Dear Editor,
Corticosteroids mitigate 28-day all-cause mortality in coronavirus disease-2019 (COVID-19) patients requiring oxygen or mechanical ventilation (meta-analysis summary odds ratio (OR), 0.66; 95%-confidence interval (95%IC), [0.53–0.82]; P < 0.001); however, mortality remains high (32.7%).1 In a previous observational cohort study, we established that an early 4-day treatment combining corticosteroid (prednisolone dose equivalent, 1.25 mg/kg/24 h) and furosemide (80 mg/day) was effective in reducing the need for mechanical ventilation and overall mortality (OR, 0.35 [0.11–1.01]; P = 0.04) in non-critically ill COVID-19 patients.2
The GRECCO-19 randomized trial suggested a benefit of colchicine in preventing clinical deterioration in hospitalized non-critically ill COVID-19 patients.3 Similarly, an observational cohort study reported that salicylate treatment was associated with reduction in intensive care unit (ICU) and mechanical ventilation requirements in hospitalized COVID-19 patients, although in-hospital death was not significantly modified.4 Moreover, prophylactic or intermediate-dose anticoagulation was highly recommended in hospitalized COVID-19 patients who are at high-risk of venous thromboembolic events (VTE).5 Specifically, direct oral anticoagulant use was shown to be associated with improved outcome.6
Based on the data discussed above and the pathophysiology of COVID-19 and its complications, i.e. thrombosis, inflammation and congestion, we hypothesized that a five-drug regimen consisting in a 5-day course of 1 mg/kg/day prednisone, 80 mg/day furosemide, 75 mg/day salicylate, colchicine (1 mg loading dose followed by 0.5 mg one hour later then 0.5 mg every 8 h as recommended to treat acute gout)7 and direct anti-Xa inhibitor such as rivaroxaban or apixaban would optimally mitigate COVID-19-attributed mortality. To address the effectiveness of this five-drug regimen, we designed an observational cohort study (COrtiCoid-Aspirin-Anticoagulant-Colchicine-LAsixⓇ, the COCAA-COLA study) including all successive non-critically ill COVID-19 patients requiring >1 L/min-oxygen and admitted to our ward between 2020/01/09 and 2020/11/30 (during the second wave in France). Patients who did not receive this regimen were treated with dexamethasone (6 mg once daily for up to 10 days)8 and low-molecular weight heparin (control group). All patients received standard of care, i.e. oxygen with flow adapted to oximetry, proton pump inhibitor, antibiotics, insulin, potassium supplementation and loperamide if needed. No antiviral or additional immunomodulatory therapy was used due to the absence of clearly demonstrated benefit. Systematic chest computed tomography angiography was performed on admission if not contra-indicated. Anticoagulants (direct anti-Xa inhibitor in the five-drug regimen-treated patients or low-molecular weight heparin in the others) were administered at prophylactic dose with the exception of patients exhibiting VTE or plasma d-dimer ≥5000 ng/mL (a threshold predicting increased VTE risk in COVID-19 patients)9 who were administered anticoagulants at therapeutic dose. Usual monitoring including pulse oximetry, electrocardiogram, finger blood sugar and daily routine chemical tests was provided.
The primary composite endpoint was requirement of high-flow oxygen therapy, non-invasive or invasive mechanical ventilation (corresponding to care escalation from ward to ICU) or 28-day mortality. The 4C Mortality Score, a risk stratification score for hospitalized COVID-19 patients, was used to predict in-hospital mortality.10 Data were expressed as median [25th-75th percentiles] or percentages. Univariate comparisons were performed using Mann-Whitney or Fisher exact tests, as appropriate. A multivariate logistic regression model was tested with the five-drug regimen as explanatory variable and adjustment for independent covariates (gender, age, body-mass index and comorbidities) to explain the outcome. Odds ratios (OR) and their 95%CI were determined. Stratified categorical data were compared using Cochran-Mantel-Haenszel tests. P-values≤0.05 were considered significant. Analyses were preformed using the R4.0 environment.
