Key Points
Question
What is the risk of progression to diabetes among older adults with prediabetes (based on glycated hemoglobin level of 5.7%-6.4%, fasting glucose levels of 100-125 mg/dL, either, or both) in a community-based population?
Findings
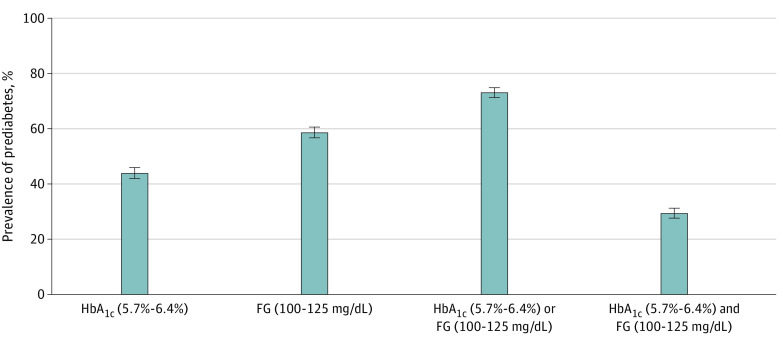
In this cohort study of 3412 older adults, the prevalence of prediabetes (mean [SD] age, 75.6 [5.2] years) was high and differed substantially depending on the definition used, with estimates ranging from 29% for glycated hemoglobin levels of 5.7% to 6.4% and fasting glucose levels of 100 to 125 mg/dL to 73% for either glycated hemoglobin levels of 5.7% to 6.4% or fasting glucose levels of 100 to 125 mg/dL. During the 6 years of follow-up, death or regression to normoglycemia from prediabetes was more frequent than progression to diabetes.
Meaning
Prediabetes may not be a robust diagnostic entity in older age.
Abstract
Importance
The term prediabetes is used to identify individuals at increased risk for diabetes. However, the natural history of prediabetes in older age is not well characterized.
Objectives
To compare different prediabetes definitions and characterize the risks of prediabetes and diabetes among older adults in a community-based setting.
Design, Setting, and Participants
In this prospective cohort analysis of 3412 older adults without diabetes from the Atherosclerosis Risk in Communities Study (baseline, 2011-2013), participants were contacted semiannually through December 31, 2017, and attended a follow-up visit between January 1, 2016, and December 31, 2017 (median [range] follow-up, 5.0 [0.1-6.5] years).
Exposures
Prediabetes defined by a glycated hemoglobin (HbA1c) level of 5.7% to 6.4%, impaired fasting glucose (IFG) level (FG level of 100-125 mg/dL), either, or both.
Main Outcomes and Measures
Incident total diabetes (physician diagnosis, glucose-lowering medication use, HbA1c level ≥6.5%, or FG level ≥126 mg/dL).
Results
A total of 3412 participants without diabetes (mean [SD] age, 75.6 [5.2] years; 2040 [60%] female; and 572 [17%] Black) attended visit 5 (2011-2013, baseline). Of the 3412 participants at baseline, a total of 2497 participants attended the follow-up visit or died. During the 6.5-year follow-up period, there were 156 incident total diabetes cases (118 diagnosed) and 434 deaths. A total of 1490 participants (44%) had HbA1c levels of 5.7% to 6.4%, 1996 (59%) had IFG, 2482 (73%) met the HbA1c or IFG criteria, and 1004 (29%) met both the HbA1c and IFG criteria. Among participants with HbA1c levels of 5.7% to 6.4% at baseline, 97 (9%) progressed to diabetes, 148 (13%) regressed to normoglycemia (HbA1c, <5.7%), and 207 (19%) died. Of those with IFG at baseline, 112 (8%) progressed to diabetes, 647 (44%) regressed to normoglycemia (FG, <100 mg/dL), and 236 (16%) died. Of those with baseline HbA1c levels less than 5.7%, 239 (17%) progressed to HbA1c levels of 5.7% to 6.4% and 41 (3%) developed diabetes. Of those with baseline FG levels less than 100 mg/dL, 80 (8%) progressed to IFG (FG, 100-125 mg/dL) and 26 (3%) developed diabetes.
Conclusions and Relevance
In this community-based cohort study of older adults, the prevalence of prediabetes was high; however, during the study period, regression to normoglycemia or death was more frequent than progression to diabetes. These findings suggest that prediabetes may not be a robust diagnostic entity in older age.
This cohort study of older adults without diabetes compares different prediabetes definitions and characterizes the risks of prediabetes and diabetes in this population.
Introduction
The prevalence of prediabetes and diabetes increases substantially with age.1 In the US, an estimated 25% of adults 65 years or older have diabetes, whereas more than 50% meet criteria for prediabetes depending on the definition used.2 Despite the high prevalence of diabetes and prediabetes among older adults, progression of hyperglycemia over time (ie, the transition from normoglycemia to prediabetes or diabetes or transition from prediabetes to diabetes) is poorly characterized in this population.
The American Diabetes Association (ADA) has highlighted the prognostic implications of hyperglycemia among older adults as a critical knowledge gap.3,4 The natural history of prediabetes in older adults is inadequately understood. Hyperglycemia among older adults is heterogeneous with regard to presentation and outcomes with implications for treatment.4,5 However, the incidence of and progression of prediabetes to diabetes in older populations are poorly characterized, and few studies6,7 have examined the prognostic implications of different definitions of prediabetes. Consensus is lacking regarding optimal prediabetes definitions, with 5 different definitions in current clinical use.8,9 An understanding of the natural history of prediabetes in later life has implications for screening, diagnosis, and management of the condition in older adults. Prognostic data are critical to understand which prediabetes definition(s)—if any—may be most useful in older populations.
We sought to compare the prevalence of prediabetes—based on glycated hemoglobin (HbA1c) levels, fasting glucose (FG) levels, either, or both—and examine progression from normoglycemia to prediabetes or diabetes and progression from prediabetes to diabetes in a community-based cohort of older adults from the Atherosclerosis Risk in Communities (ARIC) Study.
