Abstract
Background:
The COVID-19 pandemic has impacted adults with chronic diseases, and their health care delivery. Patterns of COVID-19–related preventive behaviors practiced by cancer survivors are unknown, including practices related to canceling doctor's appointments. We evaluated COVID-19–related preventive behaviors among cancer survivors in the United States.
Methods:
We used nationally representative data of 10,760 U.S. adults from the COVID-19 Impact Survey. We defined cancer survivors as those with a self-reported diagnosis of cancer (n = 854, 7.6%). We present frequencies and χ2 tests to evaluate COVID-19–related preventive behaviors among cancer survivors. We estimated determinants of canceling doctor's appointments among cancer survivors using Poisson regression models.
Results:
Cancer survivors were more likely to practice preventive behaviors, including social distancing (93%, χ2 P < 0.001), wearing a face mask (93%, χ2 P < 0.001), and avoiding crowded areas (84%, χ2 P < 0.001) compared with adults without cancer. Cancer survivors were more likely to cancel doctor's appointments (41%, χ2 P < 0.001), whereas they were less likely to cancel other social activities such as work (19%, χ2 P < 0.001) and school-related (13%, χ2 P < 0.001) activities. After adjustment for covariates, while non-Hispanic (NH)-Black cancer survivors were less likely to cancel a doctor's appointment compared with NH-White cancer survivors, cancer survivors aged 18 to 29, who were female, and who had least one comorbid condition were more likely.
Conclusions:
Cancer survivors are adhering to recommended preventive behaviors. Cancer survivor's continuity of care may be impacted by COVID-19, specifically young adults, females, and those with existing comorbid conditions.
Impact:
Insights into cancer survivors whose care may be most impacted by COVID-19 will be valuable toward surveillance and survivorship of U.S. cancer survivors.
Introduction
The World Health Organization (WHO) declared COVID-19 as a Public Health Emergency of International Concern in January 2020 (1). In the United States, the first confirmed case of COVID-19 was reported on January 20, 2020, in the state of Washington (2). Since the onset of the pandemic, preventive behaviors against COVID-19 have been promoted through the mainstream media based on recommendations from prominent public health agencies, such as the WHO and the Centers for Disease Control and Prevention (CDC; Atlanta, GA; ref. 3). Behaviors such as hand washing, maintaining 6-feet distance from others (i.e., social distancing), and avoiding large crowds have been touted as the population's main preventive strategies in the absence of a vaccine or medication to directly combat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the infection that leads to COVID-19 (4, 5). In addition, due to the surge of patients with COVID-19 in hospitals and clinics, canceling nonessential visits to the doctor's office has been recommended by the CDC to mitigate the risk of exposure.
However, the potential implications of the recommendation to cancel nonessential visits to the doctor in the population are unknown, as there is a gap in understanding among the general population between what is elective and what is essential, leading to high uncertainty among patients with a fear of contracting SARS-CoV-2 (6). Among those that may be most impacted by this recommendation are also those at high risk of contracting COVID-19, such as the elderly and those with existing chronic conditions (7,8,9,10,11,12). The COVID-19 pandemic has highly impacted the delivery of care to those with chronic conditions as providers may often opt for treatment with lower efficacy but lower risk of hospitalization to mitigate the risk of exposure. Since the start of the pandemic, health care providers have had to make critical decisions on prioritization of care among those with chronic conditions (13,14,15).
Cancer survivors, defined as those living after a diagnosis of cancer (16), may be most impacted by the shift in clinical care due to the potential immediate threat of certain malignancies and the significance of surveillance (17,18,19,20,21,22,23). Cancer and cancer-related treatment frequently cause immunosuppression, and adults with cancer have excess mortality risk from SARS-CoV-2. The magnitude of risk among adults with cancer is still unknown; however, early reports suggest a substantial risk of death associated with COVID-19 infection, perhaps highest among those older than 60 years and those with compromised lung function (24, 25). Because of the high risk among cancer survivors, preventive behaviors are critical to reducing the risk of developing COVID-19. However, data on adherence to recommended preventive behaviors among cancer survivors are limited. More specifically, national data on the impact of continuity of care among cancer survivors during the COVID-19 pandemic are also scarce. Our objective was to evaluate COVID-19–related preventive behaviors among cancer survivors using a nationally representative sample of U.S. adults. We further examined behaviors related to canceling or postponing activities, specifically doctor's appointments. Insights into the impact of the COVID-19 pandemic on the continuity of care among cancer survivors will be valuable toward surveillance and survivorship of adults with cancer in the United States.
Materials and Methods
COVID-19 Impact Survey
Data for these analyses were obtained from the publicly available COVID-19 Household Impact Survey, conducted by NORC at the University of Chicago for the Data Foundation. The COVID-19 Household Impact Survey is a philanthropic effort to provide national and regional statistics about physical health, mental health, economic security, and social dynamics in the United States (26). The survey is designed to provide weekly estimates of the U.S. adult (ages 18 and older) household population nationwide and for 18 regional areas including 10 states (CA, CO, FL, LA, MN, MO, MT, NY, OR, and TX) and eight Metropolitan Statistical Areas (Atlanta, Baltimore, Birmingham, Chicago, Cleveland, Columbus, Phoenix, and Pittsburgh). Currently, data from week 1 (April 20–26, 2020), week 2 (May 4–10, 2020), and week 3 (May 30–June 8, 2020) are available, which were merged for this analysis.
