Abstract
Repeated sprint exercise can interfere with intramuscular redox balance and cause systemic oxidative stress and muscle damage. There is growing evidence that molecular hydrogen counteracts oxidative and/or inflammatory responses. Therefore, we investigated the effects of molecular hydrogen-rich water (HW) on muscle performance and oxidative stress markers induced by strenuous exercise. A single-blind, crossover, randomized controlled trial has been designed. Eight male volunteers completed two 3-day consecutive exercise tests under two conditions: HW and placebo water (PW). The exercise test included a countermovement jump, maximal voluntary isometric contraction of knee extensors, and sprint cycling. The sprint cycling exercise was comprised three repetitions of 10-second maximal pedaling against a resistance of 7.5% body mass and 110-second active rest (no-load pedaling). Before and after the exercise test, participants drank the 500 mL of HW (5.14 ± 0.03 ppm in H2 concentration) or PW (0.00 ± 0.00 ppm). At 7 hours before the first exercise test (Day 1), as baseline, and 16 hours after the exercise test on each day, blood samples were obtained. Exercise performances in both conditions were not significantly different over 3 consecutive days. In PW trial, relative changes in biological antioxidant potential/diacron-reactive oxygen metabolites, as an index of systemic antioxidant potential, from baseline gradually decreased as the day passed. However, HW suppressed the reduction in biological antioxidant potential/diacron-reactive oxygen metabolites observed in PW. Drinking HW contributed to the maintenance of the redox status during consecutive days of strenuous exercise and might help prevent accumulative muscular fatigue. The study was approved by the Human Research Ethics Committee of the University of Yamanashi, Japan (approval No. H26-008) on December 17, 2014.
Keywords: biological antioxidant potential, diacron-reactive oxygen metabolites, ergogenic aid, molecular hydrogen, oxidative damage, single blinded cross-over design, sprint cycling, straight days of vigorous exercise
INTRODUCTION
In situations where high-intensity exercise is performed, the increase in oxygen consumption may result in the generation of reactive oxygen species (ROS).1 The balance between exercise-induced ROS and defense system by intrinsic or extrinsic antioxidants plays crucial roles in maintaining physiological function (energy metabolism, mitochondrial function, and muscle buffering). However, intensive exercise disturbs redox homeostasis leading to oxidative stress, and excessive oxidative stress can cause muscle fatigue, inflammation, and diseases in various tissues.1,2,3 These problems may get exacerbated, especially in athletic competitions and training camps, in situations where strenuous exercise is performed on consecutive days. Thus, it is important to maintain the antioxidant defense capacities in these situations to protect the athletes’ performance and protect them from oxidative damages.
For approximately 30 years, antioxidant supplementation has been applied as a defense strategy against exercise-induced oxidative damages.4 Although there are numerous antioxidants that are exogenously absorbable, molecular hydrogen (H2) has been shown to have anti-oxidative and/or anti-inflammatory potential.5,6,7 Moreover, as yet, no adverse effects of H2 have been reported.5 Furthermore, there is a growing body of evidence that suggest that H2 can attenuate exercise-induced oxidative stress.8,9,10,11,12 It has been reported that H2 attenuates the acute intensive exercise-induced elevation in oxidative stress responses in humans9 and Thoroughbred horses.10 Furthermore, several previous studies have also indicated that H2 can improve muscle fatigue13,14 and soreness,15,16 and optimize immune16 and inflammatory responses.11,14 Based on these findings, we hypothesized that H2 supplementation might increase antioxidant potential, enabling the maintenance of muscle performance and redox homeostasis during consecutive days of vigorous exercise. In order to test this, we examined the ergogenic effects of H2-rich water (HW) in healthy young males who performed 3 straight days of repeated strenuous exercise tests.
PARTICIPANTS AND METHODS
Participants
In total, eight healthy and physically active males (age, 19.4 ± 0.3 years; height, 174.3 ± 2.4 cm; body weight, 67.6 ± 2.2 kg; values are means ± standard error) who were able to perform the high-intensity exercise test were recruited for the present study. The participants had no history of cardiovascular or respiratory diseases. After receiving a detailed explanation of the experimental procedure, each participant singed an informed consent form. All experimental procedures were approved by the Human Research Ethics Committee of the University of Yamanashi (approval No. H26-008) on December 17, 2014 and were performed in accordance with the guidelines of the Declaration of Helsinki. This study followed the CONsolidated Standards Of Reporting Trials (CONSORT) Statement.
