Abstract
Background
COVID-19 is associated with both venous and arterial thrombotic complications. While prophylactic anticoagulation is now widely recommended for hospitalized patients with COVID-19, the effectiveness and safety of thromboprophylaxis in outpatients with COVID-19 has not been established.
Study Design
PREVENT-HD is a double-blind, placebo-controlled, pragmatic, event-driven phase 3 trial to evaluate the efficacy and safety of rivaroxaban in symptomatic outpatients with laboratory-confirmed COVID-19 at risk for thrombotic events, hospitalization, and death. Several challenges posed by the pandemic have necessitated innovative approaches to clinical trial design, start-up, and conduct. Participants are randomized in a 1:1 ratio, stratified by time from COVID-19 confirmation, to either rivaroxaban 10 mg once daily or placebo for 35 days. The primary efficacy end point is a composite of symptomatic venous thromboembolism, myocardial infarction, ischemic stroke, acute limb ischemia, non-central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality. The primary safety end point is fatal and critical site bleeding according to the International Society on Thrombosis and Haemostasis definition. Enrollment began in August 2020 and is expected to enroll approximately 4,000 participants to yield the required number of end point events.
Conclusions
PREVENT-HD is a pragmatic trial evaluating the efficacy and safety of the direct oral anticoagulant rivaroxaban in the outpatient setting to reduce major venous and arterial thrombotic events, hospitalization, and mortality associated with COVID-19.
COVID-19 has rapidly emerged as the world's most pressing infectious threat. The novel severe acute respiratory syndrome coronavirus-2 (SARS Co-V-2) responsible for this condition has proven to be readily transmissible, with significant morbidity and a high case fatality rate1. SARS Co-V-2 has further demonstrated wide-ranging systemic effects, including significant immunologic, pulmonary, gastrointestinal, cardiac, and neurologic manifestations.2 , 3 A particularly concerning risk that has emerged with COVID-19 is the development of an activated coagulation system associated with macrovascular and microvascular thrombosis and overall poor prognosis.4., 5., 6., 7. The incidence of venous or arterial thrombotic events in hospitalized patients may be as high as 1 in 6, and up to 1 in 3 in patients requiring intensive care depending on whether surveillance imaging for asymptomatic venous thromboembolism (VTE) is performed.5 , 7 , 8 Due to this pronounced hypercoagulable state, attention has focused on antithrombotic treatment to reduce morbidity and mortality in COVID-19. Retrospective analyses suggest lower mortality rates for hospitalized patients with COVID-19 who received prophylactic anticoagulation, compared to those who did not.9 , 10 Preliminary reports from ongoing prospective trials suggest improved outcomes with therapeutic heparin in moderately ill,11 but not in critically ill,12 adults hospitalized with COVID-19. Current expert guidance includes prophylactic-dose anticoagulant treatment to decrease the risk of thrombotic complications in hospitalized patients with COVID-19.13., 14., 15.
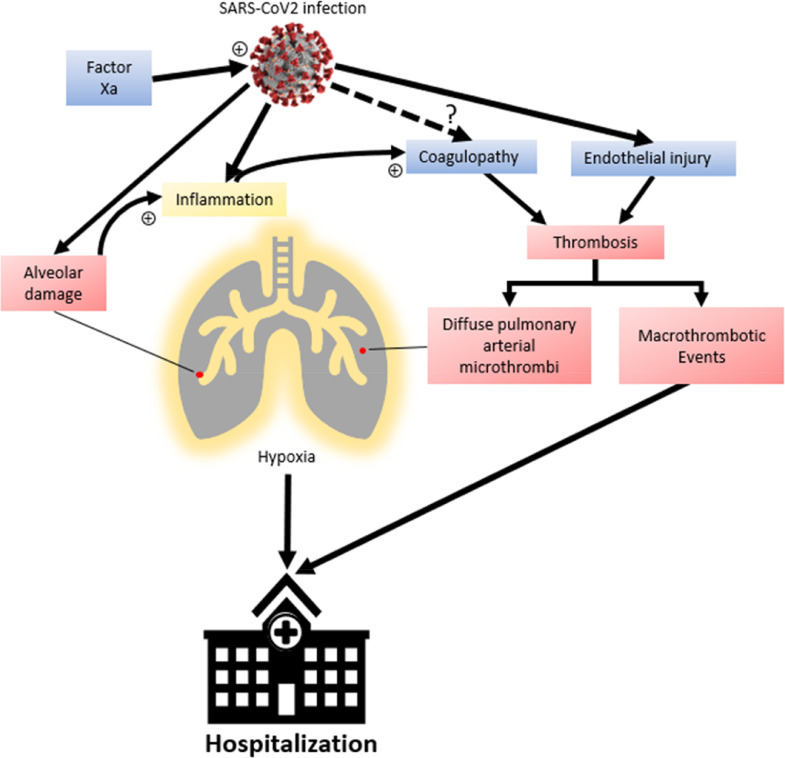
While acknowledging the potential benefit of post-hospitalization thromboprophylaxis, expert opinion and guidance statements have disagreed on the need for primary thromboprophylaxis in outpatients with COVID-19 with thrombotic risk factors.16., 17., 18. The underlying mechanisms of the hypercoagulable state in patients with COVID-19 are not clear.17 A key question is: when in the course of SARS-Co-V-2 infection does thrombotic risk reach a critical, yet modifiable point? There are data supporting activated thrombin as a key pathogenetic driver of pulmonary compromise in COVID-19. Fibrinogen and D-dimer concentrations are often already elevated at the time of hospital admission,4 , 19 and elevated D-dimer concentrations are found in almost half of hospitalized patients with nonsevere disease.20 Additionally, up to half of venous thromboembolic events in hospitalized patients in one series were diagnosed within the first 24 hours of admission.8 We hypothesize that the increased risk of thrombotic events, attributable to a thrombotic-inflammatory status associated with reduced mobility, begins prior to severe clinical manifestations of COVID-19, and includes patients who do not require hospitalization. Multiple autopsy series have reported venous thromboembolism and widespread pulmonary microthrombi in decedents with COVID-19,21., 22., 23., 24., 25., 26. suggesting a role of direct endothelial injury in the development of COVID-19 pulmonary manifestations (Figure 1 ). Therefore, we hypothesize that intervening to decrease thrombotic risk earlier in the course of COVID-19, especially in patients with known risk factors for thrombosis, will significantly decrease thrombotic complications and reduce disease progression to the point where hospitalization could be avoided.
