Abstract
Background
Studies regarding the impact of Parkinson's disease (PD) on quality of life (QOL) have reported conflicting results, and the underlying QOL domains require further study. In order to understand the association between PD and QOL, we conducted this meta‐analysis to systematically compare QOL between PD patients and healthy controls.
Method
The PubMed, PsycINFO, EMBASE, and Web of Science databases were systematically searched. Data were analyzed using the random‐effects model.
Results
Twenty studies covering 2707 PD patients and 150,661 healthy controls were included in the study. Compared with healthy controls, PD patients had significantly poorer QOL overall and in most domains with moderate to large effects sizes. Different QOL measures varied in their association with quality of life, with the Parkinson's Disease Questionnaire‐39 (PDQ‐39) having the largest effect size (standard mean difference, SMD = −1.384, 95% CI: −1.607, −1.162, Z = 12.189, P < 0.001), followed by the Europe Quality of Life Questionnaire‐visual analogue scale (EQ‐VAS) (SMD = −1.081, 95% CI: −1.578, −0.584, Z = −4.265, P < 0.001), Europe Quality of Life Questionnaire‐5D (EQ‐5D) (SMD = −0.889, 95% CI: −1.181, −0.596, Z = −5.962, P < 0.001), and the Short‐form Health Survey (SF) scales (physical dimension: SMD = −0.826, 95% CI: −1.529, −0.123, Z = −2.303, P = 0.021; mental dimension: SMD = −0.376, 95% CI: −0.732, −0.019, Z = −2.064, P = 0.039).
Conclusion
PD patients had lower QOL compared with healthy controls in most domains, especially in physical function and mental health. Considering the negative impact of poor QOL on daily life and functional outcomes, effective measures should be developed to improve QOL in this population.
Keywords: comparative study, meta‐analysis, Parkinson's disease, quality of life
Improving the QOL in patients with PD is an important concern, which can provide reference for decisions of policymakers and clinicians. PD patients had significantly poorer QOL, with moderate to large effect sizes in most domains. Moreover, different QOL measures had moderating effects on the results.
1. INTRODUCTION
Parkinson's disease (PD) is a neurodegenerative disease having an overall prevalence ranging from 1 to 2 per 1000 people. 1 , 2 PD is a chronic, progressive, age‐related disorder, which is rare in young people, but whose prevalence reaches up to 4% in older adults. 2 PD is characterized by various motor dysfunctions, such as bradykinesia, rigidity, gait freezing, resting tremor, and postural reflex impairment, 3 as well as neuropsychological dysfunctions, such as depression, fatigue, cognitive decline, and sleep disturbance, 4 all of which negatively affect patients' quality of life (QOL).
The World Health Organization (WHO) defined QOL as “an individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns.” 5 QOL encompasses physical, psychological, autonomy, cognitive, social relations, and environmental factors. 5 , 6 To improve the QOL of PD patients, it is important to understand how various QOL domains differ in PD patients and healthy controls. Some comparative studies on QOL in PD patients have been conducted, but the findings are mixed, especially the extent of differences between PD patients and controls in different domains. For instance, compared with healthy controls, some studies found that PD patients had an overall lower QOL, 7 , 8 , 9 , 10 , 11 , 12 while other studies did not find significant differences in QOL domains of physical health, 8 , 13 mental health, 9 emotional function, 10 environment, 11 and social relations. 12 Major correlates of QOL in PD include comorbid depressive symptoms, and PD severity and subtypes. 14 Gait impairments, adverse effects of medications, and psychosocial dysfunction are contributing factors to poor QOL. 15 To the best of our knowledge, no systematic review or meta‐analysis has compared QOL between PD patients and healthy controls that also drilled into various domains. The main objectives in this systematic review and meta‐analysis were as follows: (a) to compare the overall and domain QOL between PD patients and healthy controls and (b) to quantify QOL differences between groups, with different standardized instruments, using the effect size statistic. We hypothesized that PD patients would have significantly lower QOL compared with healthy controls.
2. METHODS
2.1. Search strategy
Two researchers (NZ and YY) independently and systematically searched the PubMed, PsycINFO, EMBASE, and Web of Science databases from their inception date until September 19, 2020, using the following search items: Parkinson disease, Parkinson's disease, life quality, health‐related quality of life, health‐related quality of life, HRQOL, case‐control, survey, cross‐sectional, and cohort. The references of relevant review articles were also searched manually for additional studies.
2.2. Inclusion and exclusion criteria
The search for relevant articles was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flowchart, 16 with the registration number CRD42020171092. The inclusion criteria are summarized by the PICOS acronym: (a) Participants: patients with PD according to study‐defined diagnostic criteria, such as the UK PD Society Brain Bank criteria 17 , 18 and the Movement Disorder Society (MDS) clinical diagnostic criteria for PD 19 ; (b) Intervention: not applicable. (c) Comparison: healthy controls; (d) Outcomes: QOL measured by standardized instruments, such as the World Health Organization Quality of Life Questionnaire (WHOQOL), Parkinson's Disease Questionnaire‐39 (PDQ‐39), and the Short‐Form Health Survey (SF); (e) Study design: comparative studies, such as case‐control and cohort studies (only the baseline data was extracted) published in English. Studies with meta‐analyzable data, ie, QOL means and standard deviations (SD), in PD patients and healthy controls were included for analyses. Studies conducted in special populations (eg, veterans) were excluded. The same two researchers (NZ and YY) screened the titles and abstracts of relevant literature and then read the full text to further assess eligibility. Any disagreement was discussed by the two above researchers, and if a consensus could not be reached, guidance was sought from a senior researcher (YTX).
2.3. Data extraction and quality assessment
Participant and study information, such as first author, publication year, sampling method, QOL measures, number of PD patients and controls, illness duration, and QOL scores, was extracted. For studies reporting QOL by a patient subgroup (eg, by gender), overall QOL was calculated by combining the QOL subgroup scores using a formula. 20 Study quality was independently assessed by the same two researchers (NZ and YY) using the Newcastle‐Ottawa Scale (NOS) in three domains: selection, comparability and exposure. 21 , 22 The NOS total score was calculated by summing up all item scores.
