Abstract
Background:
Drug resistance in cancer cells is a major challenge for anti-cancer therapy. Circular RNA (circRNA) circ_0003998 has been identified as an important regulator in the chemoresistance development of non-small cell lung cancer (NSCLC). The purpose of this study was to investigate the molecular basis underlying the resistance control of circ_0003998 in NSCLC.
Methods:
The levels of circ_0003998, miR-136-5p and coronin 1C (CORO1C) were gauged by the quantitative real-time polymerase chain reaction (qRT-PCR) or western blot. Cell viability, colony formation and apoptosis were evaluated by the Cell Counting Kit-8 (CCK-8), colony formation and flow cytometry assays, respectively. Targeted relationships among circ_0003998, miR-136-5p and CORO1C were confirmed by the dual-luciferase reporter and RNA immunoprecipitation (RIP) assays. Animal studies were performed to evaluate the function of circ_0003998 in vivo.
Results:
Our data indicated that circ_0003998 expression was associated with NSCLC resistance to docetaxel (DTX). The knockdown of circ_0003998 promoted DTX sensitivity, suppressed cell colony formation, and enhanced cell apoptosis of A549/DTX and H1299/DTX cells in vitro. Moreover, circ_0003998 knockdown hampered tumor growth and enhanced DTX sensitivity in vivo. Mechanistically, circ_0003998 directly targeted miR-136-5p, and miR-136-5p was a molecular mediator of circ_0003998 function in vitro. Furthermore, CORO1C was a functionally important target of miR-136-5p in regulating DTX-resistant NSCLC cell colony formation, apoptosis and DTX sensitivity in vitro. Additionally, circ_0003998 modulated CORO1C expression by working as a miR-136-5p sponge.
Conclusion:
Our present work identified that circ_0003998 regulated DTX-resistant NSCLC cell colony formation, apoptosis and DTX sensitivity at least partially by controlling CORO1C expression by sponging miR-136-5p, illuminating a rationale for developing circ_0003998 as a therapeutic target of chemoresistant NSCLC.
Keywords: NSCLC, chemoresistance, circ_0003998, miR-136-5p, CORO1C
Introduction
Non-small cell lung cancer (NSCLC) remains the leading cause of cancer morbidity and mortality worldwide.1 Chemotherapy agents, including docetaxel (DTX), have been widely used for NSCLC treatment.2-5 Nonetheless, the development of drug resistance has become a major problem in successful NSCLC treatment.6 Hence, understanding the molecular determinants underlying the regulation of NSCLC chemoresistance will provide the basis for molecularly targeted therapeutics.
Covalently closed circular RNAs (circRNAs) are natural RNA circles that are highly present in the cytoplasm of eukaryotic cells.7 Some circRNAs have been implicated in the tumorigenesis and chemoresistance development of NSCLC by acting as microRNA (miRNA) sponges.8-10 For instance, Dong et al. demonstrated that circ_0076305 enhanced the development of cisplatin resistance in NSCLC by sequestering miR-296-5p.11 Zhou et al. identified that circ_0004015 functioned as a driver in the development of drug resistance of NSCLC by sponging miR-1183.12 As for circ_0003998, an overexpressed circRNA in NSCLC, it was identified as a potential oncogene in NSCLC development.13 More interestingly, circ_0003998 was highlighted as an important modulator in the development of chemoresistance of NSCLC.14 Nevertheless, the molecular basis underlying the resistance control of circ_0003998 in NSCLC remains unknown.
miRNAs are important regulators in the tumorigenesis and chemoresistance development of NSCLC.15 Xie et al. uncovered that miR-136-5p, an underexpressed miRNA in NSCLC, was associated with the outcome of these patients.16 Geng et al. showed that miR-136-5p was involved in circ_0014130-mediated regulation in NSCLC.17 However, the precise parts of miR-136-5p in NSCLC chemoresistance have not been explored. Furthermore, it is still unclear whether miR-136-5p can act as a downstream effector of circ_0003998 function in the chemoresistance development of NSCLC.
Here, we identified that circ_0003998 regulated DTX-resistant NSCLC cell colony formation, apoptosis and DTX sensitivity by the miR-136-5p/coronin 1C (CORO1C) axis, providing a novel mechanism of circ_0003998 in NSCLC chemoresistance.
Materials and Methods
Patients and Tissue Samples
From September 2016 to April 2018, 25 patients with primary NSCLC and 25 recurrent NSCLC patients after cure with DTX-based chemotherapy were recruited at Shandong Provincial Third Hospital. During the treatment with DTX-based chemotherapy, the efficacy was assessed every 2 weeks. The follow-up information was recorded every 3 months after treatment. According to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1), when the sum of lesion diameter increased by 20% compared with the initial baseline or the sum of lesion diameter increased more than 5 mm, the disease was identified as a recurrent NSCLC. The clinicopathologic features of these patients were provided in Table 1. The study samples comprised 80 tissue samples, including 25 primary NSCLC tissues (sensitive), 25 recurrent NSCLC tissues (resistant) and 30 healthy lung tissues (normal) from 30 random cases of these patients. These fresh samples were collected after surgery and rapidly immersed in RNAlater solution (Invitrogen, Burlington, ON, Canada), and subsequently stored at −80°C. All subjects signed written informed consent, and this study was approved by the Ethics Committee of Shandong Provincial Third Hospital.
Table 1.
Correlation Between Circ_0003998 Expression and the Clinicopathologic Features of These Patients.
| Features | All cases | circ_0003998 expression | P value | |
|---|---|---|---|---|
| Low level (n = 25) | High level (n = 25) | |||
| Gender | ||||
| Male | 37 | 15 | 22 | 0.2888 |
| Female | 13 | 10 | 3 | |
| Ages | ||||
| ≤65 | 42 | 22 | 20 | 0.7019 |
| >65 | 8 | 3 | 5 | |
| Smoking history | ||||
| Yes | 32 | 13 | 19 | 0.1398 |
| No | 18 | 12 | 6 | |
| TNM stage | ||||
| I-II | 28 | 19 | 9 | 0.0096* |
| III-IV | 22 | 6 | 16 | |
| Metastasis | ||||
| M0 | 30 | 10 | 20 | 0.0086* |
| M1 | 20 | 15 | 5 | |
| Lymph Node metastasis | ||||
| N0 | 26 | 17 | 9 | 0.0465* |
| N1 | 24 | 8 | 16 | |
TNM, tumor node metastasis; *P < 0.05 by Chi-square test.