AmeriSpeak sample
Funded and operated by NORC at the University of Chicago, AmeriSpeak is a probability-based panel designed to be representative of the U.S. household population. During the initial recruitment phase of the AmeriSpeak panel, randomly selected U.S. households were sampled using area probability and address-based sampling, with a known, nonzero probability of selection from the NORC National Sample Frame. These sampled households were then contacted by U.S. mail, telephone, and field interviewers (face-to-face). The panel provides sample coverage of approximately 97% of the U.S. household population. Those excluded from the sample include people with P.O. Box only addresses, some addresses not listed in the USPS Delivery Sequence File, and some newly constructed dwellings. Although most AmeriSpeak households participate in surveys by web, non-internet households were able to participate in AmeriSpeak surveys by telephone. Households without conventional internet access but having web access via smartphones were allowed to participate in AmeriSpeak surveys by web. AmeriSpeak panelists participate in NORC studies or studies conducted by NORC on behalf of governmental agencies, academic researchers, and media and commercial organizations. Interviews were conducted in English and Spanish. Interviews were conducted with adults age 18 and over representing the 50 states and the District of Columbia. Panel members were randomly drawn from AmeriSpeak. In households with more than one adult panel member, only one was selected at random for the sample. Invited panel members were given the option to complete the survey online or by telephone with a NORC telephone interviewer. The number of participants invited and percentage of interviews completed by week are as follows: 11,133 invited with 19.7% interviews completed during week 1; 8,570 invited with 26.1% interviews completed (week 2); and 10, 373 invited with 19.7% interviews completed (week 3). Panelists were offered a $5 monetary incentive for completing the survey. The analytic sample includes 10,760 adults nationwide. The final analytic sample were weighted to reflect the U.S. population of adults aged 18 years and over. The demographic weighting variables were obtained from the 2020 Current Population Survey. The count of COVID-19 deaths by county was obtained from USA Facts.
Multimode address-based sample
Data for the regional estimates were collected using a multimode address-based sample (ABS) approach that allowed residents of each area to complete the interview via the web or with a NORC telephone interviewer. In four states (CA, FL, NY, and TX), the AmeriSpeak panel design yields representative state samples. As such, AmeriSpeak panelists who reside in each of these four states are combined with the ABS sample to generate region-level estimates. For each geographic area, an iterative raking process is used to adjust for any survey nonresponse as well as any noncoverage or under and oversampling. Demographic weighting variables were obtained from the 2018 American Community Survey. The weighted data reflect the population of adults age 18 and over in each region.
Primary outcome
The primary outcome for this analysis was participants' response (yes/no) to the following question: “Which of the following measures, if any, are you taking in response to the coronavirus?” Participants were able to select all that applied from a list of 19 options. We focused on the following options: canceled a doctor appointment; visited a doctor or hospital; canceled or postponed work activities; canceled or postponed school activities; canceled or postponed dentist or other appointments; avoided some or all restaurants; worked from home; studied from home; canceled or postponed pleasure social or recreational activities; stockpiled food or water; avoided public or crowded places; prayed; avoided contact with high-risk people; washed or sanitized hands; kept 6-feet distance from those outside my household; stayed home because I felt unwell; and wiped packages entering my home.
Primary predictor
The primary predictor for this analysis was participants' self-report of cancer. Participants were asked to reply “yes, no, or not sure” to the following question: “Has a doctor or other health care provider ever told you that you have any of the following: Diabetes; High blood pressure or hypertension; Heart disease, heart attack or stroke; Asthma; Chronic lung disease or COPD (chronic obstructive pulmonary disease); Bronchitis or emphysema; Allergies; a Mental health condition; Cystic fibrosis; Liver disease or end-stage liver disease; Cancer; a Compromised immune system; or Overweight or obesity.” We defined those who selected “Cancer” as a cancer survivor.
Covariates
The following covariates were included in the multivariable analyses: age (18–29, 30–44, 25–59, 60+), sex (male, female), race/ethnicity [non-Hispanic (NH)-White, NH-Black, Hispanic, NH-other], education [no high school (HS) diploma, HS graduate or equivalent, some college, baccalaureate degree or above], employment status (employed/unemployed), household income (<$50,000, $50,000–<$100,000, ≥$100,000), population density (rural, suburban, urban), insurance status and type, comorbid conditions, and symptoms experienced in the last 7 days. Population density was determined based on the 2010 U.S. Census data.
Statistical analysis
Descriptive statistics are displayed in percentages among all respondents unless otherwise labeled and include a margin of error of ±3.0 percentage points at the 95% confidence intervals (CI) among all adults. χ2 tests were used for bivariate comparison of physical symptoms experienced in the last 7 days and preventive behaviors against the COVID-19 pandemic among cancer survivors compared with adults without cancer. To estimate determinants of cancer survivors canceling doctor's appointments, we calculated prevalence ratios with Poisson regression using robust estimation of standard errors (27,28,29). Potential variables for inclusion in the model were assessed using available sociodemographic variables and age-adjusted Poisson regression analysis. Because of the exploratory nature of this analysis using a predictive framework, an arbitrary P value of <0.10 was used as criteria to include the variable in the multivariable Poisson regression model. For multivariable Poisson regression models, adjusted prevalence ratios (aPR), and 95% CIs for each independent variable were calculated. We conducted sensitivity analyses to evaluate the association of canceled school or childcare among female cancer survivors who canceled their doctor's appointments using Poisson regression. In addition, P < 0.05 was used as the level of significance. Collinearity was assessed using the variance inflation factor to ensure a strong linear relationship among independent variables included in the model was not present. On the basis of the exploratory nature of this analysis, we did not include an adjustment for multiple comparisons (30). All statistical analyses were conducted using Stata IC 15.1 (StataCorp LLC, College Station, TX). Sampling weights were applied to provide results that were nationally representative of the U.S. adult population.
Results
Table 1 summarizes the descriptive characteristics of the analytic sample overall and by self-reported cancer diagnosis. Overall, cancer survivors (7.6%) were mostly 60 years and above (65%), female (52%), and NH-White (75%). About one third of cancer survivors were employed, held a Baccalaureate degree or above, and had a household income of $75,000 and above. Most cancer survivors resided in urban areas (66%). The most common insurance plans among cancer survivors included Medicare (56%), employer-sponsored insurance (47%), and Medicaid (30%). About 9% of cancer survivors were dually eligible for both Medicare and Medicaid.
