Abstract
Background
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), most frequently presents with respiratory symptoms, such as fever, dyspnea, shortness of breath, cough, or myalgias. There is now a growing body of evidence that demonstrates that severe SARS-CoV-2 infections can develop clinically significant coagulopathy, inflammation, and cardiomyopathy, which have been implicated in COVID-19–associated cerebrovascular accidents (CVAs).
Case Report
We report an uncommon presentation of a 32-year-old man who sustained a large vessel cerebellar stroke associated with a severe COVID-19 infection. He presented with a headache, worse than his usual migraine, dizziness, rotary nystagmus, and dysmetria on examination, but had no respiratory symptoms initially. He was not a candidate for thrombolytic therapy or endovascular therapy and was managed with clopidogrel, aspirin, and atorvastatin. During hospital admission he developed COVID-19–related hypoxia and pneumonia, but ultimately he was discharged to home rehabilitation.
Why Should an Emergency Physician Be Aware of This?
We present this case to increase awareness among emergency physicians of the growing number of reports of neurologic and vascular complications, such as ischemic CVAs, in otherwise healthy individuals who are diagnosed with SARS-CoV-2 infection. A brief review of the current literature will help elucidate possible mechanisms, risk factors, and current treatments for CVA associated with SARS-CoV-2.
Keywords: cerebellar infarction, ischemic stroke, cerebrovascular accident, coagulopathy, prothrombotic state, COVID-19, SARS-CoV-2
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the primary cause of the ongoing coronavirus disease 2019 (COVID-19) pandemic. COVID-19 most frequently presents with respiratory symptoms, such as fever, dyspnea, cough, or myalgias (1). There is now mounting evidence that demonstrates that SARS-CoV-2 infections can give rise to a wide range of neurologic symptoms. These include headache, seizures, dizziness, ataxia, encephalitis, ageusia, anosmia, neuralgia, Guillain-Barre syndrome, acute disseminated encephalomyelitis, and cerebrovascular accidents (CVAs) (2, 3, 4, 5).
Strokes in young, healthy adults are less commonly observed and comprise approximately 10–15% of the overall number of strokes in the United States (6). However, strokes have also been observed in patients diagnosed with COVID-19 (2, 3, 4, 5,7). Observational research has demonstrated that SARS-CoV-2 infection has been associated with a coagulopathic state and a variety of prothrombotic sequelae (8, 9, 10, 11, 12, 13, 14). We report an unusual case of a large cerebellar CVA in a young man who presented to a community emergency department (ED) with a headache and dizziness and without initial respiratory symptoms that are typical of COVID-19 infections.
Case Report
A 32-year-old man with a history of tension-type migraine headache presented to a community ED with headache, generalized weakness, dizziness, nausea, and vomiting that started at 8:00 am the previous day. The patient described a severe, sudden-onset headache located over the left temple and sudden acute vertigo. His headache was constant and more severe than his normal migraine headaches. The patient denied fever, changes in vision, rash, or neck stiffness. He had no chest pain, palpitations, shortness of breath, dyspnea on exertion, or cough. He had not tried any medication and reported no palliative or provoking symptoms. He denied any tobacco use or illicit drug or prescription drug use. He was living with his parents who had both reportedly tested positive for COVID-19.
On arrival to the ED, his initial vital signs were blood pressure 130/89 mm Hg, heart rate 77 beats/min, respiratory rate 26 breaths/min, oxygen saturation 100% on room air, and body mass index of 28.7 kg/m2 (overweight). Overall, the patient was well-appearing and in mild distress. He was alert and oriented to person, place, year, and situation. His rapid alternating movements and gait were normal. His neurologic examination was notable for mild decrease sensation over the left temple, left upper extremity ataxia and dysmetria, and rotary nystagmus. The patient's vertigo and nystagmus worsened with the Dix-Hallpike maneuver when his head was turned to the left. He reported mild relief of symptoms with the Epley maneuver.
