ABSTRACT
Emerging evidence suggests that the gut microbiota may interact with the host brain and play pivotal roles in the pathogenesis of neuropsychiatric disorders. However, the mechanism underlying reciprocal interactions along the microbiota-gut-brain axis in depression remains unclear. In this study, a murine model of chronic restraint stress (CRS) was established to investigate the metabolic signaling of tryptophan (Trp) neurotransmission at the intestinal and central levels in depression. The results showed that CRS mice displayed depression- and anxiety-like behaviors. Additionally, kynurenine (Kyn) and its metabolites, an important Trp metabolic pathway, were strongly activated in the brain. Intriguingly, the Kyn toxic signaling was exacerbated in the gut, especially in the colon. Indoleamine 2,3-dioxygenase (IDO), a rate-limiting enzyme responsible for Kyn metabolic pathway initiation, was significantly upregulated in the brain and gut in CRS mice compared with control mice, promoting transfer of Trp metabolic pathway to Kyn signaling. Additionally, administration of IDO inhibitor, 1-methyl-tryptophan (1-MT), partially rescued CRS-induced depression- and anxiety-like changes. Moreover, the enhanced intestinal permeability mediated by CRS allowed toxic metabolites to “leak” into the bloodstream. The microbiome profiles of CRS mice displayed obviously altered taxonomic composition and negative correlations were observed between Enterorhabdus, Parabacteroides and Kyn levels in the brain. Reciprocal crosstalk between the brain and gut was further validated by citalopram treatment, IDO inhibitor and microbiota intervention, which counteracted depression-like behavior, Kyn metabolic signaling and microbiota composition in CRS mice. Meanwhile, Parabacteroides treatment affected Trp metabolism in mouse hippocampus, manifesting as elevated concentration of 5-HT as well as ratio of 5-HT to Trp. These results suggest that long-term stress disrupts Kyn metabolism and endocrine function along the gut-brain axis, accompanied by the disrupted homeostasis of certain microbiota, which collectively contribute to the development of depression-like behavior.
KEYWORDS: Depression, tryptophan metabolism, serotonin, kynurenine, microbiota
Introduction
Depression, the third leading contributor to global disease burden, is now one of the most prevalent psychiatric disorders and a severe public health problem worldwide that has attracted great attention in recent years.1–3 There are myriad possible causes of depressive disorders, including genetic vulnerability, physical health problems, stifling life events, etc., which makes the clinical diagnosis of depression difficult. Currently, depression is still mainly diagnosed by subjective symptoms based on the Hamilton Depression Scale, which is far from adequate to accurate classification and treatment.4,5 Therefore, it is of great value to explore objective diagnosis indicators and potential mechanisms of depression.
Neurochemical imbalances, especially the neurotransmitter imbalances in the tryptophan (Trp) pathway, underlie the pathophysiology of mood disorders.6 One major metabolite of Trp is serotonin (5-hydroxytryptamine, 5-HT), which modulates cognition, reward, physiological processes, etc.7,8 5-HT is mainly located in the gastrointestinal tract and brain, with a few circulating in peripheral blood.9 The pathophysiology of depression has been closely linked to low levels of 5-HT.10–12 Reciprocally, selective serotonin reuptake inhibitors (SSRIs), the commonly prescribed antidepressants in clinical treatment, target the serotoninergic system by inhibiting the uptake of 5-HT and increasing its synaptic level.13,14 Notably, disturbances in Trp metabolism can activate the kynurenine (Kyn) pathway, contributing to alterations in kynurenic acid (Kna), 3-hydroxykynurenine (3-HK) and quinolinic acid (QA),15 which are remarkably associated with psychiatric diseases such as depression.16 Figure. S1 represents the detailed Trp pathway and its major metabolites.
The brain-gut axis is a bidirectional communication network between the brain and gut where Trp metabolism plays an important role in both the central nervous system (CNS) and the enteric nervous system (ENS). It may be more appropriate to specify this axis as the “microbiota-gut-brain axis” due to the active role of microbiota in the function of the nervous system through its interaction with the gut-brain axis.17 Accumulating reports pointed to the influence of the Trp pathway far beyond the traditional focus on its role in only the CNS, but also in the ENS.18,19 Trp is a biosynthetic precursor of a large number of microbial and host metabolites, thus the gut microbiota may be involved in neuropsychiatric disorders partially through the modulation of circulating Trp availability directly through cell-to-cell interactions or indirectly through metabolites.20 Therefore, targeting Trp metabolism in the gut is an actionable therapeutic approach to exploit microbiota as potential probiotics. To be specific, the Kyn pathway in immune and epithelial cells can be activated through IDO1 mediated by microbiota. Similarly, the 5-HT production pathway in enterochromaffin cells (ECs) via Trp hydroxylase 1 (TPH1) can also be activated. However, the connections between microbiota and depression-like behaviors, available Kyn metabolism and crucial rate-limiting enzymes in the gut-brain axis, however, remains poorly understood.
In the present study, to fully investigate Trp metabolism, especially the Kyn metabolic profile, and the link between Kyn metabolites and gut bacteria in depression, we examined the influence of chronic restraint stress (CRS) with or without the administration of the antidepressant citalopram on behavioral changes, microbiota diversity, the Trp pathway metabonomic profile and the expression of rate-limiting enzymes in the microbiota-gut-brain axis. Our findings might provide potential bacterial targets and new theoretical basis for the early intervention of depression.
Results
CRS-induced anxiety- and depression-like behavioral changes in mice
The simplified experimental scheme is shown in Figure 1a. After the first 2 weeks of model establishment, obvious anxiety- and depression-like behaviors were observed among the mice subjected to daily CRS compared with the control group (Fig. S2A-F). Specifically, the immobility time in the FST was obviously lengthened (Fig. S2A) and the sucrose consumption ratio in the SPT was lessened in CRS mice when compared with CTL mice. Moreover, time spent in the center field and open arms in the OPT (Fig. S2C and S2E) and EPM (Fig. S2D and S2F) were markedly decreased in CRS mice as compared with control mice.
Figure 1.

CRS-induced anxiety- and depression-like behavioral changes in mice. (a) Protocol diagram of the time course related to the experimental procedure and behavioral tests. The effects of the chronic administration of vehicle or citalopram on behavioral responses in CTL or CRS mice are shown with (b) immobility time (s) in the FST, (c) sucrose consumption ratio (%) in the SPT, (d) open arms duration (%) in the EPM test and (e) center time (s) and (f) total track length (mm) in the OFT. Values represent the mean ± S.E.M. of 8 animals per group. *p < .05 and **p < .01 indicate significant differences vs. the CTL+PBS group, and #p < .05 and ##p < .01 indicate significant differences vs. the CRS+PBS group
After the following 3 weeks of PBS or citalopram administration, the anxiety- and depression-like behaviors induced by chronic stress were reversed by citalopram, manifesting as the decreased time of immobility in the FST (Figure 1b) and the increased sucrose consumption in the SPT (Figure 1c) in the CRS+CITA group compared with the CRS+PBS group. Meanwhile, the time spent in the open arms in the EPM (Figure 1d and Fig. S2H) and the time spent in the center of the OFT (Figure 1e and Fig. S2G) were increased as well (though statistically insignificant) in citalopram treatment, showing a trend of recovery to the control level. There were no significant differences in total track length in the OFT (Figure 1f). These behavioral tests, taken together, indicated the effective establishment of the mouse CRS model and SSRI citalopram administration.