Table 1.
Characteristics of COVID Impact Survey respondents (n = 10,760), a nationally representative survey of the United States, stratified by cancer diagnosis (April–June 2020).
| Total | Cancer survivorsa | Respondents never diagnosed with cancer | ||||
|---|---|---|---|---|---|---|
| Col % | 95% CI | Col % | 95% CI | Col % | 95% CI | |
| Age | ||||||
| 18–29 | 20.5 | 19.3–21.9 | 3.0 | 1.8–4.9 | 22.0 | 20.7–23.4 |
| 30–44 | 25.3 | 24.2–26.4 | 9.4 | 6.9–12.6 | 26.6 | 25.5–27.8 |
| 45–59 | 24.3 | 23.2–25.4 | 23.0 | 19.4–27.0 | 24.4 | 23.2–25.5 |
| 60+ | 29.9 | 28.8–31.1 | 64.7 | 60.3–68.9 | 27.0 | 25.8–28.2 |
| Sex | ||||||
| Male | 48.4 | 47.0–49.7 | 47.6 | 43.2–51.9 | 48.4 | 47.0–49.8 |
| Female | 51.6 | 50.3–53.0 | 52.4 | 48.1–56.8 | 51.6 | 50.2–53.0 |
| Marital status | ||||||
| Married/living with partner | 57.3 | 55.9–58.6 | 57.0 | 52.6–61.3 | 57.3 | 55.9–58.7 |
| Widowed/divorced/separated | 18.5 | 17.5–19.5 | 31.2 | 27.3–35.4 | 17.4 | 16.4–18.4 |
| Never married | 24.2 | 23.0–25.5 | 11.8 | 9.1–15.3 | 25.3 | 24.0–26.6 |
| Race/ethnicity | ||||||
| White, NH | 62.8 | 61.5–64.2 | 75.3 | 71.0–79.1 | 61.8 | 60.4–63.2 |
| Black, NH | 11.6 | 10.8–12.5 | 11.6 | 8.7–15.4 | 11.6 | 10.7–12.5 |
| Hispanic | 16.5 | 15.4–17.7 | 8.3 | 6.1–11.3 | 17.2 | 16.1–18.4 |
| Other, NH | 8.5 | 7.7–9.3 | 4.0 | 2.9–5.7 | 8.9 | 8.0–9.8 |
| Employed in the past 7 days | 49.9 | 48.6–51.3 | 31.9 | 28.1–36.2 | 51.5 | 50.1–52.9 |
| Education | ||||||
| No HS diploma | 9.6 | 8.7–10.7 | 6.4 | 4.4–9.3 | 9.9 | 8.9–11.0 |
| HS graduate | 28.1 | 26.8–29.5 | 30.3 | 26.0–34.9 | 28.0 | 26.6–29.4 |
| Some college | 27.7 | 26.7–28.8 | 27.9 | 24.7–31.4 | 27.7 | 26.7–28.8 |
| Baccalaureate or above | 34.5 | 33.3–35.7 | 35.4 | 31.3–39.7 | 34.4 | 33.2–35.7 |
| Household income | ||||||
| <$50,000 | 45.5 | 44.2–46.9 | 48.5 | 44.2–52.9 | 45.3 | 43.9–46.7 |
| $50,000–<$100,000 | 32.1 | 30.9–33.4 | 26.9 | 23.4–30.8 | 32.6 | 31.3–33.9 |
| ≥$100,000 | 22.4 | 21.3–23.5 | 24.5 | 20.9–28.5 | 22.2 | 21.0–23.4 |
| Population density | ||||||
| Rural | 9.0 | 8.3–9.8 | 13.4 | 10.4–17.0 | 8.7 | 8.0–9.4 |
| Suburban | 18.7 | 17.7–19.7 | 20.0 | 16.9–23.6 | 18.6 | 17.6–19.6 |
| Urban | 72.3 | 71.1–73.4 | 66.6 | 62.3–70.7 | 72.7 | 71.5–73.9 |
| Insurance type or health coverage plans | ||||||
| Purchased plan | 17.1 | 16.1–18.2 | 20.2 | 17.0–23.8 | 16.9 | 15.8–18.0 |
| Employer-sponsored | 51.9 | 50.6–53.3 | 47.0 | 42.6–51.4 | 52.3 | 50.9–53.7 |
| TRICARE | 4.8 | 4.3–5.4 | 6.8 | 4.8–9.5 | 4.7 | 4.2–5.2 |
| Medicaid | 23.5 | 22.3–24.6 | 30.3 | 26.1–34.8 | 22.9 | 21.7–24.1 |
| Medicare | 25.4 | 24.3–26.6 | 56.1 | 51.6–60.4 | 22.8 | 21.7–24.0 |
| Dually eligible (Medicare and Medicaid) | 10.1 | 8.9–11.3 | 9.0 | 7.9–10.3 | 23.5 | 17.8–30.2 |
| Veterans affairs (VA) | 4.4 | 4.0–4.9 | 8.8 | 6.6–11.6 | 4.1 | 3.6–4.6 |
| Indian health service | 1.2 | 0.9–1.6 | 0.3 | 0.1–0.8 | 1.3 | 0.9–1.7 |
| No insurance | 8.8 | 8.0–9.6 | 3.0 | 1.8–4.9 | 9.2 | 8.4–10.1 |
a2.46% of participants either chose not sure, skipped, or refused when asked about their chronic conditions, including cancer.