2.4. Statistical analysis
Data were analyzed with the Comprehensive Meta‐analysis software, version 2.0 (CMA; https://www.meta‐analysis.com/). Data were combined across studies using the same QOL measure, which varied from one study to another. Physical and mental/psychological domains were measured separately with the WHOQOL and SF scales; thus, domain scores were pooled for each scale. For studies without SDs for QOL data, the SDs of other studies were averaged as previously done. 23 Standardized mean differences (SMDs) in QOL between PD patients and healthy controls were calculated to estimate effect size. As a guide, SMDs of 0.2, 0.5, and 0.8 were considered small, moderate, and large effect sizes, respectively. 24 Taking into account differences in sampling methods, study characteristics, and assessment tools, random‐effects models were used to synthesize data. 25 Heterogeneity was assessed with Q and I square statistics. An I2 value of 50 percent or more 20 indicated significant heterogeneity in which case possible sources of heterogeneity between subgroups were explored based on: (a) QOL measures (WHOQOL vs. SF scales vs. PDQ‐39 vs. Europe Quality of Life Questionnaire‐5D (EQ‐5D) vs. Europe Quality of Life Questionnaire‐visual analogue scale (EQ‐VAS)) and (b) QOL domains (physical health vs. mental/psychological health). Each subgroup was required to consist of at least 3 studies. If there were 10 or more studies, funnel plots were created and Egger's Rank test was conducted to assess possible publication bias. 26 The significance level for meta‐analytic outcomes was set at 0.05 with two‐tailed tests.
3. RESULTS
3.1. Literature selection
Figure 1 shows the result of the literature search. In total, 5950 studies were identified in target databases and 2 other studies were retrieved from reference lists. The final sample included in the meta‐analysis consisted of 20 studies with 2707 PD patients and 150,661 healthy controls. 8 , 41
Figure 1.
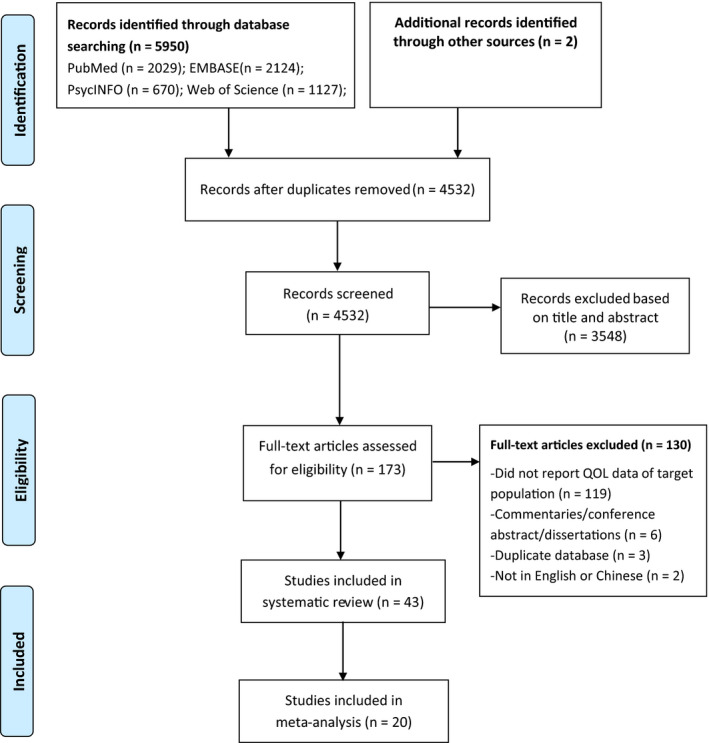
PRISMA flowchart
3.2. Study characteristics and quality assessment
Key characteristics of included studies are summarized in Table 1. They were published between 1995 and 2020, and the sample size ranged from 33 to 144,692. The details of study quality assessment are presented in Table S1.
Table 1.
Characteristics of studies included in this systematic review
| First author | References | Study site (Country) | Assessment of QOL | N total | N PD | N Controls | PD patients | Controls | NOS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (Mean ± SD) | Male (%) | Disease duration, year (Mean ± SD) | Age (Mean ± SD) | Male (%) | |||||||||
| 1 | Adewusi et al, 2018 | 27 | UK | SF‐36 | 104 | 52 | 52 | 68.1 ± 8.4 | 73.1 | 8.6 ± 5.9 | 66.8 ± 10.0 | 73.1 | 6 |
| 2 | Arun et al, 2011 | 28 | India | WHOQOL‐BREF | 76 | 46 | 30 | 65.5 ± 9.4 | 67.4 | 4.3 ± 3.5 | 62.4 ± 8.4 | 70.0 | 6 |
| 3 | Baig et al, 2015 | 48 | UK | EQ‐5D | 1056 | 769 | 287 | 67.7 ± 9.5 | 66.1 | 2.9 ± 1.9 | 65.3 ± 10.0 | 47.7 | 6 |
| 4 | Barber et al, 2017 | 49 | UK | EQ‐5D | 415 | 119 | 296 | 66.9 ± 9.1 | 70.6 | / | 64.9 ± 10.2 | 49.0 | 7 |
| 5 | Benli et al, 2016 | 61 | Turkey | IPSS | 79 | 39 | 40 | 69.8 ± 7.4 | 74.4 | 5.4 ± 3.5 | 68.0 ± 7.7 | 67.5 | 6 |
| 6 | Chotinaiwattarakul et al, 2011 | 29 | USA | SF‐36 | 226 | 134 | 92 | 70.7 ± 10.0 | 65.7 | / | 64.5 ± 9.9 | 27.2 | 5 |
| 7 | Chu and Tan, 2018 | 30 | Malaysia | PDQ‐39 | 109 | 54 | 55 | 66.8 ± 7.4 | 45.0 | / | 65.3 ± 7.5 | 51.0 | 6 |
| 8 | Dogan et al, 2015 | 55 | Turkey | PDQ‐39 | 171 | 86 | 85 | 64.3 ± 11.4 | 53.5 | / | 63.5 ± 10.7 | 51.8 | 7 |
| 9 | Fan et al, 2018 | 31 | UK | EQ‐5D/EQ‐VAS | 1650 | 1050 | 600 | 62.6 ± 7.5 | / | / | 59.7 ± 7.2 | / | 5 |
| 10 | Fonseca et al, 2015 | 80 | Brazil | QOL‐AD | 58 | 31 | 27 | 68.8 ± 10.4 | 67.7 | / | 74.0 ± 6.5 | 37.0 | 6 |
