Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), may trigger cytokine storm which is responsible for a highly pro inflammatory state in severe cases [1]. COVID-19 is also associated with an endotheliopathy characterized by the increase of von Willebrand factor (VWF) [2] and soluble endothelial markers of activation in plasma from patients with COVID-19 [3,4]. VWF is released from endothelial cells as ultra large VWF multimers (ULVWF) highly adhesive to platelets. To regulate this adhesive function, the metalloprotease with thrombospondin type 1 repeats, member 13 (ADAMTS 13) cleaves ULVWF in the circulation. The high increase in VWF activity and antigen levels reported in patients with COVID-19 is quite similar to those observed in septic patients [5]. In the latter, ADAMTS 13 deficiency has been reported as a prognostic factor for mortality. A moderate decrease of the activity of ADAMTS 13 was reported in patients with severe COVID-19 in the absence of detectable anti-ADAMTS 13 antibodies [6,7]. However, the antigen level of ADAMTS 13 has not been measured in these series. We aimed to measure the antigen level of ADAMTS 13 to appreciate the nature of the deficiency (quantitative or functional). Then, as the proinflammatory cytokine IL-6, greatly increased in COVID-19, may inhibit the proteolytic activity of ADAMTS 13 [8], we aimed to assess the impact of ADAMTS 13 deficiency on the von Willebrand factor multimeric pattern in patients with COVID-19.
We studied 70 patients with COVID-19 pneumonia confirmed by RT-PCR on a nasopharyngeal swab. We measured ADAMTS 13 antigen (Quantikine ELISA human ADAMTS 13, Bio-Techne, France), and plasma VWF activity and antigen (HemosIL Von Willebrand factor activity and antigen assays, Instrumentation Laboratory, France). VWF multimer analysis was performed with Hydragel 5 von Willebrand multimers kit (Sebia, France) using the Sebia Hydrasis 2 instrument. According to the preanalytical recommendations of the Groupe d'étude sur l'hémostase et la thrombose (GEHT), all the assays were performed on samples which have undergone one freeze-thaw cycle except for the determination of the ADAMTS 13 antigen level (two cycles). The blood samples were obtained the day of the admission of patients in our hospital. Patients were categorized into three groups according to whether they had been admitted to an intensive care unit (ICU) or a non-ICU department, or they went home. The study was approved by the local ethics committee of the Foch Hospital (reference 20-07-15).
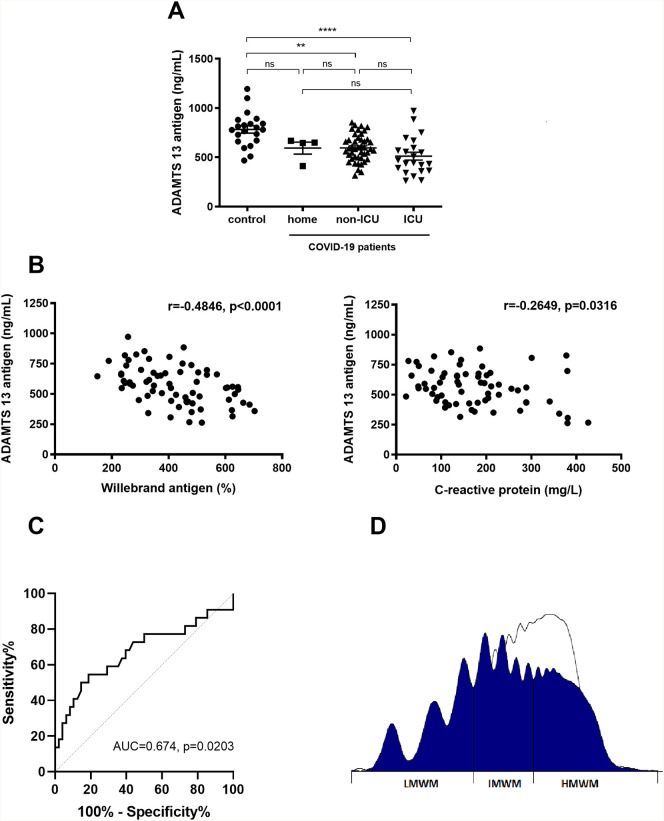
We observed a marked increase in VWF activity and antigen in patients with COVID-19 hospitalized in non-ICU or ICU department compared to the control group composed of healthy hospital workers (Table 1 ). ADAMTS 13 antigen concentrations were significantly lower in patients with COVID-19 hospitalized in non-ICU or ICU department than in controls (Fig. 1A). The ADAMTS 13 antigen deficiency was clearly associated with VWF release as suggested by the significant negative correlation between antigen concentration of ADAMTS 13 and VWF antigen (Spearman r = −0.4846, p < 0.0001) (Fig. 1B left panel). ADAMTS 13 was also correlated with the systemic inflammation since ADAMTS 13 antigen levels were negatively correlated with C-reactive protein (Spearman r = −0.2649, p = 0.0316) (Fig. 1B right panel). Then, we studied