Cell Culture
H1299 and A549 NSCLC cells were obtained from American Type Culture Collection (ATCC, Manassas, USA) and cultivated in RPMI-1640 medium (Invitrogen) with 10% fetal calf serum (FCS, Biosera, Boussens, France) as reported.18 Human bronchial 16HBE cell line (Procell, Wuhan, China) was used as a control of non-tumor cells and propagated using standard protocols provided by Procell. 293 T cells (ATCC) were cultivated in maintain medium provided by ATCC for dual-luciferase reporter assays.
DTX-resistant NSCLC cells (A549/DTX and H1299/DTX) were established in our laboratory by treating A549 and H1299 cells with gradually increasing concentrations of DTX (Sigma-Aldrich, Steinheim, Germany; starting from 20 ng/L and progressively increasing the concentration up to 10 µg/L) more than 9 months until they acquired the ability to grow in the presence of DTX at the same rate as parental cells in the absence of the drug. To maintain the resistance phenotype, additional 10 µg/L of DTX was used in the cell medium.
Lentiviral Transduction and Transient Transfection of Cells
Lentiviral particles harboring shRNA specific for circ_0003998 (sh-circ, GeneChem, Shanghai, China) were used to silence circ_0003998, and shRNA lentiviruses (sh-NC) were used as the negative control. To establish a stable circ_0003998 knockdown cell line, A549/DTX cells were incubated with viral supernatant for 8 h in the presence of 8 µg/mL of polybrene (Sigma-Aldrich). The cells with positive transduction were selected with 2 µg/mL of puromycin (Sigma-Aldrich) over 72 h.
Transient transfection was carried out using Lipofectamine 3000 (Invitrogen) with 50 nM of the indicated oligonucleotides or 100 ng of plasmids as per the manufacturing guidance. CORO1C overexpressing plasmid (oe-CORO1C) was constructed by cloning human CORO1C sequence (Accession: NM_014325.4, Genewiz, Suzhou, China) into a pcDNA3.1 vector (Invitrogen) with BamH I and Xho I sites, and the nontarget plasmid (vector) was used as a control. All oligonucleotides including circ_0003998-siRNA (si-circ) or the nontarget-siRNA (si-NC), miR-136-5p inhibitor or negative control inhibitor (inhibitor NC), the mimic of miR-136-5p (miR-136-5p mimic) or mimic control (mimic NC) were synthesized by Genecreate (Wuhan, China) and their details were presented in Supplement Table 1. Cells were harvested 48 h post transfection for further analyses.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA isolated with TriPure Isolation Reagent (Roche, Mannheim, Germany) averaged 54 µg/mL (A260/A280 = 1.92) when quantified by a Bioanalyzer (BD Bioscience, Stockholm, Sweden). To quantify circ_0003998 and CORO1C mRNA expression, cDNA was synthesized in a 25 µL reaction with 500 ng RNA using cDNA Synthesis Kit (Fermentas, Leon-Rot, Germany). The quantification of miR-136-5p was done using miScript II RT Kit (Qiagen, Crawley, UK) for cDNA synthesis. All qRT-PCR reactions were run on a 480 II Cycler (Roche) using SYBR Green Master (Fermentas) with specific primers (Supplement Table 1). Results were normalized to the expression of reference gene β-actin or U6 and calculated by the 2- ΔΔCt method.19
Cell Viability Assay for the IC50 Value
Transfected cells (10,000 cells/well) were plated into 96-well plates for 12 h and then stimulated for 24 h with DTX at 50, 100, 200, 400, 600 and 800 µg/L. 10 µL of reaction solution was added into per well based on the manufacturing protocols (Cell Counting Kit-8, CCK-8, Beyotime, Shanghai, China), followed by the incubation at 37°C for 3 h before reading absorbance at 450 nm with a M200 Pro microplate reader (Tecan, Grödig, Austria). Using the measured viability curves, the IC50 values for DTX in cells were determined.
Colony Formation Assay
Transfected cells were seeded in 12-well plates at a density of 150 cells per well. Subsequently, the wells were placed in a 5% CO2, 37°C incubator for 14 days. The colonies (over 50 cells) were scored under a microscope (Leica, Wetzlar, Germany) after being stained with 1% crystal violet (Beyotime).
Cell Apoptosis Analysis Based on Flow Cytometry
Approximately 10,000 transfected cells per sample were used for apoptosis assays based on the double-staining with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) as per the manufacturing protocols (Annexin V-FITC/PI Detection Kit, BD Bioscience). The apoptotic cells were gauged by a BD AccuriC6 flow cytometer (BD Bioscience) with AccuriC6 software. The apoptotic cells were calculated by determining the sum of early (Annexin V+/PI-) and late (Annexin V+/PI+) apoptotic cells.
Western Blot
Total protein was extracted from cells and tissues with RIPA lysis buffer (Invitrogen) and western blot was conducted using standard methods as reported.20 Primary antibodies including anti-BCL2 associated X (anti-bax, ab262929; dilution 1:1,000), anti-proliferating cell nuclear antigen (anti-PCNA, ab220208; dilution 1:1,000), anti-P-glycoprotein (anti-P-pg, ab235954; dilution 1:1,000), anti-multidrug resistance-associated protein 1 (anti-MRP1, ab32574; dilution 1:500), anti-CORO1C (ab96266; dilution 1:2,000) and anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH, ab157156; dilution 1:1,000) were used as recommended by the manufacturers (Abcam, Cambridge, UK). Anti-rabbit and anti-mouse IgG (ab205718 and ab97046, Abcam; dilution 1:10,000 and 1:10,000) labeled by horseradish peroxidase were used as secondary antibodies. Immunoreactive bands were developed with a Chemiluminescent Kit (Bio-Rad, Kidlington, UK).
Dual-Luciferase Reporter Assay
The targeted miRNAs of circ_0003998 were predicted by the target-prediction web circinteractome. The molecular targets of miR-136-5p were searched by the starBase v.3 online database. The fragment of circ_0003998 harboring the wild-type target sequence for miR-136-5p and CORO1C 3′UTR sequence were individually inserted into a pmirGLO vector (Promega, Mannheim, Germany). Mutations of the target sites were generated using the GeneArtTM Site-Directed Mutagenesis System (Invitrogen). 293 T cells of ∼70% confluence were cotransfected with 100 ng of the indicated reporter constructs and mimic NC or miR-136-5p mimic at 50 nM. After 48 h transfection, luciferase activities were gauged by the Dual-Luciferase Assay System in a Luminometer (Promega).
