Abstract
Background
Paediatric inflammatory multisystem syndrome (PIMS) temporally associated with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) (PIMS-TS) is a rare clinical syndrome associated with a multiorgan system dysfunction, especially acute cardiac injury, and mandates a higher level of care.
Aim of Review
To investigate cardiac manifestations, treatment characteristics, and outcomes of PIMS-TS.
Key Scientific Concepts of Review
Twenty-six studies were included with 1228 pooled subjects, with a mean age of 8.6 years, which were dominated by male gender (53%), and African ethnicity (31%). 732 (38%) patients were reactive on a serological test, and 457 patients (45%) were positive on SARS-CoV-2 RT-PCR. ST-segment abnormalities were the most common ECG findings (16%, n/N: 34/212). Various markers of troponin and the pooled mean of BNP and NT-pro-BNP levels were elevated. Cardiomegaly and pericardial effusion (21.8%, n/N: 164/751) were the most common chest X-ray findings. In echocardiography, the majority of patients' left ventricular ejection fraction was reduced (59.0%, n/N: 180/305), with pericardial effusion/ pericarditis seen the most (17.44%, n/N: 221/1267), and Z score ≥ 2 in 28% (n/N: 42/139). Cardiac MRI findings were consistent with acute myocarditis. Intravenous immunoglobulin, corticosteroids, and vasoactive drugs were frequently utilized. The mean length of stay was 6 days, with most patients (71%, n/N: 834/1163) were admitted to the ICU. However, the overall prognosis was favorable, with 98% alive (n/N: 1235/1260), and more than 50% of patients experienced recovery of left ventricular systolic functions at discharge (116 out of 206 patients).
Keywords: COVID-19, SARS-CoV-2, PIMS-TS, Kawasaki, Toxic shock syndrome
1. Introduction
Coronavirus disease of 2019 (COVID-19) is a highly contagious disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Although mostly asymptomatic, this disease can present with life-threatening clinical presentation [1,2]. COVID-19 is also known to disproportionately affect the elderly and patients with cardiovascular comorbidities [[3], [4], [5]]. On the contrary, the majority clinical presentation of COVID-19 in the pediatric population tends to be mild [6,7].
However, an emerging report from the United Kingdom (UK) of hyperinflammatory syndrome mimicking Kawasaki disease (KD) and toxic shock syndrome alerted pediatricians worldwide [8]. Later, this novel entity was designated as a pediatric inflammatory multisystem syndrome (PIMS) temporally associated (TS) with SARS-CoV-2 (PIMS-TS) [[9], [10], [11]]. PIMS-TS was associated with multiple organ involvement, with variable presentations. Of interest, the heart was also affected by this syndrome, and multiple studies had reported the cardiac consequences of PIMS-TS [[12], [13], [14]].
Nonetheless, these reports could not depict the entire clinical spectrum of cardiac manifestations of PIMS-TS, mainly due to the limited number of patients. Therefore, we performed a systematic review to delineate this novel clinical entity better.
2. Methods
2.1. Systematic review
We performed a systematic review of the literature following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [15] to identify all studies that describe cardiac manifestations of patients with PIMS-TS.
2.2. Information sources and search strategy
We performed a comprehensive literature search through PubMed, Europe PMC, Cochrane library, Proquest, and EBSCOhost. We limited search results from January to October 2020. To construct search strategy, we used free keywords comprised of Paediatric AND (multisystem inflammatory disease OR multisystem inflammatory syndrome OR Kawasaki-like) AND (COVID-19 OR SARS-CoV-2) AND (Cardiac OR Heart OR Myocarditis OR Troponin OR NT-pro-BNP OR Creatine Kinase OR Electrocardiography OR Chest X-ray OR Chest Radiography OR Cardiac Ultrasound OR Echocardiography OR Cardiac Magnetic Resonance Imaging). Also, we searched google scholar and relevant articles from the reference of included studies. We finalized the search on 1 October 2020.
2.3. Inclusion and exclusion criteria
The inclusion criteria for studies were studies that reported cardiac manifestations of patients with PIMS-TS in terms of one of the following: signs and symptoms of cardiac involvement, cardiac biomarkers (brain natriuretic peptide [BNP], n-terminal brain natriuretic peptide [NT-pro-BNP], creatine kinase-myocardial band [CK-MB], troponin [I/ T/ non-specific], electrocardiography findings, chest radiography (CXR), echocardiography, and cardiac magnetic resonance imaging (CMR).
We excluded non-English articles, articles without full-text availability, articles that did not describe cardiac manifestations of PIMS-TS, case reports and case series with less than 10 patients, and letters that did not report PIMS-TS cases. Exceptions will be made for case series <10 patients with important findings, such as CMR/ Echocardiographic studies. Data extracted from these studies will be only the CMR/Echocardiographic findings.
