Abstract
Joint trauma leads to post-traumatic inflammation with upregulation of inflammatory cytokines and degradative enzymes. If severe enough, this response can lead to irreversible post-traumatic osteoarthritis. Interleukin-10 (IL-10), a cytokine with potent anti-inflammatory effects, has been shown to have chondroprotective effects. A gene therapy approach using a vector to overexpress IL-10 in the joint represents a feasible method of delivering sustained high doses of IL-10 to post-traumatic joints. We hypothesized that an AAV5 vector overexpressing IL-10 would result in rapid and sustained IL-10 expression following direct intra-articular injection and that this increase would not be reflected in systemic circulation. In addition, we hypothesized that intra-articular AAV5-IL-10 injection would not induce a local inflammatory response. Twelve horses were assigned to either treatment (AAV5-IL-10-injected) or control (PBS-injected) groups. Middle carpal joints were injected with 1012 vector genomes/joint or phosphate-buffered saline (PBS) alone (3 mL). Serial synovial fluid samples were analyzed for inflammatory changes, IL-10 concentration, and vector genome copy number. Serum samples were also analyzed for IL-10 concentration and vector genome copy number. Synovial membrane was collected on day 84. Synovial fluid IL-10 was significantly increased within 48 h of AAV5-IL-10 injection and remained increased, compared to PBS-injected joints, until day 84. Serum IL-10 was not different between groups. Vector administration did not cause a significant synovial inflammatory response. Vector genomes were detectable in the plasma, synovial fluid, and synovial membrane of AAV5-IL-10-injected horses only. IL-10 has the potential to modulate the articular inflammatory response, thereby protecting cartilage from degradation and osteoarthritis. This study demonstrates the feasibility and efficiency of intra-articular AAV5-IL-10, and future studies investigating the chondroprotective effects of IL-10 in inflamed joints in vivo are warranted.
Keywords: osteoarthritis, arthritis, gene therapy, AAV, IL-10, immunomodulation
Introduction
Osteoarthritis (OA), a progressive and degenerative joint disease, is estimated to affect more than 30 million people in the United States, accounting for 16.5 billion dollars in combined health care costs in 2013.1,2 Post-traumatic osteoarthritis (PTOA) is defined as OA that develops after known joint injury and/or cartilage damage and is especially prevalent in athletes and the veteran population of the U.S. armed forces.3,4 If sufficient, joint injury incites a post-traumatic inflammatory cascade with upregulation of inflammatory and catabolic cytokines by activated synoviocytes and chondrocytes, including increased production of interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α).5,6 Intra-articular inflammation leads to loss of homeostasis in the extracellular matrix (ECM) of the articular cartilage. Catabolic cytokines downregulate production of the matrix proteins collagen type II and aggrecan while upregulating production of degradative enzymes, including matrix metalloproteinases and aggrecanases.5,7 Currently there are no effective disease-modifying OA drugs that halt or reverse OA; therefore, a therapeutic intervention that mitigates the acute post-traumatic inflammatory cascade could be greatly beneficial.
Interleukin-10 (IL-10), a cytokine with potent anti-inflammatory properties, is produced mainly by immune cells. Recently, IL-10 has been shown to play a role in cartilage disorders due to downregulation of OA cytokines, specifically IL-1β, TNF-α, IL-6, and PGE2.8–10
IL-10 has also been shown to have antiapoptotic effects on chondrocytes in vitro11 and to support connective tissue ECM homeostasis through downregulation of matrix metalloproteinases.8,9 Previous in vitro studies in our laboratory have demonstrated mitigation of the inflammatory cascade associated with overexpression of IL-10 by an adeno-associated virus (AAV) vector.10 Chondroprotective effects of IL-10 have also been demonstrated in vivo in several murine models of inflammatory arthritis.12,13
Gene therapy may be an ideal therapy for joint disease as therapeutic proteins can be produced locally, limiting the widespread effects of systemically administered vectors. In addition, gene therapy provides long-term transgene expression resolving the need for repeat intra-articular injection of traditional therapeutics.14 Although several viral vectors have been investigated, AAV-mediated gene therapy for joint disease has generated the greatest interest among musculoskeletal researchers due to its lack of pathogenicity, minimal immunogenicity, capacity for long-term transgene expression, and ability to transduce dividing and nondividing cell populations, even those within a thick ECM.15,16 Previous studies, in our laboratory and others, have demonstrated efficient transduction of synovial tissues with AAV vectors.16–18
The objectives of this study were to investigate the intra-articular response to vector injection, including quantifying the viral vector and transgene production in the joint over time. We also sought to investigate the systemic distribution of the transgene product and the viral vector. We hypothesized that direct intra-articular injection of AAV5-IL-10 would lead to rapid and sustained expression of IL-10; however, systemic increases in IL-10 would not be detectable. We also hypothesized that vector genomes would be detectable in AAV5-injected horses and would not be detectable in phosphate-buffered saline (PBS)-injected horses.
Materials and Methods
Animals
Twelve systemically healthy adult horses between the ages of 3 and 13 years (median age, 9 years) were enrolled in the study, consisting of nine Thoroughbreds and three Standardbreds of which six were castrated males and six were females. Horses were randomly divided into two treatment groups: PBS (n = 6) and AAV5-IL-10 (n = 6). All animal procedures were approved by the Institutional Laboratory Animal Care and Use Committee at the University of Pennsylvania.
