ABSTRACT
SARS-CoV-2, the virus causing COVID-19, is a single-stranded RNA virus belonging to the order Nidovirales, family Coronaviridae, and subfamily Coronavirinae. SARS-CoV-2 entry to cellsis initiated by the binding of the viral spike protein (S) to its cellular receptor. The roles of S protein in receptor binding and membrane fusion makes it a prominent target for vaccine development. SARS-CoV-2 genome sequence analysis has shown that this virus belongs to the beta-coronavirus genus, which includes Bat SARS-like coronavirus, SARS-CoV and MERS-CoV. A vaccine should induce a balanced immune response to elicit protective immunity. In this review, we compare and contrast these three important CoV diseases and how they inform on vaccine development.
KEYWORDS: SARS, coronaviruses, SARS-CoV-2, MERS, pneumonia, emerging diseases
Introduction
Coronaviruses (CoV) are enveloped, positive-sense, single-stranded RNA viruses of the family Coronaviridae with spikes around its spherical body (corona = crown).1–4CoV is the recognized cause of mild respiratory tract infections in humans.5–7 The first two HCoVs, HCoV-229E and HCoV-OC43 have been known since the 1960s.8 The viruses are subdivided into four genera on the basis of genotypic and serological characters which are Alpha-, Beta-, Gamma, and Delta-coronavirus,9,10 and among them CoVs in the first two genera infect humans.11–13Seven CoVs are known to infect humans, three of them seriously, viz., SARS (severe acute respiratory syndrome, China, 2002), MERS (Middle East respiratory syndrome, Saudi Arabia, 2012), and SARS-CoV-2 (2019–20).Which are beta-coronaviruses (beta-CoVs).12 The viral fusion protein is critical in enveloped virus entry to cells in that it mediates the membrane fusion reaction.14–18 CoV entry into target cells is performed by the spike (S) envelope glycoprotein, which mediates both host cell receptor binding and membrane fusion.19,20The history of major epidemic diseases since 1900 is shown in Table 1. The three main CoV outbreaks and their origin is indicated in Table 2. Important steps that allow CoV entry are shown in Table 3. Taxonomy of the CoV family is presented in Figure 1. Host information and distribution of SARS CoVs in GenBank are presented in Table 4.
Table 1.
History of epidemics since 1900
| Year | Outbreak |
|---|---|
| 1918 | Great flu pandemic |
| 1976 | Legionnaires disease |
| 1993 | Hanta virus pulmonary syndrome |
| 1994 | Hendra virus infection |
| 1997 | H5N1 influenza infection |
| 1999 | Nipah virus encephalitis/pneumonitis |
| 2002 | Severe acute respiratory syndrome (SARS) |
| 2012 | Middle east respiratory syndrome (MERS) |
| 2019 | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) |
Table 2.
Three main coronavirus outbreaks and their origin
| Coronavirus outbreak | Origin |
|---|---|
| SARS | Civets (although, the virus originated in bats, and civets consider as intermediary) |
| MERS | Camels (The virus also came from bats) |
| SARS-CoV-2 | Malayan pangolins (armadillo-like mammals) (the virus also came from bats) |
Table 3.
Important steps allow the virus entry.20.
| (a) Bind to a target host cell, typically via interactions with cellular receptors. |
| (b) Fuse its envelope with a cellular membrane, either at the plasma membrane or through the endocytic pathway. |
| (c) Deliver its genetic material inside the cell. |
Figure 1.
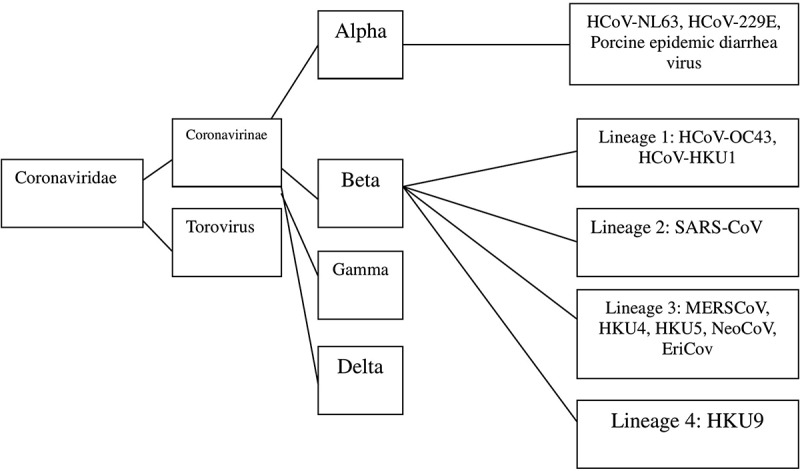
Taxonomy of the coronaviridae family
Table 4.
Host information and distribution of SARSr-CoVs available in GenBank.21.
| Provinces | Bat species |
|---|---|
| Guangdong | Rhinolophus sinicus |
| Guangxi | Rhinolophus pearsonii, Rhinolophus sinicus |
| Guizhou | Rhinolophus rex, Rhinolophus sinicus |
| Hebei | Rhinolophus ferrumequinum |
| Henan | Rhinolophus ferrumequinum |
| Hong Kong | Rhinolophus sinicus |
| Hubei | Rhinolophus ferrumequinum, Rhinolophus macrotis, Rhinolophus sinicus |
| Jilin | Rhinolophus ferrumequinum |
| Shaanxi | Rhinolophus pusillus |
| Shanxi | Rhinolophus ferrumequinum |
| Taiwan | Rhinolophus monoceros |
| Yunnan | Aselliscus stoliczkanus, Rhinolophus affinis, Rhinolophus ferrumequinum, Rhinolophus sinicus |
| Zhejiang | Rhinolophus monoceros, Rhinolophus pearsonii, Rhinolophus sinicus, Rhinolophus thomasi |
This manuscript aims to review SARS, MERS, and SARS-CoV-2 while considering their similarities and differences and possible methods to prevent their infection.
Severe acute respiratory syndrome (SARS)
The contagious and sometimes fatal severe acute respiratory syndrome (SARS) is a respiratory illness which first appeared in China in 2002, and it spread worldwide, mostly by unsuspecting travelers.22–24 SARS-CoV belongs to the Beta-coronavirus family but has a “b” lineage. Other members of this family are Arteriviridae, Mesoniviridae, and Roniviridae.25 It is similar to other coronaviruses in both virion structure and genome organization with a single-stranded, plus-sense RNA.26,27 CoVs are single-stranded RNA viruses which belong to the order Nidovirales, family Coronaviridae, and subfamily Coronavirinae,28–30 and have been classified into four major groups: α-CoVs, β-CoVs, γ-CoVs, and δ-CoVs with 17 subtypes.31 It has been reported, SARS-CoV, like other coronaviruses, is an RNA virus which replicates in the cytoplasm, and the virion envelope contains at least three structural proteins, S, E, and M, embedded in the membrane, and also like other coronaviruses, SARS-CoV encodes several group-specific proteins, termed 3a, 3b, 6, 7a, 7b, 8, and 9.32–34 Deletion of the small envelope (E) protein modestly reduces SARS-CoV growth in vitro and in vivo,35–37 which may result in an attenuated virus. SARS 8b, known as X5 is predicted to be a soluble protein with 84 amino acids and an estimated size of 9.6 kDa.38 It showed minor homology to the human coronavirus E2glycoprotein precursor.39 SARS-CoV encodes an exceptionally high number of accessory proteins that bear little resemblance to accessory genes of other coronaviruses.40–43 Like other coronaviruses, SARS-CoV is an inefficient inducer of IFN-β response in cell culture system44 and is sensitive to the antiviral state induced by IFNs.45,46 Two functional domains of S protein, S1 and S2 located in the N- and C-terminal regions, respectively, of the S protein are conserved among the coronaviruses.47 The S protein in coronaviruses is major antigenic determinants that induce immune response in the hosts.48–50 The S protein of transmissible gastroenteritis virus contains four major antigenic sites (A–D), and site A on the S1 subunit is the main inducer of neutralizing Abs.51–53 Ab responses to SARS-CoV can be developed in SARS patients; but, its antigenic determinants remain to be elucidated.54 But, in other coronaviruses, deletion of E results in either complete absence of infectious virus or a severe reduction in titer.55,56 DeDiego et al.57 reported that E protein is responsible in a significant proportion of the inflammasome activation and the associated inflammation elicited by SARS-CoV in the lung parenchyma, and the inflammation may lead to edema accumulation which cause acute respiratory distress syndrome (ARDS). E protein contains several active motifs despite its small size, between 76 and 109 amino acids depending on the CoV.58,59 The most important characteristics of SARS are shown in Table 5. Possible origins of SARS-CoV-2 and homologous analysis of SARS-CoV-2 (NC_045512) and six other Coronavirus strains isolated from different hosts in China are shown in Tables 6 and 7, respectively. Most relevant clinical similarities and differences between SARS-CoV and SARS-CoV-2 are presented in Table 8.
Table 5.
The most important characteristic of SARS
| 1. The first international disease epidemic of the twenty-first century. |
| 2. It is the first time this Coronavirus has been found in humans. |
| 3. It was the first public health challenge for international communities. |
| 4. The first disease that globalization visibly exacerbated its spread in a short time and contributed significantly to its end. |
Table 6.
Possible origins of SARS-CoV-2.60.
|
Table 7.
Homologous analysis of SARS-CoV-2 (NC_045512) and six other Coronavirus strains isolated from different hosts in China (%).61.
| Isolate | Host | Complete genome | ORF1ab | N | S |
|---|---|---|---|---|---|
| SARS coronavirus civet020 (AY572038) | Civet | 73.58 | 79.23 | 87.79 | 71.41 |
| Bat SARS-like coronavirus As6526 (KY417142) | Aselliscus stoliczkanus | 74.58 | 79.23 | 87.55 | 68.17 |
| Bat SARS-like coronavirus Rs4874 (KY417150) | Rhinolophus sinicus | 71.98 | 79.18 | 87.94 | 71.29 |
| Alphacoronavirus Mink/China/1/2016 (MF113046) | Mink | 34.97 | 38.47 | 33.70 | 30.89 |
| Bat coronavirus isolate RaTG13 (MN996532) | Rhinolophus affinis | 93.7 | 96.5 | 96.9 | 92.86 |
| Pangolin coronavirus (MT084071) | Manis javanica | ? | ? | 95 | 90 |
Note: N, N protein. S, spike protein. ?, Sequence of Pangolin coronavirus (MT084071) is not completed in this part of genome.
