Abstract
POEMS syndrome is a rare multisystem disease associated with an underlying plasma cell disorder. Its name is an acronym for peripheral neuropathy (P), endocrinopathy (E), organomegaly (O), monoclonal plasma cell proliferative disorder (M), and skin changes (S). This case report describes a patient with POEMS syndrome who presented with progressive fatigue and numbness in the lower extremities. Initially, the patient was erroneously diagnosed with diabetes and diabetic peripheral neuropathy because of the endocrinopathy associated with POEMS syndrome. After a second hospitalization, the patient was diagnosed with POEMS syndrome and recovered with alkylator therapy and a peripheral blood stem cell transplant. The patient’s overall condition was improved at the 1-year follow-up. POEMS syndrome should be considered if a patient presents with endocrinopathy and unexplained peripheral neuropathy.
Keywords: POEMS syndrome, endocrine system disease, polyneuropathy, case report, misdiagnosis, plasma cell disorder
Shi H et al. POEMS syndrome misdiagnosed as diabetes
Shi H, Jiang XH, Wang L, Zhou JY. Missed diagnose of POEMS syndrome with the onset of progressive fatigue: a case report.
Core tip
We herein report a case of POEMS syndrome in a patient who presented with progressive fatigue and numbness in the lower extremities. The patient was initially diagnosed with diabetes and diabetic peripheral neuropathy. We report this case to enhance the awareness of POEMS syndrome, especially among endocrinologists. When clinicians encounter patients with coexisting endocrinopathy, such as diabetes, and unexplained peripheral neuropathy or multisystem disorders, certain examinations must be performed to rule out POEMS syndrome. We also herein discuss several endocrine abnormalities associated with POEMS syndrome.
Introduction
POEMS syndrome, also known as Crow-Fukase syndrome, Takatsuki syndrome, or osteosclerotic myeloma, is a rare paraneoplastic syndrome caused by an underlying plasma cell disorder. Coined by Bardwick et al.1 in 1980, POEMS syndrome is characterized by polyneuropathy, endocrinopathy, organomegaly, monoclonal plasma cell proliferative disorder, skin changes, and other features. A diagnosis does not require the presence of all five features. Other symptoms related to POEMS syndrome include papilledema, extravascular volume overload, sclerotic bone lesions, and thrombocytosis/erythrocytosis (PEST); and increasing concentration of vascular endothelial growth factor (VEGF); a predisposition toward thrombosis; and abnormal pulmonary function tests.2 Consequently, patients are often misdiagnosed with diabetic peripheral neuropathy or hypothyroidism at their first visit to the endocrinology department. We herein describe a patient who presented with progressive fatigue and numbness in the lower extremities and was misdiagnosed with diabetes and diabetic peripheral neuropathy.
Case presentation
Chief complaints
In March 2017, a 60-year-old man was admitted with progressive fatigue and numbness in the lower extremities.
History of present illness
Before his presentation to our institution, he had been diagnosed with cerebral infarction at the left basal ganglia at a local hospital in August 2016. Based on an elevated fasting serum glucose concentration, he was diagnosed with diabetes mellitus and treated with oral hypoglycemic agents. As a result, his serum blood glucose concentration gradually declined. After the acute phase of cerebral infarction, he developed progressive fatigue and numbness in his lower extremities. These symptoms worsened during the next several months until he had difficulty walking and using the stairs. He also developed weight loss with a decreased appetite, constipation, dry skin, reduced sweating, and insomnia. In March 2017, he presented with progressive fatigue in the lower extremities and was admitted to the Third Affiliated Hospital of Soochow University.
Personal and family history
The patient took aspirin intermittently because of his history of cerebral infarction. He had quit smoking 10 years previously. His personal history contained nothing remarkable. He had no family history of related hereditary diseases.
