literature pertaining to BKV-related HC therapy.40
The majority of the supporting literature for anti-BKV therapies is in the form of small case series and case reports. It is possible that due to publication bias there might be over-reporting of positive outcomes. There is a scarcity of prospective high-quality evidence to guide the treatment of BKV-related HC.
It is difficult to make comparisons across the studies due to considerable heterogeneity in terms of study population, severity of HC among the patients included, and the definitions for clinical and virological response. The consensus on the definition for refractory BKV-related HC is also not clear.
The evidence for optimal timing and sequencing of available treatment options is limited.
Many studies have included grades 1 and 2 HC patients who could have possibly responded to general supportive measures alone, rather than any specific treatment.
BKV infection is unaffected by the commonly used antiviral drugs such as ganciclovir, valganciclovir, ribavirin and foscarnet.
Successful treatment outcomes have been reported among patients who have been given other BKV-specific therapies, either concomitantly or sequentially. Hence, it is difficult to ascertain the exact clinical benefit attributable to each one of them.
The broader applicability of some of the treatment alternatives will be limited by their high cost, limited availability, peculiar complications and limited experience.
Fluoroquinolones
Ciprofloxacin and levofloxacin have been some of the earliest agents that were used for the management of BKV-related HC. It is believed that fluoroquinolones restrict viral replication in the urothelial cells through the inhibition of topoisomerase enzyme.41 Some studies have shown a reduction in BK viruria and viremia with the use of fluoroquinolones.42,43 However, there is no clear evidence to suggest whether it translates into a clinical improvement. Their effectiveness has not been evaluated in clinical trials and their role in the management of BKV-related HC is doubtful.17
Intravenous cidofovir
Cidofovir is a broad-spectrum antiviral drug that has been most extensively used in this setting. It is an analogue of cytosine nucleotide and its use as an intravenous injection with probenecid in different dosing schedules (0.5–5 mg/kg weekly) has yielded variable clinical and viral response rates. The response rate observed across retrospective case series varies from 60% to 86%.40,44,45 Renal dysfunction is the main adverse effect observed with intravenous cidofovir, with reported incidences of up to 50%.44 Grades 3 and 4 BKV-related HC warrant treatment with cidofovir till clinical response or a maximum of 4 weeks. In a recent retrospective study from Canada, at least a partial response was observed in nearly two-thirds of the patients who had severe (grades 3 or 4) BKV-related HC, with comparable results among patients receiving intravenous or intravesical cidofovir, and one-third achieved complete response. Renal toxicity was observed in one-third of the patients, and it was observed regardless of the mode of administration (intravenous versus intravesical).29 Recently, comparable results with low-dose cidofovir (0.5–1.0 mg/kg weekly) without probenecid have been reported.
Considering the available literature, it can be concluded that the optimal dosing schedule, route of administration and timing for the administration of cidofovir in BKV-related HC patients are yet to be defined. Larger multicentre prospective studies with cidofovir are needed to answer these questions.
Brincidofovir
Brincidofovir (CMX001), a prodrug of cidofovir, is a broad-spectrum antiviral drug. It is an oral drug that has shown in vitro activity against various double-stranded DNA viruses including BKV, and it rarely causes renal dysfunction.46 The anecdotal reports support its efficacy in BKV-related nephropathy after allogeneic HSCT.47,48 However, its limited availability and insufficient data on its efficacy in BKV-related HC make brincidofovir less preferable for BKV-related HC.
Hyperbaric oxygen therapy
Hyperbaric oxygen therapy (HBOT) involves the supplementation of nearly 100% oxygen to the patient in a high-pressure chamber usually for 1.5–2 h. HBOT has shown effectiveness in facilitating wound healing among patients with necrotising infections, burns, radiation injuries, etc. As an adjunctive treatment, HBOT administration to BKV-related HC patients results in the generation of oxygen gradient between healthy tissues and damaged urothelium that attracts macrophages to the damaged urothelial mucosa. Local secretion of cytokines, upregulation of fibroblast growth factor (FGF), vascular endothelium-derived growth factor (VEGF) and downregulation of nitric oxide (NO) promotes vasoconstriction, angiogenesis, and urothelial repair.49,50 Savva-Bordalo et al. described clinical resolution with HBOT in 15 out of 16 patients (94%) with refractory BKV-related HC.51 The growing body of evidence and overall safety favour HBOT as adjunctive treatment among BKV-related HC patients.
