Abstract
The heart consumes circulating nutrients to fuel lifelong contraction, but a comprehensive mapping of human cardiac fuel use is lacking. We used metabolomics on blood from artery, coronary sinus, and femoral vein in 110 patients with or without heart failure to quantify the uptake and release of 277 metabolites, including all major nutrients, by the human heart and leg. The heart primarily consumed fatty acids and, unexpectedly, little glucose; secreted glutamine and other nitrogen-rich amino acids, indicating active protein breakdown, at a rate ∼10 times that of the leg; and released intermediates of the tricarboxylic acid cycle, balancing anaplerosis from amino acid breakdown. Both heart and leg consumed ketones, glutamate, and acetate in direct proportionality to circulating levels, indicating that availability is a key driver for consumption of these substrates. The failing heart consumed more ketones and lactate and had higher rates of proteolysis. These data provide a comprehensive and quantitative picture of human cardiac fuel use.
The heart generates copious adenosine 5′-triphosphate (ATP), almost all through oxidative phosphorylation in mitochondria (1). A continuous supply of oxygen and nutrients is thus vitally needed. Heart failure is a leading cause of death worldwide, and the failing heart is often described as an “engine out of fuel” that fails to use circulating nutrients to satisfy its metabolic demands (2). Understanding how the heart handles fuels during health and disease is thus foundational to the rational development of new heart failure therapies.
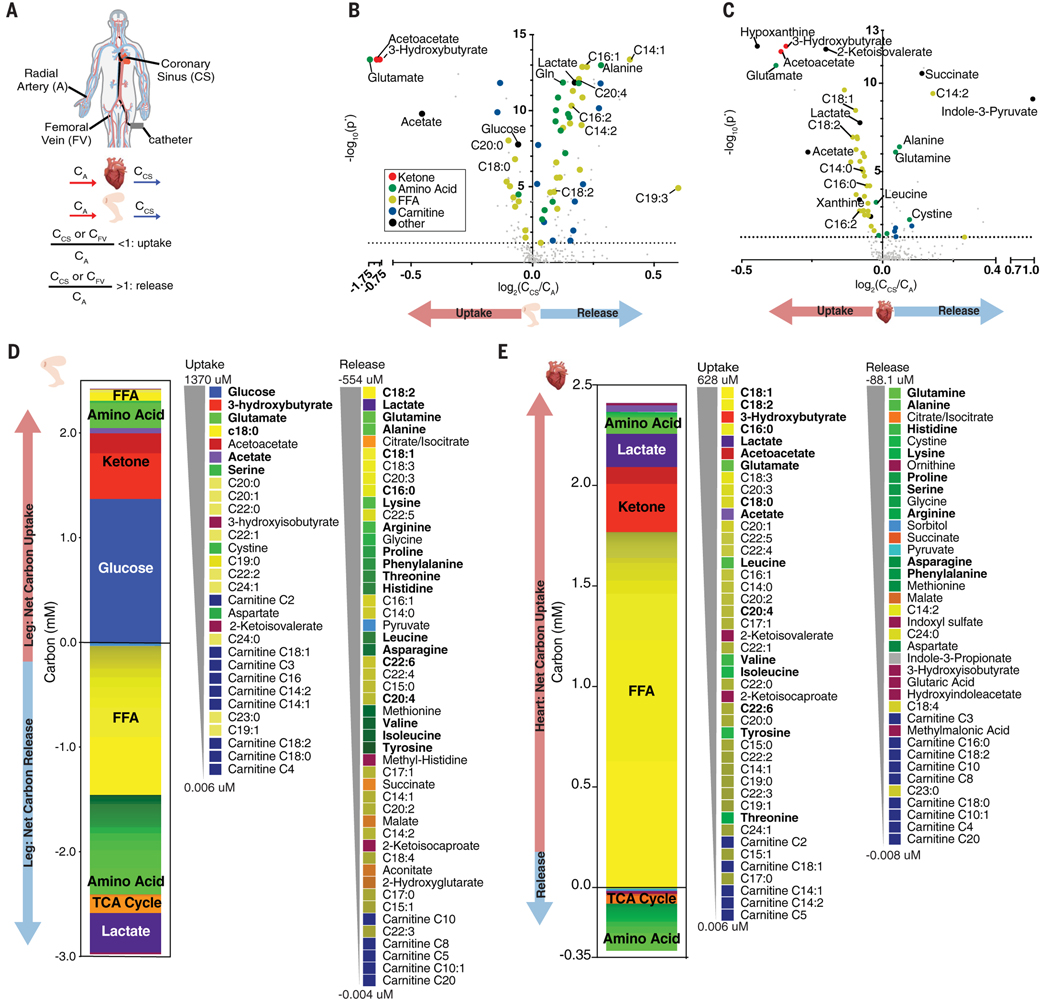
We measured arteriovenous (A-V) gradients of circulating metabolites across the human heart and leg by simultaneously sampling blood from radial artery, coronary sinus, and femoral vein (Fig. 1A). We enrolled 87 patients who were undergoing elective percutaneous catheter ablation of atrial fibrillation, had a left ventricular ejection fraction (LVEF) >50%, and had no history of heart failure (fig. S1A). Patient characteristics were representative of the US middle-aged population (table S1). A total of 600 known metabolites were measured using liquid chromatography–mass spectrometry, 277 of which were reliably detected in the plasma. By comparing the abundance of these metabolites in the artery (CA) versus vein (CCS or CFV for coronarysinus and femoral vein, respectively) (Fig. 1A), we identified statistically significant uptake or release of 117 metabolites across the leg and 65 across the heart (Fig. 1B and fig. S1B). Our CFV/CA data confirmed all 10 previously reported metabolites taken up or released by the human arm without anesthesia (3). We further identified an additional 107 metabolites that were significantly altered across the human leg (table S2).
Fig. 1. Human A-V metabolomics reveal distinct fuel profiles of the heart and leg.