RNA Immunoprecipitation (RIP) Assay
The assays were done based on the reported by Li et al.,21 using anti-Argonaute2 (anti-Ago2, ab252812) and anti-IgG (ab109489) antibodies. Briefly, cell lysates were incubated with protein-G magnetic beads-coupled anti-Ago2 or IgG antibody for 6 h at 4°C. The beads were harvested, and bound RNA was isolated to measure the enrichment levels of circ_0003998 and miR-136-5p.
Animal Studies
Animal experiments were carried out based on the protocol approved by the Animal Ethics Committee of Shandong Provincial Third Hospital. Twenty-four female BALB/c nude mice (7-week-old, ALF Biotechnology, Nanjing, China) were randomly assigned to 4 groups (n = 6 per group): sh-NC + PBS (NS), sh-circ + PBS (NS), sh-NC + DTX or sh-circ + DTX. The subcutaneous xenograft model was established by implanting sh-NC-transduced or sh-circ-infected A549/DTX cells (6 × 106 cells) into the right flanks of nude mice. When the xenograft tumors reached about 100 mm3, the nude mice were administrated with DTX (5 mg/kg) or PBS (NS) via tail vein injection every 3 days. Tumor volume (mm3) was measured every 3 days and calculated as follows: length (L) × width (W)2 × 0.5. After 22 days of implantation, the xenograft tumors were harvested from the sacrificed mice for weight and expression analysis.
Statistical Analysis
Results were expressed as the mean ± standard deviation from 3 biological replicates × 2 technical replicates. Statistical significance of all tests in different groups was determined by a Student’s t-test (2-sided), Mann-Whitney U test or 1-way analysis of variance (ANOVA). The Spearman rank correlation was used to evaluate the correlation among circ_0003998, miR-136-5p and CORO1C mRNA levels in NSCLC samples. The significance was accepted at P < 0.05.
Results
Circ_0003998 Expression Was Associated With DTX Resistance of NSCLC
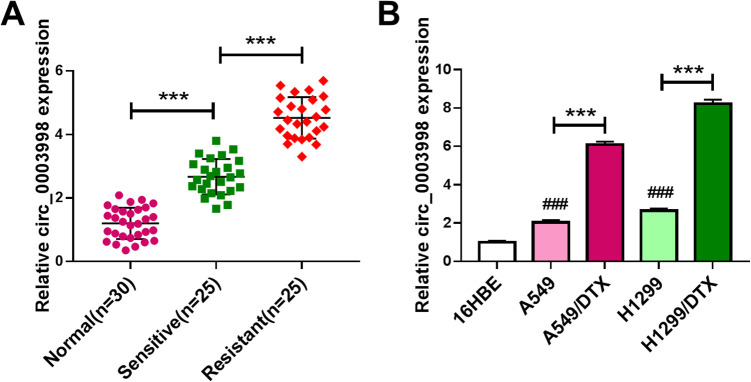
Firstly, we examined the expression pattern of circ_0003998 in NSCLC tissues and cells. By contrast, circ_0003998 level was significantly increased in NSCLC tissues and cells (Figure 1A and B). To observe the involvement of circ_0003998 in NSCLC chemoresistance, we generated 2 DTX-resistant NSCLC cell lines (A549/DTX and H1299/DTX). Of interest, circ_0003998 expression was higher in DTX-resistant tissues and cells than that in the corresponding sensitive counterparts (Figure 1A and B). Additionally, circ_0003998 expression was associated with tumor TNM stage, tumor metastasis and lymph node metastasis (Table 1).
Figure 1.
Circ_0003998 expression was associated with NSCLC resistance to DTX. Circ_0003998 expression by qRT-PCR in 25 primary NSCLC tissues (sensitive), 25 recurrent NSCLC tissues (resistant) and 30 matched healthy lung tissues (normal) (A), 16HBE, A549, H1299, A549/DTX and H1299/DTX cells (B). ***P < 0.001 or ### P < 0.001.
Circ_0003998 Knockdown Repressed Cell Colony Formation and Promoted Apoptosis and DTX Sensitivity of A549/DTX and H1299/DTX Cells In Vitro
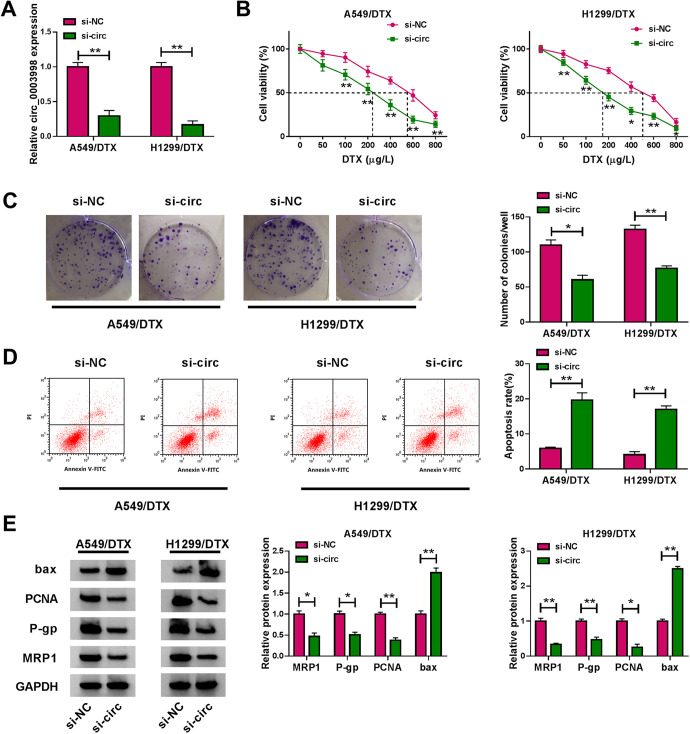
We then carried out loss-of-function analyses in vitro by silencing circ_0003998 with siRNA specific to circ_0003998 (si-circ). qRT-PCR data showed that compared with the si-NC control, the transfection of si-circ prominently down-regulated circ_0003998 level in the 2 DTX-resistant NSCLC cell lines (Figure 2A). Interestingly, circ_0003998 knockdown remarkably reduced the IC50 value for DTX in the 2 cell lines (Figure 2B). Moreover, circ_0003998 silencing significantly inhibited cell colony formation (Figure 2C) and promoted cell apoptosis (Figure 2D). Additionally, the depletion of circ_0003998 strikingly decreased the levels of PCNA, MRP1 and P-gp in both cell lines (Figure 2E). Conversely, circ_0003998 silencing dramatically elevated the pro-apoptotic protein bax expression (Figure 2E).