His initial complete blood count (CBC) had white count of 6.85 × 103/μL (range 4.80–10.7 × 103/μL), hemoglobin 15.7 g/L (range 14.1–16.6 g/L), hematocrit 48.7% (range 41.0–48.0%), platelet count 366 × 103/μL (range 130–400 × 103/μL), and lymphocytes 9.7% (range 18.0–45%). The differential had an elevation of atypical lymphocytes 8.0% (range 0.0–2.0%). The comprehensive metabolic panel was unremarkable except for glucose 121 mg/dL (range 70–110 mg/dL) and a slight elevation in the alanine aminotransferase at 88 U/L (range 0–55 U/L). The SARS-CoV-2 nasopharyngeal polymerase chain reaction test Abbott ID NOW COVID-19 assay (Abbott Diagnostics Scarborough, Inc, Scarborough, ME) was positive. In addition, his d-dimer was elevated to 2443 ng/mL (range 0–230 ng/mL), C-reactive protein was elevated to 1.7 mg/dL (range 0.0–0.9 mg/dL), and lactic dehydrogenase was elevated to 860 U/L (range 313–618 U/L). His alcohol level was < 10 mg/dL (range 0–10 mg/dL) and the urine drug screen was negative for opiates and illicit drugs. A computed tomography scan of the head (CT head) without contrast was performed.
Prior to the final read of the CT head without contrast, the tele-neurology service was also consulted due to the patient's vertigo, ataxia, and rotary nystagmus. The patient was treated for a possible complicated migraine headache, or benign positional peripheral vertigo with ketorolac 30 mg i.v., metoclopramide 10 mg i.v., and dexamethasone 6 mg i.v. in the ED. Despite treatment, the patient's vertigo, ataxia, and rotary nystagmus was persistent and severe. The results of the CT head without contrast (Figure 1 ) then showed a large left cerebellar nonhemorrhagic infarction. The radiologist also noted an 8-mm hyperattenuated lesion within the left anterolateral aspect of the foramen magnum, which was concerning for a thrombosed aneurysm within the region of the left posterior inferior cerebellar artery, with an associated high-density focus in the distal left vertebral artery. The basilar artery was unremarkable. Geographic hypoattenuation throughout the left inferior cerebellar hemisphere was also noted, concerning for ischemic change (see Figure 1). A magnetic resonance imaging (MRI) brain with and without contrast was performed shortly thereafter and confirmed the same cerebellar infarct (see Figure 2, Figure 3 ).
Figure 1.
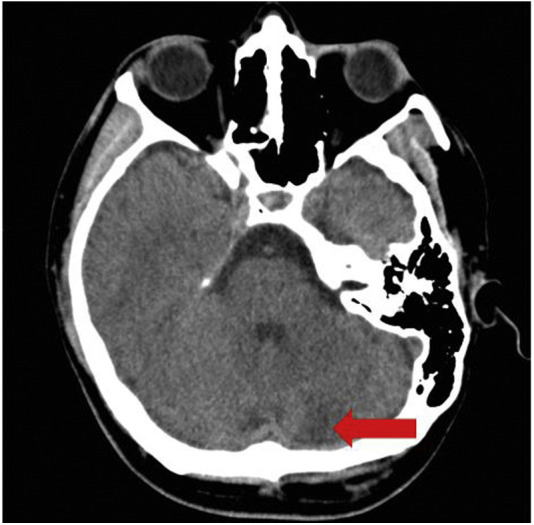
Computed tomography head without contrast. There is a moderately large patchy area of low attenuation involving the inferior two-thirds of the left cerebellum (arrow) suggesting an acute nonhemorrhagic infarction of the left posterior cerebral artery territory. There appears to be a thrombus within the left vertebral artery. There is no acute intracranial hemorrhage, no white matter ischemic changes, or demyelination identified.
Figure 2.
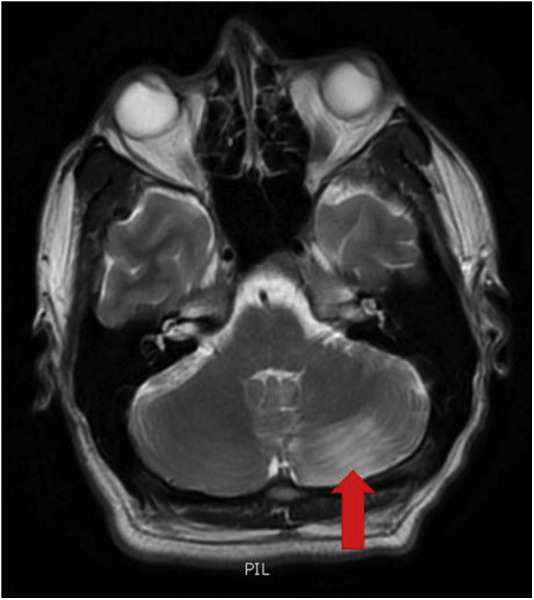
Magnetic resonance imaging of the brain without contrast shows the acute nonhemorrhagic infarction of the inferior two-thirds of the left cerebellum. There is a moderately large acute nonhemorrhagic infarction of the inferior two-thirds of the left cerebellum (arrow) corresponding to the left posterior inferior cerebellar artery territory. There is no acute intracranial hemorrhage.
