Abstract
Psoriatic arthritis (PsA) is a chronic inflammatory disease belonging to the family of spondyloarthropathies (SpA). PsA commonly aggravates psoriasis of the skin and frequently manifests as an oligoarthritis with axial skeletal involvement and extraarticular manifestations including dactylitis, enthesitis, and uveitis. The weight of genetic predisposition to psoriasis and PsA is illustrated by the concordance rates in monozygotic twins which clearly demonstrate that genomics is insufficient to induce the clinical phenotype. The association of PsA with several single nucleotide polymorphisms (SNPs) at the IL23R locus and the involvement of Th17 cells in the immunopathogenesis of PsA clearly put the IL-23/IL-17 axis in the spotlight. The IL-23 and IL-17 cytokines have a pivotal role in the chronic inflammation of the synovium in PsA and are also prominent in the skin lesions of those with PsA. In this review, we focus on the genetic association of the IL-23/IL-17 axis with PsA and the contribution of these master cytokines in the pathophysiology of the disease, highlighting the main cell types incriminated in PsA and their specific role in the peripheral blood, lesional skin and joints of patients. We then provide an overview of the approved biologic drugs targeting the IL-23/IL-17 axis and discuss the advantages of genetic stratification to enhance personalized therapies in PsA.
Keywords: genetics, psoriatic arthritis, IL17, IL23, SNPs (single nucleotide polymorphisms), therapy
Introduction
Psoriatic arthritis (PsA) is a common inflammatory disease affecting the joints and it is usually accompanied by plaque psoriasis (Ps) (1). PsA occurs in up to 30% of patients with psoriasis (particularly those with nail involvement) and affects from 0,05 to 0,25% of the general population (2), making it the second most common form of chronic inflammatory arthritis after rheumatoid disease. To address the therapeutic choices and to envision the potential musculoskeletal and dermatological phenotypes, the GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis) group identified six disease domains, i.e. peripheral arthritis, enthesitis, dactylitis, axial involvement, skin and nail psoriasis. Among these, peripheral arthritis and enthesitis are dominant and found in the vast majority of patients while the prevalence of axial PsA increases with disease duration, occurring in less than 5% of early referrals and up to 25–70% of patients with long-term disease course for PsA (2). PsA is in some cases characterized by axial skeletal involvement, along with the more frequent oligoarthritis with mostly peripheral and asymmetric manifestations (3).
The pathogenetic link between psoriasis and PsA is highly representative of the mechanistic hypotheses of disease pathogenesis. Psoriatic skin is featured by hyperplasia of the epidermis and of the stratum corneum, infiltration of the epidermis by neutrophilic granulocytes (called Munro’s micro abscesses) and infiltration of the dermis by T-cells, dendritic cells (DCs), and macrophages, which leads to the clinical features of raised erythematous silvery plaques (4). In a similar fashion, PsA is characterized by chronic inflammation which causes bone erosion and bone loss, but also new bone formation around the affected joints (5).
The increased number of osteoclasts found in the synovium in PsA is remarkably similar to rheumatoid arthritis (RA) and the persistent inflammatory synovitis causes progressive joint damage due to synovitis and erosion of articular cartilage, visible as radiological damage in almost half of the patients (6). The exaggerated inflammatory response lead to enthesitis, with the crucial contribution of IL-17 producing T-cells and enthesal resident cells, expressing IL-23R (7, 8) with biomechanical stress, HLA-B27, and a permissive microbiome as necessary factors (9).
The phenotypic features of PsA suggest that some of the genetics and molecular mechanisms of the disorder are shared across various different types of inflammatory diseases, including psoriasis, ankylosing spondylitis (AS), inflammatory bowel disease, and Behçet disease (10–12). Extra-articular manifestations of PsA include inflammation of the gastrointestinal tract (with a higher risk of inflammatory bowel disease) and the eye (uveitis), along numerous metabolic and neoplastic disturbances (13).
In this review we will address the contribution of genetics to susceptibility to PsA and its subsequent progression, with special emphasis on the IL-23/IL-17 axis and the genes and cells that this involves. We are well aware that genetics represents only a necessary but insufficient player in the disease etiology, as recapitulated by the low concordance rates in monozygotic twins for PsA. Of note, however, the same rates are for psoriasis among the highest in chronic inflammatory or autoimmune diseases.
PsA Diagnosis
The diagnosis of PsA is largely based on features and the CASPAR criteria (14) are used in the research setting for more formal; classification purposes. In contrast to rheumatoid arthritis there are no specific markers or autoantibodies for the diagnosis of PsA: it is seronegative for both rheumatoid factor and antibodies to cyclic citrullinated peptides (CCP) unlike rheumatoid arthritis where these are positive in approximately 80% of cases. Overall PsA is equally distributed between males and females but axial manifestations (spondylitis) are about three times more common in males (15). PsA patients experience substantial functional impairment, decreased quality of life and reduced life expectancy, which highlights the importance of prompt implementation of appropriate treatment. Genetics and molecular studies have led to a better understanding of the etiology of the disease and in future may allow the development of more targeted individual approaches to therapy.
Laboratory tests typically but not invariably show increased raised inflammatory markers, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), whereas rheumatoid factor (RF) and CCP antibodies are negative, in contrast to rheumatoid arthritis (16). Recent studies have also shown a possible association with anti-LL37 (cathelicidin antimicrobial peptide) autoantibodies (17).
There is an association with the Human Leukocyte Antigen (HLA)-B27 that is most prevalent in those with sacroiliitis and axial skeletal involvement. Of particular interest, about 85% of PsA patients with bilateral sacroiliitis are positive for HLA-B27 in contrast to only 22% of cases with unilateral sacroiliitis (18, 19). Conversely, HLA-B17/Cw6 haplotype (strongly associated with psoriasis itself) is mostly associated with oligoarthritis (20).
HLA-B27 is the key genetic marker of ankylosing spondylitis (AS), commonly shared with axial-PsA (21). Although the prevalence of HLA-B27 is lower in patients with PsA (20%), it has been demonstrated that axial-PsA patients are significantly HLA-B27 positive compared to patients without axial involvement (P < 0.001) (22, 23).
Radiological imaging of PsA patients may show signs of both bone erosion and new bone formation with bone proliferation mostly found in the metacarpal and metatarsal bones and joints. Formation of new bones is mostly asymmetric, with deformities arising at digits and peripheral joints. Joint damage affects mostly the hands, wrists, feet, ankles, knees, and shoulders. Syndesmophytes may develop in the skeleton and calcification may appear at the entheses, specifically at the insertion sites (24). In contrast to rheumatoid arthritis, PsA may show different manifestations in the same anatomical site with osteolysis and bone deposition detected in the same hand. Dactylitis may be accompanied by bone erosion or new bone formation as well (25). In the spine the degree of new bone formation can be graded using scoring systems, such as the Bath Ankylosing Spondylitis Radiology Index (BASRI) and the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) (26, 27).
The Genetics, Epigenetics, and Immunopathogenesis of PsA
There is a strong genetic component to psoriasis and PsA. Further, there is a significant degree of overlap in genetic predisposition between psoriasis, PsA, AS and the inflammatory bowel diseases (ulcerative colitis and Crohn Disease) (21, 28).
The genetic contribution to diseases like psoriasis and PsA has classically been investigated through twin studies. The concordance rate for psoriasis in MZ twins is between 20 to 64%, indicating that genetic factors account for roughly 70% of the population variance in the susceptibility to psoriasis (18). In polygenic diseases, there has also been an increasing focus on monozygotic (MZ) twins in order to assess the influence of epigenetics. This is true also for psoriasis and PsA (29). In addition to genetic predisposition, the onset and progression of both psoriasis and PsA appear to be influenced by both the environment and epigenetics factors (30).
