Abstract
Collegiate football athletes are subject to repeated traumatic brain injuriesthat may cause brain injury. The hippocampus is composed of several distinct subfields with possible differential susceptibility to injury. The aim of this study is to determine whether there are longitudinal changes in hippocampal subfield volume in collegiate football. A prospective cohort study was conducted over a 5-year period tracking 63 football and 34 volleyball male collegiate athletes. Athletes underwent high-resolution structural magnetic resonance imaging, and automated segmentation provided hippocampal subfield volumes. At baseline, football (n = 59) athletes demonstrated a smaller subiculum volume than volleyball (n = 32) athletes (−67.77 mm3; p = 0.012). A regression analysis performed within football athletes similarly demonstrated a smaller subiculum volume among those at increased concussion risk based on athlete position (p = 0.001). For the longitudinal analysis, a linear mixed-effects model assessed the interaction between sport and time, revealing a significant decrease in cornu ammonis area 1 (CA1) volume in football (n = 36) athletes without an in-study concussion compared to volleyball (n = 23) athletes (volume difference per year = −35.22 mm3; p = 0.005). This decrease in CA1 volume over time was significant when football athletes were examined in isolation from volleyball athletes (p = 0.011). Thus, this prospective, longitudinal study showed a decrease in CA1 volume over time in football athletes, in addition to baseline differences that were identified in the downstream subiculum. Hippocampal changes may be important to study in high-contact sports.
Keywords: cornu ammonis, dentate gyrus, entorhinal cortex/perirhinal cortex
Introduction
Repeated concussive and subconcussive high-velocity impacts may contribute to brain injury and cognitive effects in athletes of high-contact sports such as football.1–7 Non-invasive brain imaging can potentially characterize the subtle changes that occur with symptomatic mild traumatic brain injury, which can have long-term consequences.2,6–11 Recent cross-sectional magnetic resonance imaging (MRI) studies have demonstrated that repetitive concussive and subconcussive injury in collegiate football athletes is associated with significantly lower hippocampal volumes compared to healthy controls.6,7 Such findings are especially relevant given that the hippocampus demonstrates neurodegenerative changes in chronic traumatic encephalopathy (CTE), a potential long-term consequence of repeated concussive and subconcussive impacts to the head.12
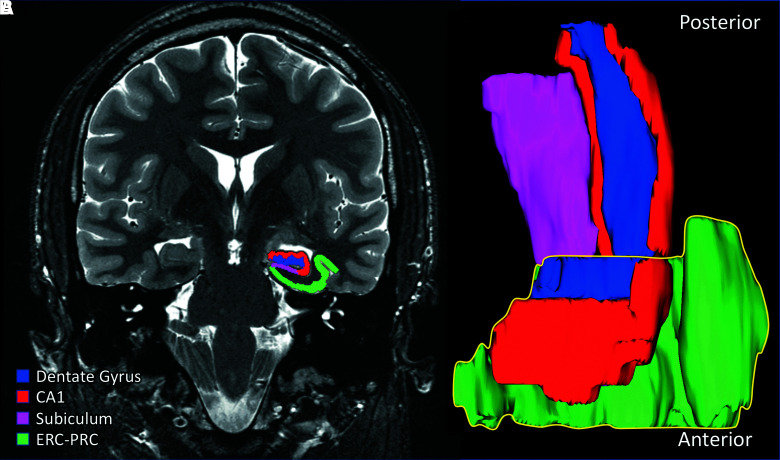
The hippocampus is a crucial structure for long-term, episodic memory formation and retrieval.13,14 It is involved in neurodegenerative disorders associated with memory decline (e.g., Alzheimer's disease [AD]) and is particularly sensitive to trauma in human studies and animal models.7,8,15,16 The hippocampal formation is comprised of several subfields (Fig. 1) that have unique connectivity, function, and selective vulnerability to disease. The most external portion of the hippocampal formation is the entorhinal and perirhinal cortices, which neighbor the hippocampus proper and are found along the length of the anterior parahippocampal gyrus. The subiculum is more internally situated and located between the hippocampus proper and entorhinal cortex (ERC). Finally, the hippocampus proper is the most internal portion and consists of, going from outside in, cornu ammonis (CA) fields 1–4 (CA1–4), followed by the dentate gyrus (DG), which lies at the center of the hippocampal hilus.
FIG. 1.
(A) High-resolution oblique coronal T2 FSE, with a representative automated segmentation of the left hippocampus using our modified atlas. (B) 3D rendering of segmentation with anterior (bottom, yellow outline) and posterior (top) subfields. 3D, three-dimensional; CA1, cornu ammonis area 1; ERC-PRC, entorhinal cortex/perirhinal cortex; FSE, fast spin echo. Color image is available online.
The literature suggests that the afferent hippocampal circuitry largely originates from the ERC, which receives inputs from the neocortex through the parahippocampal cortex and perirhinal cortex (PRC). The ERC sends projections through the subicular pyramidal layer into the DG, forming the perforant pathway, and additionally sends direct projections to CA1.17,18 The DG, in turn, sends outputs to CA4 and CA3 in the hilus by the mossy fibers.19–22 CA3 then projects to both CA123 and the fornix24 through the Schaffer collaterals, whereas CA4 projects downstream to the CA1 by the endfolial pathway.18,25,26 CA1 and the subiculum both send efferent outputs back to the ERC, forming a complete circuit loop.24,27
This subfield-specific circuitry, cytoarchitecture, and morphology give rise to unique function and differential contributions to memory encoding and retrieval. For example, the ERC and PRC have been shown as critical for the integration of novel contextual features of the environment with object identity to form durable episodic memories in humans and rats.28,29 Degeneration in the perirhinal-thalamic pathways has been shown to produce deficits in judgement of familiar context during recall and recognition.30 The subiculum has been found to be more activated during recollection of learned episodes.31–34 CA1 is thought to be critical for autobiographical retrieval in addition to spatial memory in humans.35,36 CA2 may be important for social memory.37 DG and CA3 are selectively activated during episodic memory formation (encoding face-name pairs and pattern matching).29,32,33 Moreover, recent findings suggest the presence of an anterior to posterior gradient in hippocampal activity during spatial learning.38 Integrating this body of literature suggests that more upstream (ERC-DG) circuitry appears more important in encoding, pattern matching, and familiarity, whereas more downstream (CA1-subiculum) circuitry appears critical and selectively activated during retrieval.
Both pre-clinical and post-mortem studies suggest that these structurally and functionally distinct subfields are selectively affected throughout the progression of different neurological diseases. The PRC and ERC (transentorhinal and adjacent regions) are thought to be one of the first supratentorial regions to show neurofibrillary pathology of AD, whereas slightly later in the course of the disease the most distinctive neuronal loss is localized downstream in CA1.39 The presence of activated microglia has been observed in the subiculum in post-mortem specimens of AD patients, but not controls.40 In epilepsy-associated hippocampal sclerosis, neuronal loss can take several forms with different outcomes, involving either the entire hippocampus, primarily the DG, or primarily CA1.41,42 Accumulation of alpha-synuclein is observed in the CA2 in patients with Lewy bodies dementia,43 and pathological stress has been associated with atrophy in the CA3.44 DG atrophy and mossy fiber degeneration have been linked to CTE45 and temporal lobe epilepsy.46 These disorders may have unique predilections for distinct subfields because of differential penetration of pathology (e.g., proteinopathies, increased/decreased circuit function, neurotransmitter imbalance, etc.) into hippocampal formation microcircuitry and cytoarchitecture.
