Abstract
Introduction:
The COVID-19 is a pandemic caused by SARS-CoV-2 which has infected over 74 million people, killing more than 1,600,000 million people around the world as of 17th December 2020. Accumulation of free radicals coupled by weakened antioxidant system leads to oxidative stress, which will further worsen respiratory diseases, COVID-19 inclusive. This study aimed to examine the levels of some antioxidants and oxidative stress markers in COVID-19 patients.
Methods:
This was a cross-sectional comparative study in which 50 COVID-19 symptomatic patients who were on admission at the COVID-19 isolation center in Jigawa, Northwestern Nigeria, were recruited. Twenty one (21) apparently healthy individuals were included as controls. Levels of antioxidant trace elements (Se, Zn, Mg, Cu and Cr), 8-isoprostaglandin F2 alpha and malondialdehyde in the plasma and erythrocytes activity of glutathione, glutathione peroxidase, superoxide dismutase and catalase were determined.
Results:
The plasma concentrations of vitamins A, C and E were significantly lower (p < 0.001) in COVID-19 patients than controls. Activities of glutathione, glutathione peroxidase, catalase and superoxide dismutase were lower in COVID-19 subjects than controls (p < 0.001). The concentrations of Se, Zn, Mg and Cu were significantly lower (p < 0.001; p = 0.039; p < 0.001; and p < 0.001), respectively, in COVID-19 patients than controls, while chromium showed no significant difference (p = 0.605). Oxidative stress marker, 8-isoprostaglandin F2 alpha, was significantly higher (p = 0.049), while malondialdehyde was lower (p < 0.001) in COVID-19 patients than controls.
Conclusion:
In conclusion, COVID-19 patients are prone to depleted levels of antioxidant substances due to their increase utilization in counterbalancing the negative effect of free radicals. Furthermore, COVID-19 infection with other comorbidities, such as malaria, hypertension and diabetes, are at higher risk of developing oxidative stress.
Keywords: COVID-19, antioxidants, free radicals, vitamin A, Nigeria
Introduction
The COVID-19 is a pandemic caused by SARS-CoV-2 which has infected over 74 million people, killing more than 1,600,000 million people globally as of 17th December 2020.1 Movement restrictions imposed by countries in order to flatten this curve has also pushed the world economy into a great depression, with an estimated heavy downturn of 5.2% in global gross domestic product (GDP), which is the biggest contraction since 1870, many countries are facing massive uncertainty and the likelihood of some of them going into recession.2 The disease, which emanate from China in 2019, is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a positive-sense single-stranded RNA virus3–5 and is taxonomically a member of the Betacoronavirus genus.4 Patients typically present with fever and cough;6 gastrointestinal tract manifestations such as diarrhea, vomiting and abdominal pain;7 and more recently, obesity is also shown to contribute to the increased morbidity in COVID-19 infections Other compounding factors are dyspnea, lymphopenia and higher plasma levels of cytokines (IL2, IL7 and IL10), GSCF, IP10, MCP1, MIP1A and interferon alpha (TNF-α).6,8
Antioxidants prevent or slow the damage to the cells caused by free radicals reactions. The neutralization activity of radical molecules by antioxidants is achieved through their scavenging power by stopping chain reactions, peroxide decomposition, metal-chelating and induction of antioxidant enzymes.9 Considerable interest has risen in the idea that oxidative stress (Os) is instrumental in the etiology of numerous human diseases. Os can arise through the increased production of reactive oxygen species (ROS) and/or because of a deficiency of antioxidant defenses and this may further worsen respiratory diseases (COVID-19 inclusive), especially when the level free radicals are high.9
Free radicals are natural by-product of aerobic cell metabolism that the body can normally handle, but in the presence of a secondary condition, such as COVID-19, the abnormally excessive level of radicals may contribute in the progression and pathogenesis of the disease due to depletion of antioxidants.10,11
As a general discussion to the issues and possible deficiency of antioxidants in COVID-19 patients, researchers ought to look at where there could be correlation between certain essential elements such as TEs, selenium (Se), zinc (Zn), copper (Cu) and manganese (Mn) in fighting COVID-19 or as dietary components or as supplementation in COVID-19 parenteral nutrition.12 Trace elements including Zn, Mn, Se and Cu play important roles in supporting the immune systems by acting as cofactors of antioxidant enzymes that protect the body from Os, also serving as component of many viral enzymes, proteases and polymerases that aid in viral infection prevention.12
This informed the need of assessing levels of antioxidants substances, trace elements, antioxidant enzymes as well as Os markers among the COVID-19 patients so that the result could incur a rather more robust and holistic approach in the fight against the devastating disease. This research, therefore, reports levels of antioxidants and Os markers among the COVID-19 patients managed in isolation center dedicated for COVID-19 patients in Jigawa, Northwestern region in Nigeria.
Materials and methods
Study design
This was a cross-sectional comparative study conducted in one of the isolation centers dedicated for COVID-19 patients in Jigawa, Northwestern Nigeria.