Study design
In order to examine whether dietary HW supplementation had a significant effect on exercise performance and oxidative stress markers during 3 consecutive days of strenuous exercise, a crossover, single-blinded, repeated measures design was adopted in this study (Figure 1). Considering the order effect, the participants were randomly allocated to two groups by permuted block method. As first trial, one group (n = 4) was assigned to HW intervention, the other group (n = 4) was assigned to placebo water (PW) intervention. After 1 week or more intervals, the participants in both groups conducted the experiments in respective other conditions as second trial. The participants were instructed not to take antioxidant supplements and alcohol, not to perform strenuous exercise, and not to receive any specific recovery treatments from 48 hours before the first exercise test to the completion of the last blood collection in each trial. Moreover, the participants were also requested to fast for 3 hours before each exercise test in order to eliminate the potential influence of diet on the physiological responses.
Figure 1.
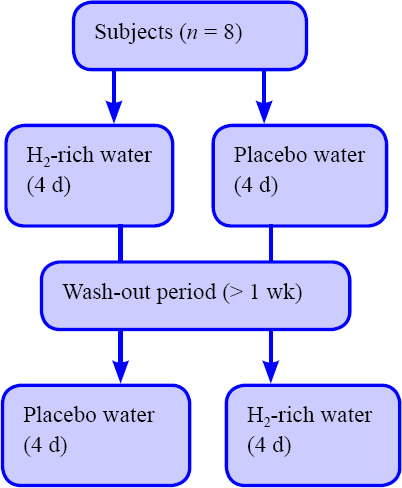
Research design.
Preparation of experimental drinking water
PW was prepared using tap water in a carbohydrate beverage container and was prepared 24 hours prior to drinking. In a similar container, HW was prepared using tap water and a commercial hydrogen-generated kit (Aquela Suisosui 7.0 ppm; Ecomo International, Fukuoka, Japan) 24 hours prior to drinking. The principle of generating hydrogen and the standardized techniques of HW preparation were followed as previously described.7 After PW and HW were generated, they were incubated in room temperature (20–22°C) for 20 minutes and stored at 4°C until required. The H2 concentration in both PW and HW was measured by a portable saturated hydrogen analyzer (DH-35A; Toa DKK, Tokyo, Japan); this revealed that the concentrations of H2 immediately before drinking were 0.00 ± 0.00, and 5.14 ± 0.03 ppm, respectively.
Experimental procedure
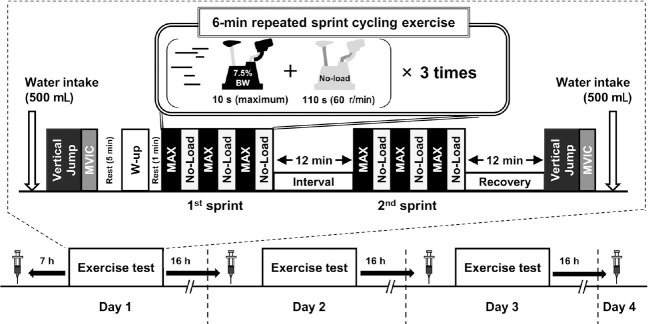
Following an initial visit to familiarize participants with the exercise mode (countermovement jump, maximal voluntary isometric contraction [MVIC] of knee extensors, and sprint cycling), the volunteers participated in two experiments (PW and HW) comprising three consecutive days of exercise test (Figure 2). After arriving at the laboratory on Day 1, participants consumed 500 mL of HW or PW within 5 minutes. Subsequently, the height of their countermovement jump and MVIC of knee extensors were assessed using a digital countermovement jump tester (Jump MD, Takei Scientific Instruments, Tokyo, Japan) and hand belt dynamometer (μTAS F-1; Anima, Tokyo, Japan), respectively.
Figure 2.
Overview of the experimental procedure.
Note: MAX: 10-second maximal pedaling; MVIC: maximum voluntary isometric contraction, No-Load: 110-second pedaling with no mechanical load; W-up: warm-up.
