Abstract
Chronic myelomonocytic leukemia (CMML) is a rare clonal stem cell disorder associated with clinical and pathologic of myelodysplasia and myeloproliferation. Systemic autoimmune/inflammatory disorders (SAID) and polyserositis have been associated with CMML. These manifestations can be observed concomitantly, shortly before diagnosis or anytime along the course of illness. We report a case of myeloproliferative CMML who presented with polyserositis and positive serology for rheumatoid arthritis. Retrospective studies of myelodysplasia/CMML have reported 15% to 25% incidence of SAID. The most commonly observed disorders include systemic vasculitis, connective tissue diseases, polychondritis, seronegative arthritis, and immune thrombocytopenia. SAID does not confer adverse prognosis in retrospective studies. Polyserositis is less common; this may result from leukemic infiltrate or result from autoimmunity. Treatment of serositis includes steroids and cytoreductive agents. Serositis may confer poor prognosis and hypomethylating therapy may improve the outcome.
Keywords: CMML, polyserositis, autoimmunity
Introduction
Chronic myelomonocytic leukemia (CMML) is a clonal stem cell disorder characterized by persistent monocytosis along with myelodysplasia (MDS) and/or myeloproliferation, and hence is classified as MDS/myeloproliferation in the World Health Organization classification. Diagnostic criteria include persistent monocytosis (≥1 × 109/L), monocyte count of ≥10% of white blood cells count (WCC) in peripheral blood, absence of other myeloproliferative syndrome, and acute leukemia. CMML is classified as myeloproliferative when the total WCC is >13 × 109 L. CMML is further stratified based on peripheral blood (PB) and bone marrow (BM) blast percentage—CMML-0 (<2% in PB and < 5% in BM), CMML-1 (<5% in PB and BM <10%), and CMML-2 (PB 5% to 19% and BM 10% to 19%). CMML is associated with high risk of transformation to acute myeloid leukemia (AML; 15% to 30%). The 5-year risk of transformation to AML in CMML-2 was as high as 63% in CMML-2 compared with 18% in CMML-1. Prognosis worsens with increasing blast count even in less aggressive disease. Overall survival in CMML-0 was significantly better than CMML-1 (31 months vs 19 months). Clinical findings may include incidental finding of monocytosis, symptoms from cytopenias, constitutional symptoms, splenomegaly, extramedullary myelomonocytic infiltrates, and symptoms from associated autoimmune and inflammatory disorders.1-3 Systemic inflammation and/or autoimmune disorders are frequently observed in patients with MDS/CMML (10% to 30%). Patients with autoimmune disorders also carry a higher risk of myeloid disorders including MDS/AML, and this can be increased by use of immunosuppressive therapy with azathioprine. There is no clear association between SIADs (syndrome of inappropriate antidiuretic hormones) and other prognostic indicators. Presence of SIADs have no impact on survival.4-9 Polyserositis is much less common and presence of serositis may be associated with adverse prognosis. Occurrence of both of these may also influence choice of therapy.10-13
Case Report
A 65-year-old female was initially evaluated in the emergency room for right upper quadrant pain; imaging studies did not confirm cholecystitis. She was treated with parenteral narcotics in short stay. WCC was elevated at 12.4 × 109/L. Absolute monocyte count was also elevated at 3.6 × 109/L, hemoglobin was 11.8gm/dL, and platelets were adequate at 392 × 109/L. She was admitted 2 weeks later with dyspnea and chest discomfort. Cardiac workup, including coronary angiogram, was negative. Computed tomography scan revealed bilateral pleural effusions and pericardial effusion (Figure 1). WCC was further elevated at 33.4 × 109/L. Rheumatoid factor was positive (54.9 IU) and anti-CCP antibody was also positive. Pleural fluid was exudative and showed monocytic infiltrate. Flow cytometry of pleural fluid could not be performed due to lack of fresh specimen. Peripheral smear confirmed monocytosis, and bone marrow biopsy was consistent with CMML-2. Janus Kinase 2 mutation and BCR/ABL translocation and fluorescence in situ hybridization analysis did not reveal CHIC2 (cysteine rich hydrophobic domain) or FIP1L1 translocation with PDGFRA (platelet-derived growth factor-α) at 4Q12. Cytogenetic analysis revealed normal karyotype (46,XX; Figures 2 and 3). Symptoms from polyserositis including chest discomfort, dyspnea, and abdominal pain subsided spontaneously and the patient was discharged with minimal nausea on tapering dose of prednisone. On evaluation, 2 weeks later, the patient’s WCC was significantly elevated at 140 × 109/L with 78% monocytes. Hemoglobin was reduced at 7.2 mg/dL, and platelet count low at 36 × 109/L. After initiation of azacitidine, the patient’s WCC and platelet count normalized and hemoglobin improved to 11 mg/dL. Despite the observed improvement in hematologic parameters, bone marrow evaluation after 5 cycles revealed transformation to AML. Cytogenetic evaluation revealed 46XX deletion (9 q13-q 22) in 20 cells (Figure 4). Molecular studies revealed mutated nucleophosmin gene. The patient underwent remission induction, following which she was maintained on decitabine. Allogenic stem cell transplant was performed as a curative treatment; she continues in excellent remission. Posttransplant course was complicated by GVHD, which is presently well controlled off the immunosuppressants. The patient did not have any recurrence of serositis-related symptoms. Positive serology for rheumatoid arthritis was not associated with joint or extra-articular manifestations of rheumatoid arthritis.