We included sixty-eight patients (age, 66years [54–75]; male/female sex-ratio, 3.5; body-mass index, 27 kg/m² [24–30]; hypertension, 46%; diabetes mellitus, 44%; cardiovascular disease, 29%; chronic lung disease, 3%). Twenty-eight patients (41%) received the five drug-therapy regimen whereas forty (59%) were included in the control group. Based on the 4C Mortality Score (10 [8–12]), predicted mortality on admission was ∼30%. No significant differences were observed between the groups regarding the clinical and biological characteristics and the predicted mortality (Table 1 ). Noteworthy, 4/40 control patients (10%) at risk of cardiogenic pulmonary edema (serum brain natriuretic peptide (BNP) ≥100 ng/mL) received furosemide.
Table 1.
Characteristics of the COVID-19 patients treated or not treated with the five-drug regimen combining prednisone, furosemide, salicylate, colchicine and direct anti-Xa inhibitor. Data are presented as percentages or medians [percentiles 25th-75th]. Comparisons were performed using Mann-Whitney or Fisher exact tests, as appropriate.
|
Patients not receiving the five-drug regimen (N = 40) |
Patients receiving the five-drug regimen (N = 28) |
P |
|
| Demographics and past medical history | |||
| Age (year) | 64 [49–73] | 68 [62–78] | 0.06 |
| Male gender, N (%) | 33 (83) | 20 (71) | 0.37 |
| Body-mass index (kg/m²) | 28 [25–31] | 26 [24–28] | 0.13 |
| Hypertension, N (%) | 16 (40) | 15 (54) | 0.32 |
| Diabetes mellitus, N (%) | 15 (38) | 15 (54) | 0.22 |
| Cardiovascular disease, N (%) | 11 (28) | 9 (32) | 0.78 |
| Chronic lung disease, N (%) | 1 (3) | 1 (4) | 1 |
| Clinical and biological parameters on admission | |||
| Symptom duration (day) | 8 [4–11] | 8 [7–10] | 0.99 |
| 4C Mortality Score | 9 [6–12] | 10 [9–12] | 0.08 |
| SpO2 at room air (%) | 92 [91–96] | 94 [91–95] | 0.65 |
| PaO2 at room air (mmHg) | 63 [58–72] | 65 [58–74] | 0.72 |
| Crazy paving area on CT-scan (%) | 50 [25–50] | 50 [25–50] | 0.75 |
| Proximal/segmental pulmonary embolism diagnosed on CT-scan, N (%) | 4 (10) | 1 (4) | 0.64 |
| C-reactive protein (mg/L) | 97 [60–165] | 86 [61–126] | 0.68 |
| Procalcitonin (µg/L) | 0,14 [0.06–0.25] | 0,11 [0.07–0.22] | 0.73 |
| White blood cells (G/L) | 7.0 [4.9–9.5] | 6.3 [4.8–7.4] | 0.28 |
| Lymphocytes (G/L) | 1.0 [0.7–1.2] | 0.9 [0.7–1.2] | 0.88 |
| Brain natriuretic peptide (ng/L) | 19 [10–52] | 38 [13–111] | 0.12 |
| Brain natriuretic peptide ≥ 100 ng/L | 8 (20) | 8 (29) | 0.56 |
| Troponin Ic high-sensitivity (ng/mL) | 9 [4–20] | 9 [4–31] | 0.90 |
