Abstract
Obesity is one of major risk factors increasing chronic diseases including type II diabetes, cardiovascular diseases, and hypertension. The effects of epigallocatechin gallate (EGCG), the major active compound in green tea, on reduced obesity and improved metabolic profiles are still controversial. Furthermore, the effects of EGCG on human adipocyte lipolysis and browning of white adipocytes have not been elucidated. This study aimed to investigate the effects of EGCG on obesity, lipolysis, and browning of human white adipocytes. The results showed that, when compared to the baseline values, EGCG significantly decreased fasting plasma triglyceride levels (P < 0.05), systolic blood pressure (P < 0.05), diastolic blood pressure (P < 0.05), and serum kisspeptin levels (P < 0.05) after 8 weeks of supplement. On the other hand, supplement of EGCG in obese human subjects for 4 or 8 weeks did not decrease body weight, body mass index, waist and hip circumferences, nor total body fat mass or percentage when compared to their baseline values. The study in human adipocytes showed that EGCG did not increase the glycerol release when compared to vehicle, suggesting that it had no lipolytic effect. Furthermore, treatment of EGCG did not enhance uncoupling protein 1 (UCP1) mRNA expression in human white adipocytes when compared with treatment of pioglitazone, the peroxisome proliferator-activated receptor γ (PPAR-γ) agonist, suggesting that EGCG did not augment the browning effect of PPAR-γ on white adipocytes. This study revealed that EGCG reduced 2 metabolic risk factors which are triglyceride and blood pressure in the human experiment. We also showed a novel evidence that EGCG decreased kisspeptin levels. However, EGCG had no effects on obesity reduction in humans, lipolysis, nor browning of human white adipocytes.
Keywords: Obesity, epigallocatechin gallate, plasma triglyceride, blood pressure, kisspeptin, human adipocytes
Impact statement
Obesity has become the worldwide problem that causes adverse health consequences in individuals. The effects of EGCG on decreased obesity and improved metabolic profiles are still inconclusive. This study revealed that EGCG decreased 2 metabolic risk factors including blood pressure and triglyceride in human obese subjects but had no effect on obesity reduction. This study also showed a novel finding that EGCG decreased kisspeptin levels in human obese subjects. The study in human adipocytes showed that EGCG had no effects on lipolysis nor browning of human white adipocytes. These findings might suggest that EGCG treatment ameliorated metabolic parameters but did not reduce obesity in obese humans. However, further studies are required to explore the relationship between the effect of EGCG on reduction of blood pressure and kisspeptin levels.
Introduction
For more than a decade, obesity is clearly identified as a common factor contributing to increased risks of morbidities including hypertension,1 cardiovascular diseases,2 diabetes mellitus,3 and dyslipidemia.4 The prevalence of obesity was rapidly increased worldwide from 1980 to 2015.5 Obesity is also known as a major risk of metabolic syndrome, high blood glucose levels, insulin resistance, dyslipidemia (high triglyceride and cholesterol levels), and high blood pressure.6,7 Obesity is associated with growth of adipocytes (adipocyte hypertrophy with excess fat accumulation in the cytosol) and hyperplasia (increased preadipocyte proliferation and differentiation).8 The adiposity reduction is revealed via diverse mechanisms including decreased adipogenesis, increased adipocyte lipolysis,9,10 and increased thermogenesis through browning of white adipocytes.11 Lipolysis is a process that triglycerides stored in adipocytes are hydrolyzed into 1 glycerol, a marker of lipolysis, and 3 free fatty acids12 causing a decrease in lipid accumulation in adipocytes. Browning of white adipocytes is the conversion of white adipocytes, which appear as spherical shape with unilocular lipid droplets and flattened nuclei,13,14 to beige adipocytes or brown-like adipocytes, characterized by polygonal shape with multilocular lipid droplets, high mitochondria content, and increased expression of uncoupling protein 1 (UCP1).15–17 The presence of UCP1, the marker of brown adipocytes,15,16 leads to heat production for thermoregulation,18 resulting in increased body energy expenditure.
Green tea is a famous beverage, made from Camellia Sinensis leaves and was reported as a preventative agent for obesity,19,20 tumor,21 inflammation, and oxidative stress.22 Green tea has been shown to prevent weight gain in rats fed with high-fat diet;23 reduce body weight, body mass index (BMI), body fat, waist circumference, hip circumference, and waist-to-hip ratio; and increase resting energy expenditure in obese human subjects.24 The major compounds in green tea are composed of caffeine, flavonols, catechins, epigallocatechin (EGC), epicatechin gallate (ECG), epicatechin (EC), and epigallocatechin gallate (EGCG).25 EGCG is an active ingredient of green tea26 which was shown to decrease visceral fat area, abdominal fat mass, total body fat mass, waist circumference, and waist-to-hip ratio in overweight and obese postmenopausal women.27 On the other hand, another study in obese women showed that EGCG did not have any effects on body weight, waist circumference, BMI, lean mass, fat mass, and resting metabolic rate.28 Furthermore, EGCG had no effects on blood glucose, insulin, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol,27,28 the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR),28 adiponectin, and leptin.27
Leptin is the well-known hormone predominantly produced by adipose cells29 and plays a major role in appetite regulation30,31 by increasing energy expenditure and decreasing food intake.32 Leptin levels are positively correlated with BMI,33 total body fat mass,34 fasting insulin, and HOMA-IR.33 Furthermore, leptin is also shown to activate sympathetic nervous system.35 Leptin injection significantly increased systolic blood pressure (SBP) in Sprague-Dawley rats36 but had no effect in humans.37 Furthermore, leptin is reported to have a positive correlation with kisspeptin in human subjects.38 Kisspeptin is a 54-amino acid peptide39 that plays a key role in reproductive regulation40 and was also associated with metabolic41–43 and cardiovascular parameters.44,45 Previous studies revealed that kisspeptin was a potent vasoconstrictor of the coronary artery, umbilical veins,44 and peripheral microvasculature.46 Leptin and kisspeptin might be involved in obesity, metabolic, and cardiovascular regulations.
In in vitro experiments, EGCG stimulated lipolysis in isolated primary adipocytes of male rats;47 reduced lipid accumulation, increased glycerol release, and increased mRNA expression of hormone-sensitive lipase (HSL) (a rate-limiting enzyme of lipolysis12) in 3T3-L1 mouse adipocytes;48 and reduced cytosol lipid contents and increased lipophagy, a form of autophagy specialized for lipid droplets degradation, in mature mouse adipocytes differentiated from C3H10T1/2 cells.49 Furthermore, EGCG increased UCP1 protein in mature mouse adipocytes differentiated from C3H10T1/2 cells,49 suggestive of its effect on adipocyte browning.
Collectively, the effects of EGCG on obesity reduction and metabolic parameters are still controversial. Furthermore, there is no study regarding EGCG effects on blood kisspeptin levels in human subjects as well as lipolysis and browning of human white adipocytes. This study aimed to (1) study the effects of EGCG on obesity reduction and improvement of metabolic parameters in obese human subjects, (2) investigate the effect of EGCG on human adipocyte lipolysis, and (3) investigate the effect of EGCG on browning of human white adipocytes.
Materials and methods
The study in obese human subjects
Subjects
Thai obese subjects (BMI ≥ 25 kg/m2), who were older than 18 years, were recruited in this study. BMI equal or more than 25 kg/m2 in Asian-Pacific populations50,51 or equal or more than 30 kg/m2 in Caucasian populations is categorized as obesity.52 The recruited subjects (n = 30) were randomly allocated into 2 groups (n = 15 per group) consisting of the EGCG-supplemented group and the placebo (starch capsule)-supplemented group. Thirty drawing cards generated from the researchers (15 cards per group) were placed in a bottle and were randomly picked from all subjects. EGCG with 95% purity was produced by the Shaanxi Jiahe Phytochem Co., Ltd, Ki’an, China (Lot. No STP-QCP-133–091) and was pharmaceutical packed in capsules by the Bangkok Lab & Cosmetic Co., Ltd, Ratchaburi, Thailand. The exclusion criteria included subjects who had metabolic diseases (e.g. hypo- or hyperthyroidism, diabetes mellitus, or Cushing syndrome), a medical condition causing weight gain known as secondary obesity (e.g. hypothalamic disorders, endocrine disorders, and some congenital conditions), and history of hypersensitivity to caffeine or tea; were menopausal, pregnant, or lactating; used weight-reducing medications in any forms and any kinds; and did regular exercise (at least 30 min per session with 3 times per week).53 There were no subjects who withdrew from the research experiment.
The study protocol
This study, a double-blind, placebo-controlled clinical trial, was done at the Department of Physiology, Faculty of Medicine Siriraj Hospital. A capsule of EGCG at a dose of 150 mg or a starch capsule was given to subjects allocated in the EGCG-supplemented group or the placebo-supplemented group, respectively, twice a day after breakfast and dinner for 8 weeks without any dietary restriction. All supplement capsules were undistinguishable in shape and appearance with neither participants nor the researchers knowing which supplement was taken by the subjects. All subjects attended laboratory experiment for 3 times which were at baseline or week 0, week 4, and week 8 for clinical assessment and anthropometric data measurements. The 8-h fasting blood samples were collected at week 0 and week 8 of the experiment. Female subjects participated each experiment on days 1–3 (the early proliferative phase) of their menstrual cycle to control the effects of sex steroids which could be the confounding factors of this study. Male subjects could participate the experiment at any available days. Throughout the entire study, all subjects were instructed to maintain their physical activity patterns and normal diet. Furthermore, all subjects received a designed calendar to mark when each supplement capsule was taken as well as record their diet 3 days before the next visit and the side effects of the intervention. For drug compliance checking, all subjects had to bring both the medication and calendar at the end of week 4 and week 8.