Table 8.
Most relevant clinical similarities and differences between SARS-CoV and SARS-CoV-2.62.
| Characteristic | SARS-CoV | SARS-CoV-2 |
|---|---|---|
| Target receptor | ACE-2 | ACE-2 |
| N protein | IFN-γ inhibitor | Unknown |
| R0 | 0.4 | 1.4–2.5 |
| Chest X-ray | Ground glass opacities | Bilateral, multilobar ground glass opacities |
| Chest CT-scan | Lobar consolidation Nodular opacities |
No nodular opacities |
| Prevention | Hand hygiene, cough etiquette | Possibly hand hygiene, cough etiquette |
| Transmission | Droplets Contact with infected individuals |
Droplets even asymptomatic ones |
| Case fatality rate (overall) | 9.6% | 2.3% |
Abbreviations: SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; N protein, Nucleocapsid protein: IFN-γ, interferon-γ; R0, R through; X-ray, radiography; CT-scan, computerized tomography.
SARS should not be confused with avian flu which is another zoonosis from the same area.It initially began in the Guangdong province in south of China in 2002–2003 which eventually involved more than 8400 people worldwide, which is around 9.5% of the total affected.63–65 The greatest number of SARS cases were in mainland China, Hong Kong, Taiwan, Singapore, Canada, respectively.66–68 Quick infection is one of the main character of SARS.69–71 The most important symptoms of SARS were fever, chills, muscle aches, headache, and diarrhea, which may lead to fever with body temperature of 38°C or higher, dry cough and shortness of breath after around one week.72–74 On the basis of former reports the features of the clinical examination found in the patients at admission were self-reported fever (99%), documented elevated temperature (85%), nonproductive cough (69%), myalgia (49%), and dyspnea (42%).75–78 SARS spread through droplets which enter the air with coughs, sneezes or talks; moreover, it may spread on contaminated objects and surfaces like doorknobs, elevator buttons, and telephones. SARS-CoV is transmitted mainly by person-to-person infection. During its outbreak, nearly 25% of people had severe respiratory failure and 10% died, and it was controlled by using public-health measures, namely, wearing surgical masks, washing hands, and isolating infected patients.79–82 Face to face contact of SARS can be divided into three groups, (1) caring for someone with SARS, (2) having contact with the bodily fluids of a person with SARS, and (3) kissing, hugging, touching or sharing eating or drinking utensils with an infected person.83–85 Almost 25% of cases developed severe pulmonary disease which may lead to death from respiratory failure.86 SARS which had flu-like signs can lead to death in severe conditions due to respiratory failure or complications consist of heart and liver failure, especially for those old people who had diabetes and hepatitis.87–89 Like the common cold, it is caused by a strain of corona CoV. Coronaviruses may lead to severe disease in animals, and it is supposed that the SARS virus might have come from animals to humans.90–92 SARS-CoV originated in wild bats and then spread to palm civets or similar mammals. Bats and Civets which are cat-like serve as foods and in folk medicines. Musk production from the scent glands of civets which is used in perfumes is another usage of civets. These mentioned animals could easily transmit the virus to humans.93
Table 9 shows the most important symptoms of SARS and MERS from the highest to the lowest in criticality, while criteria for disease control and prevention case definition of SARS are presented in Table 10. Case classification of SARS is shown in Table 11.
Table 9.
The most important symptoms of SARS and MERS from the highest to the lowest
| SARS | MERS |
|---|---|
| Fever more than 30°C | Fever |
| Dry cough | Cough |
| Sore throat | Shortness of breath |
| Problems in breathing, such as shortness of breath, inability to maintain oxygenation (hypoxia) | Sore throat |
| Headache | Diarrhea |
| Body aches and muscles pain | Head and body aches |
| Loss of appetite | Vomiting |
| Malaise | Chest pain/tightness |
| Night sweats and chills | Running nose |
| Confusion | Altered conscious/confusion |
| Rash | Sweating |
| Nausea, vomiting and diarrhea | Abdominal pain |
| Weakness | Weakness/fatigue |
| Fever | Dizziness |
| Poor appetite | Loss of appetite |
| Respiratory distress syndrome (ARD or ARDS) | Shivering |
| Attacking the alveoli (air sacs) in the lungs | |
| Kidney failure | |
| Inflammation of the heart sac (pericarditis) | |
| Sever systematic bleeding from disruption of clotting system (disseminated intravascular coagulation) | |
| Reduced lymphocyte cell counts (lymphopenia) | |
| Inflammation of the arteries (vasculitis) |
Table 10.
Criteria for disease control and prevention case definition of SARS.94.
| Clinical Criteria | Characteristics |
|---|---|
| Asymptomatic or mild respiratory illness | |
| Moderate respiratory illness | Temperature of > 100.4°F(>38° C) |
| One or more clinical findings of respiratory illness (e.g. cough, shortness of breath, difficulty breathing, or hypoxia) | |
| Severe respiratory illness | Temperature of >100.4°F (>38°) |
| One or more clinical findings of respiratory illness (e.g. cough, shortness of breath, difficulty breathing, or hypoxia) | |
| Radiographic evidence of pneumonia | |
| Respiratory distress syndrome | |
| Autopsy findings consistent with pneumonia or respiratory distress syndrome without an identifiable cause | |
| Epidemiologic Criteria | Travel (including transit in an airport) within 10 days onset of symptoms to an area with current or previously documented or suspected community transmission of SARS |
| Close contact within 10 days of onset of symptoms with a person known or suspected to have SARS | |
| Laboratory Criteria | |
| Confirmed | Detection of antibody to SARS-CoV in specimens obtained during acute illness or >21 days after illness onset |
| Detection of SARS-CoV RNA by RT-PCR confirmed by a second PCR assay, by using a second aliquot of the specimen and a different set of PCR primers | |
| Isolation of SARS-CoV | |
| Negative | Absence of antibody to SARS-CoV in convalescent serum obtained >21 days after symptom onset |
| Undetermined | Laboratory testing either not performed or incomplete |
Table 11.
Case classification of SARS
| Under investigation | A person who has been referred to the public health service for possible SARS-CoV infection |
| Suspected case | A person with all of these following: (a) Sudden onset of high fever, > 38°C (b) One or more of the following respiratory symptoms: cough, sore throat, shortness of breath, and difficulty in breathing (c) Showing symptoms within 10 days of either traveling to one of the suspected areas of SARS or being in close contact with a person who has traveled to those areas |
| Probable case | (a) A suspected case with chest X-ray findings of pneumonia or adult respiratory distress syndrome (b) A person with an unexplained respiratory illness resulting in death, with a postmortem examination demonstrating the pathology or respiratory distress syndrome without an identifiable cause |
| Confirmed case | A clinically compatible illness which is confirmed by laboratories |
| Not a case | A case that has been investigated and subsequently found not to meet the case definition |
No medication has been proven to treat SARS effectively, but oxygen therapy and tracheal intubation and mechanical ventilation to support life until recovery begins is useful for patients in severe cases.95 The most useful ways to control SARS pandemic are public-health and infection-control measures.96–105 Peiris106 confirmed that its rapid mobilization and coordination of relevant expertise when it faced with a global emerging disease threat, which highlighted its needs for improved international regulations governing the reporting of and response to unusual infectious-disease syndromes. Circulating air with high-efficiency particulate air (HEPA) filter to decontaminate, wearing masks and isolating a patient in a single room and wearing a gown, gloves, eye shield, and mask or a portable air purifier which filters out small infectious particles (N95 mask) for staffs are necessary. Hui and Chan107 found that horseshoe bats are implicated in the emergence of novel coronavirus infection in humans. Ding et al.108 indicated that in addition to viral spread through a respiratory route, SARS-CoV in the intestinal tract, kidney, and sweat glands maybe excreted via feces, urine, and sweat, so leading to virus transmission. The three-dimensional structure result indicates that the nsp2 protein of GD strain is high homologous with 3 CL(pro) of SARS-CoV urban strain, 3CL(pro) of transmissible gastroenteritis virus and 3CL(pro) of human coronavirus 229E strain, which further suggests that nsp2 protein of GD strain possesses the activity of 3CL(pro).109,110 Rabenau et al.111 showed that SARS-CoV can be inactivated easily with commonly used disinfectants. Cao et al.112 showed that SARS transmission changes in its epidemiological characteristics and SARS outbreak distributions show palpable clusters on both spatial and temporal scales, also its transmission features are affected by spatial heterogeneity. Lessons learned from the SARS outbreak and concerns identified by WHO because of SARS are presented in Table 12. Duration of clinical phases of the mild and moderately severe variants of severe acute respiratory syndrome is shown in Table 13.
Table 12.
Lessons learned from the SARS outbreak and concerns identified by WHO because of SARS.113–116.
| Lesson | Means | Concerns |
|---|---|---|
| The capacity of global alerts to improve awareness and vigilance | Wide support by responsible press and amplified by electronic communications | Inadequate surge capacity in hospitals and public health systems |
| The advantage of quick detection and reporting | Immediate reporting of initial cases by South Africa and India | Healthcare providers themselves being the victims of the disease |
| The successful containment that can be achieved by readying health services with preparedness plans and campaigns to guard against imported cases | Climate of high alert that was established after reports of the disease became known | Shortage of expert staff to coordinate national and global responses to a rapidly evolving public health emergency |
| The value of immediate political commitment at the highest level | The experience in Vietnam, where the government took immediate measures to protect its people | In some cases, the need for hasty construction of new facilities; in other cases, hospitals being closed |
| The ability of even developing countries to triumph over a disease when reporting is prompt and open and when rapid case detection, immediate isolation and infection control, and vigorous contact tracing are put in place | The appeal by Vietnam, where WHO assistance was requested quickly and fully supported | The power of poorly understood infectious diseases to incite widespread public anxiety and fear, social unease, economic losses, and unwarranted discrimination |
Table 13.
Duration of clinical phases of the mild and moderately severe variants of severe acute respiratory syndrome112.
| Phase Respiratory |
||||
|---|---|---|---|---|
| Time | Prodrome | Early | Late | Recovery |
| From onset, days | 0 | 2–7 | 8–12 | 14–18 |
| Duration, days | 2–7 | 1–10 | 5–10 | 5–7 |
The Middle East Respiratory Syndrome Coronavirus (MERS-CoV)
The middle East respiratory syndrome coronavirus (MERS-CoV) is a zoonotic beta coronavirus which can infect various kinds of animals such as humans, camels, and bats.118–123 It belongs to the Beta-coronavirus genus, of the coronavirus family.124,125 It was first discovered in September 2012 as the cause of death in a patients who had died of severe pneumonia in June 2012 in Jeddah, Saudi Arabia,126,127 and 1,348 cases of MERS-CoV infection confirmed globally, with at least 479 related deaths until June 23, 2015.128 It has exported from the Middle East to other countries even countries in Asia, Europe, and North America.128–133 The most important characteristics of MERS-CoV infections are shown in Table 14.
