Abstract
Background:
Cervical lymph nodes metastases are one of the most significant prognostic factors in patients with laryngeal carcinoma, whether treatment by surgery or by radiotherapy. The current study retrospected the postoperative radiotherapy of locally advanced supraglottic and glottic laryngeal carcinoma (at a greater risk of lymph node metastasis) to determine the effect of radiotherapy excluding cervical level Ⅳ lymph nodes.
Methods:
Patients of supraglottic type and glottic type were irradiated with level Ⅳ from January 2012 to June 2013, without level Ⅳ from July 2013 to December 2014, according to physicians’ decision. Ninety-three patients were selective neck irradiation (SNI) of levels Ⅱ-Ⅳ (Group A) and 87 patients were SNI of levels Ⅱ and Ⅲ (Group B). The comparison between Group A and Group B was made with observation of clinical risk of recurrence and radiation complications, as well as overall survival (OS), progress-free survival (PFS) and regional nodal recurrence-free survival.
Results:
No remarkable difference was observed in the distribution of recurrence, levels of relapse, OS, PFS and regional nodal recurrence-free survival between the 2 groups (p > 0.05). Mean radiation dose at level Ⅳ, thyroid and cervical esophagus showed significant difference between the 2 therapeutic groups (p < 0.01). As regard radiation complications, no significant difference was found in radiation dermatitis of any grade between the 2 groups (p > 0.05). However, there was remarkable difference in clinical hypothyroidism and radiation esophagitis between Group A and Group B (p < 0.05).
Conclusions:
Radiotherapy after surgery omitting level Ⅳ may improve the quality of life in patients with locally advanced supraglottic and glottic laryngeal carcinoma, won’t worsen the prognosis as well.
Keywords: supraglottic laryngeal carcinoma, glottic laryngeal carcinoma, radiation therapy, radiation complication, selective neck irradiation
Introduction
Laryngeal carcinoma is one of the most common malignant carcinoma and comprises around 25% of all head and neck cancers,1 of which glottic type and supraglottic type separately accounts for more than 50% and 30%∼40%.2,3 Cervical lymph node metastasis is one of the most significant prognostic factors in patients with laryngeal carcinoma.4 The cervical lymph node metastasis rate of supraglottic carcinoma is about 55%, most of which are located at level Ⅱ followed by level Ⅲ.5,6 The incidence of metastasis in patients with glottic carcinoma has been reported to happen approximately 80% at level Ⅱ and nearly 20% at level Ⅲ.7 Although it has very low incidence of lymph node positivity within level Ⅳ, some head and neck specialists still keep on surgical options for laryngeal carcinoma generally including levels Ⅱ–Ⅳ.8,9 In fact, more than 90% of laryngeal carcinoma requiring selective cervical dissection only needs to be treated with levels Ⅱ and Ⅲ.10,11 For radiation therapist, level Ⅳ is considered in a radiation setting for routine treatment with locally advanced laryngeal carcinoma, thus it remains a dilemma to including level Ⅳ or not.
The complications of cervical radiotherapy at level Ⅳ of laryngeal carcinoma are mainly including thyroid dysfunction, radiation esophagitis and dermatitis. If selective neck irradiation (SNI) of levels Ⅱ and Ⅲ, without level Ⅳ, it may reduce the radiation complications associated with these lesions, so as to minimize the pain of the patients. The rate of cervical lymph node metastasis in patients with glottic carcinoma is about 5% in T1-T2 and about 20% in T3-T4.12 Locally advanced head and neck disease carries a high risk of metastasis with a poor prognosis.13 Since all stages of supraglottic laryngeal carcinoma and locally advanced glottis carcinoma are prone to cervical lymph node metastasis, to reduce heterogenous in this study, we only retrospected post-operative patients of supraglottic type and glottic type of locally advanced stages Ⅲ-Ⅳ.
Patients and Methods
Patient Characteristics
This retrospective study was approved by the Ethics Committee of our hospital. Informed consent was obtained from all patients prior to treatment. The patients included: locally advanced supraglottic type and glottic type (stage Ⅲ and stage Ⅳ without distant metastasis), requiring radiotherapy after modified or selective radical neck dissection, without chemotherapy. All the patients, performed larynx enhanced CT, chest CT, color Doppler ultrasound of liver, bone scan or PET-CT before surgery. All the patients, undergone pretreatment physical, endoscopic and radiological examinations, had no abnormal thyroid function before radiotherapy, treated with IMRT, collected from January 2012 to December 2014 and follow-up ended in December 2019. Patients of supraglottic type and glottic type were irradiated with level Ⅳ from January 2012 to June 2013, without level Ⅳ from July 2013 to December 2014, according to physicians’ decision over their clinical practice over time: selective neck irradiation (SNI) of level Ⅱ, Ⅲ and Ⅳ (Group A) and SNI of level Ⅱ, Ⅲ (Group B). Of the included patients, 75 were diagnosed with supraglottic carcinoma and 105 were diagnosed with glottic carcinoma, respectively. Demographics and treatment characteristics of SNI of level Ⅳ or not were summarized in Table 1. Twenty-three women and 157 men were included in this analysis. There was no significant difference between Group A and Group B in gender and age (p > 0.05). Other baseline disease characteristics and treatment of pre-radiotherapy were showed generally balanced between the 2 groups (p > 0.05). The distribution of involved positive lymph nodes after surgery confirmed by biospy between Group A and Group B was shown in Table 2. No significant difference was found between the 2 groups (p > 0.05). There was no positive lymph nodes in level Ⅳ, but very few cases in level Ⅵ.