2.4. Study selection
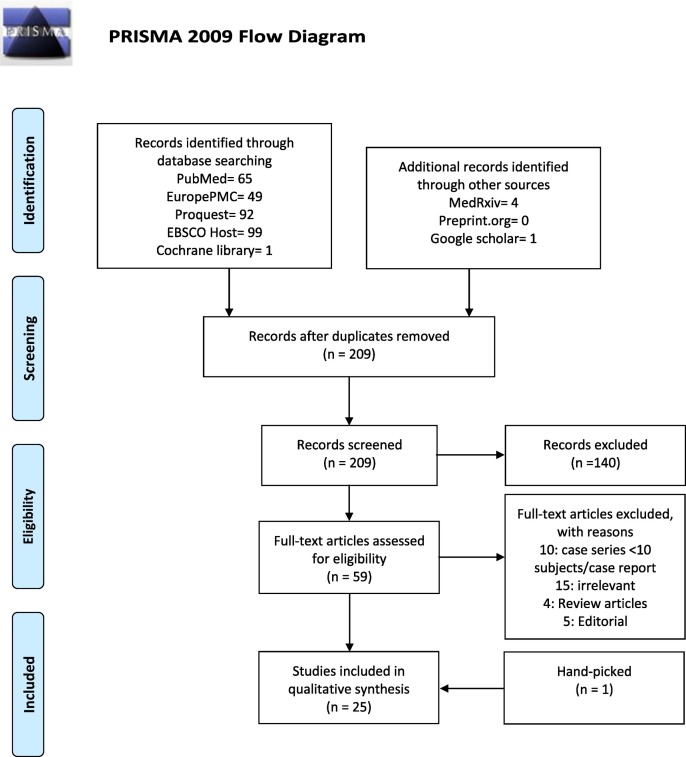
Two independent reviewers (JH and ICSP) screened the titles and abstracts for full-text eligibility and applied protocol inclusion and exclusion criteria to the full-text publication. Any discrepancies between the two reviewers were discussed with third and fourth reviewers (SL and DSM). Fig. 1 depicts the study selection flowchart.
Fig. 1.
Study profile.
2.5. Data extraction
We collected the data regarding the first author name, date of patient recruitment, country, study type, demographic characteristics (age, sex, ethnicity), SARS-CoV-2 PCR and serological status, and cardiac manifestations (sign and symptoms of cardiac involvement, brain natriuretic peptide [BNP], n-terminal brain natriuretic peptide [NT-proBNP], creatine kinase [CK], troponin [I/ T/ non-specific], electrocardiography findings, chest radiography (CXR), echocardiography, cardiac magnetic resonance imaging (CMR), treatment characteristics, and outcomes. Outcomes consist of death/alive, ICU admission, length of stays, and left ventricular ejection fraction (LVEF) improvement at discharge. Data other than the mean (SD) is transformed using a calculator if available using a calculator available online (http://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html), then we combine all of the mean (SD)s into a single mean (SD) value.
3. Results
In total, we identified 1288 patients from 26 articles that reported cardiac manifestations of PIMS-TS (Fig. 1) [13,14,[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]].
3.1. Population characteristics and SARS-CoV-2 test results of patients with PIMS-TS
Further detail on population characteristics and SARS-CoV-2 results are provided in Table 1 .
Table 1.
Population characteristics and SARS-CoV-2 test results.
| Variable | Number of study | n/N | Mean (SD) | % | |
|---|---|---|---|---|---|
| Age | Pooled Mean (SD) | 23 | 1188/1188 | 8.6 (5.5) | 100 |
| Gender | Male | 24 | 689/1288 | 53.5 | |
| Female | 599/1288 | 46.5 | |||
| Ethnicity | White | 13 | 173/1063 | 16.27 | |
| Black | 331/1063 | 31.14 | |||
| Asian | 64/1063 | 6.02 | |||
| Hispanic | 235/1063 | 22.11 | |||
| multiple | 35/1063 | 3.29 | |||
| Other | 52/1063 | 4.89 | |||
| Missing | 173/1063 | 16.27 | |||
| Positive | 870/1072 | 81.16 | |||
| PCR | Negative | 17 | 202/1072 | 18.84 | |
| NA | 0 | 0 | |||
| Serology | IgM (−) IgG (−) | 14 | 2/10 | 20.00% | |
| IgM (+) IgG (−) | 0 | 0% | |||
| IgM (−) IgG (+) | 12/26 | 46.15% | |||
| IgM (+) IgG (+) | 3/10 | 30.00% | |||
| IgG (+) | 44/51 | 86.27% | |||
| SARS-CoV-2 Antibody (+) | 266/902 | 29.49% | |||
SD; standard deviation, ADHD; attention deficit hyperactivity disorder, NA; not available, Ig; immunoglobulin, SARS-CoV-2; severe acute respiratory syndrome coronavirus 2.
3.1.1. Baseline characteristics
Of 1288 patients with PIMS-TS, 689 (53.49%) were male. The mean age of patients with PIMS-TS was 8.6 (5.5) years. Of thirteen studies that reported data on ethnicity (n = 1063), 331 (31.14%) were Black, 235 (22.11%) were Hispanic, 173 (16.27%) were White, 64 (6.02%) were Asian, 52 (4.89%) were from other ethnicities, 35 (3.29%) had mixed race.
3.1.2. Comorbidities
Of 17 studies, with pooled subjects of 1117, comorbidities were present in 354 patients. The three most common comorbidities were obesity (n = 209), chronic lung disease (n = 62), overweight (n = 39) [13,16,17,19,[21], [22], [23], [24], [25],28,29,31,32,[35], [36], [37], [38]].
3.1.3. SARS-CoV-2 RT-PCR and serology results
Of 24 studies that reported data on SARS-CoV-2 reverse-transcriptase polymerase chain reaction (n = 1020), 563 patients were negatives, 457 patients were positives [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]]. Meanwhile, a positive serology test of any kind (IgM, IgG, IgA, and non-specific SARS-CoV-2 antibody test) with a negative PCR can be seen in 458 subjects [[16], [17], [18], [19], [20], [21], [22], [23],[27], [28], [29],[36], [37], [38]].
3.2. Cardiac manifestations of PIMS-TS
A full description of cardiac manifestations is available in Table 2 .