Study subjects
Study population comprises 50 consecutive patients who were diagnosed and admitted due to COVID-19 with or without comorbidity and 21 apparently healthy individuals. Only symptomatic patients were included and those on any antioxidant supplements were excluded.
Specimen collection
Five milliliters of blood were collected from the participants; 3 mL was delivered in lithium heparin container and the serum was obtained after centrifuging at 15,000 r/min for 5 min, remaining 2 mL of the blood was preserved in Eppendorf tube at −20°C for determination of Se, Zn, Mg, Cu and Cr, while the erythrocyte part was washed and centrifuged at 2000g for 15 min for assessment of glutathione (GSH) and glutathione peroxidase (GPx).
Molecular detection of COVID-19: SARS COV-2 N and ORF1ab genes were selected as amplification target regions. Specific primers and fluorescent probes were designed for the detection of 2019 Novel Coronavirus RNA in the specimens. The extraction of RNA was performed using Life River extraction kits and the protocols were observed accordingly. The RNA templates suspended in 1.5 mL DNase/RNase-free tube were used for the detection of N and ORF1ab genes. The master mix was prepared according to the manufacturer’s (DAAN gene) instruction as follows: 17 µL of solution A (ORF1a/N PCR reaction) and 3 µL of solution B (ORF1a/N PCR reaction).
Body mass index
Body mass index (BMI) was calculated by dividing weight in kg by the square of the height in meters of the participants (weight (kg) (m2)).
Analysis of antioxidant vitamins
Serum vitamins A, C and E levels were colorimetrically determined in accordance with method of Bassey et al.,13 Roe and Kuether,14 and Nield and Pearson,15 respectively.
Antioxidant enzymes determination
Serum levels of superoxide dismutase (SOD) and catalase were determined using enzyme-linked immunoassay (ELISA) purchased from Elabscience (14780 Memorial Drive Suite 216, Houston, Texas), while erythrocyte GPx activity was measured by commercial RANSEL kits (Randox Laboratories, Crumlin, Northern Ireland, UK) and erythrocyte-reduced GSH levels were assayed in accordance with method described by Beutler et al.16
Determination of trace elements
Serum zinc and selenium were colorimetrically determined according to the method of Johnson et al.17 and Gardiner et al.,18 respectively, while copper levels were measured using the Randox kit purchased from Randox Laboratories, Crumlin, Northern Ireland, UK—colorimetric copper (CU2340). The method of Ginder et al.19 was used accordingly for the assessment of manganese in the study subjects.
Determination of Os markers
ELISA technique and thiobarbituric acid (TBA) according to Nadigaret al.20 was used in the determination of 8-isoprostaglandin F2 alpha (8-iso-PGF2α) and malondialdehyde (MDA), respectively. For 8-iso-PGF2α assay, an antibody to 8-iso-PGF2α was incubated in pre-coated microtiter plate wells, treated samples and standards were mixed with an 8-iso PGF2α-horseradish peroxidase (HRP) conjugate and simultaneously added to the wells. Free 8-iso-PGF2α and 8-iso-PGF2α-HRP conjugate compete for binding to the antibody bound to the plate; the HRP activity resulted in color development after subsequent incubation and washing; and the color formed is proportional to the amount of 8-iso-PGF2α conjugate bound to the plate and inversely proportional to the amount of free 8-iso-PGF2α in the samples or standards.
Statistical analysis
The summary data were analyzed using IBM SPSS version 26. The results were presented as mean ± standard deviation. Student’s t-test was used to determine the differences in means between COVID-19 patients and control group; abnormally distributed data were analyzed using Mann–Whitney U test. Significance in difference was set at p < 0.05.
Duration of the study
The study lasted for about 3 months from April 2020 to June 2020.
Results
Socio-demographic characteristics
The mean (±standard deviation (SD)) age of COVID-19 patients and the control group in this study were 43.8 ± 13.8 and 35.8 ± 6.8 years, respectively. Male-to-female ratio of COVID-19 infected and control group were 2.33:1 and 1.1:1, respectively, and this constituted about 70% males and 30% females in COVID-19 infected subjects and 51% males and 49% females in controls group. BMIs of two groups were within normal limits. Majority (64%) of the COVID-19 patients had mild symptoms and ten (20%) patients presented with moderate symptoms, while eight (16%) patients were classified as severe cases. Comorbidities were present in 38% of patients, with malaria (24%) being the most common, followed by hypertension (8%) and diabetes (6%) (Table 1).
Table 1.
Socio-demographic characteristics of the study subjects.
| Parameters | Participants | |
|---|---|---|
| COVID-19 (n = 50) | Controls (n = 21) | |
| Mean age (year) | 43.8 ± 13.8 | 35.8 ± 6.8 |
| Gender | ||
| Males | 35 (70%) | 11 (52.4%) |
| Females | 15 (30%) | 10 (47.6%) |
| BMI (kg/m2) | 21.9 ± 2.2 | 21.1 ± 1.1 |
| Symptoms | ||
| Mild | 32 (64%) | 0 (0%) |
| Moderate | 10 (20%) | 0 (0%) |
| Severe | 08 (16%) | 0 (0%) |
| Comorbidities | ||
| None | 31 (62%) | 0 (0%) |
| Hypertension | 06 (12%) | 0 (0%) |
| Diabetes | 04 (8%) | 0 (0%) |
| Malaria | 09 (18%) | 0 (0%) |
BMI: body mass index; NA: not applicable; n: number of subjects.
