Abstract
Degeneration of photoreceptors is a major cause of blindness. Identifying new methods for the generation of photoreceptors offers valuable options for a cell replacement therapy of blindness. Here, we show that primary adult human retinal pigmented epithelium (hRPE) cells were directly converted to postmitotic neurons with various properties of photoreceptors by the neurogenic transcription factor ASCL1 and microRNA124. At Day 8 after the induction of ASCL1 and miRNA124 expression in hRPE cells, 91% of all cells were Tuj1+, and 83% of all cells were MAP2+ neurons. The cone photoreceptor marker L/M-opsin, the rod photoreceptor marker rhodopsin, and the generic photoreceptor marker recoverin were expressed in 76%, 86%, and 92% of all cells, respectively. Real-time quantitative PCR measurements showed significant and continuous increases in the expression of photoreceptor markers phosducin and recoverin, rod cell markers phosphodiesterases 6 b and arrestin S-antigen, and cone cell markers L/M-opsin and S-opsin in three independent lines of primary hRPE cells at different days of transdifferentiation. Transmission electron microscopy of converted neurons showed disc-like structures similar to those found in photoreceptors. While the converted neurons had voltage-dependent Na+, K+, and Ca2+ currents, light-induced change in membrane potential was not detected. The study demonstrates the feasibility of rapid and efficient transdifferentiation of adult hPRE cells to neurons with many properties of photoreceptors. It opens up a new possibility in cell replacement therapy of blindness caused by photoreceptor degeneration.
Keywords: Retinal pigmented epithelium, photoreceptors, reprogramming, transdifferentiation, ASCL1, miRNA124
Impact statement
The degeneration of photoreceptors is a leading cause of blindness. Retinal pigment epithelium (RPE) cells are mitotic cells that support the function of photoreceptors. We found that lentivirus-mediated overexpression of ASCL1 and microRNA124 directly converted primary adult human RPE cells to postmitotic neurons with many properties of photoreceptors. This study identifies a new method toward the generation of human photoreceptors and provides a new avenue in cell-based therapies for blindness caused by photoreceptor degeneration.
Introduction
Photoreceptors are specialized sensory neurons in the retina. Being terminally differentiated, they cannot be regenerated. Degeneration of photoreceptors, such as by light damage, genetic mutations, or aging, is a leading cause of blindness.1 Because photoreceptor cells cannot renew after damage, there has been significant interest in cell replacement therapy to treat retinal diseases caused by injured photoreceptors. Previous investigations have differentiated bone marrow stem cells, embryonic stem cells, and induced pluripotent stem cells (iPSCs) to photoreceptors.2–6 The non-neural retinal pigment epithelium (RPE) and the neural retina originate from the same structure—the optic vesicle. Their similar developmental origins and proximity suggest that it might be feasible to reprogram RPE cells directly to photoreceptors. The proliferative potential of RPE cells and their ability to transdifferentiate to other types of retinal cells in amphibians7 make them an attractive cell source to explore their conversion to photoreceptors.
The proneural transcription factor achaete-scute homolog 1 (ASCL1) plays a significant role in initiating the development of neuronal lineages and promoting neural differentiation by regulating the expression of a large subset of genes.8,9 It is a pioneer transcription factor that can access heterochromatin in fibroblasts to induce their transdifferentiation to neurons.10 The combination of Ascl1, Brn2, and Myt1l converts mouse embryonic and postnatal fibroblasts into neurons in vitro rapidly and efficiently. These converted cells express neuron-specific proteins and show electrophysiological traits indicative of neurons.11 Expression of the microRNAs, miRNA9/9*, and miRNA124 in human fibroblasts induces their conversion into neurons. Co-expression of ASCL1 and Myt1l with miRNA9/9* and miRNA124 further enhances the rate of conversion and maturation of induced neurons.12
In this study, we examined the ability of neurogenic factors ASCL1 and miRNA124 to reprogram human RPE cells to photoreceptors. We found that expression of ASCL1 and miRNA124 induced the rapid and efficient acquisition of various photoreceptor properties. The converted neurons expressed a series of photoreceptor genes and possessed structures reminiscent of photoreceptor discs. Our results showed the effectiveness of ASCL1 and miRNA124 in the transdifferentiation of human RPE cells into postmitotic neurons with many characteristics of photoreceptors.
Materials and methods
Chemicals and plasmids
Dorsomorphin dihydrochloride was purchased from Tocris. Purmorphamine, CHIR99021, SB431542, and Y27632 were obtained from Reagents Direct. Recombinant human NGF, GDNF, BDNF, and TGFβ3 were obtained from PeproTech. FUW-tetO-LoxP, pMD2.G, and psPAX2 were purchased from Addgene. Human ASCL1 (GenBank accession BC031299) was obtained from Open Biosystems and then subcloned into FUW-tetO-LoxP. Human miRNA124 (MIMAT0000422) was amplified from human genomic DNA, then subcloned into FUW-tetO-LoxP. FUW-LoxP-M2rtTA was purchased from Addgene.