CRS-induced neurotransmitter metabolic disorders in Trp pathway in the brain and serum
As depicted in Figure 2a, the mice in the CRS+PBS group displayed lower levels of 5-HT, a lower ratio of 5-HT/Trp, and higher ratios of Kyn/Trp and 5-HIAA/5-HT in the PFC, which was partially reversed in the CRS+CITA group. In the hippocampus, Kyn and Kyn/Trp ratio were remarkably increased in the CRS+PBS group compared with the CTL+PBS group, which could also be alleviated by antidepressants (though statistically insignificant). The Trp-5-HT pathway exhibited no significant changes except for a decrease in downstream metabolite 5-HIAA in the CRS+PBS group (Figure 2b), implying the switch of Trp metabolism from the 5-HT pathway to the Kyn pathway.21 In the serum, the Trp levels were significantly reduced in the CRS+PBS-treated group, while the Kyn, 3-HAA levels and the Kyn/Trp ratio were significantly increased. Additionally, 3-HK, a Kyn metabolite, was increased, while the 5-HT level was decreased although the results were statistically insignificant (Figure 2c). These results demonstrated that the metabolism of Trp was disordered and more inclined to convert to the Kyn pathway in the PFC, hippocampus and serum of the CRS mice. Notably, citalopram administration, to some extent, reversed the changes caused by chronic stress but did not exert any effect on these neurotransmitters in control mice, indicating that it played a role only under stress-induced neurotransmitter imbalance. Additionally, neurotransmitters in the Tyrosine pathway and some amino acids that have been proven to have potential links with depression were also detected in our metabolomic platform (Supplementary Table 1–3).22,23
Figure 2.

CRS induced disrupted neurotransmitter metabolism in Trp pathway in the brain and serum. Differences in Trp and its metabolites in the (a) PFC, (b) hippocampus and (c) serum are shown. Values represent the mean ± S.E.M. of 10 animals per group. *p < .05 and **p < .01 indicate significant differences vs. the CTL+PBS group, and #p < .05 and ##p < .01 indicate significant differences vs. the CRS+PBS group
Changes in Trp metabolism contributed to anxiety- and depression-like behavioral changes in mice
To demonstrate the contribution of altered Trp metabolism to behavioral changes, another two groups of CTL and CRS mice treated with IDO inhibitor, 1-methyl-tryptophan (1-MT) were used (Fig. S3). The activity of Kyn pathway in the CRS+1-MT group was attenuated when compared with the CRS+PBS group, which could be verified by the decreased levels of Kyn and Kyn/Trp (Fig. S3A). In the FST and SPT, CRS+1-MT mice exhibited an evident reduction of immobility (Fig. S3B) and increase of sucrose consumption (Fig. S3C) respectively. Moreover, administration of 1-MT increased the duration in open arms in the EPM though the result statistically insignificant (Fig. S3D). Compared with the CRS+PBS group, extended time spent in center area was observed in CRS+1-MT mice (Fig. S3E), albeit the total track length difference was not significant (Fig. S3F). Intriguingly, by the FST assay, an increased immobility in CTL+1-MT mice was observed when compared with the CTL+PBS mice (Fig. S3B), meanwhile, the decrease in center time (Fig. S3E) as well as total distance in the OFT (Fig. S3F) was shown in CTL+1-MT mice, implying that some other unknown effects of 1-MT may be involved in these processes.
CRS altered the key rate-limiting enzymes of the Trp pathway in the brain
It revealed a significantly decreased expression of TPH2 and increased level of IDO1 in mice subjected to chronic stress in the PFC (Figure 3a and Figure 3b). However, the effect on IDO1 could be effectively reversed by citalopram, and a trend toward increased TPH2 was also observed following citalopram treatment. Mice in the CRS+PBS group showed a pronounced increase in MAO A expression (Figure 3a and Figure 3b) and a decrease in MAO B expression (Fig. S2I and S2J). Moreover, a significant decrease in brain-derived neurotrophic factor (BDNF) accompanied by decreased tropomyosin receptor kinase B (TrkB) expression was observed in the PFC of the CRS+PBS mice (Figure 3a and Figure 3b).
Figure 3.
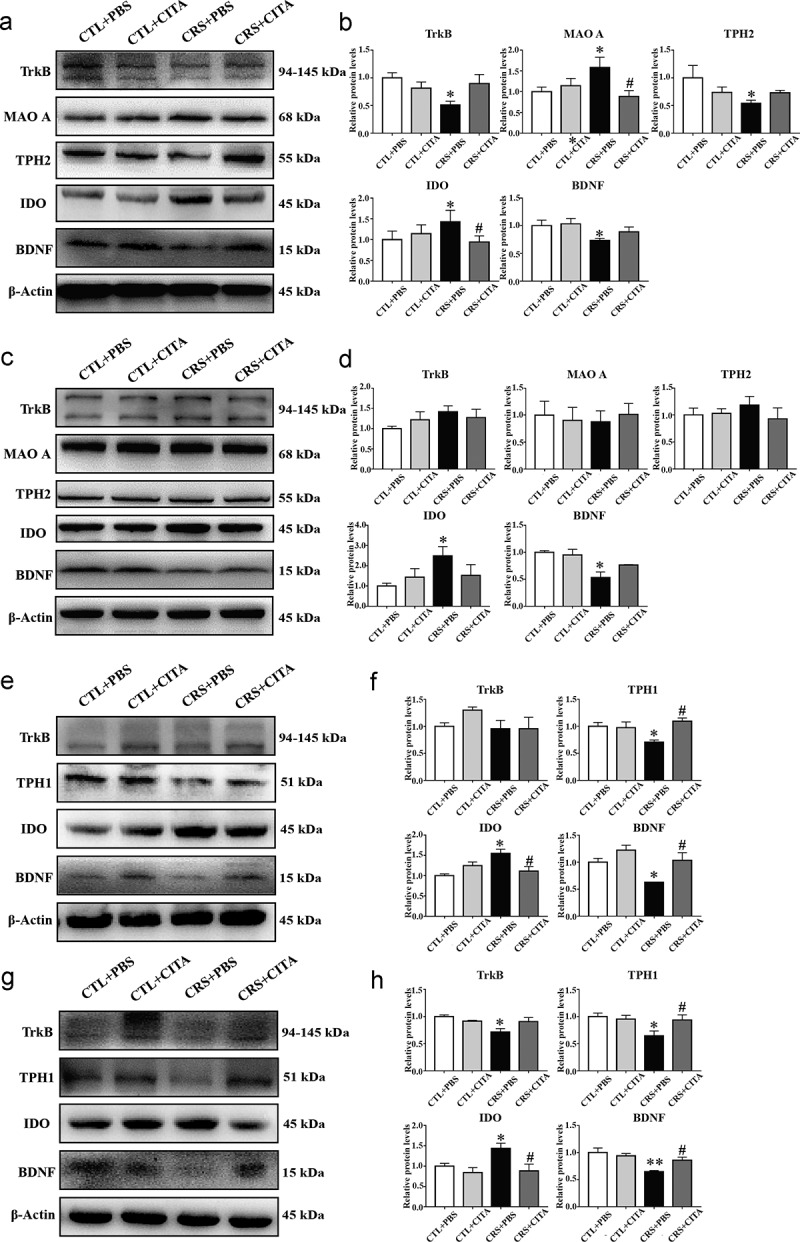
CRS-induced enzymatic alterations in the Trp pathway in the brain and gut. Western blot analysis showed the expression of rate-limiting enzymes that induced Trp metabolism and the BDNF-TrkB pathway in the (a, b) PFC, (c, d) hippocampus, (e, f) ileum and (g, h) colon. Values represent the means ± S.E.M. *p < .05 and **p < .01 indicate significant differences vs. the CTL+PBS group, and #p < .05 and ##p < .01 indicate significant differences vs. the CRS+PBS group
In accordance with the results of PFC in CRS+PBS mice, the expression of hippocampal IDO was markedly increased, while BDNF was obviously decreased (Figure 3c and Figure 3d). Intriguingly, some hippocampal proteins such as TPH2, MAO A and MAO B (Fig. S2K and S2L) displayed no significant alterations in varied treated groups.
