Abstract
Low-temperature hydrothermal epitaxial growth and topochemical conversion (TC) reactions offer unexploited possibilities for the morphological engineering of heterostructural and non-equilibrium shape (photo)catalyst particles. The hydrothermal epitaxial growth of SrTiO3 on Bi4Ti3O12 platelets is studied as a new route for the formation of novel nanoheterostructural SrTiO3/Bi4Ti3O12 platelets at an intermediate stage or (100)-oriented mesocrystalline SrTiO3 nanoplatelets at the completed stage of the TC reaction. The Bi4Ti3O12 platelets act as a source of Ti(OH)62– species and, at the same time, as a substrate for the epitaxial growth of SrTiO3. The dissolution of the Bi4Ti3O12 platelets proceeds faster from the lateral direction, whereas the epitaxial growth of SrTiO3 occurs on both bismuth-oxide-terminated basal surface planes of the Bi4Ti3O12 platelets. In the progress of the TC reaction, the Bi4Ti3O12 platelet is replaced from the lateral ends toward the interior by SrTiO3, while Bi4Ti3O12 is preserved in the core of the heterostructural platelet. Without any support from noble-metal doping or cocatalysts, the SrTiO3/Bi4Ti3O12 platelets show stable and 15 times higher photocatalytic H2 production (1265 μmol·g–1·h–1; solar-to-hydrogen (STH) efficiency = 0.19%) than commercial SrTiO3 nanopowders (81 μmol·g–1·h–1; STH = 0.012%) in pH-neutral water/methanol solutions. A plausible Z scheme is proposed to describe the charge-transfer mechanism during the photocatalysis.
Keywords: hydrothermal epitaxial growth, topochemical conversion, perovskites, SrTiO3, Aurivillius-phase layer structures, Bi4Ti3O12, hydrogen evolution
1. Introduction
Utilizing sunlight to drive chemical reactions over semiconductor photocatalysts represents a promising strategy to overcome the world’s problems related to energy shortages and environmental pollution. The production of storable, green H2 fuel from water-splitting reactions addresses these challenges.1−6 At the moment, the process still suffers from too-low efficiencies to become economically viable. However, recent findings about the importance of heterojunctions,3 mesocrystallinity,6 type of exposed facets,2 and preferential orientation7 for improved photocatalytic performance increasingly promote an interest in the morphological engineering of functional nanostructures. In particular, the integration of two different functional materials with different band gaps and band-edge positions has attracted a great deal of scientific and technological attention.3,8 Such heterojunction systems lead to an improvement of the photocatalytic efficiency by enhancing the photogenerated charge carriers’ separation.3 However, designing heterostructural photocatalysts in terms of achieving the target characteristics and boosted photocatalytic performance remains challenging. The same is true for the creation of single-phase photocatalyst particles with morphologies that are different from the thermodynamic equilibrium crystal shape. The engineering of the functional characteristics of the particles based on an understanding of the nucleation and growth can ensure that rational morphological design prevails over serendipity.
Topochemical conversion (TC) reactions from Aurivillius perovskite platelets (Bi4Ti3O12 and MBi4Ti4O15 (M = Sr, Ba)) in molten salts (NaCl/KCl) were intensively studied for the preparation of MTiO3 perovskites with non-equilibrium, platelet-shape crystallites.9−12 However, slow ionic diffusion in the solid-state lattice at much lower hydrothermal synthesis temperatures (100–200 °C) provides a better insight and understanding of the reactions at the interface compared to that in molten salt TC.9,13 Hydrothermal TC reactions initiated by the epitaxial growth of a new phase on the precursor (template) particles enable the formation of heterostructures at an intermediate state of the transformation or after complete conversion with the formation of new-phase particles with a preserved morphology and having a crystallographic relationship with the parent phase.13−15 In the field of hydrothermal TC reactions, Kalyani et al. performed an in-depth study of the epitaxial growth of SrTiO3 on anatase (TiO2) nanowires and their complete TC to SrTiO3 mesocrystalline nanowires.14 Exploring the TC reaction mechanisms for various template precursors will help us to engineer more complex heterostructures in the future.
In this study, we present an example of employing the TC reaction concept in the rational design of new heterostructural and mesocrystalline nanoparticles under hydrothermal conditions. We have studied the hydrothermal epitaxial growth of SrTiO3 on Bi4Ti3O12 template platelets with the intermediate formation of new nanoheterostructural SrTiO3/Bi4Ti3O12 platelets and after the complete transformation formation of (100)-oriented SrTiO3 mesocrystalline nanoplatelets.
One of the reasons for the selection of the SrTiO3/Bi4Ti3O12 heterostructure was the interesting photocatalytic properties of the individual materials.2,5,7,16 SrTiO3 meets the thermodynamic criteria for an overall photocatalytic water-splitting reaction in terms of the appropriate band-edge positions and bandgap.3,7,17 Several efficient H2-evolution photocatalysts based on SrTiO3 were developed using various design strategies, aiming to improve the light-harvesting capabilities and the charge-carrier separation.2,7,18−24 For example, Zhang et al. prepared (100)-oriented SrTiO3 mesocrystalline superstructural platelets by hydrothermal topotactic epitaxy from a TiO2 mesocrystalline precursor template that consisted of assembled anatase nanocrystals with a dominant exposure of [001] facets.7 The authors proved that these (100)-oriented SrTiO3 platelets exhibited 3 times higher photocatalytic efficiency for H2 evolution compared to conventional disordered SrTiO3 systems. The abovementioned study revives the interest in further photocatalytic investigations of SrTiO3 nanostructures with controlled morphologies and orientations. Similar to SrTiO3, Bi4Ti3O12 was also explored as a photocatalyst for H2 generation. However, according to several reports, pure Bi4Ti3O12 does not exhibit an outstanding H2 evolution activity,16,25 although modifications such as reduction (Bi4Ti3O12–x)16 and substitution with Cr (Bi4Ti3–xCrxO12)25−27 were confirmed to enhance considerably visible-light photocatalytic H2 evolution from water/methanol solutions (2–4 times). For both types of modified Bi4Ti3O12, the improvement was ascribed to the narrowing of the band gap and the decreased recombination of the photogenerated charges.16,25
The other motivation for choosing the SrTiO3/Bi4Ti3O12 system is related to the presence of similar perovskite units in both phases,28 which promises the successful epitaxial growth of SrTiO3 on Bi4Ti3O12. The first attempt to prepare Bi4Ti3O12/SrTiO3 composite microplatelets was made by Zhao et al.,29 who also used a combination of molten-salt-synthesized Bi4Ti3O12 platelets and alkaline hydrothermal conditions for the growth of SrTiO3. However, their Bi4Ti3O12 platelets were larger (side length: 10–15 μm, 1–2 μm in this study). The major difference was in the hydrothermal step where the titanium precursor (tetrabutyl titanate) was added for the formation of SrTiO3 where the titanium was not proposed to originate from the dissolution of Bi4Ti3O12, as in our study. Accordingly, the reported morphology of the 10–15 μm Bi4Ti3O12 platelets with sub-micrometer attachments29 was completely different from the SrTiO3/Bi4Ti3O12 heterostructural platelets described in this study. Therefore, we believe that the presented SrTiO3/Bi4Ti3O12 composite nanostructures are unique and their functional properties are worth investigating. Our study is focused on a detailed microstructural examination of the platelets at different stages of TC in order to understand the SrTiO3/Bi4Ti3O12 interface and gain a detailed insight into the mechanism of hydrothermal epitaxial growth and the TC reaction. The epitaxial growth of SrTiO3 on a layered structure of Bi4Ti3O12 is expected to be more complex and illustrative than the epitaxial growth on mono metal oxides. Moreover, the anisotropic shape of the primary Bi4Ti3O12 platelets with different dissolution rates of the basal and lateral surfaces is an interesting characteristic of this system that also influences the morphological evolution. The described TC mechanism provides general guidelines for the morphological engineering of nanoheterostructures through hydrothermal epitaxial growth. The study emphasizes the key parameters that must be considered for the selection of a heterostructural system, which include the structural matching at the interface, the thermodynamic stability and solubility of the involved materials, and the supersaturation. A light-induced, good, and stable H2 production rate from a pH-neutral solution established these novel, noble-metal-free SrTiO3/Bi4Ti3O12 nanoheterostructural platelets as promising candidates in the field of photocatalytic H2 evolution.
2. Experimental Section
2.1. Synthesis Conditions
2.1.1. Chemicals and Materials
All the chemicals were of analytical grade and were used as received without further purification. In the syntheses, the following reagents were involved: KCl (Sigma-Aldrich, ≥99.0%), NaCl (Merck, ≥99.7%), TiO2 (P25, Degussa), Bi2O3 nanopowder (Sigma Aldrich, 99.8%), HNO3 (VWR, 68%), SrCl2·6H2O (Sigma-Aldrich, ≥99.0%), NaOH (Fisher Chemicals, ≥98.7%). Water used for the study was purified with a system to produce ultrapure water (Purelab Option-Q7, ELGA). Commercially available SrTiO3 nanopowder was used as a reference photocatalyst (Sigma-Aldrich, 20–40 nm).
2.1.2. Synthesis of Bi4Ti3O12 Template Platelets
Bi4Ti3O12 platelets were synthesized in molten KCl/NaCl salt using Bi2O3 (1.9453 g) and TiO2 (0.5 g) nanopowders as the starting materials. To synthesize the Bi4Ti3O12 platelets via the molten-salt route, the molar ratio of NaCl:KCl:Bi2O3:TiO2 was optimized as 50:50:2:3. The synthesis was performed at 800 °C with a holding time of 2 h with heating and cooling rates of 10 °C/min. Details of the procedure and selected parameters are described elsewhere.30 After the synthesis, the Bi4Ti3O12 platelets were separated from the salt by washing with ultrapure water. To ensure the complete removal of any surface contamination,12 the platelets were soaked in 2-M HNO3 for a short time (5 min) and washed again with ultrapure water. The product particles were freeze-dried to obtain a powder with well-separated platelets.