Cell culture
Adult primary human RPE (hRPE) cells were provided by Dr. Sally Temple at Neural Stem Cell Institute, Rensselaer, NY. These mature primary cells are directly isolated from postmortem human eyes.13 Our experiments on these cells are not human subject research. All RPE cells were cultured on 10-cm dishes in RPE medium14,15 that consisted of equal parts of MEM-α modification (Sigma-Aldrich, St. Louis, MO) and DMEM/F12 (Gibco), 5% fetal bovine serum (HyClone, Logan, UT), N1 (1×; Sigma-Aldrich), nonessential amino acids (1×, Gibco), sodium pyruvate (1×, Gibco), GlutaMAX-I (2 mM; Gibco), triiodothyronine (0.013 μg/L; Sigma-Aldrich), hydrocortisone (20 ng/mL; Sigma-Aldrich), taurine (250 μg/mL; Sigma-Aldrich), and nicotinamide (1 mM; Sigma-Aldrich). RPE cells were passaged using TrypLE (Invitrogen). Reprogrammed neurons were cultured on 12-well plates or glasses slides coated with matrigel (Sigma-Aldrich) in neural induction medium.16
Direct conversion of hRPE cells by ASCL1 and microRNA124
All hRPE cells were cultured without antibiotics. Mycoplasma was not detected using PCR. Collection of lentivirus and infection of hRPE cells were conducted as described previously.17 HEK293FT cells (Invitrogen) were cultured at 4–6 × 106 cells in a 10 cm cell culture dish. They were transfected with 10 μg of FUW-tetO-LoxP-hASCL1, or 10 μg of FUW-tetO-LoxP-hmiRNA124, or 10 μg of FUW-LoxP-M2rtTA accompanied with 7.5 μg of psPAX2 and 2.5 μg of pMD2.G using Lipofectamine 2000 (Invitrogen). Lentiviruses were collected from 16 to 64 h after transfection. Elisa kit detecting p24 (ZeptoMetrix Corporation, Buffalo, NY) was used to titer virus.
After hRPE cells were passaged twice, they were plated at a density of 1 × 104 cells/cm2. One day later, the cells were infected for 16–20 h with the indicated combinations of lentivirus (human ASCL1, human miRNA124, and M2rtTA each at MOI 20) with 8 μg/mL polybrene (Sigma-Aldrich, St. Louis, MO). Medium containing the virus was removed after 16 h and replaced with DMEM/F12. After 24 h, the medium was changed to neural induction media (DMEM/F12, 1 × NEAA, 1 × N2 supplements, 1 × B27, 20 ng/mL NGF, 20 ng/mL GDNF, 20 ng/mL BDNF, 1 ng/mL TGFβ3, 1 μM dorsomorphin, 10 μM SB431542, 3 μM CHIR99021, 2 μM purmorphamine, 0.2 mM vitamin C, 10 μM ROCK inhibitor Y27632, and 1 μg/mL doxycycline). After six days, the medium was changed to neural maturation media (neural induction medium without dorsomorphin, SB431542, CHIR99021, purmorphamine, and doxycycline). Medium was changed every two days. Most experiments were performed on Day 8.
EdU labeling
At Day 6 after DOX induction of transgene overexpression, 10 µM EdU was added into medium to incubate with cells for 24 h. After cells were washed three times with medium, they were incubated in fresh medium until cells were fixed at Day 12 in PBS containing 4% paraformaldehyde for 20 min. Cell were permeabilized with 0.1% Triton X-100 for 20 min at room temperature, first stained with polyclonal TuJ1 antibody overnight, and then stained with anti-rabbit IgG Alexa Fluor 546 for 1 h at room temperature. EdU was detected in a solution containing 4 mM copper sulfate, 40 mM sodium ascorbate, and 20 µM Alexa Fluor 488 azide, 20 mM Tris, pH =7.6. Cells were counterstained with DAPI to identify nuclei.
Real-time quantitative RT-PCR
Total cellular RNA was extracted using RNeasy Mini kit (Qiagen). Reverse transcription was performed with iScript cDNA synthesis kit (Bio-Rad). RT-PCR was performed with iQ SYBR Green Supermix (Bio-Rad) using CFX96 Touch™ Real-time PCR detection System (Bio-Rad) for total reaction volumes of 20 µl. Each sample was done in triplicates. PCR conditions included an initial denaturation step of 4 min at 95°C, followed by 40 cycles of 1 min at 94°C, 1 min at 60°C, and 1 min at 72°C. The average threshold cycle (Ct) values were subtracted by the average Ct values for GAPDH. This difference denoted as ΔCt was used to derive the fold change, which is defined as 2−ΔCt × 106. Primers for the examined genes are listed in Table 1.
Table 1.
