Abstract
Background:
Fowl adenoviruses (FAdVs) associated with certain clinical diseases including inclusion body hepatitis (IBH) have become of considerable importance in the poultry industry. Currently, an increasing number of IBH outbreaks in different parts of Iranian poultry industries is a growing concern. Infectious bursal disease virus (IBDV) or chicken infectious anemia virus (CIAV) have historically been incriminated as predisposing factors for FAdVs to cause IBH. Furthermore, some have speculated whether IBDV vaccine strains impact on IBH clinical manifestation. The present report assesses the potential predisposing role of IBDV, CIAV, and infectious bursal disease) IBD( vaccine strains for FAdVs in the course of an IBH occurrence in the field.
Case description:
90000 day-old broiler chickens with the same parent source were housed, at 4 day-interval, in two commercial farms in Shiraz, Iran. Increased mortality with lesions of hepatitis, suggestive of IBH, started in the primitive farm right after blind prescription of IBD vaccine at the age of 12-days-old. Consequently, IBD vaccination was postponed for the apparently healthy chickens of the other farm in which chickens were monitored for the occurrence of IBH afterwards. Laboratory examination was followed by histopathology and polymerase chain reaction (PCR) on liver, cloacal bursa, and thymus samples to determine the involvement of FAdV, IBDV, and CIAV in the occurrence of the disease.
Findings/treatment and outcome:
No evidence was found to support the predisposing role of neither IBD vaccination nor IBDV/CIAV infection in this IBH occurrence. The results also demonstrated a primary role of the FAdV-11 as a causal agent of the IBH occurrence.
Conclusion:
The findings suggest that certain FAdVs are pathogenic enough to primarily induce IBH in young broilers.
Key Words: CIAV, Fowl adenovirus, IBDV, IBD vaccination, IBH
Introduction
Inclusion body hepatitis (IBH) is a world-wide distributed disease characterized by sudden onset of mortality with the most striking lesions in livers of the affected birds. Principally, 2-10% mortality in 3-7 weeks old chickens has been attributed to the disease. However, mortality as high as 30% and outbreaks in chickens as young as one-week-old have been reported (Schachner et al., 2018 ▶). The condition was first described as an avian hepatic inclusion body in 1963 with unknown significance (Helmboldt and Frazier, 1963 ▶). In later years, fowl adenoviruses (FAdVs) were attributed to the disease and perceived as opportunistic pathogens causing IBH in concurrent infection by immunosuppressive agents such as infectious bursal disease virus (IBDV) and chicken infectious anemia virus (CIAV) (Rosenberg et al., 1974 ▶; Fadley et al., 1976 ▶). However, later, documented reports of IBH outbreaks in absence of predisposing agents (Christensen and Saifuddin, 1989 ▶; Gomis et al., 2006 ▶; Nakamura et al., 2011 ▶; Steer et al., 2011 ▶) strengthened the primary role FAdV in the etiology of the IBH. Apart from CIAV and IBDV, there have been speculations (Lim et al., 2011 ▶) on the association of infectious bursal disease (IBD) vaccine strains to IBH, especially in countries in where hot or intermediate IBD vaccines are introduced in broiler flocks.
Since the first official report of IBH in Iran (Hosseini and Morshed, 2012 ▶), there have been indications of a rise in disease incidence in different provinces of the country (Nateghi et al., 2014 ▶; Khodakaram-Tafti et al., 2016 ▶; Morshed et al., 2017 ▶). Hence, IBH emerged as an economically important disease of the local poultry industries and renewed interests in further investigation of the disease. So far, studies about IBH in Iran are focused on the detection and classification of the casual FAdV, while information about the role of the predisposing agents in the disease outbreaks is limited. In the present report, we described the occurrence of IBH in two flocks of commercial broiler chickens derived from the same parent source and investigated the possible predisposing role of IBD vaccination and subclinical IBDV/CIAV infections.