An elevated antioxidant parameters with increased Os markers
Antioxidant vitamins A, C and E were determined among the study subjects and the values of those infected with COVID-19 are statistically lower than the controls. It was further noted that 74% and 64% of COVID-19 patients were deficient with vitamins C and E, respectively, while 58% deficiency of vitamin A was noted. Antioxidant enzymes measured in this study included GSH, GPx, SOD and catalase; the results showed that their serum levels were significantly different between COVID-19 and controls subjects. Accordingly, all the trace elements assayed, with the exception of chromium, were significantly lower when compared between two groups. MDA and 8-iso-PGF2α levels were measured as markers of free radicals production in COVID-19 patients and controls, both were significantly different (p = 0.049 and p < 0.0001), respectively (Table 2).
Table 2.
Plasma levels of antioxidants and oxidative stress markers among COVID-19 and control groups.
| Parameters | Participants | p value | ||
|---|---|---|---|---|
| COVID-19 (n = 50) | Controls (n = 21) | |||
| Antioxidant vitamins | Vitamin A (µg/dL) | 26.5 ± 2.3 | 28.0 ± 1.1 | <0.001 |
| Vitamin C (mg/dL) | 0.33 ± 0.43 | 0.44 ± 0.32 | <0.001 | |
| Vitamin E (mg/dL) | 0.63 ± 0.05 | 0.87 ± 0.06 | <0.001 | |
| Antioxidant enzymes | GSH (mg/gHb) | 2.1 ± 0.39 | 2.7 ± 0.24 | <0.001 |
| GPx (U/gHb) | 32.5 ± 2.3 | 38.5 ± 2.8 | <0.001 | |
| SOD (U/mL) | 1.73 ± 0.39 | 2.84 ± 0.38 | <0.001 | |
| Catalase (MU/L) | 112.5 ± 3.0 | 109.0 ± 4.3 | <0.001 | |
| Antioxidant elements | Manganese (mg/dL) | 1.64 ± 0.36 | 2.10 ± 0.35 | <0.001 |
| Zinc (µg/dL) | 58.1 ± 7.0 | 64.9 ± 6.2 | 0.390 | |
| Selenium (ng/dL) | 25.3 ± 2.4 | 29.1 ± 1.9 | 0.000 | |
| Copper (µg/dL) | 128.3 ± 7.7 | 136.5 ± 5.3 | <0.001 | |
| Chromium (mg/L) | 2.2 ± 0.40 | 2.2 ± 0.59 | 0.605 | |
| Oxidative stress markers | 8-iso-PGF2α (pg/mL) | 83.2 ± 7.2 | 54.6 ± 5.9 | 0.049 |
| MDA (mmol/L) | 4.9 ± 0.50 | 3.40 ± 0.21 | <0.001 | |
MDA: malondialdehyde; GSH: glutathione; SOD: superoxide dismutase.
Negative correlation with most antioxidants against Os marker 8-iso-PGF2α in COVID-19 patients
A Pearson product-moment correlation was conducted to examine the relationships between plasma levels of 8-iso-PGF2α and all the antioxidant parameters among COVID-19. The results revealed moderate negative correlation for vitamin A (r = −0.405, n = 50, p = 0.004), vitamin C (r = −0.605, n = 50, p = 0.004) and vitamin E (r = −0.486, n = 50, p < 0.001). Other moderate negative association between 8-iso-PGF2α in COVID-19 noted are zinc, SOD, catalase, Mn, Se and Cu. However, MDA was positively correlated (r = 0.488, n = 50, p = 0.341) with 8-iso-PGF2α (Table 3).
Table 3.
Pearson correlation coefficient test of antioxidants against 8-iso-PGF2α in COVID-19 patients.
| Parameters | Correlation (r) | p value |
|---|---|---|
| Vitamin A (µg/dL) | −0.405** | 0.004 |
| Vitamin C (mg/dL) | −0.605** | 0.004 |
| Vitamin E (mg/dL) | −0.486** | 0.001 |
| GSH (mg/gHb) | 0.044 | 0.763 |
| GPx (U/gHb) | −0.093 | 0.522 |
| SOD (U/mL) | −0.143* | 0.322 |
| Catalase (MU/L) | −0.464** | 0.001 |
| Manganese (mg/dL) | −0.315** | 0.026 |
| Zinc (µg/dL) | −0.391** | 0.005 |
| Selenium (ng/dL) | −0.011 | 0.448 |
| Copper (µg/dL) | −0.154 | 0.285 |
| Chromium(mg/L) | 0.188 | 0.190 |
| MDA (mmol/L) | 0.488 | 0.341 |
GSH: glutathione; SOD: superoxide dismutase
Decreased concentrations of antioxidant vitamins with higher levels of 8-iso-PGF2α in COVID-19 co-infected group compared with COVID-19 naïve
Lower levels of antioxidant parameters (vitamins A, C and E) was noted among COVID-19 co-infected with malaria, hypertension and diabetes compared with COVID-19 naïve. However, higher values of 8-iso-PGF2α were recorded in co-infected group compared with COVID-19 naïve (Table 4).