Leflunomide
Leflunomide is a pyrimidine synthesis inhibitor that has been used for BKV-related post-transplant infections by virtue of its ability to disrupt BKV replication and virion assembly in preclinical studies.52 Contrary to its use for BKV-associated nephropathy in renal transplant recipients, there are limited studies on leflunomide in BKV-related HC among HSCT recipients, with approximately 50% of patients attaining complete response.53,54 Chen et al. reported a 50% complete response rate and a 35.7% partial response rate with leflunomide in a case series of 14 patients.53 The use of leflunomide was well tolerated in these patients and no progression of GVHD was observed during the study. Park et al. reported a 50% complete response with leflunomide in four severe BKV-related HC patients, without substantial gastrointestinal adverse events or cytopenias.55
Cellular immunotherapy
The therapeutic use of T-lymphocytes, derived from the original donor (donor lymphocyte infusion (DLI)) or healthy seropositive individuals, for post-transplant complications related to viral infections has been explored in the recent past. There is growing literature that supports the utility of cellular immunotherapy in BKV-related HC patients. In a case report, the benefit of DLI was described in an allogeneic HSCT recipient who had developed refractory BKV-related HC and nephropathy after matched unrelated donor (MUD) transplant.56 The authors attributed the clinical improvement to the expansion of BKV-specific T-cells after DLI. Given the substantial risk of GVHD, the use of DLI for the management of post-transplant infections is often considered controversial and is reserved for refractory cases.57 To overcome the risk of GVHD, virus-specific cytotoxic T-lymphocytes (CTLs) have lately been studied as therapeutic tools against post-transplant BKV infections. Two studies have reported the safety and efficacy of such ‘adoptively transferred’ CTLs in BKV-related HC patients. In a phase II study, Tzannou et al. reported a 92% cumulative clinical response rate with single infusion of ‘off-the-shelf’ CTLs among patients who had failed at least two prior lines of conventional treatment.58 These CTLs were demonstrated to have functional persistence till 12 weeks.58 Papadopoulou et al. reported a 94% sustained clinical and virological response with adoptive transfer of virus-specific CTLs among allogeneic HSCT recipients who had evidence of multiple concomitant viral infections. Six out of seven BKV-related HC patients attained clinical benefit with virus-specific CTLs.59 With the improvement in the available technology, it is anticipated that adoptive cellular therapies will become more affordable and improve the treatment of post-transplant viral infections, including BKV.60
Mesenchymal stromal cells
Mesenchymal stromal cells (MSCs) isolated from umbilical cord blood have been used as one of the treatment modalities for severe BKV-related HC. It has been hypothesised that their potential for self-renewal and differentiation enables them to repair the inflamed and injured bladder mucosa following intravenous infusion.61 Tong et al. recently reported a remarkable clinical response among all 13 paediatric patients (100%) who were treated with intravenous infusion of MSCs derived from umbilical cord blood, without any significant toxicity.62 These patients exhibited similar overall survival and GVHD incidence compared to those who did not receive MSCs.62
Other agents
Vidarabine
Vidarabine is a purine analogue that restricts BKV replication via the inhibition of viral DNA polymerase. It use as a parenteral drug in the setting of BKV-related HC has been described in the form of case reports.63,64 Its antiviral activity against BKV has not yet been confirmed and there are no published reports on this molecule in the past 20 years.
Oestrogens
Anecdotal reports support the protective effect of oestrogens on bladder mucosa in BKV-related HC patients, possibly via exerting a stabilising effect on bladder microvasculature.65
Clotting factors
The off-label use of recombinant clotting factor concentrates, including factors XIII and VII, has been described in case reports and small case series.66,67 The authors have suggested that these clotting factors facilitate the mucosal recovery through stabilisation of the fibrin clot. They indirectly promote fibroblast proliferation and collagen synthesis. Their role in this setting is restricted by limited availability, high cost and doubtful efficacy.
Intravenous immunoglobulin
The clinical use of intravenous immunoglobulin (IVIG) for BKV-related HC has been described by Aldiwani et al. in a paediatric patient who also received concomitant intravenous ciprofloxacin.40 IVIG use was possibly inspired by the fact that IVIG preparations contain antibodies against a wide variety of viruses, including BKV.40 The guidelines do not recommend the use of IVIG in the management of BKV-related HC.17
Keratinocyte growth factor
Palifermin, a recombinant keratinocyte growth factor (KGF), has been used occasionally as a salvage therapy in combination with other treatment options for refractory grade 3 or 4 BKV-related HC. Its use is based on the premise that KGF promotes urothelial cell proliferation and repair.68,69 Despite a favourable safety profile and ease of administration, its high cost and restricted availability make it unsuitable as a rescue agent.