Figure 3.
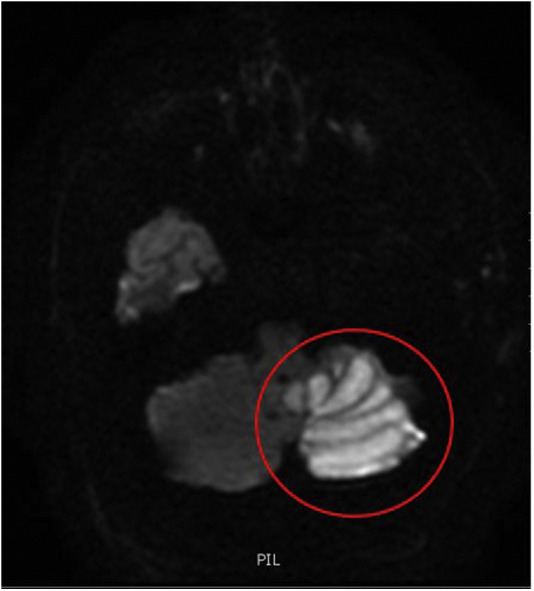
Magnetic resonance imaging head without contrast, Diffusion weight imaging demonstrates a moderately large area of true diffusion restriction involving the lower two-thirds of the left cerebellum (circled) consistent with acute nonhemorrhagic cerebellar infarction. No acute intracranial hemorrhage. There is no hemosiderin deposition evident within the brain parenchyma.
Hospital Course
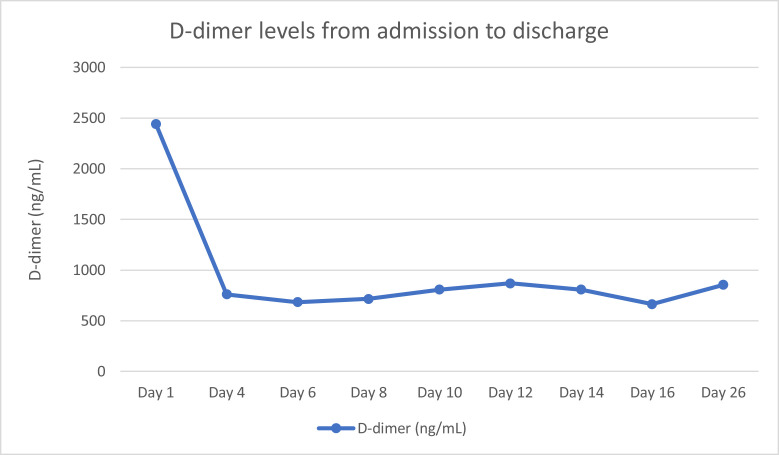
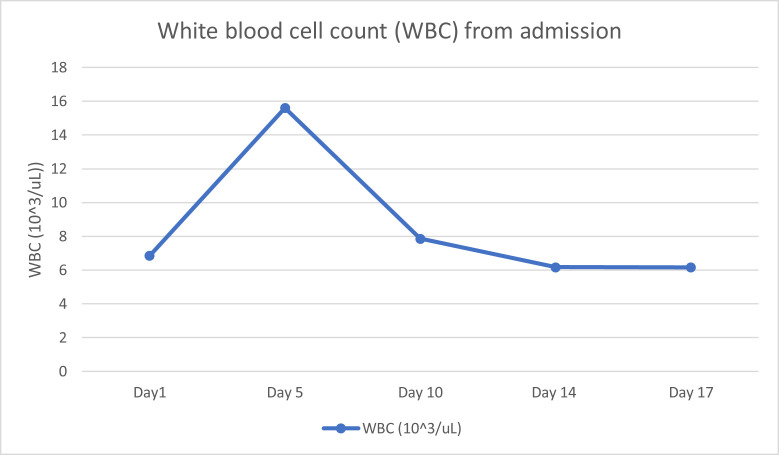
The tele-neurology service was consulted again due to the findings of both the CT head and the MRI head without contrast (Figure 1, Figure 2), which were concerning for left-sided cerebellar infarct. Because his stroke symptoms started more than 48 h prior to arrival, thrombolytics and endovascular interventions were not recommended. The National Institute of Health Stroke Scale was not recorded during his ED stay or his discharge. He was admitted and started on clopidogrel 600 mg, aspirin 324 mg, and 80 mg of atorvastatin. An initial screening chest x-ray study showed no evidence of pneumonia, consolidation, or any pulmonary edema (Figure 4 ). However, on day 5 of admission, he developed symptoms consistent with hypoxia secondary to COVID-19 pneumonia and was given supplemental oxygen, but he did not require invasive ventilation. The patient did receive a course of remdesivir when he developed COVID-19 pneumonia. The basic metabolic panel and CBC were performed throughout his admission within the first 2 weeks. However, the CBC differential was only performed sporadically. No other laboratory abnormalities were noted except for the consistently elevated d-dimer (Figure 5 ), elevated white blood cell count (WBC), and lymphopenia on day 5 when the patient was found to have COVID-19 pneumonia. Figure 6 shows the sharp increase of his WBC to 15.6 × 103/μL (range 4.80–10.7 × 103/μL) with 79.8% (range 44–72%) neutrophils and 8.6% (range 18.0–45%) lymphocytes on day 5, which normalized by day 10 to WBC of 7.86 × 103/μL (range 4.80–10.7 × 103/μL).