CRS-induced intestinal neurotransmitter disorders and enzymatic alterations in the Trp pathway
In Table 1 and Table 2, the levels of Trp and its metabolites in mouse duodenum, jejunum, ileum, colon and cecum are shown. Although it could be detected throughout the intestinal tract, Trp was mainly taken up in the small intestine and there were no great differences among the four groups. Moreover, the catabolites of Trp, 5-HT, could only be detected in the duodenum, jejunum and colon. Intriguingly, 5-HT was decreased in the duodenum and colon of mice subjected to CRS, and the effect was more pronounced in the colon. In addition, Kyn in the ileum of the CRS+PBS group was substantially increased compared to that in the ileum of the CTL+PBS mice, and this effect could be ameliorated by citalopram treatment in the colon of the CRS mice. A similar metabolic pattern was observed in the duodenum, though no significant differences were shown. Moreover, 3-HK was increased under chronic stress and partially decreased after citalopram treatment in all intestinal segments. However, no obvious changes in the neurotransmitters in the jejunum and cecum were observed in any of the four groups.
Table 1.
Concentrations of Trp and the related metabolites in the small intestine (duodenum, jejunum and ileum, ng/g)
| Compounds | CTL+PBS | CTL+CITA | CRS+PBS | CRS+CITA | |
|---|---|---|---|---|---|
| Duodenum | TRP | 27800.00 ± 2988.07 | 32914.29 ± 2723.22 | 34420.00 ± 8085.32 | 34540.00 ± 5256.96 |
| KYN | 393.08 ± 142.67 | 43.74 ± 14.56 | 512.19 ± 240.35 | 382.73 ± 108.48 | |
| 3-HK | 31.62 ± 14.45 | 62.85 ± 12.81 | 82.82 ± 29.76 | 56.47 ± 10.37 | |
| 3-HAA | 2.03 ± 0.27 | 3.20 ± 0.58 | 3.52 ± 1.04 | 1.40 ± 0.33 | |
| 5-HT | 1617.23 ± 376.24 | 901.74 ± 208.79 | 870.01 ± 220.47 | 885.49 ± 198.13 | |
| 5-HIAA | 1620.96 ± 281.65 | 888.26 ± 112.69 | 1778.46 ± 349.49 | 1393.74 ± 265.21 | |
| NAS | 9.70 ± 0.79 | 9.55 ± 0.66 | 8.80 ± 0.76 | 10.03 ± 1.10 | |
| MLT | 34.17 ± 3.18 | 33.62 ± 1.80 | 32.77 ± 3.47 | 35.09 ± 3.47 | |
| 5-HT/TRP | 0.064 ± 0.015 | 0.032 ± 0.009 | 0.021 ± 0.008* | 0.032 ± 0.009 | |
| KYN/TRP | 0.014 ± 0.005 | 0.001 ± 0.0004 | 0.018 ± 0.009 | 0.015 ± 0.005 | |
| Jejunum | TRP | 37610.20 ± 6452.10 | 49798.10 ± 17118.97 | 42209.67 ± 10969.77 | 39519.81 ± 10578.33 |
| KYN | 267.90 ± 77.96 | 335.91 ± 63.52 | 261.01 ± 63.40 | 298.33 ± 78.12 | |
| 3-HK | ND | ND | ND | ND | |
| 3-HAA | ND | ND | ND | ND | |
| 5-HT | 47.74 ± 15.03 | 35.45 ± 7.74 | 40.63 ± 12.96 | 45.29 ± 28.58 | |
| 5-HIAA | 483.24 ± 68.17 | 437.75 ± 97.81 | 567.26 ± 80.94 | 490.27 ± 92.62 | |
| NAS | 2.34 ± 0.28 | 2.24 ± 0.58 | 1.98 ± 0.18 | 1.91 ± 0.28 | |
| MLT | 1.62 ± 0.23 | 1.87 ± 0.41 | 1.91 ± 0.31 | 1.46 ± 0.27 | |
| 5-HT/TRP | 0.0022 ± 0.0008 | 0.0016 ± 0.0004 | 0.0017 ± 0.0005 | 0.0024 ± 0.0012 | |
| KYN/TRP | 0.0083 ± 0.0026 | 0.0135 ± 0.0037 | 0.0101 ± 0.0037 | 0.0147 ± 0.0043 | |
| Ileum | TRP | 30649.80 ± 5780.49 | 47924.94 ± 12970.18 | 34823.15 ± 6096.86 | 34188.81 ± 6619.53 |
| KYN | 156.97 ± 30.96 | 115.75 ± 21.91 | 303.08 ± 116.43* | 221.31 ± 39.23 | |
| 3-HK | 5.96 ± 1.09 | 12.30 ± 4.63 | 19.88 ± 6.26 | 17.33 ± 5.39 | |
| 3-HAA | 37.62 ± 3.66 | 44.80 ± 6.69 | 34.51 ± 7.78 | 32.73 ± 9.38 | |
| 5-HT | ND | ND | ND | ND | |
| 5-HIAA | 677.19 ± 98.69 | 628.70 ± 148.44 | 575.35 ± 59.31 | 969.00 ± 231.76 | |
| NAS | 2.10 ± 0.18 | 2.28 ± 0.33 | 1.81 ± 0.32 | 1.81 ± 0.50 | |
| MLT | 1.80 ± 0.20 | 1.85 ± 0.28 | 1.59 ± 0.36 | 1.57 ± 0.40 | |
| 5-HT/TRP | / | / | / | / | |
| KYN/TRP | 0.0061 ± 0.0017 | 0.0046 ± 0.0013 | 0.0151 ± 0.0050* | 0.0101 ± 0.0025 |
Values represent the mean ± S.E.M (ng/g).
ND indicates not detected.
*p < 0.05 and **p < 0.01 indicate significant differences vs. the CTL+PBS group, and #p < 0.05 and ##p < 0.01 indicate significant differences vs. the CRS+PBS group.
Table 2.