Figure 1.
Coagulopathy and COVID-19 pathogenesis. Coagulopathy and diffuse pulmonary microthrombi have been documented in COVID-19. While coagulopathy is a known consequence of inflammatory changes, it is unclear if SARS-Co-V-2 independently affects hypercoagulability. Coagulopathy, along with viral endothelial injury, leads to diffuse pulmonary microthrombi which may potentiate pulmonary injury in addition to alveolar damage from SARS-Co-V-2 infection as well as macrothrombotic events. Factor Xa can also play a role in cell entry and infection by SARS-Co-V-2, and therefore viral propagation. Outpatient anticoagulation with rivaroxaban, a specific Factor Xa inhibitor, has the potential to prevent thromboembolic events as well as pulmonary microthrombi and progression of pulmonary insufficiency in COVID-19, reducing the need for hospitalization.
Direct oral anticoagulants (DOACs) are favored due to their oral administration, selective coagulation factor inhibition, lack of required blood monitoring, and safety profile relative to vitamin K antagonists.27 Early observations of lower than expected mortality in subjects on DOACS with chronic atrial fibrillation who contract COVID-19 suggested that anticoagulation may benefit patients with COVID-19 in the prehospital setting.10 , 28 DOACs may be a preferred choice over other anticoagulant options for post-hospital discharge from COVID-19 with other indications for therapeutic thromboprophylaxis.27
Rivaroxaban, a selective factor Xa inhibitor, has been investigated in a comprehensive cardiovascular development program, establishing risk reduction of venous and arterial thrombotic events in a variety of indications.29., 30., 31., 32., 33., 34., 35. Rivaroxaban has shown benefit in decreasing the risk of venous thromboembolic events in medically ill inpatients with elevated thrombotic risk and low risk of bleeding, including those with pneumonia and sepsis, starting in-hospital and continuing during the posthospital discharge period.32 , 34 , 36 Outpatients with COVID-19 may represent a similar medically ill population that could benefit from thromboprophylaxis with rivaroxaban, but the need for, and the net clinical benefit of, anticoagulation at an early stage of COVID-19 is currently unknown. Given the potential role of factor Xa in the pathogenesis of coronavirus morbidity and mortality, rivaroxaban may have benefits in preventing the progression to severe COVID-19.37 The PREVENT-HD trial is designed to evaluate the efficacy and safety of prophylactic-dose rivaroxaban in outpatients with symptomatic COVID-19 infection with at least one additional thrombotic risk factor.
Study design and population
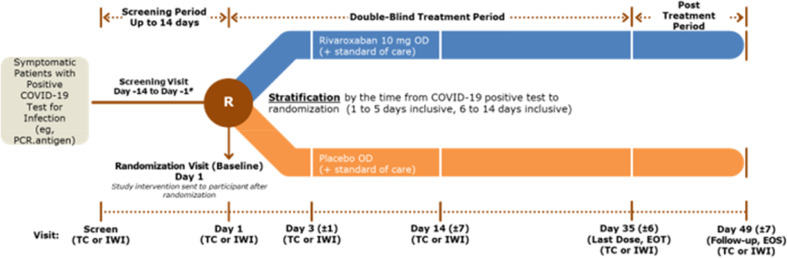
PREVENT-HD is a multicenter, randomized, double-blind, placebo-controlled, pragmatic, event-driven phase 3 study to assess the efficacy of rivaroxaban 10 mg once daily in preventing venous and arterial thrombotic events, hospitalization, and death in outpatients with symptomatic COVID-19. The trial is being conducted at large integrated health care delivery networks to facilitate centralized patient recruitment and follow-up, and to enable a pilot study of alternative methods of data collection through integration of a cloud-based data capture tool with electronic medical records (EMR). The study design incorporates several innovative processes intended to reduce exposure of health care providers to SARS Co-V-2, monitor the safety of study participants, and enhance the efficiency of study conduct using advanced informatics integration (Table 1 ). The study design is shown in Figure 2 .
Table 1.
Innovative aspects of PREVENT-HD trial conduct
| Objective | Tactic |
|---|---|
| Reduce exposure of health care providers to SARS Co-V-2 | • Remote, electronic consent • No physical examinations • No in-person visits • No study-required laboratory testing • Study medication couriered/shipped from central depot to participant's home • 100% remote site monitoring |
| Monitor safety of study participants | • Structured remote interview by trained coordinators to identify potential endpoints, adverse events • Study personnel contact information to participants; 24/7 availability of a site physician • Messaging to primary care providers of randomized subjects with information about the study and contact information for study personnel |
| Enhance the efficiency of study conduct | • Identify potential eligible patients by daily (real time) electronic medical records (EMR) data extraction • Use of advanced informatics system exchange for extraction or integration of specified variables (eg, clinical laboratory values and visit information) from subjects’ EMR records into a parallel clinical database; this data integration will facilitate recruitment and monitoring of participant safety and outcomes • Site-level classification of endpoints, based on EMR review, by centrally trained adjudicators, without a centralized adjudication committee |
Figure 2.
PREVENT-HD study design. EOS, End of study; EOT, end of treatment; IWI, Interactive Web Interface; OD, once daily; PCR,polymerase chain reaction; TC, telephone contact.
The inclusion criteria are designed to enroll a representative population of symptomatic adult outpatients with COVID-19 with at least one additional risk factor for thrombosis and with a low bleeding risk, and for whom the initial treatment plan does not include hospitalization. Study participants must be at least 18 years of age, have documented COVID-19, have symptoms attributable to COVID-19, and have an additional risk factor for venous or arterial thromboembolic disease, including elevated D-dimer level, thrombophilia, prior venous thromboembolism, history of cancer, coronary artery disease, peripheral artery disease, cerebrovascular disease or ischemic stroke; or a risk factor associated with worse COVID-19 outcomes (age ≥60 years of age, body mass index ≥35 kg/m2, or history of heart failure or diabetes requiring medication). Key exclusion criteria include convalescent infection (eg, positive COVID antibody test or other serology test at least 2 weeks following acute infection), conditions that pose an increased risk of bleeding (eg, bronchiectasis, active cancer undergoing treatment, active gastroduodenal ulcer, significant bleeding in the prior 3 months and use of dual antiplatelet therapy),38 and recent or planned therapy with medications that significantly impact rivaroxaban concentrations or bleeding risk, including anticoagulants. A complete listing of inclusion and exclusion criteria is provided in Table 2 .