(A) Blood was sampled simultaneously from the radial artery (A), coronary sinus (CS), and femoral vein (FV), and metabolite uptake or release was determined. (B and C) Volcano plot of metabolite abundance in the FV (B) or CS (C) relative to (A). P values were derived from one-sample Wilcoxon test and then Benjamini-Hochberg corrected (P*). Dotted line indicates P* = 0.05. (D and E) Net A-V carbon balance across the leg (D) and heart (E) shown in order of greatest to least average absolute carbon uptake or release.
Table S3 lists cardiac- and leg-specific uptake and release of abundant, high-turnover fuels. Both organs efficiently extracted ketones, acetate, and glutamate (up to ~67%). Although the leg took up glucose, there was no net average glucose uptake by the heart, unlike prior reports (4, 5), and cardiac glucose uptake did not correlate with plasma insulin or insulin resistance(fig. S2), perhaps reflecting the fasted state of patients. The heart consumed all free fatty acid (FFA) species, whereas the leg primarily took up saturated FFAs and released unsaturated FFAs, likely from subcutaneous adipose depots (table S4 and fig. S3). The heart secreted most essential amino acids, indicating active proteolysis (table S3).
To quantify the net contribution of each metabolite to carbon balance, we measured absolute arterial concentrations of the most abundant metabolites (tables S5 to S7) and used publicly reported concentrations for the remaining ones (6). From the concentrations and molecular formula of each metabolite, we calculated the absolute carbon uptake or excretion of each metabolite, measured as micromoles per liter of blood passing through the heart or leg (Fig. 1, D and E). The leg obtained ~90% of carbons as glucose and ketone bodies, whereas it released most carbons as FFAs, lactate, and amino acids (Fig. 1D). About half of the released FFA was linoleate (C18:2), an abundant essential FFA, indicative of active lipolysis from adipose depots. Many released amino acids were also essential, reflecting muscle proteolysis. The total release of carbons was 15% higher than uptake, indicating a net loss of leg mass during fasting.
In contrast to the leg, the heart obtained most carbons from FFAs (Fig. 1E), accounting for >70% of carbons (1750 μM) extracted from the circulation [~99 μM FFA, similar to that previously measured by 11C-palmitate and positron emission tomography (7) equivalent to ~1.4 μmol/min/g of FFA-derived carbons (8)]. This rate may be underestimated because FFAs can also accumulate in venous plasma when liberated from lipoprotein particles (9). Only a negligible portion of FA carbons were converted to acylcarnitines and secreted (< 0.25 μM carbons, ~0.01%), indicating nearly complete oxidation of FFAs. Ketones accounted for ~15% of myocardial carbon uptake (Fig. 1E). Acetate, the most abundant short-chain FA produced by gut microbiota (10), accounted for ~2% of myocardial carbon uptake and was avidly taken up by the heart (Fig. 1E and table S3). Acetate may be used as fuel and as an epigenetic modifier through histone acetylation (11, 12).