Methods
Study Design
The ARIC Study is a community-based cohort that began in 1987-1989 when participants were 45 to 64 years of age.10 Several clinic visits have occurred. Visit 5 occurred in 2011-2013, when participants were 71 to 90 years of age, and was attended by 6538 participants; visit 5 serves as the baseline for this study. Visit 6 occurred in 2016-2017 (approximately 5-6 years later). Vital status was identified through semiannual follow-up telephone calls to proxies, state records, and National Death Index linkage. The institutional review boards at each study site approved the study. Participants (and proxies, where required) provided written informed consent. Data were not deidentified. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Of the 6538 participants who attended visit 5, we excluded 2250 (34%) with a history of diagnosed diabetes (self-reported physician diagnosis or glucose-lowering medication use by visit 5) and 308 participants with HbA1c levels of 6.5% or higher (to convert to proportion of hemoglobin, multiply by 0.01) or FG levels of 126 mg/dL or higher (to convert to millimoles per liter, multiply by 0.0555). We also excluded 367 participants who were missing HbA1c or FG measurements at visit 5 or 6, 1 participant with an implausible FG level (12 mg/dL), 176 who fasted for less than 8 hours, and 5 without follow-up information. Because of small numbers, we also excluded 10 participants who self-identified as having a race/ethnicity other than Black or White and 9 Black participants at the Maryland and Minnesota study centers. Our analytic study population included 3412 participants who attended visit 5 (eFigure 1 in the Supplement), of whom 2089 (61%) attended visit 6, 915 (27%) were alive but did not attend visit 6, and 408 (12%) died before visit 6 (see eTable 1 in the Supplement for participant characteristics by visit 6 attendance).
Measurements of Glycemia
The HbA1c levels were measured in whole blood with the Tosoh G7 automated high-performance liquid chromatography analyzer (Tosoh Bioscience), standardized to the Diabetes Control and Complications Trial assay. Glucose was measured in serum using the hexokinase method on the Olympus AU400e analyzer (Beckman Coulter Inc). The laboratory intra-assay coefficient of variation based on blind duplicates at visit 5 was 1.3% for HbA1c and 5.7% for FG.
Definitions of Prediabetes at Baseline
In this population without diagnosed diabetes at visit 5, we categorized participants according to ADA definitions for prediabetes based on HbA1c levels (5.7%-6.4%) and/or FG levels (100-125 mg/dL). We also defined prediabetes as having HbA1c levels of 5.7% to 6.4% or impaired FG (IFG) criteria or both HbA1c levels of 5.7% to 6.4% and IFG (confirmatory definition). In secondary analyses, we examined 2 additional (international) thresholds for defining prediabetes: HbA1c levels of 6.0% to 6.4% endorsed by the International Expert Committee (IEC)11 and FG levels of 110 to 126 mg/dL endorsed by the World Health Organization (WHO).12
Incident Total Diabetes and Diagnosed Diabetes
Possible outcomes are summarized in eFigure 2 in the Supplement. We defined incident total diabetes based on a self-reported physician diagnosis, glucose-lowering medication use, HbA1c levels of 6.5% or higher, or FG levels of 126 mg/dL or higher using data collected at the follow-up visit (January 1, 2016, to December 31, 2017) or semiannual telephone calls (through December 31, 2017). In a secondary analysis, we limited incident cases of diabetes to those based solely on a self-reported physician diagnosis or glucose-lowering medication use (ie, diagnosed diabetes).
Statistical Analysis
We reported characteristics of participants at baseline (visit 5, 2011-2013) according to categories of HbA1c and FG. We evaluated the cumulative incidence and incidence rates (per 1000 person-years) of total diabetes, diagnosed diabetes, and mortality during the approximately 6-year follow-up period. We calculated sensitivity, specificity, positive predictive value, and negative predictive value of the different prediabetes definitions for identifying 6-year risk of total diabetes and diagnosed diabetes.
For both incident total and diagnosed diabetes, we calculated person-years from baseline to the date of diabetes ascertainment (date of visit 6 or follow-up telephone call), last date of contact, date of death, or December 31, 2017, whichever came first. For mortality, we calculated person-years from baseline to date of death or December 31, 2017.
We used Cox proportional hazards regression to generate hazard ratios (95% CIs) for the associations of baseline prediabetes with risk of total diabetes, diagnosed diabetes, and mortality adjusted for age, sex, and race-center (Maryland White, Minnesota White, Mississippi Black, North Carolina White, and North Carolina Black). We used the Fine and Gray method to incorporate competing risk of death in the cumulative incidence function and generated subhazard ratios.13
A total of 408 persons (12%) died before visit 6, and 915 (27%) were alive but did not attend visit 6. Because these individuals may have been more likely to have diabetes than those who did attend visit 6, we conducted a sensitivity analysis using an inverse probability of attrition weighting approach14,15 to account for dropout because of death or nonparticipation at the follow-up visit (see eTable 1 in the Supplement for characteristics according to follow-up and vital status). The attrition weights were derived using visit 5 HbA1c levels, FG levels, and clinical risk factors to estimate the probabilities of death and participation at the follow-up visit (eMethods in the Supplement).
All analyses were conducted using Stata software, version 16.1 (StataCorp LLC). P < .05 was considered statistically significant.
Results
A total of 3412 participants without diabetes (mean [SD] age, 75.6 [5.2] years; 2040 [60%] female; and 572 [17%] Black) attended visit 5 (2011-2013, baseline). Prediabetes prevalence differed based on the definition used: 1490 participants (44%) had HbA1c-defined prediabetes (5.7%-6.4%), 1996 (59%) had IFG (FG, 100-125 mg/dL), 2482 (73%) met either HbA1c-defined or IFG criteria, and 1004 (29%) met both HbA1c and IFG criteria (Figure 1). The prevalence of prediabetes was 15% according to the IEC HbA1c definition (6.0%-6.4%) and 23% according to the WHO definition (FG, 110-126 mg/dL) (eFigure 3 in the Supplement). Compared with participants with HbA1c levels less than 5.7%, participants with prediabetic HbA1c levels (5.7%-6.4%) were more likely to be Black or have an FG level of 100-125 mg/dL (Table 1). Half of the participants with IFG had prediabetic HbA1c levels. Nonparticipation at the follow-up visit did not differ according to baseline prediabetes status (eTable 1 in the Supplement).
Figure 1. Prevalence of Prediabetes in Older Adults in the Atherosclerosis Risk in Communities Study According to Glycated Hemoglobin (HbA1c) and Impaired Fasting Glucose (FG) Definitions.