Table 2.
PREVENT-HD inclusion/exclusion criteria
| Inclusion criteria |
| 1. Male or female (according to their reproductive organs and functions assigned by chromosomal complement) |
| 2. 18 years of age or older |
| 3. COVID-19 positive diagnosis by locally obtained viral diagnostic test (eg, PCR). This may be nasal swab or saliva test or other available technology to demonstrate current infection. (Note: this is not an antibody test or serology test that just indicate prior exposure to the disease. In the case of multiple positive COVID-19 PCR tests, only the date of the first test may be used). |
| 4. Confirm that participant is known to health system, with at least 1 contact in EMR prior to screening |
| 5. Symptoms attributable to COVID-19 (eg, fever, cough, loss of taste or smell, muscle aches, shortness of breath, and fatigue) |
| 6. Initial treatment plan does not include hospitalization |
| 7. Presence of at least 1 additional risk factor: |
| i. Age ≥60 years |
| ii. Prior history of venous thromboembolism (VTE) |
| iii. History of thrombophilia |
| iv. History of coronary artery disease (CAD) |
| v. History of peripheral artery disease (PAD) |
| vi. History of cerebrovascular disease or ischemic stroke |
| vii. History of cancer (other than basal cell carcinoma) |
| viii. History of diabetes requiring medication |
| ix. History of heart failure |
| x. Body mass index ≥35 kg/m2 |
| xi. D-dimer > upper limit of normal for local laboratory (within 2 weeks of the date of the COVID-19 test and prior to randomization) |
| 8. Must provide consent via eConsent indicating that he or she understands the purpose of, and procedures required for, the study and is willing to participate in the study, including follow up |
| 9. Willing and able to adhere to the lifestyle restrictions specified in the protocol |
| Exclusion criteria |
| 1. Increased risk of bleeding such as i) significant bleeding in the last 3 months, ii) active gastroduodenal ulcer in the last 3 months, iii) history of bronchiectasis or pulmonary cavitation, iv) need for dual antiplatelet therapy or anticoagulation, v) prior intracranial hemorrhage, vi) known severe thrombocytopenia (platelet count <50 × 109/L), or vii) active cancer and undergoing treatment |
| 2. Any illness or condition that in the opinion of the investigator would significantly increase the risk of bleeding (eg, recent trauma, recent surgery, severe uncontrolled hypertension, gastrointestinal cancer, renal failure requiring dialysis, severe liver disease, known bleeding diathesis) |
| 3. Known allergies, hypersensitivity, or intolerance to rivaroxaban or its excipients |
| 4. Positive COVID-19 antibody or serology test after 2-week period of acute, symptomatic COVID-19 infection |
| 5. Known diagnosis of triple positive (ie, positive for lupus anticoagulant, anticardiolipin, and anti-beta 2-glycoprotein I antibodies) antiphospholipid syndrome |
| 6. Recently taken, or required to take, any disallowed therapies as noted in the protocol (Disallowed Concomitant Therapy) before the planned first dose of study intervention or required during the study. For example, the need for the use of strong cytochrome P450 (CYP) 3A4 inhibitor or inducer per local prescribing information |
| 7. Received an investigational intervention (including investigational vaccines) or used an invasive investigational medical device within 30 days before the planned first dose of study intervention or is currently enrolled in an experimental, investigational study (Note: participation in an observational registry is allowed) |
| 8. Women who are pregnant or breastfeeding and women of childbearing potential without proper contraceptive measures |
Subjects are identified through site specific EMR searches to notify coordinators of potential subjects having a recent positive test result for COVID-19. At some sites, such searches are supplemented with additional inclusion and exclusion criteria verifiable through the EMR. Potential subjects are then called to verify eligibility and to assess interest. Consent is generally obtained electronically, while the site communicates directly by phone or by virtual meeting platforms. For some participants unable to provide consent electronically, the consent is verified using paper forms.
Participants meeting all inclusion and no exclusion criteria may be randomized from the date of the positive COVID-19 test until 14 days later, inclusive. All participants will be randomized in a 1:1 ratio to receive either rivaroxaban 10 mg or matching oral placebo once daily for 35 days through use of a central computerized interactive voice/web response system that conceals study drug assignment from investigators. Randomization will be further stratified by the time from positive diagnostic of COVID-19 test to randomization, into an early-dosed cohort (1-5 days) and a later-dosed cohort (6-14 days). The protocol also allows capping of enrollment of participants with certain risk factors to enable an adequate assessment of subgroups with particular risk factors. Enrollment began in August 2020 and will continue to an expected total of approximately 4,000 participants randomized to achieve the target number of end point events. The study is being performed in accordance with all local laws and regulations, and with the ethical principles of the Declaration of Helsinki, and the International Council on Harmonization Good Clinical Practice guidelines. The study protocol and informed consent have been reviewed and approved by the responsible health authorities and Institutional Review Boards for all participating study sites.