Concentrations of Trp and the related metabolites in the colon and cecum (ng/g)
| Compounds | CTL+PBS | CTL+CITA | CRS+PBS | CRS+CITA | |
|---|---|---|---|---|---|
| Colon | TRP | 9078.27 ± 862.38 | 10532.81 ± 2615.49 | 9918.90 ± 1907.19 | 9130.50 ± 1790.92 |
| KYN | 267.72 ± 20.83 | 255.97 ± 31.56 | 265.94 ± 46.51 | 157.55 ± 7.66# | |
| 3-HK | 9.24 ± 1.49 | 10.40 ± 5.79 | 14.12 ± 8.15 | 5.87 ± 1.45 | |
| 3-HAA | 33.68 ± 2.84 | 31.71 ± 5.94 | 26.20 ± 3.68 | 16.08 ± 0.92 | |
| 5-HT | 734.94 ± 120.13 | 628.03 ± 92.54 | 403.60 ± 59.65* | 607.80 ± 127.28 | |
| 5-HIAA | 5969.89 ± 805.63 | 7269.28 ± 1665.47 | 4923.71 ± 1563.07 | 4941.89 ± 708.27 | |
| NAS | 5.19 ± 1.33 | 2.85 ± 0.64 | 7.00 ± 3.41 | 3.93 ± 0.76 | |
| MLT | 2.35 ± 0.53 | 2.18 ± 0.76 | 1.45 ± 0.34 | 1.18 ± 0.24 | |
| 5-HT/TRP | 0.10 ± 0.02 | 0.07 ± 0.02 | 0.04 ± 0.01* | 0.08 ± 0.02 | |
| KYN/TRP | 0.025 ± 0.002 | 0.033 ± 0.006 | 0.039 ± 0.005* | 0.023 ± 0.004# | |
| Cecum | TRP | 5256.90 ± 599.84 | 6195.00 ± 424.35 | 4015.40 ± 454.69 | 4719.66 ± 432.26 |
| KYN | 196.28 ± 11.34 | 157.44 ± 11.46 | 169.95 ± 9.60 | 166.44 ± 14.13 | |
| 3-HK | 8.41 ± 0.94 | 7.77 ± 1.58 | 10.53 ± 4.92 | 7.47 ± 1.34 | |
| 3-HAA | 10.97 ± 4.62 | 4.32 ± 0.91 | 15.71 ± 1.20 | 16.32 ± 2.98 | |
| 5-HT | ND | ND | ND | ND | |
| 5-HIAA | 1651.11 ± 896.67 | 1612.30 ± 266.94 | 754.41 ± 215.56 | 861.13 ± 145.86 | |
| NAS | 3.51 ± 0.60 | 6.68 ± 1.15 | 7.16 ± 2.39 | 6.71 ± 1.34 | |
| MLT | 1.30 ± 0.21 | 1.45 ± 0.28 | 1.55 ± 0.22 | 1.61 ± 0.30 | |
| 5-HT/TRP | / | / | / | / | |
| KYN/TRP | 0.044 ± 0.008 | 0.026 ± 0.002 | 0.045 ± 0.003 | 0.039 ± 0.005 |
Values represent the mean ± S.E.M (ng/g).
ND indicates not detected.
*p < 0.05 and **p < 0.01 indicate significant differences vs. the CTL+PBS group, and #p < 0.05 and ##p < 0.01 indicate significant differences vs. the CRS+PBS group.
To clarify the intestinal Trp pathway alterations induced by chronic stress, the expression of critical rate-limiting enzymes in the ileum (Figure 3e and Figure 3f) and colon (Figure 3g and Figure 3h) were examined. Compared to the CTL+PBS group, the CRS+PBS group mice exhibited decreased expression of TPH1 and increased level of IDO1 activity in both the ileum and colon, which could be reversed by citalopram treatment. These results strongly supported the changes in the 5-HT/Trp and Kyn/Trp ratios detected by instrumental analysis. Meanwhile, the level of BDNF in the ileum and colon of the PBS-treated CRS mice was significantly decreased, and this effect could be partially rescued by citalopram.
CRS-induced deleterious changes in intestinal histomorphology and integrity
As shown in Fig. S4A, stress and PBS-treated animals displayed a significant loss of crypt architecture, villus degeneration and infiltration of inflammatory cells when compared with the CTL group. Intriguingly, these histopathological changes could be reversed by citalopram treatment. The detection of zonula occludens-1 (ZO-1) in the ileum (Fig. S4B) and colon (Fig. S4C) further supported the enhancement of intestinal permeability mediated by chronic stress.
CRS-induced gut dysbiosis
Principal coordinates analysis revealed major differences between control and restraint mice (Figure S5A). Moreover, a decrease in bacterial alpha diversity (though statistically insignificant), as indicated by the ACE and Simpson index (Figure 4a) was observed in the CRS+PBS group compared with the CTL+PBS group, while citalopram treatment failed to restore the relative abundance and diversity. Taxonomic shifts were also investigated; at the shallowest phylum level (Figure 4b and Figure 4c), different groups displayed changes although the murine gut microbiota were all dominated by Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria. Increased ratios of Firmicutes to Bacteroidetes and Actinobacteria to Proteobacteria (Figure 4b), though not significant, gave an indication of the overall microbiota dysbiosis in the stress-received mice. Further analysis did identify some clear differences among the four groups. At the family level (Figure 4d), the relative abundances of Sutterellaceae, Ruminococcaceae and Desulfovibrionaceae were all reduced, and the abundance of Lactobacillaceae was markedly increased in restraint animals. Interestingly, citalopram treatment, whether in restraint or control groups, also affected the microbiota composition of Ruminococcaceae, Desulfovibrionaceae and Lactobacillaceae to levels similar to those observed for CRS animals. In contrast, the effects of chronic stress on the Verrucomicrobiaceae, Rikenellaceae, Lachnospiraceae and Bacteroidaceae families were reversed by citalopram. At the genus level (Figure 4e-Figure 4m), higher relative abundances of the genera Akkermansia (Figure 4e) and Anaerofustis (Figure 4f), and significantly reduced Parabacteroides , Lachnospiraceae (Figure 4k), Ruminococcus (Figure 4l) and one species of Unclassified_Ruminococcaceae (Figure 4m) were observed in PBS-treated restraint mice, and these changes could be partially reversed by citalopram. Notably, citalopram administration affected microbiota composition as compared with PBS-treated controls, especially in Parabacteroides and Ruminococcus(Figure 4j and 4l). In addition, Bacteroides (Figure 4h),(Figure 4j) seemed to be affected by the combined effects of chronic stress and citalopram administration with higher relative abundance in CRS+CITA group than in the other groups.
Figure 4.
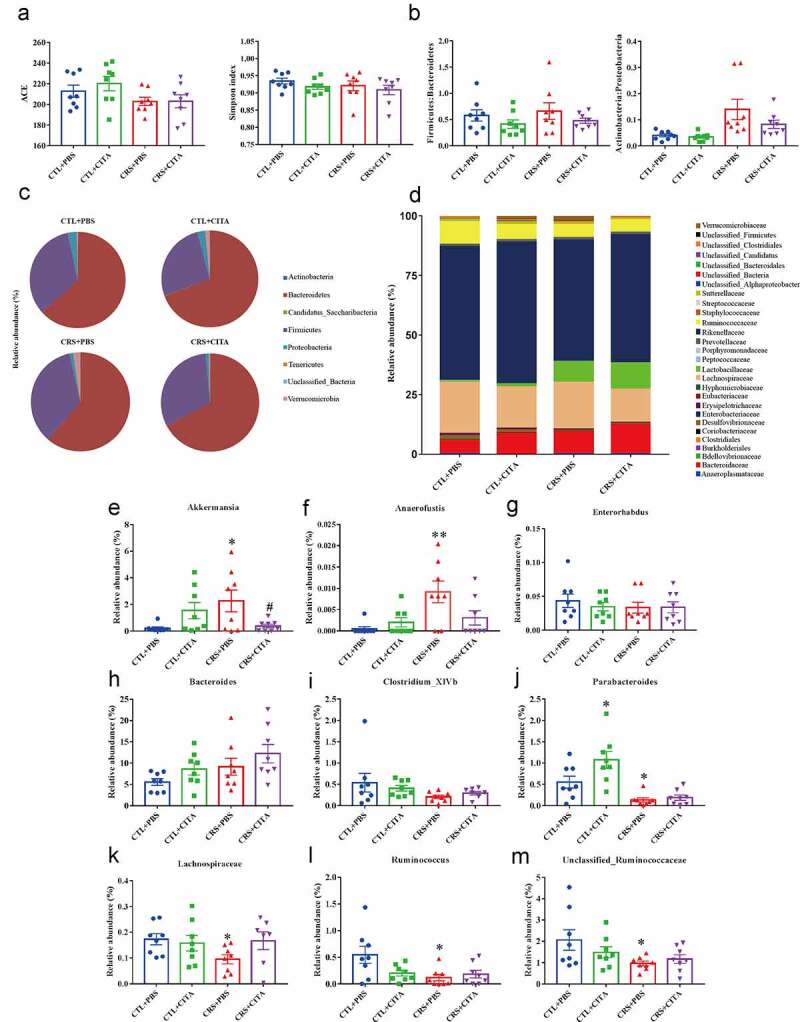
CRS-induced gut dysbiosis. (a) ACE and Simpson index describing microbial α diversity. (b) Firmicutes:Bacteroidetes and Actinobacteria:Proteobacteria ratios. (c) Relative microbial abundances at the phylum level. (d) Stacked bar-chart showing microbiota composition at the family level. (e-m) Relative abundances of obviously changed genera. Values represent the mean ± S.E.M. *p < .05 and **p < .01 indicate significant differences vs. the CTL+PBS group, and #p < .05 and ##p < .01 indicate significant differences vs. the CRS+PBS group
To further elucidate the potential roles of the microbiota-gut-brain axis, correlations were assessed between the representative values of behavioral changes, neurotransmitter levels in the PFC and alterations in microbiota (Figure 5). At the genus level, the microbiota that significantly differed between groups were highly correlated with the expression of Trp and its metabolites. Notably, Ruminococcus was positively correlated with Trp expression in the PFC, implying the role it may exert in Trp circulation along the microbiota-gut-brain axis.24 Positive correlations of Coprobacillus and Enterorhabdus with Trp were also observed. Additionally, Enterorhabdus exhibited a negative correlation with Kyn, indicating its potential role in Kyn metabolism. The 5-HT and 5-HIAA levels in the PFC were both negatively correlated with the relative abundance of Akkermansia, indicating the negative relationship between Akkermansia and the serotoninergic signaling pathway. Notably, Parabacteroides exhibited a specific role in Trp signaling since it showed a positive correlation with 5-HT but displayed a negative association with Kyn. These results, taken together, provide potential evidence for the association between gut dysbiosis, Kyn signaling and depression-like behavioral changes. Further studies could focus on Enterorhabdus and Parabacteroides for their strong correlations with both Kyn signaling and depression-like behavioral changes.