Figure 4.
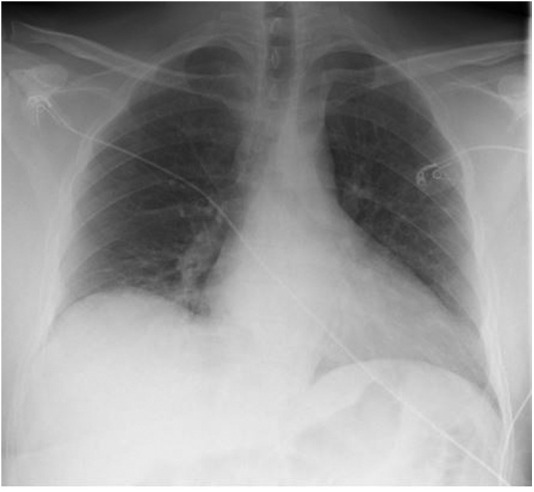
Anterior posterior view chest x-ray study is negative for coronavirus disease 2019 pneumonia on admission.
Figure 5.
d-dimer from admission to discharge.
Figure 6.
WBC from admission day 1 to day 17. The increase of WBC correlates with the patient's diagnosis of hypoxia secondary to coronavirus disease 2019 pneumonia on day 5.
Additional laboratory screening was positive for phosphatidyl serine IgM antibodies only, but all other antiphospholipid syndrome (APS) antibodies were negative. All other coagulopathic laboratory tests, such as protein C and protein S, antithrombin, factor V, von Willebrand Factor, and factor VIII were normal. In addition, the lipoprotein (a) cholesterol was < 10 mmol/L (normal < 75 mmol/L). The echocardiogram and bubble study were unremarkable. His symptoms of nystagmus and vertigo continued to improve and he was discharged on clopidogrel 75 mg daily and atorvastatin 80 mg daily. He was also ambulatory with a walker on day 13 awaiting transfer to a rehabilitation facility.
Routine laboratory values were not reported daily after day 17 of admission. Only SARS-CoV-2 and d-dimer were monitored every other day until 1 week prior to discharge. Nearby rehabilitation facilities required two serial negative SARS-CoV-2 tests for acceptance. However, the patient continued to have positive tests for SARS-CoV-2 while continuing his rehabilitation at the hospital. He was discharged on day 26 with his symptoms markedly improved while awaiting home rehabilitation. There were no additional records from outpatient follow-up to indicate that he had any additional laboratory tests to further investigate coagulopathies or vasculitis.
