Abstract
Background
Large fat embolus is a rare but potential reversible cause of ischaemic stroke.
Methods and results
We describe the neurosurgical management of a complete right internal carotid artery occlusion due to a large fat embolus, caused by a mitral valve replacement.
Conclusion
Knowledge of acute cerebral ischaemia due to large fat embolism and its hallmark ‘hypodense artery’ is mandatory. Extracranial to intracranial bypass is a feasible rescue treatment after failure of endovascular embolectomy.
Keywords: stroke, neurosurgery
Introduction
Cerebral fat embolism (CFE) is the deposition of lipid droplets or fatty tissue in the cerebral blood circulation.1
Microvascular manifestations are most common, typically occurring after trauma or orthopaedic surgery, with multiple fat microemboli migrating to the lungs, or, in the presence of a patent foramen ovale, to the brain.1 The neurological findings vary greatly, ranging from no signs or symptoms to coma.2
Macrovascular manifestations of CFE are extremely rare and are almost exclusively of iatrogenic origin during cardiac surgery, for example when cannulating the aorta for a cardiopulmonary bypass, aortic clamping, median sternotomy or dislodged pericardial or epicardial fat.3 Recently, another cause has been reported where large fat emboli occurred secondary to the injection of facial fat in aesthetic procedures resulting in migration via extracranial to intracranial collaterals.4
Imaging confirmation of CFE is preferably made with MRI, where a typical ‘starfield’ appearance is seen on DDiffusion weighted Imaging (DWI) sequences.5 On CT brain, it is more difficult to visualise microvascular CFE; however, larger fat emboli can be seen as hypodense intra-arterial nodules (density of about 60 HU), the so-called hypodense artery sign.6
We describe the management of a patient with a right internal carotid artery (ICA) occlusion due to a fat embolus, caused by an open mitral valve replacement. This case report illustrates two key points. First, due to the rarity of large vessel CFE, it can be misdiagnosed, resulting in a crucial loss of time. Second, although controversial, surgery can have an important role in the treatment of acute cerebral large vessel occlusion.
Case description
A 69-year-old patient underwent an open mechanical mitral valve replacement, seemingly an uncomplicated procedure. The patient awoke in the intensive care unit with a Glasgow Coma Scale (GCS of 9 (E2V2M5) and a complete left hemiplegia (National Institutes of Health Stroke Scale (NIHSS) of 25). An urgent CT brain and CT angiography (CTA) was performed that showed a low density abnormality on CT in the right distal internal carotid artery (ICA) and first segment of the middle cerebral artery (M1) (figure 1A and B), without evidence of cerebral infarction. On the arterial sequences, no filling of the right M1 was seen; the first segment of the right anterior cerebral artery (A1) and the posterior communicating artery (Pcom) were patent. This was initially misdiagnosed as an air embolus and the patient was urgently transferred for hyperbaric therapy, which was initiated 10 hours after surgery. Because there was no clinical improvement, the imaging was reviewed and the diagnosis of fat embolus was made. Multiple attempts at endovascular retrieval were then undertaken, but no adequate reperfusion could be obtained. Only a small channel was able to be formed around the fat embolus (Thrombolysis in Cerebral Infarction Scale, Grade 1). It was considered that the vessel was likely to reocclude given the residual stenosis and thrombogenicity of the fat embolus.
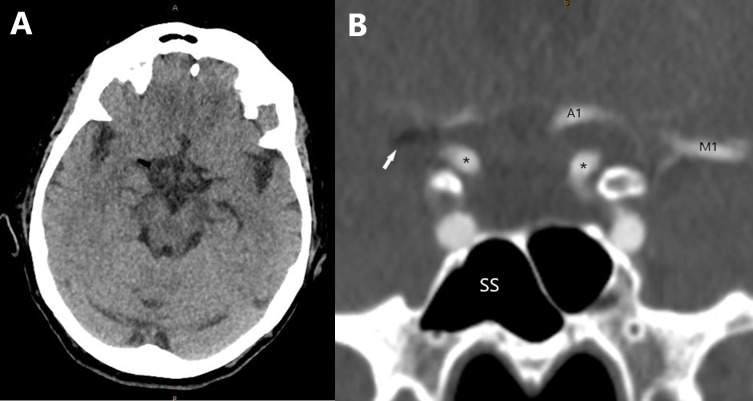
Figure 1.
(A) Axial CT brain, showing a hypodense right distal MCA. (B) Close up coronal CT angiography, showing a normal left configuration with patent left M1, A1 and terminal ICA (*), on the right, we see a fat embolus (white arrow) extending in the right M1, with no contrast enhancing distal of the embolus. Furthermore, we clearly see the difference in density between the air in the sphenoid sinus (SS) and fat embolus. ICA, internal carotid artery.