Table 4.
Plasma levels of antioxidants and oxidative stress markers among COVID-19 and control groups.
| Analytes | Comorbidities | |||
|---|---|---|---|---|
| Malaria | Hypertension | Diabetes | None | |
| Vitamin A (µg/dL) | 24.04 ± 1.40 | 24.65 ± 1.40 | 24.33 ± 0.83 | 27.90 ± 1.63 |
| Vitamin C (mg/dL) | 28.97 ± 2.55 | 27.58 ± 2.42 | 29.10 ± 1.82 | 35.88 ± 2.78 |
| Vitamin E (mg/dL) | 0.60 ± 0.05 | 0.59 ± 0.03 | 0.56 ± 0.05 | 0.65 ± 0.05 |
| 8-iso-PGF2α (pg/mL) | 88.00 ± 8.25 | 92.75 ± 5.13 | 90.80 ± 4.00 | 79.53 ± 4.24 |
8-iso-PGF2α: 8-isoprostaglandin F2 alpha; none: COVID-19 naïve.
Receiver operating characteristics to predict the sensitivity of some markers
Receiver operating characteristics (ROC) curves of vitamin E, SOD and zinc was determined to predict the specificity and sensitivity of these variables for deficiency of antioxidants and increased Os among COVID-19. This was done by plotting sensitivity (x-axis) against 1-specificity (y-axis). Accordingly, the area under the ROC curve (AUC) calculated for vitamin E, SOD and zinc are 0.840, 0.765 and 0.853, respectively (Figure 1).
Figure 1.
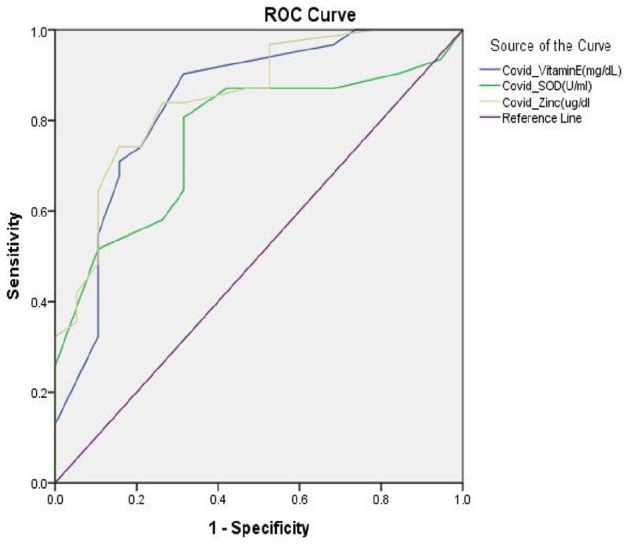
Receiver operating characteristics (ROC) curves of vitamin E, SOD and zinc among COVID-19 patients.
Discussion
This study examined the levels of antioxidants vitamins (vitamins A, C and E), enzymes (GSH, GPx SOD and Catalase), trace elements (manganese, zinc, selenium, cupper and chromium) and some Os markers (PGF2α and MDA) in COVID-19 patients and apparently healthy controls.
The effect of COVID 19 on plasma levels of vitamins A, C and E has been investigated by the current study. Vitamins C and E were significantly lower in COVID 19 patients when compared with healthy control. Vitamin A, however, only showed a slight decrease in COVID 19 patients compared to controls. These findings are similar to that of Care et al.,21 who reported extremely low levels of vitamin C in COVID-19 patients. This study also supports the report of Chris22 that antioxidants vitamins levels are reduced in SARS-COV-2 infection due to their scavenging effect on ROS. Shakoor et al.23 also reported the low levels of antioxidant vitamins in COVID 19 patients, and hence suggested that strategies aimed at improving the levels of these vitamins may be useful in these patients. These observed lower values of antioxidant vitamins in COVID 19 subjects may be due overproduction of ROS and a deprived antioxidant system. It is well recognized that infections increase Os by typically activating phagocytes which produce ROS.24 Vitamins C and E being renowned antioxidants and to some extent vitamin A scavenge for these ROS and counteract their effects. In this process of scavenging, the level of these vitamins in the plasma becomes depleted.25 Vitamins C and E are found to be helpful in cytokine storms and cellular injury which are the outcomes of SARS-COV-2 and other viral infections. Vitamins C and E act as strong antioxidants and help to scavenge the ROS, which is why Jan et al.26 regarded these vitamins as helpful in SARS-Cov-2 and other viral infections. The reduced level of these vitamins observed in this study is owing to their role in scavenging ROS as they are being used up in the process.