Case description
In December 2018, 90000 day-old broiler chickens were supplied from a breeder flock in the north of Iran to be housed in two commercial broiler farms (designated as A and B) under the same operation in Shiraz, Iran. Day-old chickens were supplied in two equal parts and arrived at farm A and B with a 4-day interval. Both farms were comprised of 4 houses with flock size varying from 10000 to 11500 birds. At 3 and 8 days of age, chicken orally received live infectious bronchitis and Newcastle disease vaccines (NDVs), respectively. A sudden increase in mortality together with lesions of hepatitis, suggestive of IBH, appeared in broiler chickens of farm A, right after blind prescription of IBD vaccine (intermediate strain) at 12 days of age. Considering the transient immunosuppressive effect of IBD vaccination and its possible impact on the onset of IBH clinical manifestation, optimum IBD vaccination timing was estimated based on maternally derived antibodies using the Deventer formula for the chickens of the farm B (data are not shown). Subsequently, postmortem examination was performed on affected 12-day-old chickens of farm A and eight apparently healthy 8-day-old chickens of farm B.
Sampling
Liver, thymus, and cloacal bursa of chickens from both farms were sampled for virology and stored frozen at -20°C. Furthermore, a small area of the liver, bursa, and thymus of the affected chickens was fixed in 10% buffered formalin solution and stored at room temperature. Clinical and pathological findings in both farms were recorded in the course of the investigation. Flocks size, age at onset of disease, and percentage of mortality are given in Table 1.
Table 1.
Flocks information and results of the virological investigation
| Farm | Flock | Flock size | Age | Mortality % | FAdVb | IBDV/CIAV co-infection |
|---|---|---|---|---|---|---|
| Farm A | A1a | 11500 | 12 | 8 | FAdV-11 | -/- |
| A2 | 11500 | 12 | 11 | FAdV-11 | -/- | |
| A3 | 10000 | 13 | 9 | FAdV-11 | -/- | |
| A4 | 12000 | 14 | 8 | FAdV-11 | -/- | |
| Farm B | B1 | 11000 | 13 | 10 | FAdV-11 | -/- |
| B2 | 11000 | 14 | 9 | FAdV-11 | -/- | |
| B3 | 12000 | 12 | 9 | FAdV-11 | -/- | |
| B4 | 11000 | 15 | 8 | FAdV-11 | -/- |
a Different houses of the farm are presented numerically, and b Conventional PCR amplifying the hexon loop-1 region of FAdV; sequencing differentiates between 12 relevant serotypes. FAdV: Fowl adenovirus, IBDV: Infectious bursal disease virus, and CIAV: chicken infectious anemia virus
Histopathology
After fixation in 10% buffered formalin, tissue samples were processed, embedded in paraffin, sectioned at 4 µm using a Microm HM 360 microtome (MicromLaborgeräte GmbH, Walldorf, Germany) and stained with haematoxylin and eosin (H&E).
Polymerase chain reaction (PCR), and sequence analysis
Viral DNAs/RNAs were simultaneously extracted from a total of 25 mg of liver, bursa of fabricius, and thymus samples, using QIAamp ® virus kits (Qiagen, Vienna, Austria), according to the manufacturer’s instructions. The extracted DNAs from liver samples were subjected to the conventional PCR amplifying the loop-1 region of the hexon gene (Meulemans et al., 2001 ▶); positive PCR products were sequenced to type the detected FAdV. Investigation of the extracted viral nucleic acids from thymus and bursa samples was carried out by primer sets targeting VP1 and VP2 genes of CIAV and IBDV, respectively (Todd et al., 1992 ▶; Lin et al., 1994 ▶). Sequencing services were provided by LGC Genomics GmbH (Berlin, Germany). Assembly and nucleotide sequence alignments were conducted with Lasergene package (DNASTAR Inc., Madison, WI, USA). Sequences were aligned using Clustal W method and phylogenetic tree was constructed with MegAlign software applying 1000 bootstrap trials. The analysis was carried out by comparing the obtained nucleotide sequences with available sequences from the GenBank database.