2.1.3. Hydrothermal TC of Bi4Ti3O12 to SrTiO3
The TC reaction of the Bi4Ti3O12 template particles to SrTiO3 platelets was carried out via the hydrothermal route. Bi4Ti3O12 particles were used as templates for the non-equilibrium plate-like growth of SrTiO3 and as well as a source of Ti, whereas SrCl2·6H2O was the source of strontium. A large excess of SrCl2·6H2O was used to ensure supersaturation conditions and promote the nucleation of SrTiO3 on both basal surface planes of the Bi4Ti3O12 platelets. First, SrCl2·6H2O was dissolved in ultrapure water, and then Bi4Ti3O12 platelets were admixed to the solution in an amount corresponding to the Sr:Ti molar ratio of 12:1. Suspensions were sonicated for 25 min followed by the addition of NaOH solutions. In the precursor suspension before the hydrothermal reaction, the concentrations of the SrCl2·6H2O, Bi4Ti3O12, and NaOH platelets were 0.0388, 0.00107, and 6 M, respectively. The hydrothermal syntheses were performed by stirring at 200 °C in a Berghof high-pressure reactor using a Teflon (PTFE) insert. The reaction time was varied from 1 to 15 h. After the hydrothermal synthesis, the product particles were separated from the reaction solution by centrifugation and washed several times with ultrapure water. The solid product was soaked in 1 M HNO3 for 5 min to remove the side products, and afterward, the particles were again repeatedly washed with ultrapure water to completely remove any traces of acid. At the end, the particles were freeze-dried to obtain the final product.
2.2. Characterization of the Samples
X-ray powder diffraction was employed using a Bruker AXS D4 Endeavor with Cu Kα radiation (1.5406 Å) for the powder samples and for the platelets cast on the Si monocrystalline substrate. The weight ratio of SrTiO3:Bi4Ti3O12 in the heterostructural platelet was estimated from the calibration curve, which was produced from XRD measurements of the mixtures of SrTiO3 and Bi4Ti3O12 platelets in various weight ratios. These XRD measurements were performed for preferentially oriented platelets cast on the Si monocrystalline substrate.
A field-emission scanning electron microscope (FE-SEM, JSM 7600 F, JEOL, Japan) was used to observe the morphology of the particles. A nanoscale analysis of the platelets was performed using a 200 kV scanning transmission electron microscope (STEM, Jeol ARM 200 CF, JEOL, Japan) equipped with an energy-dispersive X-ray spectrometer (EDXS, Jeol Centurio 100). Samples of platelet-like particles for the STEM analyses were prepared using two approaches. For observations along the shorter zone axis of the platelets, the powdered sample was sonicated in absolute ethanol and a droplet of the suspension was applied to the lacey, carbon-coated copper grid. This resulted in a spontaneous alignment along the preferential orientation with the largest surface parallel to the carbon film substrate. The thickness of the platelets of up to 100 nm allowed STEM analyses without any further thinning. For edge-on observations of the platelet-like particles with a side length between 1 and 2 microns, the particles had to be thinned to electron transparency. This was accomplished by embedding the powders in epoxy resin and further mechanical and ion milling (Gatan PIPS Model 691, USA).
The Brunauer–Emmett–Teller (BET) surface areas of the powders were measured by nitrogen adsorption with a Micromeritics Gemini II 2370 nitrogen-adsorption apparatus (Norcross, GA).
Band-gap energies of the synthesized platelets were determined from their diffuse reflection spectra with BaSO4 as a reference. The measurements in the ultraviolet and visible (UV–vis) spectral ranges were performed with an integrating sphere and a UV–vis spectrophotometer (Shimadzu UV-3600, Tokyo, Japan). Photoluminescence (PL) spectra of the samples were recorded using a Synergy H1 microplate reader with monochromator optics (Bio-Tek, U.S.A.) at an excitation wavelength of 320 nm.
2.2.1. Photocatalytic H2 Evolution
The photocatalytic H2 evolution measurements were carried out in a 50 mL quartz round-bottom flask at ambient temperature and atmospheric pressure using mixing to achieve the particle suspension. A commercial solar simulator equipped with a Xenon arc lamp (300 W, Newport) and an AM 1.5G filter was used as the light source. In a typical photocatalytic measurement, 20 mg of photocatalyst was suspended in 40 mL of aqueous solution containing 25 vol % methanol and the suspension was sonicated for 30 min to obtain a well-dispersed particle suspension. Before light irradiation, the quartz flask was sealed with a rubber septum and purged with a nitrogen flow for 40 min to remove the excess oxygen in the reaction mixture. Finally, the sealed quartz flask was placed under light irradiation. All the photocatalysts were subjected to 4 h of light irradiation, and the H2 evolution was measured periodically every hour. The generated gas composition (1 mL) was analyzed with a gas chromatograph (GC, SRI-8610C) equipped with a thermal conductivity detector (TCD), and high-purity nitrogen was used as the carrier gas.
3. Results and Discussion
The transformation of Bi4Ti3O12 into SrTiO3 under hydrothermal conditions is governed by the chemistry at the interface and the concentrations of the dissolved titanium and strontium species (supersaturation). In this particular TC reaction, the Bi4Ti3O12 platelets act as a source of titanium and as the substrate for the epitaxial growth of SrTiO3. To control and direct the growth of SrTiO3 on the surface of the Bi4Ti3O12 platelets, the characteristics of the latter must be studied first.
3.1. Structural Studies of Bi4Ti3O12 Template Platelets
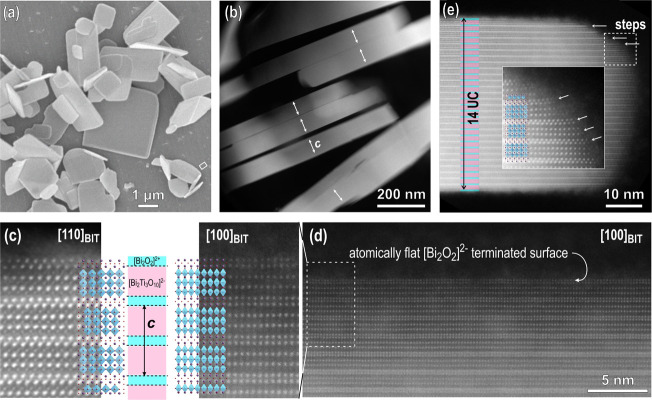
In the first step of our investigations, we characterized the Bi4Ti3O12 platelets down to the atomic scale. Knowledge about the morphology, the termination of the Bi4Ti3O12 crystallites, and the nature of the surface after applying different treatment procedures following the synthesis in molten salt (and after washing with water or acid) is important for selecting the optimum strategy for the treatment of the synthesized Bi4Ti3O12 powders and gives fundamental knowledge for steering and understanding the heterogeneous nucleation of SrTiO3 on Bi4Ti3O12 templates. Orthorhombic Bi4Ti3O12 platelets grown in NaCl/KCl molten salt at 800 °C for 2 h are shown in Figure 1a. The crystallites have a typical tabular or platelet-like morphology with a side length of up to a few microns and a thickness of well below a micron.30 The platelet-like morphology represents the equilibrium shape of Bi4Ti3O12 reflecting its layered crystal structure. High-angle annular dark field (HAADF)–STEM analysis of the Bi4Ti3O12 platelets after the removal of the residual salt by soaking in water and additionally in 2 M HNO3 for a short time is shown in Figure 1b–e. A low-magnification image of edge-on-oriented Bi4Ti3O12 platelets (Figure 1b) shows that most of the crystallites have a thickness below 100 nm (1 unit cell = 3.2882 nm; PDF: 01-084-6889). The morphology of the Bi4Ti3O12 platelets indicates a significantly faster growth in the direction of the layers and a slower thickening perpendicular to the layers, typical for minerals with a layered structure.
Figure 1.
(a) SEM image of Bi4Ti3O12 template particles. (b) Low-magnification HAADF-STEM image of edge-on-oriented Bi4Ti3O12 platelets. Arrows denote the c axes. (c) Atomic-resolution Z-contrast images of platelets in the [100] Bi4Ti3O12 and [110] Bi4Ti3O12 orientations taken near the surface of the particles with overlaid structural models. The platelets are terminated by the [Bi2O2]2+ layer. (d) Atomically flat (smooth) surface of the platelets as a result of layer-by-layer growth in molten salt. (e) Edge of a platelet with a thickness of 14 unit cells (UCs) (+ an additional [Bi2O2]2+ layer) with steps. The magnified region shows weakly bonded adatoms on the exposed surface on the lateral side of the platelet.
Atomic-resolution STEM was used to investigate the termination of the platelets. Figure 1c shows HAADF-STEM images of Bi4Ti3O12 platelets oriented along the [100] Bi4Ti3O12 and [110] Bi4Ti3O12 zone axes. The experimental images are overlaid with an atomic model of Bi4Ti3O12 (PDF: 01-084-6889) showing the layered structure composed of [Bi2O2]2+ sheets and pseudo-perovskite [Bi2Ti3O10]2– blocks. Both images were recorded near the surface of the platelets and reveal that the crystallites are terminated by [Bi2O2]2+ sheets. Several crystallites were examined and the analyses confirmed that all the Bi4Ti3O12 platelets have this termination on both basal-plane surfaces. Observations of the crystallites at lower magnifications also showed that the basal-plane surface of the Bi4Ti3O12 platelets is atomically flat on a large scale (Figure 1d), perhaps even across the whole crystallite since the presence of steps and terraces on the basal surface were never observed in the TEM. In contrast to the basal-plane surfaces, the lateral surfaces of the crystallites contain growth steps where the TiO6 octahedra are exposed (Figure 1e). HAADF-STEM images recorded at the edge of the crystallite also imply weaker bonding of the adatoms on the exposed surface of the lateral side of the platelet (see the inset in Figure 1e). These basic differences between the basal and lateral surfaces reflect the layer-by-layer growth mode and probably influence the Bi4Ti3O12 dissolution rates in different crystallographic orientations. It is expected that the dissolution of the Bi4Ti3O12 platelets will proceed faster from the lateral stepped surface.31 The [Bi2O2]2+-terminated Bi4Ti3O12 platelets with atomically flat basal-plane surfaces are an ideal substrate for the epitaxial growth of the perovskite SrTiO3 phase due to the good match of the two phases in the [001]Bi4Ti3O12||[100]SrTiO3 and {110}Bi4Ti3O12||{100}SrTiO3 orientational relationship. The lattice spacings of {110}Bi4Ti3O12 and {100}SrTiO3 are 0.3842 and 0.3905 nm, meaning that the structural match of both phases is good, a prerequisite for successful epitaxial growth.