Genome-Wide Association Studies and Array-Based Technologies
PsA is a polygenic immune-mediated disease: genome-wide association studies (GWAS) have identified many genes/genomic loci increasing susceptibility for PsA, many of which are also common to psoriasis uncomplicated by arthritis; these include HLA-A, HLA-B, HLA-C, IL23R, CSF2 (Colony Stimulating Factor 2 or granulocyte-macrophage colony stimulating factor), TRAF3IP2 (TRAF3 Interacting Protein 2), NOS2 (Nitric Oxide Synthase 2) (31, 32). The results of GWAS suggest that there are PsA-specific genetic variants which are independent of those previously identified in isolated psoriasis, specifically near IL23R and TNFAIP3 (TNFα Induced Protein 3) (33).
GWAS are very informative for common and low frequency variants but they do not identify rare variants. Exome chips, such as the Illumina Exome BeadChip, allow the identification of coding variants and detection of rare SNPs (34). A very good alternative is the ImmunoChip, such as the Illumina Infinium genotyping chip, which contains 196,524 polymorphisms (718 small insertion deletions, 195,806 SNPs) and it is a “low-cost” option. It is designed to perform deep replication of major inflammatory and autoimmune diseases and fine mapping of established GWAS significant loci (35). In 2015, Bowes and colleagues used the ImmunoChip array to fine-map immune-related susceptibility loci including the known psoriasis risk loci, to define new PsA susceptibility loci. They identified a specific PsA variant at the IL23R locus, and a new PsA-specific association at chromosome 5q31 (36).
These associations may indicate roles for certain signaling pathways that are specific to the pathogenesis of PsA rather than psoriasis itself, but further investigation is needed to clearly understand their contribution.
PsA-Associated Genetic Variants and Their Immune-Pathological Role
Antigen presentation by MHC proteins is pivotal to acquired immunity, and MHC alleles are strongly associated genetically with both psoriasis and PsA. HLA class I alleles are associated with increased susceptibility for PsA, but the PsA-associated HLA alleles differ from those reported in psoriasis (37). For example, the association of HLA-C*06, which is consistently associated with psoriasis, is only very weakly associated with PsA. Conversely HLA-B*08, HLA-B*27, HLA-B*38, and HLA-B*39 are the HLA alleles most consistently associated with PsA (38).
HLA-B*27, which is the pre-eminent genetic association with AS, is also consistently associated with PsA but not with psoriasis, thereby indicating different pathogenetic mechanisms in the two conditions despite the obvious disease-in-disease bias for case ascertainment. HLA-B*27 is especially associated with axial skeletal disease and the strength of this association tends to show an inverse relationship with the number of peripheral joints involved (39).
Several other genes involved in the immune response are also associated with PsA. Thus, there are also several other non-HLA genetic associations involving components of the MHC class I antigen processing and presentation pathway: these include ERAP1 and ERAP2 (Endoplasmic Reticulum Amino-Peptidase-1 and -2). Others are involved in the innate immune response and the initiation of the immune reaction (e.g. TLR4 - Toll-Like Receptor 4), or the differentiation and function of CD8+ T-cells (e.g. RUNX3 - Runt-related transcription factor 3) (31, 40, 41). Many of these genes are also associated with increased susceptibility to AS, highlighting once again the considerable overlap between these two disorders in terms of their genetic susceptibility (42).
The GWAS era has also highlighted several PsA-associated single nucleotide polymorphisms (SNPs) located in IL-23A, IL-23R, IL-12B, TYK2 (Tyrosine Kinase 2), and TRAF3IP2 (which is a downstream target of the IL-17 receptor - IL-17R), implying a pivotal role for the IL-23/IL-17 axis in the pathogenesis of PsA disease pathogenesis (see Table 1 ) (5, 43). A crucial role for IL-17 family is undisputed as the levels of IL-17 and IL-17R were found increased in both psoriatic skin and synovial fluid of patients with PsA (44).
Table 1.
Genetic variants related to the IL-23/IL-17 axis found associated with PsA through GWAS or consistently identified in targeted analysis studies.
| Chromosome | Gene | SNP ID | Odds ratio | Function |
|---|---|---|---|---|
| 1p31.3 | IL-23R | rs11209026 rs12044149 |
0.6#
1.4$ |
Th-17 signaling |
| 2q32.2 | STAT4 | rs7540214 | N/A | Mediating response to IL-12 and Th-17/Th-1 differentiation |
| 5q33.3 | IL12B | rs2082412 rs6887695 rs4921482 rs3212227 rs918520 |
1.4 1.3@ 1.4$ 1.4@ 1.5* |
Th-17 and Th-1 differentiation |
| 12q13.3 | IL23A | rs2020584 | N/A | Th-17 signaling |
| 17q21.2 | STAT3 | rs744166 | Mediating response to IL-12 and Th-17/Th-1 differentiation | |
| 5q33.1 | TNIP1 | rs8177833 | 1.8* | NFkB signaling |
| 6q21 | TRAF3IP2 | rs33980500 rs13190932 |
1.7* N/A |
Th-17 signaling and NFkB signaling |
| 19p13.2 | TYK2 | rs35251378 | 1.4* | NFkB, Interferon and Th-17 signaling |
*Odds Ratio (OR) from Stuart PE et al. Am J Hum Genet. 2015 3; 97(6): 816–836.
#OR from Zhu K et al. Inflamm. Res. 2012; 61, 1149–1154.
$OR from Bowes et al. Nat Commun. 2015 5; 6: 6046.
@OR from Filer et al. Arthritis Rheum 2008; 58(12):3705-9.
The contribution of the IL-23/IL-17 axis has greatly advanced our understanding of the pathogenesis of PsA. Th-17 cells produce the pro-inflammatory cytokine IL-17, and all the elements of the Th17 pathway, including MMP3 (Matrix Metalloproteinase 3), CCL1 and CCL20 (Chemokine Ligand 1 and 20) and IL6. The majority of these pro-inflammatory cytokines are upregulated in the blood, synovium, and skin of PsA patients (45, 46). A recent study has identified CXCR6 as a marker for IL-17+CD8+, specialized cells found in the synovial fluid of PsA patients. The presence of CXCR6+IL-17+CD8+ cells in PsA synovium may explain their contribution to the inflammatory environment (47).
IL-23 promotes the survival and expansion of Th-17 cells through its receptor IL23R and the related downstream signaling pathway, which is crucial to Th-17-mediated diseases like PsA. In 2009, a study conducted by Gladman group showed a protective effect for the rs11209026 SNP in IL-23R (encoding the loss-of-function 381Gln allele in the cytoplasmic tail of IL23R) in a Canadian PsA cohort (48). This SNP is also associated with disease severity, while another variant, rs12044149, showed an independent peak of association with PsA, after conditioning for the top SNP associated with psoriasis overall (rs9988642) (36).
Th17-mediate inflammatory response also involves the signaling adaptor TRAF3IP2 (TRAF 3-interacting protein 2), which is downstream of IL-17R. The PsA-associated TRAF3IP2 variant rs33980500 alters the binding of the TNF receptor-associated factor 6 (TRAF6), which modulates immunoregulatory signals (49, 50).
IL-23 and IL-17 also have major effects on the activation of osteoclasts, which are the main responsible for bone erosion and where the cytokine RANKL (receptor activator of nuclear factor kappa-β ligand) is a critical factor in the promotion of osteoclasts differentiation. RANKL is also expressed by Th-17 lymphocytes and synovial fibroblasts. The dogma that Th17 cells were the primary responsible for bone resorption, was recently challenged in animal studies where cell specific deletion of RANKL indicated that RANKL expression was limited to synovial fibroblasts and B cells (51).