Sequence of primers used in qRT-PCR.
| Genes | Primer sequence |
|---|---|
| PDC | Forward: TCAAAGGAACGAGTCAGCAGReverse: CTGCTGCAAGGCATGTTAAA |
| PDE6b | Forward: CAGTGATGAACACCGACACCReverse: ATTTGACCAGGTCCAGTTCG |
| Recoverin | Forward: TAACGGGACCATCAGCAAGReverse: CCTCGGGAGTGATCATTTTG |
| SAG | Forward: CTGATCCGCAAAGTACAGCAReverse: TCAGCGTCTTGGTCAAAGTG |
| GAPDH | Forward: ACCACAGTCCATGCCATCACReverse: TCCACCACCCTGTTGCTGTA |
| L/M-opsin | Forward: GTCACTGCATCCGTCTTCACAAATGGGReverse: ATCTCACATTGCCAAAGGGCTTGCAC |
| S-opsin | Forward: CTTCCTTATAGGGTTCCCACTCAATGCCReverse: GAAGTTGCCGAAGGGCTTACAGATGAC |
Immunocytochemistry
Reprogrammed cells in multi-well plates were fixed in 4% paraformaldehyde in PBS for 20 min at room temperature. Cells were permeabilized with 0.1% Triton X-100 in PBS for 20 min. After incubation in 5% BSA in PBS for 1 h, cells were stained in primary antibody overnight at 4°C and then incubated in secondary antibody for 2 h at room temperature in dark. Information on primary and second antibodies is listed in Table 2. Fluorescence images were taken on a Leica AF6000 inverted fluorescence microscope. Positive cells were counted with five independent frames at 10× magnification for each condition.
Table 2.
Antibodies used in the study.
| Antibody | Catalog # | Vender | Dilution |
|---|---|---|---|
| TuJ1 | AB78078 | Abcam | 1:1000 |
| Polyclonal TuJ1 | PA5-85639 | Invitrogen | 1:1000 |
| Mouse monoclonal MAP2 | sc-74421 | Santa Cruz | 1:1000 |
| Chicken polyclonal MAP2 | ab5392 | abcam | 1:1000 |
| Recoverin | AB5585 | EMD Millipore | 1:1000 |
| Rhodopsin | MAB5356 | EMD Millipore | 1:1000 |
| Opsin | AB5405 | EMD Millipore | 1:1000 |
| NeuN | ab104224 | abcam | 1:1000 |
| Donkey anti-Rabbit IgG (H + L), Alexa Fluor 488 | A21206 | Thermo Fisher | 1:2000 |
| Donkey anti-Rabbit IgG (H + L), Alexa Fluor 546 | A10040 | Thermo Fisher | 1:2000 |
| Donkey anti-Mouse IgG (H + L), Alexa Fluor 546 | A10036 | Thermo Fisher | 1:2000 |
| Donkey anti-Mouse IgG (H + L), Alexa Fluor 488 | A21202 | Thermo Fisher | 1:2000 |
| Goat anti-Chicken IgG (H + L), Alexa Fluor 546 | A11040 | Thermo Fisher | 1:2000 |
Electron microscopy
Converted cells were harvested at Day 10 of reprogramming. Following centrifugation at 1000 r/min for 5 min, cell pellet was immersed in 2.5% glutaraldehyde, then fixed with 1% osmium tetroxide. A series of graded ethanol was used to dehydrate cells. Afterwards, cells were embedded in araldite, then stained with 4% uranyl acetate for 20 min and 0.5% lead citrate for 10 min. Ultra-thin sections (50 nm) were observed with a transmission electron microscope (1200 EX II; JEOL, Tokyo, Japan).
Electrophysiology
Voltage-dependent ionic currents were recorded in converted cells at Day 7 and Day 14 using a previous protocol.16 Cells (held at −70 mV) were perfused with artificial cerebrospinal fluid (ACSF), and voltage levels were implemented from −90 to +50 mV at 10 mV increments. Whole-cell patch recordings were made using either β-escin or amphotericin forming perforated patches. Cells were held at −70 mV in voltage clamp or current clamped at −50 mV and bathed in Tyrode solution. Light stimuli were provided by a 1 s green LED stimulus (530 nm peak) of varying intensities. Neither current signals nor voltage responses were detected in response to light stimulation, but voltage-gated inward and outward currents were observed when cells were stepped to −30, −10, and +10 mV (n = 7).
Statistical analyses
All statistical analyses were performed with Origin (OriginLab, Northampton, MA). Data are expressed as mean ± SD (Standard deviation of measurement). The difference between two groups was assessed by two-tailed Student's t-tests. P-value <0.05 was considered to be statistically significant.
Results
Direct conversion of hRPE cells to postmitotic neurons using ASCL1 and miRNA124
As shown in the timeline of the conversion (Figure 1(a)), adult hRPE 252 cells were plated on Day−3. On the following day, cells were infected with reprogramming lentiviruses expressing ASCL1 or miRNA124 for 16–20 h. Our previous studies have shown that cell cycle synchronization at G1 significantly facilitates the direct conversion of human fibroblasts to induced dopaminergic neurons16 and induced serotonergic neurons.18 Thus, we induced cell cycle arrest at the G1/S checkpoint by serum withdrawal for 24 h. At the onset of reprogramming (Day 0), the cells were cultured in neural induction medium16 (DMEM/F12, 1 × NEAA, 1 × N2 supplements, 1 × B27, 20 ng/mL NGF, 20 ng/mL GDNF, 20 ng/mL BDNF, 1 ng/mL TGFβ3, 1 μM dorsomorphin, 10 μM SB431542, 3 μM CHIR99021, 2 μM purmorphamine, 0.2 mM vitamin C, 10 μM ROCK inhibitor Y27632) with 1 μg/mL doxycycline (DOX) to induce the overexpression of viral transgenes for six days. The cells were then maintained in neural maturation medium (neural induction medium without dorsomorphin, SB431542, CHIR99021, purmorphamine) in the absence of DOX.