Demographic and anthropometric data and blood pressure measurement
The clinical data including age, body weight, height, BMI, waist circumference, hip circumference, total body fat percentage, and total body fat mass were collected. Total body fat mass and total body fat percentage were estimated by TANITA®. Waist circumference was measured at the umbilicus level of subjects in the standing position with silent breathing.54 A previous study showed that measurement of waist circumference by technicians at midpoint between the superior borders of the iliac crest and the last rib (World Health Organization standard)55 was comparable with at the umbilicus, and there was no significant clinical advantage in terms of blood pressure of waist circumference measurement between these sites.54 However, measurement of waist circumference at the umbilicus was considered as the easier site54 and could be consistently measured among different visits. In this study, the measurement of waist circumference at the umbilicus was selected because it is the most noticeable landmark and could be assured that the same location was measured in every visit. Hip circumference was measured at the widest region of the buttocks.55 SBP and diastolic blood pressure (DBP) were assessed by a sphygmomanometer in the supine position after a 30-min bed rest.
Hormonal assay
Blood levels of fasting insulin, glucose, HDL cholesterol, LDL cholesterol, total cholesterol, triglycerides, creatinine, blood urea nitrogen (BUN), direct bilirubin, total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured by the central laboratory at the Department of Clinical Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand. The enzymatic hexokinase method was used to determine fasting blood glucose, and the sandwich immunoassay technique using electrochemiluminescence immunoassay was used to analyze fasting blood insulin. The enzymatic colorimetric method was used to measure levels of total, HDL, and LDL cholesterols, triglyceride, and total and direct bilirubin. The urease/glutamate dehydrogenase-coupled enzymatic technique and the modification of the ultraviolet enzymatic method by Oliver and Rosalk were used to determine BUN and creatinine, respectively. The enzymatic reaction method was used to measure ALT and AST. The HOMA-IR (the insulin resistance index)56 was calculated as the following formula: HOMA-IR = (fasting glucose (mg/dL) × fasting insulin (µU/mL))/405. The quantitative insulin sensitivity check index (QUICKI) (the insulin sensitivity index)56 was calculated as the following formula: QUICKI = 1/((log(fasting insulin µU/mL) + log(fasting glucose mg/dL)).
Analysis of serum leptin, kisspeptin, and adiponectin levels
Serum leptin and adiponectin levels were determined by commercial enzyme-linked immunosorbent assay kits, while serum kisspeptin was measured by commercial enzyme immunoassay kits (Phoenix Pharmaceuticals, CA, USA) as per the protocol recommendation. The range of detection was 0.313–20 ng/mL for leptin, 0–100 ng/mL for kisspeptin, and 0.15–10 ng/mL for adiponectin. The minimum detectable concentration was 0.313 ng/mL for leptin, 0.05 ng/mL for kisspeptin, and 0.15 ng/mL for adiponectin. Serum samples were diluted with assay buffer with 1:5 dilution for leptin, 1:2 dilution for kisspeptin, and 1:5,000 dilution for adiponectin. The intra-assay and interassay variations were 6.66% and 6.74%, respectively, for leptin; 6.38% and 7.85%, respectively, for kisspeptin; and 2.33% and 3.55%, respectively, for adiponectin. The optical density (O.D.) was read by the Synergy HT Multi-Detection Microplate Readers (BioTek Instruments, Inc., Winooski, VT, USA) at 450 nm.
The study in human adipocyte culture
Human preadipocytes (HPAd) (Cell Applications, CA, USA) were used in this study. Then, HPAd were cultured with preadipocyte growth medium (Cell Applications, CA, USA) with 1% penicillin/streptomycin (Corning, NY, USA) supplementation in 5% CO2 humidified incubator at 37 °C. The medium was changed every 2 days until 100% confluent was reached. Then, the HPAd were induced differentiation by adipocyte differentiation medium (Cell Applications, CA, USA) with 1% penicillin/streptomycin supplementation which was changed every 3 days for 15 days. The cells were differentiated into mature human adipocytes (HAd) at day 15 which was shown with lipid droplets accumulated in the cells. We confirmed preadipocyte differentiation by detecting lipid filling into adipocytes by Oil Red O staining and measuring mRNA expression of peroxisome proliferator-activated receptor γ (PPAR-γ), an adipocyte differentiation marker,57,58 by real-time polymerase chain reaction (PCR) before starting experiments (the data are not shown).
The lipolysis experiment
HPAd (55,000 cells) were grown and treated with adipocyte differentiation medium on round cover glass in 12-well plates. Before starting the experiment, mature HAd were treated with serum-free medium without phenol red (Corning, NY, USA) for 2-h starvation. Then, the cells were treated for 24 h with vehicle (0.01% dimethyl sulfoxide [DMSO]), the positive control (10 µM isoproterenol), and EGCG (0.001, 0.01, 0.1, 1, and 10 μM). Each experiment was performed in triplicate. Glycerol release, the marker of lipolysis, was measured in the medium by the Lipolysis Colorimetric Assay Kit (Sigma-Aldrich, MO, USA). The range of glycerol detection was 0–10 nmol/μL. The O.D. was read at 570 nm by the Synergy HT Multi-Detection Microplate Readers (BioTek Instruments, Inc., VT, USA). The fat area assay (the actual fat levels determined with Oil Red O) measured by the total area of fat droplets determined with Oil Red O staining of adipocytes per image was used to represent the lipid content in adipocytes because it was the accurate and quick method and had a very strong positive correlation (R = 0.8787, P < 0.0001) with fat accumulation in differentiated adipocytes.59 The round cover glass was removed out of the culture plate after Oil Red O staining, placed on glass slides, and then scanned with 20× magnification by the ScanScope® XT machine for the whole slide. The AxioVisionVR software Release 4.8.2 (Carl Zeiss AG, Oberkochen, Germany) was used to analyze total fat area of the scanned images. Then, the glycerol concentrations in samples normalized by total fat area (glycerol concentrations/total lipid) were used to represent the glycerol release for each experiment.
Browning of human white adipocytes
HPAd (100,000 cells) were cultured and treated with differentiation medium in 6-well plates. Mature HAd were treated with vehicle (0.01% DMSO), 3 µM pioglitazone (PPAR-γ agonist), 0.1 μM EGCG, 1 μM EGCG, 3 µM pioglitazone combined with 0.1 μM EGCG, and 3 µM pioglitazone combined with 1 μM EGCG, together with the serum-free medium for 7days. The medium was changed every 3 days. Each experiment was done in triplicate. RNA of the harvested cells was extracted and kept at –70 °C until analysis.
Real-time PCR analysis
Real-time PCR was used to quantify mRNA expressions of PPAR-γ, UCP1, and GAPDH. In short, the TRIzol® reagent (Invitrogen, CA, USA) was used to extract total RNA as per the protocol recommendation. One mg of total RNA was converted to complementary DNA (cDNA) by reverse transcription using the iScript cDNA Synthesis Kit (Bio-Rad, CA, USA). Real-time PCR was carried out using the reagents from the biotechrabbit QPCR Green Master Mix LROX, 2× kit (biotechrabbit, Berlin, Germany). As GAPDH mRNA expression in human adipocytes was not changed under various experimental conditions,60 it was used as the reference gene in this study. The primer sequences, which were designed by authors and blasted to check primer specificity by using nucleotide sequences published in PubMed database, are shown as follows:
PPAR-γ (NM_015869)
Forward 5′-AAAGTGCAATCAAAGTGGAGCC-3′
Reverse 5′-CAAACCTGATGGCATTATGAGAC-3′
UCP1 (NM_021833)
Forward 5′-GCTCCAGGTCCAAGGTGAATG-3′
Reverse 5′-CAATGAATACTGCCACTCCTCCA-3′
GAPDH (NM_002046)
Forward 5′-GCCAGCCGAGCCACATC-3′
Reverse 5′-GCTCCTGGAAGATGGTGATGG-3′
The CFX96 Real-Time PCR Detection System (Bio-Rad, CA, USA) was used to perform real-time PCR amplification under the following conditions: Uracil DNA glycosylase (UDG) treatment at 50 °C for 2 min, Taq DNA polymerase activation at 95 °C for 3 min, 40 cycles of DNA denaturing at 95 °C for 15 s, annealing for 30 s at 57 °C for UCP1 and GAPDH and 54 °C for PPAR-γ, and extension at 65 °C for 30 s. Each condition was performed in duplicate. No template control was used as a negative control. The 2–ΔCT method was used as a comparative technique of quantification.
Statistical analysis
The Shapiro–Wilk test was performed to test for normality. In the human study, data were shown as the percentile 25th, median, percentile 75th, and boxplot since the mean values of parameters of the control subjects were presented in another study. Comparisons of body weight, BMI, waist circumference, hip circumference, total body fat percentage, total body fat mass, SBP, and DBP among at baseline, week 4, and week 8 in the same treatment were performed by the repeated-measures analysis of variance (ANOVA) followed by the Fisher’s Least Significant Difference (LSD) analysis. For normal distributed data including total bilirubin and creatinine of the EGCG-treated group; plasma AST, BUN, estimated glomerular filtration rate (eGFR), cholesterol, LDL, glucose, and QUICKI of the placebo- and EGCG-treated groups; and plasma HDL and serum leptin of the placebo-treated group, comparisons between at baseline and at week 8 of the same treatment were performed by the paired sample t-test (as shown with t values in Table 2, Figures 1to 3). For non-normal distributed data including total bilirubin, ALT, creatinine of the placebo group; direct bilirubin, plasma triglyceride, insulin, HOMA-IR, serum kisspeptin, and serum adiponectin of the placebo- and EGCG-treated groups; and plasma HDL and serum leptin of the EGCG-treated group, comparisons between at baseline and at week 8 of the same treatment were performed by the non-parametric test (Wilcoxon Signed-Rank Test). Comparisons between EGCG and placebo at week 8 were performed by the analysis of covariance adjusted for the baseline values of each treatment. In the human adipocyte experiments, data are expressed as mean ± standard error of the mean (SEM). Comparisons between groups of treatments were performed by the one-way ANOVA after checking for homogeneity of variance, followed by the LSD analysis. P value < 0.05 was considered as statistical significance.