In PsA, bone deposition is crucial as bone resorption. This process arises from the aberrant proliferation, differentiation, and activity of osteoblasts, and several signaling pathways are involved, such as the bone morphogenetic protein (BMP) and the WNT signaling pathway, such as DKK1, and Sclerostin which are relevant for both PsA and AS (52).
The abnormal proliferation of keratinocytes promoting epidermal hyperplasia (53), also emphasizes the predominant functional role of the IL23/IL-17 axis in PsA pathogenesis and in PsA inflammatory cascade.
Lastly, Al Mossawi and colleagues recently demonstrated a marked increase of specific subsets of CD4+ and CD8 + T-cells producing GM-CSF in the blood and synovium of PsA patients. They also demonstrated an increased number of double-positive IL17/GM-CSF for CD4+, CD8+, γδ, and NK (natural killer) cells. The CSF2 locus encoding GM-CSF, is also strongly associated genetically with PsA. Overall these results suggest a functional link between GM-CSF and the IL-17/IL-23 axis, opening important potential avenues for the treatment of PsA, including those targeting GM-CSF directly (54).
Cellular Mechanisms in PsA: The Multifaceted Role of IL-17 and IL-23
The contribution of the IL-23/IL-17 axis to the development of PsA will be discussed in this section, highlighting Th-17 biology and the production of a pro-inflammatory milieu in the skin and in the synovium, and how this leads to the activation of osteoclasts, which are responsible for bone degradation, and of keratinocytes and neutrophils, which are implicated in the epidermal hyperplasia.
Cell Activation and Innate Immunity
Inflammation is one of the hallmarks of PsA and activation of the innate immune response is one of the physiological triggers leading to the inflammatory cascade. The transcription factor NF-κB has a major role in this cascade, as it promotes the transcription of several proinflammatory cytokine target genes. Downstream signaling, such as IFNs (Interferons) and TNFα strongly contribute to the immune response in PsA (55).
Several genes involved in these NF-κB pathways show genome-wide significant association with PsA, including REL (a subunit of NF-κB), NOS2, and TNFAIP3 (31, 56). The IL-23/IL-17 axis might also influence the activation of the NF-kB pathway in other ways; these include the adaptor protein Act1, which when is phosphorylated binds to TRAF6 to activate the NF-κB activator protein 1 (AP-1), or the CCAAT-enhancer- binding protein (C/EBP) cascade (57).
Interferon signaling also plays a key functional role in the pathogenesis of PsA (58). It is therefore of interest that several interferon-related genes, including TYK2, IFNL1R, and PTPN22, exhibit genome-wide significant association with PsA (59). TYK2, for instance, encodes a tyrosine kinase that is involved in initiating IFNα signaling and, as mentioned above, it is also an activator of IL-23R.
TNFα clearly plays a major role in the inflammatory process of PsA as shown by the presence of raised levels of this cytokine at the sites of inflammation and the dramatic responses to treatment with anti-TNF biologics (60). It has a major role in innate immunity, inducing the production of inflammatory chemokines leading to the accumulation of activated T-cells, neutrophils, and monocytes. It is of some interest that significant association for psoriasis and PsA has been found for the SNPs rs80267959, rs1800629, and rs361525 at the TNFA locus (61).
Cell Activation and Adaptive Immunity
The pathogenesis of PsA involves components of both the innate and adaptive immune system. Many different cell types are involved in these pathophysiological processes, including T-cells, neutrophils, keratinocytes, and synoviocytes (62).
The master cytokine in the pathogenesis of PsA is IL23. IL-23 is a heterodimer composed of two subunits p19 and p40 subunits, which bind to IL23R. While p19 is unique to IL-23, the p40 subunit is shared with IL-12 (63). The binding of IL-23 to IL23R leads to the recruitment of JAK2 and TYK2 kinases, which are able to activate IL23R (and its cognate IL12RB1), phosphorylate STAT3 (Signal Transducer and Activator of Transcription 3) and induce RORyT (RAR-related orphan receptor gamma) to promote the differentiation, survival, and expansion of Th-17 cells. IL-23 is essential not only in inducing the Th-17 phenotype but also in defining the level of pathogenicity (64).
Th-17 cells are among the possible drivers of the pathogenesis of PsA and the effector molecules they release are able to trigger different target cells such as osteoclasts, macrophages, and synovial fibroblasts. IL-17 is the major cytokine produced by Th-17 cells, gd T cells and other various immune cells. IL-17 A is a homodimer disulfide-linked glycoprotein and it is the most widely studied member of the IL-17 cytokines family, which includes also IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F (65). IL-17A and IL-17 F share 55% of homology and they can form heterodimers, which bind the receptor IL-17R. IL-17R is a dimeric complex consisting of two subunits, IL-17RA and IL-17RC subunits (66). The differential binding affinity of IL-17 for IL-17RA and IL-17RC is still not well define, especially under inflammatory conditions (67).
Th-17 cells differentiate from naïve T-cells depending on a combination of different cytokines along three distinct pathways (68): (i) IL-6 and Transforming Growth Factor-β (TGFβ) (in addition IL-1β and TNFα); (ii) IL-21 and TGFβ; (iii) IL-1β, IL-6, and IL-23 (69). Th-17 cells are characterized by the expression of the transcription factor RORyT with a specific gene signature which in addition to IL17 also includes IL6, TNF, CSF2, CCL20, and IL23R (69).
T-cell subsets triple-positive for IL-17, GM-CSF, TNF, or IFN-γ were found increase in PsA synovial tissue. These enriched polyfunctional cells were also found associated with disease activity index (70).
Recently, an elegant study by Taams’ group demonstrated that IL-17+CD8+T cells may contribute to inflammation and disease persistence in PsA. These cells were found increased in patients’ synovium and have a Th-17 resembling transcriptomic profile characterized by high expression of CXCR6 (47).
JAK and STAT are the key signaling pathways transducing the cytokine signals in Th-17 cells (63). Following the inflammatory cascade mediated by IL-1β and IL-23, Th-17 cells release specific pro-inflammatory cytokines such as IL-17, GM-CSF, and IL-22. The function of Th-17 cells is tightly dependent on the balance of the effector molecules they produced, such as IL-23 and TGF-β, and on those cytokines promoting cell differentiation and maintenance (IL-23).
The effects of IL-23 on bone are conflicting. IL-23, in a Th-17 independent way, up-regulates the expression of the nuclear factor kappa-β ligand RANKL-receptor RANK in osteoclast precursors (from monocyte lineage cells), favoring osteoclast differentiation and proliferation (64, 71). IL-23 also induces the production of GM-CSF, which is an inhibitor of the differentiation of osteoclasts, thus limiting bone resorption (72). RANKL expression is also found in synovial fibroblasts where its deletion plays a key role in bone erosion (51).
Neoangiogenesis also appears to have an important part in the pathogenesis of PsA; new blood vessels are prominent in the synovial histology of the condition. It appears that the IL-17/IL-23 axis might play a role in the angiogenic process. As we have described, IL-17 produced by Th-17 cells up-regulates proinflammatory cytokines and prompts the recruitment of neutrophils and macrophages and endothelial cell migration. Further, keratinocytes, stimulated by IL-17 ligands, start to differentiate and proliferate aberrantly, producing proinflammatory adenosine monophosphate (AMP), chemokines, and angiogenic factors such as vascular endothelial growth factor (VEGF) (73).