3.2. Background for the Selection of the TC Synthesis Conditions
The TC reaction of the Bi4Ti3O12 platelets to SrTiO3 particles with a preserved, plate-like morphology under hydrothermal conditions is expected to proceed by the dissolution of Bi4Ti3O12 and the concurrent precipitation of SrTiO3 on the surface of the Bi4Ti3O12 platelets with the only source of titanium ions being the dissolving Bi4Ti3O12 crystals, whereas the concentration of Sr2+ ions is controlled based on the amount of strontium salt (SrCl2·6H2O).
The lack of relevant thermodynamic data for Bi4Ti3O12 limits the theoretical predictions for its dissolution and for the formation of equilibrium compounds (SrTiO3, Bi2O3, and Bi12TiO20) as a function of the physicochemical conditions (pH, synthesis temperature (T), and concentrations of ions). Hence, the first approximate experimental conditions for the formation of SrTiO3 from Bi4Ti3O12 were established based on the reported thermodynamic modeling for the crystallization of SrTiO3 from TiO2 under hydrothermal conditions and previous empirical studies of SrTiO3 growth on different titanate precursors.13−15,32 Lencka and Riman32 calculated a phase-stability diagram for the Sr–Ti hydrothermal system of anatase and hydrous TiO2 gel. Later, Kalyani et al.13 extended the diagram to rutile. Their results show that the formation of SrTiO3 from titanate precursors requires a basic pH, where the Ti(OH)62– ions are the predominant aqueous titanium species.13−15,32,33 Considering that Sr(OH)2 exhibits a high solubility in aqueous media at higher temperatures of 100 °C ≤ T ≤ 300 °C, the formation of SrTiO3 under hydrothermal conditions is presented using the following equation:13,15
| 1 |
In our system, under alkaline conditions, the Sr2+ ions from the dissolved SrCl2 precipitate first as Sr(OH)2, which then dissolves at higher temperatures (100 °C ≤ T ≤ 200 °C). The Ti(OH)62– species form presumably by the dissolution of Bi4Ti3O12 in alkaline media according to eq 2.
| 2 |
It is expected that the precipitation of the SrTiO3 on Bi4Ti3O12 platelets (heterogeneous nucleation) also proceeds following eq 1. According to the theory of heterogeneous nucleation, the energy for the formation of a critical nucleus is proportional to the third power of the interfacial free energy and inversely proportional to the square of the supersaturation.34 In other words, the energy barrier for the nucleation of SrTiO3 on Bi4Ti3O12 is lowered by the close structural match between the Bi4Ti3O12 substrate and the precipitating SrTiO3 phase and by the higher concentrations of Ti(OH)62– and Sr2+ ions (supersaturation). Taking into account the orientation relationship of [001]Bi4Ti3O12||[100]SrTiO3 and {110}Bi4Ti3O12||{100}SrTiO3, the theoretical lattice match between Bi4Ti3O12 and SrTiO3 is good. The supersaturation (eq 3) in our system is defined as the ratio between the product of the activities of aqueous species immediately before the SrTiO3 formation and the solubility product Ks, which is the reciprocal of the equilibrium constant of eq 1:13
| 3 |
In our reaction system, the concentration of Ti(OH)62– is a complex function of Bi4Ti3O12 dissolution and SrTiO3 precipitation. Therefore, possibilities for the direct control of the supersaturation in terms of Ti(OH)62 are limited. In contrast, tailoring of the supersaturation with respect to the Sr2+ ions is easily feasible with the initial amount of strontium salt. To ensure the supersaturation conditions for SrTiO3 formation, the selected strontium concentration with respect to the whole titanium content was higher than that required by the SrTiO3 stoichiometry. The optimal concentration of Bi4Ti3O12 was determined during our preliminary experiments to be 0.00107 M.30 This relatively low concentration was also selected to avoid the eventual precipitation of bismuth titanium compounds (e.g., Bi12TiO20) that would compete with SrTiO3 for the Ti(OH)62– species.
Before studying the Bi4Ti3O12-to-SrTiO3 transformation, the stability of the initial Bi4Ti3O12 template platelets at 200 °C and in highly alkaline conditions (6 M NaOH) without the presence of Sr2+ ions was verified. The solubility and dissolution rates of the Bi4Ti3O12 platelets in the alkaline media should be moderate to prevent their complete dissolution and the disintegration of the substrate for epitaxial growth, as was observed in some other systems.35,36 A qualitative evaluation of the stability of the Bi4Ti3O12 platelets under applied alkaline hydrothermal conditions (6 M NaOH, 200 °C/15 h) in the absence of SrCl2 revealed no significant change in the platelet’s average side length, while the general platelet-like morphology was well preserved in spite of the harsh conditions. The major difference was observed on the lateral surfaces where the beginning of the exfoliation was observed. This is a result of the limited incongruent dissolution of [Bi2O2]2+ sheets and the pseudo-perovskite [Bi2Ti3O10]2– blocks from the lateral direction (Figure S1, Supporting Information). Considering the low concentration of Bi4Ti3O12 platelets (0.00107 M), their solubility in 6 M NaOH is relatively low. Nevertheless, under similar hydrothermal conditions in the presence of dissolved Sr2+ ions, it is assumed that SrTiO3 formation according to eq 1 is the driving force for Bi4Ti3O12 dissolution. The transformation of the initial Bi4Ti3O12 template particles to the SrTiO3 platelets was studied for a system with a strontium content that is 12 times higher than required by the SrTiO3 stoichiometry. The strontium concentration (0.0388 M) was 4 times larger than in our previous study.30 With a higher supersaturation, we aim to decrease the energy barrier for the nucleation of SrTiO3 and promote its growth over the whole basal-plane surfaces of Bi4Ti3O12 platelets and consequently ensure that the SrTiO3/Bi4Ti3O12 heterostructural and final SrTiO3 particles maintain the platelet-like shape of the initial template.13,34
3.3. Mechanistic Interpretation of the Bi4Ti3O12-to-SrTiO3 TC Process
The progress of the hydrothermal TC reaction was first inspected by XRD. Figure 2 and Figure S2 (Supporting Information) show the XRD patterns of the acid-washed platelets (free of side products) after different reaction times. The XRD patterns of the initial Bi4Ti3O12 platelets with a high (001) preferential orientation are also shown in Figure 2 and Figure S2 for comparison. The formation of the SrTiO3 was already observed after 1 h of the hydrothermal reaction (6 M NaOH, Sr/Ti = 12). The amount of SrTiO3 compared to Bi4Ti3O12 increased with a prolongation of the reaction time. Only SrTiO3 with a (100) preferential orientation and no Bi4Ti3O12 were detected after 15 h (Figure 2 and Figure S2). In the figures, SrTiO3 and Bi4Ti3O12 are labeled as STO and BIT, respectively.
Figure 2.
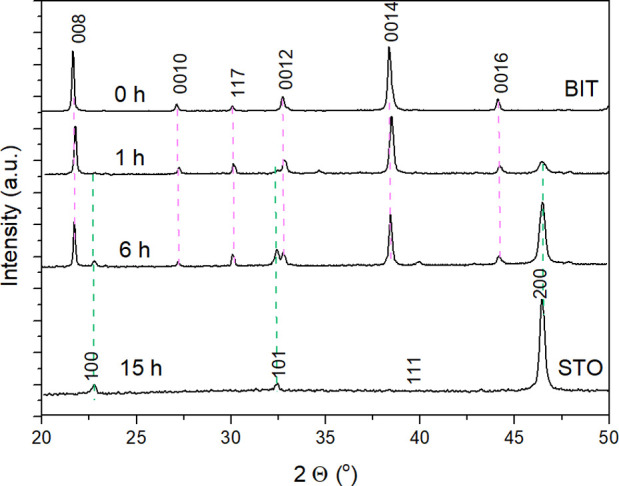
XRD patterns of the HNO3-washed platelets (cast on Si monocrystalline substrate) after different times of the TC reaction (200 °C, 6 M NaOH, Sr/Ti = 12).
The side-products can carry valuable information about the TC mechanism. An insight into all the reactions accompanying the TC of Bi4Ti3O12 to SrTiO3 was obtained with an XRD analysis of the whole reaction product (Figure S3, Supporting Information). The results revealed the formation of SrTiO3, SrCO3, and Bi2O3. SrCO3 formed through a reaction of Sr(OH)2 with carbonate impurities in the NaOH chemical and with atmospheric CO2. The formation of SrCO3 also continued after the completed reaction and the opening of the autoclave when the alkaline suspension with excessive and unreacted Sr(OH)2 is exposed to the atmosphere for a longer time. The formation of bismuth oxide, on the other hand, is a result of condensation of bismuth hydroxide Bi(OH)3,37 which forms during the Bi4Ti3O12 dissolution. No bismuth titanium compounds (e.g., Bi12TiO20) were detected. This proves that the dissolved titanium is consumed for the crystallization of SrTiO3 and not for the formation of bismuth titanium compounds (e.g., Bi12TiO20). Single-phase SrTiO3 was obtained after the dissolution of the side-products in 1 M HNO3 (Figure 2 and Figure S2, Supporting information). A deeper insight into the process of the transformation from the initial Bi4Ti3O12 platelets to SrTiO3 was obtained by a microstructural investigation of the samples after the different times for the TC reaction. The partially and fully transformed platelets were examined by SEM and STEM from top and cross-sectional views. An SEM image of the powdered sample after 1 h of reaction is shown in Figure 3a. The initial morphology of the Bi4Ti3O12 platelets is clearly preserved; however, the particles appear to have a core-rim structure. The XRD pattern of the platelets (cast from the isopropanol suspension of the platelets on the Si monocrystalline substrate) revealed the presence of Bi4Ti3O12 and SrTiO3 phases with preferential (001) and (100) orientations, respectively (Figure 2).