Figure 1.
Reprogramming hRPE cells to neurons using ASCL1 and miRNA124.(a) Using the reprogramming timeline, hRPE cells were plated in RPE medium on Day −3. One day later (Day −2), cells were infected with reprogramming lentiviruses as indicated for 16–20 h. Then, cell cycle was synchronized to the G1 phase by serum withdrawal for 24 h. Reprogramming started at Day 0 by exposing synchronized hRPE cells in Neural Induction Medium and doxycycline (DOX) to induce the expression of viral transgenes. After six days, cells were maintained in neural maturation medium. (b–e) Representative images of hRPE 252 cells (b), and hRPE 252 cells converted by ASCL1 [A] (c), miRNA124 [m] (d), or ASCL1 plus miRNA124 [Am] (e) on Day 8. Scale bar, 50 μm. (f–l‴) Immunostaining at Day 8 for DAPI, Tuj1, and MAP2 (with a mouse monoclonal antibody) in hRPE cells (f–f‴), and hRPE transduced with ASCL1 (g–g‴), miRNA124 (h–h‴), or ASCL1 plus miRNA124 (i–i‴). Scale bar, 100 μm. (j) Quantification of the percentages of Tuj1+ cells in DAPI+ cells or MAP2+ cells in DAPI+ cells at Day 8. *, # P < 0.05, unpaired, two-tailed Student’s t-tests, vs. to A or m, respectively. n = 6 wells from three independent experiments. (k–k″) Cells at Day 6 of conversion were treated with 10 µM EdU for 24 h and fixed at Day 12 for costaining of TuJ1 (kʹ), EdU (k″), and DAPI (k). (l–m‴) Fixed cells at Day 12 were costained with a chicken polyclonal antibody against MAP2 (l″ and m″) together with antibodies against Tuj1 (lʹ) or NeuN (mʹ), and DAPI (l‴ and m‴). (A color version of this figure is available in the online journal.)
We initially compared the individual and combined effects of ASCL1 and miRNA124 on reprogramming primary hRPE 252 cells. hRPE cells have cobble stone morphology and contain many pigment granules inside the cell body (Figure 1(b)). After eight days of reprogramming (Day 8), hRPE transduced by ASCL1 [A] (Figure 1(c)) or ASCL1 plus miRNA124 [Am] (Figure 1(e)) demonstrated neurite-like processes. In contrast, neurite-like processes were much less abundant in hRPE converted with miRNA124 [m] alone (Figure 1(d)). To assess reprogramming efficiency, we performed immunostainings for the pan-neuronal marker β3-tubulin (Tuj1) and the mature neuronal marker microtubule-associated protein 2 (MAP2) on Day 8. These markers would provide indications on how well reprogramming occurs, as Tuj1 expression emerges in immature neurons and MAP2 appears in mature neurons. Nuclear staining with DAPI highlights all cells in the culture for quantification purpose. The parental hRPE 252 cells did not express Tuj1 or MAP2 (Figure 1(f) and (f‴)). Meanwhile, cotransduction by ASCL1 and miRNA124 significantly increased the generation of Tuj1+ cells (90.6 ± 3.4% of all cells) and MAP2+ neurons (82.6 ± 3.9% of all cells) over the levels induced by ASCL1 alone (75.6 ± 3.2% for Tuj1+, 53.7 ± 4.9 for MAP2+) or miRNA124 alone (67.5 ± 4.4% for Tuj1+, 2.0 ± 0.8% for MAP2+) (P < 0.05, unpaired, two-tailed Student's t-tests; Figure 1(i) and (j)). Because miRNA124 enhanced the reprogramming efficiency of ASCL1, we used both ASCL1 and miRNA124 [Am] in all subsequent experiments.
To confirm that the converted cells were indeed postmitotic neurons, we treated the cells at Day 6 with 10 µM EdU (5-ethynyl-2ʹ-deoxyuridine) for 24 h to label dividing cells, which incorporate EdU into genomic DNA. When cells were fixed at Day 12 and stained for EdU, very few EdU+ cells were found and none of them was Tuj1+ (Figure 1(k) and (k″)). This is consistent with previous studies showing that overexpression of ASCL1 drives cells out of cell cycle.16 To substantiate the results, we used a different antibody against MAP2 and found the co-localization of MAP2 and Tuj1 (Figure 1(l) and (l‴)) and the co-localization of MAP2 and another mature neuronal marker, NeuN (Figure 1(m) and (m‴)) in converted cells at Day 12. Together, these results unequivocally demonstrated that Am converted RPE cells to postmitotic neurons.