Table 1. Participant Characteristics According to HbA1c-Defined Prediabetes and IFG in Older Adults, the ARIC Study, 2011-2013 (Visit 5)a.
| Characteristic | HbA1c categoryb | FG categoryc | ||
|---|---|---|---|---|
| Normoglycemia (HbA1c <5.7%) | Prediabetes (HbA1c 5.7%-6.4%) | Normoglycemia (FG <100 mg/dL) | Prediabetes (FG 100-125 mg/dL) | |
| No. (%) of participants | 1400 (56) | 1097 (44) | 1035 (41) | 1462 (59) |
| HbA1c | ||||
| Median (range), % | 5.4 (3.4-5.6) | 5.9 (5.7-6.4) | 5.5 (3.4-6.4) | 5.7 (3.4-6.4) |
| <5.7% | 1400 (100) | 0 | 672 (65) | 728 (50) |
| 5.7%-6.4% | 0 | 1097 (100) | 363 (35) | 734 (50) |
| FG | ||||
| Median (range), mg/dL | 100 (65-125) | 104 (76-125) | 94 (65-99) | 107 (100-125) |
| <100 mg/dL | 672 (48) | 363 (33) | 1035 (100) | 0 |
| 100-125 mg/dL | 728 (52) | 734 (67) | 0 | 1462 (100) |
| Age, mean (SD), y | 75.1 (5.0) | 75.5 (5.2) | 75.4 (5.2) | 75.2 (5.1) |
| Sex | ||||
| Male | 594 (42) | 446 (41) | 343 (33) | 697 (48) |
| Female | 806 (58) | 651 (59) | 692 (67) | 765 (52) |
| Race/ethnicity | ||||
| White | 1227 (88) | 838 (76) | 840 (81) | 1225 (84) |
| Black | 173 (12) | 259 (24) | 195 (19) | 237 (16) |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; FG, fasting glucose; HbA1c, glycated hemoglobin; IFG, impaired fasting glucose.
SI conversion factors: to convert HbA1c to proportion of hemoglobin, multiply by 0.01; glucose to millimoles per liter, multiply by 0.0555.
Data are presented as number (percentage) of participants unless otherwise indicated.
Normoglycemia is defined as an HbA1c level less than 5.7% without total diabetes and prediabetes as an HbA1c level of 5.7% to 6.4% without total diabetes.
Normoglycemia is defined as an FG level less than 100 mg/dL without total diabetes and prediabetes as an FG level of 100 to 125 mg/dL without total diabetes.
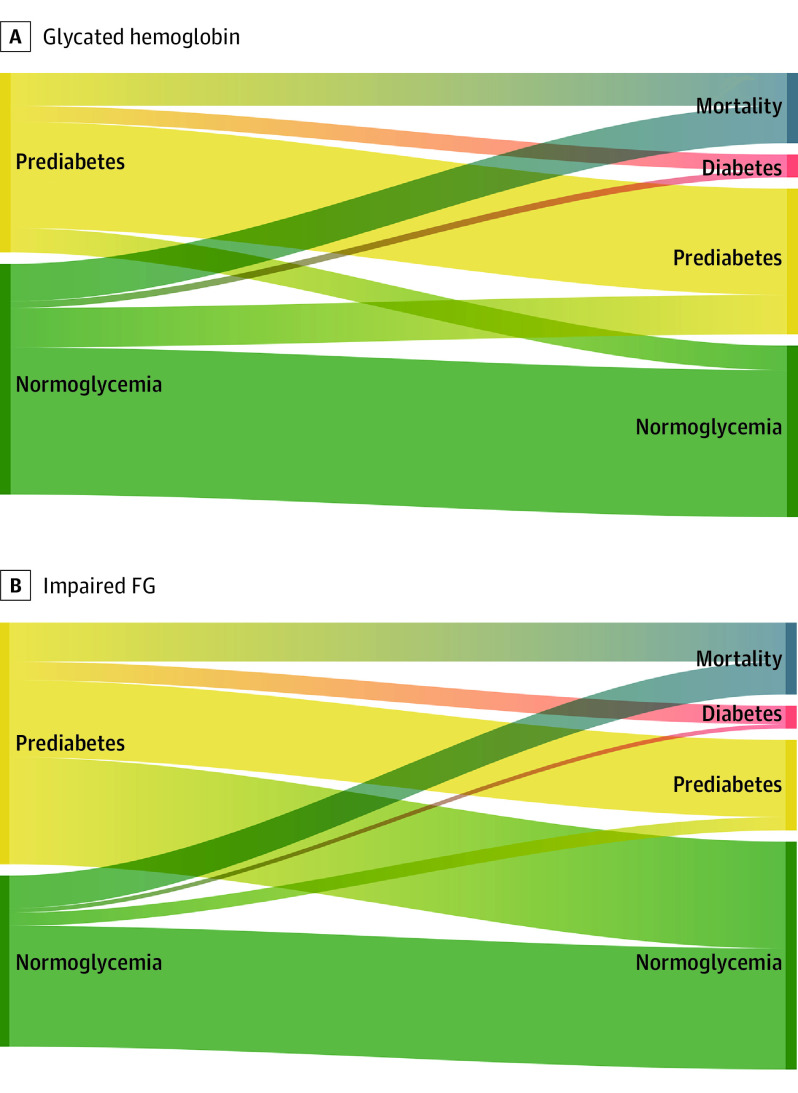
A total of 2497 participants attended visit 6 (2016-2017) or died by the end of follow-up (December 31, 2017). During the median (range) follow-up of 5.0 (0.1-6.5) years, there were 156 incident total diabetes cases, 118 incident diagnosed diabetes cases, and 434 deaths. A total of 893 participants (64%) with HbA1c levels less than 5.7% at visit 5 (baseline, 2011-2013) had HbA1c levels less than 5.7% at the follow-up visit (2016-2017), 239 (17%) progressed to prediabetic levels of HbA1c (5.7%-6.4%), 227 (16%) died, and 41 (3%) developed diabetes (Figure 2 and eTable 2 in the Supplement). Among participants with prediabetic HbA1c levels (5.7%-6.4%) at baseline, 645 (59%) had no change in status, 207 (19%) died, 148 (13%) regressed to normoglycemia, and 97 (9%) progressed to diabetes. The prevalence of HbA1c-defined prediabetes was higher among Black participants (259 [60%]) than White participants (838 [41%]) (eTable 2 in the Supplement). Progression from HbA1c-defined prediabetes (5.7%-6.4%) to diabetes was similar across age groups (≥75 vs <75 years of age: 39 [7%] vs 58 [11%]; P = .06) and sex (men vs women: 36 [8%] vs 61 [9%]; P = 0.52) but was higher in Black older adults compared with White older adults (29 [11%] vs 68 [8%]; P = .04).