Intravesical therapy
Cidofovir
The use of intravesical cidofovir has been studied in BKV-related HC with the intention to obviate its nephrotoxic effects. Its use at a 2.5–5 mg/kg weekly dose in small case series has provided variable clinical and virological response rates, ranging from 0 to 100%.70 In a recent retrospective study, it yielded comparable response rates to intravenous cidofovir (62% versus 66%).29 Contrary to conventional belief, this study also reported a higher incidence of renal dysfunction with intravesical cidofovir (33%). Bladder spasm and local discomfort are the commonly reported adverse effects with intravesical cidofovir. Recently, Tooker et al. reported a 88% clinical response in the largest series of BKV-related HC patients (n = 33) who were treated with intravesical cidofovir.70 Greater baseline urine BK viral load (>100 million copies/mL) and HC severity (grades 2–4) predicted inferior response to intravesical therapy.70
Fibrin glue
The endoscopic application of fibrin glue (FG) for the management of refractory BKV-related HC has been studied with encouraging results. Tirindelli et al. reported the largest study on the clinical utility of plasma-derived FG in 35 adult patients with severe refractory BKV-related HC.71 Complete clinical response was achieved by 29 out of 35 patients at day 14 of therapy and the overall survival of these patients was comparable to those allogeneic HSCT recipients who did not develop HC.71–73
Platelet-rich plasma
Platelet-rich plasma (PRP), also known as autologous platelet gel, is a leukocyte-free suspension of the patient’s own platelets in plasma. The various growth factors secreted by the platelets present in PRP [such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and transforming growth factor-β (TGF-β)] and the plasma proteins (such as fibrin and fibronectin) are believed to expedite repair across various tissues in the body. Recently, Masieri et al. reported their experience with intravesical PRP in 10 allogeneic HSCT recipients with refractory grades 3 or 4 HC.74
Formalin
Exposure of the bladder mucosa to formalin causes the precipitation of proteins which facilitates bleeding cessation. However, it leads to exacerbation of suprapubic pain, bladder scarring and subsequently bladder contractures that subsequently result in vesico-ureteric reflux.75 The literature pertaining to intravesical formalin use for BKV-related HC is limited to case reports and it is generally reserved for refractory patients with intractable symptoms.39
Alum
Intravesical alum application also causes the precipitation of cellular proteins that have a haemostatic effect on bladder mucosa. Its use in BKV-related HC has been inspired by its efficacy in radiation-induced HC cases. Neurological complications may arise from the systemic absorption of alum.76 Patients with pre-existing renal failure have a higher risk of aluminium toxicity.4
Prostaglandins
Intravesical prostaglandin E2 (PGE2) has been used in the past and it is believed that prostaglandins cause bleeding cessation through the stimulation of platelet aggregation and vasoconstriction.77
Surgical treatment
Selective vesical artery embolisation
Selective vesical artery embolisation has been studied in a limited number of refractory BKV-related HC patients where it was successful in stopping the ongoing bleed.78 Recent results with the selective embolisation of vesical arteries suggest a 80% response rate in patients with refractory HC.79 The potential complications with this procedure include urinary bladder necrosis, gluteal paresis and skin necrosis.
Supravesical urinary diversion
Urinary diversion by bilateral percutaneous nephrostomy possibly protects the injured bladder mucosa from the detrimental effects of urokinase and bladder distension which might hamper clot formation and urothelial repair.80
Cystectomy
The surgical removal of the bladder is generally reserved as a last-ditch effort for refractory cases because most of these patients are severely immunosuppressed and have cytopenias. There are only a few published case reports on favourable outcomes with cystectomy.81
Therapeutic advances
As the understanding regarding the pathogenesis of BKV-related HC is evolving, new potential therapeutic targets are expected to emerge.
Recently, Schneidewind et al. reported the successful use of an innovative three-dimensional (3D) organotypic cell culture model for studying BKV life cycle. Such culture methods will highlight the interactions between BKV and human urothelial cells which might unveil the unknown nuances associated with BKV pathogenesis. Furthermore, they might facilitate the in vitro testing of antiviral drugs against new potential therapeutic targets.82
In a recent experimental study, a novel 3D cell culture approach was used to study BKV pathogenesis and potential new therapeutic targets. The results favoured the involvement of the STAT3 pathway in the pathogenesis of BKV infection, and IL-11 was recognised as the potential therapeutic target. The utilisation of antibody against IL-11 showed promising in vitro activity against BKV. Although tocilizumab (an anti-IL-6 antibody) did not yield significant results in this study, its role in the treatment of BKV infection will need detailed further exploration, as IL-6 is an important component of the STAT3 pathway.83 Likewise, it may be expected that further experimental studies focussed on the STAT3 pathway in BKV infection might unveil more potential therapeutic targets.