Treatment protocol and follow-up procedures
Treatment selection
Study drug provided to participants is double-blinded rivaroxaban 10 mg or matching placebo taken once daily. This dose and duration of rivaroxaban was selected for PREVENT-HD primarily based on previous results in medically ill populations,32 , 34 , 38 , 39 suggesting that the 10 mg once daily dose of rivaroxaban is the minimally effective dose needed to reach the trough level to prevent venous thromboembolic events in medically ill populations with high thrombotic risk and low risk of bleeding, including those with pneumonia and sepsis.36 Furthermore, the 10 mg once daily dose is FDA-approved for VTE prophylaxis following hip and knee arthroplasty and has trough levels approximating the 2.5 mg twice daily dose,40 which has been previously shown to be effective in preventing thrombotic events in patients with cardiovascular disease.33 , 35 Though the 2.5 mg twice daily dose has shown efficacy in preventing both arterial and venous thrombotic events in these studies, the 10 mg once daily dose was favored for the current study given the efficacy demonstrated previously in similar medically ill populations. While there is a dearth of evidence that DOACs reduce the risk of arterial thrombosis in medically ill populations, recent emerging evidence suggests non-hemorrhagic stroke prevention with betrixaban41 and rivaroxaban42 in this population. In the MARINER study, a placebo controlled study of extended thromboprophylaxis in medically ill patients with rivaroxaban, 10 mg of rivaroxaban reduced the secondary composite end point of symptomatic VTE, myocardial infarction, non-hemorrhagic stroke, and cardiovascular death by 28% compared with placebo without a significant increase in major bleeding.42 Doses greater than 10 mg once daily of rivaroxaban have been associated with increased bleeding risk when used for secondary prophylaxis.43 Therefore, 10 mg once daily dose was selected to optimally balance the prevention of both venous and arterial thrombotic risk with the risk of excess bleeding.
Concomitant therapies
The protocol allows treatment with all clinically-recommended therapies according to local practice, including any prescribed medications intended to inhibit SARS Co-V-2 activity. Drugs that interact with rivaroxaban to either significantly increase or decrease rivaroxaban concentrations are prohibited; such medications include combined P-glycoprotein and strong inhibitors of CYP3A4, or combined P-glycoprotein and strong CYP3A4 inducers. Of importance, dexamethasone, commonly used in the treatment of COVID-19, is not a prohibited medication. Medications that significantly increase the risk of bleeding in the setting of rivaroxaban therapy, such as anticoagulants, dual antiplatelet regimens, or high-dose antiplatelet monotherapy (eg, aspirin >162 mg daily, clopidogrel >75 mg daily, or ticlopidine >250 mg daily), are also prohibited. Single antiplatelet agents including these drugs at lower doses, ticagrelor, and prasugrel are allowed, with the caution that participants should be monitored for increased risk of bleeding. If any prohibited medication is clinically indicated, blinded study drug is to be discontinued while the prohibited medication is taken.
Visit schedule and follow-up
Participants are randomized on study Day 1 and will take blinded study medication once daily through to study Day 35. All study activities will occur remotely, including screening for eligibility, consent, and follow-up. Following randomization, participants are automatically flagged for drug dispensation through an automated system. A centralized drug depot sends study drug directly to the patient's home by the next day. Delivery is confirmed by telephone contact on Day 3. To further minimize potential study related COVID-19 exposures, subjects receive detailed instructions for safe self-disposal of any unused study drug rather than being required to return drug to the site.
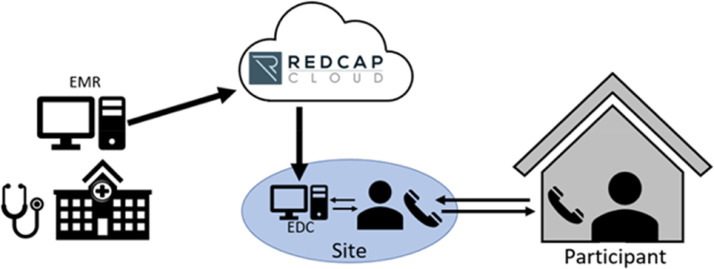
Participants will undergo virtual study visits on Day 1 (defined as Day of randomization), and on Days 3, 14, 35, and 49. At each follow-up contact, participants are assessed for study drug adherence (via questioning start/stop dates and missed doses of study drug), adverse events including bleeding, and any potential study end point events. To aid in site follow-up of subjects, PREVENT-HD will leverage the REDCap Cloud platform to integrate with each site's local EMR system (Figure 3 ). This integration will facilitate the transfer (or direct import) of data pertaining to demography, medical history, medications, encounters, diagnostic procedures, blood transfusions, and laboratory values into a parallel clinical database to facilitate the monitoring of subject health status and outcomes. As thromboprophylaxis is now recommended for all hospitalized COVID-19 patients,10 upon hospitalization, participants will discontinue blinded study medication and will receive standard of care open label thromboprophylaxis per guidelines. Upon discharge, participants may be continued on extended open label thromboprophylaxis if it is the practice of the institution or may go back on blinded study medication if that is not the case.
Figure 3.
Data flow in PREVENT-HD. Study data are collected remotely by site staff. Key data flow in daily to weekly from the local hospital electronic medical records (EMR), through REDCap Cloud, to a parallel clinical database. Data from EMR can be used in real time to identify eligible subjects to consent remotely, and to monitor for outcome events in enrolled participants. Site staff conduct virtual follow-up visits by phone or telehealth and enter data from outside the hospital system into electronic case report forms. Participants do not need to leave home through the duration of the study.
Pilot study on EMR data reliability
The EMR domains selected for the pilot study were purposely chosen to be generic and applicable to future clinical studies (eg, health care or hospital encounters, demographics, medications, labs, prior medical, or surgical diagnoses). This component of the trial is intended to explore new methods of data acquisition that may expand central monitoring capabilities and improve the efficiency of site monitoring by reducing or eliminating the requirement to perform on-site or remote source data verification. The pilot will also explore whether EMR data can replace manual transcription of study data into the clinical database for selected fields. For this study, all data will also be manually entered by site personnel into a conventional electronic data capture system for regulatory purposes and to allow for comparisons.
Study end points
The primary efficacy end point is a composite including symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non-central nervous system (non-CNS) systemic embolization, all-cause hospitalization, and all-cause mortality up to Day 35. Because many of the components of this end point are typically associated with hospitalization, the study end point represents significant morbidities that are meaningful to an outpatient population. Secondary and exploratory efficacy end points are further listed in Table 3 and include emergency department visits and need for supplemental oxygen therapy. The primary safety end point is fatal and critical-site bleeding according to the International Society on Thrombosis and Haemostasis definition. Additional safety end points will include International Society on Thrombosis and Haemostasis major bleeding and nonmajor clinically relevant bleeding.
Table 3.
