Abstract
Aims
To evaluate the quality‐of‐life (QOL) impairment and identify the possible risk factors in patients with tuberous sclerosis complex (TSC) in China.
Methods
The parent proxy‐report PedsQL 4.0 Generic Core Scales were administered to 124 caregivers of children with TSC (aged 2‐18 years). For comparison, the survey was also conducted in a demographically group‐matched sample of healthy controls (HCs) (aged 2‐18 years).
Results
A total of 124 children with TSC and 206 HCs were recruited. The mean parent proxy‐report total scale score, physical health summary score, and psychosocial health summary score for children with TSC were 65.0 (SD 19.7), 77.6 (SD 22.9), and 58.0 (SD 21.3), respectively, compared with the HC values of 83.6 (SD 14.3), 87.2 (SD 16.9), and 82.8 (SD 15.9). There were statistically significant differences between the two groups (P < .0001). TSC2 mutation (P = .033), epilepsy (P = .011), seizure before 2 years old (P = .001), course of epilepsy (more than 2 years) (P = .001), high reported seizure frequency (more than once a month) (HRSF) (P = .007), multiple antiepileptic drugs (≥2) (P = .002), intellectual disability (ID) (mild and moderate ID, P < .0001, and severe and profound ID, P < .0001), and TANDs (P < .0001) (ADHD, P = .004; agoraphobia, P = .007; and social anxiety disorder, P < .0001) were closely related to lower QOL scores.
Conclusion
This study is the first large cohort study on QOL in children with TSC in China. The results of the PedsQL 4.0 indicated that the QOL of children with TSC is significantly lower than that of HCs. TSC2 mutation, epilepsy, early onset, long disease course and HRSF, ID, and TANDs are risk factors for poor QOL.
Keywords: child, epilepsy, generic core scale, health, PedsQL, TSC
The quality of life of children with TSC is significantly lower than that of HCs. TSC2 mutation, epilepsy, early onset, long disease course and high reported seizure frequency (more than once a month), intellectual disability, and tuberous sclerosis–associated neuropsychiatric disorders are risk factors for poor quality of life.
1. INTRODUCTION
Tuberous sclerosis complex (TSC, OMIM #191100, #613254), a genetic disease with an autosomal dominant inheritance, affects those who have it across their life span and occurs in approximately 1 in 5000‐10 000 live births. 1 , 2 , 3 In our previous study, 4 epilepsy affected 85.06 percent of our cohort of 174 patients diagnosed with TSC. Intellectual disability is a primary feature of TSC, affecting 44‐70 percent of patients in population‐based reports. The prevalence of significant behavioral problems among children with TSC ranges from 40 to 90 percent. 5 , 6 , 7 However, only a few studies have assessed the associated total disease burden and quality of life (QOL).
As one of the most widely used health‐related quality‐of‐life measures for patients aged 2‐18 years, the Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales are stable, reliable, repeatable, and suitable for general and disease‐specific populations. 8 , 9 , 10 , 11 , 12 This cross‐sectional study was designed to (a) investigate the QOL of Chinese patients with TSC (aged 2‐18 years); (b) compare their QOL with that of healthy controls (HCs); and (c) more precisely explore risk factors for lower QOL scores.
2. MATERIALS AND METHODS
2.1. Subjects
During the inclusion period (June 2019‐December 2019), all children with TSC aged 2‐18 years and their parents received an invitation from Children's Hospital of Fudan University. A diagnosis of TSC was based on the latest diagnostic criteria for TSC. 13 The only exclusion criterion was an insufficient understanding of the Chinese language or unwillingness to participate in the study. A total of 206 healthy individuals aged 2‐18 years were recruited from schools to create an age‐ and sex‐matched control group.
This study was subject to approval by the ethics committee of the Children's Hospital of Fudan University (2018‐No.26). Written informed consent and verbal assent were provided by all parents and participants (aged ≥ 12 years).
2.2. Data collection
2.2.1. Standardized questionnaire
Demographic characteristics (sex, birth date, ethnicity, years of education of the parents, monthly household income, and urban/rural residence) and clinical data (age at onset of epilepsy, course of epilepsy, antiepileptic drugs, seizure frequency, family history, multisystem clinical manifestations of TSC, etc) were assessed by a standard structured questionnaire.
2.2.2. PedsQL 4.0 generic core scales
The Mandarin Chinese version of the PedsQL 4.0 parent proxy report was used to assess the QOL of Chinese children with TSC.
There are four age‐group scales specific to children aged 2‐4, 5‐7, 8‐12, and 13‐18 years, and these are used to assess parents' perceptions of the QOL of children with TSC. The 21‐item (2‐4 years)/23‐item (5‐18 years) parent proxy‐report PedsQL 4.0 consists of the following 4 domains: (a) physical functioning (8 items), (b) emotional functioning (5 items), (c) social functioning (5 items), and (d) school functioning (5 items). The reliability and validity of the Chinese translations of the PedsQL 4.0 have been demonstrated in Chinese children. 14 , 15 , 16 , 17 , 18 , 19 Emotional, school, and social functioning can also be united into a psychosocial health domain. Each item is rated using a 5‐point response scale from 0 = "never is a problem" to 4 = "almost always a problem." The weights of the items are equal, the score is reversed, and the linear conversion is 0‐100 points (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0). Thus, the higher the function score is, the better the QOL is. Face‐to‐face interviews were conducted by trained neurologists to collect the data.