IL-23 can also promote epidermal hyperplasia activating the proliferation of keratinocytes (increasing the expression of keratin 16) and by acting synergistically with IL-17 promotes the recruitment of neutrophils and the infiltration of IL-22 and IL-17 producing-cells into the lesioned skin (74). The response of keratinocytes and endothelial cells among others to IL-17 and IL-22 stimulation leads to the upregulation of chemokines such as CXCL1 and CCL20, pro-inflammatory cytokines, and anti‐microbial peptides, such as LL‐37 and β‐defensins. IL-17 and IL-22 both promote keratinocyte proliferation and the recruitment of macrophages and neutrophils; they also decrease the expression of adhesion molecules (i.e. selectins and integrins) thus favoring the disruption of the skin barrier. IL-17 can also stimulate the expression of endothelial markers such as P- and E-selectins and adhesion molecules, including ICAM-1 (Intracellular Adhesion Molecule 1) and VCAM-1 (Vascular Cellular Adhesion Molecule 1), which enhances the mobilization of neutrophils (75).
Lastly, the expression of IL-17 and IL-23 is increased in the synovium (76, 77). Gene expression analysis of PsA synovium reveals a gene signature closer to PsA skin than to rheumatoid synovium (78). The recruitment of pathogenic IL-23/IL-17- producing CD4+ T-cells has been demonstrated to be higher in the joints, while the IL-17/IL-22 producing CD4+ T-cells are strongly detected in the skin and in the circulation (79, 80).
Therapeutic Approaches in PsA
Since the development of biologic therapies, the ultimate target for the treatment of any patient with psoriasis and/or PsA in modern times is complete remission (81). Initially these targeting TNFα were used with great effect but much effort has also been made to develop biological drugs targeting the IL-23/IL-17 axis. This axis, as specified previously explained, offers several plausible drug targets, such as the p40 subunit of the IL-23/IL-12 receptor, the p19 subunit of IL-23R, IL-17A and its specific receptor IL-17R (82).
In the treatment of PsA non-steroidal anti-inflammatory drugs (NSAID) and synthetic disease modifying antirheumatic drugs [sDMARDs, such as methotrexate (MTX), leflunomide, and sulfasalazine] remain the first-line therapies but biological molecules (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) are used if therapy with NSAID and DMARDs fails to control the disease adequately.
Biologics (such as etanercept, infliximab, adalimumab, golimumab, certolizumab, ustekinumab, and secukinumab) or synthetic drugs (apremilast, tofacitinib and ixekizumab) are given in specific circumstances (62). The different drugs used in PsA (and in other conditions) with their mechanism of action are shown in Table 2 .
Table 2.
Overview of the current treatments in PsA and related mechanism of action.
| Category | Molecule | Mechanism of action | Approval and use | ACR20 in PsA (%) |
|---|---|---|---|---|
| sDMARDs | Methotrexate | Anti-metabolic | RA, Ps, and PsA | 32–40 |
| Leflunomide | Inhibitor of pyrimidine synthesis | RA, Ps, and PsA | 34 | |
| bDMARDs | Etanercept | TNFα blocker | AS, RA, and Ps | 60–65 |
| Infliximab | TNFα blocker | AS, RA, Ps, and PsA | 65 | |
| Adalimumab | TNFα blocker | Ps and PsA | 58 | |
| Golimumab | TNFα blocker | PsA, AS, and RA | 76 | |
| Certolizumab | TNFα blocker | RA and Ps | 52-63 | |
| Brodalumab | IL-17RA inhibitor | Ps and PsA | 39 | |
| Ustekinumab | IL-12/IL-23 inhibitor | Ps and PsA | 42–50 | |
| Secukinumab | IL-17A inhibitor | AS, Ps, and PsA | 54 | |
| Guselkumab | IL-23 inhibitor | Ps, PsA | 58 | |
| Ixekizumab | IL-17A inhibitor | Ps, and PsA | 60 | |
| tsDMARDs | Apremilast | PDE4 inhibitor | Ps and PsA | 31 |
| Tofacitinib | JAK-1/3 inhibitor | PsA | 50–53 |
sDMARDS, synthethic disease modifying anti-rheumatic drug; bDMARDs, biologic disease modifying anti-rheumatic drug; tsDMARDS, targeted synthetic disease modifying drug; ACR20, American College of Rheumatology 20 response; Ps, psoriasis; IL, interleukin; PDE4, phosphodiesterase type 4; TNFα, tumor necrosis factor α (15, 62, 82, 83).
A combination of two or more of these drugs is often administered in complex immunological diseases like PsA, and often results in increased efficacy compared with monotherapy (83).
In recent few years, a number of guidelines have been developed for the clinical assessment and management of PsA; these include recommendations from GRAPPA, EULAR (European League Against Rheumatology) and ACR/NPF (American College of Rheumatology/National Psoriasis Foundation) (84, 85). For instance, EULAR recommends NSAID and local glucocorticoid injections, especially for enthesitis, in the early phase of the disease. If adverse prognostic factors are present or treatment fails, the administration of sDMARDs, such as MTX (or alternatively leflunomide or sulfasalazine) is recommended (86). If these fail to control the disease adequately or are poorly tolerated the administration of TNF inhibitors should be considered either in combination with DMARDs or not. Biologics should also be used in those with prominent axial disease or severe enthesitis. The continued use of TNF inhibitors should be evaluated carefully according to the patient’s response and a switch to alternative biologics may be considered either where there is no initial benefit (primary failure) or where the response is lost after a period of time (secondary failure) perhaps due to the host generation of antibodies to the biologic (81).
Targeting the IL-17/IL-23 Pathway
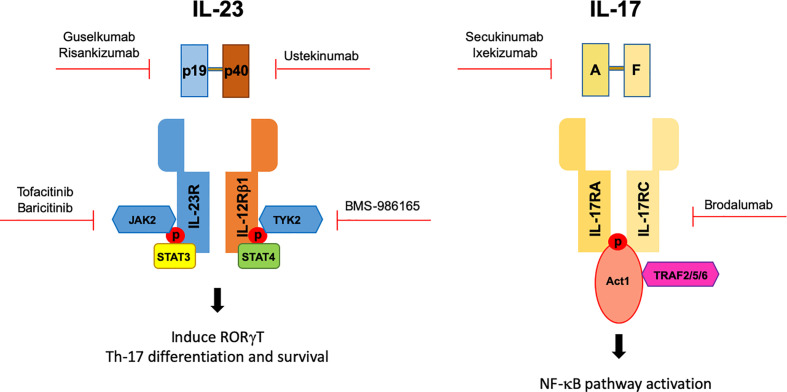
The IL-17/IL-23 pathway and Th-17 cells have become a favorite target in PsA in recent years. For this purpose, several biological drugs have been developed ( Figure 1 ). Ustekinumab, which is a monoclonal antibody targeting p40 subunit of IL-12/IL-23 has been used for skin and nail psoriasis but also for peripheral PsA in those patients who not respond not to DMARDs (87).
Figure 1.
Genetics studies allowed the identification of the IL-23/IL-17 axis having a crucial functional role in PsA pathogenesis. Genetics lead to the development of biological drugs targeting the IL-23/IL-17 axis in PsA. Red arrow indicates the target of different biologics.