Table 14.
The most important characteristics of MERS-CoV infections
|
Routine measures for travelers to help preventing the spread of viruses are hand washing, personal hygiene, avoid contacts with sick people, and animals, and covering the mouth with a tissue when coughing or sneezing and dispose properly the used tissue.137 MERS-CoV is most likely derived from an ancestral reservoir bats.143–146 Except for some cases in Korea in 2015, 82% of infections have occurred in Saudi Arabia, and the human mortality rate of MERS-CoV infection was nearly 35%.147–149 It has been reported that patients with severe diseases have at least one underlying condition, including diabetes, hypertension, chronic cardiac disease, and chronic renal disease.150,151 Human to human transmission has been facilitated in healthcare settings152,153 with the contribution of hospital-based transmission of MERS estimated at about 80% using an epidemic model.154 MERS outbreak was found in the Republic of Korea since 2015,155 which showed the importance risk of importing MERS and escalate global spread of MERS and damage to both economic and public health activities,156,157 which show the importance of travel restrictions for infected countries.158,159 The key receptor for MERS-CoV infection which is dipeptidyl peptidase 4(DPP4), is widely distributed on human endothelial and epithelial cells.147 CoV entry is initiated by the binding of the spike protein (S) to cell receptors, specifically, DDP4 and angiotensin converting enzyme 2 (ACE2) for MERS-CoV and SARS-CoV, respectively. Its genome is a single-stranded RNA which encodes 10 proteins including two replicase polyproteins (open reading frames [ORF], 1 ab and 1 a), three structural proteins (E, N, and M), a surface glycoprotein (S, spike) which comprises S1 and S1, and five nonstructural proteins (ORF 3, 4a, 4b, and 5).160–162 The subunit S1 is composed of four different core domain,163 and the domain S1B binds to the host-cell receptor dipeptidylpeptidase 4 (DPP4),162,164,165 while the domain S1A binds to sialoglycans which increased infection of human lung cells by MERS-CoV.166 It has been concluded that the MERS-S protein is known to represent a key target for the development of new therapeutics and includes of a receptor-binding subunit S1 and a membrane-fusion subunit S2.167 The roles of S protein in receptor binding and membrane fusion make it a perfect target for vaccine and antiviral development.168 It has been shown that vaccines based on the S protein can induce antibodies to block virus binding and fusion or neutralize virus infection.169–171 The subunit S2 includes the fusion peptide, two heptad repeats and a transmembrane domain, which mediate fusion of the virus with the cell membrane.172,173 Antibodies against MERS-CoV have been found among both dromedary camel populations and camel-exposed humans, which have been recognized as the source of multiple MERS case importations around the world.174,175 Previous studies have investigated viral vector-based vaccines,176–180 subunit vaccines,181–183 and DNA vaccines,184–188 but there is no clinically approved vaccine for MERS-CoV. Among these vaccines, viral vectors or DNA immunization successfully generated neutralizing antibodies and protected against infection.
The 5/end of the genome contains the rep1a and rep1b genes, which encode the viral replicase-transcriptase.At the 3/end of genome, four structural protein, namely spike (S), envelope (E), membrane (M), and nucleocapsid (N) protein and five accessory proteins (ORF3, ORF4a, ORF4b, ORF5 and ORF8) make up 10 kb.189–191 Kasem et al.192 and Dighe et al.193 showed that camels are a main reservoir for the maintenance of MERS-CoVs, and they are an important source of human infection with MERS. Al-Tawfiq et al.194 found that MERS-CoV was a rare cause of community acquired pneumonia and other viral causes such as influenza which are more common. Alfaraj et al.195 found that different factors contributed to increase mortality rate for MERS-CoV patients, and one of the most important factor is usage of corticosteroid and a continuous renal replacement therapy (CRRT). Corman et al.196 found that the kit is important tool for assisting in the rapid diagnosis, patient management, and epidemiology of suspected MERS-CoV cases. Yoon et al.197 found that 6,8-difluoro-3-isobutyryl-2-((2,3,4-trifluorophenyl)amino) quinolin-4(1 H)-one (6 u) shows high inhibitory influence and low toxicity activities which is from 3-Acyl-2-phenylamino-1,4-dihydroquinolin-4(1 H)-one derivatives. Qiu et al.198 indicated that hMS-1 might be developed as an effective immunotherapeutic agent to cure patients infected with MERS-CoV, especially in emergent cases. Early MERS-CoV diagnosis may require more sensitive risk assessment tools to reduce avoidable delays, specifically those related to patients and health system.199–204 Alqahtani et al.205 noted that still many people have lack of accurate understanding about MERS-CoV transmission and prevention, and more studies need to examine the knowledge and practices among public and workers about MERS-CoV.206–209 Close contacts include airplane setting, household setting, household setting who also visited the patient in hospital, healthcare setting.210,211 Al-Tawfiq and Auwaerter212 suggested proper infection control procedures, prompt recognition, isolation and management of suspected cases are important parameters for MERS prevention. Kim213 mentioned the importance of protecting healthcare providers from severe both physical and psychological stress. Al-Tawfiq et al.214 reported notable increase in costs of the healthcare system because of increase in utilization of surgical masks, respirators, soap, and alcohol-based hand sanitizers Douglas et al.215 indicated that the extent of MERS-CoV adaptation determines the minimal infectious dose needed to achieve severe respiratory disease. Nikiforuk et al.216 reported that viral infectious clone system may shorten time between emergence of a novel viral pathogen and construction of an infectious clone system. Ebihara et al.217 found that virus infectious clone systems allow for expression of a homogenous virus population within mammalian cell culture from a sequence of DNA or RNA. Letko et al.218 demonstrated that MERS-CoV spike can utilize multiple paths to rapidly adapt to novel species variation in DPP4. Zhang et al.219 found that MERS-4 neutralizes MERS-CoV by indirect rather than direct competition with DPP4. MERS-CoV nanoparticle vaccine produced high titer anti-S neutralizing antibody and protected mice from MERS-CoV infection in vivo. Mustafa et al.220 suggested application of antimicrobial peptides (AMPs) as alternative therapeutic agents against MERS-CoV infection. Baharoon and Memish221 emphasized on balance in application of both vaccination and antiviral therapeutics, also they highlighted the importance of avoid mechanism of escape mutant virus strains and improve activity against divergent virus strains. Widagdo et al.222 reported vaccination of dromedary camels is an appropriate way to decrease human MERS cases. Comparison between SARS and MERS-CoV in respect to their virology, epidemiology and clinical outcomes are shown in Table 15. The differences between MERS-CoV and SARS-CoV in symptoms, signs, laboratory tests, and chest film are indicated in Table 16.
Table 15.
Comparison between SARS and MERS-CoV in respect to their virology, epidemiology, and clinical outcomes.224.
| MERS-CoV | SARS | |
|---|---|---|
| Virology | Betacoronavirus lineage 2 C | Betacoronavirus lineage 2B |
| Receptor | hDPP4 | ACE2 |
| Genome size | 29.9 kb | 29.3kb |
| Source | Not yet confirmed, camel is the likely host | Civet Cat |
| Epidemiology | Limited human to human transmission, the disease is mostly localized in the Middle East | Human to human transmission is well-recognized, affected many countries but spared the Middle East |
| Ro | 2–3 (for Jeddah 3.5–6.7, for Riyadh 2–2.8) | Variable, ranges from 2 to 6 |
| Superspreading event | Not known | Reported |
| M:F | 1.74:1 | 0.75:1 |
| Median age (range) in years | 48 (1–99) | Less than a third had |
| Mean incubation period in days (range) | 5 (2–15) | Comorbidities |
| Comorbidities | Three quarter of the patients had comorbidities | Less than a third has Comorbidities |
| Clinical presentation | Unpredictable and erratic clinical course ranging from asymptomatic illness to severe pneumonia | A typical biphasic clinical course |
| Hemoptysis | More common | Less common |
| Respiratory failure | Presents relatively early | Presents relatively late |
| Travel association | Limited travel-associated exposure | Recognized travel-associated exposure |
| Time from symptom onset to hospitalization | 0–16 days | 2–8 days |
| Median time from symptom onset to death | 12 days | 21 days |
Table 16.
The difference between MERS-CoV and SARS-CoV in symptoms, signs, laboratory tests, and chest film.225.
| Symptoms | MERS-CoV | SARS-CoV |
|---|---|---|
| Headache | + | ++ |
| Fever and chills | +++ | ++ |
| Prominent fatigue | + | - |
| Myalgias | ++ | +++ |
| Dry cough | +++ | ++ |
| Shortness of breath | +++ | ++ |
| Sore throat | + | + |
| Nausea/vomiting | + | + |
| Diarrhea | ± | ± |
| Abdominal pain | ± | - |
| Hemoptysis | ± | - |
| Signs | ||
| Tachycardia | + | + |
| Conjunctival suffusion | + | - |
| Diminished breath sounds | + | + |
| Acute renal failure (ARF) | ± | - |
| Laboratory tests | ||
| Normal WBC count | - | |
| Leukopenia | + | - |
| Relative lymphopenia | +++ | +++ |
| Thrombocytopenia | ++ | +++ |
| Elevated serum transaminases | + | ± |
| Elevated ldh | + | ++ |
| Elevated cpk | ++ | - |
| Chest film | ||
| Normal/minimal basilar infiltrates (early) | - | + |
| Unilateral infiltrates (late) | + | - |
| Pleural effusion | +++ | ++ |
| Cavitation | + | - |
| ARDS (severe cases) | - | - |
Enhanced infection control measures which are effective in controlling nosocomial outbreaks and Middle East Respiratory Syndrome CoVs are shown in Tables 17 and 18, respectively.
Table 17.
Enhanced infection control measures that were effective in controlling nosocomial outbreaks.226–235.
|
Table 18.