Adeno-associated viral vector production
Equine IL-10 was derived from the liver of a 2-year-old Thoroughbred horse immediately following euthanasia. The sequence was amplified by polymerase chain reaction (PCR) using primers for equine IL-10 (GenBank Accession No. U38200.1). Full-length equine IL-10 cDNA was subcloned into the rAAV transfer plasmid pHpa-trs-SK using SacII and Not sites. The transgene was flanked by inverted terminal repeats and under control of the CMV promoter. Self-complementary AAV5-IL-10 was generated by the University of North Carolina Vector Core in HEK293 cells using the triple plasmid transfection method.19 The three plasmids included the following: (i) rAAV-IL-10, (ii) the AAV rep and cap genes, and (iii) the adenovirus helper virus. The AAV5-IL-10 vector was purified by column chromatography, and viral titers were determined using semiquantitative dot blot.
Intra-articular injections
Intra-articular injection was performed in all horses following sedation with xylazine hydrochloride (0.4 mg/kg, intravenous). Middle carpal joints were clipped and aseptically prepared. A 21-gauge, 1.5-inch needle was inserted into the dorsolateral aspect of the joint with the limb held in flexion. Synovial fluid was collected from the joint before injection with 1 × 1012 viral genomes in 3 mL of sterile PBS or 3 mL of PBS. Horses in the AAV5-IL-10 treatment group were randomly assigned to have one middle carpal joint injected with AAV5-IL-10 and the contralateral middle carpal joint injected with PBS. Horses in the PBS treatment group only had one middle carpal joint (randomly assigned) injected with PBS.
Effects of intra-articular injection
Physical examinations and joint circumference measurements were performed before initial injection on day 0 and then repeated on days 1, 2, 4, 7, 14, 28, 56, and 84. Physical examinations included heart rate, respiratory rate, and rectal temperature. Joint circumference was measured at the level of the middle carpal bone 4 cm distal to the lateral styloid process. Synovial fluid was collected immediately before AAV5-IL-10 injection and then on days 1, 2, 4, 7, 14, 28, 56, and 84. Synovial fluid was aliquoted, and one aliquot was immediately submitted for nucleated cell count (NCC) and total protein (TP) concentration analysis, one aliquot was stored at −80°C for cytokine analysis, and one aliquot was stored at −80°C for future vector genome quantification. Whole blood was also collected in a serum blood collection tube immediately before AAV injection and then on days 1, 2, 4, 7, 14, 28, 56, and 84. After 30 min, whole blood was centrifuged at 3,000 g for 10 min at 4°C. Serum was collected, placed in a new tube, and stored at −80°C for future analysis. The concentration of IL-10, IL-1β, IL-6, IL-17a, TNF-α, and IFN-γ in synovial fluid was quantified using a fluorescent bead-based multiplex assay (Luminex, Austin, TX) using anti-equine antibodies (Millipore Sigma, Burlington, MA). Serum was analyzed for IL-10 concentration using a fluorescent bead-based single-plex assay (Luminex).
Synovial membrane analysis
On day 84, horses were sedated with xylazine hydrochloride (0.4 mg/kg, intravenous), and synovial membrane biopsies were performed in the dorsal joint space of middle carpal joints. Following sterile preparation and injection of a local anesthetic (2% mepivacaine), a scalpel was used to incise the skin and joint capsule of the dorsolateral aspect of the middle carpal joint. A Ferris-Smith rongeur was used to collect synovium. Samples were fixed in 4% paraformaldehyde or snap frozen in liquid nitrogen and stored at −80°C until RNA analysis. Fixed synovial membrane samples were embedded in paraffin, sectioned, and stained with hematoxylin and eosin for scoring using a previously published microscopic grading system (Table 1).20 Frozen synovial membrane samples were pulverized in liquid nitrogen using a multiple sample stainless steel biopulverizer and hammer (BioSpec Products, Inc., Bartlesville, OK). The Qiagen RNeasy Fibrous Tissue Mini Kit (Qiagen, Germantown, MD) was then used to complete RNA isolation. For all samples, RNA concentration and purity were quantified using a UV microspectrophotometer (NanoDrop™ One; Thermo Fisher Scientific, Waltham, MA). Complementary DNA was prepared using a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA) and an Eppendorf Mastercycler (Hamburg, Germany). Real-time quantitative PCR was performed using TaqMan™ Master mix and the Applied Biosystems™ QuantStudio™ 6 Flex Real-Time PCR System (Thermo Fisher Scientific). The primers and probe for IL-10 were obtained from Thermo Fisher Scientific's proprietary equine-specific gene expression assay database (ARCE46U). All samples were run in triplicate using 18S as a reference gene. Data were quantified using ΔΔCt comparisons.
Table 1.