the role of ADAMTS 13 concentrations to predict the severity of the disease using the receiver operating characteristic (ROC) curve analysis. We identified ADAMTS 13 below 559 ng/mL as potential inclusion criteria for ICU entrance (AUC 0.674, 95% CI 0.523–0.825, p = 0.0203). Using this cut-off, the ROC curve yielded a sensitivity of 68.2% (95% CI 47.3–83.6) and a specificity of 60.4% (95% CI 46.3–73.0) (Fig. 1C). VWF multimeric pattern was analyzed in 10 patients and showed a decrease in high-molecular-weight multimers (HMWM) (36.0 [33.5–36.7] %, reference intervals 45–67%) in 8 patients associated with a relative increase in intermediate (IMWM) and low-molecular-weight multimers (LMWM) (36.8 [35.1–42.3] %, reference intervals 22–36% and 27.9 [26.5–28.5] %, reference intervals 9–21%, respectively) (Fig. 1D). In these 8 patients the median concentration of ADAMTS 13 antigen was 432 [303–592] ng/mL.
Table 1.
Comparison of biological parameters in patients with COVID-19 according to the department of admission or the prognosis.
| Median (IQR) | Control |
Home |
Non-ICU |
ICU |
Survivor |
Non-survivor |
|---|---|---|---|---|---|---|
| n = 21 | n = 4 | n = 44 | n = 22 | n = 55 | n = 15 | |
| VWF activity (%) (N:50–160%) | 105 (92–139) | 221 (161–448) (158–506)a |
329⁎⁎⁎⁎ (243-393) | 355⁎⁎⁎⁎ (297–416) | 329 (244–380) | 391 (276–448) |
| VWF antigen (%) (N:50–150%) | 116 (100–153) | 297 (170–604) (149–685)a |
390⁎⁎⁎⁎ (288–512) | 456⁎⁎⁎⁎ (402–493) | 407 (286–499) | 463 (348–517) |
| VWF ratio (activity/antigen) (N > 0.7) | 0.92 (0.84–1.0) | 0.75 (0.73–0.99) (0.72–1.06)a |
0.79⁎⁎⁎ (0.73-0.85) | 0.78⁎⁎ (0.72–0.88) | 0.78 (0.72–0.85) | 0.81 (0.77–0.85) |
| ADAMTS 13 antigen (ng/mL) (N:439–1155 ng/mL) | 808 (715–884) | 648 (471–663) (412–668)a |
588⁎⁎ (495-681) | 458⁎⁎⁎⁎ (364–615) | 584 (480–682) | 453 (392–596) |
Results are expressed as median and interquartile range (between brackets). We compared the biological variables between the four groups (control, home, non-ICU and ICU) using the Kruskal-Wallis test followed by Dunn's posttest. Between survivors and non survivors quantitative variables were compared using the Mann-Whitney U test. A P-value of <0.05 was considered to be statistically significant.
ICU Intensive care unit.
p < 0.01 versus control group.
p < 0.001 versus control group.
p < 0.0001 versus control group.
For this small group, in addition to median (IQR) results were expressed as total range (min-max).
Fig. 1.
(A) ADAMTS 13 antigen concentration in patients with COVID-19 compared to 21 healthy controls. ADAMTS 13 concentration were compared between the four groups (control, home, non-ICU and ICU) using the Kruskal-Wallis test followed by Dunn's posttest. ** p < 0.01 versus control group; ****p < 0.0001 versus control group, (B) Correlation between ADAMTS 13 antigen level and Willebrand antigen or C-reactive protein. Correlations were assessed using the Spearman coefficient correlation test. (C) ROC curve analysis of ADAMTS 13 level to predict ICU admission. (D) Distribution of von Willebrand factor multimers in a representative plasma from patient with COVID-19 (dark area) using agarose gel electrophoresis, compared to control (white area). VWF antigen of 517%, ADAMTS 13 antigen of 263 ng/mL, densitometry LMWM 28.1% (reference intervals 9–21%), IMWM 37.9% (reference intervals 22–36%) and HMWM 34% (reference intervals 45–67%).