Table 1.
Demographics and Treatment Characteristics.
| Group A (n = 93) | Group B (n = 87) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Categories | Supraglottic | Glottic | Total | Supraglottic | Glottic | Total | p value |
| Gender | Female | 7(7.5%) | 4(4.3%) | 11(11.8%) | 6(6.9%) | 6(6.9%) | 12(13.8%) | 0.694 |
| Male | 32(34.4%) | 50(53.8%) | 82(88.2%) | 30(34.5%) | 45(51.7%) | 75(86.2%) | ||
| Age, years | — | 63.1 ± 4.83 | 65.0 ± 3.78 | 64.2 ± 4.34 | 64.2 ± 5.74 | 65.4 ± 3.02 | 64.9 ± 4.37 | 0.286 |
| Site of primary tumor | — | 39(41.9%) | 54(58.1%) | 93 | 36(41.4%) | 51(58.6%) | 87 | 0.940 |
| Postoperative pathologic | T2 | 2(2.2%) | 0 | 2(2.2%) | 4(4.6%) | 0 | 4(4.6%) | 0.381 |
| T stage (AJCC 7th) | T3 | 25(26.9%) | 36(38.7%) | 61(65.6%) | 23(26.4%) | 36(41.4%) | 59(67.8%) | |
| T4 | 12(12.9%) | 18(19.3%) | 30(32.2%) | 9(10.4%) | 15(17.2%) | 24(27.6%) | ||
| Postoperative pathologic | N0 | 2(2.2%) | 25(26.9%) | 27(29.1%) | 4(4.6%) | 26(29.9%) | 30(34.5%) | 0.587 |
| N stage (AJCC 7th) | N1 | 4(4.3%) | 9(9.7%) | 13(14.0%) | 2(2.3%) | 8(9.2%) | 10(11.5%) | |
| N2a | 6(6.4%) | 4(4.3%) | 10(10.7%) | 5(5.7%) | 4(4.6%) | 9(10.3%) | ||
| N2b | 15(16.1%) | 10(10.7%) | 25(26.9%) | 13(14.9%) | 9(10.3%) | 22(25.3%) | ||
| N2c | 8(8.6%) | 4(4.3%) | 12(12.9%) | 9(10.3%) | 2(2.3%) | 11(12.6%) | ||
| N3 | 4(4.3%) | 2(2.2%) | 6(6.5%) | 3(3.4%) | 2(2.3%) | 5(5.7%) | ||
| Overall stage※ | Ⅲ | 6(6.4%) | 29(31.2%) | 35(37.6%) | 6(6.9%) | 28(32.2%) | 34(39.1%) | 0.842 |
| Ⅳa-b | 33(35.5%) | 25(26.9%) | 58(62.4%) | 30(34.5%) | 23(26.4%) | 53(60.9%) | ||
| Lead time#, days | — | 25.8 ± 7.23 | 28.1 ± 5.54 | 27.1 ± 6.37 | 26.3 ± 6.76 | 26.1 ± 6.24 | 26.2 ± 6.42 | 0.329 |
| Neck dissection | Modified radical | 11(11.8%) | 7(7.5%) | 18(19.3%) | 13(14.9%) | 7(8.0%) | 20(23.0%) | 0.551 |
| Selective radical | 28(30.1%) | 47(50.5%) | 75(80.6%) | 23(26.4%) | 44(50.6%) | 67(77.0%) | ||
Group A = selective neck irradiation of level Ⅱ—Ⅳ; Group B = selective neck irradiation of level Ⅱ and Ⅲ.
※According to the 7th UICC/AJCC staging system.
# The time between surgery and the first fraction of IMRT.
Table 2.