Table 2 summarizes symptoms experienced in the past 7 days and preventive behaviors practiced by respondents against COVID-19 across categories of prior cancer diagnosis. In the past 7 days, cancer survivors reported most frequently experiencing the following symptoms: fever (18%), muscle or body aches (17%), sneezing (17%), and sore throat (17%). Compared with those without cancer, cancer survivors were more likely to report experiencing the following symptoms in the past 7 days: muscle or body aches (χ2 P < 0.001), sneezing (χ2 P = 0.02), sore throat (χ2 P = 0.01), nausea or vomiting (χ2 P = 0.01), and shortness of breath (χ2 P = 0.01).
Table 2.
Symptoms in the last 7 days and preventive behaviors against COVID-19 of COVID Impact Survey respondents (n = 10,760), a nationally representative survey of the United States, stratified by cancer diagnosis (April–June 2020).
| Total | Cancer survivors | Respondents never diagnosed with cancer | |||||
|---|---|---|---|---|---|---|---|
| Col % | 95% CI | Col % | 95% CI | Col % | 95% CI | Pa | |
| Physical symptoms experienced in the last 7 days | |||||||
| Fever | 16.2 | 15.2–17.2 | 18.4 | 15.0–22.2 | 16.0 | 14.9–17.0 | 0.19 |
| Muscle or body aches | 12.9 | 12.1–13.9 | 17.4 | 14.1–21.2 | 12.6 | 11.7–13.5 | 0.00 |
| Sneezing | 13.6 | 12.7–14.6 | 17.1 | 14.1–20.6 | 13.3 | 12.4–14.4 | 0.02 |
| Sore throat | 13.5 | 12.7–14.5 | 17.2 | 14.2–20.7 | 13.2 | 12.3–14.2 | 0.01 |
| Nausea or vomiting | 12.7 | 11.8–13.6 | 16.5 | 13.5–20.1 | 12.4 | 11.5–13.3 | 0.01 |
| Shortness of breath | 12.3 | 11.5–13.2 | 16.0 | 13.1–19.5 | 12.0 | 11.2–12.9 | 0.01 |
| Runny or stuffy nose | 14.2 | 13.3–15.2 | 15.7 | 12.8–19.0 | 14.1 | 13.1–15.2 | 0.34 |
| Fatigue or tiredness | 13.4 | 12.5–14.4 | 15.5 | 12.6–19.0 | 13.2 | 12.3–14.3 | 0.15 |
| Headaches | 14.3 | 13.4–15.2 | 15.2 | 12.3–18.7 | 14.2 | 13.3–15.2 | 0.53 |
| Cough | 13.6 | 12.7–14.6 | 11.7 | 9.3–14.7 | 13.8 | 12.8–14.8 | 0.18 |
| Preventive behaviors against COVID-19 | |||||||
| Washed or sanitized hands | 90.2 | 89.4–90.9 | 93.9 | 91.5–95.6 | 89.9 | 89.0–90.8 | 0.00 |
| Kept 6-feet distance from those outside my household | 84.1 | 83.1–85.0 | 92.7 | 90.2–94.6 | 83.5 | 82.4–84.5 | <0.001 |
| Worn a face mask | 85.0 | 84.1–85.9 | 92.1 | 89.9–93.8 | 84.3 | 83.3–85.3 | <0.001 |
| Avoided public or crowded places | 76.2 | 75.1–77.3 | 83.8 | 80.5–86.7 | 75.7 | 74.5–76.9 | <0.001 |
| Avoided some or all restaurants | 70.6 | 69.4–71.8 | 80.4 | 77.0–83.4 | 69.9 | 68.6–71.1 | <0.001 |
| Canceled or postponed pleasure, social, or recreational activities | 64.8 | 63.5–66.0 | 73.8 | 69.8–77.5 | 64.4 | 63.0–65.7 | <0.001 |
| Avoided contact with high-risk people | 58.6 | 57.2–59.9 | 67.6 | 63.4–71.5 | 57.9 | 56.5–59.3 | <0.001 |
| Canceled a doctor appointment | 30.9 | 29.7–32.1 | 41.3 | 37.0–45.6 | 29.9 | 28.7–31.2 | <0.001 |
| Canceled or postponed dentist or other appointment | 36.1 | 34.9–37.3 | 40.7 | 36.5–45.0 | 35.7 | 34.4–37.0 | 0.03 |
| Worked from home | 31.6 | 30.4–32.8 | 23.9 | 20.4–27.8 | 32.3 | 31.1–33.6 | <0.001 |
| Canceled or postponed work activities | 27.9 | 26.7–29.1 | 18.7 | 15.7–22.1 | 28.6 | 27.4–29.9 | <0.001 |
| Visited a doctor or hospital | 9.7 | 9.0–10.5 | 14.2 | 11.4–17.6 | 9.4 | 8.6–10.2 | <0.001 |
| Canceled or postponed school activities | 20.1 | 19.0–21.2 | 13.4 | 10.4–17.1 | 20.3 | 19.2–21.5 | 0.001 |
| Stayed home because I felt unwell | 10.7 | 9.9–11.6 | 8.6 | 6.5–11.4 | 10.8 | 9.9–11.7 | 0.15 |
Note: Bolded values <0.05.
aP value based on χ2 test.