Figure 2. Flowchart (Sankey Plot) Depicting Glycemia Progression, Regression, and Mortality in Older Adults According to Prediabetes Definitions at Baseline .
A, Of the participants with glycated hemoglobin (HbA1c) levels of 5.7% to 6.4% at visit 5, 645 (59%) had HbA1c-defined prediabetes at visit 6, 97 (9%) progressed to diabetes, 148 (13%) regressed to normoglycemia (HbA1c, <5.7%), and 207 (19%) died. Of the participants with HbA1c levels less than 5.7% at visit 5, 239 (17%) progressed to HbA1c-defined prediabetes at visit 6, 41 (3%) progressed to diabetes, 893 (64%) had normoglycemia at visit 6, and 227 (16%) died. B, Of the participants with impaired fasting glucose (FG levels of 100-125 mg/dL) at visit 5, 467 (32%) had impaired FG at visit 6, 112 (8%) progressed to diabetes, 647 (44%) regressed to normoglycemia (FG, <100 mg/dL), and 236 (16%) died. Of the participants with normoglycemia (FG, <100 mg/dL) at visit 5, 80 (8%) progressed to impaired FG (FG, 100-125 mg/dL) at visit 6, 26 (3%) progressed to diabetes, 731 (71%) had normoglycemia at visit 6, and 198 (19%) died.
A total of 731 participants (71%) with normal FG levels (<100 mg/dL) at baseline had normal FG levels at the follow-up visit, 80 (8%) progressed to IFG, 198 (19%) died, and 26 (3%) developed diabetes. Among participants with IFG-defined prediabetes at baseline, 647 (44%) regressed to normoglycemia (FG, <100 mg/dL), 467 (32%) stayed in the IFG category, 236 (16%) died, and 112 (8%) progressed to diabetes. Progression from IFG to diabetes was comparable across age (≥75 vs <75 years: 44 [6%] vs 68 [9%]; P = .12) and sex (men vs women: 48 [7%] vs 64 [8%]; P = .30) but was more common among Black older adults than White older adults (27 [11%] vs 85 [7%]; P = .004).
After adjustment for age, sex, and race-center, prediabetes at baseline—by any definition—was associated with incident total diabetes and incident diagnosed diabetes (Table 2). The highest absolute risk for total diabetes was observed among those with HbA1c levels of 5.7% to 6.4% and FG levels of 100 to 125 mg/dL (confirmatory definition), with an incidence rate (per 1000 person-years) of 26.5. Incidence rates for progression to total diabetes were higher for those with prediabetes HbA1c levels of 5.7% to 6.4% than for those with IFG (incidence rate, 22.8 vs 19.0), but the CIs overlapped. For diagnosed diabetes, the highest absolute risk was observed among those with HbA1c levels of 5.7% to 6.4% and FG levels of 100 to 125 mg/dL (incidence rate, 17.7). Incidence rates for diagnosed diabetes were lower than for total diabetes (Table 2).
Table 2. Incidence Rates and Adjusted Hazard Ratios (95% CIs) for Incident Total Diabetes, Incident Diagnosed Diabetes, and Mortality According to Prediabetes Status at Baseline in Older Adults, the ARIC Study (2011-2017).
| Prediabetes criterion | No. of events/participants | Incidence rate per 1000 person-years (95% CI) | HR (95% CI)a | sHR (95% CI)a,b |
|---|---|---|---|---|
| Incident total diabetesc | ||||
| Normoglycemia (HbA1c <5.7%) | 45/1400 | 7.1 (5.3-9.5) | 1 [Reference] | 1 [Reference] |
| Prediabetes (HbA1c 5.7%-6.4%) | 111/1097 | 22.8 (18.9-27.5) | 3.16 (2.22-4.48) | 3.14 (2.21-4.45) |
| Normoglycemia (FG <100 mg/dL) | 31/1035 | 6.7 (4.7-9.5) | 1 [Reference] | 1 [Reference] |
| Prediabetes (FG 100-125 mg/dL) | 125/1462 | 19.0 (15.9-22.6) | 2.95 (1.98-4.38) | 3.02 (2.04-4.49) |
| Normoglycemia (HbA1c <5.7% and FG <100 mg/dL) | 7/672 | 2.3 (1.1-4.8) | 1 [Reference] | 1 [Reference] |
| Prediabetes (HbA1c 5.7%-6.4% or FG 100-125 mg/dL) | 149/1825 | 18.2 (15.5-21.4) | 8.12 (3.80-17.35) | 8.16 (3.82-17.44) |
| Normoglycemia (HbA1c <5.7% or FG <100 mg/dL) | 69/1763 | 8.7 (6.9-11.0) | 1 [Reference] | 1 [Reference] |
| Prediabetes (HbA1c 5.7%-6.4% and FG 100-125 mg/dL) | 87/734 | 26.5 (21.5-32.7) | 2.96 (2.16-4.07) | 3.01 (2.19-4.12) |
| Incident diagnosed diabetesd | ||||
| Normoglycemia (HbA1c <5.7%) | 40/1400 | 6.3 (4.6-8.6) | 1 [Reference] | 1 [Reference] |
| Prediabetes (HbA1c 5.7%-6.4%) | 78/1097 | 16.0 (12.8-20.0) | 2.53 (1.72-3.72) | 2.52 (1.71-3.71) |
| Normoglycemia (FG <100 mg/dL) | 27/1035 | 5.8 (4.0-8.5) | 1 [Reference] | 1 [Reference] |
| Prediabetes (FG 100-125 mg/dL) | 91/1462 | 13.8 (11.2-16.9) | 2.52 (1.64-3.89) | 2.58 (1.67-3.99) |
| Normoglycemia (HbA1c <5.7% and FG <100 mg/dL) | 7/672 | 2.3 (1.1-4.8) | 1 [Reference] | 1 [Reference] |
| Prediabetes (HbA1c 5.7%-6.4% or FG 100-125 mg/dL) | 111/1825 | 13.6 (11.3-16.4) | 6.22 (2.89-13.37) | 6.27 (2.92-13.47) |
| Normoglycemia (HbA1c <5.7% or FG <100 mg/dL) | 60/1763 | 7.6 (5.9-9.7) | 1 [Reference] | 1 [Reference] |
| Prediabetes (HbA1c 5.7%-6.4% and FG 100-125 mg/dL) | 58/734 | 17.7 (13.6-22.8) | 2.32 (1.61-3.34) | 2.35 (1.64-3.37) |
| All-cause mortality | ||||
| Normoglycemia (HbA1c <5.7%) | 227/1400 | 30.6 (26.9-34.9) | 1 [Reference] | NA |
| Prediabetes (HbA1c 5.7%-6.4%) | 207/1097 | 35.9 (31.3-41.1) | 1.07 (0.88-1.29) | NA |
| Normoglycemia (FG <100 mg/dL) | 198/1035 | 36.9 (32.1-42.4) | 1 [Reference] | NA |
| Prediabetes (FG 100-125 mg/dL) | 236/1462 | 30.2 (26.6-34.3) | 0.83 (0.68-1.00) | NA |
| Normoglycemia (HbA1c <5.7% and FG <100 mg/dL) | 117/672 | 33.3 (27.8-40.0) | 1 [Reference] | NA |
| Prediabetes (HbA1c 5.7%-6.4% or FG 100-125 mg/dL) | 317/1825 | 32.8 (29.4-36.6) | 0.94 (0.76-1.17) | NA |