Numbers of Lymph Nodes Involvement According to the 2 Therapeutic Groups Before Radiotherapy.
| Group A | Group B | |||||||
|---|---|---|---|---|---|---|---|---|
| Lymph node | Side | Supraglottic | Glottic | Total | Supraglottic | Glottic | Total | p value |
| Level Ⅱ | Ipsilateral | 107(66.5%) | 54(33.5%) | 161 | 97(64.2%) | 54(35.8%) | 151 | 0.339 |
| Level Ⅱ | Contralateral | 23(74.2%) | 8(25.8%) | 31 | 19(82.6%) | 4(17.4%) | 23 | |
| Level Ⅲ | Ipsilateral | 45(72.6%) | 17(27.4%) | 62 | 35(71.4%) | 14(28.6%) | 49 | |
| Level Ⅵ | 1(25.0%) | 3(75.0%) | 4 | 1(33.3%) | 2(66.7%) | 3 | ||
| Total | 176(68.2%) | 82(31.8%) | 258 | 152(67.3%) | 74(32.7%) | 226 | ||
Group A = selective neck irradiation of level Ⅱ—Ⅳ; Group B = selective neck irradiation of level Ⅱ and Ⅲ.
Target Volume Delineations for IMRT
All the patients received intensity-modulated radiotherapy (IMRT) in our institution. Gross Tumor Volume tumor bed (GTVtb): sketched the gross tumor according to the localization of CT, laryngoscope and PET-CT (part of patients) before resection. Clinical Target Volume 1 (CTV1): the high-risk clinical target area, including 10 mm outside of GTVtb, avoided scaling out while encountering bone and cavity anatomical barrier, and the involving area of positive lymph nodes before resection in the neck. Clinical Target Volume 2 (CTV2): low risk clinical target area, including 5 mm outside of CTV1 and the whole larynx, avoided scaling out while encountering anatomical barrier, wherein the small target group also included cervical levels Ⅱ and Ⅲ. Meanwhile, the lower boundary of the target area was 20 mm more than it of the cervical positive lymph nodes (except that the only one case in this study reached 3 mm below the inferior boundary of the cricoid cartilage, the target area of the other patients did not reach the level Ⅳ). Besides, the large target group also included cervical levels Ⅱ, Ⅲ and Ⅳ. Planning Target Volume (PTV): margins of 3 mm was added to the GTVtb to generate PTV-GTVtb. Margins of 3 mm was added to the CTV1 to generate PTV1 and to the CTV2 to generate PTV2.
Radiotherapy Dose
Radiation was delivered via 6-MV photon field. The single dose of PTV-GTVtb was 2.0 Gy and the total dose was 60Gy-66 Gy (NCCN Clinical Practice Guidelines in OncologyTM Head and Neck Cancers. Version.1.2010). The single dose of PTV1 was 2 Gy and the total dose was 60 Gy. The single dose of PTV2 was 1.8 Gy and the total dose was 54 Gy. SNI was undergone to bilateral levels Ⅱ-Ⅳ or levels Ⅱ and Ⅲ. Radiation dose in level Ⅳ, thyroid and cervical esophagus between the 2 groups was shown by the dose volume histogram (DVH) chart.
Observation of Radiation Complications
The fasting serum levels of Thyroid Stimulating Hormone (TSH), Free Triiodothyronine (FT3) and Free Thyroxine (FT4) were measured at the end of radiotherapy, 6 months and 12 months after radiotherapy by eclectro-chemiluminescence immunoassay (ECLI). TSH > 4.2 μ IU / ml was diagnosed subclinical hypothyroidism. FT4 < 12pmol/L or FP3 < 3.1pmol/L was diagnosed clinical hypothyroidism. Each patient was investigated weekly during and after radiotherapy by a radiation oncologist who was blinded to the group of patients assigned to him. Acute esophageal toxicity (symptoms less than 3 months) was assessed by review of the time parameters concerning beginning or seizing of symptoms. The RTOG toxicity scale was used to grade the side effects. In grade 1, the patient has mild dysphagia or odynophagia; may require topical anesthetic, nonnarcotic agents, or soft diet. In grade 2, the patient has moderate dysphagis or odynophagia; may require narcotic agents or puree/liquid diet. In grade 3, the patient has severe dysphagia or odynophagia with dehydration or weight loss (>15% from pretreatment baseline) requiring nasogastric feeding tube or hyperalimentation. In grade 4, the patient has complete obstruction, ulceration, perforation, or fistula. As for radiation skin injury, it was recorded according to the RTOG radiodermatitis scoring (grade 1: follicular, faint or dull erythema, epilation, dry desquamation, decreased sweating; grade 2: tender or bright erythema, patchy moist desquamation, moderate edema; grade 3: confluent, moist desquamation other than skin folds, pitting edema; grade 4: ulceration, hemorrhage, necrosis).
Follow-Up
Follow-up was scored from the completion of operation to first documented clinical disease progression or first documented regional nodal recurrence or the last visit or death. All patients were reexamined every 2 to 3 months after radiotherapy and every 4 to 6 months in the third year. Physical examination and laryngoscope were performed every time. Larynx and chest CT and liver color Doppler ultrasound were done once or twice a year. PET-CT could be considered in the clinical suspected recurrence or metastasis. The relapsed number of cervical levels was counted at the first recurrence in each patient.