Figure 2.
Knockdown of circ_0003998 suppressed cell colony formation and enhanced apoptosis and DTX sensitivity of DTX-resistant NSCLC cells. A549/DTX and H1299/DTX cells were transfected with si-NC or si-circ (si-circ_0003998) for 48 h. A, qRT-PCR for circ_0003998 expression in transfected cells. B, CCK-8 assay for the viability of transfected cells after 24 h treatment with the indicated concentration of DTX. C, Colony formation assay for cell colony formation. D, Flow cytometry for cell apoptosis. E, Western blot for PCNA, MRP1, bax and P-gp levels in transfected cells. *P < 0.05 or **P < 0.01.
Circ_0003998 Directly Interacted With miR-136-5p
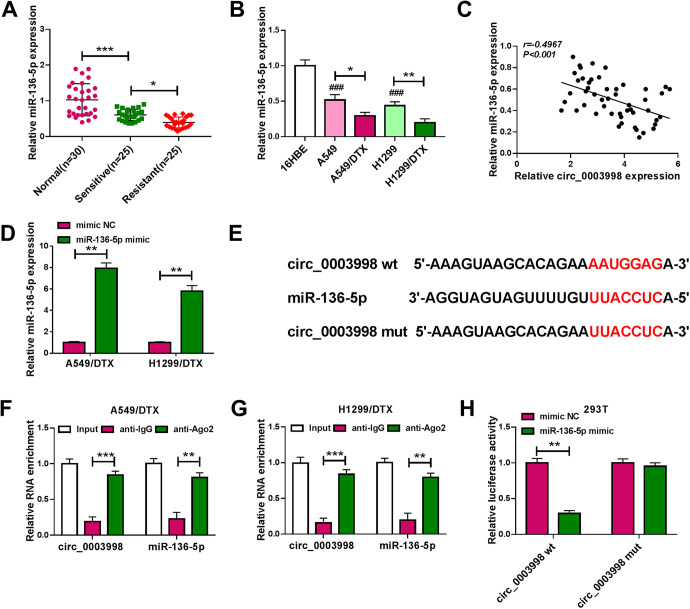
The data of qRT-PCR revealed that miR-136-5p was significantly underexpressed in NSCLC tissues and cells (Figure 3A and B). Moreover, in contrast to the sensitive counterparts, miR-136-5p expression was remarkably down-regulated in DTX-resistant NSCLC tissues and cells (Figure 3A and B). Of interest, a strong inverse correlation between miR-136-5p and circ_0003998 expression was discovered in NSCLC tissues (Figure 3C). The transfection efficiency of miR-136-5p mimic was gauged by qRT-PCR (Figure 3D). More intriguingly, the target-prediction web circinteractome22 revealed a putative target sequence (AAUGGAG) for miR-136-5p within circ_0003998 (Figure 3E). RIP experiments showed that the enrichment levels of circ_0003998 and miR-136-5p were synchronously elevated by anti-Ago2 antibody in the RNA-induced silencing complex (RISC)23 (Figure 3F and G), implying their endogenous interaction in the 2 DTX-resistant NSCLC cell lines. When we cloned circ_0003998 fragment harboring the putative miR-136-5p binding sites into a luciferase vector, the cotransfection of the reporter construct (circ_0003998 wt) and miR-136-5p mimic into 293 T cells produced lower luciferase activity than the cells cotransfected with mimic NC control but the site-directed mutation of the seed region (circ_0003998 mut) prominently abolished the repressive impact of miR-136-5p overexpression (Figure 3H), demonstrating the validity of the target sequence for interaction.
Figure 3.
Circ_0003998 in DTX-resistant NSCLC cells directly interacted with miR-136-5p. The level of miR-136-5p by qRT-PCR in 25 primary NSCLC tissues (sensitive), 25 recurrent NSCLC tissues (resistant) and 30 matched healthy lung tissues (normal) (A), 16HBE, A549, H1299, A549/DTX and H1299/DTX cells (B). (C) Correlation between miR-136-5p expression and circ_0003998 level in 50 NSCLC tissues using Spearman test. (D) miR-136-5p expression by qRT-PCR in A549/DTX and H1299/DTX cells transfected with mimic NC or miR-136-5p mimic. (E) Schematic of the putative target sequence for miR-136-5p within circ_0003998 and the mutation of the seed region. (F and G) The levels of circ_0003998 and miR-136-5p by qRT-PCR in cell lysates incubated with anti-IgG or anti-Ago2 antibody. (H) Dual-luciferase assays in 293 T cells cotransfected with mimic NC or miR-136-5p mimic and circ_0003998 wt or circ_0003998 mut. *P < 0.05, **P < 0.01, ***P < 0.001 or ### P < 0.001.
MiR-136-5p Was a Molecular Mediator of Circ_0003998 in Regulating Cell Colony Formation, Apoptosis and DTX Sensitivity In Vitro
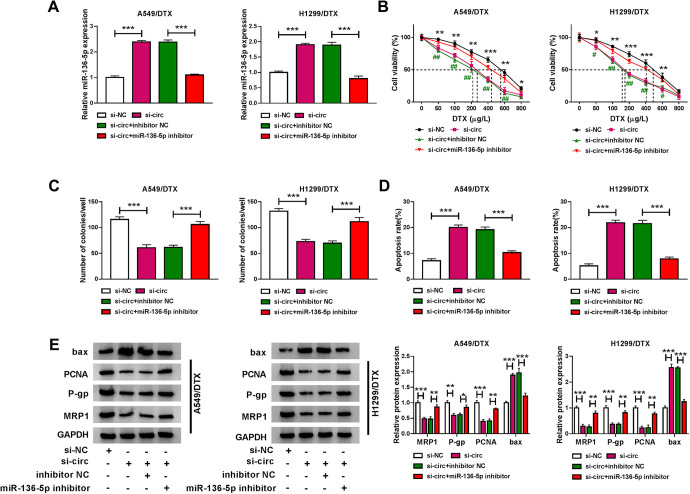
To determine whether miR-136-5p was involved in circ_0003998-mediated regulation in the progression and chemosensitivity of DTX-resistant NSCLC cells, we reduced miR-136-5p expression in si-circ-transfected cells. By contrast, the transfection of miR-136-5p inhibitor significantly abolished si-circ-mediated miR-136-5p up-regulation in the 2 DTX-resistant NSCLC cell lines (Figure 4A). Further analyses showed that the reduced expression of miR-136-5p dramatically reversed circ_0003998 knockdown-mediated IC50 value decrease (Figure 4B), colony formation inhibition (Figure 4C) and apoptosis promotion (Figure 4D). Furthermore, the reduced miR-136-5p expression dramatically abrogated the impact of circ_0003998 silencing on PCNA, P-gp, MRP1 and bax expression (Figure 4E).