Previous in vivo human imaging studies suggested an inverse relationship between multiple concussive impacts with whole hippocampal and subfield volumes. Singh and colleagues demonstrated that collegiate football players had significantly smaller hippocampi compared to age-matched healthy controls, observing a larger effect in players with a history of concussion.6 The same group investigated the link between subfield volumes and kynurenine metabolites in the same cohort, observing smaller DG and CA2–3 subfields in football players, along with lower kynurenine levels compared to controls.7 However, previous investigations of hippocampal volumetry in collegiate football athletes have all been cross-sectional, often utilizing control groups without comparable intensity of exercise and educational stage, which can affect hippocampal volume differences between groups.47,48 A study combining hippocampal subfield volumetry with a longitudinal evaluation of participation in football compared to a control sport may sensitively identify subtle volumetric changes over time associated with high-contact sports. The aim of this study is to determine whether there are baseline differences and longitudinal changes in hippocampal subfield volumes in collegiate football compared to volleyball.
Methods
Study population
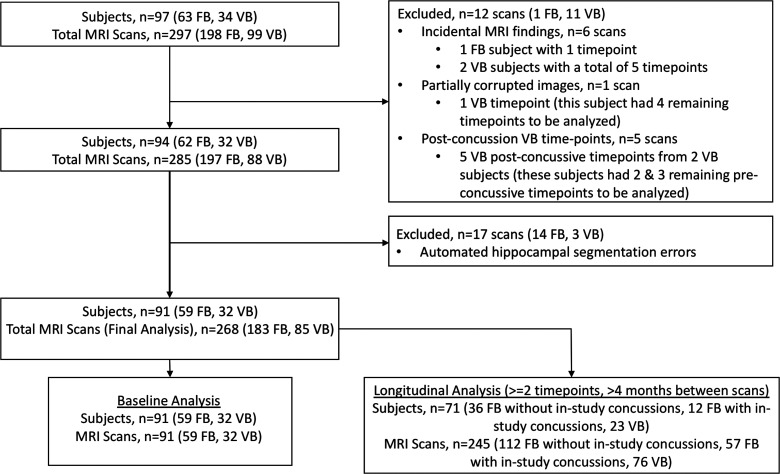
The study was introduced to members of the team in conjunction with athletic trainers. Athletes were required to be in collegiate football or volleyball and have no self-reported history of brain surgery, severe brain injury, or major neurological, psychiatric, or substance abuse disorder. All interested athletes were enrolled. We prospectively enrolled 63 high-contact (football) and 34 low-contact (volleyball) collegiate athletes over the course of 5 years (Fig. 2), in accord with the Institutional Review Board and Health Insurance Portability and Accountability Act. Athletes underwent brain MRI at the start of their respective seasons, within 24–96 h after a concussion (when possible with the athlete's schedule), and after the last season of sporting participation. During the first year of this study, athletes were additionally imaged at the end of their season. Incidences of concussion in football during the study period were identified by the athletic trainers. In total, 18 concussions occurred in 15 football athletes, 3 of whom had two concussions. None involved loss of consciousness, and mean return to play was 10.3 days (excluding 1 athlete who did not return to play). Post-concussion MRI was captured for 13 of these 18 concussions (72%) among 12 football athletes, 1 of whom had two concussions with post-concussion MRI. In 2 of the other post-concussion MRI subjects, the first imaging time point was post-concussion (1 and 13 days, respectively) without previous baseline imaging. Each athlete thus had one to eight MRI time points spanning a maximum of 4 years (Fig. 3), resulting in 198 football and 99 volleyball MRI scans, for a total of 297 scans (Fig. 2).
FIG. 2.
Enrollment figure demonstrating exclusion criteria and final baseline and longitudinal analysis populations. FB, football athletes; MRI, magnetic resonance imaging; VB, volleyball athletes.
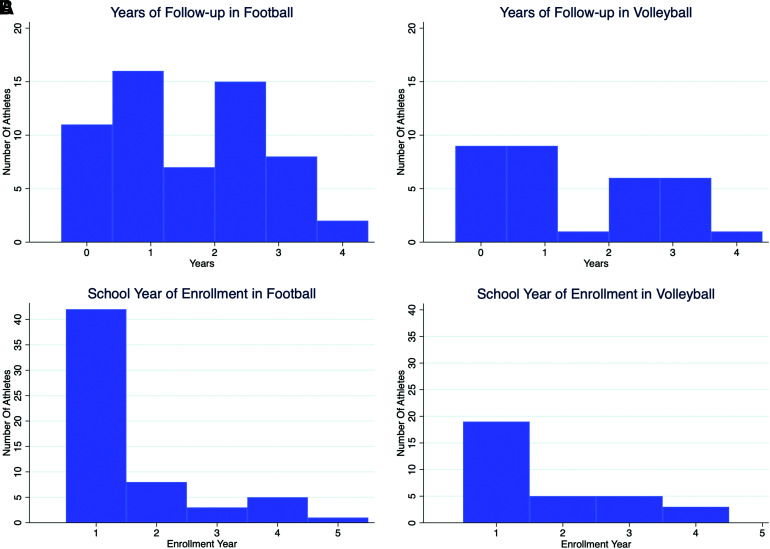
FIG. 3.
(A) Distribution of years of follow-up included in final analysis for both athlete groups, with 48 of 63 football subjects (76%) and 23 of 34 volleyball subjects (68%) having two or greater time points more than 4 months apart, (B) Distribution of enrollment year for each sport, where the year denotes the first year in school (starting with freshmen year) of usable data (i.e. with a usable hippocampal segmentation) for each subject. Color image is available online.
There were 2 volleyball athletes that suffered concussions: 1 during volleyball and the other incidental. All subsequent post-concussive imaging for these 2 volleyball athletes, which consisted of two and three time points, respectively, was excluded from the primary analyses, except where specified, to avoid the potential effect of concussions on hippocampal volumes in the control cohort. A board- and Certificate of Added Qualification–certified neuroradiologist with 9 years of post-residency experience blindly examined all scans for incidental abnormalities, resulting in exclusion of 3 athletes (each with one, two, and three time points, respectively). A single time point from 1 athlete had partially corrupted images precluding analysis. After excluding these 12 scans (Fig. 2), we analyzed a total of 197 football and 88 volleyball scans from 62 football and 32 volleyball athletes. Our football population represents approximately one half the size of a collegiate football team.
Additional information obtained for football athletes included the self-reported number and timing of past concussions, the number of years of playing football, the current position played, and a pre-first-season Standardized Concussion Assessment Tool (SCAT) evaluation.49 One football athlete and 1 volleyball athlete did not provide years of tackle football experience. The SCAT evaluation included only the cognitive assessment, balance, coordination, and standardized assessment of concussion portions of the SCAT II, with a maximum score of 61.49
Image acquisition
Using a 3-Tesla scanner (GE MR 750; GE Healthcare, Milwaukee, WI) and an eight-channel receive head coil, we acquired T1-weighted axial (inversion recovery fast spoiled gradient echo brain volume imaging, 1 × 1 × 1 mm, repetition time [TR] 7.9 ms, echo time [TE] 3.1 ms, number of excitations [NEX] 1, acquisition time 5.1 min) and high-resolution coronal T2-weighted images, perpendicular to the long axis of the hippocampus (coronal oblique fast spin echo [FSE], resolution 0.39 × 0.52 × 2 mm, 0.2 mm skip, TR 5000–15,000, TE 95–109, echo train length 25, matrix 512 × 384, NEX 2, 32–40 slices, one to two acquisitions, acquisition time 4.4–5.8 min). For 29 athlete scans, the coronal T2 FSE was judged to be corrupted by motion artifact in real time and was repeated.
Hippocampal subfield segmentation
We used Automated Segmentation of Hippocampal Subfields (ASHS; Penn Image Computing & Science Lab, http://picsl.upenn.edu/software/ashs/), which performs multi-atlas label fusion and machine-learning correction to obtain reliable segmentations of the hippocampal subfields and adjacent cortices (Fig. 1).50 We manually modified the segmentations of the 29 University of Pennsylvania Memory Center atlas brains included with the software and built a new atlas both to separate anterior from posterior hippocampus (which may be functionally different)51 and improve the segmentation of the subiculum.50 The hippocampal atlas was edited by three raters with 1, 6, and 20 years of neuroimaging and segmentation experience. For each segmentation, the most posterior aspect of the uncus was demarcated as the most posterior aspect of all anterior hippocampal structures.52 Additionally, the subiculum was adjusted to be thicker along the posterior hippocampus. The atlas was rebuilt with the new segmentations and run on all athletes. The separation of anterior and posterior substructures was incomplete after atlas-based segmentation, with a few pixels of anterior subfields bleeding into posterior slices and vice versa. A histogram was composed of anterior and posterior pixels within each slice for each segmentation, and the inflection point was used to automatically determine the slice that demarcated the boundary between a given athlete's anterior and posterior hippocampal subfields.