| 11 | Greene and Camicioli, 2007 | 32 | Canada | EQ‐5D | 101 | 51 | 50 | 71.5 ± 4.7 | 58.8 | 8.7 ± 4.4 | 71.5 ± 4.8 | 58.0 | 7 |
| 12 | Gustafsson et al, 2015 | 60 | Sweden | LiSat‐11 | 2567 | 1432 | 1135 | / | 64.0 | / | / | 60.5 | 7 |
| 13 | Haapaniemi et al, 2004 | 56 | Finland | 15D | 2985 | 256 | 2729 | / | / | / | / | / | 5 |
| 14 | Hariz and Forsgren, 2011 | 10 | Sweden | SF‐36 | 130 | 99 | 31 | 69.0 ± 9.8 | 54.5 | / | 67.4 ± 6.6 | 54.8 | 6 |
| 15 | Hendred and Foster, 2016 | 11 | USA | WHOQOL‐BREF | 156 | 96 | 60 | 62.4 ± 5.3 | 55.2 | 5.0 ± 4.3 | 61.7 ± 5.9 | 48.3 | 7 |
| 16 | Hobson and Meara, 2018 | 33 | UK | EQ‐5D | 268 | 166 | 102 | 74.2 ± 8.6 | 73.5 | 13.2 ± 8.8 | 74.8 ± 6.6 | 59.8 | 5 |
| 17 | Jakobsson et al, 2012 | 34 | Sweden | SF‐12 | 3795 | 136 | 3659 | 70.5 ± 7.9 | / | 5.0 ± 4.9 | 85.7 ± 6.1 | / | 5 |
| 18 | Jenkinson et al, 1995 | 44 | UK | SF‐36 | / | 146 | >=103 | / | / | / | / | / | 5 |
| 19 | Kang et al, 2012 | 35 | USA | SF‐36/PDQ‐39 | 33 | 15 | 18 | 65.7 ± 12.3 | 73.3 | / | 60.3 ± 13.5 | 50.0 | 6 |
| 20 | Karlsen et al, 1999 | 58 | Norway | NHP | 333 | 233 | 100 | 73.6 ± 8.4 | 49.4 | 6.3 ± 5.3 | 72.8 ± 8.2 | 50.0 | 7 |
| 21 | Kasten et al, 2012 | 42 | Germany | WHOQOL‐BREF | 255 | 128 | 127 | 63.0 ± 10.5 | 60.2 | 7.8 ± 6.3 | 59.0 ± 12.0 | 52.0 | 5 |
| 22 | Larsen et al, 2000 | 59 | Norway | NHP | 261 | 161 | 100 | / | / | / | / | / | 5 |
| 23 | Paolucci et al, 2018 | 8 | Rome | SF‐36 | 396 | 29 | 367 | 66.1 ± 8.9 | / | 4.0 ± 2.1 | / | / | 6 |
| 24 | Park et al, 2014 | 13 | Korea | PDQ‐39 | 182 | 93 | 89 | 65.1 ± 9.8 | 41.9 | 3.5 ± 3.1 | 70.1 ± 6.0 | 51.1 | 6 |
| 25 | Pohar and Jones, 2009 | 57 | Canada | HUI3 | 111,968 | 261 | 111,707 | 68.9 ± 19.0 | 55.9 | 7.3 ± 13.6 | 44.8 ± 8.5 | 49.0 | 5 |
| 26 | Prasuhn et al, 2017 | 12 | Germany | WHOQOL‐BREF | 327 | 69 | 258 | 68.0 ± 9.6 | 60.9 | / | 63.7 ± 7.1 | 48.4 | 6 |
| 27 | Quittenbaum and Grahn, 2004 | 45 | Sweden | SF‐36 | 152 | 57 | 95 | 70.1 ± 8.8 | 64.9 | / | 70.1 ± 8.3 | 69.5 | 7 |
| 28 | Reuther et al, 2007 | 50 | Germany | EQ‐5D/EQ‐VAS/PDQ‐39/PDQL | / | 145 | / | 67.3 ± 9.6 | 66.9 | 9.3 ± 7.4 | / | / | 5 |
| 29 | Riazi et al, 2003 | 46 | UK | SF‐36 | 2283 | 227 | 2056 | / | 60.0 | / | / | 45.0 | 5 |
| 30 | Santos Garcia et al, 2019 | 81 | Spain |
PQ‐10 /EUROHIS‐QOL8 |
901 | 694 | 207 | 62.6 ± 8.9 | 60.3 | 5.5 ± 4.4 | 61.0 ± 8.3 | 49.5 | 7 |
| 31 | Schrag et al, 2000 | 51 | UK | EQ‐5D/EQ‐VAS/ PDQ‐39/SF‐36 | / | 97 | / | 73.0 ± 11.3 | 51.5 | 5.8 ± 4.9 | / | / | 6 |
| 32 | Swinn et al, 2003 | 38 | UK | EQ‐5D/EQ‐VAS | 118 | 77 | 40 | 62.8 ± 10.8 | 66.2 | 12.3 ± 5.3 | 60.2 ± 11.1 | 67.5 | 6 |
| 33 | Tamás et al, 2014 | 52 | Hungary | EQ 5D/EQ‐VAS/ PDQ‐39 | 831 | 110 | 721 | 63.3 ± 11.3 | 63.6 | 8.2 ± 5.8 | / | / | 6 |
| 34 | Valeikiene et al, 2008 | 39 | Lithuania | WHOQOL‐100 | 120 | 54 | 66 | 69.5 ± 6.8 | 53.7 | / | 68.5 ± 6.7 | 51.5 | 6 |
| 35 | Vela et al, 2016 | 53 | Spain | EQ‐5D/EQ‐VAS | 174 | 87 | 87 | 46.9 ± 9.1 | 60.9 | / | 45.6 ± 8.6 | 54.7 | 6 |
| 36 | Vossius et al, 2009 | 9 | Norway | SF‐6D | 371 | 199 | 172 | 67.7 ± 9.1 | 60.8 | / | 67.5 ± 9.1 | 60.0 | 7 |
| 37 | Vescovelli et al, 2019 | 43 | Europe | A general QOL question | 103 | 50 | 53 | 70.6 ± 7.5 | 70 | / | 69 ± 8.7 | 69.8 | 6 |
| 38 | Winter et al, 2010 | 40 | Russia | EQ‐5D/EQ‐VAS | 200 | 100 | 100 | 68.9 ± 7.0 | 38.0 | 6.7 ± 5.1 | 68.9 ± 58.7 | 38 | 7 |
| 39 | Winter et al, 2011 | 54 | Italy | EQ‐5D/EQ‐VAS | / | 70 | / | 65.0 ± 8.5 | 58.6 | / | / | / | 5 |
| 40 | Yamabe et al, 2018 | 47 | Japan | SF‐6D | 144,692 | 133 | 144,559 | 61.4 ± 14.3 | 54.1 | / | 48.2 ± 15.3 | 51.6 | 5 |
| 41 | Yoon et al, 2017 | 41 | Korea | PDQ‐39 | 125 | 89 | 36 | 68.5 ± 7.9 | 52.8 | 2.84 ± 3.21 | 65.2 ± 10.8 | / | 7 |
| 42 | Pusswald et al, 2019 | 37 | Austria | SF‐36 | 61 | 41 | 20 | 61.6 ± 8.87 | 50 | / | 64.44 ± 5.48 | 32 | 7 |