Microscopic grading system for synovial membrane histology
| Outcome Parameter | Score | Description |
|---|---|---|
| Cellular infiltration | 0 | No mononuclear cells in the section |
| 1 | Occasional small areas of mononuclear cells throughout the section | |
| 2 | Mild presence of mononuclear cells in 25% of the section | |
| 3 | Moderate presence of mononuclear cells in 25–50% of the section | |
| 4 | Marked presence of mononuclear cells in greater than 50% of the section | |
| Vascularity | 0 | Normal |
| 1 | Slight increase in vessels in focal locations throughout the section | |
| 2 | Mild increase in number and dilatation of vessels in focal locations throughout the section | |
| 3 | Moderate increase in number and dilatation of vessels up to 50% of the section | |
| 4 | Marked increase in number and dilatation of vessels in greater than 50% of the section | |
| Intimal hyperplasia | 0 | None |
| 1 | Villi with 2–4 rows of intimal cells within the section | |
| 2 | Villi with 4–5 rows of intimal cells over 25–50% of the section | |
| 3 | Villi with 4–5 rows of intimal cells over 50% of the section | |
| 4 | Villi with 5 or greater rows of intimal cells over 50% of the section | |
| Subintimal edema | 0 | No edema |
| 1 | Slight edema detected within section | |
| 2 | Mild edema within 25% of the section | |
| 3 | Moderate edema within 25–50% of the section | |
| 4 | Marked edema in greater than 50% of the section | |
| Subintimal fibrosis | 0 | Normal |
| 1 | Slight increase in fibrosis within the section | |
| 2 | Mild increase in fibrosis in 25% of the section | |
| 3 | Moderate increase in fibrosis in 25–50% of the section | |
| 4 | Marked increase in fibrosis in greater than 50% of the section |
Microscopic grading system for synovial membrane histology in the carpal osteochondral fragment model.20
Vector genome quantification
Whole blood (10 mL) was collected on days 0, 1, 2, 4, 7, 14, 28, 56, and 84 in a sodium heparin blood tube, and plasma was separated by centrifugation (3,000 g for 10 min at 4°C). Cell free DNA was extracted from the supernatant using MagMAX™ Cell-Free DNA Isolation Kit. Synovial fluid collected on days 0, 1, 2, 4, 7, 14, 28, 56, and 84 was centrifuged at 3,000 g for 5 min, transferred to a fresh tube, and then genomic DNA was extracted using MagMAX Cell-Free DNA Isolation Kit (Thermo Fisher Scientific).
DNA was also isolated from synovial membrane collected on day 84. Synovial membrane (20 g) was sectioned into small pieces using a sterile scalpel blade, and DNA extraction was then performed using DNeasy Blood & Tissue Kits (Qiagen). For all samples, DNA concentration and purity were quantified using a UV microspectrophotometer (NanoDrop™; Thermo Fisher Scientific).
The absolute copy number of AAV5-IL-10 was determined using a standard curve. The primers were designed using Primer Express™ Software v3.0.1 (Thermo Fisher Scientific). The forward primer 5′-TCTAGGCCTGTACGGAAGTGTTACT-3′ and the reverse primer 5′-AGCAGTGCTGAGCTGTGCAT-3′ were designed to span the AAV5 genome and the IL-10 transgene. Standard nucleotide fragments were prepared by PCR and purified using E-Gel Precast Agarose Electrophoresis System (Thermo Fisher Scientific) and QIAquick Gel Extraction Kit (Qiagen). A 10-fold serial dilution series of standards was used to generate standard curves (1 × 101–1 × 108 molecules per reaction). Quantitative PCRs were performed in triplicate on a 7500 real-time PCR instrument (Thermo Fisher Scientific).
Statistical analyses
Normality was assessed by visual inspection of histograms for a Gaussian distribution and a Shapiro–Wilk test before statistical analysis. p < 0.05 was considered to be statistically significant. Continuous values are expressed as mean ± standard error of the mean (SEM) unless otherwise stated. A mixed effects model was used to compare changes in synovial fluid composition and synovial fluid and serum cytokine concentration over time. Horse was considered a random effect, and day treated as a categorical variable to allow for the nonlinear effect of time. Treatment and interaction between treatment group and categorical time were considered fixed effects. Post hoc pairwise comparisons were made with Tukey's method adjustment for multiplex comparisons. Statistically significant differences between the treatment groups on individual days were also determined using a Student's t-test for normally distributed data or a Wilcoxon rank sum test for data that was not normally distributed. Differences were considered significant when p < 0.05. All statistical analyses were performed using STATA 15 (StataCorp LLC, College Station, TX).
Results
Intra-articular AAV5-IL-10 injection leads to long-term, local transgene expression
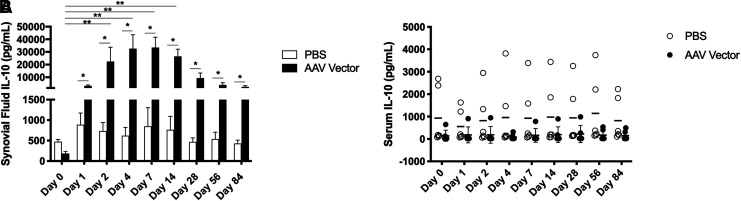
IL-10 concentration in synovial fluid from AAV5-IL-10-injected joints was elevated compared to its concentration in synovial fluid collected before injection and from PBS-injected joints as early as 1 day after injection. IL-10 concentration was significantly increased over baseline on days 2, 4, 7, and 14. When the two treatment groups were compared on each individual day, IL-10 concentration was significantly increased in AAV5-IL-10-injected joints compared to PBS-injected joints on days 1, 2, 4, 7, 14, 28, 56, and 84 (Fig. 1A). IL-10 concentration in the contralateral middle carpal joint of AAV5-IL-10 treated horses was not significantly different than baseline or control horses at any time point. Despite abundant IL-10 production within the middle carpal joint space, no increases in serum IL-10 were detected in AAV5-IL-10-injected horses compared to PBS-injected horses (Fig. 1B).