Statistical Analysis
Group A and group B were compared by means of the chi-square, Fisher’s test, or nonparametric test. Comparison of radiation dose between the 2 groups was analyzed using the independent samples t-test. The rates of overall survival (OS), progress-free survival (PFS) and regional nodal recurrence-free survival were estimated using the Kaplan-Meier method. OS was calculated from the date of completion of operation to the date of death at the time of last contact. Meanwhile date for patients who were alive or lost to follow-up was defined to be censored. PFS was calculated from the date of completion of operation to the date of progression at the time of last tumor imaging. Meanwhile date for patents without disease progression or who were lost to follow-up was defined to be censored. Regional nodal recurrence-free survival was calculated from the date of completion of operation to the date of neck lymph node relapse at the time of the last pathological diagnosis. Meanwhile date for patents without neck lymph node relapse or who were lost to follow-up was defined to be censored. Survival distributions of the 2 groups were compared by the log-rank test. Differences with p-values < 0.05 were considered statistically significant. Statistical calculations were performed using SPSS software, version 18.0.
Results
Comparison of the Characteristics of Recurrence and Lymph Node Spread Between SNI Levels Ⅱ-Ⅳ Group and SNI Levels Ⅱ and Ⅲ Group
Of the 93 patients in SNI levels Ⅱ-Ⅳ group (Group A), 52(55.9%) had no recurrence, by contrast, 53(60.9%)had no recurrence out of 87 patients in SNI levels Ⅱ and Ⅲ group (Group B). Forty-one patients (44.1%) in Group A had tumor recurrences, of which 24(25.8%) patients were found to develop local recurrence, 14(15.1%) developed distant metastases, 22(23.7%) developed ipsilateral metastases, and 4(4.3%) developed bilateral neck metastases. A total of 34 patients (39.1%) in Group B had tumor recurrences, of which 23(26.4%) patients were found to develop local recurrence, 11(12.6%) developed distant metastases, 19(21.8%) developed ipsilateral metastases, and 3(3.4%) developed bilateral neck metastases. No statistically significant difference was observed between the 2 groups (p > 0.05), as shown in Table 3. It also lists the distribution (sub) levels of nodal relapse. The number of patients with recurrence by neck level Ⅱ was 19(20.4%) ipsilateral and 4(4.3%) contralateral metastases in Group A, as well as 18(20.7%) ipsilateral and 3(3.4%) contralateral metastases in Group B. Nine of the 93 patients with recurrence by neck level Ⅲ in Group A, and 4(4.6%) in Group B. A total of 9(5.0%) patients had level Ⅲ lymph nodes be involved, accompanied by positive nodes at level Ⅱ. Three(1.7%) patients had positive nodes simultaneously at level Ⅵ and level Ⅱ. No relapsed positive nodes was found in level Ⅳ. There was no significant difference between Group A and Group B in the distribution levels of relapse (p > 0.05).
Table 3.
Recurrence According to the 2 Therapeutic Groups.
| Group A (n = 93) | Group B (n = 87) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Categories | Supraglottic | Glottic | Total | Supraglottic | Glottic | Total | p value |
| Recurrence by distribution | NO | 20(21.5%) | 32(34.4%) | 52(55.9%) | 21(24.1%) | 32(36.8%) | 53(60.9%) | 0.622 |
| Local | 0 | 6(6.5%) | 6(6.5%) | 2(2.3%) | 6(6.9%) | 8(9.2%) | ||
| Ipsilateral neck | 8(8.6%) | 2(2.2%) | 10(10.8%) | 5(5.7%) | 2(2.3%) | 7(8.0%) | ||
| Bilateral neck | 0 | 0 | 0 | 1(1.1%) | 0 | 1(1.1%) | ||
| Distant metastasis | 4(4.3%) | 3(3.2%) | 7(7.5%) | 1(1.1%) | 2(2.3%) | 3(3.4%) | ||
| Local + Ipsilateral neck | 5(5.4%) | 4(4.3%) | 9(9.7%) | 4(4.6%) | 2(2.3%) | 6(6.9%) | ||
| Local + Bilateral neck | 0 | 2(2.2%) | 2(2.2%) | 0 | 1(1.1%) | 1(1.1%) | ||
| Local + Ipsilateral neck + Distant metastasis | 1(1.1%) | 2(2.2%) | 3(3.2%) | 2(2.3%) | 4(4.6%) | 6(6.9%) | ||
| Local + Bilateral neck + Distant metastasis | 1(1.1%) | 1(1.1%) | 2(2.2%) | 0 | 1(1.1%) | 1(1.1%) | ||
| Local + Distant metastasis | 0 | 2(2.2%) | 2(2.2%) | 0 | 1(1.1%) | 1(1.1%) | ||
| Recurrence by neck level | 0.567 | |||||||
| Ipsilateral | Ⅱ | 8(8.6%) | 4(4.3%) | 12(12.9%) | 7(8.0%) | 6(6.9%) | 13(14.9%) | |
| Ⅲ | 2(2.2%) | 1(1.1%) | 3(3.2%) | 1(1.1%) | 0 | 1(1.1%) | ||
| Ⅱ + Ⅲ | 4(4.3%) | 2(2.2%) | 6(6.5%) | 2(2.3%) | 1(1.1%) | 3(3.4%) | ||
| Ⅱ + Ⅵ | 0 | 1(1.1%) | 1(1.1%) | 1(1.1%) | 1(1.1%) | 2(2.3%) | ||
| Contralateral | Ⅱ | 1(1.1%) | 3(3.2%) | 4(4.3%) | 1(1.1%) | 2(2.3%) | 3(3.4%) | |
Group A = selective neck irradiation of level Ⅱ—Ⅳ; Group B = selective neck irradiation of level Ⅱ and Ⅲ.