Following these measurements, a standardized warm-up (5 minutes of submaximal pedaling against 2.0% of the participants’ body weight with a pedaling frequency of 80 r/min) was performed on an electromagnetically braked cycle ergometer (Powermax V, Combi, Japan). Then, the participants carried out two sets of sprint cycling exercise consisting of three successive “10-second maximal pedaling and 110-second no-load pedaling (80 r/min).” The load of maximal pedaling corresponded to 7.5% of the participants’ body weight. The resting interval between the two sets of sprint cycling was set for 12 minutes. Two minutes after the completion of the second set of sprint cycling, capillary blood samples (0.3 μL) were taken from the fingertip using a disposable lancet (Safe-T-Pro-Plus; Roche Diagnostics, Tokyo, Japan) for the measurement of blood lactate concentration. The countermovement jumps and MVIC were reassessed after a 12-minutes recovery following the completion of the second set. Thereafter, the participants drank 500 mL of either HW or PW within 5 minutes. On Days 2 and 3, the same series of experimental protocols were repeated at the same clock time as Day 1. Fasted blood samples were collected from an antecubital vein 7 hours before the first exercise test (Day 1), and 16 hours after the completion of each exercise test (Days 2, 3, and 4). Whole blood was centrifuged for 15 minutes (3000 r/min, 4°C) and serum samples were separated and stored at –80°C.
Evaluation of exercise performances
The height of the countermovement jumps and the value of the MVIC of knee extensors were evaluated twice before and after the sprint cycling on Day 1 to Day 3. The highest values of these variables before and after the sprint cycling were averaged on each experimental day. With regards to the MVIC, the values of the right and left legs were further averaged.
The six peak and mean power outputs recorded during 10-second maximal pedaling (two sets of three repetitions) were averaged on each experimental day.
Analysis of blood samples
Blood lactate concentration was measured using an automated lactate analyzer (Lactate Pro2; Arkray, Tokyo, Japan).
We analyzed diacron-reactive oxygen metabolites (d-ROMs) and the biological antioxidant potential (BAP) as oxidative stress markers as previously described10,13,15,17; this is an estimation method of free radicals, including ROS. For the detection of d-ROMs, the reaction of N,N-diethyl-para-phenylenediamine with hydrogen peroxide in the serum was measured by the colorimetric change at an absorbance of 505 nm by the specific free radical analyzer (FREE carpe diem, Wismerll Co., Ltd., Tokyo, Japan). The d-ROM values were presented as Carratelli units based on the calculated absorption. The values of BAP in serum were also determined using the same apparatus, in which the reduction of FeCl3 was detected as the disappearance of the raddish colour.18 In addition, the values of total antioxidant potential in serum was expressed as BAP/d-ROMs.
Statistical analysis
All data are presented as the mean ± standard error. Concerning the oxidative stress markers (d-ROMs, BAP, and BAP/d-ROMs), the ratio of changes at Days 2, 3, and 4 relative to the baseline levels at Day 1 were indicated, as well as their absolute values. Two-way repeated measures analysis of variance (ANOVA) was applied to the main effects (water and day) and the interaction (water × day). If the ANOVA confirmed a significant interaction, a paired t-test was conducted between two different water conditions within same day and one-way repeated measures ANOVA was performed within the same water condition. With regards to multiple comparisons, the P-value was adjusted according to Benjamini-Hochberg’s procedure, and we revealed the adjusted P-value as a result. A value of P < 0.05 or adjusted P < 0.05 was considered statistically significant.
RESULTS
Exercise performance and blood lactate accumulation in physically active males
Table 1 shows the alteration in exercise performance and blood lactate concentration in the exercise tests on Day 1 to Day 3. No significant interaction and main effects of water and day were observed with respect to the height of the countermovement jump (interaction, F2,14 = 1.28 , P = 0.31, η2p = 0.15; water, F1,7 < 0.01, P = 0.93, η2p < 0.01; day, F2,14 = 0.79, P = 0.47, η2p = 0.10), and MVIC of knee extensors (interaction, F2,14 < 0.01, P = 0.99, η2p < 0.01; water, F1, 7 = 1.05, P = 0.34, η2p = 0.13; day, F2,14 = 0.42, P = 0.67, η2p = 0.06). For the peak and mean power outputs during sprint cycling, no significant interaction (water × day) was observed (peak power, F2,14 = 0.11, P = 0.90, η2p = 0.01; mean power, F2,14 = 1.95, P = 0.18, η2p = 0.22). Moreover, there was no significant main effect with respect to water (peak power, F1,7 = 2.17, P = 0.18, η2p = 0.24; mean power, F1,7 = 1.70, P = 0.23, η2p = 0.20), but there was a significant effect observed with regards to day (peak power, F2,14 = 5.03, P = 0.02, η2p = 0.42; mean power, F2,14 = 4.10, P = 0.04, η2p = 0.37). Multiple comparisons revealed that both the peak and mean power outputs at Day 2 were significantly greater than those at Day 1 (peak power, adjusted P = 0.02; mean power, adjusted P = 0.03). There were no significant interaction and main effects of water and day in terms of blood lactate concentration after the second set of sprint cycling (interaction, F2,14 = 2.69, P = 0.10, η2p = 0.28; water, F1,7 = 0.12, P = 0.74, η2p = 0.02; day, F2,14 = 1.00, P = 0.39, η2p = 0.13).