Two large clinical trials, PHOENIX 1 and 2, assessed the efficacy of ustekinumab in psoriasis patients (88) while PSUMMIT-1 and -2 examined its efficacy and safety in PsA (89). Post-hoc analyses confirmed the efficacy of ustekinumab not only on skin but also in improving PsA rheumatological manifestations and radiographic progression (90). The efficacy of ustekinumab in axial involvement for PsA or in axial SpA is believed to be marginal and the development programs have been thus discontinued. However, the final word on the efficacy of IL23 blockers in SpA remains uncertain as recent data on guselkumab showed efficacy on patient reported outcomes for PsA axial manifestations (91) and a recent phase IV study on ustekinumab reported that this drug is frequently used in patients with axial PsA (92). Inhibition of IL-17A has been achieved using secukinumab, a human monoclonal antibody targeting IL-17A, with efficacy in both PsA and ankylosing spondylitis (93, 94). In the treatment of PsA secukinumab is effective for dactylitis, enthesitis, skin and nail lesions, but its effects on joint disease is rather less, as shown in FUTURE 2 and 3 trials (95).
Targeting either IL-17 or its receptor in PsA patients include besides secukinumab, also ixekizumab and brodalumab. The efficacy of ixekizumab (also targeting IL-17A) was demonstrated in reducing active disease and radiologic progression in joints, as well as fulfilling the PsA criteria of skin response, as demonstrated in the head-to-head SPIRIT study (96). Furthermore, brodalumab, which is a human monoclonal antibody human anti-IL17RA, a pan inhibitor of IL-17A, IL-17F, and IL-25 is currently used in the treatment of psoriasis where shows a complete clearance of moderate-to-severe psoriasis (97). Brodalumab efficacy and safety was also assessed in PsA patients (98).
DISCOVER-1 and -2, a double-blind, randomized, placebo-controlled phase 3 trials proved the efficacy of Guselkumab a human monoclonal antibody specifically binding the p19 subunit of IL-23. The study has shown a substantial improvement in biological naïve patients with active disease, in particular in decreasing IL-17A, IL-17F, and CRP serum levels by week 16 achieving Psoriasis Score and Severity Index, PASI75 (99, 100).
Overall, following the blockade of the IL-23/IL-17 axis, clinical trials for Ps and PsA showed a good amelioration of the skin lesions while the joint response was much lower. A larger percentage of patients achieved the PASI75, PASI90 and PASI100 compared to proportion of patients fulfilling the ACR20, ACR50, or ACR70 criteria of response (101).
Discussion and Concluding Remarks: How Genetics Might Be Crucial In Identifying Credible Therapeutic Targets
PsA is a complex polygenic disease with a genetic contribution that overlaps with other related conditions such as psoriasis, AS and IBD. Genetic variants associated with a specific disorder have the power to highlight genes or pathways that may contain credible targets for drug therapy. This is well exemplified for the IL-17/IL-23 axis and Th-17 cells with the development of biological drugs blocking IL-17 (i.e Secukinumab in AS), or IL-23 (i.e. Ustekinumab in psoriasis/PsA). Unfortunately, the process is challenging (102).
The first crucial point following a GWAS is to accurately assign associated SNPs to the genes they regulate in order to define credible pathways. For this purpose, several experimental functional assays have been developed. Functional disease-associated SNPs may affect the binding of transcription factors or the enrichment of regulatory markers: this is currently evaluated with in vitro Electrophoretic Mobility Shift Assays and ex vivo with Chromatin ImmunoPrecipitation). SNPs may have an effect on gene expression (evaluated with expression quantitative trait loci, eQTL studies) or on chromosome looping (assessed via chromosome conformation assays). Genome editing techniques are performed to define the consequences of harboring a risk variant on cellular function.
Second, these experiments must be performed considering cell-specificity (i.e. specific tissue or cell type) and condition-specificity (i.e. different stimulatory conditions) to provide significant insights into the pathogenesis of a specific disease and develop targeted therapies.
This approach will increase our understanding in defining credible pathways, specific genes, and cell populations for targeted therapy. The better molecular stratification we can achieve (for instance, patients with enrichment of associated SNPs in the IL‐23/IL‐17 pathway may respond more effectively to secukinumab or ustekinumab), the better the design of personalized therapeutic strategy will be. The final goal will be an advanced use of SNPs as pharmacogenetic markers, in order to define credible pathways to target and predict response to biological therapy (i.e. HLA-C*06 as a pharmacogenetic marker in response to Ustekinumab) (103).
Author Contributions
MV, VH, BW, and CS conceived the manuscript. MV, VH, CD, MC, BW, and CS drafted the manuscript, and all the authors revised the final version prior to submission. All authors contributed to the article and approved the submitted version.
Funding
CS is funded by Ministero degli Esteri (Italia), grant ITALY–CHINA - SCIENCE AND TECHNOLOGY COOPERATION: PGR05455. MV is funded by Versus Arthritis#21428, CD is funded by Versus Arthritis#22198.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors sincerely apologize to those authors whose work is not cited due to space constraints.
References
- 1. Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheumat (1973) 3(1):55–78. 10.1016/0049-0172(73)90035-8 [DOI] [PubMed] [Google Scholar]
- 2. Ogdie A, Weiss P. The Epidemiology of Psoriatic Arthritis. Rheumat Dis Clinics North Am (2015) 41(4):545–68. 10.1016/j.rdc.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marinoni B, Ceribelli A, Massarotti MS, Selmi C. The Th17 axis in psoriatic disease: pathogenetic and therapeutic implications. Auto Immun Highlights (2014) 5(1):9–19. 10.1007/s13317-013-0057-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suzuki E, Mellins ED, Gershwin ME, Nestle FO, Adamopoulos IE. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev (2014) 13(4-5):496–502. 10.1016/j.autrev.2014.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Rielly DD, Jani M, Rahman P, Elder JT. The Genetics of Psoriasis and Psoriatic Arthritis. J Rheumatol Suppl (2019) 95:46–50. 10.3899/jrheum.190119 [DOI] [PubMed] [Google Scholar]
- 6. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheumat Dis (2005) 64(Suppl 2):ii14–7. 10.1136/ard.2004.032482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med (2012) 18(7):1069–76. 10.1038/nm.2817 [DOI] [PubMed] [Google Scholar]
- 8. Araujo EG, Schett G. Enthesitis in psoriatic arthritis (Part 1): pathophysiology. Rheumatol (Oxford) (2020) 59(Supplement_1):i10–i4. 10.1093/rheumatology/keaa039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scher JU, Littman DR, Abramson SB. Microbiome in Inflammatory Arthritis and Human Rheumatic Diseases. Arthritis Rheumatol (2016) 68(1):35–45. 10.1002/art.39259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bowes J, Barton A. The genetics of psoriatic arthritis: lessons from genome-wide association studies. Discov Med (2010) 10(52):177–83. [PubMed] [Google Scholar]