Physical examination
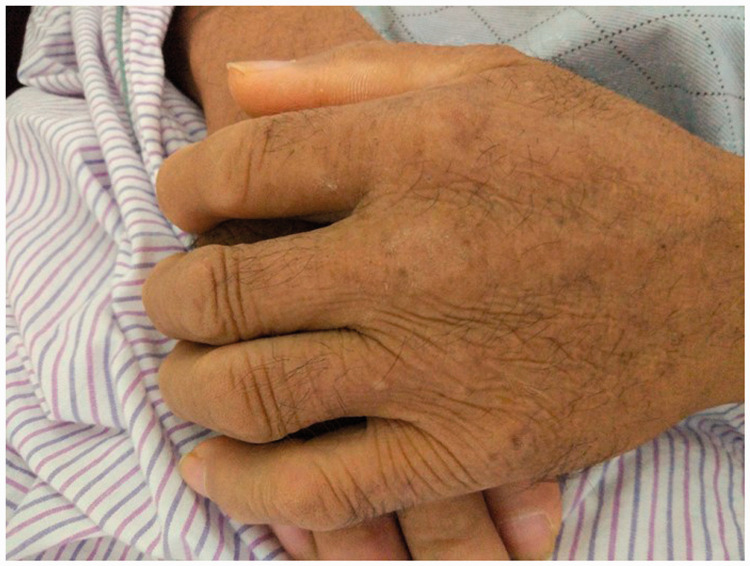
Physical examination revealed generalized hyperpigmentation of the skin, enlarged hands, and coarsened skin (Figure 1). Superficial lymph nodes were not palpable. The breath sounds in the lower lungs were slightly weakened, and the heart sounds were normal. He had hypoesthesia to light touch and reduced pin-prick sensation below the knees as well as lack of knee and ankle reflexes. His muscle strength grade was 4/5 in the upper extremities and 4/5 in the lower extremities.
Figure 1.
Enlarged hands and coarsened skin.
Laboratory examinations
The patient’s cardiac, liver, and renal functions were normal. His glycosylated hemoglobin concentration was 7.0% (reference range, <6.5%), and his fasting serum glucose concentration was 7.55 mmol/L (reference range, 3.9–6.1 mmol/L), indicating mild glucose control. The patient had a high serum estradiol concentration (158 pg/dL; reference range, <40 pg/dL) with normal luteinizing hormone and follicle-stimulating hormone concentrations. Thyroid examination showed a decreased free T3 concentration (8.93 pmol/L; reference range, <10 mg/dL), which indicated the possibility of low T3 syndrome and other consumptive diseases. His 24-hour concentrations of urinary cortisol, serum cortisol (8:00–16:00), and corticotropin (8:00–16:00) were normal, and his cortisol concentration was suppressed in response to an overnight 1-mg dexamethasone suppression test, which ruled out Cushing’s syndrome. Because of his enlarged hands and coarsened skin, a growth hormone suppression test was performed. The growth hormone concentration at 0, 30, 60, 90, and 120 minutes was 0.98, 1.37, 1.34, 1.22, and 1.02 ng/mL, respectively; insulin-like growth factor 1 (IGF-1) was negative.
Imaging examinations
Cranial magnetic resonance imaging (MRI) was performed to rule out the possibility of acromegaly. Cranial MRI showed an ischemic cavity in the left frontal lobe, brain atrophy, and segmental stenosis of the left posterior cerebral artery. Electromyography showed severe multiple peripheral neuropathy affecting both the upper and lower extremities. A vibration perception threshold test showed that the patient’s superficial sense was almost normal but that his deep sense was abnormal, indicating a high risk of neuropathic ulcers. A positron emission tomography–computed tomography (PET-CT) scan showed hypermetabolic foci in the T5–6, T8, and T11–L4 vertebral bodies and the 4th, 7th, and 10th left ribs; thus, sclerosing bone disease and an old infarction in the left thalamus were considered (Figure 2). Abdominal CT showed a small amount of fluid in the pericardial cavity, a small amount of fluid in the right pleural cavity, and multiple stones in the gallbladder. Color Doppler echocardiography showed no abnormalities.
Figure 2.
Positron emission tomography–computed tomography scan. The scan showed hypermetabolic foci in the vertebral bodies and ribs.
Primary diagnosis
The patient was initially diagnosed with diabetes, diabetic peripheral neuropathy, and cerebral infarction sequelae. For treatment of the diabetic peripheral neuropathy, the patient began pharmacologic therapy comprising α-lipoic acid, aldose reductase inhibitors, mecobalamin, and pancreatic kininogenase enteric-coated tablets. His clinical condition improved after the pharmacologic therapy. However, the patient gradually lost interest in most activities, including sex and sports, and he experienced sleep disturbances. He was given sleeping pills and antidepressants because of insomnia and depression, and his symptoms were gradually relieved. Within 3 months after discharge from the hospital, he gradually developed pain in his lower limbs. He experienced difficulty standing on his heels and unsteadiness while walking. He eventually required a walking stick, and he continued losing weight and had a poor appetite.