Table 1.
Alteration in exercise performance and blood lactate concentration over 3 consecutive days of exercise in males
| Day 1 | Day 2 | Day 3 | Two-way analysis of variance | |||
|---|---|---|---|---|---|---|
| Interaction | Water | Day | ||||
| Height of countermovement jump (cm) | ||||||
| PW | 56.9±1.4 | 57.4±1.0 | 59.3±1.2 | n.s. | n.s. | n.s. |
| HW | 57.8±1.1 | 58.6±1.1 | 57.6±0.8 | |||
| MVIC of knee extensors (N) | ||||||
| PW | 285.2±10.9 | 286.2±10.3 | 280.6±10.3 | n.s. | n.s. | n.s. |
| HW | 273.8±15.1 | 274.6±14.0 | 270.2±15.8 | |||
| Peak power output during 10 s maximal pedaling (W) | ||||||
| PW | 902.0±35.4 | 909.2±36.0 | 911.8±34.7 | n.s. | n.s. | P < 0.05 |
| HW | 894.2±32.4 | 903.9±33.9 | 902.7±33.0 | |||
| Mean power output during 10 s maximal pedaling (W) | ||||||
| PW | 812.7±33.8 | 825.3±33.7 | 827.5±33.4 | n.s. | n.s. | P < 0.05 |
| HW | 813.7±30.8 | 818.0±33.0 | 817.6±32.6 | |||
| Blood lactate concentration (mM) | ||||||
| PW | 15.5±1.0 | 14.1±0.9 | 16.1±1.0 | n.s. | n.s. | n.s. |
| HW | 15.3±1.1 | 15.1±1.0 | 14.5±0.7 | |||
Note: Blood lactate concentration was assessed 2 minutes after the completion of the second set of sprint cycling. Values are expressed as mean ± standard error. With regards to the peak and mean power outputs in 10-second maximal pedaling, significant main effects of day were found. Post-hoc analysis revealed that the values of the peak and mean power outputs at Day 2 were significantly greater than those at Day 1 (both, adjusted P < 0.05). HW: Molecular hydrogen-rich water; MVIC: maximum voluntary isometric contraction; n.s.: not significant; PW: placebo water.
Oxidative stress responses in physically active males
Table 2 demonstrates the changes in serum oxidative stress markers (d-ROMs, BAP, and BAP/d-ROMs) throughout the experiment. In terms of d-ROMs, no significant interaction was identified between water and day (F3,21 = 2.69, P = 0.10, η2p = 0.28); however, a significant main effect for day (F3, 21 = 3.24, P = 0.04, η2p = 0.32), without water (F1,7 = 3.78, P = 0.09, η2p = 0.35) was observed. Multiple comparison indicated that the values of d-ROMs at Day 4 were significantly higher than those at Day 3 (adjusted P = 0.03). Additionally, there was a trend for interaction with regard to BAP (F3,21 = 2.75, P = 0.07, η2p = 0.28), but no significant main effects of water and day were detected (water, F1,7 = 1.59, P = 0.25, η2p = 0.19; day, F3,21 = 0.34, P = 0.80, η2p = 0.05). With regards to the BAP/d-ROMs, a significant interaction between water and day was found (F3, 21 = 3.24, P = 0.04, η2p = 0.32); the BAP/d-ROMs in HW trial on Day 1 to Day 3 were significantly lower than PW trial (P < 0.05). Moreover, the BAP/d-ROMs in PW trial were significantly reduced from those recorded on Day 3 and Day 4 (adjusted P < 0.01).
Table 2.