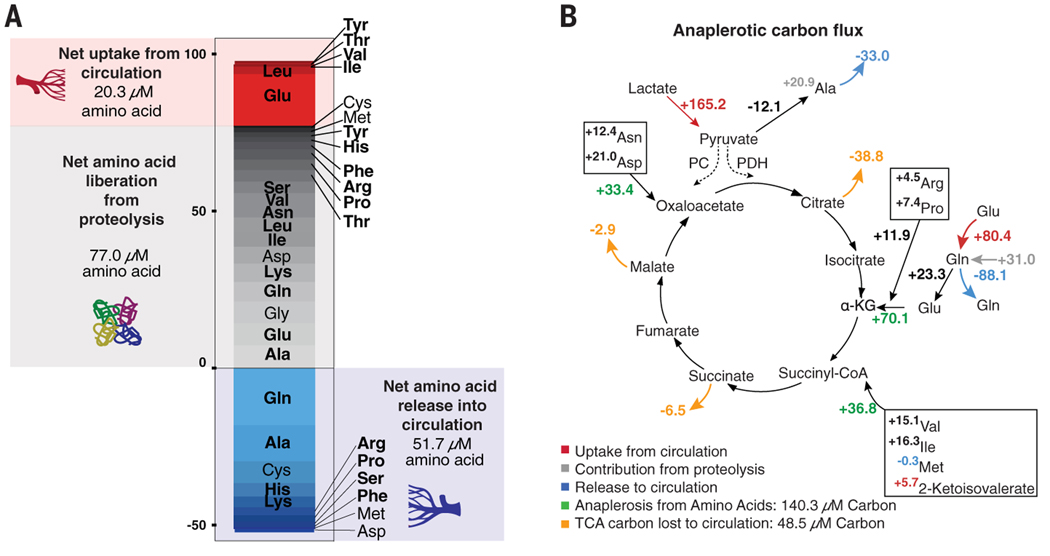
The heart secreted most amino acids (Fig. 1E and table S3), contributing to a large net negative nitrogen balance of 108 μM (P < 0.0001) (fig. S4) and indicating active proteolysis. Glutamine and alanine were the most abundant amino acids secreted. Glutamine release was nearly equimolar to glutamate uptake, suggesting active exchange of glutamate for glutamine for nitrogen removal. Lactate uptake likely supports nitrogen release as alanine through pyruvate transamination (13). Amino acids with greater nitrogen content (i.e., the ratio of nitrogen to carbon atoms) were strongly excreted (fig. S5A), further supporting the notion that the heart actively releases nitrogen as amino acids (14–16). We calculated a net cardiac proteolysis rate of 81 μM amino acid equivalents (AA-Eqs) (Fig. 2A; see the materials and methods for details of calculations), i.e., 0.06 μmol of AA-Eqs/min/g cardiac tissue. By comparison, the rate of proteolysis in the leg was 0.006 μmol of AA-Eqs/min/g. Cardiac proteolysis thus occurs at ~10× the rate in the leg, equivalent to ~1.5 g of protein over 12 hours, or just over 2% of that of cardiac protein (17, 18). Human cardiac protein is thus actively consumed and produced across fasting-feeding cycles.
Fig. 2. Cardiac nitrogen release reveals net amino acid liberation from proteolysis.
(A) Calculated cardiac sources of free amino acids (uptake from circulation is shown in red, liberation from proteolysis in gray) and released amino acids (shown in blue). Shading is proportional to the quantity of amino acid uptake of secretion. (B) Calculated anaplerotic carbon input from amino acid consumption exceeds carbon released as TCA cycle intermediates. Anaplerotic contribution from lactate through pyruvate carboxylase (PC) could not be determined (dashed lines). All numbers are micromoles of carbon. Non-anaplerotic amino acids (leucine) and amino acids not catabolized in heart (histidine, phenylalanine, and tyrosine) were excluded. PDH, pyruvate dehydrogenase.