This study also assesses the levels of erythrocyte reduced GSH, erythrocytes GPx, plasma SOD and catalase activities in COVID-19 patients and controls. The levels of erythrocytes GSH, GPx catalase and plasma SOD were observed to be lower in COVID-19 patients when compared with controls. In a similar study conducted by Dworzanski et al.27 on patients with Epstein–Barr virus, decreased activities of SOD and GPx were reported. Strycharz-Dudziak28 also reported similar decreases in the activities of these antioxidant vitamins in patients with viral disease; however, our findings contradicts Strycharz-Dudziak28 report with higher levels of catalase in COVID-19 compared to control group. The study has also demonstrated that partial reduction of O2 in oxidative processes generates superoxide which is acted upon by SOD converting it to hydrogen peroxide (H2O2) which may subsequently react forming hydroxyls (OH) through the Fenton reaction. GSH/GPX and CAT are important antioxidant components in neutralizing these oxidative processes. Qin et al.29 reported that SARS-COV-2 infection activates the phagocytic cells which cause ROS to be excessively produced, while the antioxidant enzymes are insufficiently present leading to a weakened antioxidant system in the face of increased ROS production. The GPx/reductase system allows reduced GSH to bind to free radicals giving oxidized GSH which is regenerated into GSH through this system.22 Studies conducted by Derouiche30 and Strycharz-Dudziak28 suggested that SARS-COV-2, similar to other RNA viruses, can trigger an Os leading to a weakened antioxidant system. People who are elderly and those inflicted with hypertension, diabetes mellitus and cardiovascular diseases are known to be in a state of Os; infection with COVID-19 in these population leads to heightened Os overwhelming the body’s antioxidant mechanisms as shown by the decreased levels of SOD, GSH and GPx in this study.
The plasma concentrations of manganese, zinc, selenium, copper and chromium were also investigated in this study. The plasma concentrations of these antioxidant trace elements (manganese, zinc, selenium and copper) were significantly lower in COVID-19 patients compared to controls. The concentration of chromium, however, in COVID-19 patients were similar to that of controls. It is well established that zinc, magnesium, selenium and copper have important roles in supporting the innate and adaptive immune systems. In the study by Taheri et al.,31 the low plasma zinc level observed in this study is similar to the report of Yasui et al.,32 who worked on the relationship between serum zinc level and critical illness of COVID-19 in Japan. Zinc is reported32 to be a vital mineral during COVID-19 infection because of its immunomodulatory, antioxidant and antiviral properties. Kardos et al.33 noted that, being an important cofactor in the activity of ZnCu-SOD, zinc is a vital component of antioxidant defense system, low levels of which affects the system and increase the Os. Barazzoni et al.34 also noted low plasma levels of zinc in COVID-19 patients and reported that the severity of the disease is aggravated by zinc deficiency which reduces lymphocyte counts and impairs their function. It is therefore suggested by Barazzoni et al.34 that strategies aimed at improving plasma zinc level can also interfere with viral replication, protein synthesis and Os, providing beneficial and therapeutic effects against COVID-19. In a similar note, the plasma lower concentration of selenium found in this study may be supported by the work of Zhang et al.,35 who showed selenium level to be low in infections related to RNA viruses such as Coxsackievirus B3 and influenza A due to weakened host’s antioxidant system that was incapable of producing sufficient antioxidant seleno-proteins for its own protection. From the above, we can deduce that infection with SARS-COV-2, being an RNA virus, is capable of eliciting such weakened antioxidant response and rendering the virus more virulence. Kardos et al.33 reported that the deficiency in selenium in viral infections unmakes the immune system of the host and also causes Os that increases the risk of mutation in the viral genome making it more virulence. These studies (Kardos et al.,33 Jinsong et al.,36 Taheri et al.31 and Zhang et al.35) in conjunction with this study lead us to assume that there is an association between selenium concentration and COVID-19 outcome. It has been observed that the death rate of patients with COVID-19 in low selenium population is higher than in those with high selenium in China.36 In this study, we observed lower plasma Mn in COVID-19 patient compared to control. There is limited data regarding the level and relationship between COVID-19 and Mn. It is, however, suggested by Saleh37 that decreased serum Mn concentrations may weaken the antioxidant mechanism by reducing the activity of Mn-SOD, leading to more Os. Mn is an essential element required for several metalloenzymes, such as SOD and pyruvate decarboxylase, which are concerned with antioxidant defense system and energy production.37 From the above, it may be safe to assume that there exist a relationship between COVID-19 and Mn concentration and that this induces increased Os in these patients as observed by this study. This study observed the decrease in plasma Cu level in COVID-19 patients compared to controls. Taheri et al.31 reported that the level of serum Cu in COVID-19 patients has not been established. However, Cu is an essential trace element in the body and it is needed for protecting DNA from Os.38,39 Cu is required for enzymes such as CuZn-SOD which is the main part of antioxidant defense system.38 Since COVID-19 is demonstrated to alter and weaken the body’s antioxidant system from the current study, we can deduce that the associated increase in Os affects the serum Cu level. Kardos et al.33 reported that the human immune system response is weak when Cu is deficient. Low serum Cu, as observed in this study, has been associated with altered immune responses and an increased frequency of infections coupled with increased Os.31 Mechanistically, Zabetakis et al.40 reported that Cu deficiency can occur following chronic TNF-α-induced inflammation of the lungs. COVID-19 being a disease associated with spike in the levels of inflammatory cytokines which lead to inflammation of the lungs, can cause Cu deficiency. So, it seems that in managing critically ill COVID-19 patients, physicians should consider copper insufficiency.38 Chromium levels in this study were found to be similar in COVID-19 patients with controls. This finding differs with that of the studies of Zeng et al.,41 who reported higher levels of chromium in severe and deceased patients with COVID-19. This difference with this study, however, may be related to the fact that the population examined by Zeng et al.41 also suffered the comorbidity of diabetes and the immune dysfunction in COVID-19. So from our study, chromium level is unaffected by COVID-19 and here we advocate for more research since there is paucity of data to ascertain the relationship between chromium and COVID-19.