Table 2.
Cardiac manifestations of PIMS-TS.
| Variable | Number of Study | n/N | Mean (SD) | % | |
|---|---|---|---|---|---|
| Sign of shock | 24 | 652/1288 | 50.62 | ||
| respiratory distress/failure | 24 | 270/1288 | 20.96 | ||
| Radiography | Normal | 9 | 25/751 | 3.33 | |
| Cardiomegaly + Pericardial effusion | 164/751 | 21.84 | |||
| Pleural effusion | 105/751 | 13.98 | |||
| focal/bilateral opacity | 169/751 | 22.50 | |||
| lung oedema | 16/751 | 2.13 | |||
| Atelectasis | 10/751 | 1.33 | |||
| interstitial abnormalities | 9/751 | 1.20 | |||
| Reactive airway disease | 2/751 | 0.27 | |||
| Electrocardiography | Junctional rhytm | 7 | 3/212 | 1.42 | |
| Sinus Bradycardia | 1/212 | 0.47 | |||
| Supraventricular extrasystole | 1/212 | 0.47 | |||
| Attenuated QRS | 1/212 | 0.47 | |||
| AV block | 5/212 | 2.36 | |||
| PR Interval abnormalities | 2/212 | 0.94 | |||
| QT abnormalities | 1/212 | 0.47 | |||
| T wave abnormalitis | 21/212 | 9.91 | |||
| Atrial Fibrillation | 1/212 | 0.47 | |||
| Ventricular arrhytmia (VT/VF/PVC) | 4/212 | 1.89 | |||
| ST abnormalities | 34/212 | 16.04 | |||
| Various Troponin Level | Troponin I | 3 | 869 (1229) | ||
| Troponin T | 3 | 75 (132) | |||
| High sensitivity troponin I | 3 | 1183 (3307) | |||
| Unspecified troponin | 7 | 1277 (10114) | |||
| Elevated troponin I | 4 | 50/67 | 74.63 | ||
| Elevated sensitivity troponin I | 3 | 61/68 | 89.7 | ||
| Elevated unspecified | 7 | 335/718 | 46.66 | ||
| BNP (pg/mL) | 4 | 4238.35 (5803.24) | |||
| NT-proBNP (pg/mL) | 10 | 13,590.78 (20,676.1) | |||
| Echocardiography | Mitral Regurgitation | 22 | 175/1174 | 14.91 | |
| Tricuspid Regurgitation | 19/1174 | 1.62 | |||
| Pericardial Effusion + pericarditis | 221/1267 | 17.44 | |||
| Myocarditis | 196/1267 | 15.47 | |||
| RV dysfunction | 17/1174 | 1.45 | |||
| Biventricular systolic dysfunction | 1/1174 | 0.09 | |||
| Coronary Echogenicity | 17/1174 | 1.45 | |||
| Coronary artery dilatation | 42/1174 | 3.58 | |||
| Coronary artery aneurysm | 45/1267 | 3.55 | |||
| Unspecified valvular regurgitation | 2/1174 | 0.17 | |||
| Coronary artery dilatation or aneurysm | 95/1174 | 8.09 | |||
| Biventricular or left ventricular dysfunction | 5/1174 | 0.43 | |||
| Ectatic coronary artery | 14/1174 | 1.19 | |||
| Myocardial dysfunction | 15/1174 | 1.28 | |||
| Unspecified coronary abnormalities | 4/1174 | 0.34 | |||
| Echocardiography | Cardiomegaly | 2/1174 | 0.17 | ||
| Regional wall motion abnormal | 3/1174 | 0.26 | |||
| Lack of distal tapering LAD coronary artery | 11/1174 | 0.94 | |||
| Dilated left ventricle | 1/1174 | 0.09 | |||
| Diastolic dysfunction | 2/1174 | 0.17 | |||
| Left ventricle dysfunction | 107/1174 | 8.45 | |||
| LVEF | > 55% | 13 | 33/305 | 10.82 | |
| < 55% | 180/305 | 59.02 | |||
| N/A | 80/305 | 26.23 | |||
| Coronary Artery Z score |
< 2 | 13 | 11/139 | 7.91 | |
| 2–2.5 | 9/139 | 6.47 | |||
| ≥ 2.5 | 18/138 | 12.95 | |||
AV; atrioventricular, BNP; brain natriuretic peptide, NT-proBNP; N-terminal-pro brain natriuretic peptide, RV; right ventricular, LVEF; left ventricular ejection fraction, N/A; not available.
3.2.1. Sign and symptoms of cardiac involvement
Of 1288 patients pooled across 24 studies, signs of shock were seen in 652 patients (50.62%). However, it is important to note that other than cardiogenic shock, vasoplegic shock was also common. Respiratory distress was found in 270 patients (20.96%) [13,[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38]].
3.2.2. Cardiac biomarkers
-
a.
Brain natriuretic peptide (BNP) and N-terminal brain natriuretic peptide (NT-pro BNP)
Data on brain natriuretic peptide (BNP) was available in 4 studies, whereas NT-proBNP was available in 10 studies. The pooled mean (SD) of the BNP level from 84 patients was 4238.35 (5803.24) pg/mL. The pooled mean (SD) of NT-pro-BNP level from 10 studies (n = 803 patients) was 13,590.78 (20,676.15) pg/mL.
-
b.