Middle East respiratory syndrome coronavirus vaccines236.
| Vaccine | Target | Use | Advantages | Problems |
|---|---|---|---|---|
| Anti-MERS-CoV monoclonal antibodies | Surface (S) glycoprotein | Passive immunization; prophylaxis or treatment at early times p.i. | High titer preparations; can be produced in large amounts | Short half-life; needs to be readministered for continued efficacy |
| Human polyclonal anti-MERS-CoV antibodies | Virus structural proteins | Passive immunization; treatment at early times p.i. | Polyclonal antibody so antibody escape unlikely; human antibody | Short half-life; needs to be readministered for continued efficacy; few MERS survivors available as donors |
| Inactivated virion vaccines | Virus structural proteins; anti-S neutralizing antibodies most important | Active immunization | High titer antibody to S protein | Response many not be long term; on challenge may induce immunopathological disease; may be ineffective in aged populations |
| Live attenuated vaccines (e.g. viruses deleted in envelope (E) protein; viruses with reduced fidelity (mutated in nsp14) | Mostly virus structural proteins | Active immunization | Generally safe; induce antibody and T-cell responses; long-term immunity | May not be safe is immunocompromised patients; may regain virulence by reversion or recombination with circulating CoV |
| Viral vector (attenuated) vaccines: poxvirus, AAV adenovirus, parainfluenza virus, rabies virus, measles virus, VSV | S protein | Active immunization | Safe: nonreplicating; induce antibody and T-cell responses | Long-term immunity, but not as long as live attenuated vaccines |
| Replicon particles (e.g. VEEV or VSV-based) | S protein or any viral protein | Active immunization | Safe; nonreplicating; induce antibody and T-cell responses; useful for mucosal immunity | Production is complex |
| Subunit vaccines (e.g. RBD of S protein) | Generally S protein | Active immunization | Safe; non-replicating; induce high antibody titers; may also induce T-cell responses | Duration of response not known |
| DNA vaccines | Generally S protein | Active immunization | Safe; induce high antibody titers and T-cell responses | Immunogenicity variable; may induce anti-DNA immune response |
Abbreviations: MERS-CoV, Middle East respiratory syndrome coronavirus; p.i., post infection; AAV, adeno-associated virus; VSV, vesicular stomatitis virus; VEEV, Venezuelan equine encephalitis virus; RBD, receptor binding domain.
SARS-CoV-2 coronavirus
The novel SARS-CoV-2 coronavirus which apparently first infected humans in Wuhan China, has caused the COVID-19 pandemic.237–239 It is called SARS-CoV-2 because of its high similarity in terms of clinical symptoms and biological nature with the causative agent of severe acute respiratory syndrome (SARS) by the International Committee on Taxonomy of Viruses,240,241 which can affect patients of all ages.242,243 Its genome sequence analysis has shown that SARS-CoV-2 belons to beta-coronavirus genus, which includes bat SARS-like coronavirus SARS-CoV and MERS-CoV.244 Its outbreak in China since December 2019 has caused so many challenges, and it has rapidly spread to many countries.245–248 It belongs to a large family of viruses which are known as coronaviruses.249 It emerged after 2003 SARS in China and the second coronavirus, which was famous as MERS in 2012 in Saudi Arabia. On the basis of nucleic acid sequence similarity, the newly identified SARS-CoV-2 is a beta-coronavirus.250,251 It is mainly associated with respiratory disease and few extrapulmonary signs.252 Epidemiological investigations showed that different animals (Bbats, pangolins, snakes) could have been intermediate hosts which facilitate the spill-over of SARS-CoV-2 as a distinct human Beta-coronavirus from bats to human population.253–257 Sun et al.258 reported that bats are considered as the natural hosts of this virus, cold temperature and low humidity provides conducive environmental conditions for prolonged viral survival in suspected regions concentrated with bats. The RBD portion of the SARS-CoV-2 S protein has evolved to effectively target a molecular feature on the outside of human cells called ACE2, a receptor involved in regulating blood pressure.
The SARS-CoV-2 S protein was found so effective at binding the human cells. The SARS-CoV-2 backbone differed substantially from those of known coronaviruses and mostly resembled related viruses found in bats and pangolins. With considering tabular comparison of SARS versus SARS-CoV-2, clinical presentation of SARS and SARS-CoV-2 are fever, dry cough, and shortness of breath; incubation period for SARS and SARS-CoV-2 are 2–7 days, 2–14 days, respectively.259 Its cycle starts when S protein binds to the cellular receptor ACE2; after receptor binding the conformation change in the S protein facilitates viral envelope fusion with the cell membrane through the endosomal pathway, and then SARS-CoV-2 releases RNA into the host cell. Genome RNA is translated into viral replicase polyproteins pp1a and 1ab, which are then cleaved into small products by viral proteinases. The polymerase produces a series of subgenomic mRNAs by discontinuous transcription and finally translated into relevant viral proteins. Both viral proteins and genome RNA are subsequently assembled into virions in the endoplasmic reticulum (ER) and Golgi, and then transported via vesicles and released out or the cell.260Kang et al.261 reported that the cause and consequence of pneumonia sill remain unknown. Previous coronavirus outbreaks have started, with humans contracting the virus after direct exposure to civets (SARS), and camels (MERS).60 Tseng et al.262 found that SARS-CoV vaccines all induced antibody and protection against infection with SARS-CoV. Most CoVs share a similar viral structure, similar infection pathway and a similar structure of the S protein,263 which has shown similar research strategies should be used for SARS-CoV-2.264,265 Matsuyama et al.266 discovered that SARS and MERS and SARS-CoV-2 infection is enhanced by TMPRSS2. Rasmussen et al.267 reported an incubation period of ~5 days (range-2–14 days), average age of hospitalized patients has been 49–56 years, with a third to half with an underlying illness, and men were more frequent among hospitalized cases (54–73%). Chan et al.268 reported person-to-person transmission of 2019 novel coronavirus in hospital and family settings, and the reports of infected travelers in other geographical regions. Khan et al.269 reported that during control the SARS-CoV-2, the entrances of residential communities, dormitories, and public places were restricted and temperature monitoring for related symptoms was done before residents entering.269–273
Chen et al.249 showed that on the basis of molecular modeling, SARS-CoV-2 RBD has a stronger interaction with angiotensin converting enzyme 2 (ACE2), and a unique phenylalanine F486 in the flexible loop plays an important role due to its penetration into a deep hydrophobic pocket in ACE2, and ACE2 can potentially bind RBD of SARS-CoV-2, making them all possible natural hosts for the virus. It might be able to bind to the angiotensin-converting enzyme2 receptor in humans.274 Luan et al.275 observed that N82 in ACE2 indicated a closer contact with SARS-CoV-2 S protein than M82 in human ACE2. RBD domain of SARS-CoV-2 interacts with human ACE2, which is why ACE2 is considered as the receptor for SARS-CoV-2.275,276Wall et al.276 showed the presence of a four amino acid residue insertion at the boundary between the S1 and S2 subunits in SARS-CoV-2 S compared with SARS-CoV and SARS-CoV S. Wall et al.276 demonstrated that SARS-CoV s murine polyclonal antibodies potently inhibited SARS-CoV-2 S mediated entry into cells which showed cross-neutralizing antibodies targeting conserved S epitopes can be elicited upon vaccination. Measures to prevent transmission are highly successful at an early stage, and in the next steps on the SARS-CoV-2 infection should be focused on early isolation of patients and quarantine.277,278 Possible origins of SARS-CoV-2 are shown in Table 25. In SARS-CoV-2, M protein is responsible for the transmembrane transport of nutrient, the bud release and the formation of envelope, S protein, attaching to hose receptor ACE2, including two subunits S1 and S2; S1 determines the virus host range and cellular tropism by RBD, and S2 mediates virus-cell membrane fusion by HR1 and HR2. N, E protein, and several accessory proteins, interfered with host immune response or unknown function. Li et al.61 reported that genome and ORF1a homology show that the virus is not the same CoV as the CoV derived from five wild animals, namely Paguma larvata, Paradoxurus hermaphrodites, Civet, Aselliscus stoliczkanus, and Rhinolophus sinicus, whereas the virus has the highest homology with Bat Coronavirus isolate RaTG13. Populations influenced by SARS-CoV-2 divided into four levels is shown in Table 19.
Table 19.
Populations influenced by SARS-CoV-2 divided into 4 levels.279.
|
Real-time reverse-transcriptase-polymerase chain reaction (rRT-PCR) may produce initial false negative results, and they have suggested that patients with typical computed tomography (CT) findings, but negative rRT-PCR results should be isolated, and rRT-PCR should be repeated to avoid misdiagnosis.280,281 Other scientists also stated that final diagnosis relies on rRT-PCR positively for the presence of coronavirus.282–284 However, there are currently no effective specific antivirals or drugs combinations supported by high-level evidence.285 Wang et al.286 introduced spiral chest computed tomography (CT) as a sensitive examination method, which can be applied to make early diagnosis and for evaluation of progression with a diagnostic sensitivity and accuracy better than that of nucleic acid detection. Liu et al.287 indicated that on the resolutive phase of the disease, CT abnormalities showed complete resolution or demonstrated residual linear opacities. Prem et al.288 noticed that restrictions on activities in Wuhan, would help to delay the epidemic peak and prevent the secondary peak. Sun et al.289 highlighted the importance and availability of public datasets to encourage analytical efforts and provide robust evidence to guide interventions. Kobayashi et al.290 found that the risk of death among young adults is higher than that of seasonal influenza, and those elderly with underlying comorbidities need additional care. Grubaugh et al.291 found that many more mutations will appear in the viral genome which these mutations may help scientists to track the spread of SARS-CoV-2. Coronaviruses have capacity to jump species boundaries and adapt to new hosts.292 Zhao et al.293 reported that the majority of patients with suspected or confirmed SARS-CoV-2 showed fever and dry cough and presented bilateral multiple mottling and ground-glass opacity on chest computed tomography scans. Lippi et al.294 showed that low platelet count is associated with increased risk of severe disease and mortality in patients with SARS-CoV-2, and it can be considered as clinical indicator of worsening illness during hospitalization. Lv et al.295emphasized at the importance of successive sampling and testing SARS-Cov-2 by RT-PCR. Xie et al.296 recommended combining the computed tomography scans and nucleic acid detection. Guo et al.297 indicated the strong influence of SARS-CoV-2 epidemic on the utilization or emergency dental services. It has been reported that remdesivir only and in combination with chloroquine or interferon beta significantly blocked the SARS-CoV-2 replication and patients were declared as clinically recovered.298–300 Some other anti-virals like Nafamostat, Nitazoxanide, Ribavirin, Penciclovir, Favipiravir, Ritonavir, AAK1, Baricitinib, and Arbidol showed moderate results when tested against infection in patients and in-vitro clinical isolates.301 Shen et al.302 consider isothermal nucleic acid amplification as a highly promising candidate method for detection of coronavirus infection, due to its fundamental advantage in quick procedure time at constant temperature without thermocycler operation. Seah and Agrawal303 found that the ability of SARS-CoV-2 to infect ocular tissue and its pathogenic mechanisms. Ghinai et al.304 classified SARS-CoV-2 into four categories: high-risk contacts, medium-high-risk contact, medium-risk contacts, low-risk contacts and no-contacts. The most important Diagnostic Criteria for SARS-CoV-2 are shown in Table 20.