Suppression of branched chain amino acid (BCAA) catabolism is implicated in maladaptive remodeling in heart failure (19–21). We calculate that BCAAs contribute 105 μM carbon equivalents to overall carbon use, or just under 5% of total carbon combustion, similar to that measured in mice (22). Further reduction of BCAA catabolism in the failing heart would thus have only a small effect on overall accessible carbons for combustion, suggesting that BCAAs affect heart failure through other mechanisms. Histidine was the most highly secreted essential amino acid (Fig. 2A and figs. S5A and S5B), suggesting that the proteins being degraded were histidine rich. Myoglobin constitutes 5 to 10% of cytosolic cardiac protein, is dispensable for baseline cardiac function (23), and is composed of 6% histidine, in contrast to most proteins, which contain only 1 to 2% histidine (24). Therefore, in addition to carrying oxygen, myoglobin may also serve as a reservoir of carbons. Other cardiac proteins may also contribute, as could sources exogenous to the heart, e.g., through macropinocytosis of albumin or other plasma proteins (25).
Paradoxically, despite high reliance on the tricarboxylic acid (TCA) cycle for ATP generation, the heart and leg release TCA cycle intermediates (Fig. 1, B to E). Tissues may export citrate, an allosteric inhibitor of FA oxidation and glycolysis (26), to prevent excessive suppression of these pathways. TCA intermediate secretion may also represent a means of clearing excess TCA cycle four-carbon units produced by amino acid breakdown. We calculated 140 μM (0.1 μmol/min/g) anaplerotic carbon flux from amino acid breakdown (Fig. 2B), well in excess of the ~50 μM (0.04 μmol/min/g) loss of TCA intermediates to the circulation. This is a likely underestimate of total anaplerosis because we could not quantify the contribution from pyruvate carboxylase (27, 28). This suggests active internal catabolism of TCA four-carbon units, e.g., through malic enzyme.
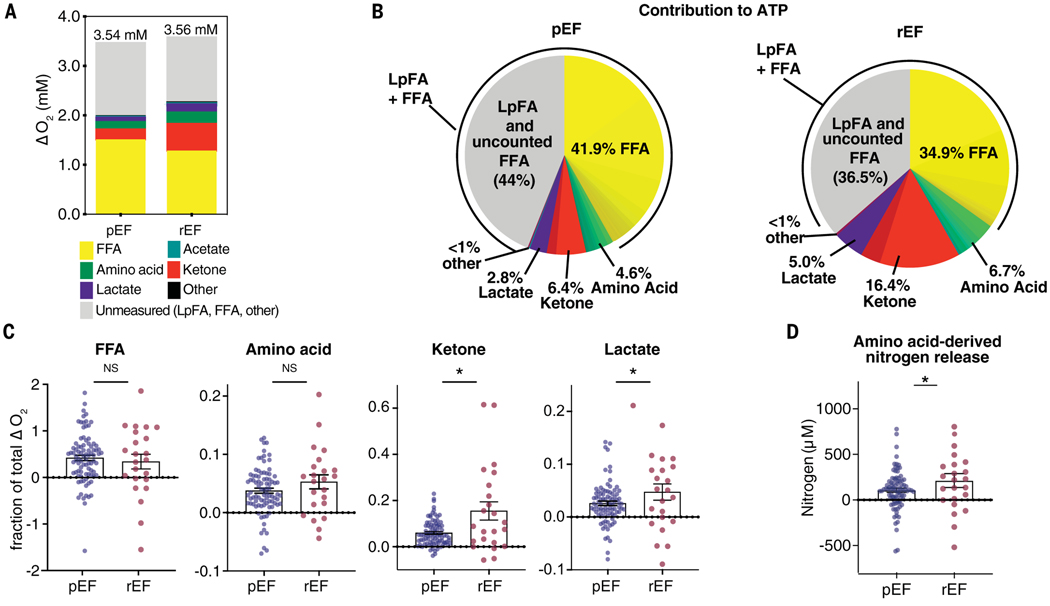
Calculating the contribution of all metabolites to cardiac oxygen consumption and ATP production (Fig. 3, A and B) implied a cardiac requirement of 3.0 mM plasma oxygen, equivalent to 2.0 mM in whole blood after correction for plasma-to-blood volume. Measured oxygen consumption was 3.5 mM (~9.5 ml O2/g/min). Thus, ~60% of measured oxygen consumption was accounted for by the measured metabolite A-V gradients (Fig. 3A). This is an underestimate because it does not account for FAs liberated from lipoproteins (29). Thus, the remaining ~40% oxygen consumption likely reflects a combination of lipoprotein-derived FAs (LpFAs) that are combusted by the heart and those that replace albumin-derived FFAs combusted by the heart. Consistent with this, unaccounted oxygen consumption correlated inversely with measured FA uptake (fig. S6, B and C). Other minor sources may include internal fuels such as triglycerides (30), FFAs liberated from epicardial adipose tissue, or oxygen consumption that does not generate ATP (e.g., conversion of hypoxanthine to uric acid by xanthine oxidase) (table S7) (31). Combustion of glycogen is not thought to occur in the heart during fasting (32–34). Assuming that ~35% of LpFAs are released into the coronary sinus, we calculated that ~57% of cardiac ATP production derives from FFAs, 6.4% from ketones, 4.6% from amino acids, 2.8% from lactate, and ~28% from LpFAs (for a total of ~85% from FAs) (Fig. 3B and table S8).