2.2.3. Statistical analysis
The statistical analyses were conducted with JMP Pro software, version 14.3.0 (SAS Institute, USA). The Shapiro‐Wilk normality test was used to test the normality of the data distribution. The unpaired t test for continuous data and the chi‐square test or Fisher's exact test for categorical data were used to test for sociodemographic differences between TSC patients and HCs. The unpaired t test was used for the comparison of the parent proxy‐report PedsQL 4.0 scores between the two groups. ANOVA F tests were used to compare the PedsQL 4.0 subscores among all of the age‐groups and different genotype groups (TSC1, TSC2, and NMI). We used multivariate linear regression models to identify risk factors for low QOL scores. Some potential confounding factors were adjusted in Models 1b, 2b, and 3b, such as sex, parents' years of education, monthly household income, and residence. A P‐value < .05 was considered significant.
3. RESULTS
3.1. Characteristics of the study population
Altogether, 124 children with TSC (median age ± standard deviation: 8.5 ± 3.7 years) who met the inclusion criteria were enrolled in the study and returned a questionnaire completed by their parents: 61 (49.2%) were boys and 63 (50.8%) were girls. A total of 206 gender‐matched (male: female = 109:97) and age‐matched (9.3 ± 4.1 years) HCs were recruited (Table 1).
Table 1.
Sociodemographic comparison of TSC patients and healthy controls
| Sociodemographic features | TSC | HCs | P‐value |
|---|---|---|---|
| N | 124 | 206 | |
| Male | 61 (49.2) | 109 (52.9) | .57 |
| Age at interview (y) | 8.5 ± 3.7 | 9.3 ± 4.1 | .08 |
| Age subgroups (y) | .99 | ||
| 2‐4 | 16 (12.9) | 27 (13.1) | |
| 5‐7 | 34 (27.4) | 57 (27.6) | |
| 8‐18 | 74 (59.7) | 122 (59.3) | |
| Father's years of education (y) | .78 | ||
| ≤9 | 43 (34.7) | 65 (31.6) | |
| 9‐12 | 28 (22.6) | 45 (21.8) | |
| >12 | 53 (42.7) | 96 (46.6) | |
| Mothers’ years of education (y) | .88 | ||
| ≤9 | 46 (37.1) | 71 (34.5) | |
| 9‐12 | 24 (19.4) | 40 (19.4) | |
| >12 | 54 (43.6) | 95 (46.1) | |
| Monthly household income (RMB) | .39 | ||
| <5000 | 21 (16.9) | 26 (12.6) | |
| 5000‐10 000 | 63 (50.8) | 119 (57.8) | |
| >10 000 | 40 (32.3) | 61 (29.6) | |
| Residence | .11 | ||
| Suburban or rural | 62 (50.0) | 122 (59.2) | |
| Urban | 62 (50.0) | 84 (40.8) |
TSC, TSC group; HCs, healthy controls
Data are presented as the median ± SD and n (%).
ANOVA F tests were used for continuous variables and chi‐square tests or Fisher's exact tests for categorical data.
All 124 patients had undergone genetic testing, and the results showed that the ratio of TSC1, TSC2, and NMI (no mutation identified) was 34:76:14. According to the DSM‐5 criteria, 81 (65.3%) patients out of 124 presented with intellectual disability (ID), with mild ID (IQ 51‐70) and moderate ID (IQ 36‐50) accounting for 33.9% (42/124) and severe ID (IQ 20‐35) and profound ID (IQ < 20) for 31.5% (39/124). Comorbid epilepsy diagnoses were found in 84.7% (105/124) of the TSC patients. Two or more seizure types were detected in 65.7% (69/105) of all patients with epilepsy. The most common type of epilepsy was focal motor epilepsy (75/105; 71.4%). As the characteristic seizure type, infantile spasms were diagnosed in 44.8% (47/105) of patients with epilepsy. Of the 124 patients with neuroimaging results available, 91.6% (114/124) had tubers or cortical dysplasias, 90.3% (112/124) had subependymal nodules (SENs), and 4.0% (5/124) had subependymal giant cell astrocytoma (SEGA). A total of 15 tuberous sclerosis–associated neuropsychiatric disorders (TANDs) were diagnosed in 95 patients aged 6‐16 years using the Mini International Neuropsychiatric Interview for patients. The incidence rates from low to high were as follows: conduct disorder (1.1%), posttraumatic stress disorder (3.2%), obsessive‐compulsive disorder (6.3%; 6/95), dysthymia (4.2%), major depressive episode (6.3%), suicide (6.3%), oppositional defiant disorder (7.4%), separation anxiety disorder (10.5%), tic disorder (15.8%), agoraphobia (16.8%), (mild) manic episodes (22.1%), specific phobia (26.3%), panic disorder (26.3%), social anxiety disorder (41.1%), and attention‐deficit/hyperactivity disorder (ADHD; 51.6%) (https://www.researchsquare.com/article/rs‐32617/v1).