Comparison of Survival Rates Between SNI Levels Ⅱ-Ⅳ Group and SNI Levels Ⅱ and Ⅲ Group
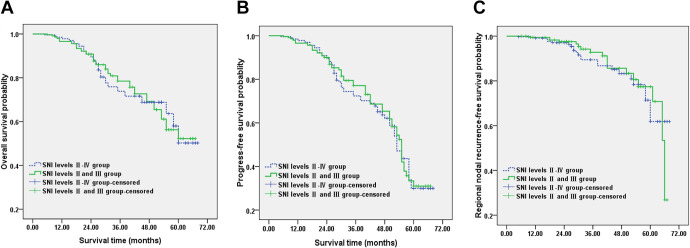
The median follow-up time was 56.8 (5-68) months in Group A and 55.8 (6-67) months in Group B. The Kaplan-Meier 5-year estimate for overall survival (OS) were 50.2% in SNI levels Ⅱ-Ⅳ group (Group A) and 52.2% SNI levels Ⅱ and Ⅲ group (Group B). Overall survival rates were not significantly different between Group A and Group B (p > 0.05, multiple intersections existed between the 2 groups) (Figure 1A). The 5-year progress-free survival (PFS) estimate rates in Group A and in Group B were 29.7% and 31.0%. The survival rates differences showed no significance (p > 0.05, multiple intersections existed between the 2 groups) (Figure 1B). The Kaplan-Meier estimate for regional nodal recurrence-free survival was showed in Figure 1C. There was no significantly difference between Group A and Group B in 5 years (p > 0.05).
Figure 1.
Comparison of the survival curve according to selective neck irradiation of level Ⅱ-Ⅳ (Group A) and selective neck irradiation of level Ⅱ, Ⅲ (Group B). A, The overall survival curve. B, The progress-free survival curve. C, The regional nodal recurrence-free survival curve.
Comparison of Radiation Complications Between SNI Levels Ⅱ-Ⅳ Group and SNI Levels Ⅱ and Ⅲ Group
DVH charts of 2 patients, irradiation including level Ⅳ and excluding level Ⅳ were separately showed in Figure 2A and Figure 2B. As shown in Table 4, in SNI levels Ⅱ-Ⅳ group (Group A), level Ⅳ lied in the radiation range, with mean radiation dose 52.6 Gy at level Ⅳ, 49.0 Gy at thyroid and 38.1 Gy at cervical esophagus. Accordingly, in SNI levels Ⅱ and Ⅲ group (Group B), level Ⅳ received 6.2 Gy of mean radiation dose, with 28.7 Gy at thyroid and 11.1 Gy at cervical esophagus. Independent samples t-test was estimated between Group A and Group B, with significant difference (p < 0.01, Table 4). There were a total of 75 patients in Group A developed thyroid dysfunction including subclinical and clinical hypothyroidism, as well as 30 in Group B. As shown in Table 5, there was significant difference in thyroid dysfunction between Group A and Group B (p < 0.05). Seventy-eight (83.9%) patients in Group A and 44 (50.6%) in Group B produced radiation esophagitis at all stages. Radiation esophagitis of Group B showed remarkable difference from Group A (p < 0.05). No significant difference was found in radiation dermatitis of any grade between Group A (93 patients) and Group B (85 patients) (p > 0.05). The above complications in each group are listed in Table 5.
Figure 2.
The dose volume histogram (DVH) chart of level Ⅳ lymph nodes (LN-4), thyroid and esophagus treated with intensity-modulated radiotherapy (IMRT). A, Selective neck irradiation of level Ⅱ-Ⅳ. B, Selective neck irradiation of level Ⅱ, Ⅲ.
Table 4.