Changes in blood oxidative stress markers over 3 consecutive days of exercise in males
| Day 1 | Day 2 | Day 3 | Day 4 | Two-way analysis of variance | |||
|---|---|---|---|---|---|---|---|
| Interaction | Water | Day | |||||
| Diacron-reactive oxygen metabolites (Carratelli unit) | |||||||
| PW | 266.5±8.9 | 268.1±9.8 | 260.6±6.8 | 273.1±9.5 | n.s. | P = 0.09 | P < 0.05 |
| HW | 297.6±17.2 | 303.4±13.8 | 283.8±18.5 | 303.5±14.3 | |||
| Biological antioxidant potential (μM) | |||||||
| PW | 2272.5±75.3 | 2291.9±80.5 | 2319.6±50.5 | 2136.0±57.2 | P = 0.07 | n.s. | n.s. |
| HW | 2149.6±64.0 | 2184.5±54.4 | 2163.6±45.7 | 2233.5±65.0 | |||
| Biological antioxidant potential/ diacron-reactive oxygen metabolites (arbitrary unit) | |||||||
| PW | 8.6±0.4 | 8.6±0.2 | 8.9±0.2 | 7.9±0.2# | P < 0.05 | – | – |
| HW | 7.3±0.3† | 7.3±0.3† | 7.8±0.4† | 7.5±0.4 | |||
Note: Values are expressed as mean ± standard error. †P < 0.05, vs. PW; #adjusted P < 0.05, vs. Day 3. For diacron-reactive oxygen metabolites, a significant main effect of day was found; post-hoc analysis revealed that the diacron-reactive oxygen metabolite values at Day 4 were significantly higher than those at Day 3 (adjusted P < 0.05). PW: Placebo water; HW: molecular hydrogen-rich water; n.s.: not significant.
Since significant differences in BAP/d-ROMs between HW and PW trials were observed on Day 1 (baseline level), the ratio of changes in oxidative stress markers (d-ROMs, BAP, and BAP/d-ROMs) on Day 2, Day 3, and Day 4 compared to those at Day 1 is shown in Figure 3. No significant interaction (F2,14 = 0.94, P = 0.41, η2p = 0.12) and main effect of water (F1,7 < 0.01, P = 0.99, η2p < 0.01) were found; however, a significant main effect of day (F2,14 = 7.43, P < 0.01, η2p = 0.52) was identified in the relative changes of d-ROMs (Figure 3A). Changes in d-ROMs were significantly reduced from Day 2 to Day 3 (adjusted P = 0.04) but were elevated again from Day 3 to Day 4 (adjusted P < 0.01). Regarding changes in BAP, no significant interaction and main effects of water and day were observed (Figure 3B; interaction, F2,14 = 3.12, P = 0.08, η2p = 0.31; water, F1,7 = 1.59, P = 0.25, η2p = 0.19; day, F2,14 = 0.48, P = 0.63, η2p = 0.07). A significant interaction was detected between water and day regarding changes in BAP/d-ROMs (Figure 3C; F2,14 = 3.83, P = 0.04, η2p = 0.35); changes in BAP/d-ROMs in PW trial were significantly decreased at Day 4 compared to those at Day 2 and Day 3 (adjusted P < 0.05, respectively). Moreover, changes in BAP/d-ROMs on Day 4 were marginally lower in PW trial than in HW trial (P = 0.08).
Figure 3.
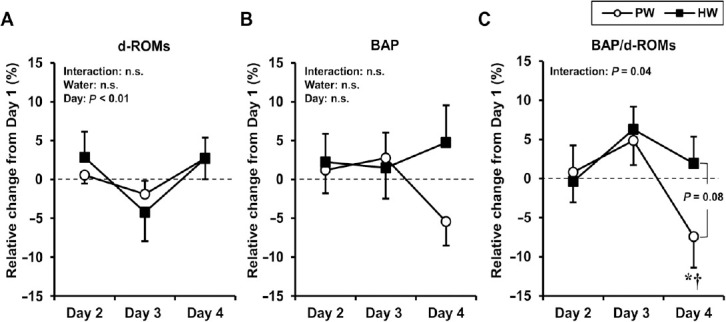
Relative changes in oxidative stress markers from baseline (Day 1) over 3 consecutive days of exercise in males.
Note: (A) d-ROMs; (B) BAP; (C) BAP/d-ROMs. Values are expressed as mean ± standard error. Two-way repeated measures analysis of variance was performed for each variable. *adjusted P < 0.05, vs. Day 2; †adjusted P < 0.05, vs. Day 3. BAP: Biological antioxidant potential; d-ROMs: diacron-reactive oxygen metabolites; HW: molecular hydrogen-rich water; PW: placebo water.
DISCUSSION
To the best of our knowledge, this is the first study to investigate whether drinking HW suppresses the accumulative muscle fatigue and oxidative damage induced by 3 straight days of strenuous exercise. A novel finding from this study was that changes in the BAP/d-ROMs relative to the baseline, as an index of systemic antioxidant potential, were decreased in PW trial but maintained by intake of HW during the 3 consecutive days of strenuous exercise.