Results
Clinical, gross, and histopathological findings
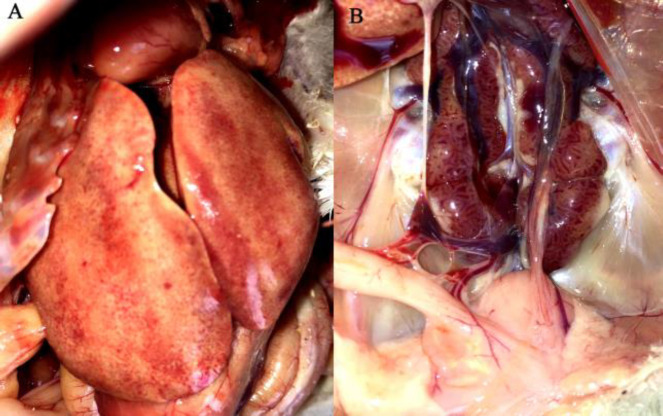
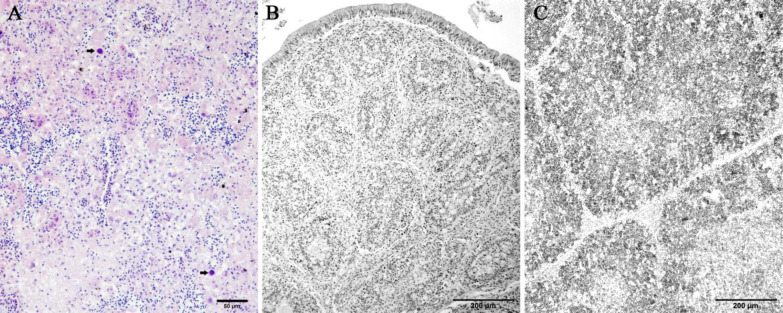
Affected chickens in farm A exhibited depression, weakness with ruffled feathers, and yellowish watery droppings. Some birds were holding their head back or lying on their sides while others died very quickly without any clinical sign. Mortality of 8-11% was recorded in different houses of the farm ( Table 1 ). Grossly, outstanding lesions were present in livers of affected chickens which were enlarged, friable, blunt edged, and mottled with petechial hemorrhages ( Fig. 1A ). In some birds, the kidneys were pale and had a mottled appearance ( Fig. 1B ). Bursa and thymus were grossly normal with no distinct evidence of atrophy for this age ( Fig. 1B ). Microscopic examination of the livers showed extensive necrotizing hepatitis characterized by loss and replacement of hepatocytes by eosinophilic remnants and accumulation of inflammatory cells in some areas. Large adenoviral inclusion bodies were present in the nuclei of hepatocytes ( Fig. 2A ). Except mild depletion of lymphocyte cells no characteristic histological lesions were found in the cloacal bursa and thymus of the affected birds ( Figs. 2B-C ).
Fig. 1.
Gross lesions associated to FAdV-11. (A) White-red marbled appearance of the enlarged liver with marked petechial hemorrhages, and (B) Swollen kidneys with mottled appearance (these findings were not as frequent as liver lesions). Size of bursa is grossly normal for this age
Fig. 2.
Histopathology of the affected chicken associated with FAdV-11. (A) Area of necrosis appears with hepatic cords disruption, eosinophilic cellular remainders, and inflammatory cells infiltration. Typical large, round, deeply basophilic intranuclear inclusion bodies are present in the necrotic area (arrows) (H&E, scale bar = 50 μm), (B) Mild loss of the follicular lymphoid cell population of the cloacal bursa (H&E, scale bar = 200 μm), and (C) Mild depletion of the lymphocytes in both the cortexes and medullae of the thymus (H&E, scale bar = 200 μm)
Before reaching the estimated IBD vaccination date, clinical signs of IBH appeared in all houses of the farm B at closely similar ages as farm A (Table 1). Overall, clinical, gross, and histopathological findings of the affected chickens in farm B were similar to what is described above for farm A.
PCR and sequencing analysis
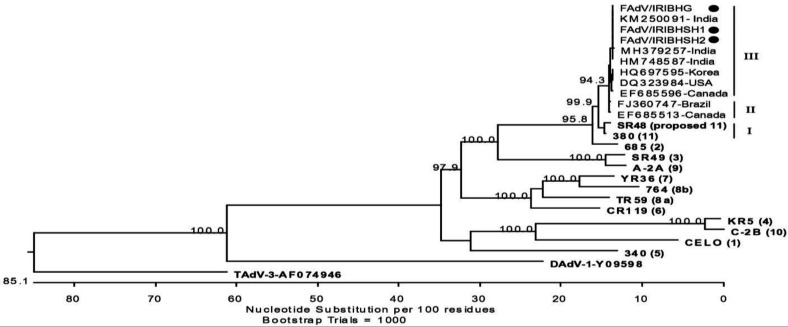
The results of PCR and sequencing are summarized in Table 1. Conventional PCR on affected liver samples from both farm A and farm B (around 12 days of age) were positive for FAdV. Sequencing of positive PCR products containing the L1 loop of the hexon gene assigned detected viruses as FAdV-11. Two FAdV-11 were 100% identical and fell into subcluster III, with 97.2 nucleotides identity with the respective prototype strain 380 based on analysis of hexon gene (Fig. 3). All liver, thymus, and cloacal bursa samples obtained from apparently healthy chickens of the farm B at 8 days of age (4 days before IBH manifestation) were negative for FAdV. Furthermore, neither the thymus and cloacal bursa samples taken from IBH affected chickens in farm A/B, nor those from healthy chickens of the farm B (8-day-old) were positive for IBDV and CIAV.