Figure 1.
(A) Mean IL-10 concentrations in synovial fluid (n = 6) and (B) serum IL-10 concentrations in individual horses. * Significant difference between two treatment groups, p < 0.05; ** significant difference from baseline, p < 0.05. IL-10, interleukin-10.
AAV5-IL-10 vector is detectable in synovial membrane and synovial fluid on day 84
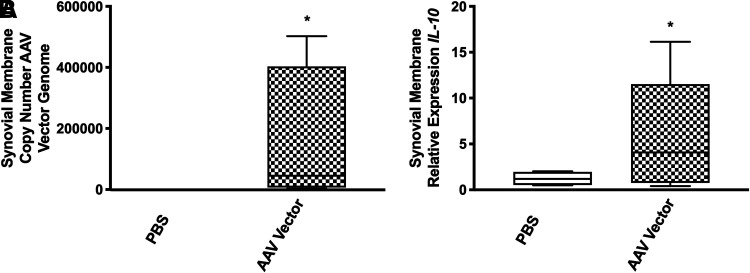
Synovial biopsies collected on day 84 were analyzed for copy number of AAV5-IL-10 vector genomes and IL-10 mRNA expression, both by quantitative reverse transcriptase-PCR. The copy number of AAV5-IL-10 vector genomes was detected in synovial membrane collected at day 84 from vector-injected horses compared to PBS-injected horses (p < 0.001) (Fig. 2A). The AAV5 vector was not detected in any of the PBS-injected horses. IL-10 expression was also significantly increased in the synovial membrane of AAV5-IL-10-injected joints at day 84 (p = 0.0036) (Fig. 2B).
Figure 2.
(A) Copy number of AAV5-IL-10 vector genomes in the synovial membrane 84 days postinjection. AAV5-IL-10 vector was not detected in the synovial membrane of PBS-injected joints. (B) IL-10 mRNA expression in the synovial membrane of PBS or AAV5-IL-10 injected horses 84 days postinjection. *p < 0.05. IL-10, interleukin-10; PBS, phosphate-buffered saline.
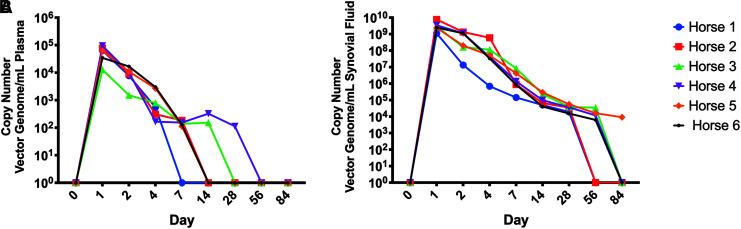
The copy number of AAV5 vectors in the plasma and synovial fluid was also assayed over time.
AAV5 vector genomes were present in the plasma of all AAV5 injected horses until day 4, with two horses having vector genomes detectable at day 14 (Fig. 3A). AAV5 vector genomes were present in synovial fluid of all AAV5 injected horses until day 28, with four horses having vector genomes detectable at day 56 (Fig. 3B). The mean copy number of AAV5 vectors in the synovial fluid was ∼105 fold greater than copy number of AAV5 vector in the plasma between days 1 and 28. No control horses had any detectable AAV5 vector genomes in plasma or synovial fluid at any time point.
Figure 3.
(A) Copy number of AAV5-IL-10 vector genomes present in plasma of vector injected horses. (B) Copy number of AAV5-IL-10 vector genomes present in the synovial fluid of vector injected horses. The AAV5-IL-10 vector was not detectable in plasma or synovial fluid from PBS-injected horses. IL-10, interleukin-10; PBS, phosphate-buffered saline.