ASCL1 and miRNA converts hRPE cells to neurons expressing photoreceptor markers
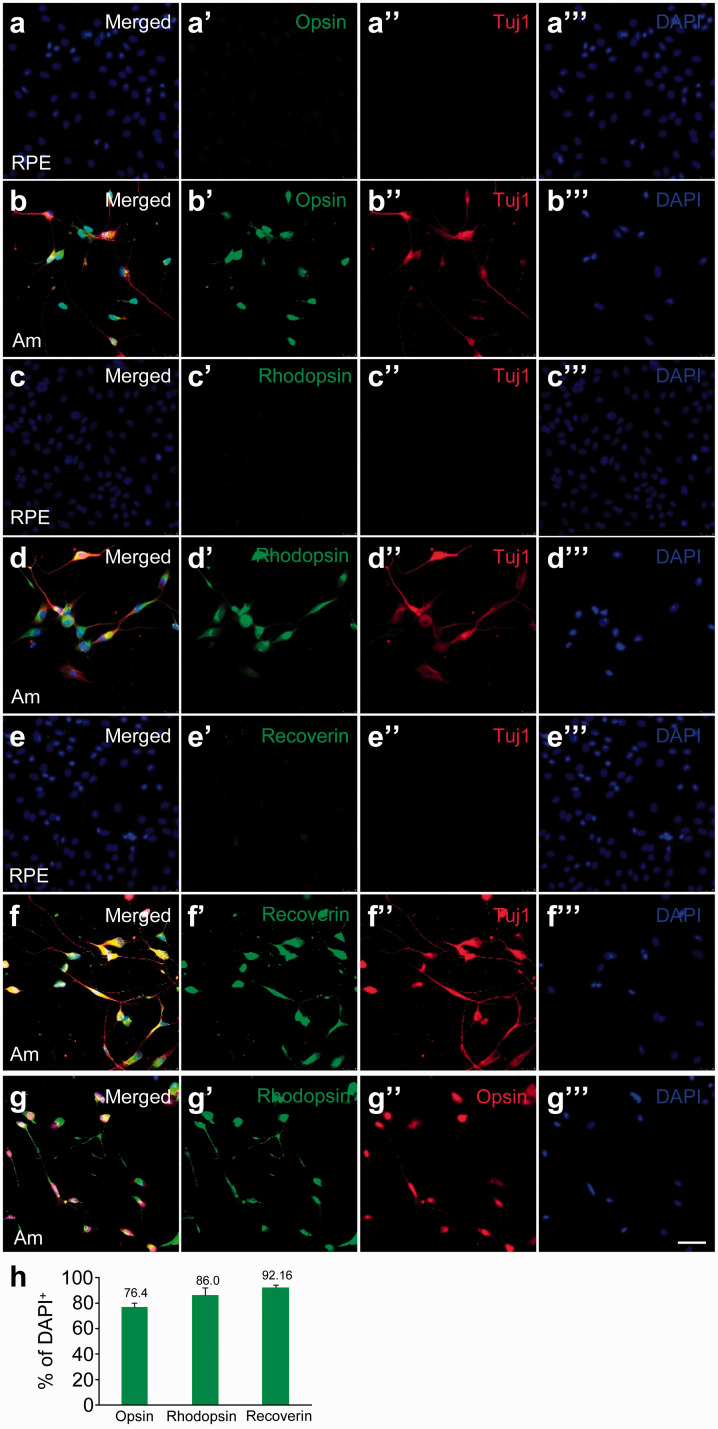
We examined whether the converted neurons express photoreceptor markers. Rods and cones, the two types of photoreceptors in human, perform phototransduction through rhodopsin and opsin, respectively. After eight days of reprogramming with ASCL1 and miRNA124, we stained the converted cells for the rod cell marker rhodopsin, the cone cell marker red/green opsin (L/M-opsin), and the pan-photoreceptor marker recoverin, which is expressed in all photoreceptors.19 While the parental hRPE 252 cells did not express opsin (Figure 2(aʹ)), rhodopsin (Figure 2(cʹ)), and recoverin (Figure 2(eʹ)), many converted cells coexpressed Tuj1 with opsin (Figure 2(bʹ)), rhodopsin (Figure 2(dʹ)), and recoverin (Figure 2(fʹ)). In contrast to native photoreceptors in vivo, the converted neurons coexpressed both rhodopsin (Figure 2(gʹ)) and opsin (Figure 2(g″)). We found that in all DAPI+ cells, 76.4 ± 3.6% expressed opsin, 86.0 ± 6.2% expressed rhodopsin, and 92.1 ± 1.6% expressed recoverin (Figure 2(h)). These results suggest that hPRE 252 cells were reprogrammed by Am to acquire some characteristics of photoreceptors, with coexpression of rhodopsin and opsin.
Figure 2.