At this stage, the neurosurgical department was contacted. As 24 hours had passed since the initial cardiac surgery, a new CTA with perfusion images was performed, which showed reocclusion of the right ICA. Perfusion images showed the infarct core at the level of the basal ganglia, defined by a prolonged Time To Maximum (Tmax) and markedly decreased Cerebral Blood Flow (CBF) and Cerebral Blood Volume (CBV). The cortex had prolonged Tmax but only moderately reduced CBF and near normal CBV (figure 2A, B and C). Clinically, the patient was unchanged. As there was a large penumbra, the patient was considered likely to benefit from reperfusion therapy by removal of the fat embolus or Superficial Temporal Artery to Middle Cerebral Artery (STA–MCA) bypass.
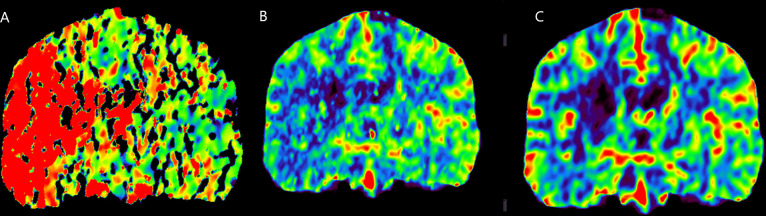
Figure 2.
(A) CT perfusion showing markedly increased Tmax at the level of the basal ganglia in the right hemisphere and at the level of the right frontal and temporal cortex. (B) CT perfusion showing markedly reduced rCBV at the level of the basal ganglia in the right hemisphere, and near normal rCBV at the level of the right frontal and temporal cortex. (C) CT perfusion showing markedly reduced rCBF at the level of the basal ganglia in the right hemisphere, and moderately reduced rCBF at the level of the right frontal and temporal cortex.
Surgical technique
A right pterional, transsylvian approach to the right ICA was performed. The fat embolus was visualised through the arterial wall at the ICA bifurcation extending into the middle cerebral artery (figure 3A). The segment was trapped using temporary clips on the internal carotid, anterior cerebral and middle cerebral arteries. An arteriotomy was performed and an organised fat embolus was found (figure 3B). The fat embolus was too adherent to the intima to be completely removed, and a subtotal piecemeal embolectomy was performed. The temporary proximal ICA clip was removed in an attempt to help in dislodging the embolus, but this was unsuccessful. At this stage, it was decided to trap the occluded segment and perform a bypass. The arteriotomy was closed. A permanent aneurysm clip was placed across the ICA just proximal to the embolus and distal to the anterior choroidal artery, proximal to A1 and M1. This was done because it was considered unsafe to reperfuse through the partially removed embolus for fear of dislodgement into the distal vasculature. The other temporary clips were removed. A superficial temporal artery to middle cerebral artery bypass graft was then performing using interrupted 10/0 nylon on a temporal branch of the middle cerebral artery. Perioperatively aspirin was started. Good flow was demonstrated in the bypass. The cross clamp time was 35 min. An intraparenchymal pressure monitoring catheter was placed and the dura, bone flap, temporal muscle and skin were closed in standard fashion.
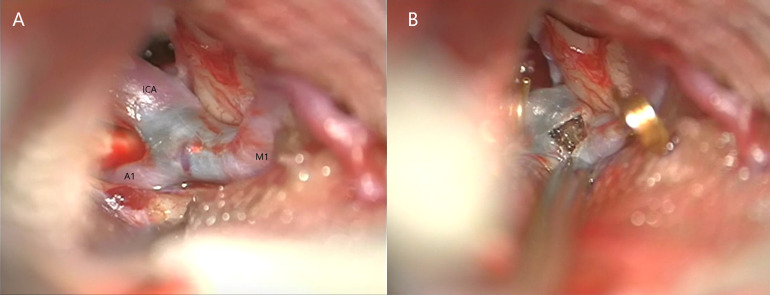
Figure 3.
(A) Intraoperative image illustrating the distal ICA, M1 and A1 segment with the obvious blue clot shining through. (B) Perioperative image showing the arteriotomy with the organised fat embolus as well as the gold temporary clips.