Subfields included were: CA1, DG (which includes CA2–4), subiculum, and ERC/PRC (which we combined into one subfield). CA4 and DG are inseparable on imaging, and CA2 and CA3 are very tiny with highly variable individual segmentations. Therefore, CA2–4 and DG were all combined into one subregion (called DG) representing a similar segment of hippocampal circuitry, which additionally reduced the number of statistical tests necessary. Similarly, ERC and PRC were combined. Automated hippocampal segmentations were manually checked for accuracy by three independent, blinded raters, and segmentations with gross errors (i.e., segmenting structures outside the hippocampal formation) were removed from analysis. In addition, we checked for visual outliers on both overall volume and volume change plots to make sure there were no segmentation errors. In total, 17 scans were excluded. The final analyses included 268 scans, consisting of 183 football and 85 volleyball scans from 59 football and 32 volleyball athletes, respectively (Fig. 2). Subfield subdivision volumes (i.e., left anterior CA1, left posterior CA1, etc.) were summed to obtain total subfield volume, and total hippocampus volume was derived by summing all subfields, including entorhinal and perirhinal cortices. To control for differences in head size,53 we estimated intracranial volume for each athlete using the longitudinal processing stream in Freesurfer software (v5.3; The General Hospital Corporation, Boston, MA; http://surfer.nmr.mgh.harvard.edu/).54,55
Statistical analysis
Subfield volumetric data exhibited normality by evaluation of regression residuals according to D'Agostino and colleagues' test for skewness and kurtosis,56 so linear regression was appropriate for subfield volumes. In contrast, demographic data did not exhibit normality, so a Wilcoxon rank-sum test was used to assess for group differences in age, body mass index (BMI), years of previous tackle football, and SCAT score at baseline. Regression coefficients were unstandardized. Standard errors were derived from a Huber-White robust variance estimate. All statistics were performed using Stata software (v15.0; StataCorp LP, College Station, TX; http://www.stata.com).
All statistical calculations on volumes were performed first on the entire hippocampal formation (all subfields bilaterally) and on the bilateral subfields (DG, CA1, subiculum, and entorhinal-perirhinal). We corrected for multiple comparisons across the four subfields using a false discovery rate (FDR) approach.57 The whole hippocampus was not corrected for because it is composed of the same four subfields. Reported p values are uncorrected. For any significant effect after FDR, secondary exploratory analyses then examined the left and right subfields separately and, for any lateralized effect, the respective anterior and posterior subfield portions.
We performed our statistical analyses with a minimum number of parameters to encapsulate the most relevant sources of variance. Given that head size is associated with hippocampal size, we included total intracranial volume in all regressions.53 Similarly, age has been included in other studies of hippocampal volume in football.6 Although both race and BMI were not equivalent between groups, we did not include either race or BMI because they individually did not contribute significantly to the model by a likelihood ratio test.
To test group differences in volume at baseline, we performed linear regression with covariates of intracranial volume and age, using a robust variance estimator. To test for the influence of two outliers on adjusted subiculum volume at baseline, we performed the same regression with these subjects removed (the football player with the lowest volume and the volleyball player with the highest volume).
Among football athletes, correlations of volumes to factors possibly related to high impacts (past history of concussions, position-based concussion risk, the number of previous years of tackle football experience, and baseline SCAT score) were each tested by four separate linear regressions with covariates of intracranial volume and age, using a robust variance estimator. Past history of concussion was included as a binary variable. Position-based concussion risk was defined as Nathanson's National Football League (NFL) reported concussion incidence (ranging from 9 to 37 per 100,000 position plays, scaled up from the original article by a factor of 100 for easy interpretability of volume change) for the current athlete position at the time of enrollment for the following position categories: linebacker (9), defensive line (9.7), offensive line (12), fullback (13), quarterback (20), defensive back (20), wide receiver (27), tight end (32), and running back (37).58 Additionally, we regressed subfield volume against literature values of Head Impact Telemetry (HIT) accelerometer-based measurements for each position, which may estimate cumulative subconcussive impacts based on player position. This included linear acceleration, rotational acceleration, and the composite HITsp measure.59,60
To determine longitudinal group differences in hippocampal volume, we performed a linear mixed-effects regression with covariates of baseline intracranial volume and age, fixed effects of sport, time, and the interaction of sport and time, and random effect of athlete. To ensure adequate temporal characterization, only athletes with two or more time points and greater than 4 months of total imaging follow-up were included in the longitudinal analysis. This resulted in a total of 245 scans that were analyzed, consisting of 169 scans from 48 football athletes (representing 76% of all football athletes enrolled) and 76 scans from 23 volleyball athletes (representing 68% of all volleyball athletes; Figs. 2 and 3). We compared football (n = 36) and volleyball (n = 23) athletes without in-study concussion to assess the effect of sport longitudinally without any component of concussion (i.e., to evaluate subconcussive injury). Next, we performed the comparison between football athletes with concussion (n = 12) and volleyball athletes. Finally, we assessed whether any significant differences from the previous two analyses remained when comparing the whole football cohort (n = 48) to the volleyball cohort. To evaluate the potential contribution of the two volleyball athletes with in-study concussions (whose two and three post-concussion time points, respectively, were excluded in the main analysis), we assessed whether significant differences remained when including these time points.
Results
Demographics
Median age showed a minimal, but statistically significant, difference between football and volleyball athletes (median age/interquartile range [IQR]: football, 18.67 [0.78] years; volleyball, 19.23 [1.67] years; p = 0.002; Table 1). BMI showed a significant difference between football and volleyball athletes (median BMI/IQR: football, 29.16 [6.78]; volleyball, 23.09 [1.81]; p = <0.001). Years of previous tackle football demonstrated an expected a significant difference between football and volleyball athletes (median years/IQR: football, 9 [4], volleyball, 0 [0] years; p = <0.001). There were 22 of 59 football and 2 of 32 volleyball athletes who had past concussions. Within football, there was no statistically significant difference in SCAT score at baseline between athletes who did not or did have a history of past concussion (median SCAT [IQR]: without concussion, 54 [6]; with concussion, 55 [3]; p = 0.309). Football and volleyball athletes had equivalent low rates of attention deficit hyperactivity disorder (ADHD) and learning disabilities. Proportionally more African Americans and fewer Caucasians played football compared to volleyball. After exclusions, years of follow-up imaging showed a bimodal distribution in both football and volleyball (Fig. 3A). Athletes were mostly freshmen at the time of study enrollment, with a similar number of upperclassmen between the sports (Fig. 3B).
Table 1.