Table 20.
The most important Diagnostic Criteria for SARS-CoV-2.305,306.
|
Human Coronaviruses and Vaccines
No licensed MERS coronavirus vaccine is currently available and the most challenges for progress to have vaccines are (a) available animal models which might not mimic human diseases,307 (b) an immune correlate or protection has not been identified and the protective immune response in natural infection is poorly understood,308 (c) there is a theoretical risk of immune enhancement during MERS coronavirus infection after vaccination possibly leading to immunopathological pulmonary eosinophilic infiltration,309 (d) demonstration of efficacy in the field will probably not be possible, necessitating alternative regulatory pathways for licensure, (e) if MERS shifts from a pattern of sporadic outbreaks to pandemic spread, it is not known whether vaccines based on current MERS coronavirus isolates will offer protection against pandemic strains. Previous work with a double inactivated SARS-CoV had shown efficacy in young mice,310 but, subsequent analysis in aged animals or with heterologous challenge showed vaccine failure and significant immune pathology.311 Not tested in experimental systems, based on reported cases, age and immune-compromised status appears to be comorbidity factors for MERS-CoV infection and lethality.312,313 Spike (S) protein of MERS-CoV is immunogenic and can induce neutralizing antibodies, which is a potential major target for vaccine development.314 It is important to prepare for large scale-manufacture of the vaccine antigen.315 In viral vaccine design, it is essential to identify the most stable and neutralizing viral receptor binding domain (RBD) fragment, while eliminating unnecessary and non-neutralizing structures as a means of immunofocusing.316 Subcutaneous vaccination of a recombinant protein containing RBD of MERS-CoV S fused with Fc of human (RBD-Fc) induced strong systemic neutralizing antibody responses in vaccinated mice.317 Spherical virus-like particles (sVLP) display the RBD of MERS-CoV are promising prophylactic candidate against MERS-CoV in a potential outbreak situation.318 Potent induction of T-cell responses by single-cycle vectors, indicate that these vectors are excellent alternatives to live-attenuated vesicular stomatitis virus (VSV).319 SARS M DNA vaccines which induce human neutralizing antibodies and human monoclonal antibodies against SARS CoV are not developed.319 SARS-CoV S DNA vaccine can generate antigen-specific humoral and cellular immune responses which may contribute to long-term protection.320 Caution should be taken in using inactivated SARS-CoV as a vaccine since it may also cause harmful immune or inflammatory responses.321 Both S-containing ecosomes and the adenoviral vector vaccine induced neutralizing antibody titers.322,323 Polypeptides originating from N or S might be a potential target for the generation of a recombinant SARS vaccine.324,325 After study of a DNA SARS-vaccine, it has been found that a combination of the vaccine-induced T-helper type 1 (Th1) immune response while the whole killed virus vaccine included T helper type 2 (Th2).326 A similar approach to a CTL vaccine design may be possible for the SARS-CoV-2 which contain multiple class I epitopes with predicted HLA restrictions consistent with broad population coverage.327 Better understanding of the pathogenesis of the infection with the covid-19 which in selected cases may lead to a similar clinical picture of macrophage activating syndrome with its associated cytokine storm may bring to an improved diagnostic measurements (Shoenfeld, 2020). Immunoreactive spike-1 proteins from SARS-CoV-2 are expressed on the surface of irradiated target I-cells, so utilizing this innovative strategy, these viral antigen-displaying decoy cells will be developed as a vaccine to protect against SARS-CoV-2 disease (Ji et al., 2020). Overview of vaccine production platforms and technologies for SARS-CoV-2 is shown in Table 21.
Table 21.
Overview of vaccine production platforms and technologies for SARS-CoV-2328.
| Platform | Target | Existing, Licensed Human Vaccines Using the Same Platform | Advantages | Disadvantages |
|---|---|---|---|---|
| RNA vaccines | S protein | No | No infectious virus needs to be handled, vaccines are typically immunogenic, rapid production possible. | Safety issues with reactogenicity have been reported. |
| DNA vaccines | S protein | No | No infectious virus needs to be handled, easy scale up, low production costs, high heat stability, tested in humans for SARS-CoV-1, rapid production | Vaccine needs specific delivery devices to reach good immunogenicity. |
| Recombinant protein vaccines | S protein | Yes for baculovirus (influenza, HPV) and yeast expression (HBV, HPV) | No infectious virus needs to be handled, adjuvants can be used to increase immunogenicity | Global production capacity might be limited. Antigen and/or epitope integrity needs to be confirmed. Yields need to be high enough. |
| Viral vector-based vaccines | S protein | Yes for VSV (Ervebo), but not for other viral vectored vaccines | No infectious virus needs to be handled, excellent preclinical and clinical data for many emerging viruses, including MERS-CoV | Vector immunity might negatively affect vaccine effectiveness (depending on the vector chosen) |
| Live attenuated vaccines | Whole virion | Yes | Straightforward process used for several licensed human vaccines, existing infrastructure can be used | Creating infectious clones for attenuated coronavirus vaccine seeds takes time because of large genome size. Safety testing will need to be extensive |
| Inactivated vaccines | Whole virion | Yes | Straightforward process used for several licensed human vaccines, existing infrastructure can be used, has been rested in humans for SARS-CoV-1, adjuvants can be used to increase immunogenicity | Large amounts of infectious virus need to be handled (Could be mitigated by using an attenuated seed virus). Antigen and/or epitope integrity needs to be confirmed |
Vaccine efficacy is measured by the ability of the antigen to raise a protective immunologic response from V and T cells after exposure to the viral agent. Vaccines can be produced by inactivation of the virus by using an attenuated or weak form of the virus or by using recombinant forms of viral components. Inactivated virus vaccines are relatively safe because they can not revert back to the live form; moreover, they are stable and may not even require refrigeration. Inactivated vaccines may usually require several doses, and some are weakly effective at stimulating an immune response.
Human coronaviruses and antibody-dependent enhancement (ADE)
The immunopathological effects of antibody-dependent enhancement (ADE) have been observed in various viral infections, characterized as antibody-mediated enhancement of viral entry and infection of a severe inflammatory response.332,333 ADE of viral entry has been observed for many viruses. It was assumed that antibodies target one serotype of viruses but only subneutralize another, leading to ADE of the latter viruses. This phenomenon has been reported in vitro and in vivo for viruses representing numerous families and genera of public health and veterinary importance, which share some common features such as preferential replication in macrophages, ability to establish persistence, and antigenic diversity. For SARS-CoV, it was suggested that antibodies against spike proteins of SARS may cause ADE effect which raised reasonable concern regarding the use of SARS-CoV vaccine and shed light on some roles in SARS pathogenesis. There are conflicting data for the role of ADE in serious coronavirus infections. Antibodies against the S protein can enhance virus uptake by cells in vitro, although the clinical relevance of these findings is conflicting. It has been reported that the rush to make a vaccine for SARS-CoV-2 may lead the infections worse, because the amin risk is the chance of inducing ADE, a process known to complicate vaccine development which has happened before in Dengue and several other diseases. For previous human coronaviruses, SARS and MERS, antibodies against the viruses have been shown to cause ADE in animal models, including non-human primates, and like those viruses, it is important to be aware of the possibility of ADE caused by SARS-CoV-2 vaccines. ADE allows the infection of phagocytic antigen-presenting (APC), such as macrophages, due to the binding of virus-bound antibodies to FcγR on their surfance.333It has been shown that neutralizing antibodies targeting the receptor-binding domains (RBD) of the MERS-CoV and SARS-CoV spike proteins, respectively, can mediate the entry of the viruses into Fc receptor-expressing human cell in vitro.334 Furthermore, T cells, believed to play an important role in controlling SARS-CoV-2 infection, are depleted in severe SARS-CoV-2,335 and this may be accelerated APC infection due to ADE.336 Recently, scientists have reported that unlike in MERS and SARS, the S-protein RBD of SARS-CoV-2 can elicit a robust neutralizing antibody response without including ADE in animal immunization studies.337
The majority of SARS-CoV-2 vaccines in development aim to elicit neutralizing antibodies against the spike protein that prevent the virus from binding ACE2 on lung cells and entering via endocytosis. Immunofocusing constitutes one of the main methods proposed to prevent ADE ad skew adaptive immunity toward protective responses, and other immunofocusing strategies consist of making undesired antibody epitopes with glycosyl groups; truncating the spike protein, and locking the antigen into conformations which display epitopes for neutralizing antibodies.
On the basis of small cohorts of SARS-CoV-2 patients, two studies have shown that an increased lgG response and a higher titer of total antibodies were associated with more severe disease,338,339 which suggestive of possible ADE in SARS-CoV-2 infection,340 but there are still some doubts over the relevance of ADE in SARS-CoV-2. In some clinical studies, it has been revealed that SARS-CoV-specific antibodies are not harmful in patients with SARS, although it has been noted that non-neutralizing coronavirus antibodies may cause ADE in feline infectious peritonitis. Such efforts have promoted investigators to remove potential ADE-promoting S protein epitopes located outside the RBD and focus on the RBD as a lead vaccine candidate.341,342There is no evidence that ADE facilitates the spread of SARS-CoV in infected hosts; in fact, infection of macrophages through ADE does not result in productive viral replication and shedding.343 Instead, internalization of virus-antibody immune complexes can promote inflammation and tissue injury by activating myeloid cells via FcRs.344 Virus introduced into the endosome through this pathway will likely engage the RNA-sensing Toll-like receptors (TLRs) TLR3, TLR7, and TLR8. Uptake of SARS-CoV through ADE in macrophages led to elevated production of TNF and IL-6. In mice infected with SARS-CoV, ADE was associated with decreased levels of the anti-inflammatory cytokines IL-10 and TGFβ and increased levels of the proinflammatory chemokines CCL2 and CCL3.345Recent studies of antibody responses in patients with SARS-CoV-2 have associated higher titers of anti-N IgM and IgG at all time points of following the onset of symptoms with a worse disease outcome.346 Furthermore, higher titers of anti-S and anti-N IgM and IgM correlated with worse clinical readouts and older age,347 suggesting potentially detrimental impacts of antibodies in some patients. But, 70% of patients who recovered from mild SARS-CoV-2 had measurable neutralizing antibodies that persisted upon revisit to the hospital.348
ADE should be given full consideration in the safety evaluation of emerging vaccines for SARS-CoV-2 and monoclonal antibodies could be used to tackle this virus.349From studies about using a MERS-CoV vaccine, it has also been proposed that neutralizing antibodies might instead induce ADE. Vaccines are a routine medical intervention performed on healthy individuals, so it is important to consider ADE seriously during vaccine development to ensure that vaccines protect individuals and do not exacerbate disease following subsequent infections. Translational considerations for SARS-CoV-2 vaccine development, and vaccine platforms for human coronaviruses are presented in Tables 22 and 23, respectively.