Fig. 3.
Phylogenetic tree based on the hexon gene of avian adenoviruses, including the FAdV-11 of this study (designated with black circles) (sequence FAdV/IRIBHG obtained from an IBH outbreak in the broiler flock of the same source located in the north of Iran). Clustal W alignment was performed for a segment corresponding to nucleotides 18,632-19,251 of the complete genome of CELO (GenBank Accession No. U46993). Reference strains are bold and numbers in parentheses indicate serotypes for the respective reference strains. Representatives of the genera Atadenovirus (DAdV-1) and Siadenovirus (TAdV-3) were used as outliers. FAdV-11 associated subclusters are shown by Roman numerals (I-III)
Discussion
In recent years, the occurrence of IBH has increased considerably in different parts of the country, especially in regions with intensive broiler production. However, it is not clear whether the increase in incidence of the disease is linked to the known predisposing factors. Suppressive agents to the chicken immune system are known to make them susceptible to secondary diseases (Hoerr, 2010 ▶). This role for IBDV and CIAV has been historically perceived as a predisposing factor for the IBH (Fadly et al., 1976 ▶; Toro et al., 2000 ▶). Moreover, there have been speculations about the effect of the transient immunosuppression through live IBDV vaccination on the pathogenicity of FAdV in young broiler chickens. Additionally, because vertical transmission is often blamed for the IBH outbreaks, the other question to be addressed is whether immunosuppressive agents are necessary for the multiplication of the vertically transmitted virus to induce pathological signs or FAdV are pathogenic enough to primarily induce IBH in young broilers. In this paper, we report a primary outbreak of IBH in young progenies of a breeder flock and exclude the role of the IBD vaccination and subclinical IBDV/CIAV infection in the disease outbreak.
The occurrence of 8-11% mortality in different houses of the investigated farms in the present study suggests an outbreak with higher severity but within the range of the previously documented mortality rate (2-10%) for IBH (Hess, 2013 ▶). An outbreak of IBH with mortality up to 30% in broiler chickens at the same age as the present case was previously reported from Australia (Christensen and Saifuddin, 1989 ▶). A higher mortality rate in an early-age IBH outbreak in the field is in agreement with age-related resistance, described for FAdV infection in the experimental setting (Schachner et al., 2018 ▶). Overall gross and histopathological findings in the course of the outbreak were consistent with the characteristics described for the IBH (Hess, 2013 ▶).
Co-infection with neither IBDV nor CIAV was found in investigated bursa and thymus samples excluding the predisposing role of these agents for this IBH outbreak. Hence, mild loss of the lymphoid cells in the thymus and cloacal bursa of the IBH-affected chickens in the present case are likely associated with the involvement of this primary lymphoid organ in the pathology of FAdV infection (Steer et al., 2015 ▶). Broiler breeders in Iran are normally vaccinated against CIAV to prevent vertical transmission of the virus and provide protection for the clinical and subclinical form of the disease. Similarly, live and killed IBD vaccines are being practiced in both breeder and broiler flocks in Iran. Moreover, measuring IBD maternal antibody titers in progenies of the respective breeder in farm B suggested a sufficient transmission of the immunity from dams to progenies. In recent years, reports from Canada, Japan, and Australia documented IBH outbreaks in absence of the predisposing role for CIAV/IBDV (Schachner et al., 2018 ▶).