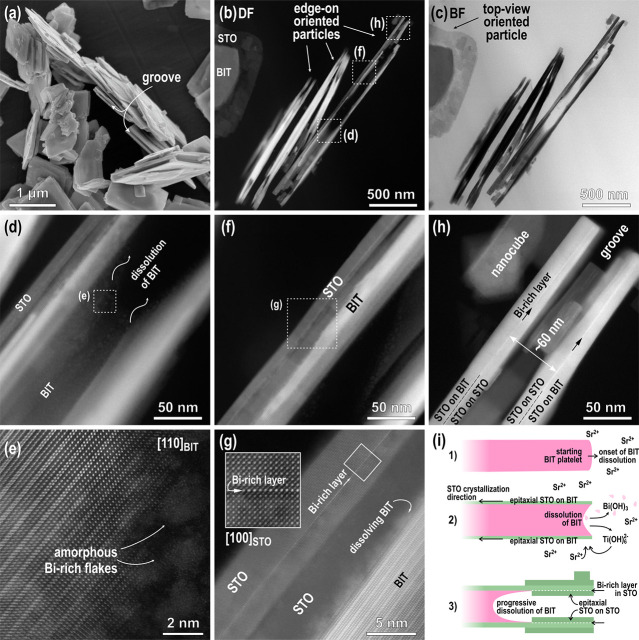
Figure 3.
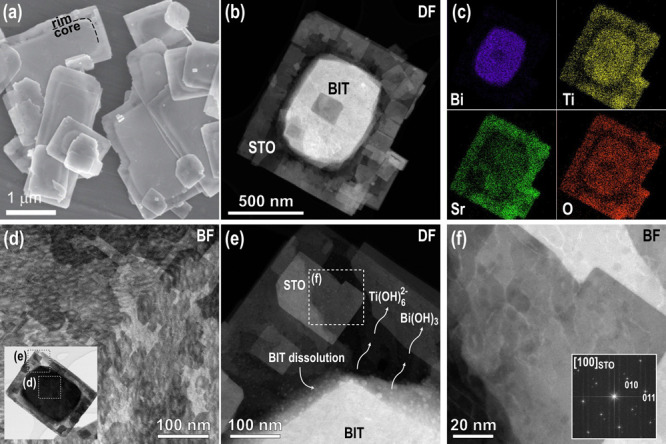
(a) SEM image of the heterostructural SrTiO3/Bi4Ti3O12 platelet with the core-rim structure after 1 h of the TC reaction at 200 °C (Sr/Ti = 12, 6 M NaOH). (b) STEM and (c) EDS analyses revealing the distribution of Bi, Ti, Sr, and O in the heterostructural platelet. (d) Close-up look with the focus set on the SrTiO3 surface layer from the marked area of the platelet in the inset. (e) Magnified region around the core-rim contact showing the decomposition of Bi4Ti3O12. (f) Higher magnification of the marked area in (e) and a fast Fourier transform (FFT) pattern in the inset.
The sample after 1 h of transformation was investigated in more detail using the HAADF-STEM. A typical particle is shown in Figure 3b, and here, the core-rim structure is even more evident. In the dark-field (DF) image, the core of the particles is much brighter, indicating a higher atomic density in the core region, whereas the rim is more electron-transparent due to the lower average atomic density or lesser thickness. More information about the chemical composition and the distribution of the elements in the particles were obtained from EDS mapping (Figure 3c) and EDS line profiles (Figure S4, Supporting Information). The results show that the core of the platelet is Bi-rich, whereas Sr is present everywhere with a higher amount in the rim. Signals from Ti and O were also detected in both parts of the platelets. The distribution of Sr all over the platelet indicates that the growth of SrTiO3 occurs on the whole area of both basal surfaces, including both surface areas over the central part (core). According to the results of the STEM/EDS analyses, the core of the partially transformed Bi4Ti3O12 platelets is mainly Bi4Ti3O12, covered by SrTiO3 on both sides, whereas the rim is newly formed SrTiO3, which replaced Bi4Ti3O12 in the dissolution–precipitation process. The platelet after 1 h of topochemical transformation can be regarded as a heterostructure of Bi4Ti3O12 and SrTiO3. Figure 3d (bright-field (BF) image) is a close-up of another partially transformed platelet in the middle region with the focus set on the surface layer. It shows the nucleation of nanosized crystallites, which occasionally show a rectangular morphology, as expected for the cubic SrTiO3 structure. The presence of regions with distinctly different gray levels suggests that the crystallization of SrTiO3 on the Bi4Ti3O12 surface occurs in several layers and that the first layer is nanocrystalline, while the crystallites in the upper layers are larger. This is also evident from Figure 3e,f where the fast Fourier transform (FFT) pattern of SrTiO3 confirms the [100] orientation. Figure 3e also shows the borderline between the rim (SrTiO3) and the core (SrTiO3/Bi4Ti3O12/SrTiO3) where (during the hydrothermal reaction) Bi4Ti3O12 disintegrates to Ti(OH)62– and Bi(OH)3 (eq 2). The Ti(OH)62– ions, exsoluted from Bi4Ti3O12, are consumed for the formation of SrTiO3 according to eq 1, while Bi(OH)3, through the condensation reactions, results in Bi2O3, as already confirmed by the XRD (Figure S3, Supporting information) and visible in the STEM as dots with bright contrast (Figure 3e). During the epitaxial crystallization of the SrTiO3 nanodomains, some amorphous bismuth oxide-rich inclusions remain captured between the SrTiO3 nanocrystallites.
An additional insight into the transformation process is obtained from the analysis of partially transformed heterostructure platelets in the edge-on orientation (Figure 4). An SEM image of the platelets in this orientation after 1 h of the TC reaction (200 °C, Sr/Ti = 12, 6 M NaOH) shows that a typical platelet contains a groove running along its edges, apparently splitting the platelet into two thinner parallel platelets (Figure 4a). A HAADF-STEM examination of the partially transformed platelets in an edge-on orientation reveals that the initial Bi4Ti3O12 platelets actually start to separate into two parallel platelets aligned with the upper and lower basal-plane surfaces of the Bi4Ti3O12 platelet. The process starts at the edges and proceeds toward the interior of the platelet (Figure 4b,c: DF–BF pair of STEM figures). One of the particles thinned to electron transparency almost along the whole area (cross-section) was investigated in more detail in three different regions—in the central part and toward the edge of the partially transformed platelet (Figure 4d–h). Figure 4d,e was taken in the central part where the particle has a sandwich structure composed of residual Bi4Ti3O12 in the middle, which is surrounded by SrTiO3 above and below the Bi4Ti3O12. A closer look at the Bi4Ti3O12 layer in this part of the platelet (Figure 4e) reveals that, here, the atomic layers of the Bi4Ti3O12 structure, the [Bi2O2]2+ sheets and the pseudo-perovskite [Bi2Ti3O10]2– blocks, are subjected to intensive dissolution. The same process was already observed in the top-view (Figure 3e). Bi4Ti3O12 disintegration is much faster from the lateral directions than from the top, as predicted on the basis of the difference of the basal and lateral surfaces with respect to the concentration of kink sites. Figure 4f,g were recorded in the region between the central part and the edge of the partially recrystallized Bi4Ti3O12 particle. Here, the epitaxial orientation relationship between SrTiO3 and Bi4Ti3O12 in [100]SrTiO3||[001]Bi4Ti3O12 is clearly visible and confirms that the orientation of SrTiO3 is dictated by the structure of the underlying Bi4Ti3O12 template. The fact that SrTiO3 growth on the Bi4Ti3O12 is epitaxial confirms that the reaction is TC.13 In this part of the crystal, the SrTiO3 layer was thinned to electron transparency (the Bi4Ti3O12 part was completely etched away in some areas) and one of the most interesting features of the SrTiO3 platelets that form during the TC transformation from Bi4Ti3O12 under hydrothermal conditions is revealed, i.e., the presence of an atomic bismuth-rich layer (Bi-rich layer), inside the SrTiO3 platelet (Figure 4g). Similarly, the STEM image of the platelet close to the edge (Figure 4h) in the section of complete transformation to SrTiO3 (rim region in Figure 3) revealed the formation of two parallel SrTiO3 platelets that both contain an atomic Bi-rich layer running along the middle part of both platelets. We believe that these Bi-rich layers correspond to the [Bi2O2]2+-terminated top layers of the initial Bi4Ti3O12 platelet.
Figure 4.
(a) SEM and (b–h) STEM micrographs of SrTiO3/Bi4Ti3O12 heterostructural platelets (mainly edge-on-oriented platelets, which were thinned to electron transparency) as obtained after 1 h of a reaction at 200 °C (6 M NaOH, Sr/Ti = 12). (b) DF and (c) BF images showing (mainly) edge-on platelets along the whole side length; (d) DF of the central part of the platelet (showing SrTiO3 layers and partially disintegrated Bi4Ti3O12 inside the groove). (e) HR image of the area marked in (d) showing the disintegration of Bi4Ti3O12. (f) Another DF of the part between the central area and the edge of the platelet. (g) Magnified area from the image (f) presenting dissolution of Bi4Ti3O12 and as-formed SrTiO3 with the incorporated Bi-rich layer (HR image of this part is in the inset). (h) DF image of the edge of the platelet with two parallel SrTiO3 platelets with incorporated Bi-rich layers. (i) Schematically shown processes of the TC reaction from Bi4Ti3O12 to SrTiO3 as reconstructed from STEM results, presented in (b)–(h).
The incorporation of the Bi-rich layer is also a consequence of the strong bonding between the termination layer of Bi4Ti3O12 and growing SrTiO3. Layer-by-layer growth (Frank–van der Merwe mechanism38), evident from our observations (Figure 3d), is also the result of strong bonding at the interface. The Bi-rich layer remains bonded to SrTiO3 even after progressive dissolution of the remaining Bi4Ti3O12 template. When the dissolution front of Bi4Ti3O12 (inside the groove) reaches the Bi-rich layer, it remains attached to the epitaxial SrTiO3 layer and the growth of the SrTiO3 also proceeds from the inner side, and the Bi-rich layer becomes a coherent part of the newly formed SrTiO3, where it is usually observed to be approximately in the middle of each SrTiO3 platelet (Figure 4h). It is obvious that the formation of two parallel platelets with an incorporated Bi-rich layer is the consequence of SrTiO3 epitaxial growth on the both bismuth-oxide-terminated basal-plane surfaces of the Bi4Ti3O12 platelets and continued SrTiO3 growth on the inner side of the Bi-rich layers (Figure 4h).