Final diagnosis
The patient visited the neurologic department of Beijing Union Medical College Hospital. During his stay there, his serum protein electrophoresis results were suggestive of monoclonal gammopathy. His serum IgG level was positive with λ light chain restriction. His serum light chain κ concentration was 1500 mg/dL (reference range, 598–1329 mg/dL), and his light chain λ concentration was 960 mg/dL (reference range, 298–665 mg/dL). His urine light chain κ concentration was 12.9 mg/dL (reference range, 0.0–5.1 mg/dL). He underwent lumbar puncture, and a bone marrow biopsy revealed megakaryocyte hyperplasia and clustering. Oligoclonal IgG bands were positive in the cerebrospinal fluid, whereas no M protein was found via serous protein electrophoresis. Unfortunately, VEGF measurement was not available in Beijing Union Medical College Hospital at the time.
This patient met both mandatory major criteria for POEMS syndrome (polyradiculoneuropathy and monoclonal plasma cell proliferative disorder), one of the three other major criteria (sclerotic bone lesions), and three of the six minor criteria (endocrinopathy, skin changes, and extravascular volume overload). The patient was diagnosed with POEMS syndrome, diabetes, and cerebral infarction sequelae.
Treatment
The patient was treated with alkylator therapy and a peripheral blood stem cell transplant. During hospitalization, he gradually stopped using oral hypoglycemic drugs. Aspirin was prescribed after discharge.
Outcome and follow-up
After discharge, the patient’s fatigue and numbness gradually improved, and he experienced no further difficulties walking and climbing stairs. Furthermore, the patient stopped losing weight and his appetite increased. His blood glucose concentration remained within the reference range during follow-up. After 5 months, the hyperpigmentation in the lower extremities gradually resolved. One year later, his glycosylated hemoglobin concentration was 5.9% (reference range, <6.5%), and his fasting serum glucose concentration was 5.7 mmol/L (reference range, 3.9–6.1 mmol/L). Thyroid examination showed no abnormalities. The patient still had a high serum estradiol concentration (72 pg/dL; reference range, <40 pg/dL).
Discussion
According to large initial reports, POEMS syndrome is widespread in Japan,3 and its incidence has been increasing in countries such as the United States, China, France, and India during the last several years.4 According to a report from the Mayo Clinic, 118 of 170 patients (69%; 95% confidence interval, 62% to 76%) with POEMS syndrome from 1960 to 2006 had a recognized endocrinopathy (89 men).5
The pathogenesis of POEMS syndrome remains unclear. An increasing VEGF concentration is strongly related to POEMS syndrome. VEGF acts on endothelial cells and induces a rapid and reversible increase in vascular permeability. VEGF is also important for angiogenesis, and disorders in VEGF may lead to ascites, edema, organomegaly, pleural effusion, and neuropathy.6 Some angiogenic factors, such as hypoxia-inducible transcription factor 1, are upstream factors that modulate VEGF production.7 However, VEGF may not be the main cause of POEMS syndrome because anti-VEGF therapy has been shown to have mixed results on curative effects.8,9 Other factors, such as some inflammatory factors, may contribute to the pathogenesis of POEMS syndrome. Wang et al.10 found that higher levels of interleukin (IL)-6 and IL-12 are related to the disease activity of POEMS syndrome. IL-6 has been shown to stimulate VEGF production.11 Kanai et al.12 also found that IL-12 is related to disease activity. In patients with POEMS syndrome, lambda light chain is restricted with restricted immunoglobulin light chain variable gene usage. Nagao et al.13 performed whole-exome sequencing, targeted sequencing, and RNA sequencing of 11 patients with POEMS syndrome and identified 20 mutations in 7 mutated genes: KLHL6, LTB, EHD1, EML4, HEPHL2, HIPK2, and PCDH10.