Fig. 3. Comparison of myocardial substrate use in patients with preserved versus reduced ejection fraction.
(A) Calculated substrate-specific contribution to total cardiac oxygen consumption. Average measured myocardial O2 consumption (ΔO2) is indicated above each bar. (B) Substrate-specific contribution to cardiac ATP generation in patients with preserved ejection fraction (pEF) versus reduced ejection fraction (rEF). (C) Proportion of total ΔO2 accounted for by the catabolism of each indicated substrate class in pEF versus rEF. (D) Net amino acid–derived nitrogen release in patients with pEF versus rEF. *P < 0.05 by t test.
We evaluated in a similar fashion 23 patients diagnosed with cardiomyopathy with an LVEF <40. Other than reduced LVEF, the cohort was similar to that with preserved LVEF (table S1). Patients with reduced LVEF had nearly tripled consumption of ketones (16.4 versus 6.4%), doubled lactate consumption (5.0 versus 2.8%), and a doubled rate of net amino acid–derived nitrogen release (212 versus 106 μM), i.e., rate of proteolysis (Fig. 3, B to D, and tables S5 to S8). Overall use of FFAs was suppressed. Increased plasma long-chain acylcarnitines have been associated with heart failure (35, 36), but tissue acylcarnitine amounts are decreased in failing hearts (37). We detected no increase in secretion of acylcarnitine by the failing heart (table S9), indicating that (i) any putative defect in FA consumption in heart failure occurred upstream of acylcarnitine production and (ii) a tissue other than the heart increases systemic acylcarnitine production.
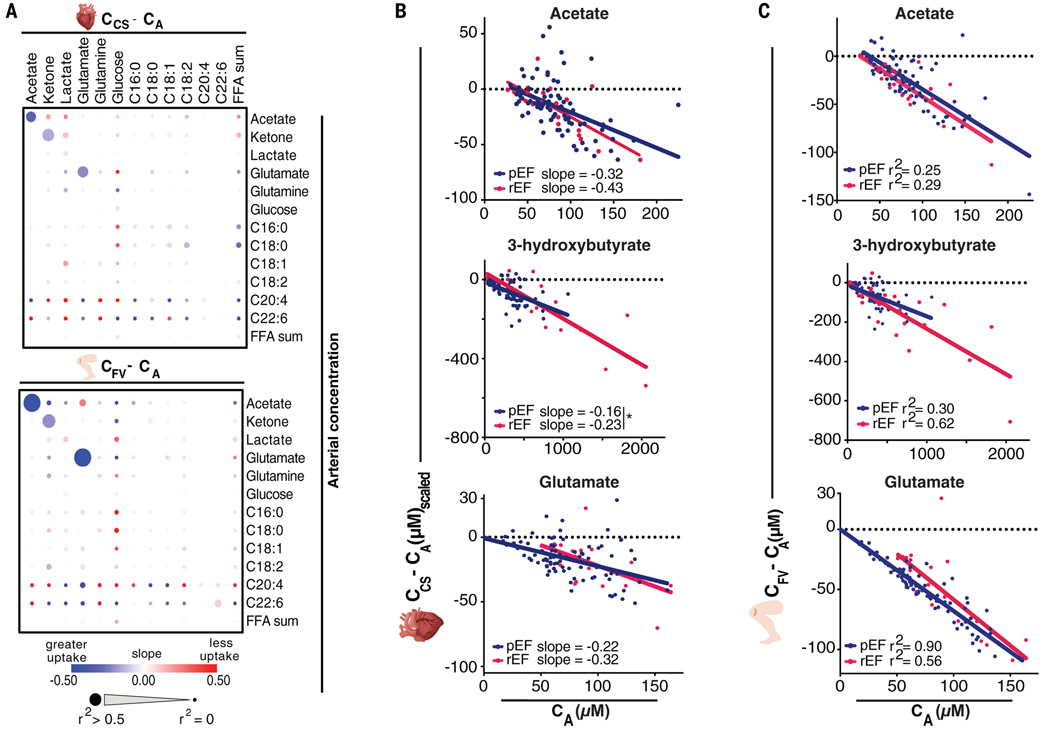
To gain insight into factors affecting fuel choice, we looked for correlations between metabolite concentrations and their use as fuel (Fig. 4A), between fractional extraction of different metabolites (fig. S8, A and B), and between fractional extraction and clinical or demographic parameters (fig. S9). Uptake of acetate, 3-hydroxybutyrate, and glutamate (but not that of glucose, lactate, or FFA) was directly proportional to circulating concentrations in both heart and leg (Fig. 4, A to C, and fig. S10C), suggesting that consumption of these three fuels is driven by substrate availability without the need for overt regulation. Moreover, their fractional uptake correlated strongly with each other (fig. S8, A and B), suggesting that it depends on tissue perfusion. In patients with reduced LVEF, all three fuels were extracted ~20% more by the heart (fig. S10, A and B), consistent with the longer transit times of blood through the heart vasculature allowing increased uptake. Thus, the increased consumption of ketones and glutamate in heart failure reflects higher plasma concentrations and lower rates of cardiac perfusion, as opposed to an inherent change in cardiac capacity to combust these fuels.