| Normoglycemia (HbA1c <5.7% or FG <100 mg/dL) | 308/1763 | 33.2 (29.7-37.1) | 1 [Reference] | NA |
| Prediabetes (HbA1c 5.7%-6.4% and FG 100-125 mg/dL) | 126/734 | 32.2 (27.1-38.4) | 0.92 (0.75-1.13) | NA |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; FG, fasting glucose; HbA1c, glycated hemoglobin; HR, hazard ratio; NA, not applicable; sHR, subhazard ratio.
Adjusted for age, sex, and race-center.
The sHRs incorporate competing risk of death.
Incident total diabetes was defined as a self-reported physician diagnosis of diabetes, glucose-lowering medication use, HbA1c level of 6.5% or higher, or FG level of 126 mg/dL or higher identified during semiannual follow-up calls or at visit 6 (2016-2017).
Incident diagnosed diabetes was defined as a self-reported physician diagnosis of diabetes or glucose-lowering medication use identified during semiannual follow-up calls or at visit 6 (2016-2017).
In models of the association of prediabetes with incident total diabetes and incident diagnosed diabetes, the associations were similar when we accounted for the competing risk of death (Table 2). When mortality was considered as the primary end point in our analyses, prediabetes (by any definition) was not significantly associated with death.
In a sensitivity analysis, alternative prediabetes definitions endorsed internationally were associated with total and diagnosed diabetes (eTable 3 in the Supplement). Incidence rates (per 1000 person-years) for total diabetes were higher than incidence rates for ADA definitions (46.2 for the IEC criterion of HbA1c levels of 6.0%-6.4% and 28.9 for the WHO criterion of FG levels of 110-125 mg/dL). Prediabetes defined according to the alternative definitions was not associated with mortality. In the sensitivity analysis that accounted for attrition (dropout or death) before visit 6 using inverse probability of attrition weighting, our results were similar, but associations were generally modestly attenuated (eTable 4 in the Supplement).
Sensitivity for identifying incident total diabetes was highest for prediabetes based on HbA1c levels of 5.7% to 6.4% or IFG, whereas specificity was highest for prediabetes based on both HbA1c levels of 5.7% to 6.4% and IFG (confirmatory definition) (Table 3). Positive predictive values for incident total diabetes based on the different prediabetes definitions were all low (<12%); negative predictive values were high (≥96%). For identifying diagnosed diabetes, sensitivity was again highest for prediabetes based on HbA1c levels of 5.7% to 6.4% or IFG (≥94%), whereas specificity was highest for prediabetes based on both HbA1c levels of 5.7%-6.4% and IFG (confirmatory definition) (Table 3). Positive predictive values for incident diagnosed diabetes were all less than 8%, and negative predictive values were high (≥97%). With the use of alternative international definitions for prediabetes, sensitivity was lower (<48%) but specificity was higher (≥78%) for both incident total and diagnosed diabetes (eTable 5 in the Supplement).
Table 3. Performance of Different Definitions of Prediabetes in Older Adults for Identifying Incident Total Diabetes and Incident Diagnosed Diabetes, the ARIC Study, 2011-2017.
| Prediabetes definition | Risk of total diabetes | Diagnostic performance for incident total diabetes, % (95% CI)a | Risk of diagnosed diabetes | Diagnostic performance for incident diagnosed diabetes, % (95% CI)b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incident total diabetes, No.a | No incident total diabetes, No. | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Incident diagnosed diabetes, No.b | No incident diagnosed diabetes, No. | Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
| Prediabetes (HbA1c) | ||||||||||||
| HbA1c 5.7%-6.4% | 1097 | 111 | 71.2 (63.4-78.1) | 57.9 (55.0-59.9) | 10.1 (55.9-59.9) | 96.8 (95.7-97.6) | 1097 | 1019 | 66.1 (56.8-74.6) | 57.2 (55.1-59.2) | 7.1 (5.7-8.8) | 97.1 (96.1-98.0) |
| HbA1c <5.7% | 1400 | 45 | NA | NA | NA | NA | 1400 | 1360 | NA | NA | NA | NA |
| Prediabetes (IFG) | ||||||||||||
| FG 100-125 mg/dL | 1462 | 125 | 80.1 (73.0-86.1) | 42.9 (40.9-44.9) | 8.5 (7.2-10.1) | 97.0 (95.8-99.0) | 1462 | 1371 | 77.1 (68.5-85.3) | 42.4 (40.4-44.4) | 6.2 (5.0-7.6) | 97.4 (96.2-98.3) |
| FG <100 mg/dL | 1035 | 31 | NA | NA | NA | NA | 1035 | 1008 | NA | NA | NA | NA |
| Prediabetes (HbA1c or IFG) | ||||||||||||
| HbA1c 5.7%-6.4% or FG 100-125 mg/dL | 1825 | 149 | 95.5 (91.0-98.2) | 28.4 (26.6-30.3) | 8.2 (6.9-9.5) | 99.0 (97.9-99.6) | 1825 | 1714 | 94.1 (88.2-97.6) | 28.0 (26.2-29.8) | 6.1 (5.0-7.3) | 99.0 (97.9-99.6) |
| HbA1c <5.7% and FG <100 mg/dL | 672 | 7 | NA | NA | NA | NA | 672 | 665 | NA | NA | NA | NA |
| Prediabetes (HbA1c and IFG) | ||||||||||||
| HbA1c 5.7%-6.4% and FG 100-125 mg/dL | 734 | 87 | 55.8 (47.6-63.7) | 72.4 (70.5-74.2) | 11.9 (9.6-14.4) | 96.1 (95.1-96.9) | 734 | 676 | 49.2 (39.8-58.5) | 68.7 (66.8-70.5) | 7.9 (6.1-10.1) | 96.6 (95.6-97.4) |
| HbA1c <5.7% or FG <100 mg/dL | 1763 | 69 | NA | NA | NA | NA | 1763 | 1703 | NA | NA | NA | NA |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; FG, fasting glucose; HbA1c, glycated hemoglobin; IFG, impaired fasting glucose; NA, not applicable.