Figure 5.
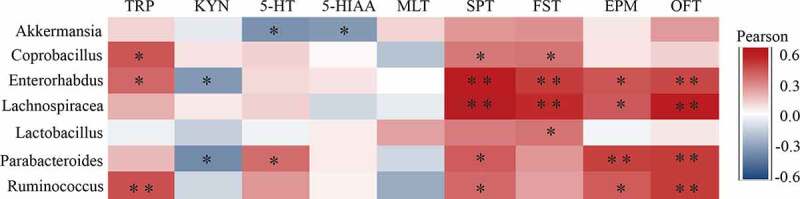
Pearson correlation heatmap focusing on the correlations between microbiota and behavioral, and neurotransmitter measurements in the PFC significantly influenced by CRS. Scale (right legend) indicates the level of positive (red) or negative (blue) correlation, and asterisks indicate significance. *p < .05, **p < .01
Parabacteroides intervention impacted behavioral changes and Trp metabolism in mice
Having determined the potential links of Parabacteroides to Trp signaling, then the potential roles of Parabacteroides in Trp metabolism and behavioral changes were examined. The Parabacteroides distasonis (PD, a species of genus Parabacteroides) was administrated to mice by oral gavage. As shown in Fig. S6A, restraint mice administrated with PD displayed elevated concentration of 5-HT as well as ratio of 5-HT to Trp in hippocampus (though statistically insignificant). On the contrary, the activated Kyn pathway was suppressed to a certain extent by PD, proved by the decreased tendency of Kyn/Trp and 3-HK. These findings revealed a potential role of PD in Trp metabolism, albeit the differences were not statistically significant. Nonetheless, the beneficial effects of this bacterium on metabolic reversal were observed.
Furthermore, the behavioral phenotypes after administration of PD were evaluated. As depicted in Fig. S6B-S6F, the depression- and anxiety-like behaviors in CRS+PD mice were partially ameliorated, since the immobility time of the FST in CRS+PD mice was markedly reduced (Fig. S6B), accompanied by the enhanced total track length in OFT when compared with those in CRS+PBS mice (Fig. S6F), implying the beneficial effects of PD on mouse depression-like behavioral changes.
Discussion
The present study demonstrated that mice subjected to CRS showed several behavioral changes akin to those seen in depression disorders, and the underlying mechanisms that may be involved in Trp metabolism through the gut-brain axis with accompanying alterations in gut microbiota composition (Fig. S5). Our findings specifically expand the understanding of the potential role of the gut microbiota in the circulating Kyn metabolic pathway in the potential “cross talk” between the gut and brain induced by chronic stress. It is of particular note that this is the first, to our knowledge, to comprehensively show detailed metabolic changes in Trp pathway in specific intestinal sections.
5-HT, one major metabolite of Trp, has been widely studied in its control of mood and behavior, and in the etiology of psychopathology.25 The lack of serotonin in the CNS (shown with TPH2 knockout mice) affected the proper wiring of the brain in a manner that may trigger the emergence of neuropsychiatric disorders.26 Clinical studies also demonstrated that the serotoninergic concentration in the serum from MDD patients was only half, or even lower, than that of healthy subjects.10 As a result, 5-HT is considered as one of the neurotransmitters that are most closely related to depression. Nonetheless, there is a paucity of reports pertaining to the relationship between depression and metabolic disorders of the whole Trp pathway, especially the Kyn pathway, another dominant physiological pathway of Trp metabolism.6 Kyn can be produced from Trp in a reaction catalyzed by the ubiquitous IDO, and then Kyn is metabolized into neuroprotective Kna or neurotoxic 3-HK and QA. Recently, great attention has been attached to the adverse effects of Kyn metabolism on the pathogenesis of many diseases, including depressive disorders.16 A population-based study revealed an increase in the Kyn/Trp ratio in the serum of depressive patients.27 Animal experiments have further proved that IDO1 inhibition ameliorated depression-like behaviors in chronic stress or LPS-induced sickness behavior models.28,29 To verify the potential roles of Kyn in mouse behavior, 1-MT, an IDO inhibitor was employed in the current study. Noteworthily, the depression- and anxiety-like behaviors in restraint mice were substantially reversed by 1-MT treatment. However, unknown effects of 1-MT were found, which seem to play a role in depressive and anxious behaviors for normal individuals. This phenomenon is unknown. We suspected that it may partially target on the Aryl hydrocarbon receptor (AhR), activated by 1-MT,30 thereby implicated in depression-like changes owing to immune/inflammatory action, which might, in turn, disturb the specific effect of 1-MT on Kyn signaling pathway.
Accordingly, based on the targeted metabolomics, the current work expanded previous work and comprehensively detected the whole Trp metabolism in the CRS mouse model. The results showed a shift in Trp metabolism toward Kyn metabolism in the serum, PFC and hippocampus of mice in the PBS-treated CRS group, while the 5-HT pathway was, to some extent, inhibited. This finding was further validated by the observation of significantly decreased expression of TPH2 and upregulation of IDO1. Taken together, these findings indicated the disruption in the homeostasis of Trp-Kyn metabolism might serve as an important factor in mediating depression-like behavior.