Detection of photoreceptor markers in hRPE 252 cells reprogrammed by ASCL1 and miRNA124. Immunostaining of hRPE 252 cells for (a–aʹ) cone cell marker L/M-opsin, (c–cʹ) rod cell marker rhodopsin, and (e–eʹ) photoreceptor marker recoverin. After eight days of reprogramming with ASCL1 and miRNA124, hRPE 252 cells were immunostained for (b–bʹ) L/M-opsin, (d–dʹ) rhodopsin, and (f–fʹ) recoverin. All cells were co-stained for (a″–f″) Tuj1 and (a‴–f‴) DAPI. (g–g‴) Merged image (g) of costaining for rhodopsin (gʹ), L/M-opsin (g″), and DAPI (g‴) in hRPE 252 cells reprogrammed with Am for eight days. Scale bar, 100 μm. (h) Quantification of the percentage of DAPI+ cells that are positive for the indicated markers at Day 8. (A color version of this figure is available in the online journal.)
Time-dependent induction of photoreceptor genes as hRPE cells are reprogrammed by Am
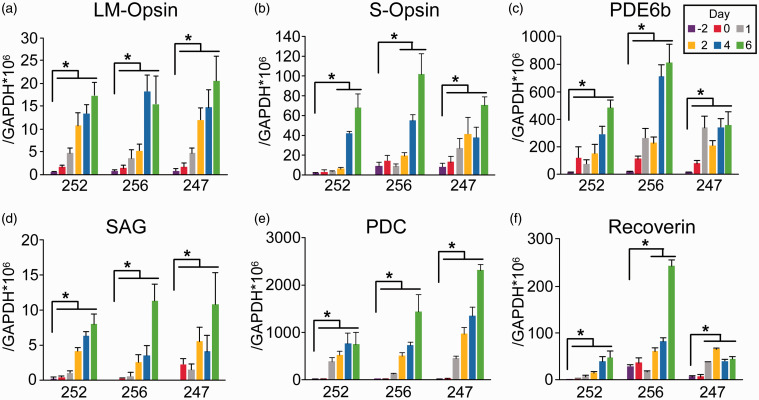
To confirm the results from immunostaining, we performed quantitative RT-PCR to measure the expression of photoreceptor-specific genes at different time points as three independent lines of adult hRPE cells (252, 256, and 247) were being reprogrammed by ASCL1 and miRNA124 (Am). We found that the expression of the cone photoreceptor markers L/M-opsin (Figure 3(a)) and S-opsin (Figure 3(b)) in the three lines of hRPE cells was significantly and consistently increased from Days −2 to 6 of reprogramming. Expression levels of the rod photoreceptor markers phosphodiesterases 6 b (PDE6b) (Figure 3(c)) and arrestin S-antigen (SAG) (Figure 3(d)) were also significantly and consistently increased from Days −2 to 6. This was also the case for the expression of the generic photoreceptor markers phosducin (PDC) (Figure 3(e)) and recoverin (Rcv) (Figure 3(f)). While each line of hRPE cells had somewhat different patterns in the induction of these marker genes, the overall trend was a substantial and continuing increase in the expression of these genes, as hRPE cells were converted to acquire photoreceptor properties.
Figure 3.
Induction of photoreceptor marker genes in multiple lines of hRPE cells reprogrammed with ASCL1 and miRNA124. Real-time quantitative RT-PCR measurements of the expression of L/M-opsin (a), S-opsin (b), PDE6b (c), SAG (d), PDC (e), and recoverin (f) in total RNA isolated from three independent lines of hRPE cells (252, 256, and 247) at Days −2, 0, 1, 2, 4, and 6 of reprogramming. Expression levels of the indicated genes were compared to that of GAPDH in the same sample. * P < 0.05, vs. Day −2, unpaired Student's t-test, n = 6 wells from three independent experiments. (A color version of this figure is available in the online journal.)
Morphological and functional analyses of converted neurons
The unique ultrastructural features of photoreceptors are densely packed membrane discs or invaginations that are embedded with components of the phototransduction pathway, such as rhodopsin and opsin.20 We performed transmission electron microscopy on hRPE 252 cells converted by ASCL1 and miRNA124 at Day 10. In these converted cells, there were many stacked, single-layered, semi-parallel membranous structures that were similar to photoreceptor discs (Figure 4(a) and (b), arrows). Cone photoreceptors have far fewer discs than rod photoreceptors have; their discs are formed by invaginations of the outer segment membrane, unlike the freely floating, stacked discs in rod photoreceptors. We did not see disc-like structures attached to the plasma membrane.
Figure 4.
Transmission electron micrographs and electrophysiology recordings of hRPE 252 cells converted by ASCL1 and miRNA124. (a, b) Transmission electron micrographs of hRPE 252 cells reprogrammed with ASCL1 and miRNA124 for 10 days. Arrowheads, photoreceptor disc-like structures. Scale bars: 500 nm in (a), 100 nm in (b). (c) In response to voltage steps, small voltage-gated Na+ currents (INa+, inward) and voltage-gated delayed rectifier K+ currents (IK+, outward) were recorded in hRPE 252 cells reprogrammed with ASCL1 and miRNA124 for seven days. (d) In response to voltage steps, large voltage-gated Na+ currents (INa+, inward), voltage-gated A-type K+ currents (IK+, outward), and voltage-gated Ca2+ currents (ICa2+, inward) were recorded in hRPE 252 cells reprogrammed with ASCL1 and miRNA124 for 14 days.
