We report the successful recovery of a patient with severe respiratory failure due to empyema with bronchopleural fistula treated with a comprehensive approach that included veno‐venous extracorporeal membrane oxygenation and a management strategy. The following management options should therefore be considered: (i) effective and safe initial drainage, (ii) extracorporeal membrane oxygenation for respiratory support for persistent respiratory failure with severe hypoxia, (iii) definitive treatment including endobronchial Watanabe spigot (EWS), which does not need to be completed (i.e., closing all fistulae) all at once, as reducing air leakage is the main goal for the first EWS (repeated EWS is required for complete bronchopleural fistula closure in patients with poor general condition such as in our case), (iv) general intensive care including nutrition and rehabilitation therapy as soon as possible.
Keywords: bronchial blocker, bronchopleural fistula, ECMO, empyema, endobronchial Watanabe spigot
Abstract
Background
Complicated empyema accompanied by bronchopleural fistula (BPF) has high mortality. The treatment strategy for severe respiratory failure due to empyema with BPF has yet to be established.
Case Presentation
A 70‐year‐old man was brought to our hospital and diagnosed with right empyema, BPF (at bronchi B4–10), and secondary left pneumonia. We initiated drainage followed by veno‐venous extracorporeal membrane oxygenation due to the severe hypoxia. First, the patient underwent endoscopic treatment with obstructive materials (known as endobronchial Watanabe spigot [EWS]) at B8–10, and was weaned off veno‐venous extracorporeal membrane oxygenation on day 7. A secondary EWS was carried out at B4–6. A combination of medical treatments (drainage, antibiotics, nutritional therapy, and rehabilitation) improved his general condition. The patient was able to leave the hospital on foot.
Conclusion
A comprehensive approach could explain the success of the medical treatment. The principal components are the repeated application of EWS as damage control.
Introduction
The incidence of empyema has increased worldwide in recent decades. 1 Complicated empyema is accompanied by bronchopleural fistula (BPF), which has high mortality and morbidity. 2 Empyema with BPF has classically been treated surgically with pneumonectomy or window opening. 3 In recent years, however, less invasive bronchoscopic treatments have been reported. 4
There has been only one reported case of a patient with severe empyema with BPF requiring veno‐venous extracorporeal membrane oxygenation (VV‐ECMO). 5 The treatment strategy for such severe respiratory failure due to empyema with BPF has not yet been established.
We report the successful recovery of a patient with severe respiratory failure due to empyema with BPF treated with a comprehensive approach that included VV‐ECMO and a management strategy.
Case presentation
A 70‐year‐old man was brought to our hospital due to fever and severe respiratory distress. His vital signs on admission were: Glasgow Coma Scale score, 14 (E4V4M6); blood pressure, 93/57 mmHg; heart rate, 138 b.p.m.; respiratory rate, 30 breaths/min; SpO2, 81% (oxygen 10 L/min through a non‐rebreathing mask); and body temperature, 38.2°C. Chest computed tomography showed right empyema and bilateral pneumonia (Fig. 1). The Acute Physiology and Chronic Health Evaluation II score was 24 and estimated non‐operative mortality was 42%.
Fig. 1.
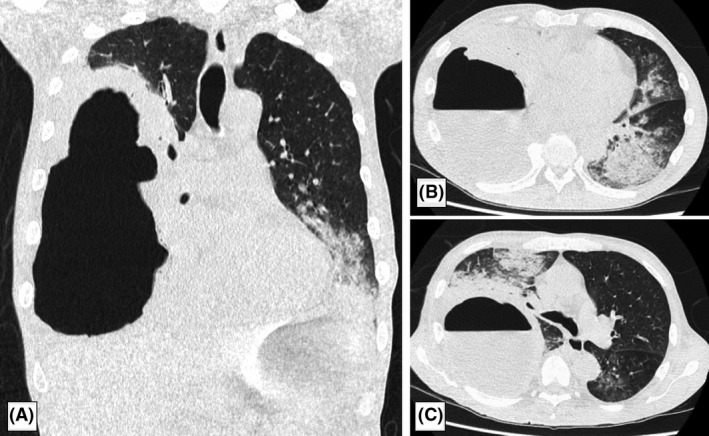
Computed tomography images on admission of a 70‐year‐old man with respiratory failure due to empyema with bronchopleural fistula.