PREVENT-HD trial outcomes
| Primary efficacy outcome |
| Time to first occurrence of a composite endpoint of symptomatic venous thromboembolism (VTE), myocardial infarction (MI), ischemic stroke, acute limb ischemia, noncentral nervous system (non-CNS) systemic embolization, all-cause hospitalization, and all-cause mortality up to Day 35 |
| Secondary efficacy outcomes |
| • Time to first occurrence of a composite end point of symptomatic VTE, MI, ischemic stroke, acute limb ischemia, non-CNS systemic embolization, and all-cause mortality up to Day 35 |
| • Time to first occurrence of all-cause hospitalization up to day 35 |
| • Time to first occurrence of symptomatic VTE up to day 35 |
| • Time to first occurrence of an emergency room (ER) visit up to Day 35 |
| • Time to first occurrence of symptomatic VTE, MI, ischemic stroke, acute limb ischemia, non-CNS systemic embolization, and all-cause hospitalization up to day 35 |
| • Incidence of participants who are hospitalized or dead from any cause on day 35 |
| • Time to all-cause mortality up to day 35 |
| Exploratory efficacy outcomes |
| • World Health Organization [WHO] Research and Development Blueprint: Novel Coronavirus Scale for Clinical Improvement over time |
| • Time to first occurrence of a component event of the primary efficacy end point (MI, ischemic stroke, acute limb ischemia, and non-CNS systemic embolization) up to day 35 |
| • The incidence of participants achieving an oxygen saturation (O2sat) below 92% on room air at rest or with ambulation up to day 35 |
| • The incidence of participants achieving an O2sat below 88% on room air at rest or with ambulation up to day 35 |
| • The incidence of participants requiring supplemental oxygen up to day 35 |
| • Time to first occurrence of the use of noninvasive ventilation or high-flow oxygen (WHO 5), Intubation and mechanical ventilation (WHO 6), or ventilation and additional organ support (vasopressors, renal replacement therapy [RRT], extracorporeal membrane oxygenation [ECMO]; WHO 7) or all-cause mortality (WHO 8) up to day 35 |
| • The incidence of participants requiring dialysis or having an estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2 on 2 measurements more than 24 hours apart up to day 35 |
| • Time to first occurrence of disseminated intravascular coagulation (DIC) up to day 35 |
| • Time to first occurrence of acute respiratory distress syndrome (ARDS) up to day 35 |
| • The incidence of occurrence of COVID digit up to day 35 |
| • Medical Resource Utilization data over time |
| Primary safety outcome |
| Time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical site and fatal bleeding on treatment (+2 days) |
| Secondary safety outcomes |
| • Time to first occurrence of ISTH major bleeding on treatment (+2 days) |
| • Time to first occurrence of nonmajor clinically relevant bleeding on treatment (+2 days) |
All primary end points and most secondary end points will undergo adjudication against trial end point definitions by trained, experienced physicians at each site who remain blinded to treatment assignment. To participate as a physician adjudicator, individuals must undergo additional training on the application of standardized event definitions. Adjudicators are instructed to contact the academic research organization for questions related to complicated cases or unanticipated circumstances, which are then logged as conventions to be applied to future cases across sites. This model of site-level adjudication allows for efficiency gains by taking advantage of full access to local EMR systems for source document review. All end point definitions used in PREVENT-HD are provided in the Appendix. Centralized oversight of local adjudicators is conducted through centralized training, use of conventions, and guidance to local adjudicators through periodic meetings to minimize site-level variability in adjudication.
Statistical considerations
Efficacy analyses will be conducted on all randomized patients using the principle of intention-to-treat up to day 35 after randomization; safety analyses will be conducted on all patients that received at least one dose of study drug until 2 days after the last dose of study medication. Treatment assignment will be balanced within a clinical site by block randomization. The randomization will be stratified by the time from COVID-19 positive test to randomization (1-5 days inclusive, 6-14 days inclusive). The primary efficacy analysis will be based on the time from randomization to the first adjudicated occurrence of any component of the primary composite end point up to day 35. The primary efficacy analysis will be done using a stratified log-rank test by the time from COVID-19 positive test to randomization with the treatment as a variable. The primary efficacy outcome will be tested at a 2-sided significance level of 5%, with appropriate apportioning of alpha to account for one planned interim analysis. Secondary end points will be analyzed hierarchically, in the order listed in Table 3, using similar time-to-event analyses described above.
The trial is event-driven such that it would require 333 confirmed first primary end point events to detect an anticipated hazard ratio of 0.70 between the study arms, with 90% power at a 2-sided 5% significance level. It was determined that the study required approximately 4,000 participants in order to have 333 patients experience a component of the primary end point over 35 days with a placebo rate of the primary outcome of 10%. If the blinded, pooled event rate proves to be lower than anticipated, enrollment of up to 5,000 participants will be considered (corresponding to an event rate in the placebo group of 7.8%, all else constant) to achieve the requisite number of events. The estimate for primary outcome event rate was estimated from unpublished data from the Northwell system (Spyropoulos AC, personal communication) suggesting a 10% hospitalization rate in unselected patients, and CDC information suggesting approximately 8% nationally in patients 18 years of age and older. The case fatality rate for unselected patients is approximately 2.3% nationally. It was also assumed that most, but not all thromboembolic events and deaths might occur after hospitalization.44., 45., 46.
An Independent Data Monitoring Committee (IDMC) is responsible for monitoring the safety of study participants throughout the trial and will perform a minimum of one interim analysis for futility or overwhelming superiority once approximately 50% of the required 333 first primary efficacy events have been observed. The stopping rule for overwhelming superiority uses an O'Brien-Fleming boundary (Z = 2.96, alpha = 0.003) approach. The IDMC may consider additional factors and request additional interim analyses (with appropriate apportionment of alpha to control the type 1 error rate) to make decisions on trial conduct.