We have previously examined the effects of cooling intervention using ice packs to the quadriceps muscle on exercise performance during 3 successive days of intense exercise.19 The results demonstrated that this cooling strategy significantly attenuated the reduction in leg muscle performances (i.e., the peak and mean power outputs in 10-second maximal pedaling and MVIC of knee extensors) that were observed in the non-cooling control trial and completely suppressed the exercise-induced increase in serum creatine kinase and lactate dehydrogenase activities during the 3 consecutive days of exercise.19 Moreover, the reduction in muscle performance might be, at least in part, due to increased ROS-induced oxidative damage.2,3 Accordingly, the present study adopted the same exercise protocol as our previous investigation, in which the 3-day accumulative fatigue could impair the muscle performance and redox status. Contrary to our expectation, any exercise performances assessed in this study were not reduced as the day passed (Table 1), or rather, the peak and mean power outputs were slightly, but significantly increased from Day 1 to Day 2, irrespective of the water effect. Although the reason for this discrepancy between these studies remains unknown, the smaller volume of exercise (4-time measurements of countermovement jumps and MVIC, and only 12-minute sprint cycling per day) might be somewhat related to the unchanged exercise performance. Indeed, one previous study suggested that a muscle damaging exercise protocol (300-time eccentric contractions) induced the reduced peak torque of isokinetic muscle contraction of knee extensors and the elevated oxidative damages estimated by increases in protein carbonyls and thiobarbituric acid-reactive substances.20 Taken together, future studies considering the exercise protocol (volume, mode, intensity, and so on) are required.
The excessive disruption in the redox status has a harmful impact on physiological homeostasis, which could be a trigger for injury, disease, and the lack of training adaptations.1,2 The BAP/d-ROMs is an index of an individual’s antioxidant potential against oxidative attack, such as that observed during vigorous exercise.21 Moreover, as a previous study has reported that HW significantly elevated serum BAP/d-ROMs,12 we measured d-ROMs and BAP in the present study. In PW trial, the relative change in serum BAP/d-ROMs was drastically reduced from Day 3 to Day 4 (Figure 2C). Although the precise underlying mechanism of the reduction in BAP/d-ROMs in PW remains unknown, we speculate that a synergistic effect of the increase in oxidative damage and the decrease in antioxidant capacity by 3-day repetition of a strenuous exercise session might be involved. Indeed, previous studies have suggested that the activity of xanthine oxidase in endothelial cells, which is known as one of the main sources for post-exercise ROS generation,22 was increased 3.2–3.5 folds by a single bout of 20-second sprint cycling 24 hours after the completion of exercise.23 In addition, the degree of activation by xanthine oxidase was negatively associated with the peak and mean power outputs during the anaerobic exercise.23 Accordingly, the increased mean and peak power outputs at Day 2 might accelerate the generation of ROS during the recovery phase after the exercise test at Day 3, which in turn, may function to elevate the serum d-ROMs from Day 3 to Day 4 (Table 2 and Figure 3A). In contrast, GSH (reduced glutathione, an antioxidant marker) was temporarily decreased by acute sprint cycling,24 and gradually decreased following consecutive days of strenuous exercise as the day passed.20 Moreover, acute intense exercise (165-km cycling competition) decreased neutrophil antioxidant enzyme activities (catalase, superoxide dismutase, and glutathione peroxidase).25 Although the exercise volume in the present study was relatively small, these findings raise the possibility that the repetition of strenuous exercise impairs these antioxidant capacities. Consequently, changes in the BAP/d-ROMs at Day 4 were significantly reduced from Day 2 and Day 3.
Interestingly, HW suppressed the reduction in BAP/d-ROMs from the baseline levels during all the experimental days. There is a growing body of evidence suggesting that H2 suppresses exercise-induced oxidative stress in both animals10,11,12 and humans9; the findings of the current study are in accordance with these previous results. Furthermore, in a recent study, HW attenuated the GSH depletion by pyocyanin (as an inducer of hydroxylradical), and thereby suppressed the increase in lipid peroxide and cellular senescence in a primary mouse embryonic fibroblast cell culture model.26 Additionally, drinking HW increased the gene expression and/or activities of cellular and blood antioxidant enzymes, including catalase, superoxide dismutase, and glutathione peroxidase.14,27,28,29,30,31 Collectively, the maintenance of BAP/d-ROMs in HW trial might be due to the attenuation of the reduction of these antioxidant potentials. However, we must acknowledge that these explanations are highly speculative, as we did not evaluate other potential oxidative stress markers. Hence, future studies are required to investigate the ergogenic and therapeutic effect of HW with extensive analysis of oxidative stress markers.