Discussion
Ischemic stroke is one of the more serious neurologic complications seen in patients with COVID-19 infection and has been observed more commonly in patients older than 55 years who have significant comorbidities (2, 3, 4, 5,9, 10, 11, 12, 13, 14, 15, 16, 17). Retrospective studies in Wuhan, China have found an incidence of 2–5% CVA in patients with COVID-19 (2,15). Ischemic strokes were more commonly observed than hemorrhagic strokes in patients with COVID-19 (3,7,15). In a case series of 6 patients with COVID-19 by Morassi and colleagues there were four (67%) ischemic strokes and two (33%) hemorrhagic strokes (3). Li and colleagues found that of their 219 patients with confirmed SARS-CoV-2, 11 (5.0%) had developed new onset of CVA after COVID-19 infection (15). Of these patients, 10 (90.9%) were diagnosed with ischemic stroke and 1 (9.1%) had intracerebral hemorrhage. Ashrafi and colleagues found 6 patients younger than 55 years with COVID-19 who were diagnosed with ischemic CVA (7). These patients presented with altered mental status, hemiplegia, hemiparesis, or dysarthria. Excluded from the study were any patients who had abnormal echocardiograms. Five of the 6 patients (83.3%) had strokes involving the middle cerebral artery and 1 patient with a basilar artery stroke. One of the patients died from the stroke (7).
A growing amount of evidence shows that patients with COVID-19 develop clinically significant coagulopathy, inflammation, and cardiomyopathy, which have been implicated in associated CVAs (15, 16, 17, 18).
Pathophysiology and Mechanisms of COVID-19 Stroke
Patients with ischemic strokes and severe SARS-CoV-2 infection are observed to have clinically significant prothrombotic states (7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17). Recently, abnormal d-dimer, prothrombin time (PT), activated partial thromboplastin time, platelets, fibrinogen, antithrombin activity, factor V, von Willebrand Factor, and factor VIII in findings have been found in patients with severe COVID-19 (18,19). A retrospective study by Helms and colleagues compared the number of significant thrombotic complications, such as arterial thrombosis, pulmonary embolisms, and CVAs, in patients with COVID-19 with acute respiratory distress syndrome (ARDS) vs. those with non–COVID-19 ARDS (19). They observed that significant thrombotic complications were more likely to be diagnosed in 27 of 150 (18%) of the patients with COVID-19 ARDS vs. 14 of the 233 (6%) non–COVID-19 ARDS patients (odds ratio [OR] 3.4; 95% confidence interval [CI] 1.7–7.3; p < 0.001) (19).
The exact pathogenesis of this hypercoagulopathy has yet to be fully elucidated, but it is postulated that it is a multifactorial process that also includes the complement pathway (18,20,21). In prior studies relating to SARS-CoV (SARS), it was observed that elevated complement (C3) activation exacerbates ARDS. Prior studies of SARS suggest that C3 inhibition may decrease the risk of inflammatory lung complications of SARS-CoV-2 (20,21). Elevated levels of complement have been observed in patients with severe SARS-CoV-2 infection. There is some histopathologic evidence to suggest that complement-driven endothelial damage on various organs due to SARS-CoV-2 infection (18,20, 21, 22). Vascular endothelium that is damaged by SARS-CoV-2 can activate the complement system and cause an overactivation of the systemic pro-inflammatory response. This is hypothesized to be part of a catastrophic positive feedback loop with the coagulation system, which in turn leads to an overactivation of the complement system (16,18,20, 21, 22). The positive feedback of the pro-inflammatory response has been observed in severe COVID-19 cases (18,20, 21, 22). In addition, known risk factors, such as diabetes, hypertension, coronary artery disease, and obesity, that have been associated with pre-existing vascular endothelial damage could also make patients with these comorbidities especially vulnerable to severe COVID-19 and associated vascular complications (18).
The most common laboratory evidence of coagulopathy in severe COVID-19 is found with elevated d-dimer (8,14,17, 18, 19,22,23). Guan and colleagues found 1099 patients with COVID-19 with a d-dimer ≥ 0.5 mg/L were more frequently observed in patients with severe disease than in those without (60% vs. 43%; p = 0.002) (23). In a retrospective analysis of 138 hospitalized patients, Wang and colleagues observed that there was a 2.5-fold increase in d-dimer level in ICU patients (n = 36) compared with non-ICU (n = 102) patients (p < 0.001) (8). Wang and colleagues also observed that those with severe COVID-19 who died, had d-dimers > 1000 mg/L compared with nonsurvivors who had d-dimer levels < 500 mg/L (p < 0.05) (8). In the retrospective cohort analysis in New York, 32 (0.9%) of the 3556 COVID-19–positive patients with strokes had higher peak d-dimer vs. stroke patients without COVID-19. Reportedly, 65% of these strokes were cryptogenic (24).