Study organization
PREVENT-HD is being conducted at up to approximately 15 sites affiliated with integrated health care delivery networks in the United States. The trial is sponsored by Janssen Research and Development, LLC (Raritan, NJ) and conducted in partnership with CPC Clinical Research (CPC; Aurora, CO), a nonprofit Academic Research Organization that is affiliated with the University of Colorado Anschutz Medical Campus. An Executive Committee (EC) is responsible for oversight of the study with unrestricted access to the necessary data to fulfill this role and will submit the results of the study for publication in a peer-reviewed journal. CPC provides administrative support to the EC, and IDMC, and helps to oversee consistency in site end point confirmation. REDCap Cloud provided oversight for defining the EMR data fields to be collected from each site and for establishing the necessary technical connections for the integration/transfer of EMR data from sites to REDCap Cloud to the study database. No direct funding was provided to support development of the current manuscript. The authors are solely responsible for drafting and editing of the manuscript, and its final contents.
PREVENT-HD is registered on clinicaltrials.gov under number NCT04508023. Major milestones for the PREVENT-HD trial include establishment of the EC in June 2020, initial protocol finalization in June 2020, first site open for enrollment in August 2020 with the first participant also randomized in August 2020. The first EMR integration was completed in October 2020. The planned interim analysis is anticipated by the second quarter of 2021, and study completion is anticipated by the end of 2021. These timelines will be highly dependent on the dynamics of the pandemic and effective implementation of public health measures and/or widely utilized effective vaccines.
Discussion
The COVID-19 pandemic has posed a unique global public health crisis. As our understanding of this disease evolves, there is increasing recognition of its severity and its association with a myriad of adverse complications including thrombotic events. Notably, thrombotic events have been reported in up to 4.5% of relatively healthy individuals with COVID-19 in the outpatient setting not requiring hospitalization.47 Rapidly developing therapeutic strategies to address these risks pose a specific challenge in an environment where traditional randomized trial models are impractical and pose safety issues such as exposure to infected individuals. Such challenges may foster variability in care and a desire to utilize non-randomized data to guide treatment with such approaches associated with important limitations.
The PREVENT-HD trial was designed to answer the hypothesis that initiation of prophylactic-dose rivaroxaban early in the course of COVID-19 infection, prior to hospitalization, will reduce the risk of thrombosis, hospitalization, and death, and to evaluate the safety of such a strategy. It has been designed to answer this hypothesis with the rigor of a double-blind, randomized event driven design, but with innovative aspects intended to improve feasibility, reduce risk to study participants and study staff, and maximize data transfer and informatics capabilities.
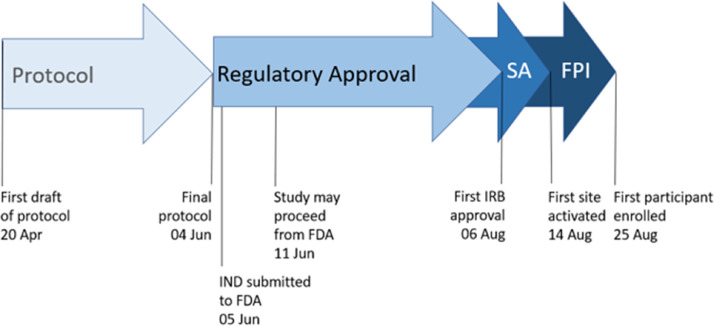
The timeline from the beginning of protocol drafting to first participant visit was only 127 days (Figure 4 ), a sharp reduction from typical trial start-up timelines.48 This timeline was facilitated by FDA review that was both expedited and accepting of a study design which emphasizes avoidance of in-person contact. The remote electronic consent process, home drug delivery, and virtual follow-up contacts allows participants with this often physically taxing and contagious disease to continue home quarantine and convalescence throughout the trial. Fully remote site monitoring further reduces interpersonal contact at the study sites. The elimination of physical contacts helps to protect the local communities from exposure to the virus and minimizes the burden and risks to a medically fragile participant population with COVID-19. Finally, PREVENT-HD gains further efficiencies by leveraging the infrastructure of integrated health care delivery network, which serves to expand the pool of patients available to recruit from while concentrating start-up activities to fewer sites (eg, contract execution, collection of regulatory documents, and implementation of training). Integration between the local EMR and the clinical database brings key data on prospective and enrolled participants immediately to the attention of the study team. The creation of a parallel dataset of selected EMR data will further enable investigation of the reliability of EMR data and determine if efficiencies can be gained by reducing or eliminating the need for manual transcription of select study data to the primary clinical study database. Site-level adjudication of end point events allows direct access to full EMR records and minimizes the site burdens of medical record collection, redaction, and central submission processing.
Figure 4.
Study start-up timeline in PREVENT-HD. To respond to the public health crisis presented by COVID-19, study start-up timelines for PREVENT-HD were accelerated. From first draft of the protocol to first participant enrolled required only 127 days. FDA, Food and Drug Administration; FPI, first participant in; IRB, Institutional Review Board; IND, Investigational New Drug; SA, site activation.
In responding to COVID-19, PREVENT-HD also responds to the recognized need to increase efficiency and decrease costs of conducting registration trials.49 , 50 Many of the design innovations employed in this trial may have application beyond the current infectious pandemic. The long-term goal is to leverage learnings necessitated by the pandemic to transform future clinical trial conduct.
In addition to the innovative approach, PREVENT HD seeks to answer an important scientific question in COVID-19. While most research is currently focused on the role of anticoagulation in hospitalized patients with severe COVID-19, it is possible that decreasing thrombotic risk earlier in the course of disease may prevent some aspects of disease progression and pulmonary injury such as microvascular thrombosis. PREVENT-HD is one of a limited number of placebo-controlled trials registered on clinicaltrials.gov examining antithrombotic interventions in outpatients with COVID-19. A smaller trial (n = 600) is also evaluating rivaroxaban versus placebo in outpatients (NCT04504032), while one larger outpatient primary thromboprophylaxis trial (the OVID trial, NCT04400799) is comparing enoxaparin 40 mg SQ daily versus placebo (N = 1000).51 The National Institute of Health trial [NCT04498273] is randomizing to 1 of 4 arms (N = 7000): apixaban 2.5 mg twice a day, apixaban 5 mg twice a day, low-dose aspirin once daily, or placebo. An additional large randomized, controlled open-label trial of enoxaparin versus no treatment is also under way (the ETHIC trial, NCT04492254).