Table 2.
The effects of EGCG on liver and kidney functions in obese subjects.
| Variables | Placebo (n = 15) |
EGCG (n = 15) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Week 8 |
(t values/NP, P values) | Baseline |
Week 8 |
(t values/NP, P values) | |||||||||
| P25 | Med | P75 | P25 | Med | P75 | P25 | Med | P75 | P25 | Med | P75 | |||
| Total bilirubin (mg/dL) | 0.35 | 0.40 | 0.70 | 0.32 | 0.39 | 0.51 | (NP, 0.589) | 0.32 | 0.48 | 0.60 | 0.30 | 0.45 | 0.59 | (1.70, 0.112) |
| Direct bilirubin (mg/dL) | 0.11 | 0.14 | 0.17 | 0.10 | 0.13 | 0.20 | (NP, 0.648) | 0.12 | 0.17 | 0.20 | 0.12 | 0.15 | 0.21 | (NP, 0.150) |
| AST (U/L) | 14.00 | 17.00 | 22.00 | 15.00 | 20.00 | 27.00 | (–0.99, 0.336) | 15.00 | 22.00 | 26.00 | 14.00 | 21.00 | 26.00 | (0.21, 0.840) |
| ALT (U/L) | 14.00 | 17.50 | 24.25 | 15.00 | 18.00 | 29.00 | (NP, 0.342) | 16.00 | 22.00 | 44.00 | 14.00 | 30.00 | 45.00 | (–0.72, 0.483) |
| BUN (mg/dL) | 8.60 | 10.70 | 12.40 | 9.00 | 10.70 | 14.60 | (–0.80, 0.435) | 9.60 | 12.10 | 15.60 | 9.80 | 12.90 | 15.40 | (–0.63, 0.535) |
| Creatinine (mg/dL) | 0.60 | 0.69 | 1.01 | 0.62 | 0.75 | 1.08 | (NP, 0.362) | 0.65 | 0.94 | 1.15 | 0.69 | 0.86 | 1.20 | (0.35, 0.730) |
| eGFR (mL/min) | 93.55 | 105.81 | 117.30 | 96.57 | 103.51 | 114.76 | (1.11, 0.285) | 87.47 | 98.63 | 114.36 | 78.92 | 103.03 | 114.36 | (–0.48, 0.640) |
EGCG: epigallocatechin gallate; NP: non-parametric test; AST: aspartate aminotransferase; ALT: alanine aminotransferase; BUN: blood urea nitrogen; eGFR: estimated glomerular filtration rate.
The liver and kidney functions of obese human subjects supplemented with placebo or EGCG at baseline and week 8 including total bilirubin, direct bilirubin, AST, ALT, BUN, creatinine, and eGFR.
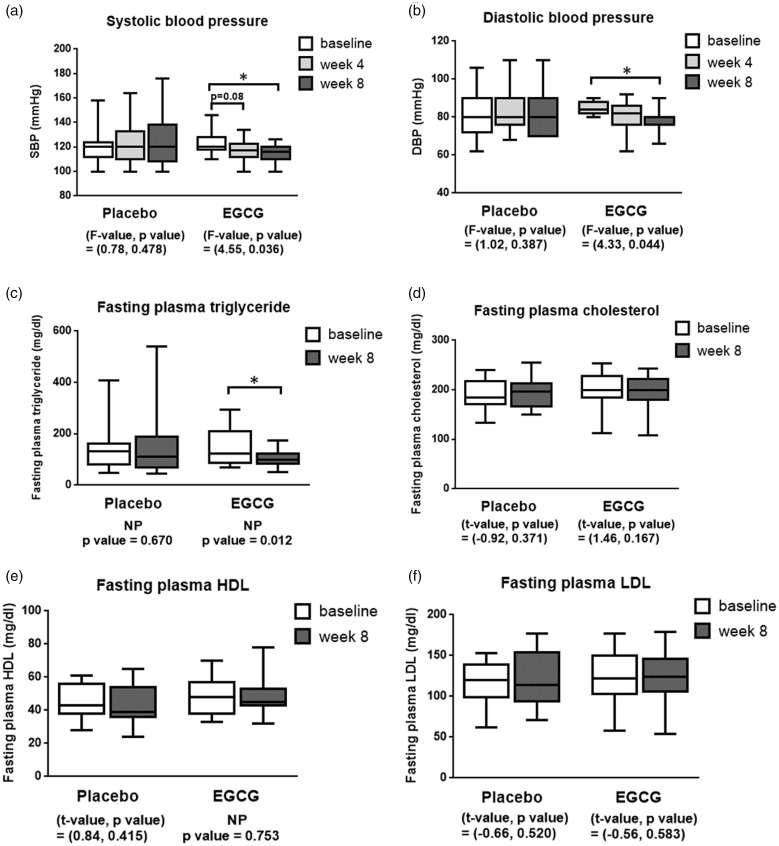
Figure 1.
The boxplots of clinical parameters before and after supplemented with placebo or EGCG for 4 and 8 weeks in obese human subjects including SBP (panel a), DBP (panel b), fasting plasma triglyceride (panel c), total cholesterol (panel d), HDL cholesterol (panel e), and LDL cholesterol (panel f). *P < 0.05.
NP: non-parametric test; EGCG: epigallocatechin gallate; SBP: systolic blood pressure; DBP: diastolic blood pressure; HDL: high-density lipoprotein; LDL: low-density lipoprotein.
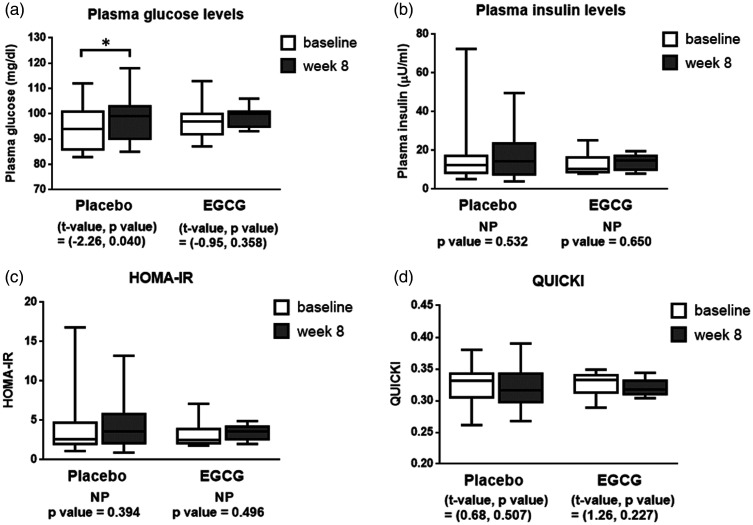
Figure 2.
The boxplots of plasma glucose levels (panel a), plasma insulin levels (panel b), HOMA-IR (panel c), and QUICKI (panel d) before and after supplemented with placebo or EGCG for 8 weeks in obese human subjects. *P < 0.05.
NP: non-parametric test; EGCG: epigallocatechin gallate; HOMA-IR: Homeostatic Model Assessment for Insulin Resistance; QUICKI: Quantitative Insulin Sensitivity Check Index.
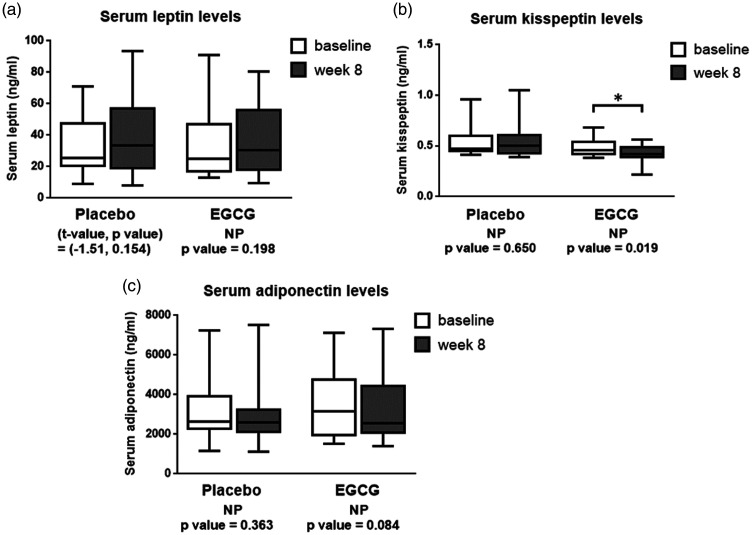
Figure 3.
The boxplots of serum levels of leptin (panel a), kisspeptin (panel b), and adiponectin (panel c) before and after supplemented with placebo or EGCG for 8 weeks in obese human subjects. *P < 0.05.
NP: non-parametric test; EGCG: epigallocatechin gallate.
Results
The effects of EGCG on obesity and clinical parameters in obese subjects
The effects of EGCG on obesity and clinical parameters in obese subjects are shown in Table 1 and Figure 1. Age was comparable between placebo- and EGCG-supplemented subjects (Table 1). Body weight, BMI, waist circumference, hip circumference, total body fat percentage, and total fat mass were comparable between the placebo- and EGCG-supplemented groups as well as among at baseline, week 4, and week 8 time points in the same supplement (Table 1). Interestingly, EGCG supplement had a trend to decrease SBP at week 4 (P = 0.08) (mean ± SEM = 117.08 ± 2.45 mmHg) and significantly decreased SBP at week 8 (P < 0.05) (mean ± SEM = 115.85 ± 1.99 mmHg) when compared to the baseline value (mean ± SEM = 122.77 ± 2.46 mmHg) (F value, P value) = (4.55, 0.036) (Figure 1a). EGCG also significantly decreased DBP (P < 0.05) at week 8 (mean ± SEM = 80.00 ± 1.79 mmHg) but not at week 4 (mean ± SEM = 82.00 ± 2.24 mmHg) of supplementation when compared to the baseline value (mean ± SEM = 84.67 ± 1.05 mmHg) (F value, P value) = (4.33, 0.044) (Figure 1b).