Figure 4.
Circ_0003998 knockdown regulated DTX-resistant NSCLC cell colony formation, apoptosis and DTX sensitivity in vitro by miR-136-5p. A549/DTX and H1299/DTX cells were transfected with si-NC, si-circ (si-circ_0003998), si-circ+inhibitor NC or si-circ+miR-136-5p inhibitor for 48 h, followed by the determination of miR-136-5p expression by qRT-PCR (A), cell viability by CCK-8 assay (B), cell colony formation by colony formation assay (C), cell apoptosis by flow cytometry (D), the levels of PCNA, P-gp, MRP1 and bax by western blot (E). *P < 0.05, **P < 0.01 or ***P < 0.001.
MiR-136-5p Directly Interacted With the 3′UTR of CORO1C
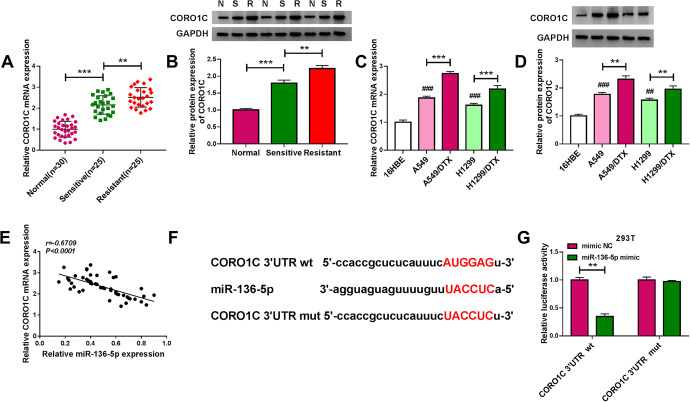
The results of qRT-PCR and western blot showed that in NSCLC tissues and cells, CORO1C expression was significantly augmented at both mRNA and protein levels (Figure 5A-D). Importantly, a more remarkable up-regulation of CORO1C level was found in DTX-resistant group compared with the corresponding sensitive group (Figure 5A-D). Moreover, a strong inverse correlation between CORO1C mRNA level and miR-136-5p expression was discovered in NSCLC tissues (Figure 5E). Using the starBase v.3 online database, a potential miR-136-5p target sequence (AUGGAG) was identified within the 3′UTR of CORO1C (Figure 5F). To validate this, we used CORO1C 3′UTR reporter constructs in dual-luciferase assays. The wild-type luciferase reporter (CORO1C 3′UTR wt) in the presence of miR-136-5p mimic caused a striking down-regulation of luciferase activity (Figure 5G). When the target sequence was mutated (CORO1C 3′UTR mut), little change was observed in luciferase with miR-136-5p mimic (Figure 5G).
Figure 5.
MiR-136-5p directly targeted the 3′UTR of CORO1C. CORO1C mRNA and protein levels by qRT-PCR and western blot in primary NSCLC tissues (sensitive/S), recurrent NSCLC tissues (resistant/R) and matched healthy lung tissues (normal/N) (A and B), 16HBE, A549, H1299, A549/DTX and H1299/DTX cells (C and D). (E) Correlation between CORO1C mRNA expression and miR-136-5p level in 50 NSCLC tissues using Spearman test. (F) Schematic of the potential miR-136-5p target sequence within the 3′UTR of CORO1C and mutated target sequence. (G) Dual-luciferase assays in 293 T cells cotransfected with CORO1C 3′UTR wt or CORO1C 3′UTR mut and mimic NC or miR-136-5p mimic. **P < 0.01, ***P < 0.001 or ### P < 0.001.
CORO1C Was a Functional Target of miR-136-5p in Regulating Cell Colony Formation, Apoptosis and DTX Sensitivity In Vitro
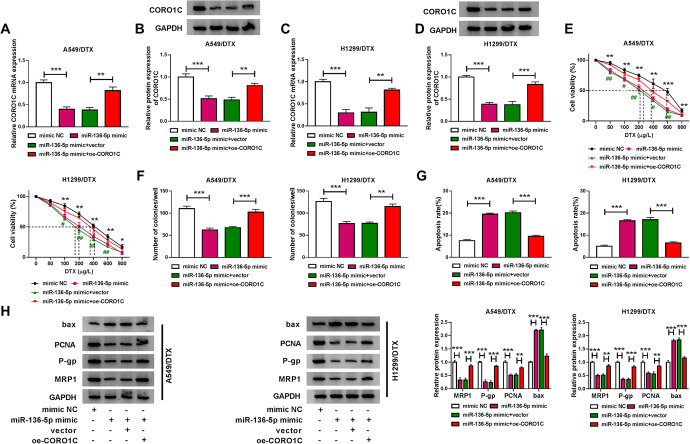
Importantly, in contrast to the mimic NC control, miR-136-5p overexpression led to a significant reduction in the expression of CORO1C at both mRNA and protein levels in the 2 DTX-resistant NSCLC cell lines (Figure 6A-D), reinforcing CORO1C as a direct target of miR-136-5p. Functional analyses showed that the elevated expression of miR-136-5p strikingly reduced the IC50 value for DTX (Figure 6E), repressed cell colony formation (Figure 6F), and promoted cell apoptosis (Figure 6G). Moreover, miR-136-5p overexpression led to a significant decrease in PCNA, P-gp and MRP1 levels, as well as a remarkable increase in bax expression (Figure 6H).