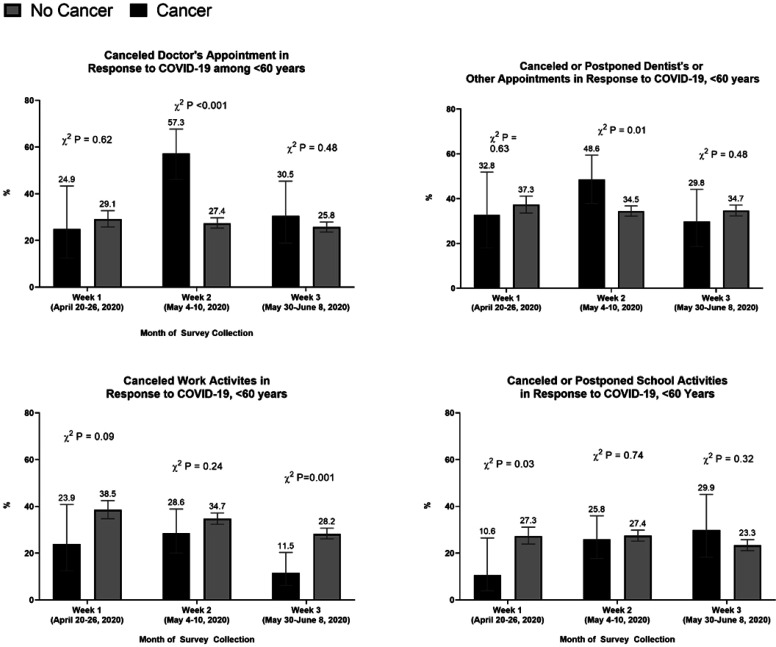
When comparing cancer survivors with the general study population, we found that cancer survivors were more likely to report washing or sanitizing their hands (94% vs. 90%, χ2 P = 0.00), maintaining social distance (93% vs. 84%, χ2 P < 0.001), wearing a face mask (92% vs. 84%, χ2 P < 0.001), avoiding public or crowded places (84% vs. 76%, χ2 P < 0.001), avoiding some or all restaurants (80% vs. 70%, χ2 P < 0.001), avoiding contact with high-risk people (68% vs. 58%, χ2 P < 0.001), and visiting a doctor or hospital (14% vs. 9%, χ2 P < 0.001). We observed that cancer survivors were also more likely to cancel a doctor's appointment (41% vs. 36%, χ2 P < 0.001), cancel or postpone a dentist or other appointment (41% vs. 36%, χ 2 P = 0.03), and cancel pleasure, social, or recreational activities (74% vs. 64%, χ 2 P < 0.001) compared with those without cancer. Between April and May, the proportion of cancer survivors that canceled a doctor or dentist's appointment increased from 35% to 52% and 36% to 49%, respectively (Fig. 1). In early June, the proportion of cancer survivors that canceled a doctor's appointment decreased to 35%; however, a significant difference between cancer survivors and other adults persisted (χ2 P = 0.02). Between April and early June, cancer survivors were less likely to cancel work activities when compared with the general population (Fig. 1). Cancer survivors were less likely to cancel school activities in April compared with other adults (7.6% vs. 21.8, χ2 P < 0.001); however, this proportion was no longer significantly different by early June. We observed similar trends when we restricted the analytic sample to adults below the age of 60 years: By early June, cancer survivors below the age of 60 years were less likely to cancel work activities (12% vs. 28%, P < 0.001; Supplementary Fig. S1).
Figure 1.
Preventive behaviors related to canceling appointments and activities in response to the COVID-19 pandemic among cancer survivors and the general adult population, COVID Impact Survey (April–June 2020).
We subsequently identified determinants of canceling a doctor's appointment among cancer survivors (Table 3). In age-adjusted analyses, we observed that cancer survivors aged 18 to 29 were more likely to cancel a doctor's appointment compared with those aged 60 years and above (PR: 1.83; 95% CI, 1.41–2.37). In addition, females (PR: 1.37; 95% CI, 1.11–1.69), and cancer survivors with at least one comorbid condition (PR: 1.47; 95% CI, 1.11–1.94) were more likely to cancel a doctor's appointment. Cancer survivors who were widowed/divorced/separated were less likely to cancel doctor's appointments compared with those who were married (PR: 0.77; 95% CI, 0.61–0.98), as well as those who were employed (PR: 0.68; 95% CI, 0.52–0.89). In addition, cancer survivors who resided in the South (age-adjusted PR: 0.75; 95% CI, 0.57–1.00) and the West (PR: 0.64; 95% CI, 0.47–0.87) were less likely to cancel doctor's appointments compared with those in the Northeast.
Table 3.
Determinants of cancer survivors canceling a doctor's appointment among COVID Impact Survey, a nationally representative survey of the United States (n = 854; April–June 2020).
| Unadjusted PR (only adjusted for age) | 95% CI | Adjusted PR | 95% CI | |
|---|---|---|---|---|
| Age | ||||
| 18–29 | 1.83 | 1.41–2.37 | 1.70 | 1.31–2.21 |
| 30–44 | 0.95 | 0.61–1.47 | 0.90 | 0.63–1.29 |
| 45–49 | 0.89 | 0.68–1.17 | 0.99 | 0.76–1.31 |
| 60+ | Ref. | Ref. | ||
| Sex | ||||
| Male | Ref. | Ref. | ||
| Female | 1.37 | 1.11–1.69 | 1.39 | 1.13–1.72 |
| Marital status | ||||
| Married/living with partner | Ref. | Ref. | ||
| Widowed/divorced/separated | 0.77 | 0.61–0.98 | 0.78 | 0.61–0.99 |
| Never married | 0.94 | 0.67–1.31 | 0.90 | 0.66–1.21 |
| Race/ethnicity | ||||
| White, NH | Ref. | Ref. | ||
| Black, NH | 0.68 | 0.43–1.07 | 0.57 | 0.34–0.97 |
| Hispanic | 1.02 | 0.72–1.44 | 1.13 | 0.83–1.54 |
| Other, NH | 0.89 | 0.57–1.39 | 1.02 | 0.67–1.56 |
| At least one comorbid conditiona | 1.47 | 1.11–1.94 | 1.38 | 1.06–1.80 |
| Region | ||||
| Northeast | Ref. | Ref. | ||
| Midwest | 0.79 | 0.58–1.06 | 0.80 | 0.61–1.05 |
| South | 0.75 | 0.57–1.00 | 0.78 | 0.60–1.02 |
| West | 0.64 | 0.47–0.87 | 0.69 | 0.51–0.95 |
| Employment status | ||||
| Not employed | Ref. | Ref. | ||
| Employed/self-employed | 0.68 | 0.52–0.89 | 0.72 | 0.56–0.93 |
| At least one COVID-19–related symptomb | 1.18 | 0.94–1.48 | — | |
| Obese/overweight | 1.13 | 0.91–1.39 | — | |
| Household income | — | |||
| <$50,000 | 0.93 | 0.72–1.21 | ||
| $50,000–<$100,000 | 1.11 | 0.85–1.45 | ||
| ≥$100,000 | Ref. | |||
| Population density | — | |||
| Rural | 1.09 | 0.79–1.52 | ||
| Suburban | 1.06 | 0.83–1.34 | ||
| Urban | Ref. | |||
aComorbid conditions include diabetes, high blood pressure, heart disease/heart attack/stroke, asthma, COPD, bronchitis or emphysema, allergies, a mental health condition, cystic fibrosis, liver disease, and a compromised immune system.