Age at the Time of First Baseline Scan, Body Mass Index, Years of Previous Football Experience, Past Concussion History, and SCAT Scores with Median, 25th and 75th Percentiles, Minimum and Maximum, as Well as Incidence of ADHD and Learning Disabilities in Football (n = 59; 37 without Past Concussion, 22 with Past Concussion) and Volleyball (n = 32)
| Football median (25th, 75th percentile) [min, max] | Volleyball median (25th, 75th percentile) [min, max] | Z (p value) | ||
|---|---|---|---|---|
| Age, years | 18.67 (18.43, 19.21) [17.91, 27.70] | 19.23 (18.79, 20.46) [18.32, 21.76] | 3.13 (0.002) | |
| Body mass index | 29.16 (26.09, 32.87) [21.70, 38.18] | 23.09 (22.50, 24.31) [19.93, 27.27] | –6.84 (0) | |
| Years of previous tackle football experience | 9 (7, 11) [4, 15] | 0 (0, 0) [0, 2] | –7.89 (0) | |
| No. of athletes with past concussion | 22/59 | 2/32 | (0.001) | |
| SCAT score (without/with past concussion) | 54 (51, 57) [42, 61] | 55 (54, 57) [51, 61] | –1.02 (0.309) | |
| No. of athletes with ADHD | 3/59 | 2/32 | (1) | |
| No. of athletes with learning disabilities | 1/59 | 0/32 | (1) | |
| Caucasian | 25/59 | 25/32 | (0.002) | |
| African American | 22/59 | 0/32 | (0) | |
| Other/mixed/unknown | 12/59 | 7/32 | (1) | |
Median age, body mass index, and years of previous football experience (followed by 25th and 75th percentiles, and minimum and maximum) compared between football and volleyball athletes. SCAT scores were compared within football only (between football athletes without past concussion [left] and with past concussion [right]). A Wilcoxon rank sum test was utilized for age, body mass index, years of previous football experience, and SCAT scores, whereas a Fisher's exact test was performed for all other rows. The one athlete with a learning disability had dyslexia.
Bold highlights p < .05 uncorrected.
ADHD, attention deficit hyperactivity disorder; SCAT, Standardized Concussion Assessment Tool.
Baseline results: Subfield volume differences between sports
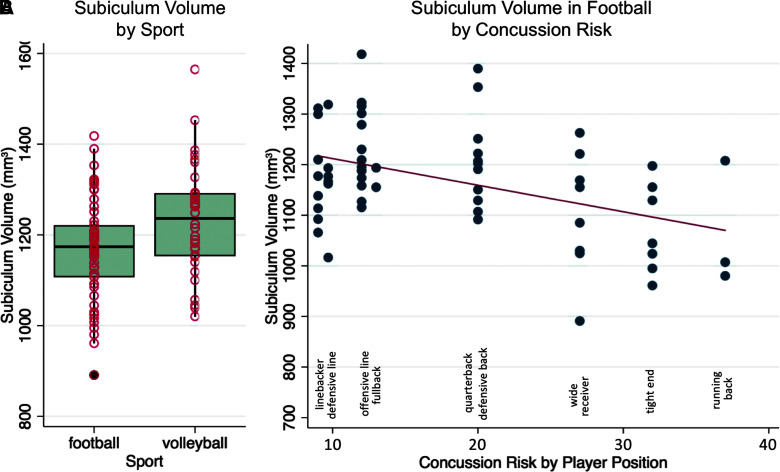
Comparing football and volleyball, total hippocampus volume was not statistically different between groups (adjusted difference = −259.58 mm3; 95% confidence interval [CI]; −642.89, 123.74]; p = 0.182; Table 2), but there was a significantly smaller subiculum volume in football athletes compared to volleyball athletes that withstood multiple comparison correction (adjusted difference = −67.77 mm3; 95% CI [−120.20, −15.33]; p = 0.012, Table 2; Fig. 4A). This effect in the subiculum was present on the right (adjusted difference = −29.81 mm3; 95% CI [−57.37, −2.24]; p = 0.034) and on the left (adjusted difference = −37.96 mm3; 95% CI [−68.66, −7.26]; p = 0.016), localizing more strongly to the posterior subiculum bilaterally (right posterior: adjusted difference = −20.41 mm3; 95% CI [−39.40, −1.42]; p = 0.035; left posterior: adjusted difference = −23.42 mm3; 95% CI [−45.43, −1.41]; p = 0.037). When outliers were removed from Figure 4A (the football player with the lowest volume and the volleyball player with the highest volume), subiculum volume was still significantly smaller in football athletes compared to volleyball athletes (adjusted difference = −67.77; 95% CI [−120.20, −15.33]; p = 0.034). Differences in other subfields were not statistically significant.
Table 2.
Adjusted Whole Hippocampus and Subfield Volume (mm3) Comparison between Football (n = 59) and Volleyball (n = 32) at Baseline
| Football mean in mm3, SD (95% CI) | Volleyball mean in mm3, SD (95% CI) | Adjusted difference in mm3 (CI) | T (p value) | R2 | d | |
|---|---|---|---|---|---|---|
| Total hippocampus | 11,704.19, 895.04 (11,472.58, 11,935.79) | 11,963.76, 869.25 (11,658.34, 12,269.19) | –259.58 (−642.89, 123.74) | –1.35 (0.182) | 0.27 | 0.29 |
| Dentate gyrus | 1873.20, 224.69 (1815.06, 1931.35) | 1960.37, 221.00 (1882.72, 2038.02) | –87.16 (−183.68, 9.35) | –1.79 (0.076) | 0.15 | 0.39 |
| CA1 | 2826.31, 295.80 (2749.77, 2902.86) | 2912.82, 286.88 (2812.02, 3013.62) | –86.51 (−212.49, 39.47) | –1.36 (0.176) | 0.16 | 0.30 |
| Subiculum | 1166.54, 110.01 (1138.08, 1195.01) | 1234.31, 124.68 (1190.50, 1278.12) | –67.77 (−120.20, −15.33) | –2.57 (0.012)a | 0.14 | 0.58 |
| Entorhinal-perirhinal | 5838.13, 505.90 (5707.22, 5969.04) | 5856.27, 583.93 (5651.09, 6061.44) | –18.14 (−262.39, 226.12) | –0.15 (0.883) | 0.22 | 0.03 |
p values for effect of sport, adjusting for age and total intracranial volume. Bolded p values are < = 0.05.
Meets multiple comparison correction across subfields by false discovery rate.
Bold highlights p < .05 uncorrected.
CA1, cornu ammonis area 1; SD, standard deviation; CI, confidence interval.
FIG. 4.
(A) Subiculum volumes at baseline by sport, regressing out the effect of head size and age, showing a reduction in the football cohort relative to volleyball. (B) Subiculum volumes in football across different concussion risks based on athlete position per 100,000 position plays, regressing out the effect of head size and age, showing that as concussion risk increases, subiculum volume decreases. Concussion risks are as follows: linebacker (9), defensive line (9.7), offensive line (12), fullback (13), quarterback (20), defensive back (20), wide receiver (27), tight end (32), and running back (37).32 Color image is available online.
Baseline results: subfield volume correlations within football
Examining just football athletes, higher position-based concussion risk had a negative relationship with total hippocampus volume (volume change for position-based risk increase = −28.67 mm3; 95% CI [−55.84, −1.50]; p = 0.039; Table 3). Subfield analysis localized this effect as strongly significant within the subiculum (volume change = −5.65 mm3; 95% CI [−8.79, −2.52]; p = 0.0007; Fig. 4B). This effect in the subiculum was present on the right (volume change = −2.28 mm3; 95% CI [−3.92, −0.64]; p = 0.007) and on the left (volume change = −3.37 mm3; 95% CI [−5.40, −1.34]; p = 0.002), localizing more strongly to the right posterior (volume change = −1.79 mm3; 95% CI [−3.07, −0.50]; p = 0.007) and left anterior subiculum (volume change = −1.31 mm3; 95% CI [−2.17, −0.46]; p = 0.003). Additionally, a relatively weak negative relationship existed between the presence of past concussions and baseline total DG volume (adjusted difference = −122.37 mm3; 95% CI [−244.53, −0.21]; p = 0.050; Table 3). Years of football experience before baseline scan and SCAT scores did not correlate significantly with whole hippocampal (p = 0.965; p = 0.611) or subfield volume (p > 0.20; p > 0.16). Literature values of position-based HIT accelerometer measurements did not correlate significantly with subfield volume after multiple comparison correction (uncorrected p > 0.045).
Table 3.