Ns: not significant; R: Spearman correlation coefficient; ICU: intensive care unit; AUC: Area under curve; LMWF: Low molecular weight multimers; IMWM: Intermediate molecular weight multimers; HMWM: High molecular weight multimers.
In our series of patients with COVID-19 we observed an imbalance between a marked increase in VWF levels associated with a moderate deficiency in ADAMTS 13. The strong VWF release related to the acute endothelial stimulation observed in both sepsis and COVID-19 could explain the consumption of the ADAMTS 13 as suggested by the negative correlation between the VWF antigen levels and the ADAMTS 13 levels. However, in patients with COVID-19, ADAMTS 13 deficiency contrasts with the decrease in HMWM associated with a relative increase of cleaved multimers of VWF suggesting that ADAMTS 13 remains functional. The decrease in HMWM may be partially explained by the consumption of larger multimers of VWF, highly adhesive to platelets as seen in the acute phase of thrombotic thrombocytopenic purpura [9]. However, the microangiopathy of COVID-19 does not resemble that of TTP, since there is no major thrombocytopenia or hemolytic anemia. In our series, we observed a thrombocytopenia <150 × 109 per L in only 23% of patients at the admission with a median of 128 × 109 per L (ranging from 63 to 148 × 109/L). Thus, the massive release of VWF related to the endothelial stimulation and the mild deficiency in ADAMTS 13 could contribute, at least locally, to the pulmonary microthrombi formation. The presence of small platelet rich thrombi within small vessels and alveolar capillaries reported by Fox et al. [10] in lung from patients with COVID-19 may supports this hypothesis. Even though the ADAMTS 13 levels are moderately decrease in patients with COVID-19, this could raise the question of supplementation in ADAMTS 13 in patients with COVID-19. VWF and the regulation of the size of its multimers by ADAMTS 13 were shown to be implicated in inflammation: ADAMTS 13 reduces VWF-mediated acute inflammation and in ADAMTS 13 knocked-out mice, an enhanced general leukocyte recruitment was observed [11]. This VWF-dependent anti-inflammatory aspect of ADAMTS 13 could be particularly interesting in patients with COVID-19.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
We would like to express our gratitude to nurses and physicians who have taken care of the patients in the ICU and departments dedicated to COVID-19 in Foch hospital. Special thanks to the laboratory technicians S Graffin and R Bironien for the von Willebrand factor multimeric pattern analysis.
References
- 1.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. Published online May. 2020;4:1–10. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smadja D.M., Guerin C.L., Chocron R., et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. May 27, 2020:1–10. doi: 10.1007/s10456-020-09730-0. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peigne V., Azoulay E., Coquet I., et al. The prognostic value of ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13) deficiency in septic shock patients involves interleukin-6 and is not dependent on disseminated intravascular coagulation. Crit. Care. 2013;17(6):R273. doi: 10.1186/cc13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinelli N., Montagnana M., Pizzolo F., et al. A relative ADAMTS13 deficiency supports the presence of a secondary microangiopathy in COVID 19. Thromb. Res. 2020;193:170–172. doi: 10.1016/j.thromres.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bazzan M., Montaruli B., Sciascia S., et al. Low ADAMTS 13 plasma levels are predictors of mortality in COVID-19 patients. Intern Emerg Med. June 18, 2020:1–3. doi: 10.1007/s11739-020-02394-0. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernardo A., Ball C., Nolasco L., et al. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104(1):100–106. doi: 10.1182/blood-2004-01-0107. [DOI] [PubMed] [Google Scholar]
- 9.Lotta L.A., Lombardi R., Mariani M., et al. Platelet reactive conformation and multimeric pattern of von Willebrand factor in acquired thrombotic thrombocytopenic purpura during acute disease and remission. J. Thromb. Haemost. 2011;9(9):1744–1751. doi: 10.1111/j.1538-7836.2011.04428.x. [DOI] [PubMed] [Google Scholar]
- 10.Fox Sharon E., Akmatbekov Aibek, Harbert Jack L., Li Guang, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir. Med. 2020 Jul;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauhan A.K., Kisucka J., Brill A., et al. ADAMTS13: a new link between thrombosis and inflammation. The Journal of Experimental Medecine. 2008;205(9):2065–2074. doi: 10.1084/jem.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]