Initial drainage was carried out on the right‐sided empyema, discharging purulent pleural effusion. Emergency endotracheal intubation was undertaken for severe hypoxia, as well as mechanical ventilation. However, adequate oxygenation was not obtained by applying high positive end‐expiratory pressure (PEEP); an air leak from the chest tube was observed, indicating right empyema with BPF. Veno‐venous ECMO was initiated due to severe hypoxia with mechanical ventilation (SpO2, 60%; PaO2, 38 mmHg; PEEP, 15 cmH2O; FIO2, 1.0). The ventilation setting during VV‐ECMO was: pressure assist‐control ventilation; PEEP, 5 cmH2O; inspiratory pressure, 10 cmH2O; FIO2, 0.4; ventilation rate, 10/min.
A bronchial blocker was inserted into the right mid‐trunk on day 3 (D3) to prevent pus flowing into the left healthy lung through a fistula, which was considered the cause of inflammation, thereby reducing but not eliminating the air leak. On D4, bronchoscopy was undertaken to identify the fistula site in detail, and forceps were inserted to check the traffic with the thoracic cavity at bronchi B4–10. Given the patient’s poor general condition, an endobronchial Watanabe spigot (EWS) was inserted at B8–10 with fibrin glue (Fig. 2A) and then another bronchial blocker in the right middle trunk to complete the procedure (Fig. 2B). Given the expected long‐term ventilator management, we carried out a tracheostomy on D6. On D7, the patient was weaned off VV‐ECMO. The air leak from the right chest tube persisted, a secondary EWS was inserted at B4–6, and the bronchial blocker was removed on D11 (Fig. 2C). Although the air leak was reduced, the fistula was not completely closed.
Fig. 2.
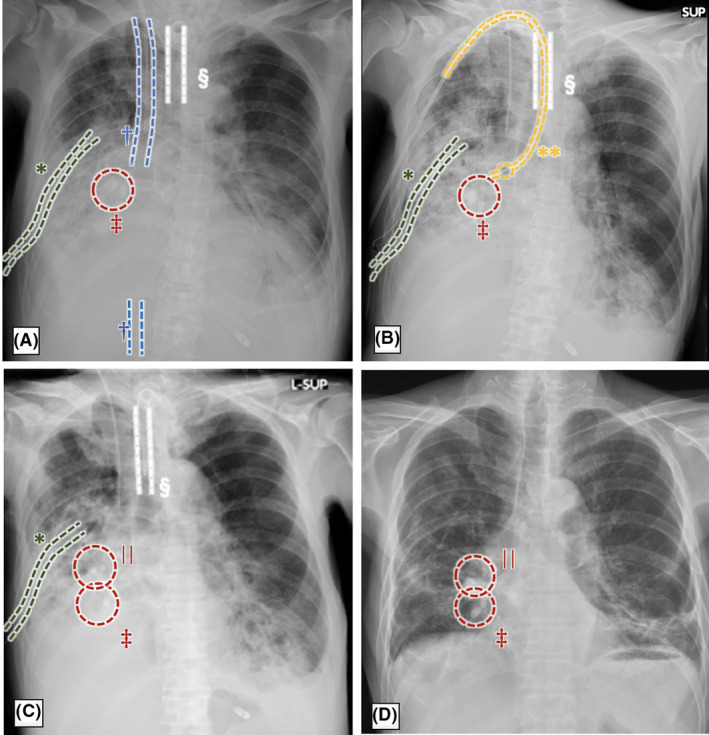
Time course of the chest X‐ray findings of a 70‐year‐old man with respiratory failure due to empyema with bronchopleural fistula. A, Day 7 after first insertion of endobronchial Watanabe spigot (EWS) at bronchi B8–10 under veno‐venous extracorporeal membrane oxygenation. B, Day 8 after bronchial blocker inserted into the right middle trunk. C, Day 12 after second insertion of EWS at B4–6. D, Day 111, last chest X‐rays before discharge. *Chest tube; †extracorporeal membrane oxygenation cannula; ‡first EWS; §tracheal cannula; **bronchial blocker; ||second EWS.