Injection of the AAV vector did not cause a significant local inflammatory response
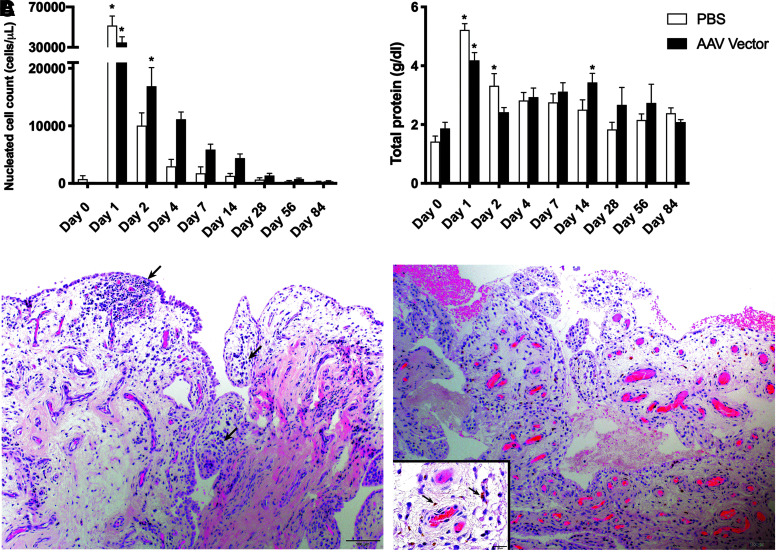
To assess the local synovial response to injection, the NCC and TP concentration in synovial fluid was assayed over time (Fig. 4A, B). In addition, joint circumference was used to assess effusion and periarticular swelling. Injection of both AAV5-IL-10 and PBS led to a very large increase in NCC and TP on day 1 compared to baseline. Before injection, mean NCC was 720 ± 620 cells/μL in the PBS treatment group and 130 ± 42.74 cells/μL in the AAV5-IL-10 treatment group. One day following injection, mean NCC was 51,550 ± 9,400.24 cells/μL in the PBS treatment group and 34,816.67 ± 5,584.39 cells/μL in the AAV5-IL-10 treatment group. NCC decreased steadily over time in both treatment groups and returned to baseline on day 2 in PBS-injected horses and on day 4 in AAV5-IL-10-injected horses. Similar to NCC, TP concentration was significantly increased compared to baseline on day 1 in both treatment groups. Before injection, mean TP was 1.42 ± 0.19 g/dL in the PBS treatment group and 1.87 ± 0.21 g/dL in the AAV5-IL-10 treatment group. One day following injection, mean TP was 5.23 ± 0.22 g/dL in the PBS treatment group and 4.18 ± 0.26 g/dL in the AAV5-IL-10 treatment group. TP returned to baseline on day 2 in AAV5-IL-10-injected horses and on day 4 in PBS-injected horses. Joint circumference was not significantly different between or within groups at any time point.
Figure 4.
(A) Nucleated cell count and (B) total protein concentration in synovial fluid over time. (C) H&E stained photomicrographs of equine synovium from PBS-injected (left panel) and AAV5-IL-10 injected (right panel) horses. Both samples comprise one to two layers of flattened to cuboidal synoviocytes supported by a loose fibromyxoid stroma with regularly interspersed blood vessels. Occasionally within the subintimal stroma are small aggregates of small lymphocytes and plasma cells (left, arrows) and scattered hemosiderin-laden macrophages (right, inset). * Significant difference from baseline, p < 0.05. H&E, hematoxylin and eosin; IL-10, interleukin-10; PBS, phosphate-buffered saline.
Synovial membrane biopsies collected on day 84 were also used to evaluate the general inflammatory response to injection (Fig. 4C). Synovial membrane collected from the middle carpal joint was scored for cellular infiltration, vascularity, intimal hyperplasia, subintimal edema, and subintimal fibrosis (Table 1). There was no significant difference in synovial membrane scores between treatment groups. The mean ± SEM total score in PBS-injected joints was 7.6 ± 2.41, while the mean ± SEM total score in AAV5-IL-10-injected joints was 9.17 ± 1.72.
Inflammatory cytokines are decreased in joints injected with AAV5-IL-10
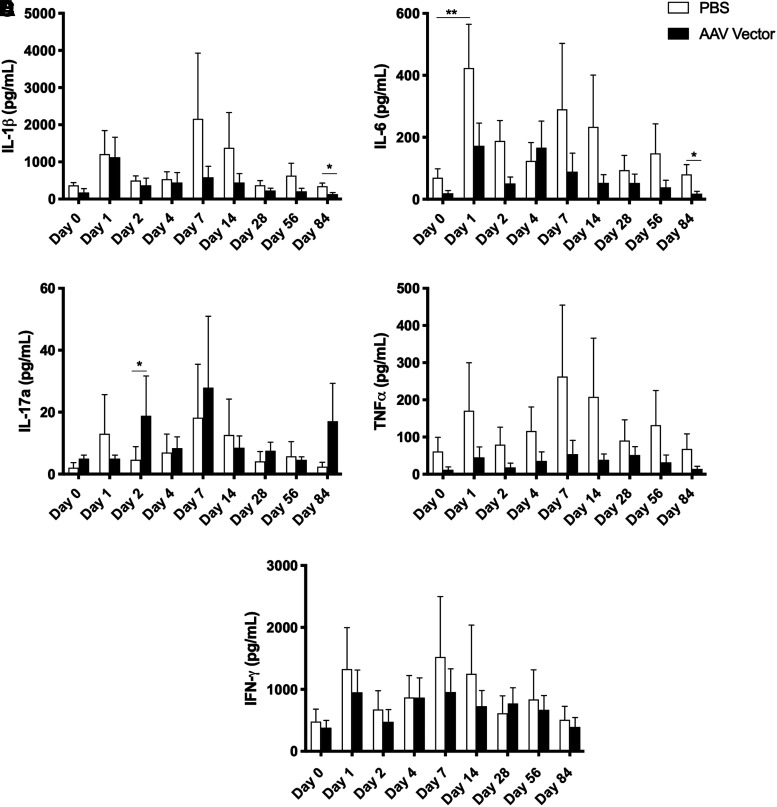
The inflammatory response to injection was further assessed by quantifying several inflammatory cytokines over time. A multiplex fluorescent bead-based assay was used to quantify the amount of IL-1β, IL-6, IL-17a, TNF-α, and IFN-γ in synovial fluid (Fig. 5).