- 11. Ellinghaus D, Jostins L, Spain SL, Cortes A, Bethune J, Han B, et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat Genet (2016) 48(5):510–8. 10.1038/ng.3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown MA, Xu H, Li Z. Genetics and the axial spondyloarthritis spectrum. Rheumatol (Oxford) (2020) 59(Supplement_4):iv58–66. 10.1093/rheumatology/keaa464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rohekar S, Tom BD, Hassa A, Schentag CT, Farewell VT, Gladman DD. Prevalence of malignancy in psoriatic arthritis. Arthritis Rheumat (2008) 58(1):82–7. 10.1002/art.23185 [DOI] [PubMed] [Google Scholar]
- 14. Zlatkovic-Svenda MI, Kerimovic-Morina D, Stojanovic RM. Psoriatic arthritis criteria evaluation: CASPAR and Modified CASPAR. Clin Exp Rheumatol (2011) 29(5):899–900. [PubMed] [Google Scholar]
- 15. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatol (Oxford) (2015) 54(1):20–8. 10.1093/rheumatology/keu237 [DOI] [PubMed] [Google Scholar]
- 16. Mahoney J, Gustafson D. The Reliability of Laboratory Testing in Diagnosing Psoriatic Arthritis: A Case Report. J Am Podiatr Med Assoc (2019) 109(6):467–70. 10.7547/17-076 [DOI] [PubMed] [Google Scholar]
- 17. Frasca L, Palazzo R, Chimenti MS, Alivernini S, Tolusso B, Bui L, et al. Anti-LL37 Antibodies Are Present in Psoriatic Arthritis (PsA) Patients: New Biomarkers in PsA. Front Immunol (2018) 9:1936. 10.3389/fimmu.2018.01936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Queiro R, Gonzalez S, Lopez-Larrea C, Alperi M, Sarasqueta C, Riestra JL, et al. HLA-C locus alleles may modulate the clinical expression of psoriatic arthritis. Arthritis Res Ther (2006) 8(6):R185. 10.1186/ar2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lopez-Larrea C, Torre Alonso JC, Rodriguez Perez A, Coto E. HLA antigens in psoriatic arthritis subtypes of a Spanish population. Ann Rheumat Dis (1990) 49(5):318–9. 10.1136/ard.49.5.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen L, Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol (2018) 178(4):854–62. 10.1111/bjd.16083 [DOI] [PubMed] [Google Scholar]
- 21. Feld J, Chandran V, Haroon N, Inman R, Gladman D. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nat Rev Rheumatol (2018) 14(6):363–71. 10.1038/s41584-018-0006-8 [DOI] [PubMed] [Google Scholar]
- 22. Jadon DR, Sengupta R, Nightingale A, Lindsay M, Korendowych E, Robinson G, et al. Axial Disease in Psoriatic Arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheumat Dis (2017) 76(4):701–7. 10.1136/annrheumdis-2016-209853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gottlieb AB, Merola JF. Axial psoriatic arthritis: An update for dermatologists. J Am Acad Dermatol (2020) 81(1):92–101. 10.1016/j.jaad.2020.05.089 [DOI] [PubMed] [Google Scholar]
- 24. Narvaez J, Narvaez JA, de Albert M, Gomez-Vaquero C, Nolla JM. Can magnetic resonance imaging of the hand and wrist differentiate between rheumatoid arthritis and psoriatic arthritis in the early stages of the disease? Semin Arthritis Rheum (2012) 42(3):234–45. 10.1016/j.semarthrit.2012.03.016 [DOI] [PubMed] [Google Scholar]
- 25. Brockbank JE, Stein M, Schentag CT, Gladman DD. Dactylitis in psoriatic arthritis: a marker for disease severity? Ann Rheumat Dis (2005) 64(2):188–90. 10.1136/ard.2003.018184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van der Heijde D, Sharp J, Wassenberg S, Gladman DD. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheumat Dis (2005) 64(Suppl 2):ii61–4. 10.1136/ard.2004.030809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lubrano E, Marchesoni A, Olivieri I, D’Angelo S, Spadaro A, Parsons WJ, et al. Psoriatic arthritis spondylitis radiology index: a modified index for radiologic assessment of axial involvement in psoriatic arthritis. J Rheumatol (2009) 36(5):1006–11. 10.3899/jrheum.080491 [DOI] [PubMed] [Google Scholar]
- 28. Robinson PC, Leo PJ, Pointon JJ, Harris J, Cremin K, Bradbury LA, et al. The genetic associations of acute anterior uveitis and their overlap with the genetics of ankylosing spondylitis. Genes Immun (2016) 17(1):46–51. 10.1038/gene.2015.49 [DOI] [PubMed] [Google Scholar]
- 29. Pedersen OB, Svendsen AJ, Ejstrup L, Skytthe A, Junker P. On the heritability of psoriatic arthritis. Disease concordance among monozygotic and dizygotic twins. Ann Rheumat Dis (2008) 67(10):1417–21. 10.1136/ard.2007.078428 [DOI] [PubMed] [Google Scholar]
- 30. Generali E, Ceribelli A, Stazi MA, Selmi C. Lessons learned from twins in autoimmune and chronic inflammatory diseases. J Autoimmun (2017) 83:51–61. 10.1016/j.jaut.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 31. Stuart PE, Nair RP, Ellinghaus E, Ding J, Tejasvi T, Gudjonsson JE, et al. Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat Genet (2010) 42(11):1000–4. 10.1038/ng.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ellinghaus E, Ellinghaus D, Stuart PE, Nair RP, Debrus S, Raelson JV, et al. Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat Genet (2010) 42(11):991–5. 10.1038/ng.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stuart PE, Nair RP, Tsoi LC, Tejasvi T, Das S, Kang HM, et al. Genome-wide Association Analysis of Psoriatic Arthritis and Cutaneous Psoriasis Reveals Differences in Their Genetic Architecture. Am J Hum Genet (2015) 97(6):816–36. 10.1016/j.ajhg.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zuo X, Sun L, Yin X, Gao J, Sheng Y, Xu J, et al. Whole-exome SNP array identifies 15 new susceptibility loci for psoriasis. Nat Commun (2015) 6:6793. 10.1038/ncomms7793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cortes A, Brown MA. Promise and pitfalls of the Immunochip. Arthritis Res Ther (2011) 13(1):101. 10.1186/ar3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bowes J, Budu-Aggrey A, Huffmeier U, Uebe S, Steel K, Hebert HL, et al. Dense genotyping of immune-related susceptibility loci reveals new insights into the genetics of psoriatic arthritis. Nat Commun (2015) 6:6046. 10.1038/ncomms8741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frischknecht L, Vecellio M, Selmi C. The role of epigenetics and immunological imbalance in the etiopathogenesis of psoriasis and psoriatic arthritis. Ther Adv Musculoskeletal Dis (2019) 11:1759720X19886505. 10.1177/1759720X19886505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haroon M, Winchester R, Giles JT, Heffernan E, FitzGerald O. Certain class I HLA alleles and haplotypes implicated in susceptibility play a role in determining specific features of the psoriatic arthritis phenotype. Ann Rheumat Dis (2016) 75(1):155–62. 10.1136/annrheumdis-2014-205461 [DOI] [PubMed] [Google Scholar]
- 39. Haroon M, Winchester R, Giles JT, Heffernan E, FitzGerald O. Clinical and genetic associations of radiographic sacroiliitis and its different patterns in psoriatic arthritis. Clin Exp Rheumatol (2017) 35(2):270–6. [PubMed] [Google Scholar]
- 40. Apel M, Uebe S, Bowes J, Giardina E, Korendowych E, Juneblad K, et al. Variants in RUNX3 contribute to susceptibility to psoriatic arthritis, exhibiting further common ground with ankylosing spondylitis. Arthritis Rheumat (2013) 65(5):1224–31. 10.1002/art.37885 [DOI] [PubMed] [Google Scholar]