We performed electrophysiological recording on converted cells at Day 7 and Day 14. In response to voltage steps applied on the cell membrane, small amount of fast-inactivating voltage-gated Na+ currents (INa+, inward) were observed, along with large voltage-gated delayed rectifier K+ currents (IK+, outward) in neurons at Day 7 (Figure 4(c)). In neurons at Day 14, much larger voltage-gated Na+ currents (INa+, inward) were seen. In addition, there were A-type voltage-gated K+ currents (IK+, transient outward), as well as voltage-gated Ca2+ currents (ICa2+, inward). These currents were similar in durations and amplitudes to what we observe in induced neurons converted from fibroblasts.16 The data showed a maturation of electrophysiological properties in the converted neurons. We tried to elicit light responses using 1 s green LED stimulus (530 nm peak) of varying intensities. There was no current or voltage response in response to light stimulation, suggesting that the complete visual phototransduction machinery is either not present or not fully functional.
Discussion
RPE cells function primarily to support the survival of adjacent photoreceptors through various housekeeping roles such as transporting nutrients, recycling outer segments, and protecting against free radicals.21 The potential for RPE cells to transdifferentiate into various retinal cell types has been documented.7 However, the conversion of RPE cells into mature and functional photoreceptors has yet to be achieved. Studies utilizing ash1, ath5, neurogenin2, and neuroD have directed non-human RPE cells towards a photoreceptor lineage, although with low yields of photoreceptor-like cells.22–26 Neurogenin1 has been shown to reprogram chick RPE into immature photoreceptors at an improved efficiency of 80%.26 In mammalian RPE cells (including human, porcine, and mouse), overexpressing SOX2, neuroD, neurogenin1, or neurogenin3 can give rise to photoreceptor-like cells.27,28
Here, we show that expression of ASCL1 and miRNA124 rapidly and efficiently converted adult hPRE cells into neurons with photoreceptor properties. ASCL1 alone exhibited significant reprogramming capacity, which was further enhanced by the addition of miRNA124 (Figure 1). Within eight days of reprogramming, approximately 80% of all cells became positive for opsin, rhodopsin, and recoverin (Figure 2). The strong and continuing induction of various photoreceptor marker genes corroborated that the hRPE cells were converted toward neurons with some features of photoreceptors (Figure 3). However, these cells co-expressed both the rod cell marker rhodopsin and the cone cell marker opsin, whereas photoreceptors in vivo have segregated expression of these two markers in rod cells and cone cells. Nevertheless, the converted neurons possessed single-layered, parallel disc-like structures resembling discs in photoreceptors. They also had voltage-gated Na+, K+, and Ca2+ currents (Figure 4), which may be related to the presence of Na+ currents in cultured hRPE cells.29 The lack of light response is not surprising, as light-induced changes in currents require elaborate signaling mechanisms with highly concentrated phototransduction molecules densely packed on photodiscs. The converted cells appear to have not acquired all the necessary components to enable light responses. Further investigations are needed to transdifferentiate human RPE cells to bona fide photoreceptors.
It is unclear how the combination of ASCL1 and miR124 converted hRPE cells to neurons with photoreceptor properties. Both RPE cells and photoreceptors are derived from the optic vesicle.30 As a pioneer transcription factor, ASCL1 activates the transcription of a variety of neurogenic genes to convert fibroblasts to neurons.10 The expression of miR124 enhances the transdifferentiation of human fibroblasts to induce neurons by ASCL1.12 As Ascl1 induces the expression of Otx2,30 a critical transcription factor for the development of photoreceptors, it is possible that expression of ASCL1 in RPE cells may convert them to photoreceptors because of their shared developmental origin. More studies are needed to understand the mechanism of the conversion. Nevertheless, the ability to directly convert human RPE cells to neurons with various properties of photoreceptors within 10 days would be quite useful for the study of diseases affecting photoreceptors. The potentials for disease modeling and cell replacement therapy will stimulate further explorations in this field.
ACKNOWLEDGEMENTS
We thank Dr. Sally Temple at Neural Stem Cell Institute for providing the adult human RPE cells.
Authors’ contributions: BL and HJ contributed equally to the study. HJ and JF: conception and experimental design; BL, H J, BZ, MS, and JF: manuscript writing and editing; BL, HJ, HL, MS, and ZY: data collection, data analysis, and preparation of figures and table; JF: financial support.
Declaration OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported in part by NIH grant NS113763 (J.F.).