This study also investigated the plasma level of Os markers, PGF2α and MDA, in COVID-19 patients and controls. PGF2α concentration in the plasma of COVID-19 patients was significantly higher than that of controls. MDA, however, in COVID-19 patients, was significantly lower than in controls. As reported in this study, COVID-19 is associated with increased oxidative processes. The increased Os causes increased lipid peroxidation30 with subsequent release of intermediates, 8-iso-PGF2α.42 This agrees with the earlier work of Muhammad et al.43 which reported an elevated 8-iso-PGF2α and decreased Alphatocopherol in COVID-19 subjects co-infected with malaria. Malaria is one of the leading causes of morbidity and mortality in Nigeria, and co-infection with COVID-19 will negatively impact the disease outcome. This study also revealed that COVID-19 co-infected with malaria was the highest comorbidity recorded; this may be due to malaria being pandemic in the region coupled with fragile healthcare delivery and poor malaria control programs. In another study on factors which increase production of ROS such as malaria, Nour Eldin et al.44 reported increased lipid peroxidation and elevated level of 8-iso-PGF2α. Similarly, our research revealed significantly increased levels of 8-iso-PGF2α in COVID-19 co-infected with malaria compared to COVID-19 subjects without co-infection, lower levels of antioxidant vitamins were also noted among the co-infected group compared to COVID-19 naïve. Muhammad et al.43 documented overproduction of proinflammatory cytokines due to sequestration of malaria parasite in vital organs may be responsible for the depletion of antioxidant parameters and consequent increase in Os markers. Angel et al.45 and Aninagyei et al.46 documented that isoprostanes are produced from arachidonic acid metabolism bound to the cell membrane when attacked by free radical or ROS activity. Among the isoprostanes, 8-iso-PGF2α is a very useful analytical tool for the assessment of endogenous lipid peroxidation. Elevated levels of MDA in COVID-19 patients compared to control group reported in the present research suggest overproduction of free radicals which in turn destroys lipid membranes with subsequent formation of MDA and 8-iso-PGF2α as by-products. This study is limited by its small sample size of control group compared to COVID-19; however, it was due to lack of apparently healthy subjects willing to participate in the research as at the time of data collection and this may not give actual relationship between the variables, same issues transpired as such our study was not age and sex-matched. Furthermore, the calculated sample size was not applied due to the fact that only 50 positive COVID-19 patients were present in the isolation center all of which consented to participate. Therefore, larger cohort study in the region or Nigeria at large concerning subject matter can be used for consensus statements if the findings correspond to our results. Finally, lack of funds limit the number of important investigation such as nutritional assessments, which should have been done.
Conclusion
In conclusion, this present research evaluated plasma levels of antioxidant vitamins, enzymes and trace elements among COVID-19 patients compared with control group. From our findings, we can conclude that COVID-19 patients are prone to depleted levels of antioxidant substances due to their increased utilization in counterbalancing the negative effect of free radicals. Furthermore, COVID-19 infection with other comorbidities, such as malaria, hypertension and diabetes, are at higher risk of developing Os. In addition to the deficiency of antioxidants and increased Os in COVID-19, there is also a gap in understanding specific micronutrient deficient in COVID-19, which can distort body’s immune function while increasing susceptibility to other infectious disease.
Research Data
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, sj-sav-1-smo-10.1177_2050312121991246 for Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: A cross-sectional comparative study in Jigawa, Northwestern Nigeria by Yahaya Muhammad, Yamuna Aminu Kani, Sani Iliya, Jafaru Bunza Muhammad, Abubakar Binji, Abdurrahman El-Fulaty Ahmad, Muhd Bashir Kabir, Kabir Umar Bindawa and Armaya’u Ahmed in SAGE Open Medicine
Acknowledgments
The authors would like to thank all the participants consented to participate in this study and they would also like to appreciate all the staffs of the isolation center in Jigawa especially medical laboratory scientists as well as rapid response team for their maximum co-operation and contribution throughout the study period.