- 41. Robinson PC, Lau E, Keith P, Lau MC, Thomas GP, Bradbury LA, et al. ERAP2 functional knockout in humans does not alter surface heavy chains or HLA-B27, inflammatory cytokines or endoplasmic reticulum stress markers. Ann Rheumat Dis (2015) 74(11):2092–5. 10.1136/annrheumdis-2015-207467 [DOI] [PubMed] [Google Scholar]
- 42. Cortes A, Hadler J, Pointon JP, Robinson PC, Karaderi T, Leo P, et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet (2013) 45(7):730–8. 10.1038/ng.2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bowes J, Orozco G, Flynn E, Ho P, Brier R, Marzo-Ortega H, et al. Confirmation of TNIP1 and IL23A as susceptibility loci for psoriatic arthritis. Ann Rheumat Dis (2011) 70(9):1641–4. 10.1136/ard.2011.150102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol (2013) 133(1):17–26. 10.1038/jid.2012.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol (2007) 8(9):950–7. 10.1038/ni1497 [DOI] [PubMed] [Google Scholar]
- 46. Pollock RA, Abji F, Liang K, Chandran V, Pellett FJ, Virtanen C, et al. Gene expression differences between psoriasis patients with and without inflammatory arthritis. J Invest Dermatol (2015) 135(2):620–3. 10.1038/jid.2014.414 [DOI] [PubMed] [Google Scholar]
- 47. Steel KJA, Srenathan U, Ridley M, Durham LE, Wu SY, Ryan SE, et al. Polyfunctional, Proinflammatory, Tissue-Resident Memory Phenotype and Function of Synovial Interleukin-17A+CD8+ T Cells in Psoriatic Arthritis. Arthritis Rheumatol (2020) 72(3):435–47. 10.1002/art.41156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rahman P, Inman RD, Maksymowych WP, Reeve JP, Peddle L, Gladman DD. Association of interleukin 23 receptor variants with psoriatic arthritis. J Rheumatol (2009) 36(1):137–40. 10.3899/jrheum.080458 [DOI] [PubMed] [Google Scholar]
- 49. Huffmeier U, Uebe S, Ekici AB, Bowes J, Giardina E, Korendowych E, et al. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat Genet (2010) 42(11):996–9. 10.1038/ng.688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bianchi E, Rogge L. The IL-23/IL-17 pathway in human chronic inflammatory diseases-new insight from genetics and targeted therapies. Genes Immun (2019) 20(5):415–25. 10.1038/s41435-019-0067-y [DOI] [PubMed] [Google Scholar]
- 51. Danks L, Komatsu N, Guerrini MM, Sawa S, Armaka M, Kollias G, et al. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheumat Dis (2016) 75(6):1187–95. 10.1136/annrheumdis-2014-207137 [DOI] [PubMed] [Google Scholar]
- 52. Paine A, Ritchlin C. Altered Bone Remodeling in Psoriatic Disease: New Insights and Future Directions. Calcified Tissue Int (2018) 102(5):559–74. 10.1007/s00223-017-0380-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kotake S, Yago T, Kawamoto M, Nanke Y. Role of osteoclasts and interleukin-17 in the pathogenesis of rheumatoid arthritis: crucial ‘human osteoclastology’. J Bone Mineral Metab (2012) 30(2):125–35. 10.1007/s00774-011-0321-5 [DOI] [PubMed] [Google Scholar]
- 54. Al-Mossawi MH, Chen L, Fang H, Ridley A, de Wit J, Yager N, et al. Unique transcriptome signatures and GM-CSF expression in lymphocytes from patients with spondyloarthritis. Nat Commun (2017) 8(1):1510. 10.1038/s41467-017-01771-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eberle FC, Bruck J, Holstein J, Hirahara K, Ghoreschi K. Recent advances in understanding psoriasis. F1000Research (2016) 5F1000 Faculty Rev-770. 10.12688/f1000research.7927.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ellinghaus E, Stuart PE, Ellinghaus D, Nair RP, Debrus S, Raelson JV, et al. Genome-wide meta-analysis of psoriatic arthritis identifies susceptibility locus at REL. J Invest Dermatol (2012) 132(4):1133–40. 10.1038/jid.2011.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, et al. NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol (2004) 24(17):7806–19. 10.1128/MCB.24.17.7806-7819.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang LJ. Type1 Interferons Potential Initiating Factors Linking Skin Wounds With Psoriasis Pathogenesis. Front Immunol (2019) 10:1440. 10.3389/fimmu.2019.01440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bowes J, Loehr S, Budu-Aggrey A, Uebe S, Bruce IN, Feletar M, et al. PTPN22 is associated with susceptibility to psoriatic arthritis but not psoriasis: evidence for a further PsA-specific risk locus. Ann Rheumat Dis (2015) 74(10):1882–5. 10.1136/annrheumdis-2014-207187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mease PJ. Tumour necrosis factor (TNF) in psoriatic arthritis: pathophysiology and treatment with TNF inhibitors. Ann Rheumat Dis (2002) 61(4):298–304. 10.1136/ard.61.4.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rahman P, Siannis F, Butt C, Farewell V, Peddle L, Pellett F, et al. TNFalpha polymorphisms and risk of psoriatic arthritis. Ann Rheumat Dis (2006) 65(7):919–23. 10.1136/ard.2005.039164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Coates LC, FitzGerald O, Helliwell PS, Paul C. Psoriasis, psoriatic arthritis, and rheumatoid arthritis: Is all inflammation the same? Semin Arthritis Rheumat (2016) 46(3):291–304. 10.1016/j.semarthrit.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 63. Boutet MA, Nerviani A, Gallo Afflitto G, Pitzalis C. Role of the IL-23/IL-17 Axis in Psoriasis and Psoriatic Arthritis: The Clinical Importance of Its Divergence in Skin and Joints. Int J Mol Sci (2018) 19(2)530. 10.3390/ijms19020530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lubberts E. The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol (2015) 11(7):415–29. 10.1038/nrrheum.2015.53 [DOI] [PubMed] [Google Scholar]
- 65. Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev (2003) 14(2):155–74. 10.1016/s1359-6101(03)00002-9 [DOI] [PubMed] [Google Scholar]
- 66. Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol (2006) 177(1):36–9. 10.4049/jimmunol.177.1.36 [DOI] [PubMed] [Google Scholar]
- 67. Wang EA, Suzuki E, Maverakis E, Adamopoulos IE. Targeting IL-17 in psoriatic arthritis. Eur J Rheumatol (2017) 4(4):272–7. 10.5152/eurjrheum.2017.17037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khan D, Ansar Ahmed S. Regulation of IL-17 in autoimmune diseases by transcriptional factors and microRNAs. Front Genet (2015) 6:236. 10.3389/fgene.2015.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Veldhoen M. Interleukin 17 is a chief orchestrator of immunity. Nat Immunol (2017) 18(6):612–21. 10.1038/ni.3742 [DOI] [PubMed] [Google Scholar]
- 70. Wade SM, Canavan M, McGarry T, Low C, Wade SC, Mullan RH, et al. Association of synovial tissue polyfunctional T-cells with DAPSA in psoriatic arthritis. Ann Rheumat Dis (2019) 78(3):350–4. 10.1136/annrheumdis-2018-214138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Okamoto K, Takayanagi H. Osteoclasts in arthritis and Th17 cell development. Int Immunopharmacol (2011) 11(5):543–8. 10.1016/j.intimp.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 72. Gravallese EM, Schett G. Effects of the IL-23-IL-17 pathway on bone in spondyloarthritis. Nat Rev Rheumatol (2018) 14(11):631–40. 10.1038/s41584-018-0091-8 [DOI] [PubMed] [Google Scholar]