Table 1.
The effects of EGCG on obesity and clinical parameters in obese subjects.
| Variables | Placebo (n = 15) |
EGCG (n = 15) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Week 4 |
Week 8 |
(F values, P values) | Baseline |
Week 4 |
Week 8 |
(F values, P values) | |||||||||||||
| P25 | Med | P75 | P25 | Med | P75 | P25 | Med | P75 | P25 | Med | P75 | P25 | Med | P75 | P25 | Med | P75 | |||
| Age (years) | 27.0 | 39.0 | 45.0 | 30.0 | 38.0 | 43.0 | ||||||||||||||
| Body weight (kg) | 64.8 | 73.3 | 82.9 | 65.6 | 73.5 | 81.7 | 65.8 | 73.9 | 81.7 | (1.21, 0.330) | 70.2 | 79.4 | 95.0 | 70.8 | 79.3 | 94.2 | 70.0 | 79.3 | 93.2 | (0.00, 0.998) |
| BMI (kg/m2) | 26.0 | 27.7 | 33.0 | 26.2 | 27.7 | 32.9 | 26.2 | 27.7 | 32.7 | (1.46, 0.269) | 25.9 | 28.4 | 35.0 | 26.0 | 28.0 | 34.8 | 25.9 | 28.3 | 34.8 | (0.03, 0.968) |
| Waist circumference (cm) | 87.0 | 94.5 | 99.0 | 87.5 | 93.5 | 97.0 | 84.0 | 93.0** | 95.5 | (7.01, 0.009) | 91.0 | 98.0 | 110.0 | 88.0 | 100.0 | 109.0 | 90.5 | 99.0 | 106.0 | (0.37, 0.697) |
| Hip circumference (cm) | 99.0 | 105.0 | 109.5 | 99.0 | 105.0 | 110.0 | 98.5 | 104.0 | 107.0 | (2.01, 0.174) | 100.5 | 106.0 | 114.0 | 98.0 | 103.0 | 114.0 | 100.0 | 104.5 | 115.5 | (1.76, 0.211) |
| Total body fat percentage (%) | 33.9 | 37.4 | 41.0 | 34.0 | 36.7 | 42.2 | 28.8 | 37.1 | 42.9 | (0.67, 0.528) | 31.1 | 33.0 | 38.5 | 30.1 | 34.9 | 39.3 | 32.0 | 34.3 | 41.3 | (0.69, 0.517) |
| Total body fat mass (kg) | 22.5 | 26.8 | 31.8 | 22.5 | 25.7 | 33.5 | 21.5 | 25.1 | 34.2 | (0.39, 0.688) | 21.4 | 26.7 | 35.3 | 21.7 | 27.7 | 38.4 | 22.5 | 28.3 | 34.7 | (0.65, 0.538) |
EGCG: epigallocatechin gallate; BMI: body mass index.
The clinical parameters of obese human subjects supplemented with placebo or EGCG at baseline, week 4, and week 8 including age, body weight, BMI, waist circumference, hip circumference, total body percentage, and total body fat mass. Data are shown as the 25th percentile (P25), median (Med), and 75th percentile (P75). **P < 0.01 compared to the baseline value.
The effects of EGCG on peripheral metabolic factors in obese subjects
The effects of EGCG on peripheral metabolic factors in obese subjects are shown in Figures 1 and 2. Interestingly, EGCG supplement for 8 weeks significantly decreased fasting plasma triglyceride levels (mean ± SEM = 105.42 ± 10.56 mg/dL) when compared to the baseline levels (mean ± SEM = 135.00 ± 21.52 mg/dL) (P < 0.05) (Figure 1c). However, levels of fasting plasma glucose, insulin, HOMA-IR, QUICKI (Figure 2a to d), total cholesterol, HDL cholesterol, and LDL cholesterol (Figure 1d to f) of the EGCG-treated group were not different between at baseline and week 8.
The effects of EGCG on serum leptin, kisspeptin, and adiponectin levels
The effects of EGCG on serum kisspeptin, leptin, adiponectin levels are shown in Figure 3. Serum leptin levels were comparable between before (mean ± SEM = 30.15 ± 5.37 ng/mL) and after 8 weeks (mean ± SEM = 37.34 ± 6.30 ng/mL) of the EGCG treatment (Figure 3a). Remarkably, serum kisspeptin of the EGCG-treated group was significantly decreased at week 8 (mean ± SEM = 0.43 ± 0.02 ng/mL) when compared to the baseline levels (mean ± SEM = 0.48 ± 0.02 ng/mL) (P < 0.05) (Figure 3b). Serum adiponectin levels of the EGCG-treated group were comparable between at baseline (mean ± SEM = 4,014.90 ± 664.78 ng/mL) and week 8 (mean ± SEM = 3,466.96 ± 479.38 ng/mL) (Figure 3c).
The effects of EGCG on blood chemistry of kidney and liver functions in obese subjects
The effects of EGCG on blood chemistry of kidney and liver functions in obese subjects are shown in Table 2. Kidney function tests including BUN (mean ± SEM = 12.77 ± 0.96 mg/dL at baseline vs. 13.14 ± 1.04 mg/dL at week 8), creatinine (mean ± SEM = 0.93 ± 0.08 mg/dL at baseline vs. 0.92 ± 0.07 mg/dL at week 8), and eGFR (mean ± SEM = 97.76 ± 5.31 mL/min at baseline vs. 98.53 ± 5.04 mL/min at week 8) as well as liver enzymes including total bilirubin (mean ± SEM = 0.52 ± 0.06 mg/dL at baseline vs. 0.45 ± 0.04 mg/dL at week 8), direct bilirubin (mean ± SEM = 0.19 ± 0.02 mg/dL at baseline vs. 0.16 ± 0.01 mg/dL at week 8), AST (mean ± SEM = 20.6 ± 1.42 U/L at baseline vs. 20.40 ± 1.70 U/L at week 8), and ALT (mean ± SEM = 29.13 ± 4.24 U/L at baseline vs. 30.53 ± 4.43 U/L at week 8) were in normal ranges and comparable between at baseline and at week 8 time points (Table 2).
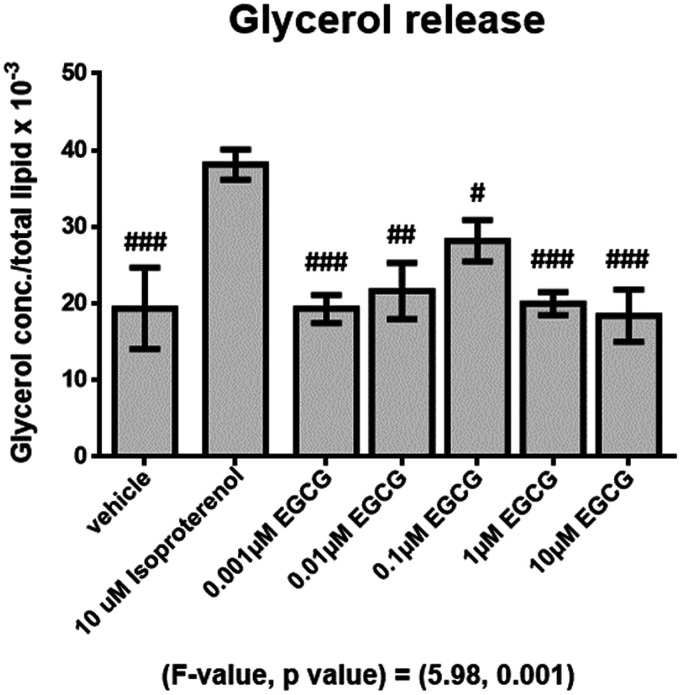
The effect of EGCG on human adipocyte lipolysis
The effect of EGCG on human adipocyte lipolysis is shown in Figure 4. The results showed that glycerol release of the isoproterenol-treated group was significantly higher (P < 0.001) than the vehicle-treated group (Figure 4). However, glycerol release of different doses of EGCG treatments was comparable with the vehicle group but was significantly lower than that of the isoproterenol-treated group (P < 0.05 all, Figure 4).
Figure 4.
Mean (±SEM) glycerol concentrations normalized to total lipid after supplemented with different doses of EGCG for 24 h, #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the isoproterenol-treated group.
EGCG: epigallocatechin gallate.