Diagnoses are often delayed because of the rarity and complexity of POEMS syndrome. Many physicians, especially those in the endocrinology department, lack an awareness of POEMS syndrome, which in turn leads to misdiagnosis. The diagnosis is made based on a composite of clinical and laboratory features. It is confirmed when both of the mandatory major criteria (polyradiculoneuropathy and monoclonal plasma cell proliferative disorder), one of the three other major criteria (Castleman disease, sclerotic bone lesions, and increasing VEGF concentration), and one of the six minor criteria (organomegaly, extravascular volume overload, endocrinopathy, skin changes, papilledema, and thrombocytosis/polycythemia) are met.14
Notably, the symptoms of POEMS syndrome arise over time rather than simultaneously, which is why the diagnosis is challenging. A detailed constellation of clinical signs and a physical examination followed by appropriate testing could help to distinguish POEMS syndrome from other diseases. Some tests are of vital importance, such as bone marrow biopsy and measurement of VEGF. According to data from the Mayo Clinic, helpful cut-offs for a diagnosis of POEMS syndrome are a plasma VEGF concentration of 200 pg/mL (specificity of 95%, sensitivity of 68%) and a serum VEGF level of 1920 pg/mL (specificity of 98%, sensitivity of 73%).5 Unfortunately, only a few hospitals in China have the ability to measure VEGF, resulting in substantial misdiagnoses of POEMS syndrome. Other novel markers, such as N-terminal propeptide of type I collagen, may serve as promising markers for the diagnosis of POEMS syndrome. According to Wang et al.,15 the best cut-off of N-terminal propeptide of type I collagen for a diagnosis of POEMS syndrome is 70 ng/mL (specificity of 91.5%, sensitivity of 80%).
Endocrinological elements are crucial in the pathogenesis of POEMS syndrome, but the real mechanisms remain poorly understood. Many of the endocrine abnormalities seen in patients with POEMS syndrome may be due to chronic illness or illnesses commonly observed in the aging population. However, few studies have compared endocrinopathies in patients with POEMS syndrome and an age- and sex-matched population with chronic diseases. Like the patient in the present study, patients with diabetes are frequently misdiagnosed with diabetic peripheral neuropathy because of the nonspecific symptoms of neuropathy. In these cases, the lack of improvement after using hypoglycemic drugs and antidepressants is usually indicative because the patients seek further help at other hospitals. As a result, the endocrinologist should consider POEMS syndrome when encountering a patient with the coexistence of diabetes and unexplained peripheral neuropathy, especially in the presence of multisystem disorders.
A study of patients with POEMS syndrome at the Mayo Clinic showed that about 84% of patients had a recognized endocrinopathy.4 The most common endocrine abnormality in patients with POEMS syndrome is hypogonadism, followed by abnormalities in thyroid and glucose metabolism and finally adrenal insufficiency. Hypogonadism is the most frequently occurring endocrinological abnormality among all other endocrinological abnormalities. Male patients tend to develop erectile dysfunction and gynecomastia, whereas female patients develop amenorrhea and galactorrhea.16 Some researchers have suggested that accelerated conversion of androgens into estrogens in patients with POEMS syndrome increases the level of serum estrogens. Increased estrogen can potentially cause hyperprolactinemia either directly or by modifying the estrogen–testosterone ratio. Others have suggested that patients with POEMS syndrome are usually ill at presentation and have a possible underlying diagnosis of hypothalamic hypogonadism.17 With respect to the cause of hyperprolactinemia, it has been suggested that higher intracranial pressure, such as that associated with empty sella syndrome, may disturb inhibitory dopaminergic pathways and in turn lead to hyperprolactinemia.18 In the present case, the patient showed an increasing estradiol concentration; however, he had no erectile dysfunction or gynecomastia.
In terms of glycometabolism, patients with POEMS syndrome often have an impaired fasting glucose concentration, impaired glucose tolerance, or diabetes. Gandhi et al.5 investigated 50 patients with POEMS syndrome, among whom 24 had diabetes or glucose intolerance. Most patients with diabetes used oral hypoglycemic drugs, while others had modest insulin requirements. Notably, some patients with POEMS syndrome take glucocorticoids, which may lead to misdiagnosis of diabetes or severe hyperglycemia. Our patient had diabetes and was treated with hypoglycemic agents. After the peripheral blood stem cell transplant, he discontinued the hypoglycemic agents.
Patients with POEMS syndrome also show thyroid gland abnormalities. In 2017, Hu et al.19 found mild primary hypothyroidism in 13 of 22 patients with POEMS syndrome (high prevalence of 59%). Furthermore, Soubrier20 found secondary (central) hypothyroidism in 2 of 10 patients with hypothyroidism and in all 25 patients with POEMS syndrome. The underlying causes of hypothyroidism need to be further elucidated.