bSymptoms include fever, chills, runny or stuffy nose, chest congestion, skin rash, cough, sore throat, sneezing, muscle or body aches, headaches, fatigue or tiredness, shortness of breath, abdominal discomfort, nausea or vomiting, diarrhea, changed or loss of sense of taste or smell, and loss of appetite.
In the fully adjusted model, those aged 18 to 29 years (aPR: 1.70; 95% CI, 1.31–2.21), females (aPR: 1.39; 95% CI, 1.13–1.72), and those with at least one comorbid condition (aPR: 1.38; 95% CI, 1.06–1.80) continued to be more likely to cancel a doctor's appointment (Table 3). Cancer survivors who were widowed/divorced/separated (aPR: 0.78; 95% CI, 0.61–0.99), resided in the West (aPR: 0.69; 95% CI, 0.51–0.95) and were employed (aPR: 0.72; 95% CI, 0.56–0.93) continued to be less likely to cancel a doctor's appointment. When compared with NH-White cancer survivors, NH-Black cancer survivors were less likely to report canceling a doctor's appointment (aPR: 0.57; 95% CI, 0.34–0.97).
As a sensitivity analysis, we focused on female cancer survivors and evaluated the association of reporting personal plans were affected by the closure of pre-K through 12th grade or childcare with canceling their doctor's appointments. Women with cancer were more likely to cancel a doctor's appointment if they reported their personal plans were affected by the closure of pre-K through 12th grade or childcare (56.7%) compared with women who did not (40.3%; χ2 P = 0.011). After adjustment for age and having at least one other comorbid condition, we found that women with cancer who reported their plans were affected by the closure of pre-K through 12th grade or childcare had a 37% higher prevalence of reporting they canceled their doctor's appointments (aPR: 1.37; 95% CI, 1.04–1.80) compared with women who did not.
Discussion
In this analysis, we found that cancer survivors are practicing many of the recommended COVID-19–related preventive behaviors, such as social distancing and staying away from crowded areas. However, cancer survivors reported experiencing symptoms that may be related to the ongoing pandemic or their cancer treatment, such as muscle or body aches, sore throat, and nausea or vomiting. In addition, we found that cancer survivors are more likely to cancel their doctor's appointments, whereas they are less likely to cancel other social activities such as work and school-related activities. The proportion of cancer patients that have canceled doctor's appointments due to COVID-19 rose from April to May, and by early June, the proportion was 34% and continued to be greater among cancer survivors compared with other adults. Younger adults aged 18 to 29 years, females, and cancer survivors with at least one other comorbid condition are more likely to cancel their doctor's appointments, whereas NH-Black cancer survivors are less likely to cancel a doctor's appointment when compared with NH-White cancer survivors. Findings from this analysis may inform strategies regarding treatment decisions among those caring for cancer survivors, specifically those with existing comorbidities.
Preventive behaviors against COVID-19 have been broadly highlighted in the media and recommended by prominent public health organizations, such as the WHO and CDC (3). A specific focus has been placed on those at high risk of infection, including the elderly and those with chronic conditions, such as cancer survivors (18,19,20,21,22,23). In the COVID-19 pandemic context, cancer survivors are of particular significance because they are generally older and frequently live with existing comorbidities (24, 31). In our study, we found that cancer survivors were often more likely to practice COVID-19–related preventive behaviors when compared with the general study population. This is not surprising due to their high-risk status. However, an interesting finding was that cancer survivors reported experiencing select COVID-19–related symptoms including shortness of breath, nausea or vomiting, sore throat, and muscle or body aches. We are unable to disentangle if these symptoms may be due to their ongoing cancer treatment, which frequently is associated with various adverse side effects (32). However, it may be concerning that despite these reported symptoms cancer survivors were more likely to cancel their doctor's appointment, and the proportion increased to 50% by May, when compared with the general adult population.
In our analysis, we identified specific demographic groups of cancer survivors that were either more or less likely to cancel a doctor's appointment. We observed that women with cancer were significantly more likely to cancel a doctor's appointment during the COVID-19 pandemic period, compared with men. This could reflect an increase in caregiver responsibilities (e.g., childcare) within the pandemic period, which poses structural barriers to maintaining appointments (33). Indeed, we observed that female cancer survivors who reported their plans being disrupted due to school or childcare closures were more likely to report canceling a doctor's appointment. Lack of childcare options coupled with an unwillingness to potentially expose children to COVID-19 to maintain the doctor's appointment may have led to this gender disparity. In addition, this gender disparity could also reflect differences in the type or stage of cancer, which may be less aggressive in women compared with men within the sample. NH-Black cancer survivors were significantly less likely to cancel a doctor's appointment during the COVID-19 pandemic period, compared with NH-White cancer survivors. This finding may be due to the advanced cancer stage at diagnosis, which was not measured in the COVID-19 Impact Survey. Previous studies have documented that NH-Black men and women are more likely to be diagnosed at a later stage for the most common forms of cancer, and have more aggressive forms of cancer, compared with NH-White adults (34,35,36). Willingness to maintain appointments may reflect the clinical characteristics of cancer, which may require more aggressive treatment (37,38,39,40,41,42). In addition, we know that NH-Black cancer survivors are also more likely to have multiple comorbidities compared with White patients with cancer; the willingness to maintain appointments among NH-Black patients with cancer may be appointments for other chronic conditions (e.g., diabetes, cardiovascular conditions; refs. 43,44,45,46,47,48).