The present study has some limitations. First, our exercise protocol did not induce decrements in exercise performance and an elevation in oxidative damages when compared to baseline. Accordingly, the magnitude of therapeutic benefits of HW in preventing muscle fatigue and in keeping adequate redox status, under the more severe exercise protocol, remains unclear. Second, unexpected differences between the two trials in the BAP/d-ROMs at Day 1 made it difficult to interpret the present results. Third, as the beverages used in the present study were blinded only for the participants, we may have under- or over-estimated the effects of HW on physiological responses. Finally, the sample size (n = 8) in the present study was quite small; since the relative change in the BAP/d-ROMs at Day 4 was marginally higher in HW trial than PW trial (Figure 2C), a significant differences in redox status between the two trials might be detected with a larger sample size.
In conclusion, HW drinking attenuated the reduction in BAP/d-ROMs during consecutive days of strenuous exercise. Our findings suggest HW drinking as a useful strategy to maintain good physical condition during athletic competition or training camps.
Acknowledgements
The authors thank all participants for their time and effort.
Footnotes
Conflicts of interest
Ecomo International supplied the hydrogen generating kits.
Financial support
None.
Institutional review board statement
The study procedures were approved by the Human Research Ethics Committee of the University of Yamanashi, Japan (approval No. H26-008) on December 17, 2014 and were performed in accordance with the guidelines of the Declaration of Helsinki.
Informed consent statement
The authors certify that they have obtained all participants’ appropriate informed consent forms. In the form the participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published.
Reporting statement
This study followed the CONsolidated Standards Of Reporting Trials (CONSORT) Statement.
Copyright transfer agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
REFERENCES
- 1.Koyama K. Exercise-induced oxidative stress: A tool for “hormesis” and “adaptive response”. J Phys Fit Sports Med. 2014;3:115–120. [Google Scholar]
- 2.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid MB. Reactive oxygen species as agents of fatigue. Med Sci Sports Exerc. 2016;48:2239–2246. doi: 10.1249/MSS.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 4.Powers SK, Radak Z, Ji LL. Exercise-induced oxidative stress: past, present and future. J Physiol. 2016;594:5081–5092. doi: 10.1113/JP270646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther. 2014;144:1–11. doi: 10.1016/j.pharmthera.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Fujita R, Tanaka Y, Saihara Y, Yamakita M, Ando D, Koyama K. Effect of molecular hydrogen saturated alkaline electrolyzed water on disuse muscle atrophy in gastrocnemius muscle. J Physiol Anthropol. 2011;30:195–201. doi: 10.2114/jpa2.30.195. [DOI] [PubMed] [Google Scholar]
- 7.Guan P, Sun ZM, Luo LF, et al. Hydrogen protects against chronic intermittent hypoxia induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 2019;225:46–54. doi: 10.1016/j.lfs.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Ostojic SM. Molecular hydrogen in sports medicine: new therapeutic perspectives. Int J Sports Med. 2015;36:273–279. doi: 10.1055/s-0034-1395509. [DOI] [PubMed] [Google Scholar]
- 9.Koyama K, Tanaka Y, Saihara Y, Ando D, Coto Y, Katayama A. Effect of hydrogen saturated alkaline electrolyzed water on urinary oxidative stress markers after an acute exercise: A randomized controlled trial. Anti Aging Med. 2008;4:117–122. [Google Scholar]
- 10.Yamazaki M, Kusano K, Ishibashi T, Kiuchi M, Koyama K. Intravenous infusion of H2-saline suppresses oxidative stress and elevates antioxidant potential in Thoroughbred horses after racing exercise. Sci Rep. 2015;5:15514. doi: 10.1038/srep15514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogueira JE, Passaglia P, Mota CMD, et al. Molecular hydrogen reduces acute exercise-induced inflammatory and oxidative stress status. Free Radical Biol Med. 2018;129:186–193. doi: 10.1016/j.freeradbiomed.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Tsubone H, Hanafusa M, Endo M, et al. Effect of treadmill exercise and hydrogen-rich water intake on serum oxidative and anti-oxidative metabolites in serum of thoroughbred horses. J Equine Sci. 2013;24:1–8. doi: 10.1294/jes.24.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoki K, Nakao A, Adachi T, Matsui Y, Miyakawa S. Pilot study: Effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med Gas Res. 2012;2:12. doi: 10.1186/2045-9912-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ara J, Fadriquela A, Ahmed MF, et al. Hydrogen water drinking exerts antifatigue effects in chronic forced swimming mice via antioxidative and anti-inflammatory activities. Biomed Res Int. 2018;2018:2571269. doi: 10.1155/2018/2571269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamura T, Gando Y, Takahashi M, et al. Effects of hydrogen bathing on exercise-induced oxidative stress and delayed-onset muscle soreness. J Phys Fit Sports Med. 2016;65:297–305. [Google Scholar]