Infectious bursal disease virus intermediate and intermediate plus vaccines are licensed and are widely used in broiler farms in Iran. During the recent surge in the number of IBH outbreaks, the co-occurrence of IBD vaccination and IBH clinical manifestation in young broiler chickens, has been a matter of debate between farmers and poultry veterinarians. While it seemed that early-age blind prescription of the IBD vaccine triggered the clinical signs of the IBH in chickens of farm A, its presumed predisposing role was ruled out later on when IBH broke out in unvaccinated chickens of farm B at closely similar ages. Moreover, the mortality rates in the course of the disease were not statistically different between vaccinated and unvaccinated chickens of farm A and B, respectively. Since IBD vaccination concurs with the occurrence of IBH in young broilers, commonly around 12-25 days of age (Lim et al., 2011 ▶; Nakamura et al., 2011 ▶; Steer et al., 2011 ▶; Morshed et al., 2017 ▶), this might be regarded as a coincidence rather than a direct etiological link to IBH pathogenesis. Nonetheless, more studies are required to elucidate the role and impact of the IBDV vaccine strains in the pathogenesis of the IBH.
While FAdV was not detected in liver samples of the healthy 8-day-old chickens of the farm B, IBH occurred at around 12 days of age in different houses of the same farm, and FAdV-11 was detected in the livers of the affected chickens. The incubation period of FAdV was shown to be very short (24-48 h) following infection under the experimental condition where gross lesion appears in the target oragn (liver) at 1-2 day(s) post-infection (Steer et al., 2015 ▶). Absence of virus in the target organ, 4 days before the manifestation of the clinical signs, might be explained under field condition where longer duration of virus multiplication before reaching target organs might be required since not all birds are simultaneously exposed to the infective dose of the virus. Furthermore, not all birds are infected at flock level and presence of the range of birds in different stages of the infection is expected.
In the present case, field data imply a strong association between IBH outbreaks in broilers and vertical transmission of the disease. It is unlikely that IBH could break out in such separated farms over a short period. Furthermore, several more concurrent IBH outbreaks were reported from progenies of the respective breeder farm in the north of Iran (one of those was used for phylogenetic analysis in this study (Fig. 3). Three FAdV-11 detected in farms A and B in Shiraz and the one in the flock located in the north of Iran were 100% identical; the only common factor was the parent flock.
Despite immunosuppressive co-agent contribution to IBH (Fadly et al., 1976 ▶; Toro et al., 2000 ▶), the present investigation is in agreement with studies pointing to IBH as a primary disease in young broilers (Reece et al., 1986 ▶; Christensen and Saifuddin, 1989 ▶; Gomis et al., 2006 ▶; Nakamura et al., 2011 ▶; Steer et al., 2011 ▶). The fact that certain FAdVs are able to primarily induce IBH might be attributable to the diverse pathogenicity of FAdV.
References
- Christensen NH, Saifuddin M. A primary epidemic of inclusion body hepatitis in broilers. Avian Dis. 1989;33:622–630. [PubMed] [Google Scholar]
- Fadly AM, Winterfield R, Olander HJ. Role of the bursa of Fabricius in the pathogenicity of inclusion body hepatitis and infectious bursal disease viruses. Avian Dis. 1976;20:467–477. [PubMed] [Google Scholar]
- Gomis S, Goodhope R, Ojkic D, Willson P. Inclusion body hepatitis as a primary disease in broilers in Saskatchewan, Canada. Avian Dis. 2006;50:550–555. doi: 10.1637/7577-040106R.1. [DOI] [PubMed] [Google Scholar]
- Helmboldt CF, Frazier MN. Avian hepatic inclusion bodies of unknown significance. Avian Dis. 1963;7:446–450. [PubMed] [Google Scholar]