Conclusion
The management of BKV-related HC among HSCT recipients is a challenging task for clinicians. It is anticipated that the number of such patients will increase in future with the overall increase in HSCT recipients globally. Development of this significant complication poses a potential threat to the survival and successful outcome of HSCT among affected patients. Due to a lack of robust high-quality data, there is no accepted standard of care for the prevention and treatment of this entity. The focus of research is gradually shifting from conventional treatment strategies towards novel strategies such as cellular immunotherapy with CTLs, and MSCs. The preliminary experience with such novel cellular therapies is encouraging. With a better understanding of the underlying pathophysiology, it is likely that new therapeutic targets might emerge in future which will completely transform the treatment of BKV-related HC.
Footnotes
Author contributions: AJ and KS planned the manuscript. AJ, KM, RS and KS contributed to the overall writing and preparation of the manuscript. All authors read and approved the final submitted version of the manuscript.
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Kundan Mishra  https://orcid.org/0000-0002-6325-2972
https://orcid.org/0000-0002-6325-2972
Kamal Kant Sahu  https://orcid.org/0000-0002-0382-6882
https://orcid.org/0000-0002-0382-6882
Contributor Information
Aditya Jandial, Department of Internal Medicine (Adult Clinical Hematology Division), Postgraduate Institute of Medical Education and Research, Chandigarh (Union Territory), India.
Kundan Mishra, Department of Clinical Hematology and Stem Cell Transplant, Army Hospital (Research & Referral) New Delhi, India.
Rajeev Sandal, Department of Radiotherapy and Oncology, Indira Gandhi Medical College Shimla, Himachal Pradesh, India.
Kamal Kant Sahu, Department of Internal Medicine, Saint Vincent Hospital, Worcester, MA 01608, USA.
References
- 1. Apperley JF, Rice SJ, Bishop JA, et al. Late-onset hemorrhagic cystitis associated with urinary excretion of polyomaviruses after bone marrow transplantation. Transplantation 1987; 43: 108–112. [DOI] [PubMed] [Google Scholar]
- 2. Arthur RR, Shah KV, Baust SJ, et al. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Engl J Med 1986; 315: 230–234. [DOI] [PubMed] [Google Scholar]
- 3. Abudayyeh A, Hamdi A, Lin H, et al. Symptomatic BK virus infection is associated with kidney function decline and poor overall survival in allogeneic hematopoietic stem cell recipients. Am J Transplant 2016; 16: 1492–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gilis L, Morisset S, Billaud G, et al. High burden of BK virus-associated hemorrhagic cystitis in patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2014; 49: 664–670. [DOI] [PubMed] [Google Scholar]
- 5. Kerbauy LN, Kerbauy MN, Bautzer V, et al. Severe hemorrhagic cystitis caused by the BK polyomavirus is associated with decreased survival post-allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2019; 21: e13101. [DOI] [PubMed] [Google Scholar]
- 6. Blackard JT, Davies SM, Laskin BL. BK polyomavirus diversity – why viral variation matters. Rev Med Virol 2020; 30: e2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghinai R, Sutak J, Saleem M, et al. BK virus nephropathy without haemorrhagic cystitis following bone marrow transplantation. Br J Haematol 2020; 188: 200. [DOI] [PubMed] [Google Scholar]
- 8. Cesaro S, Facchin C, Tridello G, et al. A prospective study of BK-virus-associated haemorrhagic cystitis in paediatric patients undergoing allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant 2008; 41: 363–370. [DOI] [PubMed] [Google Scholar]
- 9. Lee YJ, Zheng J, Kolitsopoulos Y, et al. Relationship of BK polyoma virus (BKV) in the urine with hemorrhagic cystitis and renal function in recipients of T cell-depleted peripheral blood and cord blood stem cell transplantations. Biol Blood Marrow Transplant 2014; 20: 1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lunde LE, Dasaraju S, Cao Q, et al. Hemorrhagic cystitis after allogeneic hematopoietic cell transplantation: risk factors, graft source and survival. Bone Marrow Transplant 2015; 50: 1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leung AY, Chan M, Cheng VC, et al. Polyoma BK viruria in patients undergoing autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 2005; 35: 1029–1030. [DOI] [PubMed] [Google Scholar]