3.2. Comparison between the TSC group and healthy controls
The mean total scale score, physical health summary score, and psychosocial health summary score for children with TSC were 65.0 (SD 19.7), 77.6 (SD 22.9), and 58.0 (SD 21.3), respectively, compared with the HC values of 83.6 (SD 14.3), 87.2 (SD 16.9), and 82.8 (SD 15.9). There were statistically significant differences between the two groups (P < .0001) (Figure 1A). The QOL of children with TSC was significantly worse than that of HCs. As shown in Figure 1B, there were also significant differences in the social, emotional, and school functioning scale scores between the two groups.
Figure 1.
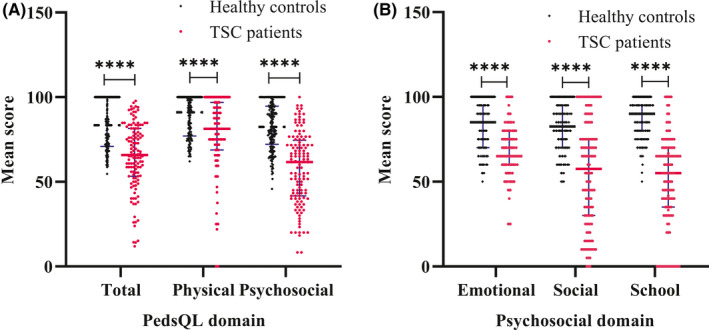
Comparison of parent proxy‐report PedsQL 4.0 scores between children with TSC and healthy controls. Data are presented as the median ± SD; ****P < .0001. Unpaired t tests or unpaired t tests with Welch's correction were used
There were no significant differences among any of the age‐groups for any of the QOL domains (P > .05). On average, the parent‐reported psychosocial health scores were significantly lower than the physical health scores (P < .0001). For the psychosocial domains, compared with the NMI group, the TSC1/TSC2 group had significantly lower scores (P = .016) (emotional functioning, P = .027; school functioning, P = .028; social functioning, P = .037).
3.3. Analysis of risk factors for lower QOL scores
Multiple linear regression models adjusted for potential confounders were used to explore the associations between QOL scores and clinical manifestations of TSC (including TANDs).
As shown in Table 2, TSC2 mutation (P = .033), epilepsy (P = .011), seizure before 2 years old (P = .001), course of epilepsy (more than two years) (P = .001), HRSF (more than once per month) (P = .007), multiple antiepileptic drugs (≥2) (P = .002), ID (mild and moderate ID, P < .0001, and severe and profound ID, P < .0001), and TANDs (P < .0001) (ADHD, P = .004; agoraphobia, P = .007; and social anxiety disorder, P < .0001) were closely related to lower QOL scores after adjusting for sex (male/female), mother's years of education (≤9/9‐12/>12), father's years of education (≤9/9‐12/>12), monthly household income (RMB) (<5000/5000‐10 000/>10 000), and residence (suburban or rural/urban) in Model 1b.
Table 2.
The associations between total scale scores and clinical manifestations of TSC (including TANDs)
| Predictor | n (%) | Mean parent proxy‐report total scale score | |||
|---|---|---|---|---|---|
| Model 1a β1 (95% CI) | P‐value | Model 1b β2 (95% CI) | P‐value | ||
| Gene | |||||
| NMI | 14 (11.3) | Ref. | / | Ref. | / |
| TSC1 | 34 (27.4) | −8.05 (−20.30, 4.20) | .200 | −7.79 (−20.87, 5.30) | .241 |
| TSC2 | 76 (61.3) | −11.55 (−22.77, −0.32) | .040* | −12.91 (−24.79, −1.03) | .033* |
| Epilepsy | |||||
| No | 19 (15.3) | Ref. | / | Ref. | / |
| Yes | 105 (84.7) | −12.86 (−22.33, −3.38) | .008* | −13.04 (−23.10, −2.99) | .011* |
| Seizure before 2 y old | |||||
| No | 38 (36.2) | Ref. | / | Ref. | / |
| Yes | 67 (63.8) | −13.28 (−21.14, −5.42) | .001* | −14.00 (−22.33, −5.68) | .001* |
| Course of epilepsy (≥2 y) | |||||
| No | 38 (36.2) | Ref. | / | Ref. | / |
| Yes | 67 (63.8) | −13.77 (−21.49, −6.05) | .001* | −13.79 (−22.02, −5.56) | .001* |
| Multiple antiepileptic drugs (≥2) | |||||
| No | 60 (49.6) | Ref. | / | Ref. | / |
| Yes | 61 (50.4) | −12.09 (−18.94, −5.24) | .001* | −11.81 (−19.10, −4.52) | .002* |
| HRSF | |||||
| No | 65 (61.9) | Ref. | / | Ref. | / |
| Yes | 40 (38.1) | −11.05 (−18.85, −3.26) | .006* | −11.33 (−19.42, −3.23) | .007* |
| IQ | |||||
| Normal | 43 (34.7) | Ref. | / | Ref. | / |
| Mild and moderate ID | 42 (33.9) | −14.93 (−21.06, −8.81) | <.0001* | −16.40 (−22.76, −10.05) | <.0001* |
| Severe and profound ID | 39 (31.5) | −33.51 (−39.75, −27.27) | <.0001* | −35.77 (−42.29, −29.25) | <.0001* |
| TANDs | |||||
| No | 16 (16.8) | Ref. | / | Ref. | / |