Incident total diabetes was defined as a self-reported physician diagnosis of diabetes, glucose-lowering medication use, HbA1c level of 6.5% or greater, or FG level of 126 mg/dL or greater identified during semiannual follow-up calls or at visit 6 (2016-2017).
Incident diagnosed diabetes was defined as a self-reported physician diagnosis of diabetes or glucose-lowering medication use identified during semiannual follow-up calls or at visit 6 (2016-2017).
Discussion
In this community-based cohort study of older adults, prediabetes was common, but its prevalence differed substantially based on the definition used. During the 6.5-year follow-up period, fewer than 12% of older adults progressed from prediabetes to diabetes, regardless of the definition of prediabetes. In addition, a substantial proportion of individuals with prediabetes at baseline regressed to normoglycemia at the follow-up visit (148 [13%] among those with HbA1c levels of 5.7%-6.4% and 647 [44%] among those with FG levels of 100-125 mg/dL). Indeed, in older adults with prediabetes, regression to normoglycemia or death was more common than progression to diabetes during the study period.
There are several definitions for prediabetes used in current clinical practice and no consensus on which definition is optimal.1 Depending on the definition, the prevalence of prediabetes in our study ranged from 29% to 73%. In secondary analyses, prediabetes prevalence based on international criteria was lower still at 15% based on the IEC criterion of HbA1c levels of 6.0% to 6.4% and 23% based on the WHO criterion of FG levels of 110 to 126 mg/dL. The various definitions and wide range in prevalence estimates pose challenges for understanding the burden of prediabetes in the population and its clinical and public health relevance. The different definitions of prediabetes also have differing performance for assessing future diabetes. For instance, this study found that specificity was highest for prediabetes based on a confirmatory definition (both HbA1c levels of 5.7%-6.4% and IFG), but specificity was lowest when based on either HbA1c levels of 5.7% to 6.4% or IFG. Conversely, sensitivity for the association with total or diagnosed diabetes was highest for prediabetes based on HbA1c levels of 5.7% to 6.4% or IFG and lowest for the confirmatory definition. The more stringent international definitions for prediabetes had higher specificity for the association with both diabetes outcomes, but sensitivity was lower. These differences in diagnostic performance have implications for screening strategies for diabetes.
The construct of prediabetes is used to identify those individuals at high risk for developing diabetes in the future. In this older population, few individuals who met the definitions of prediabetes progressed to diabetes. Most prior studies1,8,16,17 on progression from prediabetes to diabetes were conducted in middle-aged populations. Comparing estimates of prediabetes progression is challenging in part because of the different definitions of prediabetes. A systematic review1 of 103 studies in primarily middle-aged adults found that the 6-year cumulative incidence of diabetes was 17% (95% CI, 14%-20%) among those with prediabetes based on HbA1c levels of 5.7% to 6.4% and was 22% (95% CI, 15%-31%) for those with IFG (FG, 100-125 mg/dL). The current study is one of the first to document progression of prediabetes to diabetes in older adults in a community-based setting.6,7,18 The results are consistent with a population-based Swedish study6 that examined the natural progression of HbA1c-defined prediabetes (5.7%-6.4%) among adults 60 years or older. During 12 years of follow-up, most participants maintained their HbA1c-defined prediabetes status, and more participants regressed to HbA1c levels less than 5.7% than progressed to diabetes.6 Taken as a whole, the current evidence suggests that cardiovascular disease and mortality should be the focus of disease prevention among older adults rather than prediabetes progression, especially in the short term (<7 years).
Progression to diabetes occurred in some participants in the study, but regression to normoglycemia was far more common, although to differing degrees depending on the classification of prediabetes at baseline (647 [44%] of individuals with IFG regressed to normoglycemia vs 148 [13%] with HbA1c levels of 5.7%-6.4%). The higher probability of regression among those with IFG vs HbA1c levels of 5.7% to 6.4% likely reflects the higher within-person variability for FG compared with HbA1c.19 Prior 5-year cumulative incidence estimates for regression to normoglycemia have ranged from 14% to 45% but have not previously been reported in older adults.1,17,20,21,22,23,24,25,26,27
Interventions in adults with prediabetes can reduce the risk of progression to diabetes. Lifestyle improvements are particularly effective in reducing diabetes risk.28,29,30,31,32 The Diabetes Prevention Program trial28,29 demonstrated that an intensive lifestyle intervention and, to a lesser extent, metformin use reduced the risk of diabetes progression in high-risk adults 25 years or older at baseline (mean [SD] age, 51 [11] years).28,29 Current ADA guidelines recommend that adults with prediabetes (meeting any 1 of the following criteria: HbA1c levels of 5.7%-6.4%, FG levels of 100-125 mg/dL, or 2-hour glucose levels of 140-199 mg/dL) be referred to a lifestyle intervention to encourage weight loss (ideally, at least 7% of initial body weight) and increases in physical activity (moderate intensity for at least 150 minutes per week). Metformin is recommended in patients younger than 60 years with a body mass index of 35 or greater (calculated as weight in kilograms divided by height in meters squared) or in women with a history of gestational diabetes.33 The findings of the current study support a focus on lifestyle improvement when feasible and safe, especially given the broader benefits of lifestyle modification beyond diabetes prevention. Given the low risk of diabetes progression in this study (especially relative to mortality risk), it is unlikely that pharmacologic intervention or other aggressive approaches to diabetes prevention in older age will provide large benefits and could have unintended harmful effects (eg, overdiagnosis, anxiety, and implications for insurance coverage).