The brain-gut axis is a complex bidirectional system between the CNS and the gastrointestinal tract. As an important neurotransmitter along the gut-brain axis, there are three main pathways of Trp metabolism mediated by microbiota in the gut: (1) microorganisms directly transform Trp into several molecules to exert their own roles; (2) the Kyn pathway in epithelial cells can be activated through IDO1 mediated by microbiota; and (3) TPH1 can metabolize Trp into the 5-HT production pathway in ECs. Intriguingly, as an important neurotransmitter in both the brain and the gut, 5-HT is mainly located in the gut where it is synthesized from Trp in the ECs. Then, the signal can be sent from the gut to extrinsic neurons and specific receptors to modulate brain activities through the microbiota-gut-brain axis. Although the blood-brain barrier (BBB) is highly selective, Trp and Kyn can directly cross it to exert notable effects on the homeostasis of neurotransmitters.31,32 In fact, the intestinal Kyn can directly cross the BBB to participate in the CNS synthesis of neuroactive metabolites. However, to date, which intestinal part involved in Kyn metabolism remains unknown. Our study, for the first time, comprehensively analyzed the chronic stress-induced metabolic effects on specific intestinal sections and validated the distribution and impact of gut neurotransmitters in both the 5-HT and Kyn pathways. Our research demonstrated the impaired 5-HT production in the colon but not in the small intestine. More strikingly, the Kyn pathway in the ileum and colon were partially activated under CRS, with increased levels of Kyn in the ileum of CRS+PBS group and decreased concentration in colon of CRS+CITA group. However, for most segments of the intestine, the general trends of Trp metabolites were not significantly changed in a predictable way among different groups, which may be ascribed to the potential roles of the inhabiting microbiota in Trp metabolism intervention. For instance, 5-HT biosynthesis from colonic enterochromaffin cells (ECs) can be promoted by indigenous spore-forming bacteria,32 similarly, as one of the downstream catabolites of Kyn, Kyna can be produced by Escherichia coli and liberated to the extracellular milieu.33 To further unravel the metabolic transition between the Kyn and 5-HT pathway, the alterations of IDO and TPH1 levels verified the hijack of Trp metabolism into the Kyn pathway through IDO overexpression, especially in the colon. The impact of increased Trp metabolism along the Kyn pathway can be viewed through the dual lens of reduced availability of 5-HT synthesis and increased production of neuroactive Kyn metabolites related to the impact in the ENS and CNS.34 Therefore, these data gave us a hint of targeting colonic IDO activation in influencing the well-balanced Trp metabolism along the gut-brain axis. Further studies would benefit from a more complete and deep mining of these crucial Kyn pathway metabolites, both in the gut and in the CNS.
The BDNF-TrkB pathway is a key pathway in regulating synaptic plasticity and neurogenesis. Reduced BDNF expression in the brain has been shown to exert a strong influence on the development of depression.35 Our results are consistent with other literatures highlighting the role of BDNF in animal models where germ-free mice receiving gut microbial colonization showed altered exploratory behavior coupled with hippocampal expression changes of BDNF,36 suggesting that the alterations in BDNF-TrkB signaling may be responsible for the development of depressive symptoms. Although few studies have focused on the intestinal BDNF signaling, our study extended to the contention that the BDNF-TrkB pathway in the gut may exert a role that is as important as that in the brain. The detailed mechanism remains unclear, and further researches are urgently needed.
Increased intestinal permeability may be induced by bacterial elements crossing the epithelial barrier, which is a noted marker for intestinal health. In the current study, a significant decrease in ZO-1 protein was observed in both the ileum and colon of CRS animals, suggesting the destruction of the intestinal barrier. Additionally, the translocation of pro-inflammatory mediators (for example, lipopolysaccharide from gram-negative gut bacteria) and the Kyn metabolites pointed out in our study into the peripheral circulation (also called “leaky gut”) and further in the brain through the gut-brain network may play a role in the pathophysiology of depression.37,38
Recently, it has become clear that the gut microbiota exert a critical role in the gut-brain axis, which can be further described as the microbiota-gut-brain axis.39–41 Intestinal dysbiosis has been linked to stress; however, great differences exist because of numerous impacting factors, including the genetic background of the animal, experimental design, environmental factors, detection methods and so on.42–44 In our study, compared with the CTL condition, long-time stress generated remarkable dysbiosis with a distinct clustering of microbiota composition. The bacterial genera Clostridium and Ruminococcus, the identified Trp-catabolizing species, were markedly decreased in CRS-treated animals and could be partially restored by citalopram. Furthermore, Ruminococcus, a very common species found in approximately 90% of adults, showed a strong positive correlation with Trp in the PFC, suggesting the potential effects on Trp metabolism along the gut-brain axis in the pathogenesis of depression.45,46 More strikingly, Lactobacillus, many strains of which have been used as probiotics to improve despair and anxiety-like behaviors,47,48 was decreased in mice subjected to CRS. These data were in accordance with evidence showing that the restoration of intestinal Lactobacillus substantially improved the behavioral abnormalities and metabolic disturbances of the mice.49 Meanwhile, chronic stress promoted a decrease of Lachnospiraceae compared with the CTL group, which further supported previous findings reporting lowered Lachnospiraceae levels in MDD patients.50 Intriguingly, a spectrum of genera in this family can degrade food fiber and produce short-chain fatty acids (SCFAs) that not only exert an anti-inflammatory effect but also play a role in enhancing the intestinal barrier. Although we did not measure the specific levels in our work, SCFA concentrations may have an impact on intestinal and central Trp metabolism. Additionally, our correlation heatmap unraveled the potential role of Enterorhabdus and Parabacteroides in the circulating Kyn metabolites along the microbiota-gut-brain axis, and few studies to date were available related to depression in these novel species. For Parabacteroides, one recent study demonstrated its potential probiotic role in alleviating obesity and metabolic dysfunctions via the production of succinate and secondary bile acids.51 In the present study, the beneficial effects of Parabacteroides on CRS-induced depression- and anxiety-like behavioral changes were observed. Meanwhile, it also partially ameliorated the levels of adverse metabolites in Kyn signaling pathway, such as Kyn and 3-HK, revealing the underlying relationships of Parabacteroides with the pathogenesis of depression by influencing the Kyn pathway in gut–brain interaction.
The intricate bidirectional interaction between the gut microbiota and stress-related behavior makes it difficult to unravel the cause or consequences of changes, which is imperative for the development of novel and accurate therapies or even preventative methods. Since the microbiota can be a source or contributor to the biosynthesis of 5-HT and Kyn metabolites, accurate temporal analysis of the changes in behavior, neurotransmitters, enzymes and microbiota during the development of depressive syndrome might reveal the mechanism and cascade of the events, which is our further research direction.
In conclusion, the current study established a stress-induced depressive model that the disturbances of neurotransmitters and gut microbiota along the gut-brain axis may be involved in. Disparities in Trp metabolites were demonstrated to be correlated with specific gut microbiota changes, implying the possibility that specific microbiome can influence the metabolic changes in the CNS and behavior through the circulating Kyn pathway along the gut-brain axis, which may lead to the pathophysiology of depression. Further studies are needed to investigate the effects of microbial intervention on depression-like changes and neurotransmitter metabolism. Our study provided new theoretical insights and potential bacterial targets for the early intervention of depression-like behavior, which would be beneficial to understanding the mechanisms of depression and other neuropsychiatric disorders.
Materials and methods
Animals and treatments
Male C57BL/6 J mice (6 weeks), obtained from the Animal Core Facility of Nanjing Medical University, were housed in groups of 5 per cage with ad libitum access to food and water in the same temperature (22 ± 1°C) and humidity-controlled (55 ± 5%) animal room with a 12 h light/dark cycle. All mice were left undisturbed for 2 weeks prior to commencing any procedures. All procedures described here were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (Approval No. IACUC-1803017).