Results
On postoperative day 1, the intracranial pressures remained stable, a CT brain and CTA showed ischaemia of the basal ganglia, but a patent bypass (figure 4). The patient was extubated. On postoperative day 2, there was a GCS of 14/15 (E4V4M6) NIHSS of 16, but a persisting complete left hemiplegia and neglect. Therapeutic anticoagulation was started postoperative day 7. Postoperative week 3, the first signs of recovery started to appear, there was motor contraction in the left lower limb (2/5) and upper limb (1/5) NIHSS of 14. After 4 weeks, the patient was transferred to a rehabilitation centre.
Figure 4.
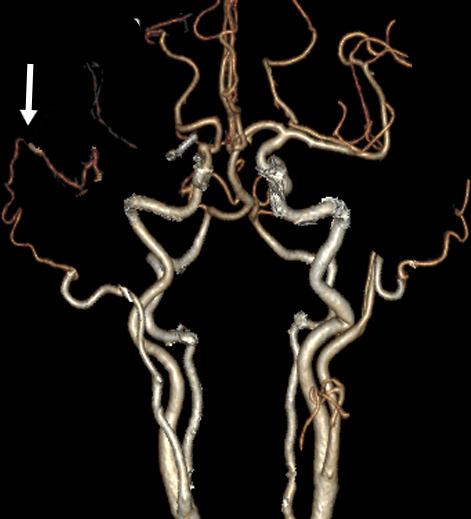
Postoperative 3D CTA, white arrow indicating the patent STA–MCA bypass. CTA, CT angiography.
At 6 months follow-up, the patient made a good recovery in the left lower limb (4-/5) and was able to walk independently; NIHSS of 9. In the left upper limb, there was no recovery. A CT brain and CTA show infarction of the basal ganglia but no infarction in the previously demonstrated penumbra (figure 5).
Figure 5.
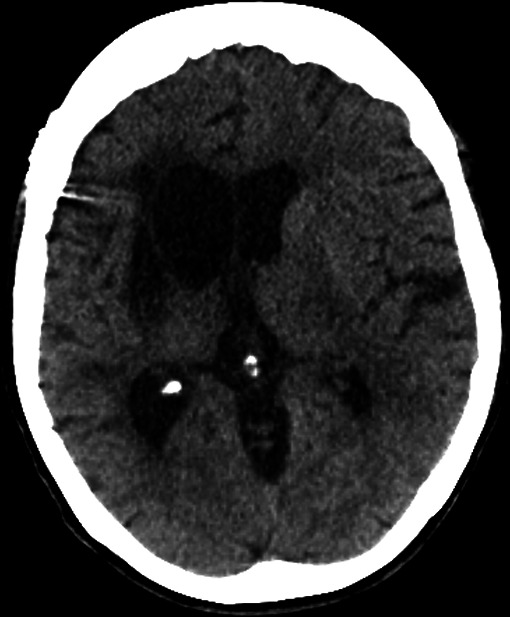
Axial CT brain, 6 months postoperatively. The well-defined right-sided basal ganglia infarct is noted with secondary right ventricular dilation.
Discussion
With only few reports of large artery occlusion due to a fat embolus, the diagnosis can be a challenge, delaying correct diagnosis and management.1 Knowledge of the hallmark hypodense artery sign and the differentiation with an air embolus, which typically has a density less than −1000 HU (vs −30 to −70 HU for fat) is mandatory.
In patients with acute or evolving stroke, outcome is related to timing of reperfusion.7 Unlike thromboembolic arterial occlusion, no guidelines exist for the management of ischaemic stroke secondary to fat emboli. Endovascular thrombectomy is now the standard of care for patients with acute ischaemic stroke secondary to acute large vessel occlusion, and seems the most appropriate first-line treatment for large fat emboli.4 Multiple successful fat emboli retrievals have been reported.4 In our case, endovascular retrieval was unsuccessful, possibly due to the time elapsed between initial onset and the endovascular attempt (24 hours).
As the patient was further deteriorating, the next treatment option could have been conservative, or an attempt for surgical reperfusion. The role of surgery in the treatment of acute ischaemic stroke is controversial. Minimally invasive and rapid surgical embolectomy has been proposed as a rescue procedure when endovascular embolectomy fails.8 9 Another surgical option in restoring blood flow if endovascular (or open) embolectomy fails is a bypass. Literature looking at Extracranial to intracranial (EC-IC) bypass for emergency cerebral revascularisation is sparse, probably provoked by the general aversion of bypass surgery after the publication of both the EC-IC bypass study and the COSS trial, and a general trend to less invasive treatment modalities.10 11 Nonetheless, as shown by Nussbaum et al and Burkhardt et al, in selected patient groups, an emergency bypass can arrest progression of stroke or result in neurological improvement.12 13
Further deterioration despite maximal standard treatment options and the presence of ‘salvageable brain’ on perfusion imaging were key in the decision making. A surgical reperfusion was thought to give the patient the best chance for a better outcome. The goal of the surgery was a complete embolectomy, with bypass as a salvage option. An adequate thrombectomy could not be obtained because of adherence of the fat to the intima of the vessel, possibly due to the time elapsed between onset and surgery, so a STA–MCA bypass was performed. Due to the involvement of a centre experienced in bypass surgery, the outcome of this patient was favourable.14 This view is supported by the rapid clinical improvement after surgery and sparing of the distal MCA territory on follow-up imaging.