Figure 6.
The effect of miR-136-5p overexpression on DTX-resistant NSCLC cell colony formation, apoptosis and DTX sensitivity in vitro was mediated by CORO1C. A549/DTX and H1299/DTX cells were transfected with mimic NC, miR-136-5p mimic, miR-136-5p mimic+vector or miR-136-5p mimic+oe-CORO1C for 48 h, followed by the detection of CORO1C mRNA and protein levels by qRT-PCR and western blot (A-D), cell viability by CCK-8 assay (E), cell colony formation by colony formation assay (F), cell apoptosis by flow cytometry (G), the levels of PCNA, P-gp, MRP1 and bax by western blot (H). *P < 0.05, **P < 0.01 or ***P < 0.001.
To determine whether CORO1C was a functionally important target of miR-136-5p, we cotransfected miR-136-5p mimic and CORO1C overexpressing plasmid (oe-CORO1C) into the 2 resistant cell lines. By contrast, oe-CORO1C transfection prominently reversed miR-136-5p overexpression-mediated CORO1C down-regulation in both cell lines (Figure 6A-D). Furthermore, CORO1C expression restoration significantly abrogated miR-136-5p overexpression-mediated IC50 value reduction (Figure 6E), colony formation suppression (Figure 6F), and apoptosis enhancement (Figure 6G). Additionally, the restored expression of CORO1C abrogated the alteration of PCNA, P-gp, MRP1 and bax levels induced by miR-136-5p overexpression (Figure 6H).
Circ_0003998 Controlled CORO1C Expression by Sponging miR-136-5p
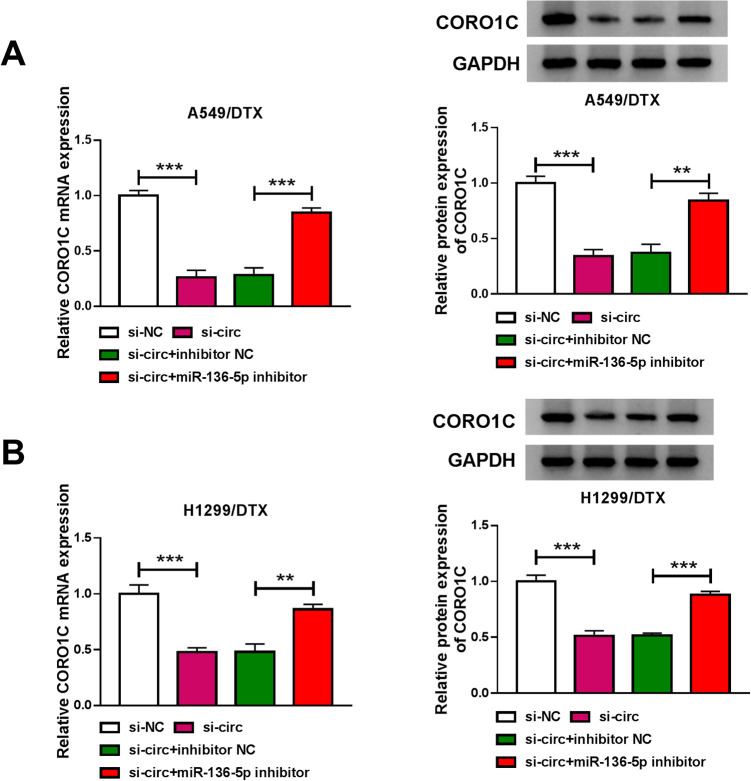
We next investigated whether circ_0003998 modulated CORO1C expression through working as a sponge. As expected, in contrast to the si-NC control, circ_0003998 knockdown led to a significant decrease in the levels of CORO1C mRNA and protein in the 2 DTX-resistant NSCLC cell lines (Figure 7A and B). Furthermore, the effect was dramatically abrogated by miR-136-5p inhibitor (Figure 7A and B).
Figure 7.
Circ_0003998 regulated CORO1C expression by sponging miR-136-5p. CORO1C mRNA and protein levels by qRT-PCR and western blot in A549/DTX (A) and H1299/DTX (B) cells transfected with si-NC, si-circ (si-circ_0003998), si-circ+inhibitor NC or si-circ+miR-136-5p inhibitor. **P < 0.01 or ***P < 0.001.
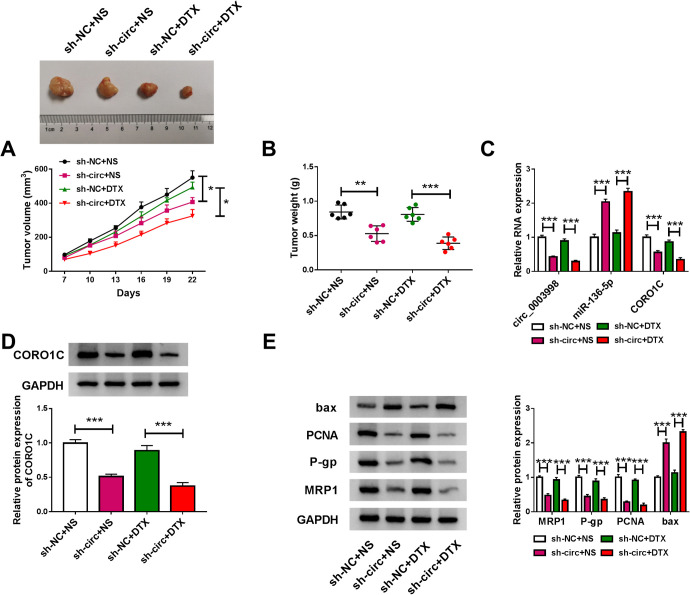
Circ_0003998 Knockdown Inhibited Tumor Growth and Promoted DTX Sensitivity In Vivo
We further determined the role of circ_0003998 in regulating A549/DTX tumor growth and DTX sensitivity in vivo. By contrast, the transduction of sh-circ led a striking inhibition in tumor growth with or without DTX administration (Figure 8A and B). Interestingly, DTX administration did not significantly affected tumor growth, and simultaneous treatment with sh-circ transduction and DTX administration resulted in a more remarkable repression in tumor growth (Figure 8A and B). Furthermore, circ_0003998 and CORO1C were significantly down-regulated and miR-136-5p was remarkably up-regulated in sh-circ-transduced A549/DTX tumors (Figure 8C and D). Additionally, western blot results showed that the levels of PCNA, P-gp and MRP1 were decreased and bax expression was increased in tumor tissues derived from sh-circ-transduced A549/DTX cells (Figure 8E).