| Yes | 95 (83.2) | −21.89 (−31.20, −12.58) | <.0001* | −21.75 (−31.59, −11.93) | <.0001* |
| ADHD | |||||
| No | 46 (48.4) | Ref. | / | Ref. | / |
| Yes | 49 (51.6) | −10.58 (−18.02, −3.15) | .001* | −11.69 (−19.57, −3.80) | .004* |
| Social anxiety disorder | |||||
| No | 57 (60) | Ref. | / | Ref. | / |
| Yes | 38 (40) | −14.64 (−21.95, −7.34) | .001* | −15.00 (−22.58, −7.43) | <.0001* |
| Agoraphobia | |||||
| No | 80 (84.2) | Ref. | / | Ref. | / |
| Yes | 15 (15.8) | −14.93 (−25.09, −4.76) | .004* | −14.73 (−25.28, −4.17) | .007* |
Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; AED, antiepileptic drugs; HRSF, high reported seizure frequency (more than once per month); ID, intellectual disability; IQ, intelligence quotient; NMI, no mutation identified; TANDs, tuberous sclerosis–associated neuropsychiatric disorders.
Data are presented as n (%).
Model 1a: unadjusted
Model 1b: adjusted for gender (male/female), mother's years of education (≤9/9‐12/>12), father's years of education (≤9/9‐12/>12), monthly household income (RMB) (<5000/5000‐10 000/>10 000), and residence (suburban or rural/urban).
As shown in Table 3, high reported HSF (P = .015), multiple antiepileptic drugs (≥2) (P < .0001), ID (mild and moderate ID, P = .001, and severe and profound ID, P < .0001), and TANDs (P < .0001) (social anxiety disorder, P = .002) were closely related to lower physical health summary scores after adjusting for sex (male/female), mother's years of education, father's years of education, monthly household income, and residence in Model 2b.
Table 3.
The associations between the physical health summary scores and clinical manifestations of TSC (including TANDs)
| Predictor | n (%) | Mean parent proxy‐report physical scale scores | |||
|---|---|---|---|---|---|
| Model 2a β1 (95% CI) | P‐value | Model 2b β2 (95% CI) | P‐value | ||
| Gene | |||||
| NMI | 14 (11.3) | Ref. | / | Ref. | / |
| TSC1 | 34 (27.4) | −0.25 (−14.61, 14.11) | .973 | −0.99 (−16.15, 14.17) | .897 |
| TSC2 | 76 (61.3) | −7.35 (20.50, 5.80) | .271 | −9.71 (−23.47, 4.06) | .165 |
| Epilepsy | |||||
| No | 19 (15.3) | Ref. | / | Ref. | / |
| Yes | 105 (84.7) | −9.59 (−20.82, 1.64) | .093 | −9.74 (−21.53, 2.05) | .104 |
| Seizure before 2 y old | |||||
| No | 38 (36.2) | Ref. | / | Ref. | / |
| Yes | 67 (63.8) | −9.42 (−19.06, 0.22) | .001* | −9.31 (−19.45, 0.83) | .071 |
| Course of epilepsy (≥2 y) | |||||
| No | 38 (36.2) | Ref. | / | Ref. | / |
| Yes | 67 (63.8) | −9.74 (−19.24, −0.24) | .045* | −8.86 (−18.89, 1.18) | .083 |
| Multiple antiepileptic drugs (≥2) | |||||
| No | 60 (49.6) | Ref. | / | Ref. | / |
| Yes | 61 (50.4) | −16.05 (−23.86, −9.24) | <.0001* | −15.36 (−23.60, −7.12) | <.0001* |
| HRSF | |||||
| No | 65 (61.9) | Ref. | / | Ref. | / |
| Yes | 40 (38.1) | −10.99 (−20.34, −1.64) | .022* | −11.99 (−21.55, −2.43) | .015* |
| IQ | |||||
| Normal | 43 (34.7) | Ref. | / | Ref. | / |
| Mild and moderate ID | 42 (33.9) | −13.54 (−22.21, −4.87) | .002* | −15.06 (−24.00, −6.13) | .001* |
| Severe and profound ID | 39 (31.5) | −27.39 (−36.22, −18.55) | <.0001* | −30.21 (−39.38, −21.04) | <.0001* |
| TANDs | |||||
| No | 16 (16.8) | Ref. | / | Ref. | / |
| Yes | 95 (83.2) | −21.89 (−31.20, −12.58) | <.0001* | −21.75 (−31.59, −11.93) | <.0001* |
| ADHD | |||||
| No | 46 (48.4) | Ref. | / | Ref. | / |
| Yes | 49 (51.6) | −8.08 (−16.61, −0.46) | .063 | −8.98 (−18.08, 0.12) | .053 |
| Social anxiety disorder | |||||
| No | 57 (60) | Ref. | / | Ref. | / |
| Yes | 38 (40) | −12.83 (−21.30, −4.35) | .003* | −13.83 (−22.59, −5.06) | .002* |
| Agoraphobia | |||||
| No | 80 (84.2) | Ref. | / | Ref. | / |
| Yes | 15 (15.8) | −9.71 (−21.46, 2.03) | .104 | −9.27 (−21.49, 2.96) | .136 |
Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; AED, antiepileptic drugs; HRSF, high reported seizure frequency (more than once per month); ID, intellectual disability; IQ, intelligence quotient; NMI, no mutation identified; TANDs, tuberous sclerosis–associated neuropsychiatric disorders.