- 12. Cesaro S, Brugiolo A, Faraci M, et al. Incidence and treatment of hemorrhagic cystitis in children given hematopoietic stem cell transplantation: a survey from the Italian Association of Pediatric Hematology Oncology–Bone Marrow Transplantation Group. Bone Marrow Transplant 2003; 32: 925–931. [DOI] [PubMed] [Google Scholar]
- 13. Copelan OR, Sanikommu SR, Trivedi JS, et al. Higher incidence of hemorrhagic cystitis following haploidentical related donor transplantation compared with matched related donor transplantation. Biol Blood Marrow Transplant 2019; 25: 785–790. [DOI] [PubMed] [Google Scholar]
- 14. Solomon SR, Solh M, Morris LE, et al. Myeloablative conditioning with PBSC grafts for T cell-replete haploidentical donor transplantation using posttransplant cyclophosphamide. Adv Hematol 2016; 2016: 9736564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanakry CG, Coffey DG, Towlerton AM, et al. Origin and evolution of the T cell repertoire after posttransplantation cyclophosphamide. JCI Insight 2016; 1: e86252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Comoli P, Hirsch HH, Ginevri F. Cellular immune responses to BK virus. Curr Opin Organ Transplant 2008; 13: 569–574. [DOI] [PubMed] [Google Scholar]
- 17. Cesaro S, Dalianis T, Hanssen Rinaldo C, et al. ECIL guidelines for the prevention, diagnosis and treatment of BK polyomavirus-associated haemorrhagic cystitis in haematopoietic stem cell transplant recipients. J Antimicrob Chemother 2018; 73: 12–21. [DOI] [PubMed] [Google Scholar]
- 18. Laskin BL, Denburg MR, Furth SL, et al. The natural history of BK polyomavirus and the host immune response after stem cell transplantation. Clin Infect Dis. Epub ahead of print 18 December 2019. DOI: 10.1093/cid/ciz1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirsch HH. BK virus: opportunity makes a pathogen. Clin Infect Dis 2005; 41: 354–360. [DOI] [PubMed] [Google Scholar]
- 20. Leung AY, Yuen KY, Kwong YL. Polyoma BK virus and haemorrhagic cystitis in haematopoietic stem cell transplantation: a changing paradigm. Bone Marrow Transplant 2005; 36: 929–937. [DOI] [PubMed] [Google Scholar]
- 21. Sahu KK, Prakash G, Khadwal A, et al. A rare case of hemorrhagic cystitis in allogeneic hematopoietic stem cell transplant patient. Indian J Hematol Blood Transfus 2016; 32 (Suppl. 1): 196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ambalathingal GR, Francis RS, Smyth MJ, et al. BK polyomavirus: clinical aspects, immune regulation, and emerging therapies. Clin Microbiol Rev 2017; 30: 503–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bedi A, Miller CB, Hanson JL, et al. Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation. J Clin Oncol 1995; 13: 1103–1109. [DOI] [PubMed] [Google Scholar]
- 24. Bogdanovic G, Priftakis P, Giraud G, et al. Association between a high BK virus load in urine samples of patients with graft-versus-host disease and development of hemorrhagic cystitis after hematopoietic stem cell transplantation. J Clin Microbiol 2004; 42: 5394–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leung AY, Suen CK, Lie AK, et al. Quantification of polyoma BK viruria in hemorrhagic cystitis complicating bone marrow transplantation. Blood 2001; 98: 1971–1978. [DOI] [PubMed] [Google Scholar]
- 26. Koskenvuo M, Dumoulin A, Lautenschlager I, et al. BK polyomavirus-associated hemorrhagic cystitis among pediatric allogeneic bone marrow transplant recipients: treatment response and evidence for nosocomial transmission. J Clin Virol 2013; 56: 77–81. [DOI] [PubMed] [Google Scholar]
- 27. Erard V, Kim HW, Corey L, et al. BK DNA viral load in plasma: evidence for an association with hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Blood 2005; 106: 1130–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cesaro S, Tridello G, Pillon M, et al. A prospective study on the predictive value of plasma BK virus-DNA load for hemorrhagic cystitis in pediatric patients after stem cell transplantation. J Pediatric Infect Dis Soc 2015; 4: 134–142. [DOI] [PubMed] [Google Scholar]