- 73. Cantatore FP, Maruotti N, Corrado A, Ribatti D. Angiogenesis Dysregulation in Psoriatic Arthritis: Molecular Mechanisms. BioMed Res Int (2017) 2017:5312813. 10.1155/2017/5312813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol (2014) 14(9):585–600. 10.1038/nri3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, et al. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol (2012) 188(12):6287–99. 10.4049/jimmunol.1200385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brentano F, Ospelt C, Stanczyk J, Gay RE, Gay S, Kyburz D. Abundant expression of the interleukin (IL)23 subunit p19, but low levels of bioactive IL23 in the rheumatoid synovium: differential expression and Toll-like receptor-(TLR) dependent regulation of the IL23 subunits, p19 and p40, in rheumatoid arthritis. Ann Rheumat Dis (2009) 68(1):143–50. 10.1136/ard.2007.082081 [DOI] [PubMed] [Google Scholar]
- 77. van Baarsen LG, Lebre MC, van der Coelen D, Aarrass S, Tang MW, Ramwadhdoebe TH, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther (2014) 16(4):426. 10.1186/s13075-014-0426-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Canete JD, Martinez SE, Farres J, Sanmarti R, Blay M, Gomez A, et al. Differential Th1/Th2 cytokine patterns in chronic arthritis: interferon gamma is highly expressed in synovium of rheumatoid arthritis compared with seronegative spondyloarthropathies. Ann Rheumat Dis (2000) 59(4):263–8. 10.1136/ard.59.4.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Belasco J, Louie JS, Gulati N, Wei N, Nograles K, Fuentes-Duculan J, et al. Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis Rheumatol (2015) 67(4):934–44. 10.1002/art.38995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Benham H, Norris P, Goodall J, Wechalekar MD, FitzGerald O, Szentpetery A, et al. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res Ther (2013) 15(5):R136. 10.1186/ar4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheumat Dis (2016) 75(3):499–510. 10.1136/annrheumdis-2015-208337 [DOI] [PubMed] [Google Scholar]
- 82. Mease PJ. Inhibition of interleukin-17, interleukin-23 and the TH17 cell pathway in the treatment of psoriatic arthritis and psoriasis. Curr Opin Rheumatol (2015) 27(2):127–33. 10.1097/BOR.0000000000000147 [DOI] [PubMed] [Google Scholar]
- 83. Braun J, Kiltz U, Heldmann F, Baraliakos X. Emerging drugs for the treatment of axial and peripheral spondyloarthritis. Expert Opin Emerg Drugs (2015) 20(1):1–14. 10.1517/14728214.2015.993378 [DOI] [PubMed] [Google Scholar]
- 84. Coates LC, Chandran V, Ogdie A, O’Sullivan D, Brooke M, Steinkoenig I, et al. International Treatment Recommendations Update: A Report from the GRAPPA 2016 Annual Meeting. J Rheumatol (2017) 44(5):684–5. 10.3899/jrheum.170144 [DOI] [PubMed] [Google Scholar]
- 85. Coates LC, Gossec L, Ramiro S, Mease P, van der Heijde D, Smolen JS, et al. New GRAPPA and EULAR recommendations for the management of psoriatic arthritis. Rheumatol (Oxford) (2017) 56(8):1251–3. 10.1093/rheumatology/kew390 [DOI] [PubMed] [Google Scholar]
- 86. Mulder MLM, Vriezekolk JE, den Broeder N, Mahler EAM, Helliwell PS, van den Hoogen FHJ, et al. Comparing methotrexate monotherapy with methotrexate plus leflunomide combination therapy in psoriatic arthritis: protocol of a randomized, placebo-controlled, double-blind clinical trial (COMPLETE-PsA). Trials (2020) 21(1):155. 10.1186/s13063-020-4097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gottlieb A, Narang K. Ustekinumab in the treatment of psoriatic arthritis: latest findings and clinical potential. Ther Adv Musculoskeletal Dis (2013) 5(5):277–85. 10.1177/1759720X13501021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Langley RG, Lebwohl M, Krueger GG, Szapary PO, Wasfi Y, Chan D, et al. Long-term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate-to-severe psoriasis: results from the PHOENIX 2 study through 5 years of follow-up. Br J Dermatol (2015) 172(5):1371–83. 10.1111/bjd.13469 [DOI] [PubMed] [Google Scholar]
- 89. Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheumat Dis (2014) 73(6):990–9. 10.1136/annrheumdis-2013-204655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kavanaugh A, Puig L, Gottlieb AB, Ritchlin C, You Y, Li S, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheumat Dis (2016) 75(11):1984–8. 10.1136/annrheumdis-2015-209068 [DOI] [PubMed] [Google Scholar]
- 91. Mease P, Helliwell P, Gladman D, Poddubnyy D, Baraliakos X, Chakravarty S, et al. OP0054 EFFICACY OF GUSELKUMAB, A MONOCLONAL ANTIBODY THAT SPECIFICALLY BINDS TO THE P19-SUBUNIT OF IL-23, ON ENDPOINTS RELATED TO AXIAL INVOLVEMENT IN PATIENTS WITH ACTIVE PSA WITH IMAGING-CONFIRMED SACROILIITIS: WEEK-24 RESULTS FROM TWO PHASE 3, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDIES. Ann Rheumat Dis (2020) 79:36–7. 10.1136/annrheumdis-2020-eular.474 [DOI] [Google Scholar]
- 92. Smolen JS, Bergmans P, Bondareva I, De Vlam K, Gremese E, Joven-Ibanez B, et al. AB0928 Ustekinumab and tnf inhibitors in psoriatic arthritis: first follow-up data from a routine care study in 8 european countries (PSABIO). Ann Rheumat Dis (2018) 77:1589–90. 10.1136/annrheumdis-2018-eular.6939 [DOI] [Google Scholar]
- 93. Coates LC, Wallman JK, McGonagle D, Schett GA, McInnes IB, Mease PJ, et al. Secukinumab efficacy on resolution of enthesitis in psoriatic arthritis: pooled analysis of two phase 3 studies. Arthritis Res Ther (2019) 21(1):266. 10.1186/s13075-019-2055-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N Engl J Med (2015) 373(26):2534–48. 10.1056/NEJMoa1505066 [DOI] [PubMed] [Google Scholar]
- 95. Nash P, Mease PJ, McInnes IB, Rahman P, Ritchlin CT, Blanco R, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther (2018) 20(1):47. 10.1186/s13075-018-1551-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheumat Dis (2017) 76(1):79–87. 10.1136/annrheumdis-2016-209709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol (2016) 175(2):273–86. 10.1111/bjd.14493 [DOI] [PubMed] [Google Scholar]
- 98. Mease PJ, Genovese MC, Greenwald MW, Ritchlin CT, Beaulieu AD, Deodhar A, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med (2014) 370(24):2295–306. 10.1056/NEJMoa1315231 [DOI] [PubMed] [Google Scholar]
- 99. Deodhar A, Helliwell PS, Boehncke WH, Kollmeier AP, Hsia EC, Subramanian RA, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFalpha inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet (2020) 395(10230):1115–25. 10.1016/S0140-6736(20)30265-8 [DOI] [PubMed] [Google Scholar]
- 100. Deodhar A, Gottlieb AB, Boehncke WH, Dong B, Wang Y, Zhuang Y, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet (2018) 391(10136):2213–24. 10.1136/annrheumdis-2018-eular.2059 [DOI] [PubMed] [Google Scholar]
- 101. Bai F, Li GG, Liu Q, Niu X, Li R, Ma H. Short-Term Efficacy and Safety of IL-17, IL-12/23, and IL-23 Inhibitors Brodalumab, Secukinumab, Ixekizumab, Ustekinumab, Guselkumab, Tildrakizumab, and Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J Immunol Res (2019) 2019:2546161. 10.1155/2019/2546161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. David T, Ling SF, Barton A. Genetics of immune-mediated inflammatory diseases. Clin Exp Immunol (2018) 193(1):3–12. 10.1111/cei.13101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Talamonti M, Galluzzo M, van den Reek JM, de Jong EM, Lambert JLW, Malagoli P, et al. Role of the HLA-C*06 allele in clinical response to ustekinumab: evidence from real life in a large cohort of European patients. Br J Dermatol (2017) 177(2):489–96. 10.1111/bjd.15387 [DOI] [PubMed] [Google Scholar]