Figure 5.
Concentrations of cytokines associated with the inflammatory response in synovial fluid over time, including (A) IL-1β, (B) IL-6, (C) IL-17a, (D) TNF-α, and (E) IFN-γ. * Significant difference between two groups, p < 0.05; ** significant difference from baseline, p < 0.05. IL, IL, interleukin; TNF-α, tumor necrosis factor-α.
IL-1β was not significantly elevated over baseline in either PBS-injected or AAV5-IL-10-injected joints. When the two treatment groups were compared on individual days, AAV5-IL-10-injected joints had significantly less IL-1β on day 84 than PBS-injected joints. IL-6 was significantly elevated over baseline in PBS-injected joints on day 1 only. At day 84, AAV5-IL-10-injected joints had significantly less IL-6 than control joints. IL-17a was significantly increased in AAV5-IL-10-injected joints compared to PBS-injected joints on day 2 only. There were no significant differences in the concentration of TNF-α or IFN-γ in AAV5-IL-10-injected or PBS-injected joints at any time point.
Discussion
This study demonstrated efficient and sustained overexpression of the immunomodulatory cytokine IL-10 following direct intra-articular injection of AAV5-IL-10 in normal equine middle carpal joints. Elevated IL-10 concentrations were present in the synovial fluid of AAV5-IL-10 injected joints starting 1 day following injection, and IL-10 concentrations remained high throughout the 84-day period, although concentrations peaked on day 7 postinjection. Despite elevated IL-10 in the synovial fluid, serum IL-10 concentrations did not differ between treated and control groups. Furthermore, AAV5-IL-10 injection did not cause a significant inflammatory response in the joint. We were also able to quantify the copy number of AAV5-IL-10 in the plasma, synovial fluid, and synovial membrane of AAV5-IL-10 injected horses.
Similar to previous studies, we found significant and persistent transgene protein synthesis over time within the AAV5-IL-10 injected joints.17,21–24 IL-10 was rapidly elevated over baseline in the synovial fluid of AAV5-IL-10 injected joints with a significant difference noted 2 days following injection. IL-10 concentration in the synovial fluid of AAV5-IL-10 injected horses was between 5- and 60-fold higher than baseline throughout the entire study period, and the concentration of IL-10 peaked 7 days following injection. The time to achieve peak concentration of the transgene (7 days) was shorter than reported by Watson Levings et al.25 In their study, intra-articular levels of the transgene eqIL-1Ra peaked at 8 weeks postinjection. The difference in the temporal expression of the transgene between studies may be associated with the AAV serotype used, as Watson Levings et al.25 injected an AAV2.5 vector and vector efficiency has been shown to be correlated with serotype.26 Previously, Hemphill et al. demonstrated superior transduction of equine ex vivo cartilage and synovium explants with AAV2 and AAV2.5 compared to AAV5.27 We chose to use AAV5 in this study based on previous in vivo studies by our group in which AAV5 was superior to AAV2 when injected intra-articularly,17 suggesting that in vivo transduction efficiency may be different than in vitro or ex vivo transduction efficiency.
Although we demonstrated elevated IL-10 concentrations over a 3-month study period, it is possible that expression would have been maintained for an even longer period based on similar studies showing intra-articular levels of AAV-mediated transgenes maintaining expression for ≥6 months.23–25 In contrast, a recent study on a helper-dependent adenovirus overexpressing eqIL-1Ra in an experimental model of PTOA in the horse reported that IL-1Ra levels returned to baseline by 5 weeks postinjection, which may suggest that AAV is a superior vector for intra-articular therapy.28 While we did not assess transduction of specific cell types, studies utilizing AAV vectors carrying reporter genes have previously shown that fibroblasts in the synovial lining are the primary cell type transduced following intra-articular injection, with a smaller number of chondrocytes in articular cartilage demonstrating transduction.16 Recently it has been shown that transduction of articular tissues, including articular cartilage, is higher in joints with OA compared to healthy joints,25 which is particularly relevant to the clinical application of intra-articular gene therapy. Future studies are warranted to study AAV5-IL-10 in joints with OA and the extent of transduction in the diseased joint.
Importantly, despite high concentrations of the IL-10 transgene being present in the joint, no perturbations of systemic IL-10 were detectable. These results provide evidence to support that utilization of gene therapy in the treatment of joint disease can avoid systemic effects of the vectors. This is especially important when the transgene has potentially broad-reaching effects, such as IL-10, which is an immunomodulatory cytokine that is involved in several signaling pathways and plays a key role in the regulation of T cells.29,30 The ability to significantly alter the joint environment without causing systemic alterations in cytokine concentrations makes gene therapy with AAV5-IL-10 quite promising.