Troponins
Among various troponin markers used as an indicator of cardiac injury (high-sensitivity [HS] troponin I, troponin I, troponin T, and unexplained specifically troponin), data showed 50 out of 67 patients (74.6%) had elevated troponin I levels [18,23,27,33], 61 out of 68 patients (89.71%) had elevated high sensitivity troponin I [25,28,31], and 335 out of 778 patients (46.6%) had elevated unspecified troponin [20,24,29,32,34,35,40]. In addition, the pooled mean (SD) of the troponin I, troponin T, and high sensitivity troponin I were 869 (1229) [18,23,33], 75.8 (132) [[36], [37], [38]], 1183 (3307) [25,28,31], respectively.
3.2.3. Chest radiography
Based on 9 studies of patients that underwent chest radiography (n = 751), 164 patients experienced cardiomegaly or pericardial effusion (21.83%), 105 patients with pleural effusion (13.98%), and 169 patients with focal or bilateral opacity (22.5%), 16 patients with lung edema (2.13%) [[17], [18], [19],21,22,28,30,33,37].
3.2.4. Electrocardiographic abnormalities
Based on 212 patients with data available on electrocardiography, pooled from 7 studies, most abnormality found was ST segment abnormalities (n = 34) 16.04%. In addition, other notable findings were abnormal T waves (n = 21) 9.91%, AV block (n = 5) 2.36%, and ventricular arrhythmia (n = 4) 1.89% [13,17,28,[33], [34], [35],38].
3.2.5. Echocardiography
Five studies comprising 139 subjects reported the coronary artery size based on the Z score [27,30,33,35,36]. The total of abnormal coronary arteries, defined as dilatation with a Z score of 2 - < 2.5, was 9 patients and an aneurysm with a Z score of >2.5 was 18 patients. Moreover, data on ejection fraction (EF) was available in 13 studies with a total of 305 patients, with more than 59.02% of them (n = 180) had EF less than 55%. In addition, there were pericardial effusion or pericarditis (n = 221), myocarditis (n = 196), mitral regurgitation (n = 175), left ventricular dysfunction (n = 107) tricuspid regurgitation (n = 19), coronary echogenicity (n = 17), right ventricular dysfunction (n = 17), and biventricular dysfunction (n = 1).) [13,14,[17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]].
3.2.6. Cardiac magnetic resonance imaging (CMR)
Only one case series [14] consists of 4 patients providing detailed descriptions of cardiac findings of PIMS-TS on MRI. There were elevations in T1 mapping values and T2-Short Tau Inversion Recovery (STIR) ratio, which suggest myocardial hyperemia and edema without evidence of fibrosis replacement at LGE at the acute disease phase. Moreover, pericardial effusions were present in 3 out of 4 patients. Nonetheless, the patient with negative pericardial effusion underwent CMR after being discharged from the hospital.
3.3. Treatment characteristics
A full description of treatment characteristics is available in Table 3 .
Table 3.
Treatment characteristics.
| Treatment | Agents | Number of Study | n/N | % |
|---|---|---|---|---|
| Immunosuppressant | IVIG | 20 | 845/1727 | 48.9 |
| Corticosteroid | 19 | 663/1259 | 52.7 | |
| Aspirin / Antiplatelet | 11 | 480/848 | 56.6 | |
| TNF-α antagonist/ Infliximab | 3 | 16/169 | 9.5 | |
| Anti CD20/ Rituximab | 1 | 1/78 | 1.3 | |
| Il-1 antagonist/ Anakinra | 9 | 30/295 | 10.2 | |
| IL-6 antagonist/ Tocilizumab | 7 | 43/207 | 20.8 | |
| Vasopressor | Vasoactive medication | 15 | 477/1010 | 47.2 |
| Fluid resuscitation | 2 | 21/36 | 58.3 | |
| Antibiotics | Broad spectrum antibiotic | 6 | 176/193 | 91.2 |
| Anticoagulants | Prophylactic anticoagulants | 4 | 62/143 | 43.4 |
| Nonspecific anticoagulants | 2 | 241/598 | 40.3 | |
| Therapeutic anticoagulants | 6 | 92/205 | 69.1 | |
| Investigative drugs | Remdesivir | 4 | 13/141 | 9.2 |
| Hydrochloroquine | 2 | 3/31 | 9.7 | |
| Diuretic | Furosemide/ diuretic | 1 | 21/33 | 63.63 |
| Oxygen therapy | Oxygen therapy | 1 | 13/27 | 48.2 |
| High flow nasal canule O2 | 5 | 44/201 | 21.9 | |
| Non-invasive ventilation | 9 | 77/342 | 22.5 | |
| Invasive ventilation | 16 | 144/504 | 27.3 | |
| Respiratory support (unspecified) | 2 | 208/614 | 33.9 | |
| Intra-aortic balloon pump | 1 | 1/33 | 3 | |
| ECMO | 6 | 21/252 | 11.2 | |
| Renal replacement therapy | 2 | 3/614 | 0.5 | |
| Antiarrhythmic | Amiodarone + Lidocaine | 1 | 1/15 | 6.7 |
| Others | Convalescent plasma | 4 | 5/73 | 6.8 |
IVIG; intravenous immunoglobulin, TNF; tumor necrosis factor, IL-1; interleukin-1, IL-6; interleukin-6, ECMO; extra corporeal membrane oxygenation.
3.3.1. Immunosuppressant and anti-inflammatory drugs
Among all patients, 845 received intravenous immunoglobulin (IVIG), 663 received corticosteroid, and 480 received aspirin [13,[16], [17], [18], [19], [20],[23], [24], [25], [26], [27], [28], [29], [30], [31],[33], [34], [35], [36], [37], [38]].