Our study has notable strengths. We were able to utilize data from a nationally representative survey of U.S. adults and therefore, obtain a representative sample of cancer survivors. However, our results should be interpreted with several limitations in mind. Chronic conditions, including a prior cancer diagnosis, were based on self-report, leading to the potential for measurement error in our definition of cancer survivor. We were unable to measure and account for important covariates such as cancer type, cancer stage, or type of cancer treatment (surgery vs. chemo vs. radiation). Important factors to consider when interpreting our results also include, if the respondent was currently undergoing cancer treatment, and if so, how long they have been undergoing treatment or time since treatment; however, these data were not available. In addition, we were unable to assess whether cancer survivors were canceling cancer-related appointments or appointments related to existing comorbidities. Importantly, data regarding the cancer survivor's symptoms and practices prior to COVID-19 were unavailable, and therefore, we were unable to conclude whether the symptoms are COVID-19 related or due to another cause. Finally, we were unable to assess whether respondents rescheduled their doctor's appointments as telehealth visits as no follow-up questions were asked regarding their reasons for canceling doctor's visits. As such, we were unable to account for potential reasons for canceled doctor's appointments, or telehealth visits, such as lack of internet access. Future qualitative research into experiences of cancer survivors during the COVID-19 pandemic to elucidate reasons for interrupted cancer care and barriers to medical care is needed.
In conclusion, we provide several insights into COVID-19–related preventive behaviors among cancer survivors. We found that cancer survivors frequently reported practicing recommended behaviors such as wearing a face mask, maintaining social distance, and avoiding crowded areas. In our study, we focused on cancer survivors reported cancelation of doctor's appointments to gain insights into the continuity of care among cancer survivors in the United States during the COVID-19 pandemic. Surveillance of cancer survivors through follow-up appointments and consistent check-ins with their care team is a pivotal aspect of cancer survivorship. However, the COVID-19 pandemic has introduced a new dynamic in clinical care, transitioning to telehealth, and limiting health care appointments to only essential appointments (49,50,51). Limited understanding in the general population of when appropriate to cancel doctor's appointments and potentially inequitable access to telehealth are factors that providers should consider, specifically among this vulnerable group (21, 52). As the COVID-19 pandemic continues in the coming months, providers will have to continue to prioritize resources and treatment, and surveillance of potential unintended consequences of these decisions should continue.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors gratefully acknowledge NORC at the University of Chicago for the Data Foundation for data collection and making the data from the COVID Impact Survey publicly available. J.Y. Islam is supported by the University of North Carolina's Cancer Care Quality Training 2T32CA116339-11. M. Camacho-Rivera is supported by the TRANSPORT – The Translational Program of Health Disparities Research Training 5S21MD012474-02.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 2387
Footnotes
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
Cancer Epidemiol Biomarkers Prev 2020;29:2583–90
Authors' Contributions
J.Y. Islam: Conceptualization, formal analysis, supervision, visualization, methodology, writing–original draft, writing–review and editing. M. Camacho-Rivera: Visualization, methodology, writing–review and editing. D.C. Vidot: Methodology, writing–review and editing.
References
- 1. Coronavirus Disease 2019 (COVID-19); [about 7 screens]. [cited 2020 May 8]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- 2.CDC COVID-19 Response Team. Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep 2020;69:465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. How to Protect Yourself & Others; [about 10 screens]. [cited 2020 May 8]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html.
- 4. Saad L. Americans still social distancing, but less vigilant. Gallup 2020. Apr 30. Available from: https://news.gallup.com/poll/309611/americans-social-distancing-less-vigilant.aspx.
- 5. Rothberger H, Wilson T, Whaley D, Rosenfeld DL, Humphrey M, Moore A, et al. Politicizing the COVID-19 pandemic: ideological differences in adherence to social distancing. PsyArXiv [Preprint]. 2020. Apr 22. Available from: https://psyarxiv.com/k23cv/.
- 6. Birch J. Which non-covid doctor's visits should you make, keep, postpone or do by telemedicine? The Washington Post. 2020. May 5. Available from: https://www.washingtonpost.com/lifestyle/wellness/doctors-appointment-coronavirus-telemedicine/2020/05/04/f7005afe-8e20-11ea-a9c0-73b93422d691_story.html.
- 7. Yancy CW. COVID-19 and African Americans. JAMA 2020;323:1891–2. [DOI] [PubMed] [Google Scholar]
- 8. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020;5:831–40. [DOI] [PubMed] [Google Scholar]
- 9. Dietz W, Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity 2020;28:1005. [DOI] [PubMed] [Google Scholar]
- 10. Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis 2020;71:896–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Lancet Diabetes & Endocrinology. COVID-19: underlying metabolic health in the spotlight. Lancet Diabetes Endocrinol 2020;8:457. [Google Scholar]
- 12. Bernstein L, Sellers FS. Patients with heart attacks, strokes and even appendicitis vanish from hospitals. The Washington Post. 2020. Apr 19. Available from: https://www.washingtonpost.com/health/patients-with-heart-attacks-strokes-and-even-appendicitis-vanish-from-hospitals/2020/04/19/9ca3ef24-7eb4-11ea-9040-68981f488eed_story.html.