Footnotes
Author contributions: All authors contribute effectively in the development of the article; Y.M., Y.A.K., S.I. and M.B.K. designed the study, collected specimens and statistically analyzed the data. A.B., J.B.M., A.E.A., K.U.B. and A.Y.A. interpreted, discussed the findings and drafted the article. All authors reviewed and approved the article prior to submission
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: An ethical approval was waived by the HREC of Jigawa State department of infectious diseases, Rasheed Shekoni Specialist Hospital Dutse, as part of the isolation center policy to test patients on these parameters (Waiver No. RSSH/GEN/226/V.1/11). The research was carried out in accordance with the Declaration of Helsinki concerning the ethical principles for medical research involving human subjects.
Informed consent: A written informed consent for inclusion into the study was obtained from each of the patients before participation in the study. They were made aware that all the information obtained will be treated confidential and would be used for research purpose only.
ORCID iDs: Yahaya Muhammad  https://orcid.org/0000-0002-7873-6283
https://orcid.org/0000-0002-7873-6283
Sani Iliya  https://orcid.org/0000-0001-7685-3147
https://orcid.org/0000-0001-7685-3147
Abdurrahman El-Fulaty Ahmad  https://orcid.org/0000-0003-1941-8346
https://orcid.org/0000-0003-1941-8346
References
- 1. World Health Organization. Novel Coronavirus (2019-nCoV). WHO Bulletin, 2020, https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf
- 2. The World Bank. The global economic outlook during the COVID-19 pandemic: a changed world, 2020, https://www.worldbank.org/en/news/feature/2020/06/08/the-global-economic-outlook-during-the-covid-19-pandemic-a-changed-world
- 3. Khaerunnisa S, Kurniawan H, Awaluddin R, et al. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints 2020; 2020: 0266. [Google Scholar]
- 4. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579(7798): 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang S, Xia S, Ying T, et al. A novel coronavirus (2019-nCoV) causing pneumonia-associated respiratory syndrome. Cell Mol Immunol 2020; 17: 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kong R, Yang G, Xue R, et al. COVID-19 Docking Server: an interactive server for docking small molecules, peptides and antibodies against potential targets of COVID-19, 2020, https://arxiv.org/abs/2003.00163v1 [DOI] [PMC free article] [PubMed]
- 7. Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol 2020; 35: 744–748. [DOI] [PubMed] [Google Scholar]
- 8. Dietz W, Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity 2020; 28(6): 1005. [DOI] [PubMed] [Google Scholar]
- 9. Ntyonga-Pono MP. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment? Pan African Med J 2020; 35(Suppl. 2): 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arvinte C, Singh M, Marik PE. Serum levels of Vitamin C and Vitamin D in a cohort of critically Ill COVID-19 patients of a North American community hospital intensive care unit in May 2020: a pilot study. Med Drug Discov 2020; 8: 100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weir EK, Thenappan T, Bhargava M, et al. Does vitamin D deficiency increase the severity of COVID-19. Clin Med 2020; 20(4): e107–e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Te Velthuis AJ, van den Worm SH, Sims AC, et al. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog 2010; 6: e1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bassey OA, Lowry OH, Brook MJ, et al. The determination of vitamin A and carotene in small quantities of blood serum. J Biol Chem 1964; 3: 166–170. [PubMed] [Google Scholar]
- 14. Reo JH, Kuether CA. Determination of ascorbic acid in whole blood and urine through the 2, 4-dinitrophenylhydrazinederivate of ascorbic acid. J Biochem 1943; 6: 1009–1012. [Google Scholar]
- 15. Neil JH, Pearson WN. Macro and micro method of determination of serum vitamin E using triflouroacetic acid. Nutr 1967; 79: 10–20. [DOI] [PubMed] [Google Scholar]
- 16. Beutler E, Duron O, Kelly BM. The improved method for the determination of blood glutathione. J Lab Clin Med 1963; 61: 882–888. [PubMed] [Google Scholar]
- 17. Johnson DJ, Djuh YY, Bruton J, et al. Improved colorimetric determination of serum zinc. Clin Chem 1977; 23(7): 1321–1323. [PubMed] [Google Scholar]
- 18. Gardiner PHE, Littlejohn D, Halls DJ, et al. Direct determination of selenium in human blood serum and plasma by electro thermal atomic absorption spectrometry. Trace Elem Med Biol J 1995; 9(2): 74–81. [DOI] [PubMed] [Google Scholar]
- 19. Ginder EM, Heth DA. Colorimeter determination with bound “Calmagite” of magnesium in human bloodserum. Clin Chem 1971; 17: 662. [Google Scholar]
- 20. Nadiger HA, Marcus SR, Chandrakala MV, et al. Malondialdehyde levels in different organs of rats subjected to acute alcohol toxicity. IJCB 1986; 1: 133–136. [Google Scholar]