| 43 | Prell et al, 2020 | 36 | German | A novel QOL questionnaire | 116 | 77 | 39 | 68.3 ± 8.90 | 55.8 | 8.8 ± 7.4 | 65.2 ± 10.1 | 25.6 | 7 |
Abbreviations: 15D, The generic 15D questionnaire; EQ‐5D, Europe Quality of Life Questionnaire‐5D; EQ‐VAS, Europe Quality of Life Questionnaire‐visual analogue scale; HUI3, The Health Utilities Index Mark 3; IPSS, The last question of the International Prostate Symptom Score; LiSat‐11, the Life Satisfaction Questionnaire; N, number; NHP, Nottingham Health Profile; EUROHIS‐QOL8, an 8‐item version of the WHOQOL‐BREF; PDQ‐39, the Parkinson's Disease Questionnaire‐39; PDQL, Parkinson's Disease Quality of Life; PQ‐10, a scale of global perceived QOL, from 0 (worst) to 10 (best); QOL‐AD, Quality of Life‐Alzhimer's Disease; SF‐12, SF‐6D, The short versions of SF scale; SF‐36, Short‐Form Health Survey (SF); WHOQOL‐100, World Health Organization Quality of Life Questionnaire; WHOQOL‐BREF, The short version of WHOQOL‐100.
3.3. QOL measurements
QOL measures involved in this systematic review are shown in Table 1. Five studies used the WHOQOL or its short version (WHOQOL‐BREF), 11 , 12 , 28 , 42 , 43 of which 3 studies with available data 12 , 28 , 39 were included in the meta‐analysis. Thirteen studies used the SF‐36, or its brief versions, such as SF‐12 and SF‐6D 8 , 9 , 10 , 47 ; 7 studies with available data were included in the meta‐analysis. Another twelve studies used EQ‐5D or EQ‐VAS 11 , 54 ; 4 studies using EQ‐5D 31 , 33 , 38 , 49 and 5 studies using EQ‐VAS 31 , 32 , 33 , 38 , 40 with available data were included in the meta‐analysis.
Four studies applied PDQ‐39 13 , 30 , 41 , 55 and all of them had available data and were included in the meta‐analysis. Other QOL measures were also used such as the generic 15D questionnaire (15D), 56 the Health Utilities Index Mark 3 (HUI3), 57 Nottingham Health Profile (NHP), 58 , 59 the Life Satisfaction Questionnaire (LiSat‐11), 60 an item of the International Prostate Symptom Score (IPSS) 61 and a newly developed QOL questionnaire. 36
Eventually, 20 studies with available data in both patient and control groups 8 , 9 , 37 , 38 , 39 , 40 , 41 , 49 , 52 were included in the meta‐analysis.
3.4. QOL comparisons by scale
Three studies employing the WHOQOL 12 , 28 , 39 were included in the meta‐analysis. Compared with healthy controls, PD patients had significantly poorer QOL in the physical domain with a large effect size (SMD = −0.866, 95% CI: ‐1.067, ‐0.665; P < 0.001), and the psychological (SMD = −0.405, 95% CI: 0.673, −0.138; P = 0.003), environmental (SMD = −0.470, 95% CI: −0.680, −0.259; P < 0.001), and social domains (SMD = −0.315, 95% CI: −0.597, −0.033; P = 0.028) with moderate effect sizes (Figure 2).
Figure 2.
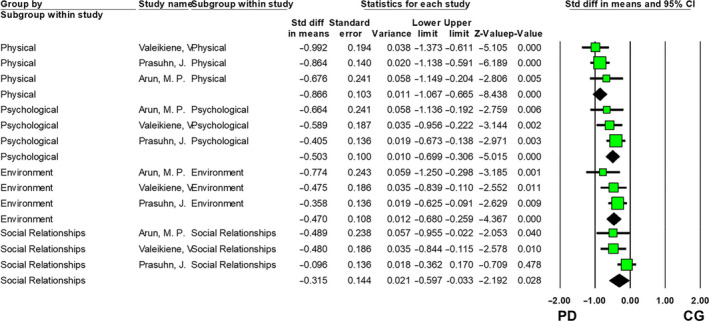
QOL comparison between PD patients and control group (CG) using WHOQOL
Seven studies utilizing the SF scales were included in the meta‐analysis. Compared with healthy controls, the patient group had significantly poorer QOL in the physical domain with a large effect size (SMD = −0.826, 95% CI: −1.529, −0.123; P = 0.021), and in the mental domain with a moderate effect size (SMD = −0.376, 95% CI: −0.732, −0.019; P = 0.039) (Figure 3).
Figure 3.
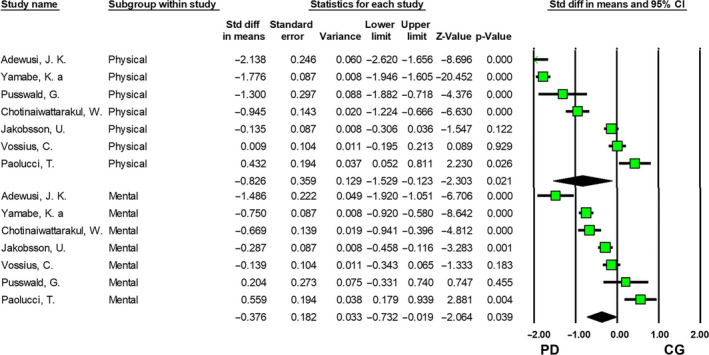
QOL comparisons between PD patients and control group (CG) using SF scales
In order to increase statistical power, we pooled the studies with available data on physical and psychological/mental QOL domains in either the WHOQOL or SF scales. Compared with healthy controls, PD patients had significantly poorer QOL in the physical QOL with a large effect size (SMD = −0.857, 95% CI: −1.394, −0.321; P = 0.002), and in the psychological/mental QOL with a moderate effect size (SMD = −0.438, 95% CI: −0.726, −0.150; P = 0.003) (Figure S1).