Fig. 4. Cardiac uptake of acetate, ketones, and glutamate primarily depends upon circulating concentrations in pEF and rEF.
(A) Relationship of A-V metabolite gradient (CV – CA) with arterial concentration of indicated metabolites by linear regression. (B) CA versus uptake of indicated metabolites by the heart after adjustment for acetate extraction [(CCS – CA)scaled; see the supplementary data]. *P < 0.05 by analysis of covariance. (C) CA versus uptake of the indicated metabolites by the leg.
In summary, we measured the uptake and secretion of 277 circulating metabolites by the human nonfailing and failing heart during fasting. FAs were the predominant cardiac fuel source. Unexpectedly, we observed little glucose uptake, perhaps a response to fasting or to general anesthesia, limitations of our study that were imposed by clinical necessity. Uptake of ketones was substantial and accentuated in heart failure. Ketone consumption has been suggested to be protective in heart failure (38), and our data suggest that delivery of ketones to the heart should be readily achievable. There was evidence of cardiac proteolysis despite the availability of circulating amino acids, which was markedly accentuated in heart failure. Whether this increased proteolysis in heart failure is adaptive or maladaptive will require further studies. The present data provide a framework of fuel use in the beating human heart and a foundation for understanding aberrant cardiac metabolism in disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff of the University of Pennsylvania
Electrophysiology Section for their enthusiastic support of this study, in particular M. Gnap, K. Conn, L. Tomczuk, and L. Czerniawski, as well as members of the Arany and Rabinowitz laboratories for insightful comments, in particular N. Yücel and S. Yang.
Funding: D.M. was supported by the NHLBI (F30 HL142186-01A1) and the Blavatnik Family Foundation, C.J. was supported by the American Diabetes Association (1-17-PDF-076), J.J.E. was supported by NIH 5T32HL007915, J.D.R. was supported by an NIH Pioneer grant (1DP1DK113643) and an NIH Diabetes Research Center grant (P30 DK019525), and Z.A. was supported by the NHLBI (HL126797) and the NIDDK (DK114103).
Footnotes
Competing interests: J.D.R. is a member of the Rutgers Cancer Institute of New Jersey and the University of Pennsylvania Diabetes Research Center, cofounder of VL54, stakeholder in Colorado Research Partners, and consultant to Pfizer, Inc. The remaining authors declare no competing interests.