It is clear from Figure 4h that the distance between the two Bi-rich layers, which actually represent a part of the [Bi2O2]2+ terminating sheets on both sides of the starting Bi4Ti3O12 platelet, is approximately 60 nm and corresponds to a typical thickness for starting Bi4Ti3O12 template platelets (Figure 1). The attachment of SrTiO3 nanocubes on both sides of the SrTiO3 layers is also occasionally observed (Figure 4h). The conversion of Bi4Ti3O12 to SrTiO3, as reconstructed from investigations of edge-on-oriented, partially recrystallized platelets, is schematically shown in Figure 4i. The dissolution of the initial Bi4Ti3O12 platelets starts from the lateral surfaces (Figure 4i, step 1) with a high concentration of atomic steps and where both types of structural units ([Bi2O2]2+ sheets and the pseudo-perovskite [Bi2Ti3O10]2– blocks) are exposed. The edging atoms are weakly bonded (Figure 1e). When the solution becomes locally saturated with Sr2+ and Ti(OH)62–, nucleation of SrTiO3 occurs in the areas with the lowest energy barrier. As noted earlier, the interfacial free energy for SrTiO3 nucleation on the basal-plane surfaces of Bi4Ti3O12 is low due to the close structural match at the interface and therefore, SrTiO3 nucleation can immediately occur when saturation conditions are achieved. The areas close to the edges are subjected to higher concentrations of Ti(OH)62– from the beginning of the reaction, and therefore, SrTiO3 nucleation starts there (Figure 4i, step 2). Then, with the progressive dissolution of the Bi4Ti3O12, the growth of SrTiO3 continues on both basal surfaces of the Bi4Ti3O12 platelet. However, when the Bi4Ti3O12 inside the groove completely dissolves to both the initially terminating Bi-rich layers, attached to the newly formed SrTiO3, the epitaxial growth of SrTiO3 also proceeds on the inner side of these Bi-rich layers and they become coherently integrated into the SrTiO3 platelets on both sides. In the end, the Bi-rich layers lie approximately in the middle of each SrTiO3 platelet half (Figure 4i, step 3; see also Figure 4h). The reactions of Bi4Ti3O12 dissolution and SrTiO3 precipitation (epitaxial growth) continue until there is complete dissolution of the Bi4Ti3O12 matrix crystal. The SrTiO3 platelets after 15 h of the reaction at 200 °C in 6 M NaOH and with a Sr:Ti ratio of 12 are shown in Figure 5. The general plate-like shape of the initial Bi4Ti3O12 template particles is well preserved (Figure 5a); however, the integrity/crystallinity of the SrTiO3 platelets reflects the specifics of the recrystallization mechanism. The final platelets usually consist of two intergrown SrTiO3 platelets, as shown by the STEM analysis of the sample after 15 h of hydrothermal treatment (Figure 5b). The presence of Bi4Ti3O12 between the SrTiO3 platelets is not observed, indicating that all the Bi4Ti3O12 molecules dissolved and Ti(OH)62–was used for the formation of SrTiO3. In the edge-on-oriented platelets, the two parallel Bi-rich atomic layers, which are a peculiarity of the studied hydrothermal TC reaction, were observed along the whole length of both SrTiO3 platelet halves (Figure 5c).
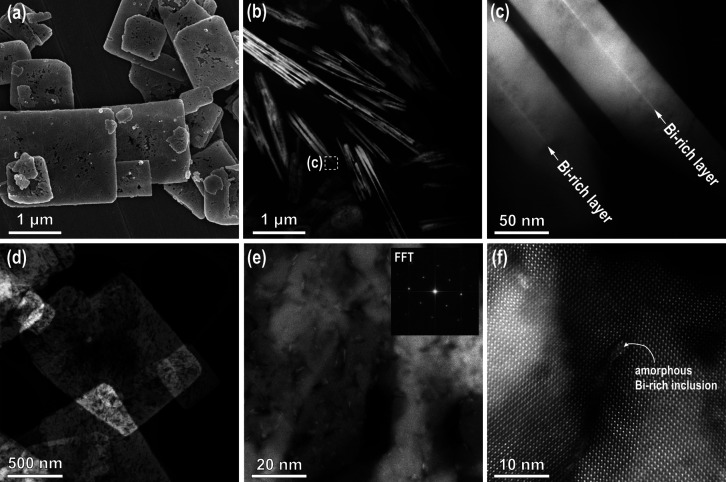
Figure 5.
(a) SEM and (b–f) STEM DF images of (b, c) edge-on oriented SrTiO3 platelets and (d–f) STEM top-view of SrTiO3 platelets, formed after 15 h at 200 °C (Sr:Ti = 12, 6 M NaOH)). (e) STEM image from the central part with FFT showing a (100) orientation in the inset (f) HR image from the central part of the SrTiO3 platelet with Bi-rich inclusion.
From our calculation, a 60 nm-thin Bi4Ti3O12 platelet would result in the formation of an approximately 42 nm-thin SrTiO3 platelet (Figure S5, Supporting Information) or two parallel 21 nm-thin platelets; however, in the process of Bi4Ti3O12 dissolution, the smallest Bi4Ti3O12 crystallites most probably dissolve and then these Ti(OH)62– species are consumed for SrTiO3 growth on the larger Bi4Ti3O12 platelets. Therefore, the typical thickness of the final SrTiO3 platelets is slightly larger and comparable to that of the initial Bi4Ti3O12 platelets.
The crystallinity of the fully transformed SrTiO3 platelets was analyzed in the top view (Figure 5d–f). A low-magnification STEM image of a thinner SrTiO3 platelet is shown in Figure 5d. The crystal is relatively dense at the edges where the transformation starts, and the porosity of the platelet increases toward the central region of the platelet. A higher-magnification STEM image taken in the central part of the platelet with the FFT calculated from the whole area is shown in Figure 5e. It is clear that the matrix consists of epitaxially oriented nanocrystallites that formed (100)-oriented SrTiO3 mesocrystalline platelets with some pores and nanosized inclusions with brighter contrast. The analysis showed that these are amorphous Bi-rich inclusions, which were trapped and overgrown by SrTiO3 during the processes of Bi4Ti3O12 dissolution and SrTiO3 crystallization. The density of the amorphous Bi-rich inclusions appears to be higher in the central part of the SrTiO3 platelets.
The observed morphological development resulted in an interesting variation of the BET specific surface area through the progress of the TC reaction. Actually, the measured BET values of the SrTiO3/Bi4Ti3O12 heterostructures increased in the first 6 h of the TC reaction, reaching a maximum at ∼20 m2·g–1, and then the BET values decreased and approached that of SrTiO3 (∼10 m2·g–1), which was still higher than the BET value of the initial Bi4Ti3O12 (2–3 m2·g–1) (Table S1, Supporting Information). The high specific surface area of the SrTiO3/Bi4Ti3O12 heterostructures is most probably related to the emerging groove and the high surface roughness of the growing SrTiO3 layers. Smoothening of the surface of the SrTiO3 platelets with the completion of the TC reaction is the reason for the lower specific surface area of the final SrTiO3 platelets compared to that of the heterostructures.
3.4. Photocatalytic Performance
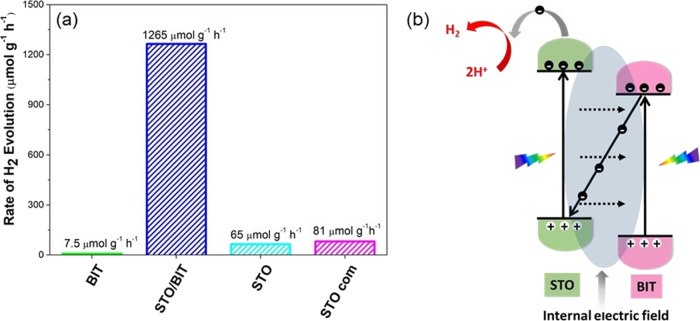
To demonstrate the potential of the developed Bi4Ti3O12, SrTiO3/Bi4Ti3O12 and mesocrystalline SrTiO3 platelets, the as-prepared materials were tested and assessed in terms of the photocatalytic activity for H2 evolution in pH-neutral aqueous media (H2O/CH3OH = 75/25). The results were compared to those involving commercial SrTiO3 nanopowders that were evaluated under the same conditions (Figure 6a). The Bi4Ti3O12 platelets with the smallest specific surface area, approximately 2–3 m2·g–1 (Supporting Information, Table S1), were found to exhibit the lowest H2 evolution rate among the studied materials, only 7.5 μmol·g–1·h–1. Mesocrystalline (100)-oriented SrTiO3 platelets (65 μmol·g–1·h–1) and commercial nanocrystalline SrTiO3 powders (81 μmol·g–1·h–1) show comparable photocatalytic activities, although the specific surface area of the platelets (10 m2·g–1) was lower than that of the commercial nanopowder (24 m2·g–1). An extraordinarily higher H2 evolution rate (1265 μmol·g–1·h–1) was observed for the heterostructural SrTiO3/Bi4Ti3O12 platelets (Figure 6a and Table S1, Supporting Information). In this study, the enhanced photocatalytic performance for H2 evolution is only presented for the heterostructural SrTiO3/Bi4Ti3O12 platelets with a SrTiO3/Bi4Ti3O12 weight ratio of 60/40 and BET = 20 m2·g–1 (Figure S6, Supporting information). We confirmed several times that the heterostructural platelets exhibiting a BET surface area of >15 m2·g–1 typically show a considerably higher H2 evolution rate than the pure SrTiO3 platelets and the commercial SrTiO3 nanopowders. The results support the important role of the heterojunction for an improvement of the photocatalytic efficiency. A systematic study of the mutual effect of the SrTiO3/Bi4Ti3O12 ratio and the specific surface area on the photocatalytic H2 evolution is beyond the scope of the present article, and the results of this research will be published in a forthcoming study. It is noteworthy that the relatively high H2 evolution rate was determined for bare nanoheterostructural SrTiO3/Bi4Ti3O12 platelets without any noble-metal doping or cocatalyst support. In terms of the H2 production rate, our heterostructures demonstrate better performance than several other noble-metal-loaded photocatalysts (Table S2, Supporting Information).19−22,39−41 Cycled measurements of the H2 evolution revealed good repeatability and reusability of the nanoheterostructural SrTiO3/Bi4Ti3O12 platelets (Figure 6 and Figure S7). The stability of the H2 evolution over the tested 24 h reaction time is similar to that reported for other SrTiO3-based photocatalysts (Figure S7).23,24
Figure 6.
(a) Rate of H2 evolution over the studied photocatalyst particles (20 mg) in 40 mL of aqueous solution with 25 vol % methanol without noble-metal cocatalysts. (b) Plausible photocatalytic H2 evolution mechanism.