In addition, cytokine inhibitors; TNF- α antagonist/ Infliximab, IL-1 antagonist/ Anakinra, and IL-6 antagonist/ Tocilizumab were sparingly used in 16 [20,35,36], 30, and 43, respectively [13,20,[26], [27], [28],31,[35], [36], [37], [38]].
3.3.2. Vasoactive drugs and volume expander
Of 1010 patients, 477 received vasoactive agents [13,[17], [18], [19],23,24,[26], [27], [28], [29], [30],[33], [34], [35],37], including dobutamine, norepinephrine, epinephrine, vasopressin, milrinone, and dopamine. In addition, volume expander/fluid resuscitation, indicated for refractory shock/ hypotension, was used in 21 patients [30,33]. Of note, patients might receive more than one vasoactive agent.
3.3.3. Antibiotics
Of 193 patients, 176 received empirical antibiotics, such as vancomycin, meropenem, ceftriaxone, cefepime, metronidazole, linezolid, and cefazolin [20,23,28,30,33,37].
3.3.4. Anticoagulants
Out of 845 patients, 92 patients were therapeutically anticoagulated, 62 patients were prophylactically anticoagulated, and 241 patients received nonspecific anticoagulants. [13,19,20,23,24,27,28,37,38]
3.3.5. Mechanical supports
Of 252 patients, 21 patients received extracorporeal membrane oxygenation (ECMO) [13,26,28,29,35,37]. Intra-aortic balloon pump (IABP) was utilized in one patient [18]. Moreover, 144 and 77 patients were invasive [13,17,21,23,24,26,[28], [29], [30], [31], [32], [33], [34], [35],37] and non-invasively ventilated [13,17,24,28,29,31,32,34,37]. Renal replacement therapy (RRT), was also utilized in three patients [19,32].
3.3.6. Other treatments
Diuretics were also utilized in 21 patients [37]. Furthermore, two putative antiviral drugs, namely remdesivir and hydroxychloroquine, were used in thirteen patients [20,27,28,37] and 3 patients [28,31], respectively.
3.4. Outcome
Of 1163 patients, 834 were admitted to ICU/PICU [13,16,17,[19], [20], [21], [22], [23],[29], [30], [31],[33], [34], [35], [36], [37], [38]]. On the contrary, of 1260 patients hospitalized, only 25 patients (1.98%) died [13,[16], [17], [18], [19], [20], [21], [22], [23],[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38]]. The mean length of stay (n = 5 studies) was 6.02 (2.93 SD) days [20,29,30,36,37]. Of 153 patients that experienced EF reduction during hospitalization, 116 were normalized after discharge. A detailed description of the outcome is provided in Table 4 .
Table 4.
Outcome.
| Variable | Number of study | n/N | Mean (SD) | % | |
|---|---|---|---|---|---|
| Admission to ICU | 14 | 200/262 | 75.5 | ||
| Length of stay (days) | 8 | 128/128 | 8 (5) | 100 | |
| Outcome | Alive | 16 | 271/274 | 98.9 | |
| Death | 3/274 | 1.1 | |||
| LVEF Improvement | Previously decreased LVEF | 7 | 91/244 | 37.3 | |
| Improved LVEF at/after discharged | 69/244 | 28.3 | |||
SD; standard deviation, ICU; intensive care unit, LVEF; left ventricular ejection fraction.
4. Discussion
The life-threatening syndrome associated with delayed COVID-19 disease and mimicking incomplete Kawasaki disease (KD) and toxic shock syndrome has been identified as PIMS-TS or a multisystem inflammatory syndrome in children (MIS-C) [[8], [9], [10], [11]]. Although rare, this syndrome remains an important source of morbidity and mortality of pediatric COVID-19 patients. In addition, the heart is one of the major organs affected by this syndrome.
In our review, the mean age (SD) was 8.6 (5.5) years old (n = 1188). In addition, African descent makes up more than 30% of all ethnicities. Contrastingly, the incidence of typical KD exclusively occurred in East Asian ethnicity, with the peak age at 2.5 years old [41]. Older children that were affected by PIMS-TS in comparison to KD might imply more developed immunity as underlying pathophysiology [42]. Indeed, one case series found that along with ferritinemia, age > 5 years were a remarkable prognosticator of a severe course [31].
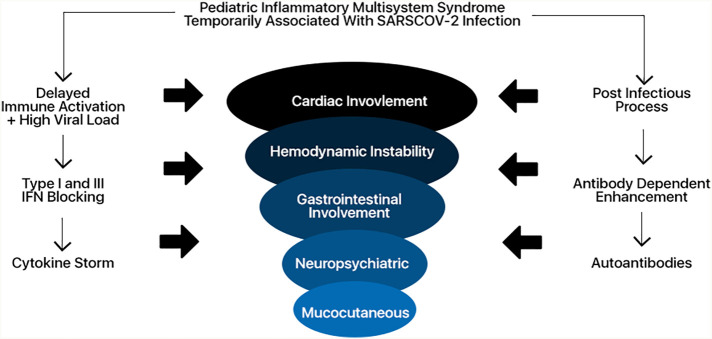
Several mechanistic underpinnings may explain the occurrence of PIMS – TS. First, SARS-CoV-2 can block type I and III interferon responses, which subsequently cause delayed cytokine storms in patients with high virus load of SARS-CoV-2 or uncontrolled viral replication [43,44]. Alternative hypothesis is proposed antibody-dependent enhancement as the mechanistic underpinnings of COVID-19 [42,45]. However, this hypothesis has several shortcomings that could not explain, such as why there is no report of an apparent clinical problem in COVID-19 patients who received convalescent plasma as a part of their treatment [42]. The third one is autoantibodies mediated injury. Several autoantibodies exclusively seen in PIMS-TS patients possibly explain the pathogenesis of PIMS-TS, as the targets of these autoantibodies are broadly expressed across tissues [46]. Another group showed that two autoantibodies commonly detected in other autoimmunities, anti-La, and anti-Jo-1, were enriched in PIMS-TS patients [47]. Thus, others have postulated that PIMS-TS is immunologically similar to acute rheumatic fever [48] (Fig. 2 ).