Of note, 2 observational case-control analyses reported no effect of preadmission exposure to either antiplatelet therapy or anticoagulant therapy prescribed for other clinical indications on presenting acute respiratory distress syndrome, intensive care unit admission rates, or mortality rates for patients admitted with COVID-19.52 , 53 However, these analyses were of nonrandomized cohorts comprised of patients already hospitalized and prone to potential bias from the underlying clinical conditions for which the antithrombotic was prescribed. A large prospective, randomized trial of outpatient anticoagulant prophylaxis such as PREVENT-HD in addition to the other large trials will provide high-quality evidence of the value of this preventative strategy.
If rivaroxaban can provide thromboembolic benefits in the outpatient COVID-19 population, another important question to be answered by PREVENT-HD is whether this benefit comes at an unacceptably elevated risk of bleeding. A disseminated intravascular coagulation-like picture can be seen late in COVID-19; while this pattern of laboratory abnormalities can be associated with a bleeding diathesis, bleeding complications in COVID-19 are generally infrequent.54 Additionally, immune thrombocytopenia has been reported in COVID-19 and can also increase the risk of bleeding.55 , 56 At this point, these potential bleeding risks appear to be uncommon in the population targeted for enrollment in PREVENT-HD, and subjects with known or expected high risk of bleeding will be excluded from the trial. Nevertheless, net clinical benefit in the outpatient population will be important to characterize.
A potential concern is completing an outpatient trial in COVID-19 with rapidly-shifting self-prevention practices, improved COVID-19 therapeutics, and the development of vaccines against SARS-CoV-2. However, there are currently limited therapeutic options for slowing disease progression in the outpatient setting; therefore, the primary outcome, which includes hospitalization, should be robust to these evolving social factors. Furthermore, vaccine distribution and administration may leave significant proportions of the population vulnerable over the next 1 to 2 years. Knowledge of therapeutic approaches to minimize morbidity and relieve stress on inpatient services will remain critical.
Summary
Patients with COVID-19 are at an increased risk of venous and arterial thrombotic events, and this tendency for thrombosis may play an important role in disease progression requiring hospitalization. The role of prophylactic anticoagulation in the pre-hospital setting has not yet been established. PREVENT-HD is a pragmatic trial, with innovative design features to respond to needs in the context of an infectious pandemic and beyond. This trial will evaluate, in symptomatic outpatients with COVID-19 with at least one additional thrombotic risk factor who are at low risk for bleeding, the efficacy and safety of the 10mg once daily dose of rivaroxaban to reduce thrombotic events, hospitalizations, and mortality.
Disclosure
Warren Capell reports research grants from Janssen Research and Development to CPC Clinical Research, during the conduct of the study; grants from Bayer Health Care to CPC Clinical Research, outside the submitted work. Elliot Barnathan is an employee of Janssen Research and Development, LLC, sponsor of the study, owns stock in the company, and reports a patent entitled "Prophylactic treatment of thrombotic events in outpatients" that is pending. Gregory Piazza reports grants from Janssen, during the conduct of the study; grants from BMS, grants from Bayer, grants from Portola, grants from BSC, outside the submitted work. Alex Spyropoulos reports grants and personal fees from Janssen, grants and personal fees from Boehringer Ingelheim, personal fees from BMS, personal fees from Bayer, personal fees from Portola, during the conduct of the study; personal fees from ATLAS Group, outside the submitted work. Judith Hsia reports grants from Janssen to CPC Clinical Research during protocol development. Scott Bull, Concetta Lipardi, Chiara Sugarmann, and Eunyoung Suh are employees of Janssen Research and Development LLC, during the conduct of the study and own stock in the company. Jaya Prakash Rao has nothing to disclose. William Hiatt reports grants from Janssen to CPC Clinical Research, but no payments to authors for work on the manuscript during the conduct of the study; grants from Northwell Health, outside the submitted work. Marc Bonaca reports grants from Janssen to CPC Clinical Research, during the conduct of the study; grants to CPC Clinical Research from Amgen, AstraZeneca, Bayer, Janssen, Merck, NovoNordisk, Pfizer, outside the submitted work.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ahj.2021.02.001.
Appendix. Supplementary materials
References
- 1.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 2.Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45 doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York City Health System. JAMA. 2020 doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billett HH, Reyes-Gil M, Szymanski J, et al. Anticoagulation in COVID-19: effect of enoxaparin, heparin, and apixaban on mortality. Thromb Haemost. 2020 doi: 10.1055/s-0040-1720978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. Available from: https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients; Accessed 26 January, 2021.
- 12.NIH ACTIV Trial of blood thinners pauses enrollment of critically ill COVID-19 patients. Available from: https://www.nih.gov/news-events/news-releases/nih-activ-trial-blood-thinners-pauses-enrollment-critically-ill-covid-19-patients; Accessed 26 January, 2021.
- 13.Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf; Accessed 26 January, 2021.