Some patients may be misdiagnosed with adrenal insufficiency because of the hyperpigmentation at the onset of disease. Bardwick et al.1 described a patient who had an isolated increasing adrenocorticotropic hormone concentration and normal cortisol concentration, and the authors considered that the normal cortisol concentration was compensatory. Prokop et al.21 described a patient who presented with two endocrine abnormalities: Addison’s disease and hypothyroidism. The patient did not present with symptoms suggestive of primary adrenal insufficiency, such as skin hyperpigmentation; this might have been becausethe patient was of African origin and did not notice deepening of his skin color or palmar lines. Patients who undergo long-term steroid treatment may develop secondary cortisol deficiency. Our patient had hyperpigmentation, but his adrenocorticotropic hormone and cortisol concentrations were with the reference ranges.
Interestingly, our patient had enlarged hands and coarsened skin; therefore, we performed a growth hormone suppression test to rule out acromegaly. His baseline growth hormone concentration was normal, but ingestion of 75 g of glucose did not depress the growth hormone concentration to <1 ng/mL. Cranial MRI was negative for a pituitary tumor. As a result, we ruled out acromegaly. Our patient was a peasant, and his long-term history of manual labor may have accounted for his enlarged hands and coarsened skin. Caimari et al.17 performed a cohort study with a systematic review of endocrinopathy in 75 patients with POEMS syndrome, and 8 patients with a high IGF-1 concentration were identified. One of these eight patients had acromegalic features, and an oral glucose tolerance test and pituitary MRI examination were negative for acromegalia. Another of the eight patients had only a mildly increased IGF-1 concentration and was also negative for acromegaly. These findings are inconsistent with previous studies and need to be further explained by future studies.
Peripheral neuropathy is always the initial presenting symptom of POEMS syndrome. Misdiagnosis often occurs because the characteristics of the neuropathy resemble those of chronic inflammatory demyelinating polyneuropathy. Li et al.18 described a patient with POEMS syndrome who was erroneously misdiagnosed with diabetic peripheral neuropathy based on a history of type 2 diabetes mellitus. Although this case was similar to our patient, the treatment differed. As mentioned above, measurement of VEGF is not available in most hospitals in China, which adds to the difficulties in achieving the correct diagnosis. Only when typical features of POEMS syndrome are recognized can the correct diagnosis be made and effective therapies initiated. The initial symptoms in our patient were progressive fatigue and numbness in the lower extremities, which we ascribed to cerebral infarction and diabetes because this seemed reasonable at that time. However, after the patient’s second hospitalization, he was finally properly diagnosed and given the correct therapy. Accordingly, it is very challenging but important to recognize this disease at an early stage.
Ischemic stroke has been increasingly recognized as a complication of POEMS syndrome and may be related to the vasculopathy caused by an elevated VEGF concentration.22–24 Cohort studies from the Mayo Clinic and Peking Union Medical College Hospital have addressed this issue in detail.25,26 Conversely, VEGF can promote the growth of new blood vessels and capillaries around ischemic blood vessels to improve the blood supply.27 Meanwhile, POEMS syndrome is a plasma cell disease. The abnormal proliferation of plasma cells leads to marked monoclonal or polyclonal albuminemia. Albuminemia and thrombocytosis cause changes in blood viscosity. Together, these changes cause vascular disease and even arterial occlusion. Our patient met the diagnostic criteria for POEMS syndrome and had no risk factors for arteriosclerosis or thrombosis. He had no family history of cerebrovascular disease. Color Doppler echocardiography excluded embolism caused by cardiogenic disease. By the time he was admitted to our hospital, the platelet level was normal because of the use of aspirin. After reviewing the literature, we believe that the onset of cerebral infarction in this case was caused by POEMS syndrome.
The treatment of POEMS syndrome is based on the extent of the plasma cell infiltration. For patients with an isolated bone lesion without clonal plasma cells found on iliac crest biopsy, radiation is recommended as the first-line therapy; however, systemic therapy is recommended for patients with disseminated disease.28 Because of the lack of published randomized clinical trials of POEMS syndrome, treatment recommendations are based on limited trial data, case series, and anecdotal data. According to the existing studies, the most effective therapeutic approach is likely to be alkylator therapy with a blood stem cell transplant. Other promising treatments include lenalidomide, thalidomide, and bortezomib, all of which can have anti-VEGF and anti-tumor necrosis factor effects. Our patient received alkylator therapy with a blood stem cell transplant, which led to substantial improvement of his condition.