- 29. Coomes EA, Wolfe Jacques A, Michelis FV, et al. Efficacy of cidofovir in treatment of BK virus-induced hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant 2018; 24: 1901–1905. [DOI] [PubMed] [Google Scholar]
- 30. Mackey MC. Intravesicular cidofovir for the treatment of polyomavirus-associated hemorrhagic cystitis. Ann Pharmacother 2012; 46: 442–446. [DOI] [PubMed] [Google Scholar]
- 31. Cesaro S, Hirsch HH, Faraci M, et al. Cidofovir for BK virus-associated hemorrhagic cystitis: a retrospective study. Clin Infect Dis 2009; 49: 233–240. [DOI] [PubMed] [Google Scholar]
- 32. Ghosh A, Tan TT, Linn YC, et al. What we learned from plasma BK-virus monitoring in allogeneic hematopoietic transplant recipients. Transplantation 2016; 100: e17–e18. [DOI] [PubMed] [Google Scholar]
- 33. Imlay H, Xie H, Leisenring WM, et al. Presentation of BK polyomavirus-associated hemorrhagic cystitis after allogeneic hematopoietic cell transplantation. Blood Adv 2020; 4: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jaiswal SR, Singhal P, Thatai A, et al. Impact of extended infusional mesna prophylaxis on the incidence of BK viruria and hemorrhagic cystitis following post-transplantation cyclophosphamide and CTLA4 Ig-based haploidentical transplantation. Ann Hematol 2020; 99: 839–845. [DOI] [PubMed] [Google Scholar]
- 35. Arango M, Cardona D. Hemorrhagic cystitis after haploidentical transplantation with post-transplantation cyclophosphamide: protective effect of MESNA continuous infusion. Biol Blood Marrow Transplant 2020; 26: 1492–1496. [DOI] [PubMed] [Google Scholar]
- 36. Knoll GA, Humar A, Fergusson D, et al. Levofloxacin for BK virus prophylaxis following kidney transplantation: a randomized clinical trial. JAMA 2014; 312: 2106–2114. [DOI] [PubMed] [Google Scholar]
- 37. Visintini C, Venturini M, Botti S, et al. Nursing management of haemorrhagic cystitis in patients undergoing haematopoietic stem cell transplantation: a multicentre Italian survey. Mediterr J Hematol Infect Dis 2019; 11: e2019051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gander R, Asensio M, Guillen G, et al. Hemorrhagic cystitis after hematopoietic stem cell transplantation: a challenge for the pediatric urologist. J Pediatr Urol 2018; 14: 366–373. [DOI] [PubMed] [Google Scholar]
- 39. Cheuk DK, Lee TL, Chiang AK, et al. Risk factors and treatment of hemorrhagic cystitis in children who underwent hematopoietic stem cell transplantation. Transpl Int 2007; 20: 73–81. [DOI] [PubMed] [Google Scholar]
- 40. Aldiwani M, Tharakan T, Al-Hassani A, et al. BK virus associated haemorrhagic cystitis. A systematic review of current prevention and treatment strategies. Int J Surg 2019; 63: 34–42. [DOI] [PubMed] [Google Scholar]
- 41. Toptas T, Kaygusuz-Atagunduz I, Kani HT, et al. Levofloxacin for the treatment of severe refractory BK virus-associated hemorrhagic cystitis in hematopoietic stem cell transplantation recipients: a report of three cases. Oncol Lett 2014; 8: 1775–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leung AY, Chan MT, Yuen KY, et al. Ciprofloxacin decreased polyoma BK virus load in patients who underwent allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2005; 40: 528–537. [DOI] [PubMed] [Google Scholar]
- 43. Randhawa PS. Anti-BK virus activity of ciprofloxacin and related antibiotics. Clin Infect Dis 2005; 41: 1366. –1367; author reply 1367. [DOI] [PubMed] [Google Scholar]
- 44. Philippe M, Ranchon F, Gilis L, et al. Cidofovir in the treatment of BK virus-associated hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2016; 22: 723–730. [DOI] [PubMed] [Google Scholar]
- 45. Perez-Huertas P, Cueto-Sola M, Escobar-Cava P, et al. BK virus-associated hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation in the pediatric population. J Pediatr Oncol Nurs 2017; 34: 13–19. [DOI] [PubMed] [Google Scholar]
- 46. Tippin TK, Morrison ME, Brundage TM, et al. Brincidofovir is not a substrate for the human organic anion transporter 1: a mechanistic explanation for the lack of nephrotoxicity observed in clinical studies. Ther Drug Monit 2016; 38: 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Papanicolaou GA, Lee YJ, Young JW, et al. Brincidofovir for polyomavirus-associated nephropathy after allogeneic hematopoietic stem cell transplantation. Am J Kidney Dis 2015; 65: 780–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reisman L, Habib S, McClure GB, et al. Treatment of BK virus-associated nephropathy with CMX001 after kidney transplantation in a young child. Pediatr Transplant 2014; 18: E227–E231. [DOI] [PubMed] [Google Scholar]