A band-structure analysis is an essential approach to provide a deep insight into the possible photocatalytic mechanism. The band-gap energy (Eg) of the constituents was calculated using the well-known Tauc method from the UV–vis diffuse reflectance spectra and Kubelka–Munk function (see calculation in the Supporting Information and Figure S8).42 The obtained band gaps for SrTiO3 and Bi4Ti3O12 were 3.23 and 3.16 eV, respectively. The conduction band (ECB) and valence band (EVB) energies, another two important factors, are calculated using the empirical formulas (see calculation in the Supporting Information).43−45 The ECB and EVB of SrTiO3 were determined to be −0.80 and 2.43 eV, while for Bi4Ti3O12, the calculations revealed an ECB = −0.21 eV and EVB = 2.95 eV. For SrTiO3, the calculated ECB (−0.8 eV) perfectly matches the reported ECB value (−0.81 eV7,17), determined by the Mott–Schottky method for single-crystalline SrTiO3.17 In contrast, in the case of Bi4Ti3O12, a larger deviation was observed between the calculated ECB (−0.21 eV) and those ECB values obtained from the Mott–Schottky plot (−0.1 eV46 and −0.41 eV47). The discrepancies are not unexpected since these experimental values were determined for two different Bi4Ti3O12 nanostructures.46,47 Namely, it is known that the experimental determination of the fundamental characteristics (e.g., ECB) of nanostructural materials is associated with a high degree of uncertainty.48 This is also true for the determination of ECB from the Mott–Schottky relationship, which is based on several assumptions and ideal conditions, which are not entirely fulfilled by nanostructures.48,49
Finally, another important parameter, the Fermi energy (EF), is determined according to the estimation that it lies at 0.3–0.1 eV below ECB for n-type semiconductors. These values are −0.51 and −0.11 eV for SrTiO3 and Bi4Ti3O12, respectively (Figure S9, Supporting Information), and also support the previous results.50,51 The studied heterostructural SrTiO3/Bi4Ti3O12 platelets can be categorized as staggered type II alignments, and for this current research, we found that the potential of water reduction (at pH 7) lies in between ECB/SrTiO3 and ECB/Bi4Ti3O12.5Figure S8c (Supporting Information) shows the modified band gap of the heterojunction, and this supports the band rearrangements as well. Under simulated-light irradiation, the constituent elements absorb photons, and as a result, electron/hole pairs are generated (Figure 6b). It is already determined that EF/SrTiO3 lies in a more negative position than EF/Bi4Ti3O12. During Fermi-level rearrangement, due to the higher Fermi energy of SrTiO3, the electrons tend to move from SrTiO3 to Bi4Ti3O12. This phenomenon causes the SrTiO3 and Bi4Ti3O12 sites to be positively and negatively charged, respectively. As a result, a weak internal electric field is generated at the solid–solid interface. Therefore, the photo-generated electrons prefer to migrate from a CB of Bi4Ti3O12 to a VB of SrTiO3 via this low-resistance pathway. This prevents the electron/hole recombination, and this study supports the possible execution of a Z-scheme transfer (Figure 6b).52,53 For this experiment, the coupling of SrTiO3 and Bi4Ti3O12 greatly facilitates the photo-generated carrier transfer and separation of electron/hole pairs under light irradiation and, as a result, the H2 evolution rate is enhanced significantly. Here, the holes at VB/Bi4Ti3O12 were consumed by the hole-scavenger methanol.54 PL spectroscopy with an excitation wavelength of 320 nm was used to evaluate the separation efficiencies of the photo-excited charge carriers in the studied photocatalyst platelets (Figure 7). The Bi4Ti3O12 platelets show strong PL emission peaks at approximately 415 and 450 nm, which is in line with the reported PL spectra of Bi4Ti3O12.25,47 As compared to pure Bi4Ti3O12, the SrTiO3/Bi4Ti3O12 heterostructures show a significantly lower PL intensity. This result suggests the inhibition of charge recombination in the SrTiO3/Bi4Ti3O12 heterostructure, resulting in an improvement of its photocatalytic activity. In contrast to Bi4Ti3O12, the SrTiO3 platelets do not show a significant visible PL, which is typical for non-defective SrTiO3.18,55
Figure 7.
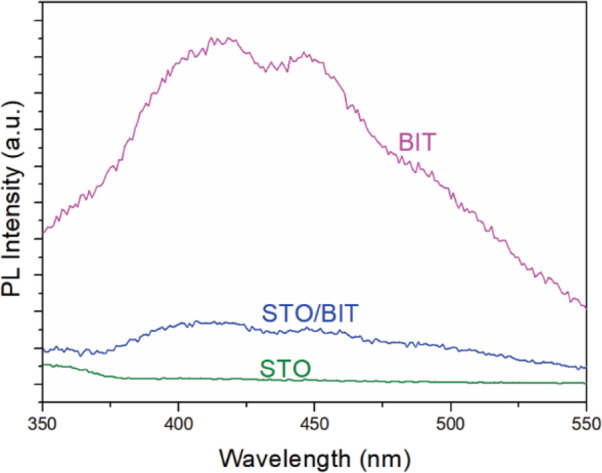
Photoluminescence (PL) spectra of the Bi4Ti3O12, SrTiO3/Bi4Ti3O12 heterostructure (SrTiO3/Bi4Ti3O12 = 60/40), and SrTiO3 platelets.
In terms of band-gap energy, the SrTiO3/Bi4Ti3O12 heterostructural platelets are, similar to SrTiO3, UV-active photocatalysts. Due to the small portion of UV light in the incident light spectra, the SrTiO3-based photocatalysts do not show a high solar-to-hydrogen (STH) efficiency. It has been reported that a modification of the SrTiO3 by doping and/or cocatalyst deposition led to a variation of the STH from 0.037 to 0.65% (Table S3, Supporting Information).22,24,56−59 The highest STH efficiency (0.65%) was reported by Domen and co-workers24 for Al-doped SrTiO3 loaded with Rh/Cr2O3 and CoOOH cocatalysts. An STH greater than 1% was demonstrated for La- and Rh-codoped SrTiO3 (H2 evolution) combined with Mo-doped BiVO4 (O2 evolution) and Au in the Z-scheme-based photocatalysts.56 In the current study, SrTiO3/Bi4Ti3O12 heterostructural platelets without any noble-metal doping or cocatalyst loading exhibit an STH efficiency of 0.19%, which is moderate but comparable to several other reported STH values for noble-metal decorated SrTiO3 photocatalysts (Table S3, Supporting Information). Considering that the SrTiO3/Bi4Ti3O12 heterostructure was evaluated for the first time in terms of photocatalytic H2 evolution, we believe that there is still room for improvement in its STH efficiency.
4. Conclusions
The epitaxial growth of SrTiO3 on Bi4Ti3O12 template platelets was studied under alkaline hydrothermal conditions at 200 °C to illustrate the TC reaction for the formation of novel SrTiO3/Bi4Ti3O12 heterostructural platelets and (100)-oriented SrTiO3 mesocrystalline platelets. In the presented TC reaction, the Bi4Ti3O12 platelets act as a source of dissolved Ti(OH)62– species and also serve as a substrate for epitaxial growth of SrTiO3. The heterogeneously layered structure of the Bi4Ti3O12 platelets with different dissolution rates of the basal and lateral surfaces results in an interesting morphological development and additionally offers a unique track and insight into the hydrothermal TC mechanism. Dissolution of the initial Bi4Ti3O12 platelet from the lateral ends into the interior and the simultaneous epitaxial growth of SrTiO3 on both bismuth-oxide-terminated basal-surface planes of the template platelet result in the formation of two parallel SrTiO3 platelets separated by a groove that deepens with the progress of the TC reaction, whereas Bi4Ti3O12 constitutes the core of the SrTiO3/Bi4Ti3O12 heterostructural platelet. When the TC reaction is completed, the newly formed platelet-like particle consists of two parallel SrTiO3 platelets, both of which have an incorporated monoatomic Bi-rich layer, the remains of the top layers of the parent Bi4Ti3O12 platelet.
The intermediate heterostructural SrTiO3/Bi4Ti3O12 and the final SrTiO3 platelets develop approximately 5–10 times higher specific surfaces (10–20 m2·g–1) than the initial Bi4Ti3O12 platelets, mainly due to the newly formed groove and the high surface roughness of the growing SrTiO3. The photocatalytic activity for the H2 evolution and the STH efficiency of the as-prepared SrTiO3/Bi4Ti3O12 platelets free of noble-metal cocatalysts are reproducible, stable, and 18 times (1265 μmol·g–1·h–1; STH = 0.19%) higher than that of the (100)-oriented SrTiO3 mesocrystalline platelets (65 μmol·g–1·h–1; STH = 0.01%) and 15 times more than that of the commercial SrTiO3 nanopowders (81 μmol·g–1·h–1; STH = 0.012%). The enhanced photocatalytic activity of the SrTiO3/Bi4Ti3O12 heterostructural platelets is explained by the efficient transfer of the photogenerated carriers from Bi4Ti3O12 to SrTiO3 and separation of electron/hole pairs at the interface. The reduced recombination of photoinduced charge carriers in the SrTiO3/Bi4Ti3O12 heterostructural platelets was confirmed by the decreased intensity of the photoluminescence.
The detailed insight into the mechanism of epitaxial growth for SrTiO3 on Bi4Ti3O12 expands the possibilities for using the hydrothermal TC reaction concept in the design of highly preferentially oriented heterostructures or mesocrystallites, involving other template particles and growing phases for the preparation of new efficient photocatalyst systems.
Acknowledgments
The authors acknowledge the M-era.Net projects (HarvEnPiez (no. 3184), contract no. C3330-17-500075, and SunToChem (no. 6081), contract no. C3330-19-252011), project no. J1-9177 and research program no. P2-0091, which were financially supported by the Ministry of Higher Education, Science and Technology and the Slovenian Research Agency, respectively. A.Č. is grateful to the Slovenian Research Agency for the financial support of her PhD study (no. PR-07596). The authors would also like to thank to Katarina Lapornik, Dominika Zorman, Tina Pečarič, and Špela Kaps for their help in the synthesis of the samples, Medeja Gec for the preparation of the samples for the TEM examinations, and Dr. Špela Kunej for performing the UV–vis spectroscopic measurements.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.0c16253.