Table 22.
Translational considerations for SARS-CoV-2 vaccine development.350.
| Stage | Translational category | Activity |
|---|---|---|
| T0 | Basic Research | Characterize antibody-dependent enhancement (ADE) mechanisms |
| Identify SARS-CoV-2 ADE-associated epitopes | ||
| Bioinformatics of SARS-CoV-2 mutations | ||
| Generating recombinant vaccine proteins | ||
| Animal models for SARS-CoV-2 vaccines | ||
| T1 | Preclinical Research | Phase 1 clinical trials |
| Assay development for human anti-SARS-CoV-2 IgG | ||
| Assay development for neutralizing versus ADE-inducing human IgG | ||
| Assess effects of vaccine on ADE induction in animal models | ||
| Modify vaccines to minimize ADE risk | ||
| T2 | Clinical Research | Phase 2/3 clinical trials |
| Consider limiting initial vaccine studies to subjects ≥20 years old | ||
| Multiplex measurement of anti-COVID IgG | ||
| Outcomes research | ||
| T3 | Clinical Implementation | Phase 4 clinical trials |
| Long-term follow-up of post-vaccinated and infected subjects for ADE | ||
| Reexamine age indications for SARS-CoV-2 vaccination | ||
| T4 | Public Health | Population-level studies of vaccine efficacy |
| Assessment of ADE-associated antibody prevalence |
Table 23.
Vaccine platforms for Human coronaviruses
| Target | MERS-CoV | Target | SARS-CoV-2 | Target | |
|---|---|---|---|---|---|
| Inactivated SARS virus (β-propiolactone, formalin, UV, irradiation) | All virus structural proteins | Live attenuated MERS-CoV (E envelope-deleted) | All virus genome component s (except E) | Inactivated virus/alum | All structural proteins of the virus |
| Live attenuated/host adapted SARS virus (E-deleted) | All genome (except the envelope protein) | Chemically for physically-Inactivated virus (MERS-CoV, rabies virus) | S glycoprotein and S1 subunit | Virus-like particle (VLP) | Unknown |
| Live attenuated recombinant virus (parainfluenza virus, Vesicular Stomatitis Virus, Venezuela equine encephalitis virus, Newcastle disease virus) | S glycoprotein Nucleocapsid (N) protein | Replication-deficient viral-vectored vaccines (Poxvirus, adenocirus, measles, rabies) | S glycoprotein or S1 subdomain (containing the receptor-binding protein) | Protein nanoparticles/Matrix-M | S glycoprotein |
| Recombinant modified vaccinia Ankara (MVA) virus | Spike (S) glycoprotein or nucleocapsid (N) protein | Soluble protein vaccines/adjuvant | S glycoprotein and fragments | Non-replicating viral sector (adenovirus, chimeric chimpanaeeadenocirus) | S glycoprotein/unknown |
| Recombinant non-replicating adenovirus (E-deleted) | S glycoprotein/nucleocapsid (N) protein | Nanoparticles | S glycoprotein | S glycoprotein/unknown | |
| DNA-based vaccines | Full spike (S) glycoprotein of fragments | DNA-based vaccines | S glycoprotein and subunits | DNA-based vaccine | S glycoprotein/unknown |
| Soluble proteins/adjuvant | Full spike (S) glycoprotein of fragments | Combination vaccines (protein and DNA) | S glycoprotein and subunits | Protein subunit | S glycoprotein and peptides/unknown |
| Virus-like particles (VLPs)/adjuvant | Spike (S) glycoprotein | Live attenuated virus | All proteins of the virus | ||
| Combination of vaccine approaches (DNA/peptide, DNA/recombinant viral vector, viral vector/peptides) | S glycoprotein or fragments | Replicating viral sector (measles, horsepox) | Unknown |
Conclusion
Severe acute respiratory syndrome (SARS) is caused by a coronavirus SARS-CoV which was started in pigs or ducks in south of China and mutated to affect humans. It was originated in Guangdong province. CoVs are single-stranded RNA viruses which belong to the order Nidovirales, family Coronaviridae, and subfamily Coronavirinae. SARS is caused by a coronavirus (SARS-CoV) which exists in bats and palm civets in Southern China. Its family is Coronaviridae, and its genus is Coronavirus. It is enveloped, helical nucleocapsid, spherical to pleomorphic, helical nucleocapsid, spherical to pleomorphic, kidney-shaped or rod-shaped particles, 100–130 nm in diameter. Its nucleic acid is linear, positive-sense, single-stranded RNA, ~29.8 kb in length. Virions sensitive to treatment with lipid solvents, nonionic detergents, formaldehyde, and oxidizing agents. The civet cat (palm civet) in Southeast Asia is likely source of introduction of the agent to humans. The SARS CoV RNA sequence found in palm civets is 99% identical to that found in palm civets is 99% identical to that found in humans. Similar RNA sequences have been found in bats, snakes and monkeys. No medication has been proven to treat SARS effectively, but oxygen therapy and tracheal intubation and mechanical ventilation to support life until recovery begins is useful for patients in severe case. The most useful ways to control SARS pandemic is public-health and infection-control measures. The most important primary measures are isolation, ribavirin, and corticosteroid therapy, mechanical ventilation, convalescent plasma, and others. It can be spread from close person-to-person contact via respiratory droplets which come in contact with skin or mucous membranes such as eyes, mouth or nose.
MERS-CoV is a zoonotic virus which can lead to secondary human infections. Dromedary camels have been recognized as the intermediate host, with closely related virus sequences in bats. MERS carries a 35% mortality rate. There is no clear and specific treatment for MERS, and person to person spread causes hospital and household outbreaks of MERS-CoV. It is the sixth coronavirus that influences human. Like other coronaviruses, it is an enveloped single-stranded RNA virus which replicates in the host-cell cytoplasm, with approximate size of 30 kb. It has structural proteins, called the E, M, and N proteins, and membrane protein called the spike (S) protein, which has important role in the virus attachment and entry into the host cells. It has been concluded that the MERS-S protein is known to represent a key target for the development of new therapeutics and includes of a receptor-binding subunit S1 and a membrane-fusion subunit S2. The subunit S1 is composed of four different core domain, and the domain S1B binds to the host-cell receptor dipeptidylpeptidase 4 (DPP4), while the domain S1A binds to sialoglycans which increased infection of human lung cells by MERS-CoV. The roles of S protein in receptor binding and membrane fusion make it a perfect target for vaccine and antiviral development. It has been showed that vaccines based on the S protein can induce antibodies to block virus binding and fusion or neutralize virus infection.
The novel SARS-CoV-2, which apparently first infected people in the city of Wuhan, spread in all over the world. Its genome sequence analysis has shown that SARS-CoV-2 belongs to beta-coronavirus genus, which includes Bat SARS-like coronavirus, SARS-CoV and MERS-CoV. On the basis of nucleic acid sequence similarity, the newly identified SARS-CoV-2 is a beta-coronavirus. The RBD portion of the SARS-CoV-2 pike proteins has evolved to effectively target a molecular feature on the outside of human cells called ACE2, a receptor involved in regulating blood pressure. The SARS-CoV-2 spike protein was found so effective at binding the human cells. In SARS-CoV-2, M protein is responsible for the transmembrane transport of nutrient, the bud release and the formation of envelope, S protein, attaching to hose receptor ACE2, including two subunits S1 and S2. It has been reported that remdesivir only and in combination with chloroquine or interferon beta significantly blocked the SARS-CoV-2 replication and patients were declared as clinically recovered. Some other anti-virals like Nafamostat, Nitazoxanide, Ribavirin, Penciclovir, Favipiravir, Ritonavir, AAK1, Baricitinib, and Arbidol showed moderate results when tested against infection in patients and in-vitro clinical isolates. Isothermal nucleic acid amplification as a highly promising candidate method for detection of coronavirus infection, due to its fundamental advantage in quick procedure time at constant temperature without thermocycler operation.
Antibody dependent enhancement of viral infection is a process by which the virus leverages the antibodies to aid its infection. ADE allows the infection of phagocytic antigen-presenting cells (APC), such as macrophages, due to the binding of virus-bound antibodies to FcγR on their surface. Identification of viral epitopes associated with ADE neutralization is effective for development of vaccines with minimum or no risk for ADE. Also, clear understanding of the cellular events after virus entry through ADE has become crucial for developing efficient intervention, and it is necessary to better understanding the mechanisms of ADE.
The vaccine effort should be guided by three imperatives: speed, manufacture and deployment at scale, and global access. Once the vaccine found to be effective, it will be distributed to millions or billions of people. The amino acid sequences of the virus like in other viruses, might have a cross-reaction with the human body sequences. Researchers should work to develop effective and safe subunit vaccines against human coronaviruses, especially SARS-CoV-2 and any other emerging coronaviruses that might cause future pandemics.
Disclosure of potential conflicts of interest
The manuscript has not been published or presented elsewhere in part of in entirety and is not under consideration by another journal. We have read and understood journal’s policies, and we believe that neither the manuscript nor the study violates any of the rules. There are no conflicts of interest to declare.
Ethical approval
Not required.