Figure 8.
Circ_0003998 knockdown repressed tumor growth and promoted DTX sensitivity in vivo. sh-NC-infected or sh-circ (sh-circ_0003998)-transduced A549/DTX cells were subcutaneously implanted into the right flanks of nude mice (n = 6 per group). When the tumors reached about 100 mm3, the nude mice were given PBS (NS) or DTX (5 mg/kg) via tail vein injection every 3 days. (A) Growth curve of the xenograft tumors. (B) Tumor weights of the xenograft tissues. The levels of circ_0003998, miR-136-5p and CORO1C mRNA by qRT-PCR (C), CORO1C protein expression by western blot (D), PCNA, P-gp, MRP1 and bax levels by western blot (E) in the excised A549/DTX tumors. *P < 0.05, **P < 0.01 or ***P < 0.001.
Discussion
Chemoresistance in cancer cells is a fundamental challenge.24 A deeper understanding of what drives resistance development would help to develop novel therapies and reverse drug resistance in NSCLC. CircRNAs have been identified as key participators in NSCLC chemoresistance, providing a new opportunity for NSCLC resistance management.8,25 Considering the enhanced effect of circ_0003998 on NSCLC tumorigenesis and chemoresistance development,13,14 we undertook to investigate the molecular basis underlying the resistance control of circ_0003998 in NSCLC.
In agreement with previous work,14 our data supported the significant up-regulation of circ_0003998 in DTX-resistant NSCLC tissues and cells. PCNA is a proliferation marker that is capable of evaluating the growth of cancer cells.26,27 P-gp and MRP1 are 2 members of ATP-binding cassette transporter proteins that play crucial roles in the development of chemoresistance in cancer cells.28,29 Our results demonstrated that the knockdown of circ_0003998 repressed cell colony formation and enhanced apoptosis and DTX sensitivity of DTX-resistant NSCLC cells in vitro and in vivo.
miR-136-5p was a strong candidate as a targeted miRNA of circ_0003998 due to the inverse correlation of their expression and the anti-tumor property16 of miR-136-5p in NSCLC. We here confirmed that circ_0003998 directly targeted miR-136-5p. MiR-136-5p has been reported to involve in the development of various cancers, such as renal cell carcinoma, cervical cancer and gastric cancer.30-32 Our data first showed that the increased level of miR-136-5p hampered DTX-resistant NSCLC cell colony formation and enhanced apoptosis and DTX sensitivity. More interestingly, we first highlighted miR-136-5p as a downstream effector of circ_0003998 function.
CORO1C, an F-actin binding protein, has been unraveled to associate with tumor invasion and metastasis.33-35 CORO1C was also found to be overexpressed in NSCLC and small-cell lung cancer.36,37 Here, we were first to demonstrate that CORO1C was a functionally important target of miR-136-5p in regulating NSCLC resistance to DTX. Liao and colleagues identified that miR-206 suppressed NSCLC malignant progression by targeting CORO1C.36 More importantly, we first pointed out the role of circ_0003998 as a regulator of CORC1C expression through miR-136-5p. Future work will build on the findings by determining how the novel mechanism regulates the functional properties of DTX-resistant NSCLC cells. With these findings, we envisioned that circ_0003998 inhibition might be a starting point of the development of circRNA-based therapies to improve the anti-NSCLC effect of DTX.
Collectively, the present work identified that circ_0003998 modulated DTX-resistant NSCLC cell colony formation, apoptosis and DTX sensitivity by the miR-136-5p/CORO1C axis. Our research highlighted a novel regulatory mechanism of circ_0003998 in NSCLC chemoresistance.
Supplemental Material
Supplemental Material, sj-pdf-1-tct-10.1177_1533033821990040 for Circ_0003998 Regulates the Progression and Docetaxel Sensitivity of DTX-Resistant Non-Small Cell Lung Cancer Cells by the miR-136-5p/CORO1C Axis by Wei Zhang, Chao Song and Xiaona Ren in Technology in Cancer Research & Treatment
Footnotes
Authors’ Note: Our study was approved by The Mercy Health Research Ethics Committee (approval no.2019995190). All patients provided written informed consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Xiaona Ren  https://orcid.org/0000-0003-2962-6114
https://orcid.org/0000-0003-2962-6114
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29(4):959–965. [DOI] [PubMed] [Google Scholar]
- 3. Bordoni R, Ciardiello F, von Pawel J, et al. Patient-reported outcomes in OAK: a phase III study of atezolizumab versus docetaxel in advanced non-small-cell lung cancer. Clin Lung Cancer. 2018;19(5):441–449. e444. [DOI] [PubMed] [Google Scholar]
- 4. Chen X, Zhao L, Kang Y, et al. Significant suppression of non-small-cell lung cancer by hydrophobic poly(ester amide) nanoparticles with high docetaxel loading. Front Pharmacol. 2018;9:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ge L, You X, Huang J, et al. Human albumin fragments nanoparticles as PTX carrier for improved anti-cancer efficacy. Front Pharmacol. 2018;9:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen W, Pang H, Liu J, et al. Epithelial-mesenchymal transition contributes to docetaxel resistance in human non-small cell lung cancer. Oncol Res. 2014;22(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–691. [DOI] [PubMed] [Google Scholar]
- 8. Xu N, Chen S, Liu Y, et al. Profiles and bioinformatics analysis of differentially expressed circrnas in taxol-resistant non-small cell lung cancer cells. Cell Physiol Biochem. 2018;48(5):2046–2060. [DOI] [PubMed] [Google Scholar]
- 9. Xiao G, Huang W, Zhan Y, Li J, Tong W. CircRNA_103762 promotes multidrug resistance in NSCLC by targeting DNA damage inducible transcript 3 (CHOP). J Clin Lab Anal. 2020;34(6):e23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang MS, Liu JY, Xia XB, et al. Hsa_circ_0001946 inhibits lung cancer progression and mediates cisplatin sensitivity in non-small cell lung cancer via the nucleotide excision repair signaling pathway. Front Oncol. 2019;9:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dong Y, Xu T, Zhong S, et al. Circ_0076305 regulates cisplatin resistance of non-small cell lung cancer via positively modulating STAT3 by sponging miR-296-5p. Life Sci. 2019;239:116984. [DOI] [PubMed] [Google Scholar]