Within Football (n = 59) Association of Baseline Whole Hippocampus and Subfield Volumes (in mm3) with the Presence of Past Concussions, the Position-Based Concussion Risk, the Years of Tackle Football Experience Before Baseline, and Baseline SCAT Score
| Past concussions (n = 22 with past concussion) | Position-based concussion risk | Years of tackle football | SCAT score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted difference in mm3 (95% CI) | T (p value) | R2 | Volume change (mm3) for position-based risk increase (95% CI) | T (p value) | R2 | Volume change (mm3) per year (95% CI) | T (p value) | R2 | Volume change (mm3) per 1 unit change in SCAT score (95% CI) | T (p value) | R2 | |
| Hippocampus | –229.71 (−711.42, 252.01) | –0.96 (0.343) | 0.29 | –28.67 (−55.84, −1.50) | –2.12 (0.039) | 0.33 | –1.94 (−90.31, 86.44) | –0.04 (0.965) | 0.26 | –18.21 (−89.49, 53.07) | –0.51 (0.611) | 0.28 |
| Dentate gyrus | –122.37 (−244.53, −0.21) | –2.01 (0.050) | 0.17 | –4.09 (−10.52, 2.34) | –1.28 (0.208) | 0.13 | -12.40 (−35.77, 10.98) | –1.06 (0.292) | 0.13 | –11.89 (−29.18, 5.40) | –1.38 (0.174) | 0.15 |
| CA1 | –58.53 (−228.20, 111.13) | –0.69 (0.492) | 0.17 | –7.74 (−16.38, 0.90) | –1.80 (0.078) | 0.20 | 0.45 (−32.07, 32.98) | 0.03 (0.978) | 0.16 | –6.46 (−27.19, 14.28) | –0.62 (0.535) | 0.16 |
| Subiculum | –41.92 (−101.35, 17.51) | –1.41 (0.163) | 0.13 | –5.65 (−8.79, −2.52) | –3.61 (<0.001)a | 0.29 | 0.83 (−9.58, 11.24) | 0.16 (0.873) | 0.09 | 0.53 (−5.86, 6.93) | 0.17 (0.868) | 0.10 |
| Entorhinal-perirhinal | –6.89 (−255.39, 0.00) | –0.06 (0.956) | 0.28 | –11.18 (−28.43, 6.07) | –1.30 (0.199) | 0.31 | 9.18 (−38.84, 57.19) | 0.38 (0.703) | 0.27 | –0.39 (−40.03, 39.24) | –0.02 (0.984) | 0.28 |
A negative difference/coefficient means decreased volumes in football are associated with the presence of past concussion, with increased concussion risk (measured in units of concussions per 100,000 position plays), with increased years of previous tackle football, and with increasing baseline SCAT score, using age and total intracranial volume as covariates. Of the 22 with past concussions, 2 subjects had two past concussions, and 1 subject had three past concussions.
Meets multiple comparison correction across subfields by false discovery rate.
Bold highlights p < .05.
SCAT, Standardized Concussion Assessment Tool; CA1, cornu ammonis area 1; CI, confidence interval.
Longitudinal results: Interaction of sport and time
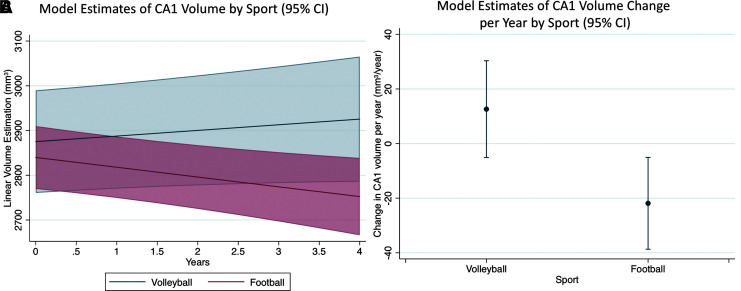
In athletes without concussion (n = 36 football, 23 volleyball), prospective longitudinal analysis of the interaction between sport and time for the whole hippocampus did not show a significant difference (coefficient = −79.26 mm3; 95% CI [−181.31, 22.78]; p = 0.128; Table 4). However, subfield analysis demonstrated a statistically significant decrease in CA1 volume in football compared to volleyball athletes that withstood multiple comparison correction (coefficient = −35.22 mm3; 95% CI [−59.67, −10.77]; p = 0.005; Fig. 5). This CA1 effect was present in the right CA1 (coefficient = −29.49 mm3; 95% CI [−47.49, −11.48]; p = 0.001), but not the left CA1 (coefficient = −5.87 mm3; 95% CI [−18.20, 6.46]; p = 0.351), further localizing to the right anterior CA1 (coefficient = −13.53 mm3; 95% CI [−24.59, −2.46]; p = 0.017). Longitudinal evaluation of CA1 among the same football athletes without reference to volleyball athletes still revealed that CA1 volume decreased over time (coefficient = −22.00 mm3; 95% CI [−38.90, −5.10]; p = 0.011), again localizing to the right CA1 (coefficient = −20.22 mm3; 95% CI [−30.23, −10.21]; p = 0.0001). Longitudinal evaluation of CA1 among volleyball athletes alone revealed no changes in CA1 volume over time (coefficient = 12.84 mm3; 95% CI [−5.22, 30.92]; p = 0.164).
Table 4.
Interaction of Sport and Time Modeling Longitudinal Whole Hippocampus and Subfield Volumes between Football Athletes (n = 36) and Volleyball Athletes (n = 23), All without In-Study Concussion
| Interaction coefficient (volume difference in mm3 per year between sports) (CI) | T (p value) | R2 | |
|---|---|---|---|
| Hippocampus | –79.26 (−181.31, 22.78) | –1.52 (0.128) | 0.50 |
| Dentate gyrus | 3.43 (−18.90, 25.76) | 0.30 (0.763) | 0.35 |
| CA1 | –35.22 (−59.67, −10.77) | –2.82 (0.005)a | 0.42 |
| Subiculum | –5.15 (−18.57, 8.27) | –0.75 (0.452) | 0.34 |
| ERC/PRC | –42.92 (−112.42, 26.57) | –1.21 (0.226) | 0.49 |
The interaction coefficient reflects the difference between sports in the per-year change in volume (i.e., the difference in slopes in Fig. 5A, equivalent to the differences in volume change per year by sport in Fig. 5B). A negative interaction coefficient means decreasing volumes in football compared to volleyball over time (all players without concussion during the study), using age at the time of baseline scanning and total intracranial volume as covariates.
Bold text indicates the statistic meets multiple comparison correction across subfields by false discovery rate.
CA1, cornu ammonis area 1; ERC/PRC, entorhinal cortex/perirhinal cortex; CI, confidence interval.
FIG. 5.
Football athletes (n = 36) show decreasing CA1 volumes with time compared to volleyball athletes (n = 23) with a significant sport by time interaction, in the absence of in-study concussion. (A) Model estimates of CA1 volume by sport over time when controlling for total intracranial volume and age, depicting a divergence of CA1 volume over time depending on sport. The shaded regions represent the 95% confidence interval (CI). (B) Model estimates of CA1 volume change over time (i.e., slope) when controlling for total intracranial volume and age, demonstrating the CA1 volume loss per year in football athletes compared to both volleyball athletes and zero, with the 95% confidence interval shown. CA1, cornu ammonis area 1. Color image is available online.
Comparing the smaller cohort of football athletes with an in-study concussion (n = 12) to volleyball athletes (n = 23), a significant interaction between sport and time was not found in CA1 (coefficient = −16.83 mm3; 95% CI [−36.77, 3.12]; p = 0.098) or any other subfields (p > 0.080). Comparing football athletes with (n = 12) versus without (n = 36) in-study concussion, a significant interaction between the presence of in-study concussions and time was also not found in CA1 (coefficient = 17.39 mm3; 95% CI [−1.66, 36.45]; p = 0.074). Expanding the analyzed cohort to include all football players (n = 48), both with and without concussions, the interaction between sport and time in CA1 was significant (coefficient = −27.15 mm3; 95% CI [−48.14, −6.17]; p = 0.011). When the two volleyball athletes with concussions were included, CA1 volume was still significantly decreasing in football compared to volleyball, with a significant interaction between sport and time (coefficient = −23.27 mm3; 95% CI [−42.43, −4.11]; p = 0.017).