Comparison of Mean Radiation Dose Between the 2 Therapeutic Groups.
| Mean radiation dose (Gy) | |||
|---|---|---|---|
| Area | Group A (n = 93) | Group B (n = 87) | p value |
| Level Ⅳ | 52.6 ± 1.45 | 6.2 ± 1.16 | 0.001 |
| Thyroid | 49.0 ± 1.49 | 28.7 ± 2.17 | 0.001 |
| Esophagus | 38.1 ± 1.29 | 11.1 ± 1.15 | 0.001 |
Group A = selective neck irradiation of level Ⅱ—Ⅳ; Group B = selective neck irradiation of level Ⅱ and Ⅲ.
Table 5.
Radiation Complications According to the 2 Therapeutic Groups.
| Group A (n = 93) | Group B (n = 87) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Categories | Supraglottic | Glottic | Total | Supraglottic | Glottic | Total | p value |
| Thyroid dysfunction | Subclinical hypothyroidism | 17(18.3%) | 19(20.4%) | 36(38.7%) | 10(11.5%) | 14(16.1%) | 24(27.6%) | 0.003 |
| Clinical hypothyroidism | 18(19.4%) | 21(22.6%) | 39(41.9%) | 2(2.3%) | 4(4.6%) | 6(6.9%) | ||
| Radiation esophagitis | Ⅰ | 14(15.1%) | 19(20.4%) | 33(35.5%) | 13(14.9%) | 15(17.2%) | 28(32.2%) | 0.006 |
| Ⅱ | 17(18.3%) | 12(12.9%) | 29(31.2%) | 3(3.4%) | 6(6.9%) | 9(10.3%) | ||
| Ⅲ | 8(8.6%) | 8(8.6%) | 16(17.2%) | 3(3.4%) | 4(4.6%) | 7(8.0%) | ||
| Radiation dermatitis | Ⅰ | 17(18.3%) | 23(24.7%) | 40(43.0%) | 20(23.0%) | 23(26.4%) | 43(49.4%) | 0.456 |
| Ⅱ | 12(12.9%) | 20(21.5%) | 32(34.4%) | 10(11.5%) | 18(20.7%) | 28(32.2%) | ||
| Ⅲ | 10(10.7%) | 7(7.5%) | 17(18.3%) | 6(6.9%) | 8(9.2%) | 14(16.1%) | ||
Group A = selective neck irradiation of level Ⅱ—Ⅳ; Group B = selective neck irradiation of level Ⅱ and Ⅲ.
Discussion
In the current study, data of locally advanced supraglottic type and glottic type patients (stages Ⅲ and Ⅳ without distant metastasis) were retrospected. No significant difference was found for the results of OS, PFS and regional nodal recurrence-free survival, which suggest that the postoperative radiotherapy omitting level Ⅳ may not worsen the prognosis and there were no discrepancy in therapeutic efficacy between the 2 groups. It is reported that the presence of lymph node metastasis before treatment is an important prognostic factor for head and neck cancer, even though the presence of one positive lymph node is considered to reduce OS by 50%.14 In this study, there is no cervical lymph node metastasis found at level Ⅳ before and after treatment, which is common in our clinical practice. Our results may supported by the following: the lymphatic fluid of supraglottic and glottic areas flows mainly into the lymph nodes of level Ⅱ and Ⅲ15; ipsilateral Levels II and III are reported to be the main regions of neck metastases16,17; lymph nodes at level Ⅳ are found extremely infrequently6,18; contralateral level Ⅳ shows no positive metastatic lymph nodes in any of the stages.11,19 Surgical reports showed that elective dissection of lymph nodes at levels Ⅱ-Ⅳ is indicated for patients with T3 and T4 laryngeal cancers.20 Besides, it is reported that metastasis at level Ⅳ may not play a role in a fatal prognosis.15
It is found in our daily work that recurrence and/or metastasis of laryngeal carcinoma after treatment may occur whether radiotherapy including level Ⅳ or not. Deleterious effect of neck recurrence on quality of life is worth of elective neck treatment, at least at the level of high-risk lymph nodes in different subgroups of patients.21 Therefore, we tend to choose patients at a greater risk of lymph node metastasis (patients of locally advanced stages Ⅲ-Ⅳ supraglottic type and glottic type) for this study. Since advanced stage laryngeal cancer often requires a multimodal treatment of surgery and radiotherapy with or without chemotherapy, and its main influencing factors for treatment are related to the primary tumor.15,22 If treatment tailored to the site of the primary cancer, prophylactic neck radiotherapy will decrease the risk of recurrence and spread to ipsilateral or bilateral nodal sites.13 Thus, the incidence of late metastatic development seems to depend on the location and the extension of the primary tumor. From our data, whether the distribution of recurrence or the level of lymph node involved, the effect of radiotherapy omitting level Ⅳ or not seems unchanged.