| D-dimer (ng/mL) | 935 [578–1402] | 870 [528–1575] | 0.92 |
| Serum creatinine (µmol/L) | 85 [71–105] | 86 [68–111] | 0.81 |
| Estimated Glomerular filtration (mL/min) | 78 [59–94] | 74 [49–91] | 0.47 |
| Additional treatments | |||
| Prophylactic/therapeutic anticoagulant, N (%) Therapeutic anticoagulant, N (%) |
40 (100) 8 (20) |
28 (100) 9 (32) |
1 0.27 |
| Aspirin, N (%) | 8 (20) | 28 (100) | < 0.0001 |
| Colchicine, N (%) | 0 (0) | 28 (100) | < 0.0001 |
| Furosemide, N (%) | 4 (10) | 28 (100) | < 0.0001 |
| Antibiotics, N (%) | 29 (73) | 17 (61) | 0.43 |
| Outcomes | |||
| Invasive or non-invasive mechanical ventilation, high-flow oxygen therapy or 28-day death, N (%) | 18 (45) | 2 (7) | 0.0009 |
| Maximal oxygen flow (L/min) | 6 [3–11] | 3 [2–4] | 0.002 |
| High-flow oxygen therapy, N (%) | 5 (13) | 1 (4) | 0.38 |
| Non-invasive mechanical ventilation, N (%) | 5 (13) | 0 (0) | 0.07 |
| Invasive mechanical Ventilation, N (%) | 6 (15) | 1 (4) | 0.21 |
| 28-day death, N (%) | 2 (5) | 0 (0) | 0.5 |
| Length of hospital stay (days) | 7 [4–9] | 7 [6–9] | 0.28 |
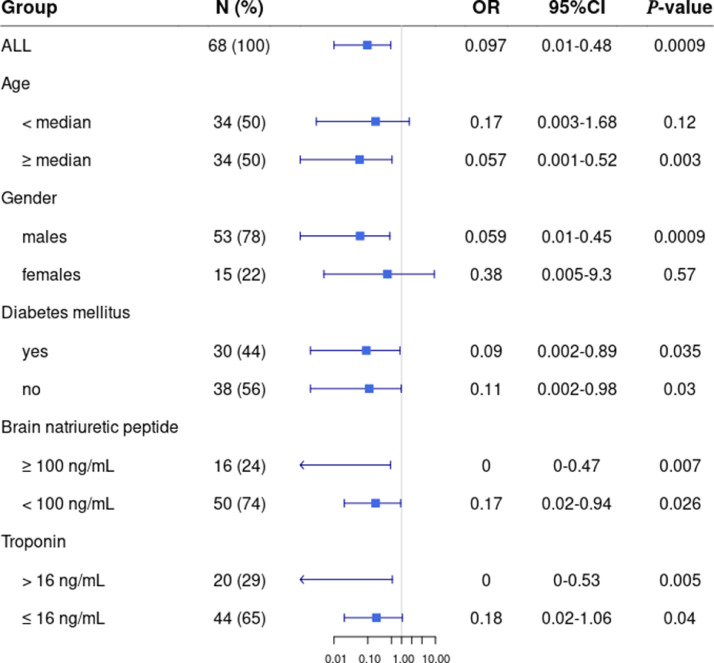
Among patients receiving the five-drug regimen, the incidence of primary composite endpoint was lower than in the control group (OR=0.097 [0.001–0.48], P = 0.0009). Multivariate analysis confirmed the significant effect of the five-drug regimen on outcome after adjustment for independent covariates, including age, body-mass index, 4C Mortality Score, high serum BNP level and high white blood cell count (OR=0.043 [0.0053–0.21], P = 0.0005). The model was significant compared to a model without the five-drug regimen (P < 0.00001). Additionally, patient subgroups were analyzed following stratification by age (using the median value as threshold), gender and risk factors including diabetes, elevated BNP (threshold, 100 ng/ml) and troponin levels (threshold, 16 ng/mL; Fig. 1 ). Remarkably, the five-drug regimen was associated with a significant reduction in primary composite endpoint in males only. Additionally, there was a stronger and more significant protective effect of our regimen in patients with elevated-BNP (OR=0.0 [0.0–0.47], P = 0.007) than in low-BNP patients (OR=0.17 [0.02–0.94], P = 0.03). Thus, the primary composite endpoint was improved in elevated- versus low-BNP patients (P = 0.0003). We observed no remarkable adverse effects attributed to the five-drug regimen except mild colchicine-related diarrhea (21%) resolved with loperamide.
Fig. 1.