The ADA guidelines recommend annual diabetes screening for adults who meet the criteria for prediabetes. In 2019, the Endocrine Society recommended that older adults with prediabetes defined by HbA1c and/or FG be further screened using a 2-hour oral glucose tolerance test to avoid underdiagnosis of diabetes.34 A previous report35 found that this strategy is likely to have little clinical and public health benefit because treatment would not change for older adults identified as having diabetes based on a 2-hour glucose test but who have an HbA1c level less than 6.5% and an FG level less than 126 mg/dL. The current study further highlights the potential futility of aggressive diabetes screening in older adults given the very low rates of diabetes progression among those with prediabetes.
Strengths and Limitations
This study has strengths, including the large, well-characterized, community-based study population with major risk factors assessed in a standardized fashion at in-person visits, including fasting blood collection at 2 time points in older age.
This study also has limitations. First, 915 (27%) of living ARIC Study participants who attended the baseline visit did not attend the follow-up visit. However, participation in the follow-up visit did not differ according to baseline prediabetes status (by any definition). Nevertheless, if participants who did not attend the follow-up visit were more likely to have diabetes than those who attended, the study may have underestimated the risk of diabetes during the follow-up period. However, in a sensitivity analysis that accounted for study attrition, the results were not appreciably different. Second, FG and HbA1c laboratory results are reported back to ARIC Study participants. Thus, participants in our study with prediabetes may have been referred by their health care practitioner or advised on lifestyle modifications at higher rates than other populations. Third, 2-hour glucose testing was not conducted in the ARIC Study. Fourth, the sample size was limited for subgroup analyses by age, race/ethnicity, or sex.
Conclusions
The prevalence of prediabetes was high among older adults; however, progression from prediabetes to diabetes was uncommon during the 6.5-year period. Indeed, regression to normoglycemia and death was more frequent than progression to diabetes from prediabetes. These findings suggest that prediabetes in older age may not be a robust diagnostic entity for predicting diabetes progression.
eMethods. Supplemental Methods
eTable 1. Baseline (Visit 5, 2011-2013) Characteristics of Participants by Attendance at Visit 6 (2016-2017)
eTable 2. Cumulative Incidence Over 6.5 Years (Maximum) of A1C-Defined and Impaired Fasting Glucose (IFG)-Defined Progression to Prediabetes, Total Diabetes, or Mortality, Overall and According to Age, Sex, and Race: The ARIC Study (2011-2017)
eTable 3. Incidence Rates and Adjusted Hazard Ratios (95% CIs) for Incident Total Diabetes, Incident Diagnosed Diabetes, and Mortality According to International Definitions of Prediabetes Status at Baseline in Older Adults: The ARIC Study (2011-2017)
eTable 4. Cumulative Incidence Over 6.5 Years Maximum and Hazard Ratios (95% CIs) for Incident Total Diabetes and Incident Diagnosed Diabetes With Inverse Probability of Attrition Weighting According to Prediabetes Status at Baseline in Older Adults: The ARIC Study (2011-2017)
eTable 5. Performance of Alternative International Definitions of Prediabetes in Older Adults for 6.5-Year Prediction of Incident Total Diabetes and Incident Diagnosed Diabetes: The ARIC Study, 2011-2017
eFigure 1. Participant Flow Chart
eFigure 2. Possible Outcomes According to Baseline Status Defined by A1C (Panel A) or Defined by Fasting Glucose (Panel B)
eFigure 3. Prevalence of Prediabetes in Older Adults in the ARIC Study According to International Expert Committee A1C 6.0-6.4% and World Health Organization Impaired Fasting Glucose (IFG, 110-125 mg/dL) Definitions
References
- 1.Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev. 2018;10:CD012661. doi: 10.1002/14651858.CD012661.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . National Diabetes Statistics Report. 2017. Accessed September 4, 2019. https://www.cdc.gov/diabetes/data/statistics/statistics-report.htm
- 3.Sinclair A, Dunning T, Rodriguez-Mañas L. Diabetes in older people: new insights and remaining challenges. Lancet Diabetes Endocrinol. 2015;3(4):275-285. doi: 10.1016/S2213-8587(14)70176-7 [DOI] [PubMed] [Google Scholar]
- 4.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650-2664. doi: 10.2337/dc12-1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halter JB, Musi N, McFarland Horne F, et al. Diabetes and cardiovascular disease in older adults: current status and future directions. Diabetes. 2014;63(8):2578-2589. doi: 10.2337/db14-0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shang Y, Marseglia A, Fratiglioni L, et al. Natural history of prediabetes in older adults from a population-based longitudinal study. J Intern Med. 2019;286(3):326-340. doi: 10.1111/joim.12920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motta M, Bennati E, Cardillo E, Ferlito L, Malaguarnera M. The value of glycosylated hemoglobin (HbA1c) as a predictive risk factor in the diagnosis of diabetes mellitus (DM) in the elderly. Arch Gerontol Geriatr. 2010;50(1):60-64. doi: 10.1016/j.archger.2009.01.012 [DOI] [PubMed] [Google Scholar]
- 8.Warren B, Pankow JS, Matsushita K, et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2017;5(1):34-42. doi: 10.1016/S2213-8587(16)30321-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Echouffo Tcheugui JB, Selvin E. Prediabetes and what it means: the epidemiological evidence. Ann Rev Public Health. Published online December 23, 2020. doi: 10.1146/annurev-publhealth-090419-102644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The ARIC Investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 11.International Expert Committee . International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327-1334. doi: 10.2337/dc09-9033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. World Health Organization; 2011. [PubMed] [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 14.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656-664. doi: 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23(1):119-128. doi: 10.1097/EDE.0b013e318230e861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt MI, Bracco PA, Yudkin JS, et al. Intermediate hyperglycaemia to predict progression to type 2 diabetes (ELSA-Brasil): an occupational cohort study in Brazil. Lancet Diabetes Endocrinol. 2019;7(4):267-277. doi: 10.1016/S2213-8587(19)30058-0 [DOI] [PubMed] [Google Scholar]