Figure 1.
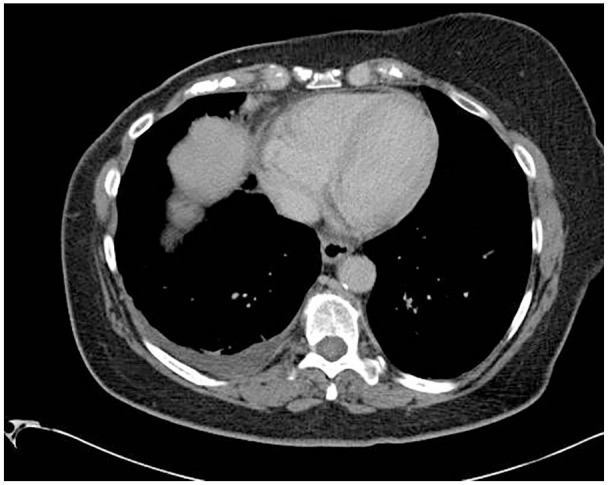
Computed tomography of the chest on day 1 with no acute findings.
Figure 2.
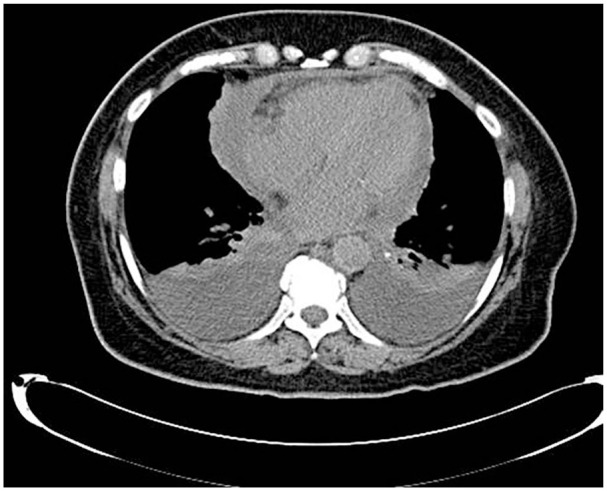
Computed tomography of the chest 2 weeks after showing polyserositis marked by bilateral pleural effusion and pericardial effusion.
Figure 3.

Peripheral smear showing increased monocytes and background neutrophils.
Figure 4.
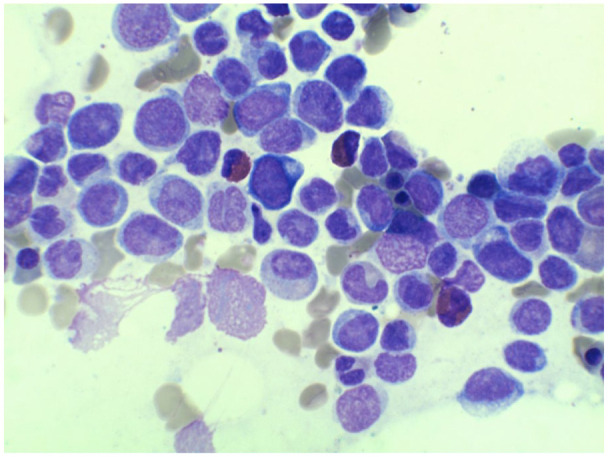
Bone marrow aspirate demonstrating increased atypical monocytes with a spectrum of maturation (including blast equivalent) and increased myeloblasts.