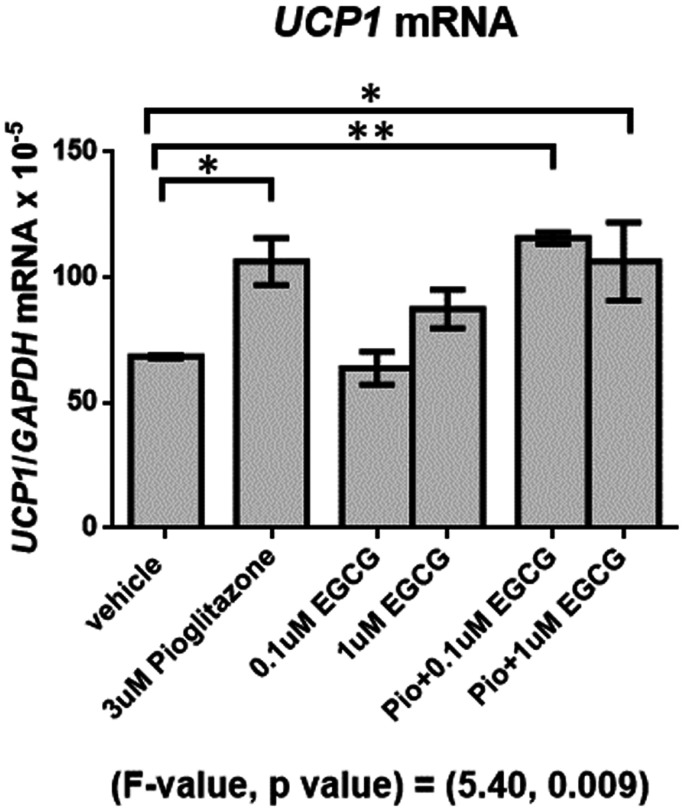
The effect of EGCG on browning of human white adipocytes
The effect of EGCG on browning of human white adipocytes is shown in Figure 5. The results showed that UCP1 mRNA was significantly higher in the pioglitazone alone (P < 0.05) and pioglitazone combined with 0.1 μM and 1 μM EGCG (P < 0.05 all)-treated groups when compared with vehicle (Figure 5). EGCG treatment at doses 0.1 and 1 μM did not increase UCP1 mRNA compared with the vehicle-treated group. EGCG at doses 0.1 and 1 μM combined with pioglitazone treatments did not enhance UCP1 mRNA expression when compared to pioglitazone treatment alone (Figure 5).
Figure 5.
Mean (±SEM) UCP1 mRNA expression normalized to GAPDH (reference gene) in different conditions of supplements for 7 days. *P < 0.05, **P < 0.01.
EGCG: epigallocatechin gallate; UCP1: uncoupling protein 1.
Discussion
This study investigated the effects of EGCG on obesity, metabolic profiles, and adipokines in obese human subjects as well as on lipolysis and browning of human white adipocytes. The study in humans showed that supplement of EGCG at the dose of 150 mg twice a day or 300 mg per day in obese human subjects for 4 and 8 weeks did not decrease body weight, BMI, total body fat mass, waist circumference, and hip circumference when compared to their baseline levels or the control group. As a result, these data suggest that EGCG had no effect on obesity reduction. These results were consistent with previous studies showing that EGCG did not decrease body weight and BMI after 12 weeks of treatment in obese postmenopausal women27 or could not prevent body weight and fat gains after treatment less than 12 months (3 and 6 months) in Down syndrome obese subjects.61 However, a previous study showed that after 12 months of treatment in Down syndrome obese subjects, EGCG was effective in preventing body weight and body fat gains which were observed in the placebo group.61 These results indicate that EGCG supplement for 8 weeks could not be effective in obesity reduction; however, a longer period of supplement might have a beneficial effect. Furthermore, our results of EGCG treatment on body composition were different from results of green tea treatment in previous studies showing that green tea extract (GTE) could reduce body weight,24,62 BMI,62 waist circumference,24 hip circumference,24 and waist-to-hip ratio24 in obese subjects after 8 weeks of treatment. The different findings might be because green tea has other compounds that can reduce body weight and body composition including caffeine63,64 or flavonoid65 causing more pronounced effect on obesity reduction.
Although EGCG had no effects on body weight and body fat, it reduced fasting plasma triglyceride levels after supplement for 8 weeks when compared to the baseline levels around 30 mg/dL (with mean ± SEM of 135.00 ± 21.52 at baseline to 105.42 ± 10.56 mg/dL at week 8). Our result was consistent with previous studies showing that green tea catechins or EGCG alone decreased triglyceride levels in overweight and obese adults after 12 weeks of consumption,66 in high-fat diet fed mice for 20 weeks treatment,67 and in Caenorhabditis elegans for 2 days treatment.68 However, the effect of EGCG supplement on triglyceride reduction was different from GTE supplement in some previous studies showing that plasma triglyceride levels were increased after supplementation of GTE (composed of 207.5 mg of EGCG, 181.1 mg of EGC, 55.1 mg of gallocatechin, 47 mg of EC, 31 mg of gallocatechin gallate, 25.6 mg of ECG, 11.9 mg of catechin, and 120.4 mg of caffeine) twice a day for 8 days in healthy male subjects69 and GTE capsules (composed of 4 mg caffeine, 843 mg of EGCG, 202 mg of ECG, 107 mg of EGC, and 107 mg of EC) twice a day for 12 months in healthy postmenopausal subjects.70 The different findings might be because of different ingredients, doses of EGCG in green tea supplement, duration of treatment, and subject characteristics. There are some possible mechanisms that could explain how EGCG decreases triglyceride levels. A study in rats showed that tea catechins inhibited the activity of pancreatic lipase dose-dependently, thereby suppressing triacylglycerol absorption and postprandial hypertriglyceridemia.71 Furthermore, another study in rat hepatoma cells revealed that EGCG decreased apoB-100 very-low-density lipoprotein (VLDL) assembly and secretion as well as reduced triglyceride secretion72 which could probably result in reduced plasma triglyceride levels but increased development of a fatty liver. As a result, we hypothesized that a decrease in triglyceride levels in the EGCG group after treatment for 8 weeks might be caused from decreased triglyceride absorption at the gastrointestinal tract and/or VLDL assembly and secretion at the liver.
Despite lower triglyceride levels were found after EGCG treatment, this did not contribute to a reduction in body weight or fat mass in these subjects. This might be because energy input of the EGCG-treated subjects was not significantly reduced enough to decrease their body weight which might be due to increased ingestion/absorption of other nutrients including carbohydrate and/or protein and/or a small degree of reduction of fat absorption compared to their energy input.
Furthermore, our study also found that SBP had a trend to be decreased after EGCG treatment for 4 weeks for 5.7 mmHg and was significantly decreased after EGCG treatment for 8 weeks for 6.9 mmHg compared to the baseline values (with mean ± SEM of 122.77 ± 2.46 mmHg at baseline, 117.08 ± 2.45 mmHg at week 4, and 115.85 ± 1.99 mmHg at week 8). DBP was significantly decreased after EGCG treatment for 8 weeks for 4.7 mmHg compared to the baseline values (with mean ± SEM of 84.67 ± 1.05 mmHg at baseline to 80.00 ± 1.79 mmHg at week 8). Our results were in accordance with a previous study in obese hypertensive subjects (BMI ≥ 30 kg/m2) showing that GTE including 208 mg of EGCG a day significantly decreased both SBP and DBP after treatment for 3 months when compared to their baseline values.73 The possible mechanisms of EGCG on decreasing blood pressure were demonstrated in many studies. EGCG was shown to stimulate production of nitric oxide from endothelium in spontaneously hypertensive rats leading to vasodilation and decreased blood pressure.74 Furthermore, EGCG was shown to counteract caffeine-induced increases in mean arterial pressure, adrenaline, and noradrenaline levels in the circulation of Wistar rats,75 indicating its properties in preventing an increase in blood pressure. In addition, EGCG and green tea polyphenols are widely recognized as antioxidants.76,77 In male Sprague-Dawley rats, GTE containing 59% EGCG decreased oxidative stress that caused hypertension induced by a high dose of angiotensin II probably through the scavenging of superoxide anion generation.78 Taken together, EGCG could reduce blood pressure effectively via many possible mechanisms.
We revealed a novel finding showing that EGCG treatment in obese subjects for 8 weeks significantly decreased serum kisspeptin levels when compared to their baseline levels and when compared to subjects treated with placebo. In addition to its well-established role in regulating reproductive function, kisspeptin may also regulate blood pressure. Kisspeptin and kisspeptin receptor (GPR54)-like immunoreactivity localizations were found in smooth muscle and endothelial cells of human coronary artery, umbilical vein, and within atherosclerotic plaque of human coronary artery.44 Kisspeptin has been revealed to be a potent vasoconstrictor of the coronary artery, umbilical veins, and peripheral microvasculature.44,46 In addition, study in rats found that kisspeptin injection decreased sodium excretion and urine flow probably leading to an increase in blood pressure.79 A previous result from our lab in female adults showed that serum kisspeptin was significantly higher in hypertensive subjects compared to non-hypertensive subjects (unpublished data). Moreover, kisspeptin levels were shown to have positive correlations with both SBP and DBP in the central precocious puberty girls45 and in female adults (unpublished data). Taken together, kisspeptin might be considered as a vasoactive substance. A reduction of kisspeptin in EGCG-treated subjects might be possibly related to a decrease in blood pressure in these subjects. However, we did not find a linear correlation between kisspeptin levels and blood pressure which is consistent with a study in pregnant women revealing that there was no correlation between plasma kisspeptin levels and both SBP and DBP.80 Moreover, a study in non-diabetic subjects reported that plasma kisspeptin levels were weakly significantly correlated with DBP (R = 0.17, P = 0.007) but not SBP (R = 0.09, P = 0.09).81 The possible mechanisms explaining a reduction in kisspeptin levels of these subjects need to be investigated.
According to the National Cholesterol Education Program Adult Treatment Panel III (NCEP/ATPIII), the criteria for diagnosis of metabolic syndrome includes the presence of any 3 of 5 conditions including (1) waist circumference equal or more than 102 cm for men or equal or more than 88 cm for women, (2) triglyceride equal or more than 150 mg/dL, (3) HDL cholesterol less than 40 mg/dL in men or 50 mg/dL in women, (4) blood pressure equal or more than 130/85 mmHg, and (5) fasting plasma glucose equal or more than 100 mg/dL or diabetes.82,83 For South Asians populations, the cut-off points of waist circumference were considered at equal or more than 90 cm for males or equal or more than 80 cm for females.83 From our results, EGCG treatment had a beneficial effect on decreases in triglyceride and blood pressure which are 2 risk factors of metabolic syndrome. It could be concluded that EGCG might be able to reduce risks of metabolic syndrome even it did not have the effect on obesity reduction.