- 12. Zhou Y, Zheng X, Xu B, et al. Circular RNA hsa_circ_0004015 regulates the proliferation, invasion, and TKI drug resistance of non-small cell lung cancer by miR-1183/PDPK1 signaling pathway. Biochem Biophys Res Commun. 2019;508(2):527–535. [DOI] [PubMed] [Google Scholar]
- 13. Yu W, Jiang H, Zhang H, Li J. Hsa_circ_0003998 promotes cell proliferation and invasion by targeting miR-326 in non-small cell lung cancer. Onco Targets Ther. 2018;11:5569–5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu W, Peng W, Sha H, Li J. Hsa_circ_0003998 promotes chemoresistance via modulation of miR-326 in lung adenocarcinoma cells. Oncol Res. 2019;27(5):623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wei X, Shen X, Ren Y, Hu W. The roles of microRNAs in regulating chemotherapy resistance of non-small cell lung cancer. Curr Pharm Des. 2018;23(39):5983–5988. [DOI] [PubMed] [Google Scholar]
- 16. Xie ZC, Li TT, Gan BL, et al. Investigation of miR-136-5p key target genes and pathways in lung squamous cell cancer based on TCGA database and bioinformatics analysis. Pathol Res Pract. 2018;214(5):644–654. [DOI] [PubMed] [Google Scholar]
- 17. Geng Y, Bao Y, Zhang W, Deng L, Su D, Zheng H. Circular RNA hsa_circ_0014130 inhibits apoptosis in non-small cell lung cancer by sponging miR-136-5p and upregulating BCL2. Mol Cancer Res. 2020;18(5):748–756. [DOI] [PubMed] [Google Scholar]
- 18. Lee J, Jang HJ, Chun H, et al. Calotropis gigantea extract induces apoptosis through extrinsic/intrinsic pathways and reactive oxygen species generation in A549 and NCI-H1299 non-small cell lung cancer cells. BMC Complement Altern Med. 2019;19(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chowdhury N, Vhora I, Patel K, et al. Liposomes co-loaded with 6-Phosphofructo-2-Kinase/Fructose-2, 6-Biphosphatase 3 (PFKFB3) shRNA plasmid and docetaxel for the treatment of non-small cell lung cancer. Pharm Res. 2017;34(11):2371–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y, Chen B, Huang S. Identification of circRNAs for miRNA targets by argonaute2 RNA immunoprecipitation and luciferase screening assays. Methods Mol Biol. 2018;1724:209–218. [DOI] [PubMed] [Google Scholar]
- 22. Dudekula DB, Panda AC, Grammatikakis I, De S, Abdelmohsen K, Gorospe M. CircInteractome: a web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol. 2016;13(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawamata T, Tomari Y. Making RISC. Trends Biochem Sci. 2010;35(7):368–376. [DOI] [PubMed] [Google Scholar]
- 24. Sharma A. Chemoresistance in cancer cells: exosomes as potential regulators of therapeutic tumor heterogeneity. Nanomedicine (Lond). 2017;12(17):2137–2148. [DOI] [PubMed] [Google Scholar]
- 25. Kong R. Circular RNA hsa_circ_0085131 is involved in cisplatin-resistance of non-small-cell lung cancer cells by regulating autophagy. Cell Biol Int. 2020;44(9):1945–1956. [DOI] [PubMed] [Google Scholar]
- 26. Juríková M, Danihel Ľ, Polák Š, Varga I. Ki67, PCNA, and MCM proteins: markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016;118(5):544–552. [DOI] [PubMed] [Google Scholar]
- 27. Wang L, Kong W, Liu B, Zhang X. Proliferating cell nuclear antigen promotes cell proliferation and tumorigenesis by up-regulating STAT3 in non-small cell lung cancer. Biomed Pharmacother. 2018;104:595–602. [DOI] [PubMed] [Google Scholar]
- 28. Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18(7):452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar A, Jaitak V. Natural products as multidrug resistance modulators in cancer. Eur J Med Chem. 2019;176:268–291. [DOI] [PubMed] [Google Scholar]
- 30. Li J, Huang C, Zou Y, Ye J, Yu J, Gui Y. CircTLK1 promotes the proliferation and metastasis of renal cell carcinoma by sponging miR-136-5p. Mol Cancer. 2020;19(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Zhao J, Yang T, Li L. LncRNA FOXP4-AS1 is involved in cervical cancer progression via regulating miR-136-5p/CBX4 axis. Onco Targets Ther. 2020;13:2347–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang S, Zhang X, Li Z, et al. Circular RNA profile identifies circOSBPL10 as an oncogenic factor and prognostic marker in gastric cancer. Oncogene. 2019;38(44):6985–7001. [DOI] [PubMed] [Google Scholar]
- 33. Lim JP, Shyamasundar S, Gunaratne J, Scully OJ, Matsumoto K, Bay BH. YBX1 gene silencing inhibits migratory and invasive potential via CORO1C in breast cancer in vitro. BMC Cancer. 2017;17(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fan L, Wei Y, Ding X, Li B. Coronin3 promotes nasopharyngeal carcinoma migration and invasion by induction of epithelial-to-mesenchymal transition. Onco Targets Ther. 2019;12:9585–9598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35. Cheng X, Wang X, Wu Z, Tan S, Zhu T, Ding K. CORO1C expression is associated with poor survival rates in gastric cancer and promotes metastasis in vitro. FEBS Open Bio. 2019;9(6):1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liao M, Peng L. MiR-206 may suppress non-small lung cancer metastasis by targeting CORO1C. Cell Mol Biol Lett. 2020;25:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujii K, Miyata Y, Takahashi I, et al. Differential proteomic analysis between small cell lung carcinoma (SCLC) and pulmonary carcinoid tumors reveals molecular signatures for malignancy in lung cancer. Proteomics Clin Appl. 2018;12(6):e1800015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-tct-10.1177_1533033821990040 for Circ_0003998 Regulates the Progression and Docetaxel Sensitivity of DTX-Resistant Non-Small Cell Lung Cancer Cells by the miR-136-5p/CORO1C Axis by Wei Zhang, Chao Song and Xiaona Ren in Technology in Cancer Research & Treatment