- 49. Lopez D, Alismail A, Tan LD. Hyperbaric oxygen therapy of an adolescent stem cell transplantation recipient with hemorrhagic cystitis and BK virus. Case Rep Pulmonol 2020; 2020: 3465412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zama D, Masetti R, Vendemini F, et al. Clinical effectiveness of early treatment with hyperbaric oxygen therapy for severe late-onset hemorrhagic cystitis after hematopoietic stem cell transplantation in pediatric patients. Pediatr Transplant 2013; 17: 86–91. [DOI] [PubMed] [Google Scholar]
- 51. Savva-Bordalo J, Pinho Vaz C, Sousa M, et al. Clinical effectiveness of hyperbaric oxygen therapy for BK-virus-associated hemorrhagic cystitis after allogeneic bone marrow transplantation. Bone Marrow Transplant 2012; 47: 1095–1098. [DOI] [PubMed] [Google Scholar]
- 52. Farasati NA, Shapiro R, Vats A, et al. Effect of leflunomide and cidofovir on replication of BK virus in an in vitro culture system. Transplantation 2005; 79: 116–118. [DOI] [PubMed] [Google Scholar]
- 53. Chen XC, Liu T, Li JJ, et al. Efficacy and safety of leflunomide for the treatment of BK virus-associated hemorrhagic cystitis in allogeneic hematopoietic stem cell transplantation recipients. Acta Haematol 2013; 130: 52–56. [DOI] [PubMed] [Google Scholar]
- 54. Schneidewind L, Neumann T, Dräger DL, et al. Leflunomide in the treatment of BK polyomavirus associated nephropathy in kidney transplanted patients – a systematic review. Transplant Rev (Orlando) 2020; 34: 100565. [DOI] [PubMed] [Google Scholar]
- 55. Park YH, Lim JH, Yi HG, et al. BK virus-hemorrhagic cystitis following allogeneic stem cell transplantation: clinical characteristics and utility of leflunomide treatment. Turk J Haematol 2016; 33: 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Orti G, Iacoboni G, Barba P, et al. Donor lymphocyte infusion for BK virus hemorrhagic cystitis and nephropathy: a case report. Bone Marrow Transplant 2019; 54: 772–774. [DOI] [PubMed] [Google Scholar]
- 57. Papadopoulos EB, Ladanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein–Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med 1994; 330: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 58. Tzannou I, Papadopoulou A, Naik S, et al. Off-the-shelf virus-specific T cells to treat BK virus, human Herpesvirus 6, Cytomegalovirus, Epstein–Barr virus, and Adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol 2017; 35: 3547–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Papadopoulou A, Gerdemann U, Katari UL, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med 2014; 6: 242ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Copelan OR, Oberlin DT. Hemorrhagic cystitis: brighter days ahead. Biol Blood Marrow Transplant 2018; 24: 1773–1774. [DOI] [PubMed] [Google Scholar]
- 61. Ringden O, Uzunel M, Sundberg B, et al. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia 2007; 21: 2271–2276. [DOI] [PubMed] [Google Scholar]
- 62. Tong J, Liu H, Zheng C, et al. Effects and long-term follow-up of using umbilical cord blood-derived mesenchymal stromal cells in pediatric patients with severe BK virus-associated late-onset hemorrhagic cystitis after unrelated cord blood transplantation. Pediatr Transplant 2020; 24: e13618. [DOI] [PubMed] [Google Scholar]
- 63. Vianelli N, Renga M, Azzi A, et al. Sequential vidarabine infusion in the treatment of polyoma virus-associated acute haemorrhagic cystitis late after allogeneic bone marrow transplantation. Bone Marrow Transplant 2000; 25: 319–320. [DOI] [PubMed] [Google Scholar]
- 64. Seabra C, Perez-Simon JA, Sierra M, et al. Intra-muscular vidarabine therapy for polyomavirus-associated hemorrhagic cystitis following allogeneic hemopoietic stem cell transplantation. Bone Marrow Transplant 2000; 26: 1229–1230. [DOI] [PubMed] [Google Scholar]
- 65. Heath JA, Mishra S, Mitchell S, et al. Estrogen as treatment of hemorrhagic cystitis in children and adolescents undergoing bone marrow transplantation. Bone Marrow Transplant 2006; 37: 523–526. [DOI] [PubMed] [Google Scholar]