To minimize the postoperative morbidity, some experts proposed a highly selective neck dissection by the omission of levels Ⅱb and Ⅳ.19,23 Indeed, for radiotherapy, there are no important organs at level Ⅱb that need to be avoided. The advantage of omitting Level Ⅳ is that it prevents complications associated with radiotherapy lesions. Neck irradiation omitting level Ⅳ can avoid irradiation of thyroid, esophagus, tracheostomy and lung tip, thereby reducing radiation damage to corresponding organs and reducing irradiation dose, as well as reducing bone marrow suppression. Thyroid gland is more sensitive to radiation, and more than half volume of thyroid gland is located in the irradiation range of level Ⅳ, so thyroid gland is extremely vulnerable to damage, resulting in disorder of metabolism of the body. The major complications of radiotherapy associated with level Ⅳ are thyroid dysfunction, radiation esophagitis and dermatitis. The results of this study showed that there was remarkable decrease in thyroid dysfunction and radiation esophagitis after radiation excluding level Ⅳ. In China, in order to simply keep the respiratory tract unobstructed and sputum aspiration of the laryngocarcinoma patients, it is advocated the use of metal tracheal cannula after tracheotomy. Mental tracheal cannula has such characteristics as cheap price, cleanable and not easy to block, so it permits a long wearing time. In the irradiation including level Ⅳ, medical staff will replace the metal tracheal cannula with a plastic tracheal cannula to facilitate radiotherapy. Neck irradiation without level Ⅳ can also avoid damage caused by tracheal cannula replacement.
It is questioned to remove level Ⅳ, probably because risk for associated morbidity may increase accordingly. However, level Ⅳ nodes are rarely the solely involved nodes in head and neck primary tumors.20 It is well-known fact that metastasis preferentially proceeds along lymph node levels and rarely bypasses or skips the succeeding level.17 In this study, 9 patients had level Ⅲ lymph nodes be involved after radiotherapy, accompanied by positive nodes at level Ⅱ, without level Ⅳ be involved. Three patients had positive nodes simultaneously at level Ⅵ and level Ⅱ (the recurrence sites of these patients all in the radiation field). Even if patients with laryngeal carcinoma had lymph node metastasis at level Ⅳ in clinical practice, large lymph nodes would appear at level Ⅲ simultaneously, and were located within 20 mm below the lower bound of level Ⅲ.
The present study has several limitations. Firstly, to lessen heterogeneous population, the retrospective design limited the collection of the stage; secondly, for the strictly matching conditions, the sample is still small; thirdly, there was discrepancy sample size between Group A and Group B, as well as between supraglottic and glottic laryngeal carcinoma. It may be feasible to omit level Ⅳ in the patients with locally advanced supraglottic and glottic laryngeal carcinoma. However, a larger prospective clinical trial might be warranted to endorse the benefit.
Conclusions
Our findings suggest that the postoperative radiotherapy omitting level Ⅳ will not worsen the prognosis in supraglottic and glottic laryngeal carcinoma. Moreover, radiotherapy without level Ⅳ may reduce radiation damage and relieve suffering of patients.
Acknowledgments
We thank our colleagues Xin Hou, Mingming Guo for their help of collecting clinical data and our friends Binghua Wang, Ping Zeng for their help of analysis of data.
Abbreviations
- CT
computerized tomography
- CTV
clinical target volume
- DVH
dose volume histogram
- ECLI
eclectro-chemiluminescence immunoassay
- FT4
free thyroxine
- FT3
free triiodothyronine
- GTVtb
gross tumor volume tumor bed
- IMRT
intensity modulated radiotherapy
- OS
overall survival
- PET-CT
positron emission tomography-computerized tomography
- PFS
progress-free survival
- PTV
planning target volume
- SNI
selective neck irradiation
- TSH
thyroid stimulating hormone
Authors’ Note: Our study was approved by The Ethics Committee of Xuzhou Medical University Affiliated Hospital (approval no. XYFY2016-YL031-02). All patients or the patients’ carer provided written informed consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Six Talent Peaks Project in Jiangsu Province (WSN-119).