Conclusion
The aim of this report is to enhance the awareness of POEMS syndrome, especially among endocrinologists. When clinicians encounter patients with a coexisting endocrinopathy, such as diabetes, and unexplained peripheral neuropathy or multisystem disorders, certain examinations are necessary to rule out POEMS syndrome. POEMS syndrome is a rare multisystem disease associated with multiple endocrinological abnormalities, both primary and secondary, that are easily misdiagnosed. The underlying pathogenesis is still unknown. As a result, detailed examinations should be performed to distinguish POEMS syndrome from other diseases such as diabetic peripheral neuropathy, hypothyroidism, and similar conditions.
Method
Briefly, laboratory parameters such as the concentrations of glycosylated hemoglobin, fasting glucose, serum hormones, urinary hormones, and IGF-1 were tested by an AU5800 Series Clinical Chemistry Analyzer (Beckman Coulter, Brea, CA, USA). Blood pressure was regularly measured with an OMRON Model HEM-752 FUZZY blood pressure monitor (Omron Company, Dalian, China). Liver imaging was performed with a LOGIQ E9 ultrasound system (GE Healthcare, Chicago, IL, USA). Cardiac function was recorded by a cardiac ultrasound system (LOGIQ E9; GE Healthcare). CT and MRI examinations were performed by a CT SOMATOM Sensation 64 (dual-source CT system) and 3.0T MRI system (Ingenia; Philips Healthcare, Best, Netherlands), respectively. PET-CT was performed for further metabolic analysis by a Biograph mCT 64 PET/CT scanner (Siemens Healthcare, Erlangen, Germany). Instruments used for the examinations in other hospitals are not mentioned here.
CARE Checklist (2016) statement
The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2016). The patient’s data have been de-identified.
Informed consent and ethics statements
Written informed consent was obtained from the patient for publication of this report and any accompanying images. The study protocol was approved by the medical ethics committee of the Third Affiliated Hospital of Soochow University.
Footnotes
Authors’ contributions: Shi H, Wang L, and Zhou JY cared for the patient, and Shi H wrote the paper. Jiang XH revised the paper. All authors approved the final version of the manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Xiaohong Jiang https://orcid.org/0000-0002-9792-0852
References
- 1.Bardwick PA, Zvaifler NJ, Gill GN, et al. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine (Baltimore) 1980; 59: 311–322. [DOI] [PubMed] [Google Scholar]
- 2.Brown R, Ginsberg L. POEMS syndrome: clinical update. J Neurol 2019; 266: 268–277. DOI: 10.1007/s00415-018-9110-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dispenzieri A. POEMS syndrome: 2019 update on diagnosis, risk-stratification, and management. Am J Hematol 2019; 94: 812–827. DOI: 10.1002/ajh.25495 [DOI] [PubMed] [Google Scholar]
- 4.Li J, Zhou DB. New advances in the diagnosis and treatment of POEMS syndrome. Br J Haematol 2013; 161: 303–315. DOI: 10.1111/bjh.12236 [DOI] [PubMed] [Google Scholar]
- 5.Gandhi GY, Basu R, Dispenzieri A, et al. Endocrinopathy in POEMS syndrome: the Mayo Clinic experience. Mayo Clin Proc 2007; 82: 836–842. DOI: 10.4065/82.7.836 [DOI] [PubMed] [Google Scholar]
- 6.D'Souza A, Hayman SR, Buadi F, et al. The utility of plasma vascular endothelial growth factor levels in the diagnosis and follow-up of patients with POEMS syndrome. Blood 2011; 118: 4663–4665. DOI: 10.1182/blood-2011-06-362392 [DOI] [PubMed] [Google Scholar]