Severe SARS-CoV-2 infection is also associated with increased systemic inflammation from infection-mediated endothelial injury through interleukin 6 and tumor necrosis factor–α, which triggers excessive thrombin production leading to microthrombi and microvascular dysfunction (10,25, 26, 27). In addition, angiotensin converting enzyme type 2 (ACE2) receptors found on endothelial cells of the blood–brain barrier can allow for viral entry into the nervous system and attack the vasculature of the nervous system, causing endothelitis (12,21,25, 26, 27). SARS-CoV-2 infection stimulates ACE2, thus increasing ATII, which causes microcirculatory vasoconstriction and endothelial dysfunction with consequent ischemia and apoptosis (12,20,21,25, 26, 27).
Patients with infections due to viral illnesses have had antiphospholipid antibodies, which may have contributed to major coagulopathic complications (28, 29, 30). Standard APS classification includes thrombosis or pregnancy morbidity and the presence of one laboratory criterion lupus anticoagulant, anticardiolipin antibodies (aCL), or β2-glycoprotein I antibodies (aβ2GPI). Then, the presence of these antibodies are measured again 12 weeks after the initial testing (31). Recently, there have been reports that have found a temporary increase of antiphospholipid antibodies in critically ill patients with COVID-19 (9, 10, 11,13,29,30). Zhang and colleagues reported confirmed severe COVID-19 cases were found to have aCL and aβ2GPI IgA and IgG (11). In critically ill patients with COVID-19 with thrombotic events, the prevalence of antiphospholipid antibodies is estimated to be between 45% and 91% (9,11, 12, 13). The presence of these various antiphospholipid antibodies has also been observed in reported cases of patients with COVID-19 with large vessel cerebral infarcts in multiple vascular territories (9, 10, 11,13).
Although not part of the formal APS criteria, a review of literature has demonstrated that even anti-phosphatidylserine (aPS) IgM antibodies have been associated with significant thrombotic events (32,33). Our patient in the case had an elevation of aPS IgM in his serum. These aPS IgMs have been observed as transient, but have been implicated in patients with severe COVID-19 with major thromboembolic complications (30,32). In a recent review of 172 hospitalized patients with COVID-19, Zuo and colleagues measured levels of aCL, aβ2GPI, and aPS/PT (30). They found that 50% of the hospitalized patients had become transiently aPL-positive. Of the 172 hospitalized patients, 18% were positive for aPS/PT IgM (30). In their systematic review, Sciascia and colleagues found that in 7000 patients from 48 studies, aPS IgM had increased the risk of thrombotic events (OR 2.3; 95% CI 1.72–3.5) (32). In comparing anti-prothrombin antibodies vs. aPS antibodies, Sciascia and colleagues observed aPS antibodies appeared to have stronger risk factor for thrombosis, both arterial or venous, than aPT (OR 5.11; 95% CI 4.2–6.3 and OR 1.82; 95% CI 1.44–2.75, respectively) (32).
In addition, there are several proposed mechanisms in SARS-CoV-2 infection that could contribute to cardiomyopathy and ischemic stroke. In their research, Wang and colleagues observed that acute cardiac injury can come from direct invasion of SARS-CoV-2 (8). Direct invasion causes inflammation and myocarditis (34). Similarly, inflammation produced from SARS-CoV-2 could be implicated in pericarditis. Myocarditis, myopericarditis, and pericarditis due to COVID-19 can predispose one to cardiac dysrhythmias that contribute to strokes (14,34,35). In addition, stress and cytokine storm can also cause dysrhythmias, which predispose patients to embolic strokes (14,34,35).