Impact of the prednisone/furosemide/colchicine/salicylate/direct anti-Xa inhibitor regimen in the different patient subgroups defined according to age (using the median value, 66.5 years, as threshold), gender, presence of diabetes mellitus, serum brain natriuretic peptide (BNP; threshold at 100 ng/mL) and troponin levels (threshold at 16 ng/mL). Odds ratio (OR) and their 95%-confidence intervals were determined . (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The GRECCO-19 trial showed improved time to clinical deterioration in hospitalized COVID-19 patients receiving colchicine; however, the benefit relied on a narrow margin of clinical significance.3 By adding colchicine to the recommended corticosteroid and anticoagulant, together with aspirin and furosemide, we succeeded in improving the outcome. The five drugs included in our regimen were given orally for a short course, paving the way for an outpatient treatment. Interestingly, the recent COLCORONA trial conducted in non-hospitalized COVID-19 patients supported colchicine-related benefit in reducing hospitalizations, need for mechanical ventilation and mortality.11
Colchicine dose regimen differed between the three studies with higher cumulative colchicine doses in the GRECCO-19 (22 mg) and COLCORONA trials (16.5 mg) compared to ours (8 mg). Using the same primary composite endpoint, our five-drug regimen significantly improved prognosis in comparison to the corticosteroid/furosemide combination of our previous study2 (P = 0.0001).
In conclusion, our data highlight the benefit and safety of an early short-course oral regimen combining prednisone/colchicine/salicylate/direct anti-Xa inhibitor/furosemide to reduce the risk of high flow oxygen need, mechanical ventilation requirement or 28-day mortality in hospitalized non-critically ill COVID-19 patients. Our preliminary observational findings should be confirmed in larger cohorts.
Ethics approval and consent to participate
This study was part of the French COVID-19 cohort registry conducted by the REACTing consortium (REsearch and ACTion targeting emerging infectious diseases) and directed by INSERM (Institut national de la santé et de la recherche médicale) and ISARIC (International Severe Acute Respiratory and Emerging Infection Consortium). Our institutional ethics committee approved the study (N°, IDRCB, 2020-A00256–33; CPP, 11–20 20.02.04.68737).
Availability of data and materials
J.-P.K. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Consent for publication
All the authors agree to publish.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Funding
F.M.J. was funded by National Institutes of Health awards (DK074970 and DK107444), American Diabetes Association COVID-19 Research Award [7–20-COVID-051] and a US Department of Veterans Affairs Merit Review Award [BX003725].
References
- 1.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kevorkian J.P., Riveline J.P., Vandiedonck C., Girard D., Galland J., Féron F., et al. Early short-course corticosteroids and furosemide combination to treat non-critically ill COVID-19 patients: an observational cohort study. J Infect. 2021;82:e22–e24. doi: 10.1016/j.jinf.2020.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deftereos S.G., Giannopoulos G., Vrachatis D.A., Siasos G.D., Giotaki S.G., Gargalianos P., et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow J.H., Khanna A.K., Kethireddy S., Yamane D., Levine A., Jackson A.M., et al. Aspirin use is associated with decreased mechanical ventilation, ICU admission, and in-hospital mortality in hospitalized patients with COVID-19. Anesth Analg. 2020 doi: 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- 5.Fröhlich G.M., Jeschke E., Eichler U., Thiele H., Alhariri L., Reinthaler M., et al. Impact of oral anticoagulation on clinical outcomes of COVID-19: a nationwide cohort study of hospitalized patients in Germany. Clin Res Cardiol. 2021:1–10. doi: 10.1007/s00392-020-01783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhury J.F., Moores L.K., Connors J.M. Anticoagulation in hospitalized patients with COVID-19. N Engl J Med. 2020;383:1675–1678. doi: 10.1056/NEJMclde2028217. [DOI] [PubMed] [Google Scholar]
- 7.Richette P., Doherty M., Pascual E., Barskova V., Becce F., Castañeda-Sanabria J., et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 8.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight S.R., Ho A., Pius R., Buchan I., Carson G., Drake T.M., et al. Risk stratification of patients admitted to hospital with COVID-19 using the ISARIC WHO Clinical Characterization Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tardif J.C., Bouabdallaoui N., L'Allier P.L., Gaudet D., Shah B., Pillinger M.H., et al. Efficacy of colchicine in non-hospitalized patients with COVID-19. Medrxiv. 2021 https://www.medrxiv.org/content/10.1101/2021.01.26.21250494v1 Available at. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
J.-P.K. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.