Electron microscopy analyses, XRD patterns, photocatalytic data, and the calculation of the band structures (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Xiong Z.; Kuang C. C.; Lin K. Y.; Lei Z.; Chen X.; Gong B.; Yang J.; Zhao Y.; Zhang J.; Xia B.; Wu J. C. S. Enhanced CO2 Photocatalytic Reduction through Simultaneously Accelerated H2 Evolution and CO2 Hydrogenation in a Twin Photoreactor. J. CO2 Util. 2018, 24, 500–508. 10.1016/j.jcou.2018.02.006. [DOI] [Google Scholar]
- Mu L.; Zhao Y.; Li A.; Wang S.; Wang Z.; Yang J.; Wang Y.; Liu T.; Chen R.; Zhu J.; Fan F.; Li R.; Li C. Enhancing Charge Separation on High Symmetry SrTiO3 Exposed with Anisotropic Facets for Photocatalytic Water Splitting. Energy Environ. Sci. 2016, 9, 2463–2469. 10.1039/c6ee00526h. [DOI] [Google Scholar]
- Afroz K.; Moniruddin M.; Bakranov N.; Kudaibergenov S.; Nuraje N. A Heterojunction Strategy to Improve the Visible Light Sensitive Water Splitting Performance of Photocatalytic Materials. J. Mater. Chem. A 2018, 6, 21696–21718. 10.1039/c8ta04165b. [DOI] [Google Scholar]
- Li D.; Yu J. C.-C.; Nguyen V. H.; Wu J. C. S.; Wang X. A Dual-Function Photocatalytic System for Simultaneous Separating Hydrogen from Water Splitting and Photocatalytic Degradation of Phenol in a Twin-Reactor. Appl. Catal., B 2018, 239, 268–279. 10.1016/j.apcatb.2018.08.010. [DOI] [Google Scholar]
- Chen S.; Takata T.; Domen K. Particulate Photocatalysts for Overall Water Splitting. Nat. Rev. Mater. 2017, 2, 17050. 10.1038/natrevmats.2017.50. [DOI] [Google Scholar]
- Li X.; Yu J.; Jaroniec M. Hierarchical Photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. 10.1039/c5cs00838g. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Ochi T.; Fujitsuka M.; Kobori Y.; Majima T.; Tachikawa T. Topotactic Epitaxy of SrTiO3 Mesocrystal Superstructures with Anisotropic Construction for Efficient Overall Water Splitting. Angew. Chem., Int. Ed. Engl. 2017, 56, 5299–5303. 10.1002/anie.201702223. [DOI] [PubMed] [Google Scholar]
- Guo E.; Yin L. Tailored SrTiO3/TiO2 Heterostructures for Dye-Sensitized Solar Cells with Enhanced Photoelectric Conversion Performance. J. Mater. Chem. A 2015, 3, 13390–13401. 10.1039/c5ta02556g. [DOI] [Google Scholar]
- Poterala S. F.; Chang Y.; Clark T.; Meyer R. J. Jr.; Messing G. L. Mechanistic Interpretation of the Aurivillius to Perovskite Topochemical Microcrystal Conversion Process. Chem. Mater. 2010, 22, 2061–2068. 10.1021/cm903315u. [DOI] [Google Scholar]
- Chang Y.; Ning H.; Wu J.; Zhang S.; Lü T.; Yang B.; Cao W. Formation Mechanism of (001) Oriented Perovskite SrTiO3 Microplatelets Synthesized by Topochemical Microcrystal Conversion. Inorg. Chem. 2014, 53, 11060–11067. 10.1021/ic501604c. [DOI] [PubMed] [Google Scholar]
- Kržmanc M. M.; Uršič H.; Meden A.; Korošec R. C.; Suvorov D. Ba1-xSrxTiO3 Plates: Synthesis through Topochemical Conversion, Piezoelectric and Ferroelectric Characteristics. Ceram. Int. 2018, 44, 21406–21414. 10.1016/j.ceramint.2018.08.198. [DOI] [Google Scholar]
- Kržmanc M. M.; Jančar B.; Uršič H.; Tramšek M.; Suvorov D. Tailoring the Shape, Size, Crystal Structure, and Preferential Growth Orientation of BaTiO3 Plates Synthesized through a Topochemical Conversion Process. Cryst. Growth Des. 2017, 17, 3210–3220. 10.1021/acs.cgd.7b00164. [DOI] [Google Scholar]
- Kalyani V.; Vasile B. S.; Ianculescu A.; Testino A.; Carino A.; Buscaglia M. T.; Buscaglia V.; Nanni P. Hydrothermal Synthesis of SrTiO3: Role of Interfaces. Cryst. Growth Des. 2015, 15, 5712–5725. 10.1021/acs.cgd.5b00770. [DOI] [Google Scholar]
- Kalyani V.; Vasile B. S.; Ianculescu A.; Buscaglia M. T.; Buscaglia V.; Nanni P. Hydrothermal Synthesis of SrTiO3 Mesocrystals: Single Crystal to Mesocrystal Transformation Induced by Topochemical Reactions. Cryst. Growth Des. 2012, 12, 4450–4456. 10.1021/cg300614f. [DOI] [Google Scholar]
- Canu G.; Buscaglia V. Hydrothermal Synthesis of Strontium Titanate: Thermodynamic Considerations, Morphology Control and Crystallisation Mechanisms. CrystEngComm 2017, 19, 3867–3891. 10.1039/c7ce00834a. [DOI] [Google Scholar]
- Zhang Y.; Chen Z.; Lu Z. A Facile Method for the Preparation of Colored Bi4Ti3O12–x Nanosheets with Enhanced Visible-Light Photocatalytic Hydrogen Evolution Activity. Nanomaterials 2018, 8, 261. 10.3390/nano8040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolts J. M.; Wrighton M. S. Correlation of Photocurrent-Voltage Curves with Flat-Band Potential for Stable Photoelectrodes for the Photoelectrolysis of Water. J. Phys. Chem. 1976, 80, 2641–2645. 10.1021/j100565a004. [DOI] [Google Scholar]
- Zhou X.; Liu N.; Yokosawa T.; Osvet A.; Miehlich M. E.; Meyer K.; Spiecker E.; Schmuki P. Intrinsically Activated SrTiO3: Photocatalytic H2 Evolution from Neutral Aqueous Methanol Solution in the Absence of Any Noble Metal Cocatalyst. ACS Appl. Mater. Interfaces 2018, 10, 29532–29542. 10.1021/acsami.8b08564. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Willard E. J.; Li H.; Wu Z.; Castro R. H. R.; Osterloh F. E. Aluminum Enhances Photochemical Charge Separation in Strontium Titanate Nanocrystal Photocatalysts for Overall Water Splitting. J. Mater. Chem. A 2018, 6, 16170–16176. 10.1039/c8ta05885g. [DOI] [Google Scholar]
- Kuang Q.; Yang S. Template Synthesis of Single-Crystal-Like Porous SrTiO3 Nanocube Assemblies and Their Enhanced Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2013, 5, 3683–3690. 10.1021/am400254n. [DOI] [PubMed] [Google Scholar]
- Puangpetch T.; Sreethawong T.; Yoshikawa S.; Chavadej S. Hydrogen Production from Photocatalytic Water Splitting over Mesoporous-Assembled SrTiO3 Nanocrystal-Based Photocatalysts. J. Mol. Catal. A: Chem. 2009, 312, 97–106. 10.1016/j.molcata.2009.07.012. [DOI] [Google Scholar]
- Wang Q.; Hisatomi T.; Ma S. S. K.; Li Y.; Domen K. Core/Shell Structured La- and Rh-Codoped SrTiO3 as a Hydrogen Evolution Photocatalyst in Z-Scheme Overall Water Splitting under Visible Light Irradiation. Chem. Mater. 2014, 26, 4144–4150. 10.1021/cm5011983. [DOI] [Google Scholar]
- Chiang T. H.; Lyu H.; Hisatomi T.; Goto Y.; Takata T.; Katayama M.; Minegishi T.; Domen K. Efficient Photocatalytic Water Splitting Using Al-Doped SrTiO3 Coloaded with Molybdenum Oxide and Rhodium–Chromium Oxide. ACS Catal. 2018, 8, 2782–2788. 10.1021/acscatal.7b04264. [DOI] [Google Scholar]
- Takata T.; Jiang J.; Sakata Y.; Nakabayashi M.; Shibata N.; Nandal V.; Seki K.; Hisatomi T.; Domen K. Photocatalytic Water Splitting with a Quantum Efficiency of Almost Unity. Nature 2020, 581, 411–414. 10.1038/s41586-020-2278-9. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Jiang X.; Zhu C.; Shi C. Chromium-Modified Bi4Ti3O12 Photocatalyst: Application for Hydrogen Evolution and Pollutant Degradation. Appl. Catal., B 2016, 199, 241–251. 10.1016/j.apcatb.2016.06.036. [DOI] [Google Scholar]
- Hou J.; Cao R.; Wang Z.; Jiao S.; Zhu H. Chromium-Doped Bismuth Titanate Nanosheets as Enhanced Visible-Light Photocatalysts with a High Percentage of Reactive {110} Facets. J. Mater. Chem. 2011, 21, 7296–7301. 10.1039/c0jm04374e. [DOI] [Google Scholar]
- Zhang H.; Chen G.; Li X. Synthesis and Visible Light Photocatalysis Water Splitting Property of Chromium-Doped Bi4Ti3O12. Solid State Ionics 2009, 180, 1599–1603. 10.1016/j.ssi.2009.10.005. [DOI] [Google Scholar]
- Wu J.; Chang Y.; Lv W.; Jiang G.; Sun Y.; Liu Y.; Zhang S.; Yang B.; Cao W. Topochemical Transformation of Single Crystalline SrTiO3 Microplatelets from Bi4Ti3O12 Precursors and their Orientation-Dependent Surface Piezoelectricity. CrystEngComm 2018, 20, 3084–3095. 10.1039/c8ce00473k. [DOI] [Google Scholar]
- Zhao W.; Wang H.; Feng X.; Jiang W.; Zhao D.; Li J. Hydrothermal Synthesis and Photocatalytic Activities of Bi4Ti3O12/SrTiO3 Composite Micro-Platelets. Mater. Res. Bull. 2015, 70, 179–183. 10.1016/j.materresbull.2015.04.032. [DOI] [Google Scholar]