Data are presented as n (%).
Model 2a: unadjusted
Model 2b: adjusted for gender (male/female), mother's years of education (≤9/9‐12/>12), father's years of education (≤9/9‐12/>12), monthly household income (RMB) (<5000/5000‐10 000/>10 000), and residence (suburban or rural/urban).
As shown in Table 4, TSC2 mutation (P = .031), epilepsy (P = .007), seizure before 2 years old (P = .001), course of epilepsy (more than 2 years) (P < .0001), HRSF (P = .012), multiple antiepileptic drugs (≥2) (P = .012), ID (mild and moderate ID, P < .0001, and severe and profound ID, P < .0001), and TANDs (P < .0001) (ADHD, P = .004; agoraphobia, P = .003; and social anxiety disorder, P = .001) were closely related to lower psychosocial health summary scores after adjusting for sex (male/female), mothers' years of education, father's years of education, monthly household income, and residence in Model 3b.
Table 4.
The associations between the psychosocial health summary scores and clinical manifestations of TSC (including TANDs)
| Predictor | n (%) | Mean parent proxy‐report psychosocial scale score | |||
|---|---|---|---|---|---|
| Model 3a β1 (95% CI) | P‐value | Model 3b β2 (95% CI) | P‐value | ||
| Gene | |||||
| NMI | 14 (11.3) | Ref. | / | Ref. | / |
| TSC1 | 34 (27.4) | −11.96 (−25.21, 1.29) | .076 | −11.19 (−25.40, 3.02) | .121 |
| TSC2 | 76 (61.3) | −13.30 (25.43, −1.16) | .032* | −14.23 (−27.13, −1.33) | .031* |
| Epilepsy | |||||
| No | 19 (15.3) | Ref. | / | Ref. | / |
| Yes | 105 (84.7) | −14.88 (−25.09, −4.66) | .005* | −15.13 (−25.98, −4.28) | .007* |
| Seizure before 2 y old | |||||
| No | 38 (36.2) | Ref. | / | Ref. | / |
| Yes | 67 (63.8) | −14.78 (−22.23, −6.33) | .001* | −16.10 (−25.04, −7.15) | .001* |
| Course of epilepsy (≥2 y) | |||||
| No | 38 (36.2) | Ref. | / | Ref. | / |
| Yes | 67 (63.8) | −15.39 (−23.68, −7.10) | <.0001* | −16.04 (−24.87, −7.21) | <.0001* |
| Multiple antiepileptic drugs (≥2 AEDs) | |||||
| No | 60 (49.6) | Ref. | / | Ref. | / |
| Yes | 61 (50.4) | −10.42 (−17.95, −2.89) | .007* | −10.28 (−18.30, −2.27) | .012* |
| HRSF | |||||
| No | 65 (61.9) | Ref. | / | Ref. | / |
| Yes | 40 (38.1) | −11.44 (−19.88, −3.00) | .008* | −11.35 (−20.16, −2.53) | .012* |
| IQ | |||||
| Normal | 43 (34.7) | Ref. | / | Ref. | / |
| Mild and moderate ID | 42 (33.9) | −15.70 (−22.16, −9.24) | <.0001* | −17.17 (−23.95, −10.39) | <.0001* |
| Severe and profound ID | 39 (31.5) | −37.22 (−43.81, −30.63) | <.0001* | −39.31 (−46.27, −32.35) | <.0001* |
| TANDs | |||||
| No | 16 (16.8) | Ref. | / | Ref. | / |
| Yes | 95 (83.2) | −24.90 (−35.02, −14.78) | <.0001* | −24.46 (−35.13, −13.80) | <.0001* |
| ADHD | |||||
| No | 46 (48.4) | Ref. | / | Ref. | / |
| Yes | 49 (51.6) | −11.76 (−19.90, −3.61) | .005* | −12.88 (−21.49, −4.27) | .004* |
| Social anxiety disorder | |||||
| No | 57 (60) | Ref. | / | Ref. | / |
| Yes | 38 (40) | −15.24 (−23.32, −7.17) | <.0001* | −15.24 (−23.62, −6.85) | .001* |
| Agoraphobia | |||||
| No | 80 (84.2) | Ref. | / | Ref. | / |
| Yes | 15 (15.8) | −17.44 (−28.52, −6.37) | .002* | −17.38 (−28.83, −5.93) | .003* |
Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; AED, antiepileptic drugs; HRSF, high reported seizure frequency (more than once per month); ID, intellectual disability; IQ, intelligence quotient; NMI, no mutation identified; TANDs, tuberous sclerosis–associated neuropsychiatric disorders.