Discussion
Polyserositis in conjunction with monocytosis could result from a large number of disorders including malignancies (hematopoietic and nonhematopoietic), rheumatoid arthritis, systemic lupus erythematosus, chronic infections, and vasculitides. Peripheral smear evaluation is the first step in distinguishing reactive monocytosis from monocytic malignancies. Morphologic evaluation of maturity should distinguish monocytes from promonocytes, which are considered to be blast equivalents (Figure 5). Dysplasia should be assessed in neutrophils, as dyserythropoiesis may be observed in rheumatoid arthritis and other collagen vascular disorders. Flow cytometry does not reliably distinguish reactive monocytes from promonocytes. However, flow cytometry can differentiate reactive monocytosis characterized by aggregation of intermediate (CD14+/CD16+; MO2), nonclassical (CD14−/CD16+; MO3) from classical (CD14+/CD16−) monocytes (MO1). Classical monocyte (≥94%) reliably differentiates CMML from reactive moncytosis of monocytes in healthy individuals and predominates in CMML. Bone marrow biopsy is indicated for unexplained persistent monocytosis to rule out myeloid malignancies (AML, CMML). Cytogenetics and molecular genetics are integral parts of bone marrow evaluation.14-20
Figure 5.
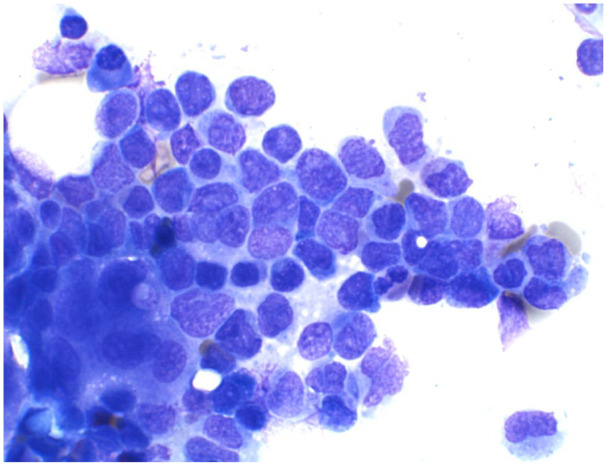
Bone marrow aspirate of acute myeloid leukemia with monocytic differentiation showing increased myeloblasts and blasts equivalents (promonocytes).
Retrospective studies have established a greater incidence of SIADs in patients with MDS/CMML compared with the general population and, in one study, compared with patients with chronic myeloid leukemia. Patients with autoimmune disease are at higher risk for development of myelodysplastic syndrome and AML; odds ratio of 1.5 increasing to 2.1 for patients who have autoimmune disease for 10 years or longer. In a large population of patients with autoimmune disorders, exposure to azathioprine was associated with 7-fold risk of myeloid neoplasm. Serologic abnormalities of rheumatoid arthritis were not associated with any articular or extra-articular manifestation of the disease in our patient. Serologic abnormalities of autoimmunity may be as high as 65% in MDS/CMML. Incidence of clinically apparent inflammatory/autoimmune disorders is 10% to 30%; autoimmune disorders can be observed concomitantly, precede the diagnosis, or occur anytime during the course of MDS/CMML. SIADs may exhibit all the classification criteria in the majority of patients, but complete criteria may be missing in a significant minority of patients. The most commonly observed disorders include systemic vasculitis, connective tissue diseases, polychondritis, seronegative arthritis, and immune thrombocytopenia. CMML was significantly associated with systemic vasculitis. Association between SIAD and other prognostic indicators is unclear; however, SIADs do not confer adverse prognosis in retrospective studies.1-9 The relationship between CMML and SIADs is not fully understood. Autoimmune/inflammatory disorders–associated chronic inflammation may be a contributing factor to the development of myeloid malignancies. Chronic inflammation is thought to lead to oncogenesis through recruitment of inflammatory cells with resultant production of reactive oxygen species, cytokines, chemokines, growth factors. Continual exposure to these inflammatory cells is thought to cause mutations, thus leading to activation of prosurvival and anti-apoptotic pathways. Chronic inflammation thus drives clonal selection, dominance, and independence, resulting in myeloid malignancies.21,22 Marrow failure is probably mediated by autoimmunity in some MDS subtypes, wherein immunosuppressive therapy has been successfully employed. Conversely, CMML may predispose patients to SIADS, through cytokine production by monocytes (interleukin (IL)-6, tumor necrosis factor-α, interferon regulatory factor-1) or by abnormality of immune population in MDS/CMML. CMML monocytes have been shown to be highly proinflammatory with upregulation of multiple inflammatory pathways including tumor necrosis factor, IL-6, and IL-17 signaling. TET2 (Tet methylcytosine dioxugenase 2) mutations are common in MDS/CMML and significantly associated with SIADs. TET enzymes modulate DNA hydroxy methylation converting 5-methylcytosine to 5-hydroxymethylcytosine, and hence promote DNA demethylation. TET are involved in normal innate and adaptive immunity, playing a critical role in establishing or maintaining immune tolerance. TET2 is highly expressed in T-helper subset, and probably modulates Th1 and Th17 cell differentiation. TET2 inhibited naive CD4 cells do not differentiation into Th1 and Th17, thereby suppressing autoimmunity. TET2 also mediates Foxp3 (forkhead box P3, a protein involved in immune system response) demethylation, which drives Treg (regulatory T cells) differentiation. Mutant TET2 cooperating with RhoA leads to aberrant CD4 proliferation and interruption of immune homeostasis. TET2 also regulates innate immunity and suppresses inflammation mediated by macrophages. TET2 loss is associated with increased expression of IL-1a, IL-6, and arginase-1 by macrophages.21,23-26