Epidemiology and Profile of Patients With COVID-19 Strokes
Several retrospective cohort studies have estimated the incidence of COVID-19–associated strokes to be 2.5–6% of the total number of patients with COVID-19 (13,35, 36, 37, 38). According to several retrospective cohort studies, COVID-19–associated CVAs were more frequently observed in patients older than 55 years and with stroke risk factors due to the increased risk of having diabetes, hypertension, hypercholesterolemia, peripheral vascular disease, prior strokes, obesity, and cardiac disease (3,7,13,15,18,27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37).
Cohort studies of CVAs associated with COVID-19 infection found that they often occurred in a much younger population. Ashrafi and colleagues reported that their patients had a mean ± standard deviation age of 43.5 ± 7.42 years (range 33–53 years). In this cohort, half were male patients who presented with respiratory symptoms and were hypoxic with O2 saturation < 92% on room air and half of the patients had comorbidities, such as hypertension and or diabetes.7 Oxley and colleagues reported 5 patients younger than 50 years with no medical history presenting with large vessel strokes and 4 of the 5 patients had an elevated d-dimer (39).
Potential Treatment and Therapy of COVID-19 Stroke
Because of the mechanism of increased coagulation, it is hypothesized that anticoagulants such as enoxaparin should help decrease coagulopathic complications in COVID patients.
There are interim guidelines that support the routine use of low-molecular-weight heparin in patients with coagulopathy (14,24). Yaghi and colleagues reported that a randomized trial of therapeutic anticoagulation vs. prophylactic anticoagulation is underway to test for safety and efficacy of enoxaparin in patients with severe COVID-19 infection–associated coagulopathy (24). In the case of ischemic stroke, if patients are within the given 3-h time frame of stroke onset, thrombolytic therapy or thrombectomy should be considered (14,40).
Limitations
We cannot establish strong causal effect of a coagulopathic state with the outcome of ischemic stroke in our patient. Migraine, in itself, increases the risk of stroke, but this is more likely in women (41). Furthermore, we do not know whether our patient truly had an underlying primary coagulopathy. Although, he did not report having a concomitant connective tissue disease or a coagulopathy to suggest that could have contributed to his hypercoagulopathic state and stroke.
Our patient had an elevation of aPS antibodies, which does not meet the diagnosis of APS. However, there are no records to re-evaluate for the presence of APS and the aPS antibodies in a 12-week span. However, recent research has observed a transient elevation of aPS antibodies in patients with COVID-19 and that these patients do have a higher risk for coagulopathic complications, including strokes (29,30,35). In addition, elevation in d-dimer and presence of aPS antibodies could be an indicator for a hypercoagulopathic state in patients with COVID-19, and might have led to our patient's stroke, as in other cases (9, 10, 11, 12,35). Regardless, we postulate that the SARS-CoV-2 infection may have been an important precipitating factor for thrombosis that resulted in our patient's cerebellar CVA.
Why Should an Emergency Physician Be Aware of This?
We report an unusual case of a large ischemic cerebellar stroke in a young man with no medical history other than migraine headaches who did not present with primary respiratory symptoms of COVID-19 at the onset of his course. Although rare, this case should raise awareness among emergency physicians of SARS-CoV-2 in otherwise low-risk patients who present with a high clinical suspicion of CVA, given the coagulopathic risk.