Four studies using the PDQ‐39 (SMD = −1.384, 95% CI: −1.607, −1.162; Figure S2), 4 studies using the EQ‐5D) (SMD = −0.889, 95% CI = −1.181, −0.596, P < 0.001; Figure S3), and 5 studies applying the EQ‐VAS (SMD = −1.081, 95% CI = −1.578, −0.584, P < 0.001; Figure S4) were meta‐analyzed separately. Compared with controls, PD patients had significantly poorer overall QOL in these analyses.
3.5. Subgroup analyses and publication bias
No significant difference was found between the WHOQOL and SF assessments regarding physical and mental QOL (Table 2). There was a significant difference between QOL measures in effect sizes (Table 2); the PDQ‐39 was associated with the largest effect size, followed by the EQ‐VAS, EQ‐5D and SF scales (Table 2). Since the minimum number of studies per measure was not met, publication bias analysis could not be undertaken.
Table 2.
Subgroup analyses of QOL between PD patients and healthy controls
| Subgroups | Categories (number of studies) | Sample size | SMD | 95% CI | I2 | P within subgroup | Q (P value across subgropus) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| PD | HC | Lower | Upper | |||||||
| Domain | Physical | WHOQOL (3) | 169 | 354 | −0.866 | −1.067 | −0.665 | 0 | <0.001 | 1.351 (P = 0.245) |
| SF (7) | 724 | 148,921 | −0.866 | −1.593 | −0.139 | 98.216 | <0.001 | |||
| Mental | WHOQOL (3) | 169 | 354 | −0.503 | −0.699 | −0.306 | 0 | 0.557 | 0 (P = 0.989) | |
| SF (7) | 724 | 148,921 | −0.598 | −0.907 | −0.289 | 89.961 | <0.001 | |||
| Measurement of QOL | / | EQ‐5D (4) | 1412 | 1098 | −0.889 | −1.181 | −0.596 | 85.787 | <0.001 | 188.353 (P < 0.001) |
| EQ‐VAS (5) | 1444 | 952 | −1.081 | −1.578 | −0.584 | 94.362 | <0.001 | |||
| PDQ‐39 (4) | 251 | 198 | −1.384 | −1.607 | −1.162 | 7.645 | 0.355 | |||
| SF(7) | 724 | 148,921 | −0.423 | −1.131 | 0.285 | 98.194 | <0.001 | |||
4. DISCUSSION
To the best of our knowledge, this was the first systemic review and meta‐analysis that compared QOL between PD and healthy controls with standardized measures and estimating group differences. PD patients had significantly poorer QOL than healthy controls overall and in most domains.
Based on the distress/protection model of QOL, QOL is determined by the overall balance between protective and distressing factors. 62 QOL is lower if distressing factors (eg, severe depressive symptoms) predominate over protective factors (eg, social support from family). Both motor and psychosocial dysfunctions and psychiatric comorbidities (eg, bradykinesia, rigidity, gait freezing, depression, fatigue, cognitive decline, and sleep disturbances associated with PD) are common in PD patients, which could lower their QOL. Certain demographic (eg, age, 11 , 29 gender, 39 education level, 11 , 63 living condition, 43 , 64 knowledge and beliefs 64 and marital status 40 ) and clinical characteristics (eg, illness duration, 55 and disease stage, 54 , 55 , 56 severity 28 , 42 , 43 and subtypes 10 , 53 ) were significantly associated with QOL in PD patients. The findings on the associations between psychiatric comorbidities and QOL in PD are conflicting. For example, depression was the strongest contributing factor for QOL in some, 11 , 12 , 28 but not all studies. 55 Anxiety, apathy, and pain are also associated with poor QOL in PD, 11 , 48 with greater effect sizes than motor‐symptoms. 48 However, the significant relationship between anxiety and poor QOL was not found in another study. 12 Similarly, the association between sleep disturbances and QOL is contested, with some studies finding a significant relationship between sleep problems and QOL, 7 , 42 , 58 but not others. 12 , 55 In addition, some studies found that REM sleep behavior disorder with reduced striatal dopamine transporter values and increased expression of PD‐related pattern may be associated with the occurrence of PD. 65 , 66 , 67 The discrepancy between studies could be partly due to differences in instruments, 68 , 69 sampling methods, disease severity, 14 , 70 effects of treatments, 71 , 72 , 73 , 74 , 75 cognitive performance, 76 and clinical presentations caused by different associated genes. 77 The limited number of studies with the same QOL measure precluded an analysis of the moderating effects of the abovementioned demographic and clinical characteristics on QOL in PD.
Subgroup analyses revealed that QOL differences between PD patients and healthy controls varied by instrument (EQ‐5D vs. EQ‐VAS vs. PDQ‐39 vs. SF scales), probably resulting from the use of different items and emphasis between scales. 78 , 79 Two types of QOL measurements were applied, generic, and disease‐specific scales. Generic scales (eg, SF scales, EQ‐5D, and EQ‐VAS) are designed for all types of populations but may not be sensitive to PD‐related QOL. A disease‐specific scale (eg, PDQ‐39) 14 is constructed for PD and detects minor differences in QOL. Hence, PD‐specific scales are clearly desirable clinical and research tools.
The strengths of this systematic review and meta‐analysis are the inclusion of comparative studies using standardized QOL measures and the large sample size (ie, 2707 PD patients and 150,661 healthy controls in the meta‐analysis) that improved statistical power and generalizability. However, several limitations should also be noted. First, different QOL measures were applied; therefore, in order to reduce heterogeneity attributable to measures, QOL was synthesized by QOL instrument. Second, some factors related to QOL, such as gender, illness duration, disease severity, health service system, and medication treatment, were not analyzed due to insufficient data in included studies. Third, causality between QOL and associated factors could not be explored due to the cross‐sectional design of the included studies. Fourth, only studies published in English were searched and limited number of studies conducted in developing countries were included.