References
- 1.Huang J, Cao Y, Du J, Bu X, Ma R, Wu C.. Printing with SARS CoV S DNA and boosting with SARS CoV S epitopes specific for CD4+ and CD8+ T cells promote cellular immune responses. Vaccine. 2007;25:6981–91. doi: 10.1016/j.vaccine.2007.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kam YW, Kien F, Roberts A, Cheung YC, Lamirande EW, Vogel L, Chu SL, Tse J, Guarner J, Zaki SR, et al. Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcγRII-dependent entry into B cells in vitro. Vaccine. 2007;25:729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu Y-K, Ali GD, Jia F, Li Q, Kelvin D, Couch RC, Harrod KS, Hutt JA, Cameron C, Weiss SR, et al. The SARS-CoV ferret model in an infection-challenge study. Virology. 2008;374:151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masters PS, Perlman S.. Coronaviridae in field,s virology. In: Knipe DM, Howley PM editors. Lippincott. Vol. 1. Philadelphia (USA): Williams & Wilkins; 2013. p. 825–58. [Google Scholar]
- 5.Kumaki Y, Wandersee MK, Smith AJ, Zhou Y, Simmons G, Nelson NM, Bailey KW, Vest ZG, Li J-K-K, Chan PK-S, et al. Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin, Urtica dioica agglutinin. Antiviral Res. 2011;90:22–32. doi: 10.1016/j.antiviral.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermingham A, Chand MA, Brown CS, Aarons E, Tong C, Langrish C, Zambon M. Severe respiratory illness caused by a novel coronavirus, in a patient transderred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012;17:20290. [PubMed] [Google Scholar]
- 7.Karypidou K, Ribone SR, Quevedo MA, Persoons L, Pannecouque C, Helsen C, Claessens F, Dehaen W. Synthesis, biological evaluation and molecular modeling of a novel series of fused 1,2,3-triazoles as potential anti-coronavirus agents. Bioorg Med Chem Lett. 2018;28:3472–76. doi: 10.1016/j.bmcl.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang L, Yan Y, Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev. 2020. http://doi.10.1016/j,tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akerstrom S, Gunalan V, Tat Keng C, Tan Y-J, Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395:1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams MJ, Carstens EB. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2012). Arch Viral. 2012;157:1411–22. doi: 10.1007/s00705-012-1299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sims AC, Burkett SE, Yount B, Pickles RJ. SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium. Virus Res. 2008;133:33–44. doi: 10.1016/j.virusres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu G, Wang Q, Gao GF. Bat-to-human: spike features determining host jump of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trend Microbiol. 2015;23(8):468–78. doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ar Gouilh M, Puechmaille SJ, Diancourt L, Vandenbogaert M, Serra-Cobo J, Roig ML, Brown P, Moutou F, Caro V, Vabret A, et al. SARS-CoV related Betacoronavirus and diverse Alphacoronavirus members found in western old-world. Virology. 2018;517:88–97. doi: 10.1016/j.virol.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman PM, Lawrence MC. The structural biology of type I viral membrane fusion. Nat Rev Mol Cell Biol. 2003;4:309–19. doi: 10.1038/nrm1076. [DOI] [PubMed] [Google Scholar]
- 15.Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat. Struct. Mol Biol. 2008;15:675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15:690–98. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White JM, Delos SE, Brecher M, Schornberg K. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit Rev Biochem Mol Biol. 2008;43:189–219. doi: 10.1080/10409230802058320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White JM, Whittaker GR. Fusion of enveloped viruses in endosomes. Traffic. 2016;17:593–614. doi: 10.1111/tra.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosch BJ, van der Zee R, de Haan CAM, Rottier PJM. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77:8801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millet JK, Whittaker GR. Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology. 2018;517:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu P, Hu B, Shi Z-L, Cui J. Geographical structure of bat SARS-related coronaviruses. Infect Genet Evol. 2019;69:224–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillissen A, Ruf BR. Severe acute respiratory syndrome (SARS). Med Klin. 2003;98:319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong N-S, Wong GWK. Epidemiology of severe acute respiratory syndrome (SARS): adults and children. Paediatr Respir Rev. 2004;5:270–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, Fung KS, Tang HL, Yan WW, Chan HWH, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pillaiyar T, Manickam M, Namasivayam V, Hayashi Y, Jung S-H. An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy. J Med Chem. 2016;59:6595–628. doi: 10.1021/acs.jmedchem.5b01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhai S, Liu W, Yan B. Recent patents on treatment of severe acute respiratory syndrome (SARS). Recent Pat Antiinfect Drug Discov. 2007;2:1–10. [DOI] [PubMed] [Google Scholar]
- 27.Hong X, Currier GW, Zhao X, Jiang Y, Zhou W, Wei J. Posttraumatic stress disorder in convalescent severe acute respiratory syndrome patients: a 4-year follow-up study. Gen Hosp Psychiatry. 2009;31:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yam LY-C, Lau AC-W, Lai FY-L, Shung E, Chan J, Wong V. Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. J Infect. 2007;54:28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang N, Tang J, Lu L, Jiang S, Du L. Receptor-binding domain-based subunit vaccines against MERS-CoV. Virus Res. 2015;202:151–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saminathan M, Chakraborty S, Tiwari R, Dhama K, Verma A. Coronavirus infection in equines: a review. Asian J Anim Vet Adv. 2014;9:164–76. [Google Scholar]
- 32.Snijder EJ, Bredenbeek PJ, Dobbe JC, Thiel V, Ziebuhr J, Poon LL, Guan Y, Rozanov M, Spaan WJ, Gorbalenya AE. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J Mol Biol. 2003;331:991–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Netland J, DeDiego ML, Zhao J, Fett C, Alvarez E, Nieto-Torres JL, Enjuanes L, Perlman S. Immunization with an attenuated severe acute respiratory syndrome coronavirus deleted in E protein protects against lethal respiratory disease. Virology. 2010;399:120–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeDiego ML, Alvarez E, Almazan F, Rejas MT, Lamirande E, Roberts A, Shieh WJ, Zaki SR, Subbarao K, Enjuanes L. A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo. J Virol. 2007;81:1701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeDiego ML, Pewe L, Alvarez E, Rejas MT, Perlman S, Enjuanes L. Pathogenicity of severe acute respiratory coronavirus deletion mutants in hACE-2 transgenic mice. Virology. 2008;376:379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohnishi K, Hattori Y, Kobayashi K, Akaji K. Evaluation of a non-prime site substituent and warheads combined with a decahydroisoquinolin scaffold as a SARS 3CL protease inhibitor. Bioorg Med Chem. 2019;27:425–35. doi: 10.1016/j.bmc.2018.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Law PYP, Liu Y-M, Geng H, Kwan KH, Waye MM-Y, Ho -Y-Y. Expression and functional characterization of the putative protein 8b of the severe acute respiratory syndrome-associated coronavirus. FEBS Lett. 2006;580:3643–48. doi: 10.1016/j.febslet.2006.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, Penaranda S, Bankamp B, Maher K, Chen MH, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–99. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 40.Shin G-C, Chung Y-S, Kim I-S, Cho H-W, Kang C. Preparation and characterization of a novel monoclonal antibody specific to severe acute respiratory syndrome-coronavirus nucleocapsid protein. Virus Res. 2006;122:109–18. doi: 10.1016/j.virusres.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narayanan K, Huang C, Makino S. SARS coronavirus accessory proteins. Virus Res. 2008;133:113–21. doi: 10.1016/j.virusres.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen TTH, Ryu H-J, Lee S-H, Hwang S, Breton V, Rhee JH, Kim D. Virtual screening identification of novel severe acute respiratory syndrome 3C-like protease inhibitors and in vitro confirmation. Bioorg Med Chem Lett. 2011;21:3088–91. doi: 10.1016/j.bmcl.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu DX, Fung TS, Chong KKL, Shukla A, Hilgenfeld R. Accessory proteins of SARS-CoV and other coronaviruses. Antivir Res. 2014;109:97–109. doi: 10.1016/j.antiviral.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spiegel M, Pichlmair A, Martinez-Sobrido K, Cros J, Garcia-Sastre A, Haller O, Weber F. Inhibition of Beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J Virol. 2005;79:2079–86. doi: 10.1128/JVI.79.4.2079-2086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spiegel M, Pichlmair A, Muhlberger E, Haller O, Weber F. The antiviral effect of interferon-beta against SARS-coronavirus is not mediated by MxA protein. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2004;30:211–13. doi: 10.1016/j.jcv.2003.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng B, He M-L, Wong K-L, Lum CT, Poon LLM, Peng Y, Guan Y, Lin MCM, Kung H-F. Potent inhibition of SARS-associated coronavirus (SCOV) infection and replication by type I interferons (IFN-alpha/beta) but not by type II interferon (IFN-gamma). J Interferon Cytokine Res. 2004;24:388–90. doi: 10.1089/1079990041535610. [DOI] [PubMed] [Google Scholar]
- 47.He Y, Zhou Y, Wu H, Luo B, Chen J, Li W, Jiang S. Identification of immunodominant sites on the spike protein of severe acute respiratory syndrome (SARS) coronavirus: implication for developing SARS diagnostics and vaccines. J Immunol. 2004;173:4050–57. doi: 10.4049/jimmunol.173.6.4050. [DOI] [PubMed] [Google Scholar]
- 48.Gallagher TM, Buchmeier MJ. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmes KV. SARS coronavirus: a new challenge for prevention and therapy. J Clin Invest. 2003;111:1605. doi: 10.1172/JCI18819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holmes KV. SARS-associated coronavirus. N Engl J Med. 2003;348:1948. doi: 10.1056/NEJMp030078. [DOI] [PubMed] [Google Scholar]
- 51.Gebauer F, Posthumus WP, Correa I, Sune C, Smerdou C, Sanchez CM, Lenstra JA, Meloen RH, Enjuanes L. Residues involved in the antigenic sites of transmissible gastroenteritis coronavirus S glycoprotein. Virology. 1991;183:225. doi: 10.1016/0042-6822(91)90135-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Posthumus WP, Lenstra JA, van Nieuwstadt AP, Schaaper WM, van der Zeijst BA, Meloen RH. Immunogenicity of peptides simulating a neutralization epitope of transmissible gastroenteritis virus. Virology. 1991;182:371. doi: 10.1016/0042-6822(91)90684-4. [DOI] [PubMed] [Google Scholar]