Fig. 2.
Mechanistic underpinnings of Pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2.
Furthermore, findings of cardiac involvement, in terms of signs and symptoms, electrocardiography, chest radiography, cardiac biomarkers as well as echocardiography in the majority of PIMS-TS patients reflect a high prevalence of myocarditis/ acute myocardial dysfunction in this population. A large number of patients with signs of shock and needed vasopressors support also corroborated this. In contrast, shock or vascular compromise affects less than 10% of patients with Kawasaki's disease [49]. Strikingly, more cardiovascular involvement is observed based on a multimodality cardiac evaluation of PIMS-TS patients, which consists of left ventricular dysfunction, myocardial edema, and coronary artery changes even after defervescence [39].
Moreover, coronary artery involvement incidence is higher (60%) compared to their Kawasaki counterparts (23–50%) [50,51]. The pattern of coronary involvement is also different from Kawasaki disease, reinforcing PIMS-TS as an entity of its own and not part of the Kawasaki disease spectrum [39].
In acute COVID-19 infection, incidences of acute cardiac injury have been well documented [4]. Regardless, a shared underlying pathomechanism of cardiac dysfunction between PIMS-TS and during acute COVID-19 infection is unlikely due to the temporal differences. This is reinforced by the cardiac MRI findings of PIMS-TS patients which showed diffuse myocardial edema without replacement fibrosis or focal necrosis [14]. Therefore, it is a distinct clinical entity and should be distinguished from the acute COVID-19 infection.
Our review also provided detailed descriptions of the treatment characteristics of PIMS-TS patients. The majority of patients received immunosuppressant, which mostly constitutes IVIG. In Kawasaki disease, treatment with IVIG is effective for the majority of cases [44,45]. Indeed, the treatment with immunoglobulin was associated with improved left ventricular systolic function in PIMS-TS [13].
Corticosteroid, which was the second most commonly used immunosuppressant, was used in 156 patients. Two studies highlighted that this immunomodulator was considered in high-risk patients with symptoms similar to an incomplete form of Kawasaki disease [13] and the presence of cytokine storm with Kobayashi score of 5 [18]. Acetylsalicylic acid was used in 90 patients. Varying doses of aspirin were also used, with 30–80 mg/Kg used for anti-inflammatory and lower dose for anti-aggregative [31]. Cytokine inhibitors which constitute TNF-α antagonist, IL-1 antagonist, and IL-6 antagonist were also sparingly used in 16 [20,35,36], 30, and 43, respectively [13,20,[26], [27], [28],31,[35], [36], [37], [38]]. Mainly due to persistent inflammatory state, respiratory distress, and extremely elevated CRP [13,31,35].
Nevertheless, high survival rates of PIMS-TS patients in the paucity of high-quality evidence should be carefully attributed to these biological agents as cytokine storm in COVID-19 is not as severe as the cytokine storm in other infectious processes. This can explain the futility of Tocilizumab (anti-IL-6 antibody) in randomized controlled trials for hospitalized COVID-19 patients [[52], [53], [54]]. Other cytokine profiles (IL1, TNF alpha, IL8) were much lower in severe-critical COVID-19 than sepsis, ARDS, or cytokine release syndrome related to Chimeric Antigen Receptor T Cell (CAR-T) cell therapy [55].
Indeed, system-level analyses of blood immune cells, cytokines, and autoantibodies of PIMS-TS patients confirmed this [46]. Nonetheless, the role of aberrant adaptive immune response activation in PIMS-TS patients is evident, as treatment with intravenous immunoglobulin (IVIG) results in the resolution of the disease [47].
The need for vasopressors and fluid resuscitation was not uncommon, whereas only 21 patients needed veno-arterial/ veno-venous ECMO, and one patient needed an intra-aortic balloon pump as mechanical circulatory supports. Of note, patients might receive more than one type of vasopressors, and comparable amounts of norepinephrine and epinephrine were used, signifying the incidence of cardiogenic and vasoplegic shock is not uncommon.
More than 90% of the total patients received empirical antibiotics. Indeed, an initial broad-spectrum antibiotic was endorsed because symptoms overlap between PIMS-TS and severe bacterial infection [46]. Therefore, empirical broad-spectrum antibiotics seem rational with the uncertainty of diagnosis. We also noted that almost half (44.2%) of patients were anticoagulated, prophylactically, or therapeutically, with the majority receiving enoxaparin. Recently, the American College of Rheumatology has published a treatment guideline for MIS-C patients, specifically antiplatelet and anticoagulation therapy. With limited evidence, this guideline can provide interim guidance for clinicians [56].
Finally, we have defined the outcome of PIMS-TS patients. The mean length of stay was 6 days, and although patients' ejection fraction was decreased (n = 153), the majority (n = 116) reverted to normal at discharge. Out of 1163 patients, 834 patients were admitted to the intensive care unit, which underscores this syndrome's critical nature. Nevertheless, this syndrome has an excellent prognosis, with 25 out of 1260 patients die. Of interest, all studies were reported in developed countries, implicating that the wide range of pharmacologic and non-pharmacologic armamentarium in treating this complex syndrome is readily available, which might not be the case in developing countries.