- 15.Zhai Z, Li C, Chen Y, et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120:937–948. doi: 10.1055/s-0040-1710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerotziafas GT, Catalano M, Colgan MP, et al. Guidance for the management of patients with vascular disease or cardiovascular risk factors and COVID-19: position paper from VAS-European Independent Foundation in Angiology/Vascular Medicine. Thromb Haemost. 2020 doi: 10.1055/s-0040-1715798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emert R, Shah P, Zampella JG. COVID-19 and hypercoagulability in the outpatient setting. Thromb Res. 2020;192:122–123. doi: 10.1016/j.thromres.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naymagon L, Zubizarreta N, Feld J, et al. Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19. Thromb Res. 2020;196:99–105. doi: 10.1016/j.thromres.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grosse C, Grosse A, Salzer HJF, et al. Analysis of cardiopulmonary findings in COVID-19 fatalities: High incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc Pathol. 2020;49 doi: 10.1016/j.carpath.2020.107263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasquez-Bonilla WO, Orozco R, Argueta V, et al. A review of the main histopathological findings in the Coronavirus Disease 2019 (COVID-19) Hum Pathol. 2020 doi: 10.1016/j.humpath.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wichmann D, Sperhake JP, Lutgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iturbe-Hernandez T, Garcia de Guadiana Romualdo L, Gil Ortega I, et al. Dabigatran, the oral anticoagulant of choice at discharge in patients with non-valvular atrial fibrillation and COVID-19 infection: the ANIBAL protocol. Drugs Context. 2020;9 doi: 10.7573/dic.2020-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harenberg J, Bauersachs R, Ageno W. Does chronic treatment with oral anticoagulants ameliorate the clinical course of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in coronavirus disease 2019 (COVID-19) Semin Thromb Hemost. 2020 doi: 10.1055/s-0040-1715091. [DOI] [PubMed] [Google Scholar]
- 29.Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373:1673–1680. doi: 10.1016/S0140-6736(09)60734-0. [DOI] [PubMed] [Google Scholar]
- 30.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 31.Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 32.Cohen AT, Spiro TE, Buller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–523. doi: 10.1056/NEJMoa1111096. [DOI] [PubMed] [Google Scholar]
- 33.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without Aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 34.Spyropoulos AC, Ageno W, Albers GW, et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med. 2018;379:1118–1127. doi: 10.1056/NEJMoa1805090. [DOI] [PubMed] [Google Scholar]
- 35.Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382:1994–2004. doi: 10.1056/NEJMoa2000052. [DOI] [PubMed] [Google Scholar]
- 36.Cohoon KP, De Sanctis Y, Haskell L, et al. Rivaroxaban for thromboprophylaxis among patients recently hospitalized for acute infectious diseases: a subgroup analysis of the MAGELLAN study. J Thromb Haemost. 2018;16:1278–1287. doi: 10.1111/jth.14146. [DOI] [PubMed] [Google Scholar]
- 37.Frydman GH, Streiff MB, Connors JM, Piazza G. The potential role of coagulation factor Xa in the pathophysiology of COVID-19: a role for anticoagulants as multimodal therapeutic agents. Thromb Haemost Open. 2020 doi: 10.1055/s-0040-1718415. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spyropoulos AC, Lipardi C, Xu J, et al. Improved benefit risk profile of rivaroxaban in a subpopulation of the MAGELLAN study. Clin Appl Thromb Hemost. 2019;25 doi: 10.1177/1076029619886022. 1076029619886022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weitz JI, Raskob GE, Spyropoulos AC, et al. Thromboprophylaxis with rivaroxaban in acutely ill medical patients with renal impairment: insights from the MAGELLAN and MARINER trials. Thromb Haemost. 2020;120:515–524. doi: 10.1055/s-0039-1701009. [DOI] [PubMed] [Google Scholar]
- 40.Mueck W, Borris LC, Dahl OE, et al. Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost. 2008;100:453–461. [PubMed] [Google Scholar]
- 41.Gibson CM, Chi G, Halaby R, et al. Extended-duration betrixaban reduces the risk of stroke versus standard-dose enoxaparin among hospitalized medically ill patients: an APEX trial substudy (Acute Medically Ill Venous Thromboembolism Prevention With Extended Duration Betrixaban) Circulation. 2017;135:648–655. doi: 10.1161/CIRCULATIONAHA.116.025427. [DOI] [PubMed] [Google Scholar]
- 42.Spyropoulos AC, Ageno W, Albers GW, et al. Post-discharge prophylaxis with rivaroxaban reduces fatal and major thromboembolic events in medically ill patients. J Am Coll Cardiol. 2020;75:3140–3147. doi: 10.1016/j.jacc.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376:1211–1222. doi: 10.1056/NEJMoa1700518. [DOI] [PubMed] [Google Scholar]
- 44.Uppuluri EM, Shapiro NL. Development of pulmonary embolism in a nonhospitalized patient with COVID-19 who did not receive venous thromboembolism prophylaxis. Am J Health Syst Pharm. 2020;77:1957–1960. doi: 10.1093/ajhp/zxaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Overstad S, Tjonnfjord E, Garabet L, et al. Venous thromboembolism and coronavirus disease 2019 in an ambulatory care setting - A report of 4 cases. Thromb Res. 2020;194:116–118. doi: 10.1016/j.thromres.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piazza G, Campia U, Hurwitz S, et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76:2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pawlowski C, Wagner T, Puranik A, et al. Inference from longitudinal laboratory tests characterizes temporal evolution of COVID-19-associated coagulopathy (CAC) Elife. 2020;9 doi: 10.7554/eLife.59209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamberti MJ, Brothers C, Manak D, Getz K. Benchmarking the Study Initiation Process. Ther Innov Regul Sci. 2013;47:101–109. doi: 10.1177/2168479012469947. [DOI] [PubMed] [Google Scholar]
- 49.Antman EM, Harrington RA. Transforming clinical trials in cardiovascular disease: mission critical for health and economic well-being. JAMA. 2012;308:1743–1744. doi: 10.1001/jama.2012.14841. [DOI] [PubMed] [Google Scholar]
- 50.Jackson N, Atar D, Borentain M, et al. Improving clinical trials for cardiovascular diseases: a position paper from the Cardiovascular Round Table of the European Society of Cardiology. Eur Heart J. 2016;37:747–754. doi: 10.1093/eurheartj/ehv213. [DOI] [PubMed] [Google Scholar]
- 51.Barco S, Bingisser R, Colucci G, et al. Enoxaparin for primary thromboprophylaxis in ambulatory patients with coronavirus disease-2019 (the OVID study): a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:770. doi: 10.1186/s13063-020-04678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sivaloganathan H, Ladikou EE, Chevassut T. COVID-19 mortality in patients on anticoagulants and antiplatelet agents. Br J Haematol. 2020;190:e192–e195. doi: 10.1111/bjh.16968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russo V, Di Maio M, Attena E, et al. Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: A multicenter observational study. Pharmacol Res. 2020;159 doi: 10.1016/j.phrs.2020.104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bomhof G, Mutsaers P, Leebeek FWG, et al. COVID-19-associated immune thrombocytopenia. Br J Haematol. 2020;190:e61–e64. doi: 10.1111/bjh.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zulfiqar AA, Lorenzo-Villalba N, Hassler P, Andres E. Immune thrombocytopenic purpura in a patient with Covid-19. N Engl J Med. 2020;382:e43. doi: 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.