In conclusion, PD patients had lower QOL compared with healthy controls in most dimensions, especially in physical function and mental health domains. Considering the negative impact of poor QOL on life and functional outcomes, factors contributing to poor QOL should be identified in longitudinal studies and effective measures should be developed to improve QOL in this population. For example, in order to improve QOL in physical function domain, physical rehabilitation together with the conventional pharmacotherapy and novel treatments, such as deep brain stimulation (DBS) surgery, could be considered. In contrast, timely adjunctive psychotherapy and psychotropic medications should be offered to appropriate PD patients in order to improve their mental health QOL.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The study was supported by The National Science and Technology Major Project for investigational new drug (2018ZX09201‐014); the University of Macau (MYRG2019‐00066‐FHS); National Nature Science Foundation of China (81520108016; 81661148045). We acknowledge the contributions of each author. The author contributions were displayed as follows, Study design: Na Zhao, Yuan Yang, Yu‐Feng Zang, Yu‐Tao Xiang. Data collection, analysis, and interpretation: Na Zhao; Yuan Yang, Ling Zhang, Qinge Zhang. Manuscript drafting: Na Zhao, Brian J. Hall, Yu‐Tao Xiang. Critical revision of the manuscript: Gabor S. Ungvari, Lloyd Balbuena.
Zhao N, Yang Y, Zhang L, et al. Quality of life in Parkinson's disease: A systematic review and meta‐analysis of comparative studies. CNS Neurosci Ther.2021;27:270–279. 10.1111/cns.13549
Zhao and Yang are contributed equally to the work.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created in this study.
REFERENCES
- 1. Muangpaisan W, Mathews A, Hori H, Seidel D. A systematic review of the worldwide prevalence and incidence of Parkinson's disease. J Med Assoc Thai. 2011;94(6):749. [PubMed] [Google Scholar]
- 2. Tysnes OB, Storstein A. Epidemiology of Parkinson's disease. J Neural Transm (Vienna). 2017;124(8):901–905. [DOI] [PubMed] [Google Scholar]
- 3. Wirdefeldt K, Adami H‐O, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol. 2011;26(1):1. [DOI] [PubMed] [Google Scholar]
- 4. Garcia‐Ruiz PJ, Chaudhuri KR, Martinez‐Martin P. Non‐motor symptoms of Parkinson's disease A review… from the past. J Neurol Sci. 2014;338(1‐2):30–33. [DOI] [PubMed] [Google Scholar]
- 5. WHO . The World Health Organization quality of life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41(10):1403–1409. [DOI] [PubMed] [Google Scholar]
- 6. Den Oudsten BL, Van Heck GL, De Vries J. Quality of life and related concepts in Parkinson's disease: a systematic review. Movem Disord. 2007;22(11):1528–1537. [DOI] [PubMed] [Google Scholar]
- 7. Park S, Kim R, Shin JH, Kim HJ, Paek SH, Jeon B. The probable REM sleep behavior disorder negatively affects health‐related quality of life in Parkinson's disease with bilateral subthalamic nucleus stimulation. Parkinsonism Relat Disord. 2020;81:136–139. [DOI] [PubMed] [Google Scholar]
- 8. Paolucci T, Iosa M, Morone G, et al. Romberg ratio coefficient in quiet stance and postural control in Parkinson's disease. Neurol Sci. 2018;39(8):1355–1360. [DOI] [PubMed] [Google Scholar]
- 9. Vossius C, Nilsen OB, Larsen JP. Health state values during the first year of drug treatment in early‐stage Parkinson's disease: a prospective, population‐based, cohort study. Drugs Aging. 2009;26(11):973–980. [DOI] [PubMed] [Google Scholar]
- 10. Hariz GM, Forsgren L. Activities of daily living and quality of life in persons with newly diagnosed Parkinson's disease according to subtype of disease, and in comparison to healthy controls. Acta Neurol Scand. 2011;123(1):20–27. [DOI] [PubMed] [Google Scholar]
- 11. Hendred SK, Foster ER. Use of the World Health Organization quality of life assessment short version in mild to moderate Parkinson disease. Arch Phys Med Rehabil. 2016;97(12):2123–2129.e2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prasuhn J, Piskol L, Vollstedt EJ, et al. Non‐motor symptoms and quality of life in subjects with mild parkinsonian signs. Acta Neurol Scand. 2017;136(5):495–500. [DOI] [PubMed] [Google Scholar]
- 13. Park HJ, Sohng KY, Kim S. Validation of the Korean version of the 39‐Item Parkinson's Disease Questionnaire (PDQ‐39). Asian Nurs Res. 2014;8(1):67–74. [DOI] [PubMed] [Google Scholar]
- 14. Soh S‐E, Morris ME, McGinley JL. Determinants of health‐related quality of life in Parkinson's disease: a systematic review. Parkinsonism Relat Disord. 2011;17(1):1–9. [DOI] [PubMed] [Google Scholar]
- 15. van Uem JM, Marinus J, Canning C, et al. Health‐Related Quality of Life in patients with Parkinson's disease–a systematic review based on the ICF model. Neurosci Biobehav Rev. 2016;61:26–34. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 17. Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol. 1993;50(2):140–148. [DOI] [PubMed] [Google Scholar]
- 18. Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta‐analysis. Neurology. 2016;86(6):566–576. [DOI] [PubMed] [Google Scholar]
- 19. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2015;30(12):1591–1601. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐analyses. Ottawa: Ottawa Hospital Research Institute; 2011. [Google Scholar]
- 22. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐analyses. Ottawa: Ottawa Hospital Research Institute; 2014. [Google Scholar]
- 23. Leucht S, Komossa K, Rummel‐Kluge C, et al. A meta‐analysis of head‐to‐head comparisons of second‐generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152–163. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 4 Cochrane: John Wiley and Sons; 2011. Available from www.training.cochrane.org/handbook [Google Scholar]
- 25. DerSimonian R, Laird N. Meta‐Analysis in Clinical Trials; 1986. [DOI] [PubMed] [Google Scholar]
- 26. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adewusi JK, Hadjivassiliou M, Vinagre‐Aragón A, et al. Sensory neuropathic symptoms in idiopathic Parkinson's disease: prevalence and impact on quality of life. Acta Neurol Belg. 2018;118(3):445–450. [DOI] [PubMed] [Google Scholar]