- 21. Care C, Chiscano-Camón L, Ruiz-Rodriguez JC, et al. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit Care 2020; 24: 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chris P. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment. PAMJ 2020; 35(Supp2): 12–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shakoor H, Feehan J, Al AS, et al. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas 2020; 143: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Derbyshire E, Delange J. COVID-19: is there a role for immunonutrition, particularly in the over 65s? BMJ Nutr Prevent Health 2020; 3: 071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Delgado-Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res 2020; 51(5): 384–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jan H, Usman H, Zainab R. COVID-19: a brief overview on the role of vitamins specifically vitamin C as immune modulators and in prevention and treatment of SARS-Cov-2 infections. Biomed J Sci Tech Res 2020; 28(3): 21580–21586. [Google Scholar]
- 27. Dworzanski J, Strycharz-Dudziak M, Kliszczewska E, et al. Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in patients with diabetes mellitus type 2 infected with Epstein-Barr virus. PLoS ONE 2020; 15(3): e0230374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strycharz-dudziak M. Total antioxidant status (TAS), superoxide dismutase (SOD), and glutathione peroxidase (GPx) in oropharyngeal cancer associated with EBV infection. Viruses 2019; 2019: 5832410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qin M, Cao Z, Wen J, et al. An antioxidant enzyme therapeutic for COVID-19, 2020, https://www.biorxiv.org/content/10.1101/2020.07.15.205211v1 [DOI] [PubMed]
- 30. Derouiche S. Oxidative stress associated with SARS-Cov-2 (COVID-19) increases the severity of the lung disease—a systematic review. J Infect Dis Epidemol 2020; 6(3): 1–6. [Google Scholar]
- 31. Taheri M, Bahrami A, Habibi P, et al. A review on the serum electrolytes and trace elements role in the pathophysiology of COVID-19. Biol Trace Elem Res 2020; 8: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yasui Y, Yasui H, Suzuki K, et al. Analysis of the predictive factors for a critical illness of COVID-19 during treatment—relationship between serum zinc level and critical illness of COVID-19. Int J Infect Dis 2020; 100: 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kardos J, Héja L, Simon Á, et al. Copper signalling: causes and consequences. Cell Commun Signal 2018; 16(1): 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barazzoni R, Bischoff SC, Krznaric Z, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr 2020: 1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang J, Taylor EW, Bennett K, et al. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr 2020; 111(6: 1297–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jinsong Z, Ethan WT, Kate B, et al. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr 2020; 111: 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saleh SAK. Serum levels of selenium, zinc, copper, manganese, and iron in prostate cancer patients. Curr Urol 2020; 14(1): 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fooladi S, Matin S, Mahmoodpoor A. Copper as a potential adjunct therapy for critically ill COVID-19 patients. Clin Nutr ESPEN 2020; 40: 90–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raha S, Mallick R, Basak S, et al. Is copper beneficial for COVID-19 patients? Med Hypotheses 2020; 142: 109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zabetakis I, Lordan R, Norton C. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients 2020; 12: 1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeng H, Yang Q, Yuan P, et al. Associations of blood essential and toxic metal(loid)s with both disease severity and mortality in patients with COVID-19: a retrospective study. Res Square 2020; 1: 1–19. [Google Scholar]
- 42. Dhama K, Latheef SK, Dadar M, et al. Biomarkers in stress related diseases /disorders: diagnostic, prognostic, and therapeutic values. Front Mol Biosci 2019; 6: 00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Muhammad Y, Aminu YK, Ahmad AE, et al. An elevated 8-isoprostaglandin F2 alpha (8-iso-PGF2α) in COVID-19 subjects co-infected with malaria. Pan Afr Med J 2020; 37: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nour Eldin EEM, Nour Eldein MM, El-Readi MZ, et al. Evaluation of the diagnostic and predicative values of 8-iso-prostaglandin F2α as a biomarker of breast cancer. Oncol Res Treat 2020; 43(10): 506–517. [DOI] [PubMed] [Google Scholar]
- 45. Angel BPL, Jadoon S, Malik A. A comprehensive review article on isoprostanes as biological markers. Biochem Pharmacol 2018; 7: 2. [Google Scholar]
- 46. Aninagyei E, Tetteh ET, Banini J, et al. Evaluation of haemato-biochemical parameters and serum levels of 8-iso-prostaglandin F2α oxidative stress biomarker in sickle cell-malaria comorbidity. J Appl Microb Res 2019; 2(1): 32–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, sj-sav-1-smo-10.1177_2050312121991246 for Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: A cross-sectional comparative study in Jigawa, Northwestern Nigeria by Yahaya Muhammad, Yamuna Aminu Kani, Sani Iliya, Jafaru Bunza Muhammad, Abubakar Binji, Abdurrahman El-Fulaty Ahmad, Muhd Bashir Kabir, Kabir Umar Bindawa and Armaya’u Ahmed in SAGE Open Medicine