Discussion
This study demonstrated that CA1 volume prospectively decreased in football athletes who did not experience concussion when compared to volleyball athletes. This longitudinal decline in CA1 volume was evident even when evaluating football athletes on their own. We found a dissociation between sports both across time and across hippocampal subfields. At baseline, there was a lower subiculum volume in athletes who played collegiate football compared to volleyball peers. Further, subiculum volumes were lower in football athletes with higher position-based concussion risk (e.g., running back, tight end, and wide receiver).
Our work builds upon cross-sectional imaging studies assessing hippocampal volume differences in football athletes compared to control groups6,7 by showing longitudinal volume changes in a prospective cohort while accounting for differences at baseline that could otherwise complicate interpretation. The lack of clear differences associated with in-study concussion suggests that cumulative subconcussive impacts may serve as an important factor contributing to hippocampal volume changes, although the smaller size of the in-study concussion cohort may partially explain this observation. The literature-based measurements of HIT accelerometers did not significantly correlate with hippocampal volume for player position, but newer accelerometers and quantitative metrics of field time used concomitantly along with imaging may better discern a potential relationship between subconcussive impacts and hippocampal subfield volume. Whereas a longitudinal divergence does suggest that the volume effect in CA1 could be attributed to sport, other possible explanations include differences associated with genetic, behavioral, and developmental factors.
The hippocampus and its subfields may exhibit selective vulnerability to disease, including subconcussive trauma.16,61 The strongest longitudinal difference found between football and volleyball athletes in CA1 appears upstream from the strongest baseline difference in the subiculum. Given the known feed-forward circuitry of the hippocampus, with CA1 projecting to the subiculum,18 this longitudinal finding suggests that changes in relatively upstream circuitry may be more recent, whereas changes in downstream circuitry may be more long term. Although we did not observe significant longitudinal differences in the DG, we found a negative correlation between past concussion history and DG volume in football athletes. This association suggests that second-order long-term effects of concussion may additionally involve more upstream circuitry at the DG, which has been implicated in CTE.45 Shorter-term (<1 year) effects may only be discernible by examining hippocampal functional connectivity or diffusivity.62 Dentate findings in other studies may demonstrate a bigger effect attributed to differences in segmentation protocols, control cohorts, or cross-sectional study design.7 Additionally, our subfield measurements may be more sensitive outside the hilus because anatomic variants, such as globularity, inject heterogeneity and uncertainty into morphometric measures, in particular involving the DG.63 CA2–3 volumes may also exhibit different trends across studies because of differences in border definitions or subfield grouping and their relatively small volumes compared to in vivo imaging resolution. Notably, both CA1 and the subiculum are important for memory retrieval, so it is possible that our imaging findings may be a precursor to subsequent episodic memory impairments reported in NFL players. 64
Challenges are present in the approach of the current study. In vivo hippocampal subfield segmentations may not uniformly correspond to anatomical boundaries defined on histology and are evolving over time.50,65 Therefore, we used high-resolution coronal T2 FSE images to best depict hippocampal anatomy, updated a standardized hippocampal subfield atlas to improve boundary precision, and blindly verified the segmentations to avoid bias. The MRI changes we observed are statistically significant, but the small approximate 0.5% CA1 volume decrement on average per year within football athletes (1% relative to volleyball athletes) is of unknown clinical significance. Although causality cannot be conclusively determined, this longitudinal study provides a more direct evaluation of volumetric changes in the hippocampus associated with football than cross-sectional studies in the literature. Given that we utilized self-reporting of individual concussion history, we cannot assess for under-reported past concussions. There was heterogeneity in the duration of longitudinal follow-up, but this was similar between sports and is inherent to long-term studies of student athletes. All subjects enrolled were males from only one institution, so future work will span institutions and sexes. Whereas concussion and football playing history was fully assessed in both groups in this study, history of participation in other high-contact sports was not assessed. Finally, whereas SCAT was used in the current study, other well-validated tools, such as Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT), can be used in future hippocampal volume studies to assess whether these changes correlate with neuropsychological function, reaction time, and memory.66, 67
In summary, we found a significant divergence between hippocampal volumes and sport over time, with volleyball (low-contact) athletes maintaining hippocampal subfield volume and football (high-contact) athletes showing gradually decreasing hippocampal subfield volume. This initial association warrants a multi-site study and underscores the need for longitudinal imaging to determine the possible neuropathological and cognitive effects of long-term participation in high-contact sports.
Acknowledgments
This research was conducted with funding from the Radiology Society of North America and American Society for Neuroradiology, the Center for Biological Imaging at Stanford, and General Electric Healthcare. Christian Thaler received individual research funding by the German Research Foundation (DFG; reference no.: TH 2235/1-1). This publication was also supported, in part, by the Pac-12 Conference's Student-Athlete Health and Well-Being Initiative. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the Pac-12 Conference or its members.
We thank Dr. Andrew Hoffman, Dr. Tony Wyss-Coray, Steven Bartlinsky, Scott Anderson, Christopher Barrett, Lexie Ross, and Eitan Gelber for their assistance with study design, recruitment, and evaluation.
Author Disclosure Statement
Dr. Zeineh receives research funding from General Electric Healthcare. Max Wintermark is a member of the GE NFL Advisory Board.
References
- 1. Shultz S.R., MacFabe D.F., Foley K.A., Taylor R., and Cain D.P. (2012). Sub-concussive brain injury in the Long-Evans rat induces acute neuroinflammation in the absence of behavioral impairments. Behav. Brain Res. 229, 145–152 [DOI] [PubMed] [Google Scholar]
- 2. Bailes J.E., Petraglia A.L., Omalu B.I., Nauman E., and Talavage T. (2013). Role of subconcussion in repetitive mild traumatic brain injury. J. Neurosurg. 119, 1235–1245 [DOI] [PubMed] [Google Scholar]
- 3. Broglio S.P., Eckner J.T., Paulson H.L., and Kutcher J.S. (2012). Cognitive decline and aging: the role of concussive and subconcussive impacts. Exerc. Sport Sci. Rev. 40, 138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Talavage T.M., Nauman E.A., Breedlove E.L., Yoruk U., Dye A.E., Morigaki K.E., Feuer H., and Leverenz L.J. (2014). Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J. Neurotrauma 31, 327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tagge C.A., Fisher A.M., Minaeva O.V., Gaudreau-Balderrama A., Moncaster J.A., Zhang X.L., Wojnarowicz M.W., Casey N., Lu H., Kokiko-Cochran O.N., Saman S., Ericsson M., Onos K.D., Veksler R., Senatorov V.V., Jr., Kondo A., Zhou X.Z., Miry O., Vose L.R., Gopaul K.R., Upreti C., Nowinski C.J., Cantu R.C., Alvarez V.E., Hildebrandt A.M., Franz E.S., Konrad J., Hamilton J.A., Hua N., Tripodis Y., Anderson A.T., Howell G.R., Kaufer D., Hall G.F., Lu K.P., Ransohoff R.M., Cleveland R.O., Kowall N.W., Stein T.D., Lamb B.T., Huber B.R., Moss W.C., Friedman A., Stanton P.K., McKee A.C., and Goldstein L.E. (2018). Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain 141, 422–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh R., Meier T.B., Kuplicki R., Savitz J., Mukai I., Cavanagh L., Allen T., Teague T.K., Nerio C., Polanski D., and Bellgowan P.S. (2014). Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA 311, 1883–1888 [DOI] [PubMed] [Google Scholar]