- 28. Arun MP, Bharath S, Pal PK, Singh G. Relationship of depression, disability, and quality of life in Parkinson's disease: a hospital‐based case‐control study. Neurol India. 2011;59(2):185–189. [DOI] [PubMed] [Google Scholar]
- 29. Chotinaiwattarakul W, Dayalu P, Chervin RD, Albin RL. Risk of sleep‐disordered breathing in Parkinson's disease. Sleep Breathing = Schlaf & Atmung. 2011;15(3):471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chu SY, Tan CL. Subjective self‐rated speech intelligibility and quality of life in patients with Parkinson's disease in a Malaysian sample. Open Publ Health J. 2018;11(1):485–493. [Google Scholar]
- 31. Fan XJ, Wang DL, Hellman B, et al. Assessment of health‐related quality of life between people with Parkinson's disease and non‐Parkinson's: using data drawn from the ‘100 for Parkinson's’ smartphone‐based prospective study. Int J Environ Res Publ Health. 2018;15(11):2358–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greene T, Camicioli R. Depressive symptoms and cognitive status affect health‐related quality of life in older patients with Parkinson's disease. J Am Geriatr Soc. 2007;55(11):1888–1890. [DOI] [PubMed] [Google Scholar]
- 33. Hobson P, Meara J. Mortality and quality of death certification in a cohort of patients with Parkinson's disease and matched controls in North Wales, UK at 18 years: a community‐based cohort study. BMJ Open. 2018;8(2):e018969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jakobsson U, Westergren A, Lindskov S, Hagell P. Construct validity of the SF‐12 in three different samples. J Eval Clin Pract. 2012;18(3):560–566. [DOI] [PubMed] [Google Scholar]
- 35. Kang P, Kloke J, Jain S. Olfactory dysfunction and parasympathetic dysautonomia in Parkinson's disease. Clin Autonomic Res. 2012;22(4):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prell T, Teschner U, Witte OW, Kunze A. Current and desired quality of life in people with Parkinson's disease: the Calman gap increases with depression. J Clin Med. 2020;9(5):1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pusswald G, Wiesbauer P, Pirker W, Novak K, Foki T, Lehrner J. Depression, quality of life, activities of daily living, and subjective memory after deep brain stimulation in Parkinson disease‐A reliable change index analysis. Int J Geriatr Psychiatry. 2019;34(11):1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Swinn L, Schrag A, Viswanathan R, Bloem BR, Lees A, Quinn N. Sweating dysfunction in Parkinson's disease. Mov Disord. 2003;18(12):1459–1463. [DOI] [PubMed] [Google Scholar]
- 39. Valeikiene V, Ceremnych J, Alekna V, Juozulynas A. Differences in WHOQOL‐100 domain scores in Parkinson's disease and osteoarthritis. Med Sci Monitor: Int Med J Exp Clin Res. 2008;14(4):Cr221‐Cr227. [PubMed] [Google Scholar]
- 40. Winter Y, von Campenhausen S, Popov G, et al. Social and clinical determinants of quality of life in Parkinson's disease in a Russian cohort study. Parkinsonism Relat Disord. 2010;16(4):243–248. [DOI] [PubMed] [Google Scholar]
- 41. Yoon JE, Kim JS, Jang W, et al. Gender differences of nonmotor symptoms affecting quality of life in Parkinson disease. Neurodegener Dis. 2017;17(6):276–280. [DOI] [PubMed] [Google Scholar]
- 42. Kasten M, Kertelge L, Tadic V, et al. Depression and quality of life in monogenic compared to idiopathic, early‐onset Parkinson's disease. Mov Disord. 2012;27(6):754–759. [DOI] [PubMed] [Google Scholar]
- 43. Vescovelli F, Sarti D, Ruini C. Well‐being and distress of patients with Parkinson's disease: a comparative investigation. Int Psychogeriatr. 2019;31(1):21–30. [DOI] [PubMed] [Google Scholar]
- 44. Jenkinson C, Peto V, Fitzpatrick R, Greenhall R, Hyman N. Self‐reported functioning and well‐being in patients with Parkinson's disease: comparison of the short‐form health survey (SF‐36) and the Parkinson's Disease Questionnaire (PDQ‐39). Age Ageing. 1995;24(6):505–509. [DOI] [PubMed] [Google Scholar]
- 45. Quittenbaum BH, Grahn B. Quality of life and pain in Parkinson's disease: a controlled cross‐sectional study. Parkinsonism Relat Disord. 2004;10(3):129–136. [DOI] [PubMed] [Google Scholar]
- 46. Riazi A, Hobart JC, Lamping DL, et al. Using the SF‐36 measure to compare the health impact of multiple sclerosis and Parkinson's disease with normal population health profiles. J Neurol Neurosurg Psychiatry. 2003;74(6):710–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamabe K, Liebert R, Flores N, Pashos C. Health‐related quality‐of‐life, work productivity, and economic burden among patients with Parkinson's disease in Japan. J Med Econ. 2018;21(12):1206–1212. [DOI] [PubMed] [Google Scholar]
- 48. Baig F, Lawton M, Rolinski M, et al. Delineating nonmotor symptoms in early Parkinson's disease and first‐degree relatives. Mov Disord. 2015;30(13):1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barber TR, Lawton M, Rolinski M, et al. Prodromal parkinsonism and neurodegenerative risk stratification in REM sleep behavior disorder. Sleep. 2017;40(8):zsx071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reuther M, Spottke EA, Klotsche J, et al. Assessing health‐related quality of life in patients with Parkinson's disease in a prospective longitudinal study. Parkinsonism Relat Disord. 2007;13(2):108–114. [DOI] [PubMed] [Google Scholar]
- 51. Schrag A, Jahanshahi M, Quinn N. How does Parkinson's disease affect quality of life? A comparison with quality of life in the general population. Mov Disord. 2000;15(6):1112–1118. [DOI] [PubMed] [Google Scholar]
- 52. Tamás G, Gulácsi L, Bereczki D, et al. Quality of life and costs in Parkinson's disease: a cross sectional study in Hungary. PLoS One. 2014;9(9):e107704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vela L, Martínez Castrillo JC, García Ruiz P, et al. The high prevalence of impulse control behaviors in patients with early‐onset Parkinson's disease: a cross‐sectional multicenter study. J Neurol Sci. 2016;368:150–154. [DOI] [PubMed] [Google Scholar]
- 54. Winter Y, von Campenhausen S, Arend M, et al. Health‐related quality of life and its determinants in Parkinson's disease: results of an Italian cohort study. Parkinsonism Relat Disord. 2011;17:265‐269. [DOI] [PubMed] [Google Scholar]