- Čontala A.; Maček Kržmanc M.; Suvorov D. Plate-Like Bi4Ti3O12 Particles and their Topochemical Conversion to SrTiO3 Under Hydrothermal Conditions. Acta Chim. Slov. 2018, 65, 630–637. 10.17344/acsi.2018.4286. [DOI] [PubMed] [Google Scholar]
- Snyder R. C.; Doherty M. F. Faceted Crystal Shape Evolution During Dissolution or Growth. AIChE J. 2007, 53, 1337–1348. 10.1002/aic.11132. [DOI] [Google Scholar]
- Lencka M. M.; Riman R. E. Thermodynamics of the Hydrothermal Synthesis of Calcium Titanate with Reference to Other Alkaline-Earth Titanates. Chem. Mater. 1995, 7, 18–25. 10.1021/cm00049a006. [DOI] [Google Scholar]
- Livage J.; Henry M.; Sanchez C. Sol-Gel Chemistry of Transition Metal Oxides. Prog. Solid State Chem. 1988, 18, 259–341. 10.1016/0079-6786(88)90005-2. [DOI] [Google Scholar]
- Li L.; Fijneman A. J.; Kaandorp J. A.; Aizenberg J.; Noorduin W. L. Directed Nucleation and Growth by Balancing Local Supersaturation and Substrate/Nucleus Lattice Mismatch. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 3575–3580. 10.1073/pnas.1712911115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxim F.; Ferreira P.; Vilarinho P. M.; Reaney I. Hydrothermal Synthesis and Crystal Growth Studies of BaTiO3 Using Ti Nanotube Precursors. Cryst. Growth Des. 2008, 8, 3309–3315. 10.1021/cg800215r. [DOI] [Google Scholar]
- Maxim F.; Vilarinho P. M.; Ferreira P.; Reaney I. M.; Levin I. Kinetic Study of the Static Hydrothermal Synthesis of BaTiO3 Using Titanate Nanotubes Precursors. Cryst. Growth Des. 2011, 11, 3358–3365. 10.1021/cg101466u. [DOI] [Google Scholar]
- Bartonickova E.; Cihlar J.; Castkova K. Microwave-Assisted Synthesis of Bismuth Oxide. Process. Appl. Ceram. 2007, 1, 29–33. 10.2298/pac0702029b. [DOI] [Google Scholar]
- Markov I. V.Crystal Growth for Beginners Fundamentals of Nucleation, Crystal Growth and Epitaxy; 2nd Editio.; World Scientific: Singapore, 2003. 10.1142/5172. [DOI] [Google Scholar]
- Huang B.-S.; Wey M.-Y. Properties and H2 Production Ability of Pt Photodeposited on the Anatase Phase Transition of Nitrogen-Doped Titanium Dioxide. Int. J. Hydrogen Energy 2011, 36, 9479–9486. 10.1016/j.ijhydene.2011.05.064. [DOI] [Google Scholar]
- Wu Z.-L.; Wang C.-H.; Zhao B.; Dong J.; Lu F.; Wang W.-H.; Wang W.-C.; Wu G.--J.; Cui J.-Z.; Cheng P. A Semi-Conductive Copper-Organic Framework with Two Types of Photocatalytic Activity. Angew. Chem., Int. Ed. 2016, 55, 4938–4942. 10.1002/anie.201508325. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Lan Z.-A.; Lin L.; Lin S.; Wang X. Overall Water Splitting by Pt/g-C3N4 Photocatalysts without Using Sacrificial Agents. Chem. Sci. 2016, 7, 3062–3066. 10.1039/c5sc04572j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makuła P.; Pacia M.; Macyk W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. 10.1021/acs.jpclett.8b02892. [DOI] [PubMed] [Google Scholar]
- Lin X. P.; Huang F. Q.; Wang W. D.; Zhang K. L. A Novel Photocatalyst BiSbO4 for Degradation of Methylene Blue. Appl. Catal., A 2006, 307, 257–262. 10.1016/j.apcata.2006.03.057. [DOI] [Google Scholar]
- Mousavi M.; Habibi-Yangjeh A.; Abitorabi M. Fabrication of Novel Magnetically Separable Nanocomposites Using Graphitic Carbon Nitride, Silver Phosphate and Silver Chloride and Their Applications in Photocatalytic Removal of Different Pollutants Using Visible-Light Irradiation. J. Colloid Interface Sci. 2016, 480, 218–231. 10.1016/j.jcis.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Cao T.; Li Y.; Wang C.; Zhang Z.; Zhang M.; Shao C.; Liu Y. Bi4Ti3O12 Nanosheets/TiO2 Submicron Fibers Heterostructures: In Situ Fabrication and High Visible Light Photocatalytic Activity. J. Mater. Chem. 2011, 21, 6922–6927. 10.1039/c1jm10343a. [DOI] [Google Scholar]
- Zhao X.; Yang H.; Li S.; Cui Z.; Zhang C. Synthesis and Theoretical Study of Large-Sized Bi4Ti3O12 Square Nanosheets with High Photocatalytic Activity. Mater. Res. Bull. 2018, 107, 180–188. 10.1016/j.materresbull.2018.07.018. [DOI] [Google Scholar]
- Liu Y.; Zhu G.; Gao J. Z.; Hojamberdiev M.; Lu H.; Zhu R.; Wei X.; Liu P. A Novel CeO2/Bi4Ti3O12 Composite Heterojunction Structure with an Enhanced Photocatalytic Activity for Bisphenol A. J. Alloys Compd. 2016, 688, 487–496. 10.1016/j.jallcom.2016.07.054. [DOI] [Google Scholar]
- Hankin A.; Bedoya-Lora F. E.; Alexander J. C.; Regoutz A.; Kelsall G. H. Flat Band Potential Determination: Avoiding the Pitfalls. J. Mater. Chem. A 2019, 7, 26162–26176. 10.1039/c9ta09569a. [DOI] [Google Scholar]
- Cardon F.; Gomes W. P. On the Determination of the Flat-Band Potential of a Semiconductor in Contact with a Metal or an Electrolyte from the Mott-Schottky Plot. J. Phys. D: Appl. Phys. 1978, 11, L63. 10.1088/0022-3727/11/4/003. [DOI] [Google Scholar]
- Schafranek R.; Li S.; Chen F.; Wu W.; Klein A. PbTiO3/SrTiO3 Interface: Energy Band Alignment and its Relation to the Limits of Fermi Level Variation. Phys. Rev. B: Condens. Matter Mater. Phys. 2011, 84, 045317 10.1103/PhysRevB.84.045317. [DOI] [Google Scholar]
- Al-Keisy A.; Ren L.; Xu X.; Hao W.; Dou S. X.; Du Y. Selective Ferroelectric BiOI/Bi4Ti3O12 Heterostructures for Visible Light-Driven Photocatalysis. J. Phys. Chem. C 2019, 123, 517–525. 10.1021/acs.jpcc.8b09816. [DOI] [Google Scholar]
- Ng B.-J.; Putri L. K.; Kong X. Y.; Teh Y. W.; Pasbakhsh P.; Chai S.-P. Z-Scheme Photocatalytic Systems for Solar Water Splitting. Adv. Sci. 2020, 1903171. 10.1002/advs.201903171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.; Xu D.; Peng T. Enhanced Photocatalytic Activity of g-C3N4 for Selective CO2 Reduction to CH3OH via Facile Coupling of ZnO:A Direct Z-scheme Mechanism. J. Mater. Chem. A 2015, 3, 19936–19947. 10.1039/c5ta05503b. [DOI] [Google Scholar]
- Denisov N.; Yoo J. E.; Schmuki P. Effect of Different Hole Scavengers on the Photoelectrochemical Properties and Photocatalytic Hydrogen Evolution Performance of Pristine and Pt-Decorated TiO2 Nanotubes. Electrochim. Acta 2019, 319, 61–71. 10.1016/j.electacta.2019.06.173. [DOI] [Google Scholar]
- Kan D.; Terashima T.; Kanda R.; Masuno A.; Tanaka K.; Chu S.; Kan H.; Ishizumi A.; Kanemitsu Y.; Shimakawa Y.; Takano M. Blue-Light Emission at Room Temperature from Ar+-Irradiated SrTiO3. Nat. Mater. 2005, 4, 816–819. 10.1038/nmat1498. [DOI] [Google Scholar]
- Wang Q.; Hisatomi T.; Jia Q.; Tokudome H.; Zhong M.; Wang C.; Pan Z.; Takata T.; Nakabayashi M.; Shibata N.; Li Y.; Sharp I. D.; Kudo A.; Yamada T.; Domen K. Scalable Water Splitting on Particulate Photocatalyst Sheets with a Solar-to-Hydrogen Energy Conversion Efficiency Exceeding 1%. Nat. Mater. 2016, 15, 611–615. 10.1038/nmat4589. [DOI] [PubMed] [Google Scholar]
- Goto Y.; Hisatomi T.; Wang Q.; Higashi T.; Ishikiriyama K.; Maeda T.; Sakata Y.; Okunaka S.; Tokudome H.; Katayama M.; Akiyama S.; Nishiyama H.; Inoue Y.; Takewaki T.; Setoyama T.; Minegishi T.; Takata T.; Yamada T.; Domen K. A Particulate Photocatalyst Water-Splitting Panel for Large-Scale Solar Hydrogen Generation. Joule 2018, 2, 509–520. 10.1016/j.joule.2017.12.009. [DOI] [Google Scholar]
- Zhao Z.; Goncalves R. V.; Barman S. K.; Willard E. J.; Byle E.; Perry R.; Wu Z.; Huda M. N.; Moulé A. J.; Osterloh F. E. Electronic Structure Basis for Enhanced Overall Water Splitting Photocatalysis with Aluminum Doped SrTiO3 in Natural Sunlight. Energy Environ. Sci. 2019, 12, 1385–1395. 10.1039/c9ee00310j. [DOI] [Google Scholar]
- Lyu H.; Hisatomi T.; Goto Y.; Yoshida M.; Higashi T.; Katayama M.; Takata T.; Minegishi T.; Nishiyama H.; Yamada T.; Sakata Y.; Asakura K.; Domen K. An Al-doped SrTiO3 Photocatalyst Maintaining Sunlight-Driven Overall Water Splitting Activity for Over 1000 h of Constant Illumination. Chem. Sci. 2019, 10, 3196–3201. 10.1039/c8sc05757e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.