- 17.Ligthart S, van Herpt TT, Leening MJ, et al. Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: a prospective cohort study. Lancet Diabetes Endocrinol. 2016;4(1):44-51. doi: 10.1016/S2213-8587(15)00362-9 [DOI] [PubMed] [Google Scholar]
- 18.Wannamethee SG, Papacosta O, Whincup PH, et al. The potential for a two-stage diabetes risk algorithm combining non-laboratory-based scores with subsequent routine non-fasting blood tests: results from prospective studies in older men and women. Diabet Med. 2011;28(1):23-30. doi: 10.1111/j.1464-5491.2010.03171.x [DOI] [PubMed] [Google Scholar]
- 19.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167(14):1545-1551. doi: 10.1001/archinte.167.14.1545 [DOI] [PubMed] [Google Scholar]
- 20.Herman WH, Pan Q, Edelstein SL, et al. ; Diabetes Prevention Program Research Group . Impact of lifestyle and metformin interventions on the risk of progression to diabetes and regression to normal glucose regulation in overweight or obese people with impaired glucose regulation. Diabetes Care. 2017;40(12):1668-1677. doi: 10.2337/dc17-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aroda VR, Knowler WC, Crandall JP, et al. ; Diabetes Prevention Program Research Group . Metformin for diabetes prevention: insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia. 2017;60(9):1601-1611. doi: 10.1007/s00125-017-4361-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perreault L, Pan Q, Schroeder EB, et al. ; Diabetes Prevention Program Research Group . Regression from prediabetes to normal glucose regulation and prevalence of microvascular disease in the Diabetes Prevention Program Outcomes Study (DPPOS). Diabetes Care. 2019;42(9):1809-1815. doi: 10.2337/dc19-0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perreault L, Temprosa M, Mather KJ, et al. ; Diabetes Prevention Program Research Group . Regression from prediabetes to normal glucose regulation is associated with reduction in cardiovascular risk: results from the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2014;37(9):2622-2631. doi: 10.2337/dc14-0656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laiteerapong N, Karter AJ, Moffet HH, et al. Ten-year hemoglobin A1c trajectories and outcomes in type 2 diabetes mellitus: the Diabetes & Aging Study. J Diabetes Complications. 2017;31(1):94-100. doi: 10.1016/j.jdiacomp.2016.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heianza Y, Hara S, Arase Y, et al. HbA1c 5.7-6.4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet (London, England). 2011;378(9786):147-155. doi: 10.1016/S0140-6736(11)60472-8 [DOI] [PubMed] [Google Scholar]
- 26.de Abreu L, Holloway KL, Kotowicz MA, Pasco JA. Dysglycaemia and other predictors for progression or regression from impaired fasting glucose to diabetes or normoglycaemia. J Diabetes Res. 2015;2015:373762. doi: 10.1155/2015/373762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vistisen D, Kivimäki M, Perreault L, et al. Reversion from prediabetes to normoglycaemia and risk of cardiovascular disease and mortality: the Whitehall II cohort study. Diabetologia. 2019;62(8):1385-1390. doi: 10.1007/s00125-019-4895-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowler WC, Fowler SE, Hamman RF, et al. ; Diabetes Prevention Program Research Group . 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677-1686. doi: 10.1016/S0140-6736(09)61457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V; Indian Diabetes Prevention Programme (IDPP) . The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289-297. doi: 10.1007/s00125-005-0097-z [DOI] [PubMed] [Google Scholar]
- 31.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537-544. doi: 10.2337/diacare.20.4.537 [DOI] [PubMed] [Google Scholar]
- 32.Tuomilehto J, Lindström J, Eriksson JG, et al. ; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343-1350. doi: 10.1056/NEJM200105033441801 [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association . Prevention or delay of type 2 diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S32-S36. doi: 10.2337/dc20-S003 [DOI] [PubMed] [Google Scholar]
- 34.LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of diabetes in older adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1520-1574. doi: 10.1210/jc.2019-00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang M, Echouffo-Tcheugui JB, Selvin E. Clinical and public health implications of 2019 endocrine society guidelines for diagnosis of diabetes in older adults. Diabetes Care. 2020;43(7):1456-1461. doi: 10.2337/dc19-2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eTable 1. Baseline (Visit 5, 2011-2013) Characteristics of Participants by Attendance at Visit 6 (2016-2017)
eTable 2. Cumulative Incidence Over 6.5 Years (Maximum) of A1C-Defined and Impaired Fasting Glucose (IFG)-Defined Progression to Prediabetes, Total Diabetes, or Mortality, Overall and According to Age, Sex, and Race: The ARIC Study (2011-2017)
eTable 3. Incidence Rates and Adjusted Hazard Ratios (95% CIs) for Incident Total Diabetes, Incident Diagnosed Diabetes, and Mortality According to International Definitions of Prediabetes Status at Baseline in Older Adults: The ARIC Study (2011-2017)
eTable 4. Cumulative Incidence Over 6.5 Years Maximum and Hazard Ratios (95% CIs) for Incident Total Diabetes and Incident Diagnosed Diabetes With Inverse Probability of Attrition Weighting According to Prediabetes Status at Baseline in Older Adults: The ARIC Study (2011-2017)
eTable 5. Performance of Alternative International Definitions of Prediabetes in Older Adults for 6.5-Year Prediction of Incident Total Diabetes and Incident Diagnosed Diabetes: The ARIC Study, 2011-2017
eFigure 1. Participant Flow Chart
eFigure 2. Possible Outcomes According to Baseline Status Defined by A1C (Panel A) or Defined by Fasting Glucose (Panel B)
eFigure 3. Prevalence of Prediabetes in Older Adults in the ARIC Study According to International Expert Committee A1C 6.0-6.4% and World Health Organization Impaired Fasting Glucose (IFG, 110-125 mg/dL) Definitions