Polyserositis is less common and thus less well studied. Polyserositis can result from malignant infiltration or alternatively from inflammation. Serosal effusions are usually exudative. Exudative serosal effusion may demonstrate benign monocytic infiltration, demonstrated by CD16 expression arguing for an inflammatory etiology. We did not measure C-reactive protein; however, C-reactive protein elevation was consistent when evaluated, arguing for underlying inflammation as well.11-13 As discussed above, monocytes in CMML demonstrate upregulation of multiple inflammatory pathways. In addition, TET2 abnormality also results in increased cytokine production by macrophages.23-26 In contrast to autoimmune manifestations, serositis may be associated with poor prognosis.13
Serositis may resolve spontaneously without therapy. Our patient showed resolution of all her serositis-related symptoms other than mild nausea before the start of prednisone therapy. Treatment of serositis includes steroid therapy and cytoreductive agents to address the underlying CMML when indicated. Detailed risk stratification studies including mutational analysis need to be undertaken prior to initiation of therapy. This risk stratification in addition to patient associated factors such as age, performance status, and comorbidities determines the therapy. Patients with high-risk disease including myeloproliferative subtype and patients with excess blasts need to be started on cytoreductive therapy. Demethylating agents are preferred over intensive chemotherapy regimens. Allogeneic stem cell transplant is the only curative option; reduced intensity conditioning enables the use in patients up to 70 years of age without excess comorbidities and good performance status. Demethylating agents may be indicated for remission induction prior to stem cell transplant.1-3 Demethylating agents may be preferred treatment for patients with serositis even in the absence of aggressive prognostic indicators.13
Autoimmune and inflammatory disorders associated with MDS/CMML respond well to steroid therapy. However, steroid dependence remains a significant issue. Demethylating agents may enable dose reduction or discontinuation of steroids in this setting.2 In conclusion, serositis and SIADs are significant complications affecting the life of patients with MDS/CMML. In addition to symptom load from SIADs and serositis, they may affect choice of therapy as well.
Footnotes
Authors’ Note: This case has been presented as a poster presentation at the American College of Physicians Arkansas chapter in September 2017 at Little Rock, Arkansas, and also at the American College of Physicians National Conference in April 2018 at New Orleans, Louisiana.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal consent was obtained from the patient regarding publication of the case report.
ORCID iDs: Anthony Kunnumpurath  https://orcid.org/0000-0001-5091-3463
https://orcid.org/0000-0001-5091-3463
Sai Prasad Desikan  https://orcid.org/0000-0003-1157-6489
https://orcid.org/0000-0003-1157-6489
References
- 1. Benton CB, Nazha A, Pemmaraju N, Garcia-manero G. Chronic myelomonocytic leukemia: fore front of the field in 2015. Crit Rev Onc Hematol. 2015;95:222-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Solary E, Itzykson R. How I treat chronic myelomonocytic leukemia. Blood. 2017;130:126-136. [DOI] [PubMed] [Google Scholar]
- 3. Itzykson R, Fenaux P, Bowen D, et al. Diagnosis and treatment of chronic myelomonocytic leukemias in adults. Recommendations from the European Hematology Association and European Leukemia Net. Hemasphere. 2018;2:e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mekinian A, Grignano E, Braun T, et al. Systemic inflammatory and autoimmune manifestations associated with myelodysplatic syndromes and chronic myelomonocytic leukemia. A French multicenter retrospective study. Rheumatology (Oxford). 2016;55:291-300. [DOI] [PubMed] [Google Scholar]