Our data showed that waist circumference, hip circumference, body fat percentage, plasma glucose, insulin, HOMA-IR, QUICKI, total cholesterol, HDL cholesterol, LDL cholesterol, leptin, and adiponectin levels were not different at week 4 or week 8 of EGCG supplement when compared with their baseline levels or control subjects. Our data suggest that EGCG had no effect on these metabolic parameters which were in accordance with a previous study.61 However, a previous study showed that EGCG supplement for 12 weeks significantly decreased waist circumference and total body fat mass27 when compared with the baseline values. Our study protocol was different from the previous study as subjects of the previous study were postmenopausal women who performed regular aerobic exercise at moderate intensity together with supplement of 300 mg per day EGCG for 12 weeks.27 The inconsistency might be from different period of treatment, exercise, age, or hormonal status.
This study revealed that supplement of 300 mg per day of EGCG for 8 weeks did not show any adverse effects on liver and kidney functions. The liver chemistry parameters including total bilirubin, direct bilirubin, AST, and ALT and kidney chemistry parameters including BUN, creatinine, and eGFR were within the normal ranges. Moreover, the mean (± SEM) values of liver and kidney functions were comparable between at baseline and week 8 time points of the EGCG treatment. These data suggest the safety of EGCG usage at the dose of 150 mg twice a day for 2 months. Subjects reported some adverse effects including headache (n = 1) and mouth ulcer (n = 1). However, previous studies showed that long-term consumption of GTE caused an increase in ALT and AST after 12 months84 and triggered toxic hepatitis after 6 months85 of supplementation in human subjects who had normal baseline levels of liver enzymes. So, it should be noted that long-term supplementation of EGCG, which is the major compound of GTE, might cause hepatic toxicity.85,86
The study in human adipocytes showed that the positive control (10 μM of isoproterenol, a non-specific β adrenergic receptors agonist) significantly increased glycerol release into the medium when compared with the vehicle-treated group, suggestive of its effect on increased lipolysis. The different doses of EGCG treatment did not increase the glycerol release in the medium after 24 h incubation, suggestive of no lipolytic effect of EGCG. These findings are consistent with data from previous studies showing that EGCG or green tea catechins treatment in differentiated 3T3-L1 mouse adipocytes did not enhance the release of glycerol into the medium after incubation for 4 or 24 h87,88 or free fatty acids after incubation for 24 h,88 indicating no lipolytic effect of EGCG. However, while EGCG caused no increase in PKA-mediated lipolysis, it increased autophagic lipolysis in mature mouse adipocytes differentiated from C3H10T1/2 cells.49 On the other hand, previous studies revealed that EGCG stimulated lipolysis in isolated primary adipocytes of male Wistar rats via extracellular signal-regulated kinases 1/2,47 reduced lipid accumulation, increased glycerol release, and increased HSL mRNA expression after incubation for 24 h in 3T3-L1 mouse adipocytes.48 The different findings might be because measurement of glycerol release of the previous studies was normalized with protein concentration in the adipocytes,47,48 while the glycerol release of our study and another previous study that showed similar result was normalized with lipid accumulation in the cells.87 In our study, the cellular lipid accumulation was used to normalize glycerol release between treatments because lipolysis occurs according to the hydrolysis of triglyceride into glycerol and free fatty acids.89 To directly determine lipolysis, normalization with triglyceride accumulation might better reflect lipolytic reaction than protein content as protein content could reflect amount of adipocytes in each culture plate but could not represent triglyceride accumulation in the cells. To determine triglyceride accumulation in our study, we performed fat area assay of Oil Red O staining per image in adipocytes to represent lipid content in adipocytes. The glycerol concentrations normalized with protein concentrations in adipocytes might not be the good method to represent glycerol release from adipocytes as protein content in the cells does not present lipid accumulation. Furthermore, we found that total area of fat droplets per image stained with Oil Red O was not different among experimental groups and had a very strong negative correlation with glycerol concentrations in the medium (R = –0.832, P < 0.001), suggestive of a negative correlation between triglyceride content and glycerol release. As a result, we conclude that EGCG had no lipolytic effect on human adipocytes.
This study also investigated the effect of EGCG on browning of human white adipocytes by detecting mRNA expression of UCP1, the marker of brown adipocytes.90 Our study showed that pioglitazone alone and pioglitazone plus 0.1 and 1 μM EGCG increased UCP1 expression in adipocytes when compared with vehicle. Pioglitazone, which is the PPAR-γ agonist, is the major regulator of adipogenesis, insulin sensitivity, and lipid metabolism as well as is involved in thermogenesis.91,92 On the other hand, treatment of EGCG did not increase UCP1 mRNA when compared with the control. Furthermore, EGCG plus pioglitazone treatment did not enhance UCP1 expression when compared to pioglitazone treatment alone. Our results indicate that EGCG did not augment the browning effect of PPAR-γ agonist on human white adipocytes. These results were similar to a previous study in adipocytes differentiated from C3H10T1/2 cells and 3T3-L1 mouse adipocytes showing that EGCG did not significantly increased UCP1 mRNA and other genes involved in mitochondrial functions of brown adipocytes.49 As a result, EGCG might not enhance the browning effect of PPAR-γ agonist on human white adipocytes. Taken together, results from the human study and adipocyte experiments indicate that EGCG had no effect on obesity reduction, lipolysis, norwhite adipocyte browning.
There were some limitations in our study including (1) energy consumption and energy expenditure were not controlled in the human study since we aimed to evaluate the effect of supplement rather than treatment, (2) the duration and dose of EGCG supplement in the human study might not be optimal, (3) the body composition was measured by TANITA® which could only estimate total body fat mass and percentage but could not measure the actual values of these factors, and (4) the histological staining of the browing experiment was not performed, so we could not confirm that EGCG had no effect on white adipocyte browning.
In conclusion, EGCG had a beneficial effect on reduction of 2 metabolic risk factors including triglyceride and blood pressure in the human experiment. We also showed a novel evidence that EGCG decreased kisspeptin levels. This study revealed that EGCG had no effects on obesity reduction in humans nor lipolysis or browning of white adipocytes. Further studies are required to disclose the association between the effect of EGCG on reduction of blood pressure and kisspeptin.
Acknowledgments
The authors would like to thank the staff of the Department of Pathology and Anatomy for photo imaging by scan scope. The authors would also like to give sincere gratitude to the staff of the Department of Clinical Pathology for collecting plasma samples of the subjects and printing laboratory results. The authors also thank Ms.Panomporn Livikorn for printing laboratory results.
Authors’ contributions: All authors have approved the submitted version and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. SC, CS, and ST participated in the conception and design of the work. SC and CS participated in the analysis and interpretation of the data and have drafted the work. SC, CS, PM, KP, IK, MC, ChS, and RS participated in the acquisition of data. All authors participated in substantively revised the work.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval : The study in humans was approved by the Siriraj Institutional Review Board of the Faculty of Medicine Siriraj Hospital, Mahidol University (si344/2558(EC2)) in full compliance with international guidelines for human research protection such as the Declaration of Helsinki, the Belmont Report, the CIOMS Guidelines, and the International Conference on Harmonization in Good Clinical Practice (ICH-GCP). The protocol of the study was successfully registered in the Thai Clinical Trials Registry (TCTR) with the identification number of TCTR20200422001 on 2020–04-21 20:31:49. All subjects signed informed consents before the study. For the studies in human adipocytes, the protocols of human adipocyte lipolysis (492/2559) and browning of human adipocytes (451/2560) were exempted from the Siriraj Institutional Review Board of the Faculty of Medicine Siriraj Hospital, Mahidol University.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0089/2558) to SC and CS, the Faculty of Medicine Siriraj Hospital Research Fund ((IO) R015932003, R0159333013, and R016132012), and the Siriraj Graduate Scholarship Type II for SC.