References
- 1.Burke R.M., Killerby M.E., Newton S., et al. Symptom profiles of a convenience sample of patients with COVID-19–United States, January—April 2020. MMWR. Morb Mortal Wkly Rep. 2020;69:904–908. doi: 10.15585/mmwr.mm6928a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morassi M., Bagatto D., Cobelli M., et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;267:2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azhideh A. COVID-19 neurological manifestations. Int Clin Neurosci J. 2020;7(2):54. [Google Scholar]
- 5.Niazkar H.R., Zibaee B., Nasimi A., Bahri N. The neurological manifestations of COVID-19: a review article. Neurol Sci. 2020;41:1667–1671. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George M.G. Risk factors for ischemic stroke in younger adults: a focused update. Stroke. 2020;51:729–735. doi: 10.1161/STROKEAHA.119.024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashrafi F., Zali A., Ommi D., et al. COVID-19-related strokes in adults below 55 years of age: a case series. Neurol Sci. 2020;41:1985–1989. doi: 10.1007/s10072-020-04521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan H., Tang X., Song Y., Liu P., Chen Y. Influence of COVID-19 on cerebrovascular disease and its possible mechanism. Neuropsychiatr Dis Treat. 2020;16:1359–1367. doi: 10.2147/NDT.S251173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Xiao M., Zhang S., et al. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridwell R., Long B., Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. 2020;38:1549.e3–1549.e7. doi: 10.1016/j.ajem.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasset C., Gedansky A., Mays M., Uchino K. Acute ischemic stroke and COVID-19. Cleve Clin J Med. 2020 doi: 10.3949/ccjm.87a.ccc042. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Li M., Wang M., et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:e000431. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spence J., Freitas G., Pettigrew L., et al. Mechanisms of stroke in COVID-19. Cerebrovasc Dis. 2020;49:451–458. doi: 10.1159/000509581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merkler A.E., Parikh N.S., Mir S., et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77(11):1–7. doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Ani F., Chehade S., Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risitano A.M., Mastellos D.C., Huber-Lang M., et al. Complement as a target in COVID-19? Nat Rev Immunol. 2020;20:343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Java A., Apicelli A.J., Liszewski M.K., et al. The complement system in COVID-19: friend and foe? JCI Insight. 2020;5(15):e140711. doi: 10.1172/jci.insight.140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Trans Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaghi S., Ishida K., Torres J., et al. SARS2-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2001–2011. doi: 10.1161/STROKEAHA.120.031606. [DOI] [PubMed] [Google Scholar]
- 25.Pons S., Fodil S., Azoulay E., Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24:353. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S.G., Fralick M., Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192(21):E583. doi: 10.1503/cmaj.200685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta A., Madhavan M.V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uthman I.W., Gharavi A.E. Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31:256–263. doi: 10.1053/sarh.2002.28303. [DOI] [PubMed] [Google Scholar]
- 29.Mackman N., Antoniak S., Wolberg A.S., Kasthuri R., Key N.S. Coagulation abnormalities and thrombosis in patients infected with SARS-CoV-2 and other pandemic viruses. Arterioscler Thromb Vasc Biol. 2020;40:2033–2044. doi: 10.1161/ATVBAHA.120.314514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo Y., Estes S.K., Ali R.A., et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12(570):eabd3876. doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devreese K.M.J., Ortel T.L., Pengo V., de Laat B., for the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies Laboratory criteria for antiphospholipid syndrome: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16:809–813. doi: 10.1111/jth.13976. [DOI] [PubMed] [Google Scholar]
- 32.Sciascia S., Sanna G., Murru V., Roccatello D., Khamashta M.A., Bertolaccini M.L. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. A systematic review. Thromb Haemost. 2014;111:354–364. doi: 10.1160/TH13-06-0509. [DOI] [PubMed] [Google Scholar]
- 33.Radin M., Foddai S.G., Cecchi I., et al. Antiphosphatidylserine/prothrombin antibodies: an update on their association with clinical manifestations of antiphospholipid syndrome. Thromb Haemost. 2020;120:592–598. doi: 10.1055/s-0040-1705115. [DOI] [PubMed] [Google Scholar]
- 34.Esposito A., Palmisano A., Natale L., et al. Cardiac magnetic resonance characterization of myocarditis-like acute cardiac syndrome in COVID-19. JACC Cardiovasc Imaging. 2020;13:2462–2465. doi: 10.1016/j.jcmg.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iguina M., Saleh A., Sayeedi I., Danckers M. Recurrent ischemic strokes in a patient with severe COVID-19 infection and phosphatidylserine antibodies. Chest. 2020;158(4):A776. [Google Scholar]
- 36.Hess D.C., Eldahshan W., Rutkowski E. COVID-19-related stroke. Transl Stroke Res. 2020 doi: 10.1007/s12975-020-00818-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Z., Kang H., Li S., et al. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. 2020;267:2179–2184. doi: 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larson A.S., Savastano L., Kadirvel R., Kallmes D.F., Hassan A.E., Brinjikji W. Coronavirus disease 2019 and the cerebrovascular-cardiovascular systems: what do we know so far? J Am Heart Assoc. 2020;9(13):e016793. doi: 10.1161/JAHA.120.016793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thachil J., Tang N., Gando S., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Øie L.R., Kurth T., Gulati S., et al. Migraine and risk of stroke. J Neurol Neurosurg Psychiatry. 2020;91:593–604. doi: 10.1136/jnnp-2018-318254. [DOI] [PMC free article] [PubMed] [Google Scholar]