ORCID iD: Jian Feng https://orcid.org/0000-0001-7630-8800
References
- 1.Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res 1998; 17:175–205 [DOI] [PubMed] [Google Scholar]
- 2.Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol 2008; 26:215–24 [DOI] [PubMed] [Google Scholar]
- 3.Farah MH, Olson JM, Sucic HB, Hume RI, Tapscott SJ, Turner DL. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development 2000; 127:693–702 [DOI] [PubMed] [Google Scholar]
- 4.Lakowski J, Welby E, Budinger D, Di Marco F, Di Foggia V, Bainbridge JWB, Wallace K, Gamm DM, Ali RR, Sowden JC. Isolation of human photoreceptor precursors via a cell surface marker panel from stem Cell-Derived retinal organoids and fetal retinae. Stem Cells 2018; 36:709–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagliardi G, Ben M'Barek K, Goureau O. Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: a pluripotent stem cell-based approach. Prog Retin Eye Res 2019; 71:1–25 [DOI] [PubMed] [Google Scholar]
- 6.Mathivanan I, Trepp C, Brunold C, Baerlocher G, Enzmann V. Retinal differentiation of human bone marrow-derived stem cells by co-culture with retinal pigment epithelium in vitro. Exp Cell Res 2015; 333:11–20 [DOI] [PubMed] [Google Scholar]
- 7.Fuhrmann S, Zou C, Levine EM. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp Eye Res 2014; 123:141–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci 2002; 3:517–30 [DOI] [PubMed] [Google Scholar]
- 9.Vasconcelos FF, Castro DS. Transcriptional control of vertebrate neurogenesis by the proneural factor Ascl1. Front Cell Neurosci 2014; 8:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vierbuchen T, Wernig M. Molecular roadblocks for cellular reprogramming. Mol Cell 2012; 47:827–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010; 463:1035–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 2011; 476:228–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salero E, Blenkinsop TA, Corneo B, Harris A, Rabin D, Stern JH, Temple S. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell 2012; 10:88–95 [DOI] [PubMed] [Google Scholar]
- 14.Hu Q, Friedrich AM, Johnson LV, Clegg DO. Memory in induced pluripotent stem cells: reprogrammed human retinal-pigmented epithelial cells show tendency for spontaneous redifferentiation. Stem Cells 2010; 28:1981–91 [DOI] [PubMed] [Google Scholar]
- 15.Hu Q, Chen R, Teesalu T, Ruoslahti E, Clegg DO. Reprogramming human retinal pigmented epithelial cells to neurons using recombinant proteins. Stem Cells Transl Med 2014; 3:1526–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Xu Z, Zhong P, Ren Y, Liang G, Schilling HA, Hu Z, Zhang Y, Wang X, Chen S, Yan Z, Feng J. Cell cycle and p53 gate the direct conversion of human fibroblasts to dopaminergic neurons. Nat Commun 2015; 6:10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Ren Y, Yuen EY, Zhong P, Ghaedi M, Hu Z, Azabdaftari G, Nakaso K, Yan Z, Feng J. Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun 2012; 3:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Z, Jiang H, Zhong P, Yan Z, Chen S, Feng J. Direct conversion of human fibroblasts to induced serotonergic neurons. Mol Psychiatry 2016; 21:62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polans AS, Witkowska D, Haley TL, Amundson D, Baizer L, Adamus G. Recoverin, a photoreceptor-specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proc Natl Acad Sci U S A 1995; 92:9176–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker SA, Kerov V. Photoreceptor inner and outer segments. Curr Top Membr 2013; 72:231–65 [DOI] [PubMed] [Google Scholar]
- 21.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev 2005; 85:845–81 [DOI] [PubMed] [Google Scholar]
- 22.Mao W, Yan RT, Wang SZ. Reprogramming chick RPE progeny cells to differentiate towards retinal neurons by ash1. Mol Vis 2008; 14:2309–20 [PMC free article] [PubMed] [Google Scholar]
- 23.Ma W, Yan RT, Xie W, Wang SZ. bHLH genes cath5 and cNSCL1 promote bFGF-stimulated RPE cells to transdifferentiate toward retinal ganglion cells. Dev Biol 2004; 265:320–8 [DOI] [PubMed] [Google Scholar]
- 24.Yan RT, Ma WX, Wang SZ. neurogenin2 elicits the genesis of retinal neurons from cultures of nonneural cells. Proc Natl Acad Sci U S A 2001; 98:15014–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan RT, Wang SZ. neuroD induces photoreceptor cell overproduction in vivo and de novo generation in vitro. J Neurobiol 1998; 36:485–96 [PMC free article] [PubMed] [Google Scholar]
- 26.Yan RT, Liang L, Ma W, Li X, Xie W, Wang SZ. Neurogenin1 effectively reprograms cultured chick retinal pigment epithelial cells to differentiate toward photoreceptors. J Comp Neurol 2010; 518:526–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezati R, Etemadzadeh A, Soheili ZS, Samiei S, Ranaei Pirmardan E, Davari M, Najafabadi HS. The influence of rAAV2-mediated SOX2 delivery into neonatal and adult human RPE cells; a comparative study. J Cell Physiol 2018; 233:1222–35 [DOI] [PubMed] [Google Scholar]
- 28.Yan RT, Li X, Huang J, Guidry C, Wang SZ. Photoreceptor-like cells from reprogramming cultured mammalian RPE cells. Mol Vis 2013; 19:1178–87 [PMC free article] [PubMed] [Google Scholar]
- 29.Wen R, Lui GM, Steinberg RH. Expression of a tetrodotoxin-sensitive Na+ current in cultured human retinal pigment epithelial cells. J Physiol 1994; 476:187–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brzezinski JA, Reh TA. Photoreceptor cell fate specification in vertebrates. Development 2015; 142:3263–73 [DOI] [PMC free article] [PubMed] [Google Scholar]