Several limitations of this review exist due to the nature of case series. First, we could not rule out the possibility of selection bias, i.e., whether all cases or only a few selected individuals are described. Second, we could not confirm whether all case-series are self-reported or through clinical records, billing, or administrative codes, in which the latter three are more reliable than the first. Therefore, ascertainment bias could not be ruled out. Third, reliable conclusions could not be drawn, and more prospective observational cohort studies are needed. Nevertheless, our systematic review compiles 26 studies, which will help with the rapid dissemination of these important findings.
In conclusion, PIMS-TS is a severe but rare clinical syndrome associated with a multiorgan system dysfunction. It mandates a higher level of care, which often needs advanced circulatory and respiratory supports. Nevertheless, when prompt recognition and appropriate treatments are available, the prognosis is generally favorable, and the cardiac function rapidly reverted to normal. Moreover, medium and long-term follow-up is needed because cardiac abnormalities persist despite defervescence and normalization of inflammatory markers. Shortly, we should be able to define better the clinical characteristics for risk stratifications, elucidate the pathomechanisms, evaluate the safety and efficacy of therapies and outcomes through results from large studies, such as the DIAMONDS and the ISARIC [57].
CRediT authorship contribution statement
Joshua Henrina: Conceptualization, Formal Analysis, Methodology, Writing – Original Draft, Project Administration
Iwan Cahyo Santosa Putra: Data Curation, Writing – Review & Editing
Sherly Lawrensia: Data Curation, Writing – Review & Editing
Della Sabrina Marta: Data Curation, Writing – Review & Editing
Ellen Wijaya: Writing – Review & Editing
Aninka Saboe: Writing – Review & Editing
Charlotte Johanna Cool: Writing – Review & Editing
Leonardo Paskah Suciadi: Writing – Review & Editing
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ppedcard.2021.101365.
Appendix A. Supplementary data
Supplementary material
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yonas E, Alwi I, Pranata R, Huang I, Lim MA, Gutierrez EJ, et al. Effect of heart failure on the outcome of COVID-19 — a meta analysis and systematic review. Am J Emerg Med 2020;0. doi: 10.1016/j.ajem.2020.07.009. [DOI] [PMC free article] [PubMed]
- 4.Santoso A., Pranata R., Wibowo A., Al-Farabi M.J., Huang I., Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moccia F., Gerbino A., Lionetti V., Miragoli M., Munaron L.M., Pagliaro P. COVID-19-associated cardiovascular morbidity in older adults: a position paper from the Italian Society of Cardiovascular Researches. Geroscience. 2020;42:1021–1049. doi: 10.1007/s11357-020-00198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castagnoli R., Votto M., Licari A., Brambilla I., Bruno R., Perlini S., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 7.Zachariah P., Johnson C.L., Halabi K.C., Ahn D., Sen A.I., Fischer A., et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a Children’s Hospital in New York City. New York JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet Lond Engl. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19 n.d. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed July 29, 2020).
- 10.Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019. 2020;COVID-19 https://emergency.cdc.gov/han/2020/han00432.asp [Google Scholar]
- 11.Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19. RCPCH n.d. https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims (accessed July 29, 2020).
- 12.Rauf A., Vijayan A., John S.T., Krishnan R., Latheef A. Multisystem inflammatory syndrome with features of atypical Kawasaki disease during COVID-19 pandemic. Indian J Pediatr. 2020 doi: 10.1007/s12098-020-03357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belhadjer Zahra, Méot Mathilde, Bajolle Fanny, Khraiche Diala, Legendre Antoine, Abakka Samya, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation n.d.;0. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed]
- 14.Blondiaux E., Parisot P., Redheuil A., Tzaroukian L., Levy Y., Sileo C., et al. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology. 2020;202288 doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G. PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biko D.M., Ramirez-Suarez K.I., Barrera C.A., Banerjee A., Matsubara D., Kaplan S.L., et al. Imaging of children with COVID-19: experience from a tertiary children’s hospital in the United States. Pediatr Radiol. 2020:1–9. doi: 10.1007/s00247-020-04830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swann O.V., Holden K.A., Turtle L., Pollock L., Fairfield C.J., Drake T.M., et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370 doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. The Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shana G-C, Bobbi B, Jessica L, Matthew EO, L C, Joseph A, et al. COVID-19-Associated Multisystem Inflammatory Syndrome in Children - United States, March–July 2020. MMWR Morb Mortal Wkly Rep 2020;69:1074–80. doi:10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed]
- 20.Davies P., Evans C., Kanthimathinathan H.K., Lillie J., Brierley J., Waters G., et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;S2352464220302157 doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blumfield E., Levin T.L. COVID-19 in pediatric patients: a case series from the Bronx. Pediatr Radiol. 2020;50:1369–1374. doi: 10.1007/s00247-020-04782-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumfield E., Levin T.L., Kurian J., Lee E.Y., Liszewski M.C. Imaging findings in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. AJR Am J Roentgenol. 2020 doi: 10.2214/AJR.20.24032. [DOI] [PubMed] [Google Scholar]