Our policy was to administer medical treatment (continuous drainage, antibiotics, nutritional therapy, and rehabilitation) and wait for the patient’s general condition to improve.
The patient underwent a fasting regimen up to D2 due to unstable circulatory dynamics. Once the circulation stabilized, enteral feeding was started from D3. On admission, the patient was undernourished (height, 172 cm; BMI, 19.5; albumin, 1.5 g/dL). The dose was increased to 2,200 kcal from D10, and the patient started oral intake on D35 after ventilator withdrawal. Respiratory rehabilitation was started on D7, and starting on D21, the patient was treated with prone position therapy. Due to the patient’s pain, we could not undertake an extended trial. However, the oxygenation gradually improved and continued until D26 when the patient left the intensive care unit.
Antibiotics were started with meropenem and vancomycin, changed to ampicillin/sulbactam on D11, and ended on D50. Parvimonas micra was detected in blood cultures, and Streptococcus milleri was detected in the purulent pleural fluid culture.
The patient’s respiratory status gradually improved. The patient underwent tracheostomy closure on D95 and was discharged on D181 on foot and by himself without having the EWS removed. (Figs. 2D,3).
Fig. 3.
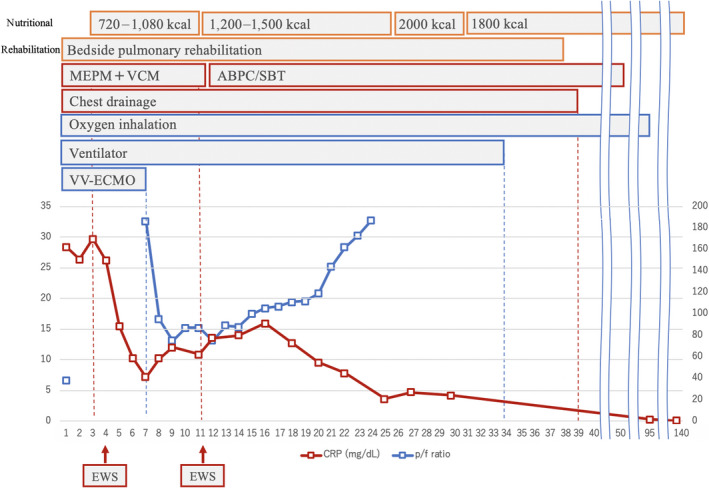
Time course of treatment of a 70‐year‐old man with respiratory failure due to empyema with bronchopleural fistula. Day 1, meropenem (MEPM) and vancomycin (VCM), chest tube, ventilation, and veno‐venous extracorporeal membrane oxygenation (VV‐ECMO) was initiated. Day 3, nutrition and rehabilitation was initiated. Day 4, endobronchial Watanabe spigot (EWS) was inserted at bronchi B8–10. Day 7, the patient was weaned off VV‐ECMO. Day 11, MEPM + VCM was changed to ampicillin/sulbactam (ABPC/SBT) and EWS was inserted at B4–6. Day 39, air leak disappeared and the chest tube was removed. Day 50, antibiotics completed. Day 95, oxygen inhalation completed. CRP, C‐reactive protein; P/F, PaO2/FIO2 ratio.
Discussion
In this case, acute phase management, including resuscitation and definitive treatment, was initiated as follows: (i) drainage with chest tube followed by VV‐ECMO as the resuscitation for initial severe hypoxia, (ii) bronchial blocker placement to control infection and air leaks, (iii) EWS to close the BPF. Appropriate nutritional management and rehabilitation therapy improved the patient’s general condition.
Kondo et al. reported a case with empyema requiring surgery during ECMO in which they replaced the chest tube due to inadequate drainage. Lung injury was suspected due to bleeding, and emergency surgery was undertaken to treat the leak and cure the empyema. However, the intraoperative procedure resulted in a new air leak. Only drainage was carried out, EWS was subsequently undertaken for fistula closure, and the patient completely recovered. 5 If initial drainage with chest tube is carried out effectively and safely, surgical treatment might not be required.