- 5. Swedeh A, Patnaik M, Alfakara D, et al. Autoimmunity in patients with chronic myelomonocytic leukemia (CMML): a frequent finding. Blood. 2012;120:4930. [Google Scholar]
- 6. Peker D, Padron E, Bennett JM, et al. A close association of history of autoimmunity with chronic myelomonocytic leukemia (CMML) in contrast to chronic myeloid leukemia (CML). Paper presented at: 54th ASH Annual Meeting and Exposition; December 8-11, 2012; Atlanta, GA. [Google Scholar]
- 7. Grignano E, Mekinian A, Braun T, et al. Autoimmune and inflammatory diseases associated with chronic myelomonocytic leukemia: a series of 26 cases and literature review. Leuk Res. 2016;47:136-141. [DOI] [PubMed] [Google Scholar]
- 8. Jacobse J, Sijpkens YWJ, van’t Wout JW, et al. Vasculitis in myelodysplastic syndrome and chronic myelomonocytic leukemia: a report of two cases. J Hematol. 2018;7:158-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ertz-Archambault N, Kosiorek H, Taylor G, et al. Association of therapy for autoimmune disease with myelodysplastic syndrome and acute myeloid leukemia. JAMA Oncol. 2017;3:936-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manoharan A. Malignant pleural effusion in chronic myelomonocytic leukemia. Thorax. 1991;46:461-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Imataki O, Watanabe N, Matsumoto K, et al. Chronic myelomonocytic leukemia presenting with polyserositis due to an immune-mediated monocyte activation. Clin Case Rep. 2014;2:42-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bourantos KL, Tsiara S, Pantell A, Milionis C, Christou L. Pleural effusion in chronic myelomonocytic leukemia. Acta Hematol. 1998;99:34-37. [DOI] [PubMed] [Google Scholar]
- 13. Remy-Neris S, Willems L, Deau B, et al. Polyserositis in the course of chronic myelomonocytic leukemia: impact of hypomethylating agents. Cancer Res Front. 2016;2:126-130. [Google Scholar]
- 14. Lynch DT, Foucar K. Ask the hematopathologists: diagnostic approach to monocytosis. Hematologist. 2016;13:4. [Google Scholar]
- 15. Lynch DT, Hall J, Foucar K. How I investigate monocytosis. Int J Lab Hematol. 2018;40:107-114. [DOI] [PubMed] [Google Scholar]
- 16. Sin S, Lewin L, Aung TY, Maung MT, et al. Approach to the patient with monocytosis. IOSR J Dental Med Sci. 2015;14:81-86. [Google Scholar]
- 17. Saxena A, Bodhke S, Kulkarni AR, et al. Acute myeloid leukemia presenting as polyserositis and leukemia cutis. Clin Cancer Investig J. 2016;5:284-286. [Google Scholar]
- 18. Zampeli E, Skopouli FN, Moutsopoulos HM. Polyserositis in the course of systemic lupus erythematosus: a case of pseudo-pseudo Meigs syndrome. J Rheumatol. 2018;45:877-878. [DOI] [PubMed] [Google Scholar]
- 19. Arasaratnam K, Judge D, Bossingham D. Rheumatoid arthritis presenting with polyserositis and fever. J Clin Rheumatol. 2020;26:e105. doi: 10.1097/RHU.0000000000968 [DOI] [PubMed] [Google Scholar]
- 20. Kitagawa J, Hara T, Tsurumi H, Kanemura N, Oyama M, Moriwaki H. Abolishment of pleural effusion as the initial manifestation of chronic myelomonocytic leukemia without chemotherapy. J Clin Exp Hematop. 2008;48:75-76. [DOI] [PubMed] [Google Scholar]
- 21. Ambinder AJ, Miller J, DeZern AE. Autoimmune disease in CMML—the chicken or the egg? Best practice and research. Clin Haematol. 2020;33:101136. [DOI] [PubMed] [Google Scholar]
- 22. Savola P, Lundgren S, Keranen MA, et al. Clonal hematopoiesis in patients with rheumatoid arthritis. Blood Cancer J. 2018;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barrett JA, Sloand E. Autoimmune mechanisms in the pathophysiology of myelodysplastic syndromes and their clinical relevance. Haematologica. 2009;94:449-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Francinin A, Pomicter AD, Yan D, et al. The transcriptome of CMML monocytes is highly inflammatory and reflects leukemia-specific and age-related alterations. Blood Adv. 2019;20:2949-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oh YJ, Shin DY, Hwang SM, et al. TET2 mutation is significantly associated with development of autoimmune disorder in patients with myelodysplastic syndrome. Arthritis Rheumatol. 2017;69(suppl 10):2905. [Google Scholar]
- 26. Smith AE, Mohammedali AM, Kulasekararaj A, et al. Next-generation sequencing of TET2 gene in 355 MDS and CMML patients reveals low-abundance mutant clones, with early origins, but indicates no definite prognostic value. Blood. 2010; 116:3923-3932. [DOI] [PubMed] [Google Scholar]