- 23.Torres J.P., Izquierdo G., Acuña M., Pavez D., Reyes F., Fritis A., et al. Multisystem inflammatory syndrome in children (MIS-C): report of the clinical and epidemiological characteristics of cases in Santiago de Chile during the SARS-CoV-2 pandemic. Int J Infect Dis. 2020;100:75–81. doi: 10.1016/j.ijid.2020.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsubara D., Kauffman H.L., Wang Y., Calderon-Anyosa R., Nadaraj S., Elias M.D., et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol. 2020;76:1947–1961. doi: 10.1016/j.jacc.2020.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamishi S., Movahedi Z., Mohammadi M., Ziaee V., Khodabandeh M., Abdolsalehi M.R., et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol Infect. 2020;148 doi: 10.1017/S095026882000196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly M.S., Valle C.W., Fernandes N.D., Cummings B.M., Lahoud-Rahme M., Chiu J.S. Multisystem inflammatory syndrome in children: cardiac biomarker profiles and echocardiographic findings in the acute and recovery phases. J Am Soc Echocardiogr. 2020;33:1288–1290. doi: 10.1016/j.echo.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jhaveri S., Ahluwalia N., Kaushik S., Trachtman R., Kowalsky S., Aydin S., et al. Longitudinal echocardiographic assessment of coronary arteries and left ventricular function following multisystem inflammatory syndrome in children. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.M R-C, E A, E B-B, S K, J R, R P, et al. Multisystem inflammatory syndrome in children related to COVID-19: A New York City experience. J Med Virol 2020. doi: 10.1002/jmv.26224. [DOI] [PMC free article] [PubMed]
- 29.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J., et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369 doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pouletty M., Borocco C., Ouldali N., Caseris M., Basmaci R., Lachaume N., et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantor A, Miller J, Zachariah P, DaSilva B, Margolis K, Martinez M. Acute Hepatitis Is a Prominent Presentation of the Multisystem Inflammatory Syndrome in Children: A Single-Center Report. Hepatology n.d.;n/a. doi: 10.1002/hep.31526. [DOI] [PMC free article] [PubMed]
- 33.Ramcharan T., Nolan O., Lai C.Y., Prabhu N., Krishnamurthy R., Richter A.G., et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK Tertiary Paediatric Hospital. Pediatr Cardiol. 2020:1–11. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimaud M., Starck J., Levy M., Marais C., Chareyre J., Khraiche D., et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capone C.A., Subramony A., Sweberg T., Schneider J., Shah S., Rubin L., et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS-C) associated with SARS-CoV-2 infection. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaushik S., Aydin S.I., Derespina K.R., Bansal P.B., Kowalsky S., Trachtman R., et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection: a multi-institutional study from New York City. J Pediatr. 2020;S0022347620307472 doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheung E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S., et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theocharis P, Wong J, Pushparajah K, Mathur SK, Simpson JM, Pascall E, et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J - Cardiovasc Imaging n.d. doi: 10.1093/ehjci/jeaa212. [DOI] [PMC free article] [PubMed]
- 40.Pereira M.F.B., Litvinov N., Farhat S.C.L., Eisencraft A.P., Gibelli M.A.B.C., de Carvalho W.B., et al. Severe clinical spectrum with high mortality in pediatric patients with COVID-19 and multisystem inflammatory syndrome. Clinics. 2020;75 doi: 10.6061/clinics/2020/e2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dietz S.M., van Stijn D., Burgner D., Levin M., Kuipers I.M., Hutten B.A., et al. Dissecting Kawasaki disease: a state-of-the-art review. Eur J Pediatr. 2017;176:995–1009. doi: 10.1007/s00431-017-2937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowley A.H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020;20:453–454. doi: 10.1038/s41577-020-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park A, Iwasaki A. Type I and Type III Interferons – induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 2020;27:870–8. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed]
- 44.Blanco-Melo D, Nilsson-Payant BE, Liu W-C, Uhl S, Hoagland D, Møller R, et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020;181:1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed]
- 45.Piram M., Bello M.D., Tellier S., Filippo S.D., Boralevi F., Madhi F., et al. Defining the risk of first intravenous immunoglobulin unresponsiveness in non-Asian patients with Kawasaki disease. Sci Rep. 2020;10:3125. doi: 10.1038/s41598-020-59972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020;183:968–981.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed]
- 47.Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F, et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020;183:982–995.e14. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed]
- 48.Buonsenso D., Riitano F., Valentini P. Pediatric inflammatory multisystem syndrome temporally related with SARS-CoV-2: immunological similarities with acute rheumatic fever and toxic shock syndrome. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanegaye J.T., Wilder M.S., Molkara D., Frazer J.R., Pancheri J., Tremoulet A.H., et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123:e783–e789. doi: 10.1542/peds.2008-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newburger J.W., Takahashi M., Beiser A.S., Burns J.C., Bastian J., Chung K.J., et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 51.Dallaire F., Fournier A., Breton J., Nguyen T.-D., Spigelblatt L., Dahdah N. Marked variations in serial coronary artery diameter measures in Kawasaki disease: a new indicator of coronary involvement. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2012;25:859–865. doi: 10.1016/j.echo.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 52.Hermine O., Mariette X., Tharaux P.-L., Resche-Rigon M., Porcher R., Ravaud P., et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med 2020;0:null. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed]
- 54.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L., et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med 2020;0. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed]
- 56.Henderson L.A., Canna S.W., Friedman K.G., Gorelik M., Lapidus S.K., Bassiri H., et al. American College of Rheumatology Clinical Guidance for multisystem inflammatory syndrome in children associated with SARS–CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol. 2020;72:1791–1805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet Lond Engl. 2020;395:1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material