Fistula closure is important in the definitive treatment of empyema with BPF. 6 Bronchopleural fistula sometimes causes pleural effusion from the fistula to the contralateral lung. 7 Surgery is often required for closure, 3 but other options with endoscopy (fibrin glue, silver nitrate cautery, coils, endobronchial stents, 2 and obstructive materials 8 ) have been developed. Appropriate ventilator management (to maintain low airway pressure) 9 and nutrition therapy contribute to BPF closure through improved systemic condition. A comprehensive approach including initial chest tube drainage, ECMO introduction, and EWS under appropriate nutritional and rehabilitation therapy could explain the successful medical treatment in this case. In particular, the main components are the repeated use of EWS as damage control treatment to reduce air leaks in the early stages and definitive treatment to completely close the BPF in later stages. One challenge for the future is that there is no verification of the long‐term safety of the placement of an EWS. 10
The following management options should therefore be considered as the strategy for treating empyema with BPF in severe respiratory failure: (i) effective and safe initial drainage, (ii) ECMO for respiratory support for persistent respiratory failure with severe hypoxia, (iii) definitive treatment including EWS, which does not need to be completed (i.e., closing all fistulae) all at once, as reducing air leakage is the main goal for the first EWS (repeated EWS is required for complete BPF closure in patients with poor general condition, such as in our case), (iv) general intensive care including nutrition and rehabilitation therapy as soon as possible.
Disclosure
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Funding information
No funding information provided.
References
- 1. Burgos J, Falcó V, Pahissa A. The increasing incidence of empyema. Curr. Opin. Pulm. Med. 2013; 19: 350–6. [DOI] [PubMed] [Google Scholar]
- 2. Batıhan G, Can CK. Bronchopleural fistula: causes, diagnoses and management. Dis. Pleura. 2020; 1–14. [Google Scholar]
- 3. Scarci M, Abah U, Solli P, et al EACTS expert consensus statement for surgical management of pleural empyema. Eur. J. Cardio. Thoracic Surg. 2015; 48: 642–53. [DOI] [PubMed] [Google Scholar]
- 4. West D, Togo A, Kirk AJB. Are bronchoscopic approaches to post‐pneumonectomy bronchopleural fistula an effective alternative to repeat thoracotomy? Interact. Cardiovasc. Thorac. Surg. 2007; 6: 547–50. [DOI] [PubMed] [Google Scholar]
- 5. Kondo T, Masaaki Sakuraya KY. A case of bronchial fistulae accompanied with empyema and ARDS (acute respiratory distress syndrome) treated with EWS (endobronchial Watanabe spigot) under VV‐ECMO. J. Japanese Soc. Intensive Care Med. 2018; 25: 155–6. [Google Scholar]
- 6. Bribriesco A, Patterson GA. Management of postpneumonectomy bronchopleural fistula: from thoracoplasty to transsternal closure. Thorac. Surg. Clin. [Internet]. 2018; 28: 323–35. [DOI] [PubMed] [Google Scholar]
- 7. Zanotti G, Mitchell JD. Bronchopleural fistula and empyema after anatomic lung resection. Thorac. Surg. Clin. [Internet]. 2015; 25: 421–7. [DOI] [PubMed] [Google Scholar]
- 8. Dalar L, Kosar F, Eryuksel E, Karasulu L, Altin S. Endobronchial Watanabe spigot Embolisation in the treatment of bronchopleural fistula due to tuberculous empyema in intensive care unit. Ann. Thorac.. Cardiovasc. Surg. 2013; 19: 140–3. [DOI] [PubMed] [Google Scholar]
- 9. Hommel M, Deja M, Von Dossow V et al Bronchial fistulae in ARDS patients: Management with an extracorporeal lung assist device. Eur. Respir. J. 2008; 32: 1652–5. [DOI] [PubMed] [Google Scholar]
- 10. Machida Y, Tanaka M, Motono N, Maeda S, Usuda K, Sagawa M. Case report successful treatment of bronchial fistula after pulmonary lobectomy by endobronchial embolization using an endobronchial Watanabe spigot. Case Rep. Pulmonol. 2015; 2015: 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