Data and materials availability: All data are available in the main text or the supplementary materials.
REFERENCES AND NOTES
- 1.Allard MF, Schönekess BO, Henning SL, English DR, Lopaschuk GD, Am. J. Physiol 267, H742–H750 (1994). [DOI] [PubMed] [Google Scholar]
- 2.Neubauer S, Engl N. J. Med 356, 1140–1151 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Ivanisevic J. et al. , Sci. Rep 5, 12757 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizuno Y. et al. , Metabolism 77, 65–72 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Wisneski JA et al. , J. Clin. Invest. 76, 1819–1827 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wishart DS et al. , Nucleic Acids Res. 46 (D1), D608–D617 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson LR et al. , Diabetes 57, 32–40 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Hutchins GD et al. , J. Am. Coll. Cardiol 15, 1032–1042 (1990). [DOI] [PubMed] [Google Scholar]
- 9.Nelson RH, Prasad A, Lerman A, Miles JM, Diabetes 56, 527–530 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Perry RJ et al. , Nature 534, 213–217 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soliman ML, Rosenberger TA, Mol. Cell. Biochem 352, 173–180 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Taylor PB, Liew CC, Basic Res. Cardiol 71, 27–35 (1976). [DOI] [PubMed] [Google Scholar]
- 13.Peuhkurinen KJ, Hassinen IE, Biochem. J 202, 67–76 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takala T, Hiltunen JK, Hassinen IE, Biochem. J 192, 285–295 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taegtmeyer H, Ferguson AG, Lesch M, Exp. Mol. Pathol 26, 52–62 (1977). [DOI] [PubMed] [Google Scholar]
- 16.Pisarenko OI, Solomatina ES, Studneva IM, Biochim. Biophys. Acta 885, 154–161 (1986). [DOI] [PubMed] [Google Scholar]
- 17.Clowes GHA Jr., Randall HT, Cha C-J, JPEN J. Parenter. Enteral Nutr 4, 195–205 (1980). [DOI] [PubMed] [Google Scholar]
- 18.Preedy VR, Paska L, Sugden PH, Schofield PS,Sugden MC, Biochem. J 250, 179–188 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun H. et al. , Circulation 133, 2038–2049 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li T. et al. , Cell Metab. 25, 374–385 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W. et al. , Am. J. Physiol. Heart Circ. Physiol 311, H1160–H1169 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Neinast MD et al. , Cell Metab. 29, 417–429.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garry DJ et al. , Nature 395, 905–908 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Romero-Herrera AE, Lehmann H, Proc. R. Soc. London Ser. B 186, 249–279 (1974). [DOI] [PubMed] [Google Scholar]
- 25.Commisso C. et al. , Nature 497, 633–637 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garland PB, Randle PJ, Newsholme EA, Nature 200, 169–170 (1963). [DOI] [PubMed] [Google Scholar]
- 27.Comte B. et al. , J. Biol. Chem 272, 26125–26131 (1997). [DOI] [PubMed] [Google Scholar]
- 28.Panchal AR et al. , Am. J. Physiol. Heart Circ. Physiol 279, H2390–H2398 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Noh H-L, Okajima K, Molkentin JD, Homma S,Goldberg IJ, Am. J. Physiol. Endocrinol. Metab. 291, E755–E760 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Banke NH et al. , Circ. Res 107, 233–241 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia Y, Zweier JL, J. Biol. Chem 270, 18797–18803 (1995). [DOI] [PubMed] [Google Scholar]
- 32.Laughlin MR, Petit WA Jr., Shulman RG, Barrett EJ,Am. J. Physiol 258, E184–E190 (1990). [DOI] [PubMed] [Google Scholar]
- 33.Schneider CA, Taegtmeyer H, Circ. Res 68, 1045–1050 (1991). [DOI] [PubMed] [Google Scholar]
- 34.Evans G, Physiol J. 82, 468–480 (1934). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad T. et al. , J. Am. Coll. Cardiol 67, 291–299 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter WG et al. , J. Am. Heart Assoc 5, e003190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedi KC Jr. et al. , Circulation 133, 706–716 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen R. et al. , Circulation 139, 2129–2141 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.