- 7. Meier T.B., Savitz J., Singh R., Teague T.K., and Bellgowan P.S. (2016). Smaller dentate gyrus and CA2 and CA3 volumes are associated with kynurenine metabolites in collegiate football athletes. J. Neurotrauma 33, 1349–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monti J.M., Voss M.W., Pence A., McAuley E., Kramer A.F., and Cohen N.J. (2013). History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front. Aging Neurosci. 5, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beauchamp M.H., Ditchfield M., Maller J.J., Catroppa C., Godfrey C., Rosenfeld J.V., Kean M.J., and Anderson V.A. (2011). Hippocampus, amygdala and global brain changes 10 years after childhood traumatic brain injury. Int. J. Dev. Neurosci. 29, 137–143 [DOI] [PubMed] [Google Scholar]
- 10. Govindarajan K.A., Narayana P.A., Hasan K.M., Wilde E.A., Levin H.S., Hunter J.V., Miller E.R., Patel V.K., Robertson C.S., and McCarthy J.J. (2016). Cortical thickness in mild traumatic brain injury. J. Neurotrauma 33, 1809–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee Y.K., Hou S.W., Lee C.C., Hsu C.Y., Huang Y.S., and Su Y.C. (2013). Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One 8, e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKee A.C., Stein T.D., Kiernan P.T., and Alvarez V.E. (2015). The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 25, 350–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bird C.M., and Burgess N. (2008). The hippocampus and memory: insights from spatial processing. Nat. Rev. Neurosci. 9, 182–194 [DOI] [PubMed] [Google Scholar]
- 14. Zeidman P., and Maguire E.A. (2016). Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat. Rev. Neurosci. 17, 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aungst S.L., Kabadi S.V., Thompson S.M., Stoica B.A., and Faden A.I. (2014). Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J. Cereb. Blood Flow Metab. 34, 1223–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Girgis F., Pace J., Sweet J., and Miller J.P. (2016). Hippocampal neurophysiologic changes after mild traumatic brain injury and potential neuromodulation treatment approaches. Front. Syst. Neurosci. 10, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Witter M.P., and Amaral D.G. (1991). Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J. Comp. Neurol. 307, 437–459 [DOI] [PubMed] [Google Scholar]
- 18. Zeineh M.M., Palomero-Gallagher N., Axer M., Grassel D., Goubran M., Wree A., Woods R., Amunts K., and Zilles K. (2017). Direct visualization and mapping of the spatial course of fiber tracts at microscopic resolution in the human hippocampus. Cereb. Cortex 27, 1779–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosene D.L., and Van Hoesen G.W. (1977). Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science 198, 315–317 [DOI] [PubMed] [Google Scholar]
- 20. Rosene D., and Van Hoesen G. (1987). The hippocampal formation of the primate brain, in: Cerebral Cortex. Jones E, Peters A. (eds). Plenum: New York, pps. 345–456 [Google Scholar]
- 21. Lim C., Blume H.W., Madsen J.R., and Saper C.B. (1997). Connections of the hippocampal formation in humans: I. The mossy fiber pathway. J. Comp. Neurol. 385, 325–351 [DOI] [PubMed] [Google Scholar]
- 22. Kondo H., Lavenex P., and Amaral D.G. (2008). Intrinsic connections of the macaque monkey hippocampal formation: I. Dentate gyrus. J. Comp. Neurol. 511, 497–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kondo H., Lavenex P., and Amaral D.G. (2009). Intrinsic connections of the macaque monkey hippocampal formation: II. CA3 connections. J. Comp. Neurol. 515, 349–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saunders R.C., and Aggleton J.P. (2007). Origin and topography of fibers contributing to the fornix in macaque monkeys. Hippocampus 17, 396–411 [DOI] [PubMed] [Google Scholar]
- 25. Lim C., Mufson E.J., Kordower J.H., Blume H.W., Madsen J.R., and Saper C.B. (1997). Connections of the hippocampal formation in humans: II. The endfolial fiber pathway. J. Comp. Neurol. 385, 352–371 [PubMed] [Google Scholar]
- 26. Parekh M.B., Rutt B.K., Purcell R., Chen Y., and Zeineh M.M. (2015). Ultra-high resolution in-vivo 7.0T structural imaging of the human hippocampus reveals the endfolial pathway. Neuroimage 112, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saunders R.C., and Rosene D.L. (1988). A comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: I. Convergence in the entorhinal, prorhinal, and perirhinal cortices. J. Comp. Neurol. 271, 153–184 [DOI] [PubMed] [Google Scholar]
- 28. Wilson D.I., Langston R.F., Schlesiger M.I., Wagner M., Watanabe S., and Ainge J.A. (2013). Lateral entorhinal cortex is critical for novel object-context recognition. Hippocampus 23, 352–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carr V.A., Viskontas I.V., Engel S.A., and Knowlton B.J. (2010). Neural activity in the hippocampus and perirhinal cortex during encoding is associated with the durability of episodic memory. J. Cogn. Neurosci. 22, 2652–2662 [DOI] [PubMed] [Google Scholar]
- 30. Aggleton J.P., and Brown M.W. (1999). Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav. Brain Sci. 22, 425–444; discussion, 444–489 [PubMed] [Google Scholar]
- 31. Gabrieli J.D., Brewer J.B., Desmond J.E., and Glover G.H. (1997). Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science 276, 264–266 [DOI] [PubMed] [Google Scholar]
- 32. Zeineh M.M., Engel S.A., Thompson P.M., and Bookheimer S.Y. (2003). Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science 299, 577–580 [DOI] [PubMed] [Google Scholar]
- 33. Eldridge L.L., Engel S.A., Zeineh M.M., Bookheimer S.Y., and Knowlton B.J. (2005). A dissociation of encoding and retrieval processes in the human hippocampus. J. Neurosci. 25, 3280–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Viskontas I.V., Carr V.A., Engel S.A., and Knowlton B.J. (2009). The neural correlates of recollection: hippocampal activation declines as episodic memory fades. Hippocampus 19, 265–272 [DOI] [PubMed] [Google Scholar]
- 35. Bartsch T., Dohring J., Rohr A., Jansen O., and Deuschl G. (2011). CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc. Natl. Acad. Sci. U. S. A. 108, 17562–17567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stevenson R.F., Zheng J., Mnatsakanyan L., Vadera S., Knight R.T., Lin J.J., and Yassa M.A. (2018). Hippocampal CA1 gamma power predicts the precision of spatial memory judgments. Proc. Natl. Acad. Sci. U. S. A. 115, 10148–10153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hitti F.L., and Siegelbaum S.A. (2014). The hippocampal CA2 region is essential for social memory. Nature 508, 88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hrybouski S., MacGillivray M., Huang Y., Madan C.R., Carter R., Seres P., and Malykhin N.V. (2019). Involvement of hippocampal subfields and anterior-posterior subregions in encoding and retrieval of item, spatial, and associative memories: longitudinal versus transverse axis. Neuroimage 191, 568–586 [DOI] [PubMed] [Google Scholar]
- 39. Braak H., and Braak E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 [DOI] [PubMed] [Google Scholar]
- 40. Ali R., Goubran M., Choudhri O., and Zeineh M.M. (2015). Seven-Tesla MRI and neuroimaging biomarkers for Alzheimer's disease. Neurosurg. Focus 39, E4. [DOI] [PubMed] [Google Scholar]