Data are presented as n (%).
Model 3a: unadjusted.
Model 3b: adjusted for gender (male/female), mother's years of education (≤9/9‐12/>12), father's years of education (≤9/9‐12/>12), monthly household income (RMB) (<5000/5000‐10 000/>10 000), and residence (suburban or rural/urban).
4. DISCUSSION
As far as we know, this study was the first cohort study of QOL in children with TSC in China. A total of 65.3% of patients presented with ID, which is why we chose the parent proxy version. The mean total scale score, psychosocial score, and physical health score for the children with TSC were significantly lower than those in the healthy population. QOL in patients with TSC was significantly compromised compared with that in the healthy group, as might be expected.
Notably, the psychosocial health scores were significantly lower than the physical health scores, which was consistent with previous studies. 20 , 21 As in previous studies, TANDs can exist at all ages. 5 The lifetime cumulative incidence of TANDs is approximately 90%. 22 The incidence of neuropsychiatric comorbidities was significantly higher in TSC patients than in HCs. 7 , 23 Our findings indicate that neuropsychiatric disorders seriously affect patients' QOL and require careful attention from neurologists. However, our results are quite different from those of previous studies: There were no significant differences in any of the QOL domains by age. 20 It was first found that the TSC1/TSC2 group reported significantly worse QOL. Similar to previous studies, TSC1/TSC2 pathogenic variants could generally cause a more severe clinical phenotype. 4 , 24 , 25 , 26 , 27 , 28
Patient QOL is affected by the interaction of all phenotypes. 29 , 30 This study is probably the most comprehensive study on risk factors for QOL to be conducted thus far. 20 , 21 , 31 Multiple linear regression models were adopted to identify possible risk factors independently related to the low proxy‐report PedsQL scores. Simple linear regression revealed that TSC2 mutation, epilepsy, seizure before 2 years old, course of epilepsy, HRSF, multiple antiepileptic drugs, ID, and TANDs could be associated with poor QOL. After adjusting for all of the possible confounding factors (sex, maternal education, paternal education, family income, and residence), HRSF, use of a greater number of antiepileptic drugs (≥2), ID, and TANDs were significant independent risk factors for all QOL domains. In addition, TSC2 mutation, epilepsy, seizure before 2 years old, and course of epilepsy ≥ 2 years were also related to lower psychosocial health scores, but not to physical health scores. In this study, neither multisystem clinical manifestations of TSC (brain, heart, skin, eyes, kidney, lung, and liver) nor the TSC1/TSC2 gene mutation type was associated with poor QOL. In agreement with previous research, we consider epilepsy an important factor affecting the QOL of children with TSC. The earlier the age of onset of epilepsy, the more antiepileptic drugs needed for refractory epilepsy, and the longer the course of epilepsy, the greater the impact on QOL. 31 Treatments for epilepsy in early life will help to improve QOL in epilepsy care. 32 , 33
In view of the diversity of clinical manifestations in TSC patients, the burden of disease is highly variable, 34 , 35 and the impact on QOL is also different. Research on risk factors will help to improve the QOL of children with TSC. Follow‐up of these children with TSC is ongoing, with additional future focus on QOL solutions for this population. Early screening, timely diagnosis, and correct intervention for risk factors are essential to improving their QOL. Psychosocial‐based interventions also represent a major opportunity to enhance QOL.
This study has several limitations, including those inherent to a single‐center cross‐sectional study design. Further prospective multicenter studies are needed to validate these results. Considering the relatively high proportion of ID, parent proxy reports were used. Although the selected reporters were close caregivers of the child, there may still be certain deviations. The MINI‐KID scale cannot be used to screen and diagnose ASD, and the age‐group is limited to those aged 6‐16 years old. In our study, epilepsy and TAND are important risk factors that affect quality of life. To reduce bias, future studies might need to compare the quality of life of children with chronic neurological and non‐neurological diseases, such as epilepsy, diabetes, narcolepsy, and psychosis, and assess the impact of sleep disorders and rehabilitation on quality of life. 8 , 36 , 37
5. CONCLUSIONS
This study was the first large cohort study of QOL in children with TSC in China. The results indicated that the QOL of children with TSC is significantly lower than that of HCs. TSC2 mutation, epilepsy, seizure before 2 years old, course of epilepsy (more than 2 years), HRSF, multiple antiepileptic drugs (≥2), ID, and TANDs were closely related to poor QOL.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
We thank the participants and Dr X Wang for help with statistical support.