- 13. Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr 2020;174:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323:1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marzorati C, Riva S, Pravettoni G. Who is a cancer survivor? A systematic review of published definitions. J Cancer Educ 2017;32:228–37. [DOI] [PubMed] [Google Scholar]
- 17. Wang H, Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol 2020;21:e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and cancer: a comprehensive review. Curr Oncol Rep 2020;22:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol 2020;21:629–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Felice F, Polimeni A, Valentini V. The impact of Coronavirus (COVID-19) on head and neck cancer patients' care. Radiother Oncol 2020;147:84–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burki TK. Cancer care in the time of COVID-19. Lancet Oncol 2020;21:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang G, Zhang H, Yang Y. Challenges and countermeasures of integrative cancer therapy in the epidemic of COVID-19. Integr Cancer Ther 2020;19:1534735420912811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang L, Xu HY, Wang Y. [Diagnostic and therapeutic strategies of lung cancer patients during the outbreak of 2019 novel coronavirus disease (COVID-19)]. Zhonghua Zhong Liu Za Zhi 2020;42:292–5. [DOI] [PubMed] [Google Scholar]
- 24. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020;23:1775–6. [DOI] [PubMed] [Google Scholar]
- 25. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. COVID Impact Survey [homepage on the Internet]. Washington (DC): The Data Foundation; 2020. [cited 2020 May 20]. Available from: https://www.covid-impact.org/. [Google Scholar]
- 27. Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 2003;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Behrens T, Taeger D, Wellmann J, Keil U. Different methods to calculate effect estimates in cross-sectional studies. A comparison between prevalence odds ratio and prevalence ratio. Methods Inf Med 2004;43:505–9. [PubMed] [Google Scholar]
- 29. Coutinho LMS, Scazufca M, Menezes PR. Methods for estimating prevalence ratios in cross-sectional studies. Rev Saude Publica 2008;42:992–8. [PubMed] [Google Scholar]
- 30. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. [PubMed] [Google Scholar]
- 31. Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol 2020;31:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Side Effects of Cancer Treatment; [about 3 screens]. [cited 2020 May 22]. Available from: https://www.cancer.gov/about-cancer/treatment/side-effects.
- 33. Wenham C, Smith J, Morgan R, Gender and COVID-19 Working Group. COVID-19: the gendered impacts of the outbreak. Lancet 2020;395:846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Valeri L, Chen JT, Garcia-Albeniz X, Krieger N, VanderWeele TJ, Coull BA. The role of stage at diagnosis in colorectal cancer black-white survival disparities: a counterfactual causal inference approach. Cancer Epidemiol Biomarkers Prev 2016;25:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol 2018;36:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin 2019;69:211–33. [DOI] [PubMed] [Google Scholar]
- 37. White A, Vernon SW, Franzini L, Du XL. Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer 2010;116:4622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst 2002;94:334–57. [DOI] [PubMed] [Google Scholar]
- 39. Lai Y, Wang C, Civan JM, Palazzo JP, Ye Z, Hyslop T, et al. Effects of cancer stage and treatment differences on racial disparities in survival from colon cancer: a United States population-based study. Gastroenterology 2016;150:1135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yedjou CG, Sims JN, Miele L, Noubissi F, Lowe L, Fonseca DD, et al. Health and racial disparity in breast cancer. Adv Exp Med Biol 2019;1152:31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hill DA, Friend S, Lomo L, Wiggins C, Barry M, Prossnitz E, et al. Breast cancer survival, survival disparities, and guideline-based treatment. Breast Cancer Res Treat 2018;170:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Newman LA. Breast cancer disparities: socioeconomic factors versus biology. Ann Surg Oncol 2017;24:2869–75. [DOI] [PubMed] [Google Scholar]
- 43. Weaver KE, Foraker RE, Alfano CM, Rowland JH, Arora NK, Bellizzi KM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv 2013;7:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gross CP, Guo Z, McAvay GJ, Allore HG, Young M, Tinetti ME. Multimorbidity and survival in older persons with colorectal cancer. J Am Geriatr Soc 2006;54:1898–904. [DOI] [PubMed] [Google Scholar]
- 45. Cuthbert CA, Hemmelgarn BR, Xu Y, Cheung WY. The effect of comorbidities on outcomes in colorectal cancer survivors: a population-based cohort study. J Cancer Surviv 2018;12:733–43. [DOI] [PubMed] [Google Scholar]
- 46. Lopez R, Agullo P, Lakshmanaswamy R. Links between obesity, diabetes and ethnic disparities in breast cancer among Hispanic populations. Obes Rev 2013;14:679–91. [DOI] [PubMed] [Google Scholar]
- 47. Lega IC, Austin PC, Fischer HD, Fung K, Krzyzanowska MK, Amir E, et al. The impact of diabetes on breast cancer treatments and outcomes: a population-based study. Diabetes Care 2018;41:755–61. [DOI] [PubMed] [Google Scholar]
- 48. Charlot M, Castro-Webb N, Bethea TN, Bertrand K, Boggs DA, Denis GV, et al. Diabetes and breast cancer mortality in Black women. Cancer Causes Control 2017;28:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wosik J, Fudim M, Cameron B, Gellad ZF, Cho A, Phinney D, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc 2020;27:957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Portnoy J, Waller M, Elliott T. Telemedicine in the era of COVID-19. J Allergy Clin Immunol Pract 2020;8:1489–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smith AC, Thomas E, Snoswell CL, Haydon H, Mehrotra A, Clemensen J, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare 2020;26:309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med 2020;382:1679–81. [DOI] [PubMed] [Google Scholar]