ORCID iD: Chantacha Sitticharoon https://orcid.org/0000-0001-8790-1300
References
- 1.Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res 2017; 122:1–7 [DOI] [PubMed] [Google Scholar]
- 2.Vecchie A, Dallegri F, Carbone F, Bonaventura A, Liberale L, Portincasa P, Fruhbeck G, Montecucco F. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med 2018; 48:6–17 [DOI] [PubMed] [Google Scholar]
- 3.8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes-2020. Diabetes Care 2020;43:S89–97 [DOI] [PubMed]
- 4.Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 2013; 5:1218–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Arnlov J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Furst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017; 377:13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han TS, Lean ME. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis 2016; 5:2048004016633371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol 2017; 960:1–17 [DOI] [PubMed] [Google Scholar]
- 8.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev 2001; 2:239–54 [DOI] [PubMed] [Google Scholar]
- 9.Yang RY, Yu L, Graham JL, Hsu DK, Lloyd KC, Havel PJ, Liu FT. Ablation of a galectin preferentially expressed in adipocytes increases lipolysis, reduces adiposity, and improves insulin sensitivity in mice. Proc Natl Acad Sci U S A 2011; 108:18696–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sergeev IN, Song Q. High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol Nutr Food Res 2014; 58:1342–8 [DOI] [PubMed] [Google Scholar]
- 11.Scheja L, Heeren J. Metabolic interplay between white, beige, brown adipocytes and the liver. J Hepatol 2016; 64:1176–86 [DOI] [PubMed] [Google Scholar]
- 12.Zechner R, Strauss JG, Haemmerle G, Lass A, Zimmermann R. Lipolysis: pathway under construction. Curr Opin Lipidol 2005; 16:333–40 [DOI] [PubMed] [Google Scholar]
- 13.Park A, Kim WK, Bae KH. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J Stem Cells 2014; 6:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saely CH, Geiger K, Drexel H. Brown versus white adipose tissue: a mini-review. Gerontology 2012; 58:15–23 [DOI] [PubMed] [Google Scholar]
- 15.Ghorbani M, Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord 1997; 21:465–75 [DOI] [PubMed] [Google Scholar]
- 16.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 2000; 279:C670–81 [DOI] [PubMed] [Google Scholar]
- 17.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010; 285:7153–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brondani LA, Assmann TS, Duarte GC, Gross JL, Canani LH, Crispim D. The role of the uncoupling protein 1 (UCP1) on the development of obesity and type 2 diabetes mellitus. Arq Bras Endocrinol Metabol 2012; 56:215–25 [DOI] [PubMed] [Google Scholar]
- 19.Zheng G, Sayama K, Okubo T, Juneja LR, Oguni I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo 2004; 18:55–62 [PubMed] [Google Scholar]
- 20.Ueda M, Ashida H. Green tea prevents obesity by increasing expression of insulin-like growth factor binding protein-1 in adipose tissue of high-fat diet-fed mice. J Agric Food Chem 2012; 60:8917–23 [DOI] [PubMed] [Google Scholar]
- 21.Kavanagh KT, Hafer LJ, Kim DW, Mann KK, Sherr DH, Rogers AE, Sonenshein GE. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J Cell Biochem 2001; 82:387–98 [DOI] [PubMed] [Google Scholar]
- 22.Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord Drug Targets 2007; 7:135–44 [DOI] [PubMed] [Google Scholar]
- 23.Lu C, Zhu W, Shen CL, Gao W. Green tea polyphenols reduce body weight in rats by modulating obesity-related genes. PLoS One 2012; 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auvichayapat P, Prapochanung M, Tunkamnerdthai O, Sripanidkulchai BO, Auvichayapat N, Thinkhamrop B, Kunhasura S, Wongpratoom S, Sinawat S, Hongprapas P. Effectiveness of green tea on weight reduction in obese Thais: a randomized, controlled trial. Physiol Behav 2008; 93:486–91 [DOI] [PubMed] [Google Scholar]
- 25.Perva-Uzunalić A, Škerget M, Knez Ž, Weinreich B, Otto F, Grüner S. Extraction of active ingredients from green tea (camellia sinensis): extraction efficiency of major catechins and caffeine. Food Chemistry 2006; 96:597–605 [Google Scholar]
- 26.Nagle DG, Ferreira D, Zhou Y-D. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry 2006; 67:1849–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill AM, Coates AM, Buckley JD, Ross R, Thielecke F, Howe PR. Can EGCG reduce abdominal fat in obese subjects? J Am Coll Nutr 2007; 26:396S–402S [DOI] [PubMed] [Google Scholar]
- 28.Mielgo-Ayuso J, Barrenechea L, Alcorta P, Larrarte E, Margareto J, Labayen I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: randomised, double-blind, placebo-controlled clinical trial. Br J Nutr 2014; 111:1263–71 [DOI] [PubMed] [Google Scholar]
- 29.Considine RV. Human leptin: an adipocyte hormone with weight-regulatory and endocrine functions. Semin Vasc Med 2005; 5:15–24 [DOI] [PubMed] [Google Scholar]
- 30.Harwood HJ., Jr. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology 2012; 63:57–75 [DOI] [PubMed] [Google Scholar]
- 31.Henry BA, Goding JW, Alexander WS, Tilbrook AJ, Canny BJ, Dunshea F, Rao A, Mansell A, Clarke IJ. Central administration of leptin to ovariectomized ewes inhibits food intake without affecting the secretion of hormones from the pituitary gland: evidence for a dissociation of effects on appetite and neuroendocrine function. Endocrinology 1999; 140:1175–82 [DOI] [PubMed] [Google Scholar]
- 32.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995; 269:543–6 [DOI] [PubMed] [Google Scholar]
- 33.Yadav A, Jyoti P, Jain SK, Bhattacharjee J. Correlation of adiponectin and leptin with insulin resistance: a pilot study in healthy North Indian population. Indian J Clin Biochem 2011; 26:193–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fors H, Matsuoka H, Bosaeus I, Rosberg S, Wikland KA, Bjarnason R. Serum leptin levels correlate with growth hormone secretion and body fat in children. J Clin Endocrinol Metab 1999; 84:3586–90 [DOI] [PubMed] [Google Scholar]
- 35.Simonds SE, Cowley MA, Enriori PJ. Leptin increasing sympathetic nerve outflow in obesity: a cure for obesity or a potential contributor to metabolic syndrome? Adipocyte 2012; 1:177–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassan MJM, Bakar NS, Aziz MA, Basah NK, Singh HJ. Leptin-induced increase in blood pressure and markers of endothelial activation during pregnancy in Sprague Dawley rats is prevented by resibufogenin, a marinobufagenin antagonist. Reprod Biol 2020; 20:184–90 [DOI] [PubMed] [Google Scholar]
- 37.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, Myers MG, Jr, Licinio J, Brown RD, Enriori PJ, O'Rahilly S, Sternson SM, Grove KL, Spanswick DC, Farooqi IS, Cowley MA. Leptin mediates the increase in blood pressure associated with obesity. Cell 2014; 159:1404–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sitticharoon C, Boonpuan V, Mitrpant C, Churintaraphan M. Determination of KISS1, KISS1R and kisspeptin in fat tissue of normal weight and obese humans and correlations between serum kisspeptin and leptin. Siriraj Med J 2017; 65:112–16 [Google Scholar]
- 39.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst 1996; 88:1731–7 [DOI] [PubMed] [Google Scholar]
- 40.Zeydabadi Nejad S, Ramezani Tehrani F, Zadeh-Vakili A. The role of kisspeptin in female reproduction. Int J Endocrinol Metab 2017; 15:e44337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu HJ, Li SJ, Pan H, Li N, Zhang DX, Wang LJ, Yang HB, Wu Q, Gong FY. The changes of serum leptin and kisspeptin levels in Chinese children and adolescents in different pubertal stages. Int J Endocrinol 2016; 2016:6790794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izzi-Engbeaya C, Comninos AN, Clarke SA, Jomard A, Yang L, Jones S, Abbara A, Narayanaswamy S, Eng PC, Papadopoulou D, Prague JK, Bech P, Godsland IF, Bassett P, Sands C, Camuzeaux S, Gomez-Romero M, Pearce JTM, Lewis MR, Holmes E, Nicholson JK, Tan T, Ratnasabapathy R, Hu M, Carrat G, Piemonti L, Bugliani M, Marchetti P, Johnson PR, Hughes SJ, James Shapiro AM, Rutter GA, Dhillo WS. The effects of kisspeptin on β-cell function, serum metabolites and appetite in humans. Diabetes Obes Metab 2018; 20:2800–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bacopoulou F, Lambrou GI, Rodanaki ME, Stergioti E, Efthymiou V, Deligeoroglou E, Markantonis SL. Serum kisspeptin concentrations are negatively correlated with body mass index in adolescents with anorexia nervosa and amenorrhea. Hormones (Athens) 2017; 16:33–41 [DOI] [PubMed] [Google Scholar]
- 44.Mead EJ, Maguire JJ, Kuc RE, Davenport AP. Kisspeptins are novel potent vasoconstrictors in humans, with a discrete localization of their receptor, G protein-coupled receptor 54, to atherosclerosis-prone vessels. Endocrinology 2007; 148:140–7 [DOI] [PubMed] [Google Scholar]
- 45.Sitticharoon C, Sukharomana M, Likitmaskul S, Churintaraphan M, Maikaew P. Increased high molecular weight adiponectin, but decreased total adiponectin and kisspeptin, in Central precocious puberty compared with aged-matched prepubertal girls. Reprod Fertil Dev 2017; 29:2466–78 [DOI] [PubMed] [Google Scholar]
- 46.Sawyer I, Smillie SJ, Bodkin JV, Fernandes E, O'Byrne KT, Brain SD. The vasoactive potential of kisspeptin-10 in the peripheral vasculature. PLoS One 2011; 6:0014671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogasawara J, Kitadate K, Nishioka H, Fujii H, Sakurai T, Kizaki T, Izawa T, Ishida H, Ohno H. Comparison of the effect of oligonol, a new lychee fruit-derived low molecular form of polyphenol, and epigallocatechin-3-gallate on lipolysis in rat primary adipocytes. Phytother Res 2011; 25:467–71 [DOI] [PubMed] [Google Scholar]
- 48.Lee MS, Kim CT, Kim IH, Kim Y. Inhibitory effects of green tea catechin on the lipid accumulation in 3T3-L1 adipocytes. Phytother Res 2009; 23:1088–91 [DOI] [PubMed] [Google Scholar]