Ding Y, Wang J, Zhou Y, et al. Quality of life in children with tuberous sclerosis complex: A pediatric cohort study. CNS Neurosci Ther.2021;27:280–288. 10.1111/cns.13473
Funding informationThe work was supported by Shanghai Municipal Science and Technology Major Project (Grant Nos. 2017SHZDZX01 and 2018SHZDZX03). The funders were not involved in research design, data collection, analysis, or interpretation.
DATA AVAILABILITY STATEMENT
The datasets used during and/or analyzed during the current study are available from the corresponding author on request.
REFERENCES
- 1. Cotter JA. An update on the central nervous system manifestations of tuberous sclerosis complex. Acta Neuropathol. 2019;139:613‐624. [DOI] [PubMed] [Google Scholar]
- 2. Lu DS, Karas PJ, Krueger DA, Weiner HL. Central nervous system manifestations of tuberous sclerosis complex. Am J Med Genet C Semin Med Genet. 2018;178:291‐298. [DOI] [PubMed] [Google Scholar]
- 3. Peron A, Northrup H. Tuberous sclerosis complex. Am J Med Genet C Semin Med Genet. 2018;178:274‐277. [DOI] [PubMed] [Google Scholar]
- 4. Ding Y, Wang J, Zhou S, et al. Genotype and phenotype analysis of Chinese children with tuberous sclerosis complex: a pediatric cohort study. Front Genet. 2020;11:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curatolo P, Moavero R, de Vries PJ. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 2015;14:733‐745. [DOI] [PubMed] [Google Scholar]
- 6. de Vries PJ, Wilde L, de Vries MC, Moavero R, Pearson DA, Curatolo P. A clinical update on tuberous sclerosis complex‐associated neuropsychiatric disorders (TAND). Am J Med Genet C Semin Med Genet. 2018;178:309‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Vries PJ, Belousova E, Benedik MP, et al. TSC‐associated neuropsychiatric disorders (TAND): findings from the TOSCA natural history study. Orphanet J Rare Dis. 2018;13:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varni JW, Limbers CA, Burwinkle TM, Bryant WP, Wilson DP. The ePedsQL in type 1 and type 2 diabetes: feasibility, reliability, and validity of the Pediatric Quality of Life Inventory Internet administration. Diabetes Care. 2008;31:672‐677. [DOI] [PubMed] [Google Scholar]
- 9. Desai AD, Zhou C, Stanford S, Haaland W, Varni JW, Mangione‐Smith RM. Validity and responsiveness of the pediatric quality of life inventory (PedsQL) 4.0 generic core scales in the pediatric inpatient setting. JAMA Pediatr. 2014;168:1114‐1121. [DOI] [PubMed] [Google Scholar]
- 10. Goldstein SL, Graham N, Warady BA, et al. Measuring health‐related quality of life in children with ESRD: performance of the generic and ESRD‐specific instrument of the Pediatric Quality of Life Inventory (PedsQL). Am J Kidney Dis. 2008;51:285‐297. [DOI] [PubMed] [Google Scholar]
- 11. Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the pediatric quality of life inventory generic core scales, multidimensional fatigue scale, and cancer module. Cancer. 2002;94:2090‐2106. [DOI] [PubMed] [Google Scholar]
- 12. Hullmann SE, Ryan JL, Ramsey RR, Chaney JM, Mullins LL. Measures of general pediatric quality of life: Child Health Questionnaire (CHQ), DISABKIDS Chronic Generic Measure (DCGM), KINDL‐R, Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales, and Quality of My Life Questionnaire (QoML). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S420‐430. [DOI] [PubMed] [Google Scholar]
- 13. Northrup H, Krueger DA. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ji Y, Chen S, Li K, et al. Measuring health‐related quality of life in children with cancer living in Mainland China: feasibility, reliability and validity of the Chinese Mandarin version of PedsQL 4.0 Generic Core Scales and 3.0 Cancer Module. Health Qual Life Outcomes. 2011;9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu T, Wu Z, Yan Z, Rou K, Duan S. Measuring health‐related quality of life in children living in HIV/AIDS‐affected families in rural areas in Yunnan, China: Preliminary reliability and validity of the Chinese version of PedsQL 4.0 generic core scales. J Acquir Immune Defic Syndr. 2010;53(Suppl 1):S111‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu HH, Li H, Gao Q. Psychometric properties of the Chinese version of the pediatric quality of life inventory 4.0 Generic core scales among children with short stature. Health Qual Life Outcomes. 2013;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeung NC, Lau JT, Yu XN, et al. Psychometric properties of the Chinese version of the Pediatric Quality Of Life Inventory 4.0 Generic Core scales among pediatric cancer patients. Cancer Nurs. 2013;36:463‐473. [DOI] [PubMed] [Google Scholar]