After acclimatization, mice were randomly divided into two groups, the control (CTL) group and the CRS group where mice were subjected to chronic restraint stress by being placed into 50 mL conical tubes with holes for air flow for 3–4 h per day for 14 consecutive days. Afterward, the two groups were further divided into two sub-groups individually:
(1) Receiving phosphate buffer solution (PBS, without Ca2+ and Mg,2+ pH 7.4) or citalopram hydrobromide (CITA, 10 mg/kg/d) daily. In other words, the following four groups were included in the second period: 1) CTL+PBS; 2) CTL+CITA; 3) CRS+PBS; 4) CRS+CITA. Dissolved in PBS, citalopram hydrobromide was prepared every day before injection and administered intraperitoneally (i.p.) for 21 d.
(2) The IDO inhibitor, 1-methyl-tryptophan (1-MT, Sigma-Aldrich, USA) was administered intraperitoneally at a dose of 15 mg/kg/d for 21 d. In this section, the CTL group and the CRS group were further sorted into two sub-groups, respectively, using PBS or 1-MT. Considering the solubility of this inhibitor, drug powder was dissolved in 0.1 M sodium hydroxide and then the pH was adjusted to 9.0 by hydrochloric acid with a dilution volume ratio of 1:1 before administration.
(3) Parabacteroides distasonis (PD, ATCC 8503) strain was cultured in blood agar medium at 37°C under anaerobic conditions (80% N2, 10% CO2, 10% H2). Then, the cell suspension for oral administration was prepared by suspending cultured bacterium in sterile PBS with a final density of 4 × 1010 CFU/mL. Mice were divided into four groups, including CTL+PBS, CTL+PD, CRS+PBS and CRS+PD. The PD groups were given 200 μL/mouse suspension solution continuously for 28 d, while the other groups received an equivalent volume of sterile PBS by gavage.
Behavioral testing
Behavioral tests (N = 8) were performed at the end of both periods. The schedule of these tests was arranged to avoid carry-over effects from the prior testing experience (in order: open field test (OFT), elevated plus maze (EPM), sucrose preference test (SPT) and forced swim test (FST); see Supplementary Materials for details). At the end of the second set of behavioral tests, all the mice were sacrificed for further evaluations. The analysis was performed blinded to the detailed experimental conditions.
Western blotting analysis
After anesthesia, each animal was sacrificed, and the brain and intestine regions of interest were collected and then immediately frozen at −80°C for subsequent analysis (N = 10). The following primary antibodies were applied: (a) rabbit anti-ZO-1 (1:2000, Proteinetch Group, Chicago, IL, United States); (b) mouse anti-Trk B (1:2000, Santa Cruz Biotechnology, CA); (c) rabbit anti-TPH (1:500, Abcam, Cambridge, MA); (d) rabbit anti-TPH2 (1:1000, Abcam); (e) mouse anti-IDO1 (1:5000, Proteinetch); (f) rabbit anti-MAOA (1:5000, Abcam); (g) rabbit anti-MAOB (1:3000, Abcam); (h) rabbit anti-TH (1:500, Abcam); (i) rabbit anti-BDNF (1:4000, Abcam). The detailed extraction and detection methods are described in the Supplementary Materials.
Microbiota analysis by 16S sequencing
Please see Supplementary Materials for detailed information related to the microbiota analysis. Briefly, samples (5 g) of fresh stool were collected into a sterile EP tube from respective stages, snap-frozen on dry ice and stored at −80°C until processing. Following DNA extraction, fecal microbiota profiling was performed by paired-end 16S rRNA gene amplicon sequencing (2 × 300), based on an Illumina MiSeq platform.
Histology
Formalin-fixed intestinal samples were embedded in paraffin, sliced into 5-μm thickness and stained with hematoxylin and eosin (H&E). Photomicrographs were scanned and viewed using Pannoramic scanner (3DHISTECH, Germany).
Measurement of neurotransmitter levels
Mice from each group (N = 10) were sacrificed, and brain (prefrontal cortex (PFC) and hippocampus), gut (duodenum, jejunum, ileum, colon and cecum) and blood samples were harvested. Blood samples were centrifuged for 10 min at 1500 g and the upper serum was collected for analysis. The gut samples were washed with PBS to remove contents. All these samples were immediately stored at −80°C until analysis. The sample pretreatment procedures were performed according to our previous work.52 See Supplementary Materials for information on instrumental sample treatment.
A UHPLC Ultimate 3000 system coupled with a Q Exactive hybrid quadrupole-orbitrap mass spectrometer was used for determination. Separation was carried out in an Acquity BHE-C18 column (100 mm×2.1 mm, 1.7 μm) at 35°C. The mobile phases were water consisted of 0.1% formic acid (component A) and acetonitrile (component B). The chromatogram was run under multistep gradient conditions at a flow rate of 0.25 mL/min. The parallel reaction monitoring (PRM) was used for quantitative analysis. Instrument control, data acquisition and analysis were performed with Thermo XCalibur 2.2 software.
Statistical analysis
Analyses were performed blinded to treatment assignments in all experiments. Statistical analyses were performed with SPSS statistics 20.0 software. The results are represented as the mean ± SEM. When normality and equal variance between sample groups were achieved, two-way ANOVA followed by Fisher’s Least Significant Difference (LSD) test was used. If failed, two-way ANOVA followed by Dunn’s correction was performed. Correlation analyses were performed using a Pearson correlation coefficient. The threshold for statistical significance was set as P < .05.
Supplementary Material
Funding Statement
Funding was provided by the National Natural Science Foundation of China (No. 81773484), Topnotch Academic Programs Project of Jiangsu Higher Education Institutions (No. PPZY2015A067) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
Author contributions
JW and RG conceptualized the project. YYD and JXY designed the study and draft the work. MFZ designed and performed all supplementary experiments in revision part. YYD, JXY and JFW contributed to literature search, data collection, analysis and interpretation. JY, WWL and LLW provided technical support and assisted with data collection as well as data interpretation. JW and RG critically revised the paper. All authors approved the final version of the paper.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, Bordin IA, Costello EJ, Durkin M, Fairburn C, et al. Grand challenges in global mental health. Nature. 2011;475(7354):27–16. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vigo DV, Kestel D, Pendakur K, Thornicroft G, Atun R.. Disease burden and government spending on mental, neurological, and substance use disorders, and self-harm: cross-sectional, ecological study of health system response in the americas. Lancet Public Health. 2019;4:e89–e96. doi: 10.1016/S2468-2667(18)30203-2. [DOI] [PubMed] [Google Scholar]
- 3.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 4.Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry. 2015;20(1):32–47. doi: 10.1038/mp.2014.163. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Du L, Bai Y, Han B, He C, Gong L, Huang R, Shen L, Chao J, Liu P, et al. CircDYM ameliorates depression-like behavior by targeting miR-9 to regulate microglial activation via HSP90 ubiquitination. Mol Psychiatry. 2018. doi: 10.1038/s41380-018-0285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silber BY, Schmitt JA. Effects of tryptophan loading on human cognition, mood, and sleep. Neurosci Biobehav Rev. 2010;34(3):387–407. doi: 10.1016/j.neubiorev.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Kraus C, Castrén E, Kasper S, Lanzenberger R. Serotonin and neuroplasticity-Links between molecular, functional and structural pathophysiology in depression. Neurosci Biobehav Rev. 2017;77:317–326. doi: 10.1016/j.neubiorev.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Dell’Osso L, Carmassi C, Mucci F, Depression MD. Serotonin and Tryptophan. Curr Pharm Des. 2016;22(8):949–954. doi: 10.2174/1381612822666151214104826. [DOI] [PubMed] [Google Scholar]