- 55. Dogan VB, Koksal A, Dirican A, Baybas S, Dirican A, Dogan GB. Independent effect of fatigue on health‐related quality of life in patients with idiopathic Parkinson's disease. Neurol Sci. 2015;36(12):2221–2226. [DOI] [PubMed] [Google Scholar]
- 56. Haapaniemi TH, Sotaniemi KA, Sintonen H, Taimela E. The generic 15D instrument is valid and feasible for measuring health related quality of life in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75(7):976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pohar SL, Jones CA. The burden of Parkinson disease (PD) and concomitant comorbidities. Arch Gerontol Geriatr. 2009;49(2):317–321. [DOI] [PubMed] [Google Scholar]
- 58. Karlsen KH, Larsen JP, Tandberg E, Maeland JG. Influence of clinical and demographic variables on quality of life in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;66(4):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Larsen JP, Karlsen K, Tandberg E. Clinical problems in non‐fluctuating patients with Parkinson's disease: a community‐based study. Mov Disord. 2000;15(5):826–829. [DOI] [PubMed] [Google Scholar]
- 60. Gustafsson H, Nordström P, Stråhle S, Nordström A. Parkinson's disease: a population‐based investigation of life satisfaction and employment. J Rehabil Med. 2015;47(1):45–51. [DOI] [PubMed] [Google Scholar]
- 61. Benli E, Ozer FF, Kaya Y, Ozcan TS, Ayyildiz A. Is there a difference between Parkinson disease patients and a control group in terms of urinary symptoms and quality of life? Turk J Med Sci. 2016;46(6):1665–1671. [DOI] [PubMed] [Google Scholar]
- 62. Ritsner M, Modai I, Endicott J, et al. Differences in quality of life domains and psychopathologic and psychosocial factors in psychiatric patients. J Clin Psychiatry. 2000;61(11):880–889; quiz 890. [DOI] [PubMed] [Google Scholar]
- 63. Cubo E, Rojo A, Ramos S, et al. The importance of educational and psychological factors in Parkinson's disease quality of life. Eur J Neurol. 2002;9(6):589–593. [DOI] [PubMed] [Google Scholar]
- 64. Li W, Reavley NJAPP. Patients' and caregivers' knowledge and beliefs about mental illness in mainland China. A systematic review. Asia‐Pacific Psychiatry. 2020:e12423. [DOI] [PubMed] [Google Scholar]
- 65. Huang Z, Jiang C, Li L, et al. Correlations between dopaminergic dysfunction and abnormal metabolic network activity in REM sleep behavior disorder. J Cereb Blood Flow and Metab. 2020;40(3):552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wu P, Yu H, Peng S, et al. Consistent abnormalities in metabolic network activity in idiopathic rapid eye movement sleep behaviour disorder. Brain: J Neurol. 2014;137(Pt 12):3122–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Holtbernd F, Gagnon JF, Postuma RB, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology. 2014;82(7):620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Balzer‐Geldsetzer M, Klotsche J, Consortium L, Dodel R, Riedel O. Quality of life in a German cohort of Parkinson's patients assessed with three different measures. J Neurol. 2018;265(11):2713–2722. [DOI] [PubMed] [Google Scholar]
- 69. Martinez‐Martin P, Jeukens‐Visser M, Lyons KE, et al. Health‐related quality‐of‐life scales in Parkinson's disease: critique and recommendations. Mov Disord. 2011;26(13):2371–2380. [DOI] [PubMed] [Google Scholar]
- 70. Karlsen KH, Tandberg E, Årsland D, Larsen JP. Health related quality of life in Parkinson's disease: a prospective longitudinal study. J Neurol Neurosurg Psychiatry. 2000;69(5):584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670–1683. [DOI] [PubMed] [Google Scholar]
- 72. Dafsari HS, Dos Santos Ghilardi MG, Visser‐Vandewalle V, et al. Beneficial nonmotor effects of subthalamic and pallidal neurostimulation in Parkinson's disease. Brain Stimul. 2020;13(6):1697–1705. [DOI] [PubMed] [Google Scholar]
- 73. You Z, Wu YY, Wu R, Xu ZX, Wu X, Wang XP. Efforts of subthalamic nucleus deep brain stimulation on cognitive spectrum: from explicit to implicit changes in the patients with Parkinson's disease for 1 year. CNS Neurosci Ther. 2020;26(9):972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cernera S, Eisinger RS, Wong JK, et al. Long‐term Parkinson's disease quality of life after staged DBS: STN vs GPi and first vs second lead. NPJ Parkinson's Dis. 2020;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rughani A, Schwalb JM, Sidiropoulos C, et al. Congress of neurological surgeons systematic review and evidence‐based guideline on subthalamic nucleus and globus pallidus internus deep brain stimulation for the treatment of patients with Parkinson's disease: executive summary. Neurosurgery. 2018;82(6):753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhou C, Guan XJ, Guo T, et al. Progressive brain atrophy in Parkinson's disease patients who convert to mild cognitive impairment. CNS Neurosci Ther. 2020;26(1):117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shen YT, Wang JW, Wang M, et al. BST1 rs4698412 allelic variant increases the risk of gait or balance deficits in patients with Parkinson's disease. CNS Neurosci Ther. 2019;25(4):422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Damiano AM, Snyder C, Strausser B, Willian MK. A review of health‐related quality‐of‐life concepts and measures for Parkinson's disease. Qual Life Res. 1999;8(3):235–243. [DOI] [PubMed] [Google Scholar]
- 79. Marinus J, Ramaker C, van Hilten JJ, Stiggelbout AM. Health related quality of life in Parkinson's disease: a systematic review of disease specific instruments. J Neurol Neurosurg Psychiatry. 2002;72(2):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fonseca LC, Tedrus GM, Rezende AL, Giordano HF. Coherence of brain electrical activity: a quality of life indicator in Alzheimer's disease? Arq Neuropsiquiatr. 2015;73(5):396–401. [DOI] [PubMed] [Google Scholar]
- 81. Santos Garcia D, Jesus S, Aguilar M, et al. COPPADIS‐2015 (COhort of Patients with PArkinson's DIsease in Spain, 2015): an ongoing global Parkinson's disease project about disease progression with more than 1000 subjects included. Results from the baseline evaluation. Eur J Neurol. 2019;1399–1407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data sharing is not applicable to this article as no new data were created in this study.