Conclusion
Knowledge of acute cerebral ischaemia due to large fat embolism and its hallmark ‘hypodense artery’ is mandatory. Extracranial to intracranial bypass can be a feasible rescue treatment after failure of endovascular embolectomy.
Footnotes
Contributors: JVDV collected data, analyzed data, wrote paper. ALP intellectual review. MJM intellectual review. MAS performed procedure described in article, contributed data, intellectual review. All coauthors were involved in the revision for intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Deidentified participant data.
References
- 1. Scarpino M, Lanzo G, Lolli F, et al. From the diagnosis to the therapeutic management : cerebral fat embolism, a clinical challenge. Int J Gen Med 2019;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scarpino M, Lanzo G, Moretti M, et al. Delayed cerebral fat embolism occurring after off-pump coronary artery bypass grafting. Cardiol J 2018;25:155–7. 10.5603/CJ.2018.0015 [DOI] [PubMed] [Google Scholar]
- 3. Abend NS, Levine JM. Hypodense middle cerebral artery with fat embolus. Neurocrit Care 2007;6:147–8. 10.1007/s12028-007-0007-y [DOI] [PubMed] [Google Scholar]
- 4. Huo X, Liu R, Wang Y, et al. Cerebral fat embolism as complication of facial fat graft: retrospective analysis of clinical characteristics, treatment, and prognosis. World Neurosurg 2018;120:249–55. 10.1016/j.wneu.2018.08.148 [DOI] [PubMed] [Google Scholar]
- 5. Zakhari N, Castillo M, Torres C. Unusual cerebral emboli. Neuroimaging Clin N Am 2016;26:147–63. 10.1016/j.nic.2015.09.013 [DOI] [PubMed] [Google Scholar]
- 6. Avila JD Hypodense artery sign in cerebral fat embolism. Pract Neurol 2017;17:304–5. 10.1136/practneurol-2017-001603 [DOI] [PubMed] [Google Scholar]
- 7. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart Association/American stroke association. Stroke 2018;49:46–110. 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 8. Park J, Hwang Y-H, Huh S, et al. Minimally invasive and rapid surgical embolectomy (MIRSE) as rescue treatment following failed endovascular recanalization for acute ischemic stroke. Acta Neurochir 2014;156:2041–9. 10.1007/s00701-014-2179-5 [DOI] [PubMed] [Google Scholar]
- 9. Hino A, Oka H, Hashimoto Y, et al. Direct microsurgical embolectomy for acute occlusion of the internal carotid artery and middle cerebral artery. World Neurosurg 2016;88:243–51. 10.1016/j.wneu.2015.12.069 [DOI] [PubMed] [Google Scholar]
- 10. Ausman JI, Diaz FG. Critique of the extracranial-intracranial bypass study. Surg Neurol 1986;26:218–21. 10.1016/0090-3019(86)90152-7 [DOI] [PubMed] [Google Scholar]
- 11. Derdeyn CP, Grubb RL, Powers WJ, et al. Extracranial-Intracranial bypass for ischemic cerebrovascular disease: what have we learned from the carotid occlusion surgery study? Neurosurg Focus 2014;36:9. [DOI] [PubMed] [Google Scholar]
- 12. Nussbaum ES, Janjua TM, Defillo A, et al. Emergency extracranial-intracranial bypass surgery for acute ischemic stroke. J Neurosurg 2010;112:666–73. 10.3171/2009.5.JNS081556 [DOI] [PubMed] [Google Scholar]
- 13. Burkhardt J-K, Winklhofer S, Fierstra J, et al. Emergency extracranial-intracranial bypass to revascularize salvageable brain tissue in acute ischemic stroke patients. World Neurosurg 2018;109:e476–85. 10.1016/j.wneu.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 14. Gunawardena M, Rogers JM, Stoodley MA, et al. Revascularization surgery for symptomatic non-Moyamoya intracranial arterial stenosis or occlusion. J Neurosurg 2019;16:1–6. 10.3171/2018.9.JNS181075 [DOI] [PubMed] [Google Scholar]