To further assess the systemic impact of intra-articular AAV5-IL-10, we sought to quantify the number of vector genomes present in the plasma, synovial fluid, and synovial membrane using PCR with primers designed to span the vector genome and the transgene. The purpose of this aim was twofold: (i) to gain quantifiable data on the diffusion of the AAV vector from the joint into systemic circulation and (ii) to determine if AAV-injected horses could be reliably distinguished from control horses over time. Although serum IL-10 concentrations were not affected in AAV-injected horses, vector genomes were detected in the plasma of all horses until day 4, with two horses having vector genomes detectable at day 14. Vector genomes were detectable in the synovial fluid until day 28 in all horses and until day 56 in 4/6 horses with the total copy number of vector genomes in the synovial fluid ∼105-fold higher than in plasma.
All horses had detectable vector genomes in the synovial membrane at day 84, although it should be noted that there was moderate interanimal variability in the number of vector genomes in the synovial membrane, with a range of 1,500–100,000 vector genomes/μg gDNA observed. This has been demonstrated in other intra-articular gene therapy studies in the horse.25 As the vector genome was not detected in any sample collected from any control horse, we determined that our method of vector quantification was reliable.
Overall, the AAV vector appeared to be an effective and clinically relevant viral vector for intra-articular injection. Importantly, this study, in addition to others, has demonstrated that direct injection of AAV into a joint does not cause a significant inflammatory response.16,17 None of the horses showed any adverse clinical effects of injection in the short- or long term, and there was no evidence of a histologic inflammatory response in the synovial membrane collected 84 days following injection. NCCs and TP concentrations had transient elevations postinjection of both PBS and AAV5-IL-10; however, rapid resolution was noted in both groups. To further assess an inflammatory response to the AAV5-IL-10 vector, several inflammatory cytokines were quantified in the synovial fluid over time, including IL-1β, IL-6, IL-17a, TNF-α, and IFN-γ. Vector injection led to significantly increased concentration of IL-17a on day 2 only with return to baseline by day 4. Although IL-17a is recognized as an important pro-inflammatory cytokine in inflammatory joint diseases, especially rheumatoid and psoriatic arthritides,31 the small transient increase in IL-17a noted in this study is not likely clinically significant. AAV5-IL-10 did not lead to increases in any other inflammatory cytokine assayed, including IFN-γ, which has been demonstrated to increase following viral activation of T cells.32 Although the objectives of this study did not include evaluation of the potential anti-inflammatory properties of IL-10, it is interesting to note that IL-1β and IL-6 were both significantly lower in AAV5-IL-10 injected joints compared to PBS injected joints. This finding may lend support to the immunomodulatory potential of IL-10; however, this will require further evaluation in joints either experimental or naturally-occurring PTOA.
The horse has been recognized as a highly relevant and robust model for human joint disease due to the similarities in cartilage thickness, biochemical composition of the ECM, and nature of subchondral bone, in addition to the species' propensity for developing OA.24,33 In addition, the large volume of synovial fluid and ease of repeated synoviocentesis provide an excellent platform for performing serial assays to assess intra-articular gene therapy.
This study provides critical information regarding the clinical application of intra-articular gene therapy. Injection of an AAV5 vector led to rapid transduction and sustained expression of the transgene without causing a significant inflammatory response. Although intra-articular levels of IL-10 were 5- to 60-fold greater than control, no perturbations of IL-10 in the systemic circulation were noted, further supporting the use of AAVs as gene therapy vectors for treatment of joint disease. The AAV5 vector was detectable in the plasma, synovial fluid, and synovial membrane of AAV-injected horses only. Based on previous in vitro results demonstrating the chondroprotective effects of IL-10 overexpression and this promising in vivo data, further investigation of articular immunomodulation with IL-10 in diseased states is warranted.
Acknowledgments
The authors acknowledge the McCabe Fund, the Pennsylvania Horse Breeders Association, and the Pennsylvania State Horse Racing Commission for their generous support of this study.
Author Disclosure
No competing financial interests exist for any of the authors.
Funding Information
This article was funded by the McCabe Fund, University of Pennsylvania.