- 9.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 10.El-Haggar SM, Eissa MA, Mostafa TM, El-Attar KS, Abdallah MS. The phosphodiesterase inhibitor pentoxifylline as a novel adjunct to antidepressants in major depressive disorder patients: a proof-of-concept, randomized, double-blind, placebo-controlled trial. Psychother Psychosom. 2018;87(6):331–339. doi: 10.1159/000492619. [DOI] [PubMed] [Google Scholar]
- 11.Jaracz J, Gattner K, Moczko J, Hauser J. Comparison of the effects of escitalopram and nortriptyline on painful symptoms in patients with major depression. Gen Hosp Psychiatry. 2015;37(1):36–39. doi: 10.1016/j.genhosppsych.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Shih J-H, Chiu C-H, Ma K-H, Huang Y-S, Shiue C-Y, Yeh T-Y, Kao L-T, Lin -Y-Y, Li I-H. Autophagy inhibition plays a protective role against 3, 4-methylenedioxymethamphetamine (MDMA)-induced loss of serotonin transporters and depressive-like behaviors in rats. Pharmacol Res. 2019;142:283–293. doi: 10.1016/j.phrs.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 13.McGlashan EM, Nandam LS, Vidafar P, Mansfield DR, Rajaratnam SMW, Cain SW. The SSRI citalopram increases the sensitivity of the human circadian system to light in an acute dose. Psychopharmacology. 2018;235(11):3201–3209. doi: 10.1007/s00213-018-5019-0. [DOI] [PubMed] [Google Scholar]
- 14.Aldrich SL, Poweleit EA, Prows CA, Martin LJ, Strawn JR, Ramsey LB. Influence of CYP2C19 metabolizer status on escitalopram/citalopram tolerability and response in youth with anxiety and depressive disorders. Front Pharmacol. 2019;10:99. doi: 10.3389/fphar.2019.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cervenka I, Agudelo LZ, Ruas JL. Kynurenines.Tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:eaaf9794. doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- 16.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am. 2017;46(1):77–89. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473–486. doi: 10.1038/nrgastro.2013.105.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20(1):14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filho AJMC, Lima CNC, Vasconcelos SMM, de Lucena DF, Maes M, Macedo D. IDO chronic immune activation and tryptophan metabolic pathway: A potential pathophysiological link between depression and obesity. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:234–249. doi: 10.1016/j.pnpbp.2017.04.035. [DOI] [PubMed] [Google Scholar]
- 21.Laurans L, Venteclef N, Haddad Y, Chajadine M, Alzaid F, Metghalchi S, Sovran B, Denis RGP, Dairou J, Cardellini M, et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med. 2018;24(8):1113–1120. doi: 10.1038/s41591-018-0060-4. [DOI] [PubMed] [Google Scholar]
- 22.Lu Q, Mouri A, Yang Y, Kunisawa K, Teshigawara T, Hirakawa M, Mori Y, Yamamoto Y, Libo Z, Nabeshima T, et al. Chronic unpredictable mild stress-induced behavioral changes are coupled with dopaminergic hyperfunction and serotonergic hypofunction in mouse models of depression. Behav Brain Res. 2019;372:112053. doi: 10.1016/j.bbr.2019.112053. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharyya S, Ahmed AT, Arnold M, Liu D, Luo C, Zhu H. Metabolomic signature of exposure and response to citalopram/escitalopram in depressed outpatients. Transl Psychiatry. 2019;9(1):173. doi: 10.1038/s41398-019-0507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis DJ, Hecht PM, Jasarevic E, Beversdorf DQ, Will MJ, Fritsche K, Gillespie CH. Sex-specific effects of docosahexaenoic acid (DHA) on the microbiome and behavior of socially-isolated mice. Brain Behav Immun. 2017;59:38–48. doi: 10.1016/j.bbi.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Young SN, Leyton M. The role of serotonin in human mood and social interaction. Insight from Altered Tryptophan Levels Pharmacol Biochem Behav. 2002;71:857–865. doi: 10.1016/s0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 26.Migliarini S, Pacini G, Pelosi B, Lunardi G, Pasqualetti M. Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Mol Psychiatry. 2013;18(10):1106–1118. doi: 10.1038/mp.2012.128. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Zheng W, Liu W, Wang C, Zhan Y, Li H, Chen L, Li M, Ning Y. Antidepressant effect of repeated ketamine administration on kynurenine pathway metabolites in patients with unipolar and bipolar depression. Brain Behav Immun. 2018;74:205–212. doi: 10.1016/j.bbi.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Laugeray A, Launay JM, Callebert J, Mutlu O, Guillemin GJ, Belzung C, Barone PR. Chronic treatment with the ido1 inhibitor 1-methyl-d-tryptophan minimizes the behavioral and biochemical abnormalities induced by unpredictable chronic mild stress in mice - comparison with fluoxetine. PLoS One. 2016;11(11):e0164337. doi: 10.1371/journal.pone.0164337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, Chen L, Lim G, Sung B, Wang S, McCabe MF, Rusanescu G, Yang L, Tian Y, Mao J. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J Clin Invest. 2012;122(8):2940–2954. doi: 10.1172/JCI61884.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunther J, Dabritz J, Limitations WE. Off-target effects of tryptophan-related ido inhibitors in cancer treatment. Front Immunol. 2019;10:1801. doi: 10.3389/fimmu.2019.01801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 32.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuc D, Zgrajka W, Parada-Turska J, Urbanik-Sypniewska T, Turski WA. Micromolar concentration of kynurenic acid in rat small intestine. Amino Acids. 2008;35(2):503–505. doi: 10.1007/s00726-007-0631-z. [DOI] [PubMed] [Google Scholar]
- 34.Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):702–721. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Waterhouse EG, Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol Cell Neurosci. 2009;42(2):81–89. doi: 10.1016/j.mcn.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609. doi: 10.1053/j.gastro.2011.04.052.. [DOI] [PubMed] [Google Scholar]
- 37.Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, Allen NB, Stuart AL, Hayley AC, Byrne ML, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 40.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 41.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56(11):1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65(3):263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 44.Szyszkowicz JK, Wong A, Anisman H, Merali Z, Audet M-C. Implications of the gut microbiota in vulnerability to the social avoidance effects of chronic social defeat in male mice. Brain Behav Immun. 2017;66:45–55. doi: 10.1016/j.bbi.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann TW, Pham H-P, Bridonneau C, Aubry C, Lamas B, Martin-Gallausiaux C, Moroldo M, Rainteau D, Lapaque N, Six A, et al. Microorganisms linked to inflammatory bowel disease-associated dysbiosis differentially impact host physiology in gnotobiotic mice. Isme J. 2016;10(2):460–477. doi: 10.1038/ismej.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. MetaHIT consortium, bork P, ehrlich SD, wang J. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oh PL, Benson AK, Peterson DA, Patil PB, Moriyama EN, Roos S, Walter J. Diversification of the gut symbiont lactobacillus reuteri as a result of host-driven evolution. Isme J. 2010;4(3):377–387. doi: 10.1038/ismej.2009.123. [DOI] [PubMed] [Google Scholar]
- 48.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, Wu M, Overall CC, Kipnis J, Gaultier A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep. 2017;7(1):43859. doi: 10.1038/srep43859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26(8):1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 51.Wang K, Liao M, Zhou N, Bao L, Ma K, Zheng Z, Wang Y, Liu C, Wang W, Wang J, et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019;26(1):222–235. doi: 10.1016/j.celrep.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 52.Yao J, Lu H, Wang Z, Wang T, Fang F, Wang J, Yu J, Gao R. A sensitive method for the determination of the gender difference of neuroactive metabolites in tryptophan and dopamine pathways in mouse serum and brain by UHPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1093-1094:91–99. doi: 10.1016/j.jchromb.2018.06.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