- 16.Kawamura T, Suzuki K, Takahashi M, et al. Involvement of neutrophil dynamics and function in exercise-induced muscle damage and delayed-onset muscle soreness: effect of hydrogen bath. Antioxidants (Basel) 2018;7:127. doi: 10.3390/antiox7100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugita M, Kapoor MP, Nishimura A, Okubo T. Influence of green tea catechins on oxidative stress metabolites at rest and during exercise in healthy humans. Nutrition. 2016;32:321–331. doi: 10.1016/j.nut.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Carratelli M, Porcaro L, Ruscica M, De Simone E, Bertelli AA, Corsi MM. Reactive oxygen metabolites and prooxidant status in children with Down’s syndrome. Int J Clin Pharmacol Res. 2001;21:79–84. [PubMed] [Google Scholar]
- 19.Koyama K, Mochizuki C, Dobashi S, Takeuchi K, Horiuchi M. Cooling attenuates the reduction in muscle performance during repeated three consecutive days of strenuous exercise. Med Sci Sports Exerc. 2015;47:196. [Google Scholar]
- 20.Sakelliou A, Fatouros IG, Athanailidis I, et al. Evidence of a redox-dependent regulation of immune responses to exercise-induced inflammation. Oxid Med Cell Longev. 2016;2016:2840643. doi: 10.1155/2016/2840643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Hashimoto J, Suzuki T, Satoh A. The effects of exercise load during development on oxidative stress levels and antioxidant potential in adulthood. Free Radical Res. 2017;51:179–186. doi: 10.1080/10715762.2017.1291939. [DOI] [PubMed] [Google Scholar]
- 22.Bloomer RJ, Goldfarb AH. Anaerobic exercise and oxidative stress: a review. Can J Appl Physiol. 2004;29:245–263. doi: 10.1139/h04-017. [DOI] [PubMed] [Google Scholar]
- 23.Wiecek M, Maciejczyk M, Szymura J, Kantorowicz M, Szygula Z. Impact of single anaerobic exercise on delayed activation of endothelial xanthine oxidase in men and women. Redox Rep. 2017;22:367–376. doi: 10.1080/13510002.2016.1238991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuevas MJ, Almar M, García-Glez JC, et al. Changes in oxidative stress markers and NF-kappaB activation induced by sprint exercise. Free Radical Res. 2005;39:431–439. doi: 10.1080/10715760500072149. [DOI] [PubMed] [Google Scholar]
- 25.Sureda A, Ferrer MD, Tauler P, et al. Intense physical activity enhances neutrophil antioxidant enzyme gene expression Immunocytochemistry evidence for catalase secretion. Free Radical Res. 2007;41:874–883. doi: 10.1080/10715760701416459. [DOI] [PubMed] [Google Scholar]
- 26.Sakai T, Kurokawa R, Hirano SI, Imai J. Hydrogen indirectly suppresses increases in hydrogen peroxide in cytoplasmic hydroxyl radical-induced cells and suppresses cellular senescence. Int J Mol Sci. 2019;20:456. doi: 10.3390/ijms20020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeBaron TW, Kura B, Kalocayova B, Tribulova N, Slezak J. A new approach for the prevention and treatment of cardiovascular disorders molecular hydrogen significantly reduces the effects of oxidative stress. Molecules. 2019;24:2076. doi: 10.3390/molecules24112076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan J, Wang D, Liu Y, et al. Hydrogen-rich water attenuates oxidative stress in rats with traumatic brain injury via Nrf2 pathway. J Surg Res. 2018;228:238–246. doi: 10.1016/j.jss.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Murakami Y, Ito M, Ohsawa I. Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PLoS One. 2017;12:e0176992. doi: 10.1371/journal.pone.0176992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasegawa S, Ito M, Fukami M, Hashimoto M, Hirayama M, Ohno K. Molecular hydrogen alleviates motor deficits and muscle degeneration in mdx mice. Redox Rep. 2017;22:26–34. doi: 10.1080/13510002.2015.1135580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J Clin Biochem Nutr. 2010;46:140–149. doi: 10.3164/jcbn.09-100. [DOI] [PMC free article] [PubMed] [Google Scholar]