References
- 1. Cisternas MG, Murphy L, Sacks JJ, et al. Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a US population-based survey. Arthritis Care Res 2016;68:574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torio CM, Moore BJ. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013: Statistical Brief #204 2013. www.ncbi.nlm.nih.gov/pubmed/27359025 (last accessed March8, 2018)
- 3. Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res 2004;7–16 [PubMed] [Google Scholar]
- 4. Arthritis and the Military: Support Arthritis Research at the Department of Defense. https://www.arthritis.org/Documents/Sections/Advocate/DoD-Leave-Behind-Green.pdf (last accessed March8, 2018)
- 5. Goldring MB, Otero M, Plumb DA, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater 2011;21:202–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olson SA, Horne P, Furman B, et al. The role of cytokines in posttraumatic arthritis. J Am Acad Orthop Surg 2014;22:29–37 [DOI] [PubMed] [Google Scholar]
- 7. Kobayashi M, Squires GR, Mousa A, et al. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum 2005;52:128–135 [DOI] [PubMed] [Google Scholar]
- 8. Moroguchi A, Ishimura K, Okano K, et al. Interleukin-10 suppresses proliferation and remodeling of extracellular matrix of cultured human skin fibroblasts. Eur Surg Res 2004;36:39–44 [DOI] [PubMed] [Google Scholar]
- 9. Reitamo S, Remitz A, Tamai K, et al. Interleukin-10 modulates type I collagen and matrix metalloprotease gene expression in cultured human skin fibroblasts. J Clin Invest 1994;94:2489–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ortved KF, Begum L, Stefanovski D, et al. AAV-mediated overexpression of IL-10 mitigates the inflammatory cascade in stimulated equine chondrocyte pellets. Curr Gene Ther 2018;18:171–179 [DOI] [PubMed] [Google Scholar]
- 11. Schulze-Tanzil G, Zreiqat H, Sabat R, et al. Interleukin-10 and articular cartilage: experimental therapeutical approaches in cartilage disorders. Curr Gene Ther 2009;9:306–315 [DOI] [PubMed] [Google Scholar]
- 12. Finnegan A, Kaplan CD, Cao Y, et al. Collagen-induced arthritis is exacerbated in IL-10-deficient mice. Arthritis Res Ther 2003;5:R18–R24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuzzocrea S, Mazzon E, Dugo L, et al. Absence of endogeneous interleukin-10 enhances the evolution of murine type-II collagen-induced arthritis. Eur Cytokine Netw 2001;12:568–580 [PubMed] [Google Scholar]
- 14. Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol 2014;10:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev 2008;21:583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watson RS, Broome TA, Levings PP, et al. scAAV-mediated gene transfer of interleukin-1-receptor antagonist to synovium and articular cartilage in large mammalian joints. Gene Ther 2013;20:670–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ortved K, Wagner B, Calcedo R, et al. Humoral and cell-mediated immune response, and growth factor synthesis after direct intraarticular injection of rAAV2-IGF-I and rAAV5-IGF-I in the equine middle carpal joint. Hum Gene Ther 2015;26:161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watson Levings RS, Smith AD, Broome TA, et al. Self-complementary adeno-associated virus–mediated interleukin-1 receptor antagonist gene delivery for the treatment of osteoarthritis: test of efficacy in an equine model. Hum Gene Ther Clin Dev 2018;29:101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drittanti L, Rivet C, Manceau P, et al. High throughput production, screening and analysis of adeno-associated viral vectors. Gene Ther 2000;7:924–929 [DOI] [PubMed] [Google Scholar]
- 20. McIlwraith CW, Frisbie DD, Kawcak CE, et al. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the horse. Osteoarthritis Cartilage 2010;18:S93–S105 [DOI] [PubMed] [Google Scholar]
- 21. Bevaart L, Aalbers CJ, Vierboom MPM, et al. Safety, biodistribution, and efficacy of an AAV-5 vector encoding human interferon-beta (ART-I02) delivered via intra-articular injection in rhesus monkeys with collagen-induced arthritis. Hum Gene Ther Clin Dev 2015;26:103–112 [DOI] [PubMed] [Google Scholar]
- 22. Kay JD, Gouze E, Oligino TJ, et al. Intra-articular gene delivery and expression of interleukin-1Ra mediated by self-complementary adeno-associated virus. J Gene Med 2009;11:605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodrich LR, Phillips JN, McIlwraith CW, et al. Optimization of scAAVIL-1ra in vitro and in vivo to deliver high levels of therapeutic protein for treatment of osteoarthritis. Mol Ther acids 2013;2:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goodrich LR, Grieger JC, Phillips JN, et al. scAAVIL-1ra dosing trial in a large animal model and validation of long-term expression with repeat administration for osteoarthritis therapy. Gene Ther 2015;22:536–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watson Levings RS, Broome TA, Smith AD, et al. Gene therapy for osteoarthritis: pharmacokinetics of intra-articular self-complementary adeno-associated virus interleukin-1 receptor antagonist delivery in an equine model. Hum Gene Ther Clin Dev 2018;29:90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pillay S, Zou W, Cheng F, et al. Adeno-associated virus (AAV) serotypes have distinctive interactions with domains of the cellular AAV receptor. J Virol 2017;91:e00391–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hemphill DD, McIlwraith CW, Samulski RJ, et al. Adeno-associated viral vectors show serotype specific transduction of equine joint tissue explants and cultured monolayers. Sci Rep 2015;4:5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nixon AJ, Grol MW, Lang HM, et al. Disease-modifying osteoarthritis treatment with interleukin-1 receptor antagonist gene therapy in small and large animal models. Arthritis Rheumatol 2018;70:1757–1768 [DOI] [PubMed] [Google Scholar]
- 29. Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683–765 [DOI] [PubMed] [Google Scholar]
- 30. Riley JK, Takeda K, Akira S, et al. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J Biol Chem 1999;274:16513–16521 [DOI] [PubMed] [Google Scholar]
- 31. Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol 2018;55:379–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martino AT, Suzuki M, Markusic DM, et al. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood 2011;117:6459–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McIlwraith CW, Frisbie DD, Kawcak CE. The horse as a model of naturally occurring osteoarthritis. Bone Joint Res 2012;1:297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]