- 7.Cerri F, Falzone YM, Riva N, et al. An update on the diagnosis and management of the polyneuropathy of POEMS syndrome. J Neurol 2019; 266: 258–267. DOI: 10.1007/s00415-018-9068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neary J, Goodwin SE, Cohen LB, et al. Alcohol misuse link to POEMS syndrome in a patient. Cancers (Basel) 2017; 9: 129. DOI: 10.3390/cancers9100129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekiguchi Y, Misawa S, Shibuya K, et al. Ambiguous effects of anti-VEGF monoclonal antibody (bevacizumab) for POEMS syndrome. J Neurol Neurosurg Psychiatry 2013; 84: 1346–1348. DOI: 10.1136/jnnp-2012-304874 [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Huang XF, Cai QQ, et al. Remarkable expression of vascular endothelial growth factor in bone marrow plasma cells of patients with POEMS syndrome. Leuk Res 2016; 50: 78–84. DOI: 10.1016/j.leukres.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Sharma S. Polyneuropathy, organomegaly, endocrinopathy, M-protein and skin changes (POEMS syndrome): a paraneoplastic syndrome. Oxf Med Case Reports 2015; 2015: 237–240. DOI: 10.1093/omcr/omv023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanai K, Sawai S, Sogawa K, et al. Markedly upregulated serum interleukin-12 as a novel biomarker in POEMS syndrome. Neurology 2012; 79: 575–582. DOI: 10.1212/WNL.0b013e318263c42b [DOI] [PubMed] [Google Scholar]
- 13.Nagao Y, Mimura N, Takeda J, et al. Genetic and transcriptional landscape of plasma cells in POEMS syndrome. Leukemia 2019; 33: 1723–1735. DOI: 10.1038/s41375-018-0348-x [DOI] [PubMed] [Google Scholar]
- 14.Keddie S, Lunn MP. POEMS syndrome. Curr Opin Neurol 2018; 31: 551–558. DOI: 10.1097/wco.0000000000000610 [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Zhou YL, Cai H, et al. Markedly elevated serum total N-terminal propeptide of type I collagen is a novel marker for the diagnosis and follow up of patients with POEMS syndrome. Haematologica 2014; 99: e78–e80. DOI: 10.3324/haematol.2013.102962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos G, Lestre S, Joao A. Do you know this syndrome? POEMS syndrome. An Bras Dermatol 2013; 88: 1009–1010. DOI: 10.1590/abd1806-4841.20132266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caimari F, Keddie S, Lunn MP, et al. Prevalence and course of endocrinopathy in POEMS syndrome. J Clin Endocrinol Metab 2019; 104: 2140–2146. DOI: 10.1210/jc.2018-01516 [DOI] [PubMed] [Google Scholar]
- 18.Li H, Huang Y, Li Y, et al. Endocrine manifestations in POEMS syndrome: a case report and literature review. BMC Endocr Disord 2019; 19: 33. DOI: 10.1186/s12902-019-0355-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu P, Luo YY, Wu J, et al. [Analysis of clinical features of 23 patients with POEMS syndrome]. Beijing Da Xue Xue Bao Yi Xue Ban 2017; 49: 985–989. [PubMed] [Google Scholar]
- 20.Soubrier M. [POEMS syndrome]. Presse Med 2007; 36: 1676–1682. [DOI] [PubMed] [Google Scholar]
- 21.Prokop J, Estorninho J, Marote S, et al. POEMS syndrome: a rare cause of adrenal insufficiency in a young male. Endocrinol Diabetes Metab Case Rep 2019; 2019: EDM190010. DOI: 10.1530/edm-19-0010 [DOI] [PubMed] [Google Scholar]
- 22.Wu SF, Zhou GQ, Zhu ZF. [A case of POEMS syndrome patient with main clinical manifestation of multiple acute cerebral infarction]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2013; 25: 753. [PubMed] [Google Scholar]
- 23.Mitsutake A, Matsumoto H, Hatano K, et al. Lenalidomide-induced ischemic cerebrovascular disease in polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes syndrome. J Stroke Cerebrovasc Dis 2018; 27: e102–e103. [DOI] [PubMed] [Google Scholar]
- 24.De Celis E, De Leciñana MA, Rodríguez-Pardo J, et al. Increased risk of ischemic stroke in multiple myeloma associated with lenalidomide treatment: a case report and review of the literature. Clin Neuropharmacol 2018; 41: 232–235. [DOI] [PubMed] [Google Scholar]
- 25.Feng J, Gao XM, Zhao H, et al. Ischemic stroke in patients with POEMS syndrome. Blood Adv 2020; 4: 3427–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupont SA, Dispenzieri A, Mauermann ML, et al. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology 2009; 73: 1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou DG, Shi YH, Cui YQ. Impact of G-CSF on expressions of Egr-1 and VEGF in acute ischemic cerebral injury. Exp Ther Med 2018; 16: 2313–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warsame R, Yanamandra U, Kapoor P. POEMS syndrome: an enigma. Curr Hematol Malig Rep 2017; 12: 85–95. DOI: 10.1007/s11899-017-0367-0 [DOI] [PubMed] [Google Scholar]