ORCID iD: Hongmin Yu https://orcid.org/0000-0001-5667-2891
https://orcid.org/0000-0001-5667-2891
References
- 1. Megwalu UC, Sikora AG. Survival outcomes in advanced laryngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;9140(9):855–860. [DOI] [PubMed] [Google Scholar]
- 2. Zhang Q, Lai FY, Guo ZM, et al. Correlation of cervical lymphatic metastasis to prognosis of glottic carcinoma: a report of 333 cases [in Chinese]. Ai Zheng. 2007;26(10):1138–1142. [PubMed] [Google Scholar]
- 3. Yang L, Guo ZM, Chen WK, Zeng ZY. Detection and clinical significance of lymphatic microvessel density in supraglottic laryngeal carcinoma [in Chinese]. Ai Zheng. 2009;28(6):637–641. [PubMed] [Google Scholar]
- 4. Johnson JT. Carcinoma of the larynx: selective approach to the management of cervical lymphatics. Ear Nose Throat J. 1994;73(5):303–305. [PubMed] [Google Scholar]
- 5. Lee NK, Goepfert H, Wendt CD. Supraglottic laryngectomy for intermediate-stage cancer: U.T. M.D. Anderson cancer center experience with combined therapy. Laryngoscope. 1990;100(8):831–836. [DOI] [PubMed] [Google Scholar]
- 6. Zhao J, Xu Y, Zhou Y, Zhou F, Liu D. Study of the lymph node micrometastasis in patients with supraglottic carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;22(18):837–839. [PubMed] [Google Scholar]
- 7. Mukherji SK, Armao D, Joshi VM. Cervical nodal metastases in squamous cell carcinoma of the head and neck: what to expect. Head Neck. 2001;23(11):995–1005. [DOI] [PubMed] [Google Scholar]
- 8. Buckley JG, MacLennan K. Cervical node metastases in laryngeal and hypopharyngeal cancer: a prospective analysis of prevalence and distribution. Head Neck. 2000;22(4):380–385. [DOI] [PubMed] [Google Scholar]
- 9. Birkeland AC, Rosko AJ, Issa MR, et al. Occult nodal disease prevalence and distribution in recurrent laryngeal cancer requiring salvage laryngectomy. Otolaryngol Head Neck Surg. 2016;154(3):473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khafif A, Fliss DM, Gil Z, Medina JE. Routine inclusion of level IV in neck dissection for squamous cell carcinoma of the larynx: is it justified? Head Neck. 2004;26(4):309–312. [DOI] [PubMed] [Google Scholar]
- 11. Lim YC, Choi EC, Lee JS, et al. Is dissection of level IV absolutely necessary in elective lateral neck dissection for clinically N0 laryngeal carcinoma? Oral Oncol. 2006;42(1):102–107. [DOI] [PubMed] [Google Scholar]
- 12. Million RR. The larynx…so to speak: everything I wanted to know about laryngeal cancer I learned in the last 32 years. Int J Radiat Oncol Biol Phys. 1992;23(4):691–704. [DOI] [PubMed] [Google Scholar]
- 13. Chow LQM. Head and neck cancer. N Engl J Med. 2020;382(1):60–72. [DOI] [PubMed] [Google Scholar]
- 14. Hingsammer L, Seier T, Ikenberg J, et al. The influence of lymph node ratio on survival and disease recurrence in squamous cell carcinoma of the tongue. Int J Oral Maxillofac Surg. 2019;48(7):851–856. [DOI] [PubMed] [Google Scholar]
- 15. Teymoortash A, Werner JA. Current advances in diagnosis and surgical treatment of lymph node metastasis in head and neck cancer. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2012;11:Doc04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karatzanis AD, Psychogios G, Waldfahrer F, et al. Management of locally advanced laryngeal cancer. J Otolaryngol Head Neck Surg. 2014;43(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eder-Czembirek C, Erlacher B, Thurnher D, Erovic BM, Selzer E, Formanek M. Comparative analysis of clinical and pathological lymph node staging data in head and neck squamous cell carcinoma patients treated at the General Hospital Vienna. Radiol Oncol. 2018;52(2):173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chone CT, Kohler HF, Magalhães R, Navarro M, Altemani A, Crespo AN. Levels II and III neck dissection for larynx cancer with N0 neck. Braz J Otorhinolaryngol. 2012;78(5):59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsushima N, Hayashi R, Shinozaki T, Tomioka T, Okano W, Ikeda M. The role of elective neck dissection for cT4aN0 glottic squamous cell carcinoma. Jpn J Clin Oncol. 2019;49(6):525–528. [DOI] [PubMed] [Google Scholar]
- 20. Kulzer MH, Branstetter BF IV. Chapter 1 Neck anatomy, imaging-based level nodal classification and impact of primary tumor site on patterns of nodal metastasis. Semin Ultrasound CT MR. 2017;38(5):454–465. [DOI] [PubMed] [Google Scholar]
- 21. Ahn PH, Mitra N, Alonso-Basanta M, et al. Nodal metastasis and elective nodal level treatment in sinonasal small-cell and sinonasal undifferentiated carcinoma: a surveillance, epidemiology and end results analysis. Br J Radiol. 2016;89(1058):20150488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elicin O, Giger R. Comparison of current surgical and non-surgical treatment strategies for early and locally advanced stage glottic laryngeal cancer and their outcome. Cancers (Basel). 2020;12(3):732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanayama N, Nishiyama K, Kawaguchi Y, et al. Selective neck irradiation for supraglottic cancer: focus on sublevel IIb omission. Jpn J Clin Oncol. 2016;46(1):51–56. [DOI] [PubMed] [Google Scholar]