- Hess M. Aviadenovirus infections. In: Swayne DE, editor. Diseases of poultry. 13th Edn. Ames: Wiley-Blackwell; 2013. pp. 290–300. [Google Scholar]
- Hoerr FJ. Clinical aspects of immunosuppression in poultry. Avian Dis. 2010;54:2–15. doi: 10.1637/8909-043009-Review.1. [DOI] [PubMed] [Google Scholar]
- Hosseini H, Morshed R. Molecular identification of fowl adenovirus associated with inclusion body hepatitis in Iran. Iran J. Virol. 2012;6:7–12. [Google Scholar]
- Khodakaram-Tafti A, Asasi K, Namazi F. Clinicopathological characteristics of acute inclusion body hepatitis outbreak in broiler chickens in Iran. Bulg. J. Vet. Med. 2016;19:163–168. [Google Scholar]
- Lim TH, Lee HJ, Lee DH, Lee YN, Park JK, Youn HN, Kim MS, Youn HS, Lee JB, Park SY, Choi IS, Song CS. Identification and virulence characterization of fowl adenoviruses in Korea. Avian Dis. 2011;55:554–560. doi: 10.1637/9730-032011-Reg.1. [DOI] [PubMed] [Google Scholar]
- Lin TL, Wu CC, Rosenberger JK, Saif YM. Rapid differentiation of infectious bursal disease virus serotypes by polymerase chain reaction. J. Vet. Diagn. Invest. 1994;6:100–102. doi: 10.1177/104063879400600119. [DOI] [PubMed] [Google Scholar]
- Meulemans G, Boschmans M, Van den Berg TP, Decaesstecker M. Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses. Avian Pathol. 2001;30:655–660. doi: 10.1080/03079450120092143. [DOI] [PubMed] [Google Scholar]
- Morshed R, Hosseini H, Langeroudi AG, Fard MHB, Charkhkar S. Fowl adenoviruses D and E cause inclusion body hepatitis outbreaks in broiler and broiler breeder pullet flocks. Avian Dis. 2017;61:205–210. doi: 10.1637/11551-120516-Reg.1. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Mase M, Yamamoto Y, Takizawa K, Kabeya M, Wakuda T, Matsuda M, Chikuba T, Yamamoto Y, Ohyama T, Takahashi K, Sato N, Akiyama N, Honma H, Imai K. Inclusion body hepatitis caused by fowl adenovirus in broiler chickens in Japan, 2009-2010. Avian Dis. 2011;55:719–723. doi: 10.1637/9813-052511-Case.1. [DOI] [PubMed] [Google Scholar]
- Nateghi E, Razmyar J, Bassami MR. Molecular characterization of avian adenoviruses in Iranian broiler flocks. Iran. J. Vet. Res. 2014;15:164–167. [Google Scholar]
- Reece RL, Barr DA, Grix DC, Forsyth WM, Condron RJ, Hindmarsh M. Observations on naturally occurring inclusion body hepatitis in Victorian chickens. Aust. Vet. J. 1986;63:201–202. doi: 10.1111/j.1751-0813.1986.tb02981.x. [DOI] [PubMed] [Google Scholar]
- Rosenberger JK, Eckroade RJ, Klopp S, Krauss WC. Characterization of several viruses isolated from chickens with inclusion body hepatitis and aplastic anemia. Avian Dis. 1974;18:399–409. [PubMed] [Google Scholar]
- Schachner A, Matos M, Grafl B, Hess M. Fowl adenovirus-induced diseases and strategies for their control–a review on the current global situation. Avian Pathol. 2018;47:111–126. doi: 10.1080/03079457.2017.1385724. [DOI] [PubMed] [Google Scholar]
- Steer PA, O’Rourke D, Ghorashi SA, Noormohammadi AH. Application of high-resolution melting curve analysis for typing of fowl adenoviruses in field cases of inclusion body hepatitis. Aust. Vet. J. 2011;89:184–192. doi: 10.1111/j.1751-0813.2011.00695.x. [DOI] [PubMed] [Google Scholar]
- Steer PA, Sandy JR, O’Rourke D, Scott PC, Browning GF, Noormohammadi AH. Chronological analysis of gross and histological lesions induced by field strains of fowl adenovirus serotypes 1, 8b and 11 in one-day-old chickens. Avian Pathol. 2015;44:106–113. doi: 10.1080/03079457.2015.1007919. [DOI] [PubMed] [Google Scholar]
- Todd D, Mawhinney KA, McNulty MS. Detection and differentiation of chicken anemia virus isolates by using the polymerase chain reaction. J. Clin. Microbiol. 1992;30:1661–1666. doi: 10.1128/jcm.30.7.1661-1666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro H, Gonzalez C, Cerda L, Hess M, Reyes E, Geisse C. Chicken anemia virus and fowl adenoviruses: association to induce the inclusion body hepatitis/hydropericardium syndrome. Avian Dis. 2000;44:51–58. [PubMed] [Google Scholar]