- 66. Demesmay K, Tissot E, Bulabois CE, et al. Factor XIII replacement in stem-cell transplant recipients with severe hemorrhagic cystitis: a report of four cases. Transplantation 2002; 74: 1190–1192. [DOI] [PubMed] [Google Scholar]
- 67. Blatt J, Gold SH, Wiley JM, et al. Off-label use of recombinant factor VIIa in patients following bone marrow transplantation. Bone Marrow Transplant 2001; 28: 405–407. [DOI] [PubMed] [Google Scholar]
- 68. Czibere A, Bruns I, Graef T, et al. Treatment of severe hemorrhagic cystitis after allogeneic stem cell transplantation with palifermin, a recombinant human keratinocyte growth factor. Biol Blood Marrow Transplant 2007; 13: 872–874. [DOI] [PubMed] [Google Scholar]
- 69. Bhaskaran S, Abu-Arja RF, Abusin G, et al. Recombinant human keratinocyte growth factor: successful treatment of severe, refractory hemorrhagic cystitis after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2014; 49: 1550–1551. [DOI] [PubMed] [Google Scholar]
- 70. Tooker GM, Stafford KA, Nishioka J, et al. Intravesicular cidofovir in the treatment of BK virus-associated hemorrhagic cystitis following hematopoietic stem cell transplantation. Ann Pharmacother 2020; 54: 547–553. [DOI] [PubMed] [Google Scholar]
- 71. Tirindelli MC, Flammia GP, Bove P, et al. Fibrin glue therapy for severe hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014; 20: 1612–1617. [DOI] [PubMed] [Google Scholar]
- 72. Bove P, Iacovelli V, Tirindelli MC, et al. Endoscopic intravesical fibrin glue application in the treatment of refractory hemorrhagic radiation cystitis: a single cohort pilot study. J Endourol 2019; 33: 93–98. [DOI] [PubMed] [Google Scholar]
- 73. Tirindelli MC, Flammia G, Sergi F, et al. Fibrin glue for refractory hemorrhagic cystitis after unrelated marrow, cord blood, and haploidentical hematopoietic stem cell transplantation. Transfusion 2009; 49: 170–175. [DOI] [PubMed] [Google Scholar]
- 74. Masieri L, Sessa F, Mari A, et al. Intravesical application of platelet-rich plasma in patients with persistent haemorrhagic cystitis after hematopoietic stem cell transplantation: a single-centre preliminary experience. Int Urol Nephrol 2019; 51: 1715–1720. [DOI] [PubMed] [Google Scholar]
- 75. Lukasewycz SJ, Smith AR, Rambachan A, et al. Intractable hemorrhagic cystitis after hematopoietic stem cell transplantation – is there a role for early urinary diversion in children? J Urol 2012; 188: 242–246. [DOI] [PubMed] [Google Scholar]
- 76. Bogris SL, Johal NS, Hussein I, et al. Is it safe to use aluminum in the treatment of pediatric hemorrhagic cystitis? A case discussion of aluminum intoxication and review of the literature. J Pediatr Hematol Oncol 2009; 31: 285–288. [DOI] [PubMed] [Google Scholar]
- 77. Laszlo D, Bosi A, Guidi S, et al. Prostaglandin E2 bladder instillation for the treatment of hemorrhagic cystitis after allogeneic bone marrow transplantation. Haematologica 1995; 80: 421–425. [PubMed] [Google Scholar]
- 78. Palandri F, Bonifazi F, Rossi C, et al. Successful treatment of severe hemorrhagic cystitis with selective vesical artery embolization. Bone Marrow Transplant 2005; 35: 529–530. [DOI] [PubMed] [Google Scholar]
- 79. Han Y, Wu D, Sun A, et al. Selective embolization of the internal iliac arteries for the treatment of severe hemorrhagic cystitis following hematopoietic SCT. Bone Marrow Transplant 2008; 41: 881–886. [DOI] [PubMed] [Google Scholar]
- 80. Ebiloglu T, Kaya E, Yilmaz S, et al. Treatment of resistant cyclophosphamide induced haemorrhagic cystitis: review of literature and three case reports. J Clin Diagn Res 2016; 10: PD15–PD16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Garderet L, Bittencourt H, Sebe P, et al. Cystectomy for severe hemorrhagic cystitis in allogeneic stem cell transplant recipients. Transplantation 2000; 70: 1807–1811. [DOI] [PubMed] [Google Scholar]
- 82. Schneidewind L, Neumann T, Plis A, et al. Novel 3D organotypic urothelial cell culture model for identification of new therapeutic approaches in urological infections. J Clin Virol 2020; 124: 104283. [DOI] [PubMed] [Google Scholar]
- 83. Schneidewind L, Neumann T, Krüger W, et al. Targeting IL-11 in the treatment of BK virus-associated haemorrhagic cystitis-a promising new approach. J Cell Mol Med 2020; 24: 9097–9100. [DOI] [PMC free article] [PubMed] [Google Scholar]