- 18. Duan X, Zhang S, Xiao N. Reliability and validity of the PedsQL™ Generic Core Scales 4.0 for Chinese children with epilepsy. Epilepsy Behav. 2012;23:431‐436. [DOI] [PubMed] [Google Scholar]
- 19. Chen YM, He LP, Mai JC, et al. Validity and reliability of Pediatric Quality of Life Inventory Version 4.0 Generic Core Scales in Chinese children and adolescents. Zhonghua Liu Xing Bing Xue Za Zhi. 2008;29:560‐563. [PubMed] [Google Scholar]
- 20. Fong CY, Ng K, Kong AN, et al. Quality of life of children with tuberous sclerosis complex. Arch Dis Child. 2019;104:972‐978. [DOI] [PubMed] [Google Scholar]
- 21. Amin S, Mallick AA, Lux A, O'Callaghan F. Quality of life in patients with Tuberous Sclerosis Complex (TSC). Eur J Paediatr Neurol. 2019;23:801‐807. [DOI] [PubMed] [Google Scholar]
- 22. de Vries PJ, Whittemore VH, Leclezio L, et al. Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND Checklist. Pediatr Neurol. 2015;52:25‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toldo I, Brasson V, Miscioscia M, et al. Tuberous sclerosis‐associated neuropsychiatric disorders: a paediatric cohort study. Dev Med Child Neurol. 2019;61:168‐173. [DOI] [PubMed] [Google Scholar]
- 24. Farach LS, Pearson DA, Woodhouse JP, et al. Tuberous sclerosis complex genotypes and developmental phenotype. Pediatr Neurol. 2019;96:58‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogórek B, Hamieh L, Hulshof HM, et al. TSC2 pathogenic variants are predictive of severe clinical manifestations in TSC infants: results of the EPISTOP study. Genet Med. 2020;22:1489‐1497. [DOI] [PubMed] [Google Scholar]
- 26. Niida Y, Lawrence‐Smith N, Banwell A, et al. Analysis of both TSC1 and TSC2 for germline mutations in 126 unrelated patients with tuberous sclerosis. Hum Mutat. 1999;14:412‐422. [DOI] [PubMed] [Google Scholar]
- 27. Hong CH, Tu HP, Lin JR, Lee CH. An estimation of the incidence of tuberous sclerosis complex in a nationwide retrospective cohort study (1997–2010). Br J Dermatol. 2016;174:1282‐1289. [DOI] [PubMed] [Google Scholar]
- 28. Garaci FG, Floris R, Bozzao A, et al. Increased brain apparent diffusion coefficient in tuberous sclerosis. Radiology. 2004;232:461‐465. [DOI] [PubMed] [Google Scholar]
- 29. Varni JW, Franciosi JP, Shulman RJ, et al. PedsQL gastrointestinal symptoms scales and gastrointestinal worry scales in pediatric patients with inflammatory bowel disease in comparison with healthy controls. Inflamm Bowel Dis. 2015;21:1115‐1124. [DOI] [PubMed] [Google Scholar]
- 30. Hulse D, Harvey AS, Freeman JL, Mackay MT, Dabscheck G, Barton SM. Clinical application of the PedsQL Epilepsy Module (PedsQL‐EM) in an ambulatory pediatric epilepsy setting. Epilepsy Behav. 2020;106:107005. [DOI] [PubMed] [Google Scholar]
- 31. Tritton T, Bennett B, Brohan E, et al. Health utilities and quality of life in individuals with tuberous sclerosis complex (TSC) who experience epileptic seizures: a web‐based survey. Epilepsy Behav. 2019;92:213‐220. [DOI] [PubMed] [Google Scholar]
- 32. Huang P, Zheng‐Dao D, Sun B‐M, et al. Bilateral anterior capsulotomy enhances medication compliance in patients with epilepsy and psychiatric comorbidities. CNS Neurosci Ther. 2019;25:824‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan JJ, Shan W, Wu JP, Wang Q. Research progress of vagus nerve stimulation in the treatment of epilepsy. CNS Neurosci Ther. 2019;25:1222‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marques R, Belousova E, Benedik MP, et al. Treatment patterns and use of resources in patients with tuberous sclerosis complex: insights from the TOSCA registry. Front Neurol. 2019;10:1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zöllner JP, Franz DN, Hertzberg C, et al. A systematic review on the burden of illness in individuals with tuberous sclerosis complex (TSC). Orphanet J Rare Dis. 2020;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inocente CO, Gustin M‐P, Lavault S, et al. Quality of life in children with narcolepsy. CNS Neurosci Ther. 2014;20:763‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lai J‐S, Nowinski CJ, Zelko F, et al. Validation of the Neuro‐QoL measurement system in children with epilepsy. Epilepsy Behav. 2015;46:209‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during and/or analyzed during the current study are available from the corresponding author on request.