- 49.Kim SN, Kwon HJ, Akindehin S, Jeong HW, Lee YH. Effects of epigallocatechin-3-Gallate on autophagic lipolysis in adipocytes. Nutrients 2017; 9:680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan WH, Yeh WT. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: an extension of Asian-Pacific recommendations. Asia Pac J Clin Nutr 2008; 17:370–4 [PubMed] [Google Scholar]
- 51.Lim JU, Lee JH, Kim JS, Hwang YI, Kim T-H, Lim SY, Yoo KH, Jung K-S, Kim YK, Rhee CK. Comparison of world health organization and Asia-Pacific body mass index classifications in COPD patients. Int J Chron Obstruct Pulmon Dis 2017; 12:2465–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Misra A. Ethnic-specific criteria for classification of body mass index: a perspective for Asian Indians and American diabetes association position statement. Diabetes Technol Ther 2015; 17:667–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrella RJ, Lattanzio CN, Demeray A, Varallo V, Blore R. Can adoption of regular exercise later in life prevent metabolic risk for cardiovascular disease? Diabetes Care 2005; 28:694–701 [DOI] [PubMed] [Google Scholar]
- 54.Brown RE, Randhawa AK, Canning KL, Fung M, Jiandani D, Wharton S, Kuk JL. Waist circumference at five common measurement sites in normal weight and overweight adults: which site is most optimal? Clin Obes 2018; 8:21–29 [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization. Waist Circumference and waist–hip ratio: report of a WHO expert consultation. Geneva: Author, 2008 [Google Scholar]
- 56.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000; 85:2402–10 [DOI] [PubMed] [Google Scholar]
- 57.Ali AT, Hochfeld WE, Myburgh R, Pepper MS. Adipocyte and adipogenesis. Eur J Cell Biol 2013; 92:229–36 [DOI] [PubMed] [Google Scholar]
- 58.Moseti D, Regassa A, Kim WK. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int J Mol Sci 2016; 17:124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dragunow M, Cameron R, Narayan P, O'Carroll S. Image-based high-throughput quantification of cellular fat accumulation. J Biomol Screen 2007; 12:999–1005 [DOI] [PubMed] [Google Scholar]
- 60.Gorzelniak K, Janke J, Engeli S, Sharma AM. Validation of endogenous controls for gene expression studies in human adipocytes and preadipocytes. Horm Metab Res 2001; 33:625–7 [DOI] [PubMed] [Google Scholar]
- 61.Xicota L, Rodriguez J, Langohr K, Fito M, Dierssen M, de la Torre R. Effect of epigallocatechin gallate on the body composition and lipid profile of Down syndrome individuals: implications for clinical management. Clin Nutr 2019; 8:30255–9 [DOI] [PubMed] [Google Scholar]
- 62.Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, Aston CE, Lyons TJ. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr 2010; 29:31–40 [DOI] [PubMed] [Google Scholar]
- 63.Coffey CS, Steiner D, Baker BA, Allison DA. Randomized double-blind placebo-controlled clinical trial of a product containing ephedrine, caffeine, and other ingredients from herbal sources for treatment of overweight and obesity in the absence of lifestyle treatment. Int J Obes Relat Obes 2004; 28:1411–9 [DOI] [PubMed] [Google Scholar]
- 64.Westerterp-Plantenga MS, Lejeune MP, Kovacs EM. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res 2005; 13:1195–204 [DOI] [PubMed] [Google Scholar]
- 65.Tominaga Y, Mae T, Kitano M, Sakamoto Y, Ikematsu H, Nakagawa K. Licorice flavonoid oil effects body weight loss by reduction of body fat mass in overweight subjects. J Health Sci 2006; 52:672–83 [Google Scholar]
- 66.Maki KC, Reeves MS, Farmer M, Yasunaga K, Matsuo N, Katsuragi Y, Komikado M, Tokimitsu I, Wilder D, Jones F, Blumberg JB, Cartwright Y. Green tea catechin consumption enhances exercise-induced abdominal fat loss in overweight and obese adults. J Nutr 2009; 139:264–70 [DOI] [PubMed] [Google Scholar]
- 67.Li F, Gao C, Yan P, Zhang M, Wang Y, Hu Y, Wu X, Wang X, Sheng J. EGCG reduces obesity and white adipose tissue gain partly through AMPK activation in mice. Front Pharmacol 2018; 9:1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J, Peng Y, Yue Y, Shen P, Park Y. Epigallocatechin-3-Gallate reduces fat accumulation in Caenorhabditis elegans. Prev Nutr Food Sci 2018; 23:214–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hodgson AB, Randell RK, Mahabir-Jagessar TK, Lotito S, Mulder T, Mela DJ, Jeukendrup AE, Jacobs DM. Acute effects of green tea extract intake on exogenous and endogenous metabolites in human plasma. J Agric Food Chem 2014; 62:1198–208 [DOI] [PubMed] [Google Scholar]
- 70.Samavat H, Newman AR, Wang R, Yuan JM, Wu AH, Kurzer MS. Effects of green tea catechin extract on serum lipids in postmenopausal women: a randomized, placebo-controlled clinical trial. Am J Clin Nutr 2016; 104:1671–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ikeda I, Tsuda K, Suzuki Y, Kobayashi M, Unno T, Tomoyori H, Goto H, Kawata Y, Imaizumi K, Nozawa A, Kakuda T. Tea catechins with a galloyl moiety suppress postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats. J Nutr 2005; 135:155–9 [DOI] [PubMed] [Google Scholar]
- 72.Li L, Stillemark-Billton P, Beck C, Boström P, Andersson L, Rutberg M, Ericsson J, Magnusson B, Marchesan D, Ljungberg A, Borén J, Olofsson SO. Epigallocatechin gallate increases the formation of cytosolic lipid droplets and decreases the secretion of apoB-100 VLDL. J Lipid Res 2006; 47:67–77 [DOI] [PubMed] [Google Scholar]
- 73.Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res 2012; 32:421–7 [DOI] [PubMed] [Google Scholar]
- 74.Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim JA, Quon MJ, Montagnani M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab 2007; 292:E1378–E87 [DOI] [PubMed] [Google Scholar]
- 75.Han JY, Kim CS, Lim KH, Kim JH, Kim S, Yun YP, Hong JT, Oh KW. Increases in blood pressure and heart rate induced by caffeine are inhibited by (-)-epigallocatechin-3-O-gallate: involvement of catecholamines. J Cardiovasc Pharmacol 2011; 58:446–9 [DOI] [PubMed] [Google Scholar]
- 76.Shi X, Ye J, Leonard SS, Ding M, Vallyathan V, Castranova V, Rojanasakul Y, Dong Z. Antioxidant properties of (-)-epicatechin-3-gallate and its inhibition of Cr(VI)-induced DNA damage and Cr(IV)- or TPA-stimulated NF-kappaB activation. Mol Cell Biochem 2000; 206:125–32 [DOI] [PubMed] [Google Scholar]
- 77.Forester SC, Lambert JD. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol Nutr Food Res 2011; 55:844–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antonello M, Montemurro D, Bolognesi M, Di Pascoli M, Piva A, Grego F, Sticchi D, Giuliani L, Garbisa S, Rossi GP. Prevention of hypertension, cardiovascular damage and endothelial dysfunction with green tea extracts. Am J Hypertens 2007; 20:1321–8 [DOI] [PubMed] [Google Scholar]
- 79.Ten SC, Gu SY, Niu YF, An XF, Yan M, He M. Central administration of kisspeptin-10 inhibits water and sodium excretion of anesthetized male rats and the involvement of arginine vasopressin. Endocr Res 2010; 35:128–36 [DOI] [PubMed] [Google Scholar]
- 80.Nijher GM, Chaudhri OB, Ramachandran R, Murphy KG, Zac-Varghese SE, Fowler A, Chinthapalli K, Patterson M, Thompson EL, Williamson C, Kumar S, Ghatei MA, Bloom SR, Dhillo WS. The effects of kisspeptin-54 on blood pressure in humans and plasma kisspeptin concentrations in hypertensive diseases of pregnancy. Br J Clin Pharmacol 2010; 70:674–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andreozzi F, Mannino GC, Mancuso E, Spiga R, Perticone F, Sesti G. Plasma kisspeptin levels are associated with insulin secretion in nondiabetic individuals. PLoS One 2017; 12:e0179834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–97 [DOI] [PubMed] [Google Scholar]
- 83.Zafar U, Khaliq S, Ahmad HU, Manzoor S, Lone KP. Metabolic syndrome: an update on diagnostic criteria, pathogenesis, and genetic links. Hormones (Athens) 2018; 17:299–313 [DOI] [PubMed] [Google Scholar]
- 84.Yu Z, Samavat H, Dostal AM, Wang R, Torkelson CJ, Yang CS, Butler LM, Kensler TW, Wu AH, Kurzer MS, Yuan JM. Effect of green tea supplements on liver enzyme elevation: results from a randomized intervention study in the United States. Cancer Prev Res (Res ) 2017; 10:571–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rohde J, Jacobsen C, Kromann-Andersen H. [Toxic hepatitis triggered by green tea]. Ugeskr Laeg 2011; 173:205–6 [PubMed] [Google Scholar]
- 86.TRaX TD. Suspected herb induced liver injury by green tea extracts: critical review and case analysis applying RUCAM for causality assessment. Jpn J Gastroenterol Hepatol 2019; 6:1–16 [Google Scholar]
- 87.Mochizuki M, Hasegawa N. Effects of green tea catechin-induced lipolysis on cytosol glycerol content in differentiated 3T3-L1 cells. Phytother Res 2004; 18:945–6 [DOI] [PubMed] [Google Scholar]
- 88.Chen S, Osaki N, Shimotoyodome A. Green tea catechins enhance norepinephrine-induced lipolysis via a protein kinase A-dependent pathway in adipocytes. Biochem Biophys Res Commun 2015; 461:1–7 [DOI] [PubMed] [Google Scholar]
- 89.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 2009; 48:275–97 [DOI] [PubMed] [Google Scholar]
- 90.Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012; 151:400–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lasar D, Rosenwald M, Kiehlmann E, Balaz M, Tall B, Opitz L, Lidell ME, Zamboni N, Krznar P, Sun W, Varga L, Stefanicka P, Ukropec J, Nuutila P, Virtanen K, Amri EZ, Enerback S, Wahli W, Wolfrum C. Peroxisome proliferator activated receptor gamma controls mature brown adipocyte inducibility through glycerol kinase. Cell Rep 2018; 22:760–73 [DOI] [PubMed] [Google Scholar]
- 92.Ma X, Wang D, Zhao W, Xu L. Deciphering the roles of PPARγ in adipocytes via dynamic change of transcription complex. Front Endocrinol (Lausanne) 2018; 9:473. [DOI] [PMC free article] [PubMed] [Google Scholar]