- 41. Blumcke I., Thom M., Aronica E., Armstrong D.D., Bartolomei F., Bernasconi A., Bernasconi N., Bien C.G., Cendes F., Coras R., Cross J.H., Jacques T.S., Kahane P., Mathern G.W., Miyata H., Moshe S.L., Oz B., Ozkara C., Perucca E., Sisodiya S., Wiebe S., and Spreafico R. (2013). International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 54, 1315–1329 [DOI] [PubMed] [Google Scholar]
- 42. Santyr B.G., Goubran M., Lau J.C., Kwan B.Y.M., Salehi F., Lee D.H., Mirsattari S.M., Burneo J.G., Steven D.A., Parrent A.G., de Ribaupierre S., Hammond R.R., Peters T.M., and Khan A.R. (2017). Investigation of hippocampal substructures in focal temporal lobe epilepsy with and without hippocampal sclerosis at 7T. J. Magn. Reson. Imaging 45, 1359–1370 [DOI] [PubMed] [Google Scholar]
- 43. Adamowicz D.H., Roy S., Salmon D.P., Galasko D.R., Hansen L.A., Masliah E., and Gage F.H. (2017). Hippocampal alpha-synuclein in dementia with Lewy bodies contributes to memory impairment and is consistent with spread of pathology. J. Neurosci. 37, 1675–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sapolsky R.M., Uno H., Rebert C.S., and Finch C.E. (1990). Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J. Neurosci. 10, 2897–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McKee A.C., Cairns N.J., Dickson D.W., Folkerth R.D., Keene C.D., Litvan I., Perl D.P., Stein T.D., Vonsattel J.P., Stewart W., Tripodis Y., Crary J.F., Bieniek K.F., Dams-O'Connor K., Alvarez V.E., and Gordon W.A.; TBI/CTE group. (2016). The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 131, 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goubran M., Bernhardt B.C., Cantor-Rivera D., Lau J.C., Blinston C., Hammond R.R., de Ribaupierre S., Burneo J.G., Mirsattari S.M., Steven D.A., Parrent A.G., Bernasconi A., Bernasconi N., Peters T.M., and Khan A.R. (2016). In vivo MRI signatures of hippocampal subfield pathology in intractable epilepsy. Hum. Brain Mapp. 37, 1103–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L., Kim J.S., Heo S., Alves H., White S.M., Wojcicki T.R., Mailey E., Vieira V.J., Martin S.A., Pence B.D., Woods J.A., McAuley E., and Kramer A.F. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 108, 3017–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Noble K.G., Grieve S.M., Korgaonkar M.S., Engelhardt L.E., Griffith E.Y., Williams L.M., and Brickman A.M. (2012). Hippocampal volume varies with educational attainment across the life-span. Front. Hum. Neurosci. 6, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCrory P., Meeuwisse W., Johnston K., Dvorak J., Aubry M., Molloy M., and Cantu R. (2009). Consensus statement on concussion in sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Phys. Sportsmed. 37, 141–159 [DOI] [PubMed] [Google Scholar]
- 50. Contijoch F., Witschey W.R., Rogers K., Rears H., Hansen M., Yushkevich P., Gorman J. I, II, Gorman R.C., and Han Y. (2015). User-initialized active contour segmentation and golden-angle real-time cardiovascular magnetic resonance enable accurate assessment of LV function in patients with sinus rhythm and arrhythmias. J. Cardiovasc. Magn. Reson. 17, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Poppenk J., Evensmoen H.R., Moscovitch M., and Nadel L. (2013). Long-axis specialization of the human hippocampus. Trends Cogn. Sci. 17, 230–240 [DOI] [PubMed] [Google Scholar]
- 52. Insausti R.A., and Amaral D.G. (2012). Hippocampal formation, in: The Human Nervous System, 2nd ed. Paxinos G., Mai J.K. (eds). Elsevier: San Diego, CA, pps. 896–942 [Google Scholar]
- 53. Barnes J., Ridgway G.R., Bartlett J., Henley S.M., Lehmann M., Hobbs N., Clarkson M.J., MacManus D.G., Ourselin S., and Fox N.C. (2010). Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage 53, 1244–1255 [DOI] [PubMed] [Google Scholar]
- 54. Salat D.H., Buckner R.L., Snyder A.Z., Greve D.N., Desikan R.S., Busa E., Morris J.C., Dale A.M., and Fischl B. (2004). Thinning of the cerebral cortex in aging. Cereb. Cortex 14, 721–730 [DOI] [PubMed] [Google Scholar]
- 55. Reuter M., Schmansky N.J., Rosas H.D., and Fischl B. (2012). Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. D'Agostino R.B., Balanger A., and D'Agostino R.B., Jr. (1990). A suggestion for using powerful and informative tests of normality. Am. Stat. 44, 316–321 [Google Scholar]
- 57. Benjamini Y., and Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Statist. Soc. B 57, 289–300 [Google Scholar]
- 58. Nathanson J.T., Connolly J.G., Yuk F., Gometz A., Rasouli J., Lovell M., and Choudhri T. (2016). Concussion incidence in professional football: position-specific analysis with use of a novel metric. Orthop. J. Sports Med. 4, 2325967115622621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Crisco J.J., Wilcox B.J., Beckwith J.G., Chu J.J., Duhaime A.C., Rowson S., Duma S.M., Maerlender A.C., McAllister T.W., and Greenwald R.M. (2011). Head impact exposure in collegiate football players. J. Biomech. 44, 2673–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Crisco J.J., Wilcox B.J., Machan J.T., McAllister T.W., Duhaime A.C., Duma S.M., Rowson S., Beckwith J.G., Chu J.J., and Greenwald R.M. (2012). Magnitude of head impact exposures in individual collegiate football players. J. Appl. Biomech. 28, 174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wolf J.A., Johnson B.N., Johnson V.E., Putt M.E., Browne K.D., Mietus C.J., Brown D.P., Wofford K.L., Smith D.H., Grady M.S., Cohen A.S., and Cullen D.K. (2017). Concussion induces hippocampal circuitry disruption in swine. J. Neurotrauma 34, 2303–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Slobounov S.M., Walter A., Breiter H.C., Zhu D.C., Bai X., Bream T., Seidenberg P., Mao X., Johnson B., and Talavage T.M. (2017). The effect of repetitive subconcussive collisions on brain integrity in collegiate football players over a single football season: a multi-modal neuroimaging study. Neuroimage Clin. 14, 708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tsai M.H., Vaughan D.N., Perchyonok Y., Fitt G.J., Scheffer I.E., Berkovic S.F., and Jackson G.D. (2016). Hippocampal malrotation is an anatomic variant and has no clinical significance in MRI-negative temporal lobe epilepsy. Epilepsia 57, 1719–1728 [DOI] [PubMed] [Google Scholar]
- 64. Hart J., Jr., Kraut M.A., Womack K.B., Strain J., Didehbani N., Bartz E., Conover H., Mansinghani S., Lu H., and Cullum C.M. (2013). Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross-sectional study. JAMA Neurol. 70, 326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wisse L.E.M., Daugherty A.M., Olsen R.K., Berron D., Carr V.A., Stark C.E.L., Amaral R.S.C., Amunts K., Augustinack J.C., Bender A.R., Bernstein J.D., Boccardi M., Bocchetta M., Burggren A., Chakravarty M.M., Chupin M., Ekstrom A., de Flores R., Insausti R., Kanel P., Kedo O., Kennedy K.M., Kerchner G.A., LaRocque K.F., Liu X., Maass A., Malykhin N., Mueller S.G., Ofen N., Palombo D.J., Parekh M.B., Pluta J.B., Pruessner J.C., Raz N., Rodrigue K.M., Schoemaker D., Shafer A.T., Steve T.A., Suthana N., Wang L., Winterburn J.L., Yassa M.A., Yushkevich P.A., and la Joie R.; Hippocampal Subfields Group. (2017). A harmonized segmentation protocol for hippocampal and parahippocampal subregions: why do we need one and what are the key goals? Hippocampus 27, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Covassin T., Elbin R.J., III, Stiller-Ostrowski J.L., and Kontos A.P. (2009). Immediate post-concussion assessment and cognitive testing (ImPACT) practices of sports medicine professionals. J. Athl. Train. 44, 639–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lepage C., Muehlmann M., Tripodis Y., Hufschmidt J., Stamm J., Green K., Wrobel P., Schultz V., Weir I., Alosco M.L., Baugh C.M., Fritts N.G., Martin B.M., Chaisson C., Coleman M.J., Lin A.P., Pasternak O., Makris N., Stern R.A., Shenton M.E., and Koerte I.K. (2018). Limbic system structure volumes and associated neurocognitive functioning in former NFL players. Brain Imaging Behav. May 19. doi: 10.1007/s11682-018-9895-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]