- 53.Enjuanes L, Sune F, Gebauer C, Smerdou C, Camacho A, Anton IM, Gonzalez S, Talamillo A, Mendez A, Ballesteros ML, et al. Antigen selection and presentation to protect against transmissible gastroenteritis coronavirus. Vet Microbiol. 1992;33:249. doi: 10.1016/0378-1135(92)90053-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li G, Chen X, Xu A. Profile of specific antibodies to the SARS-associated coronavirus. N Engl J Med. 2003;349:508. doi: 10.1056/NEJM200307313490520. [DOI] [PubMed] [Google Scholar]
- 55.Kuo L, Masters PS. The small envelope protein E is not essential for murine coronavirus replication. J Virol. 2003;77:4597–608. doi: 10.1128/JVI.77.8.4597-4608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortego J, Ceriani JE, Patino C, Plana J, Enjuanes L. Absence of E protein arrests transmissible gastroenteritis coronavirus maturation in the secretory pathway. Virology. 2007;368(2):296–308. doi: 10.1016/j.virol.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeDiego ML, Nieto-Torres JL, Jimenez-Guardeno JM, Regla-Nava JA, Castano-Rodriguez C, Fernandez-Delgado R, Usera F, Enjuanes L. Coronavirus virulence genes with main focus on SARS-CoV envelope gene. Virus Res. 2014;194:124–37. doi: 10.1016/j.virusres.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeDiego MK, Nieto-Torres JL, Jimenez-Guardeno JM, Regla-Nava JA, Alvarez E, Oliveros JC, Zhao J, Fett C, Perlman S, Enjuanes L. Severe acute respiratory syndrome coronavirus envelope protein regulates cell stress response and apoptosis. PLoS Pathog. 2011;7:e1002315. doi: 10.1371/journal.ppat.1002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nieto-Torres JL, DeDiego ML, Verdia-Baguena C, Jimenez-Guardeno JM, Regla-Nava JA, Fernandez-Delgado R, Castano-Rodriguez C, Alcaraz A, Torres J, Aguilella VM, et al. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014. http://dx.doi.10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li T. Diagnosis and clinical management of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: an operational recommendation of Peking Union Medical College Hospital (V2.0). Emerg Microbes Infect. 2020;9:582–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ceccarelli M, Berretta M, Venanzi Rullo E, Nunnari G, Cacopardo B. Editorial- difference and similarities between severe acute respiratory syndrome (SARS)- Coronavirus (CoV) an SARS-CoV-2. Would a rose by another name smell as sweet? Eur Rev Med Pharmacol Sci. 2020;24:2781–83. [DOI] [PubMed] [Google Scholar]
- 63.Chee YC. Severe acute respiratory syndrome (SARS)-150 days on. Ann Acad Med Singapore. 2003;32:277–80. [PubMed] [Google Scholar]
- 64.Kissoon N. The threat of severe acute respiratory syndrome (SARS). West Indian Med J. 2003;52:91–94. [PubMed] [Google Scholar]
- 65.Thomas PA. Severe acute respiratory syndrome (SARS): a bolt from the blue. Indian J Med Microbiol. 2003;21:150–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chowell G, Fenimore PW, Castillo-Garsow MA, Castillo-Chavez C. SARS outbreaks in Ontario, Hong Kong and Singapore: the role of diagnosis and isolation as a control mechanism. J Theor Biol. 2003;224:1–8. doi: 10.1016/S0022-5193(03)00228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lang Z-W, Zhang L-J, Zhang S-J, Meng X, Li J-Q, Song C-Z, Sun L, Zhou Y-S, Dwyer DE. A clinicopathological study of three cases of severe acute respiratory syndrome (SARS). Pathology. 2003;35(6):526–31. doi: 10.1080/00313020310001619118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruiz-Contreras A. Case report: caring for suspected severe acute respiratory syndrome (SARS) patients. Dis Manage Response. 2003;1(3):71–75. doi: 10.1016/S1540-2487(03)00045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawkey PM, Bhagani S, Gillespie SH. Severe acute respiratory syndrome (SARS): breath-taking progress. J Med Microbiol. 2003;52:609–13. doi: 10.1099/jmm.0.05321-0. [DOI] [PubMed] [Google Scholar]
- 70.Stohr K. World Health Organization multicentre collaborative network for severe acute respiratory syndrome (SARS) diagnosis. A multi-centre collaboration to investigate the cause of severe acute respiratory syndrome. Lancet. 2003;361:1730–33. doi: 10.1016/S0140-6736(03)13376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoheisel G, Luk WK, Winkler J, Gillissen A, Wirtz H, Liebert Y, Hui DS. Severe acute respiratory syndrome (SARS). Med Klin. 2007;101(12):957–63. doi: 10.1007/s00063-006-1127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patrick DM. The race to outpace severe acute respiratory syndrome (SARS). Can Med Assoc J. 2003;168:1265–66. [PMC free article] [PubMed] [Google Scholar]
- 73.Shin G-C, Chung Y-S, Kim I-S, Cho H-W, Kang C. Antigenic characterization of severe acute respiratory syndrome-coronavirus nucleocapsid protein expressed in insect cells: the effect of phosphorylation on immunoreactivity and specificity. Virus Res. 2007;127:71–80. doi: 10.1016/j.virusres.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hui DSC, Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am. 2019;33(4):869–89. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 76.Chan-Yeung M, Yu WC. Outbreak of severe acute respiratory syndrome in Hong Kong special administrative region: case report. BMJ. 2003;326:850–52. doi: 10.1136/bmj.326.7394.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leung WK, To K-F, Chan PKS, Chan HLY, Wu AKL, Lee N, Yuen KY, Sung JJY. Enteric involvement of severe acute respiratory syndrome associated coronavirus infection. Gastroenterology. 2003;125:1011–17. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhong NS. The clinical diagnosis and treatment of SARS at present. Zhong Guoyi Xuelun Tanbao. 2003;4:29. [Google Scholar]
- 79.Aronin SI, Sadigh M. Severe acute respiratory syndrome. Conn Med. 2004;68:207–15. [PubMed] [Google Scholar]
- 80.Shen B, Cheng SKW, Lau KK, Li HL, Chan ELY. The effects of disease severity, use of corticosteroids and social factors on neuropsychiatric complaints in severe acute respiratory syndrome (SARS) patients at acute and convalescent phases. Eur Psychiatr. 2005;20:236–42. doi: 10.1016/j.eurpsy.2004.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lien T-C, Sung C-S, Lee C-H, Kao H-K, Huang Y-C, Liu C-Y, Perng R-P, Wang J-H. Characteristic features and outcomes of severe acute respiratory syndrome found in severe acute respiratory syndrome intensive care unit patients. J Crit Care. 2008;23:557–64. doi: 10.1016/j.jcrc.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmad A, Krumkamp R, Reintje R. Controlling SARS: a review on China,s response compared with other SARS-affected countries. Trop Med Int Health. 2009;14(1):36–45. doi: 10.1111/j.1365-3156.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang W, Ruan S. Simulating the SARS outbreak in Beijing with limited data. J Theor Biol. 2004;227:369–79. doi: 10.1016/j.jtbi.2003.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Derrick JL, Gomersall CD. Protecting healthcare staff from severe acute respiratory syndrome: filtration capacity of multiple surgical masks. J Hosp Infect. 2005;59:365–68. doi: 10.1016/j.jhin.2004.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bryce E, Copes R, Gamage B, Lockhart K, Yassi A. Staff perception and institutional reporting: two views of infection control compliance in British Columbia and Ontario three years after an outbreak of severe acute respiratory syndrome. J Hosp Infect. 2008;69:169–76. doi: 10.1016/j.jhin.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsui WMS. Coronavirus is the cause of the severe acute respiratory syndrome (SARS) outbreak in Hong Kong and worldwide. Adv Anat Pathol. 2003;10(4):236. doi: 10.1097/00125480-200307000-00008. [DOI] [Google Scholar]
- 87.Tong TR. Severe acute respiratory syndrome coronavirus (SARS-CoV). Perspect Med Virol. 2006;16:43–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang H, Rao S, Jiang C. Molecular pathogenesis of severe acute respiratory syndrome. Microbes Infect. 2007;9:119–26. doi: 10.1016/j.micinf.2006.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong CKK, Lai V, Wong YC. Comparison of initial high resolution computed tomography features in viral pneumonia between metapneumovirus infection and severe acute respiratory syndrome. Eur J Radiol. 2012;81:1083–87. doi: 10.1016/j.ejrad.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poon LLM, Chan KH, Nicholls JM, Zheng BJ, Yuen KY, Guan Y, Peiris JSM. Characterization of a novel coronavirus responsible for severe acute respiratory syndrome. Int Congr Ser. 2004;1263:805–08. doi: 10.1016/j.ics.2004.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li G-M, Li Y-G, Yamate M, Li S-M, Ikuta K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. 2007;9:96–102. doi: 10.1016/j.micinf.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hafeez R, Aslam M, Aman S, Tahir M. Severe acute respiratory syndrome (SARS): a deadly disease. Ann King Edw Med Univ Pak. 2016;10(1). [Google Scholar]
- 93.Wang ZG, Xu SP, Zhang YJ. The possible origin of recent human SARS coronavirus isolate from China. Acta Virol. 2006;50:211–13. [PubMed] [Google Scholar]
- 94.Centers for disease control and prevention: updated interim US case definition for severe acute respiratory syndrome (SARS). URL: http://www.cdc.gov/ncidod/sars/casedefinition.htm
- 95.Woodhead M, Ewig S, Torres A. Severe acute respiratory syndrome (SARS). Eur Respir J. 2003;21:739–40. doi: 10.1183/09031936.03.00035403. [DOI] [PubMed] [Google Scholar]
- 96.Dolan SA. A new disease emerges: severe acute respiratory syndrome (SARS). J Spec Pediatr Nurs. 2003;8(2):75–76. doi: 10.1111/j.1744-6155.2003.tb00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wenzel RP, Bearman G, Edmond MB. Lessons from severe acute respiratory syndrome (SARS): implications for infection control. Arch Med Res. 2005;36:610–16. doi: 10.1016/j.arcmed.2005.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu C, Wang J, Wang L, Cao C. Spatial pattern of severe acute respiratory syndrome in-out flow in 2003 in mainland China. BMC Infect Dis. 2014. doi: 10.1186/s12879-014-0721-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parashar UD, Anderson LJ. Severe acute respiratory syndrome: review and lessons of the 2003 outbreak. Int J Epidemiol. 2004;33:628–34. doi: 10.1093/ije/dyh198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nie Q-H, Luo X-D, Hui W-L. Advances in clinical diagnosis and treatment of severe acute respiratory syndrome. World J Gastroenterol. 2003;9(6):1139–43. doi: 10.3748/wjg.v9.i6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fujii T, Nakamura T, Iwamoto A. Current concepts in SARS treatment. J Infect Chemother. 2004;10:1–7. doi: 10.1007/s10156-003-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheung TMT, Yam LYC, Lau ACW, Kong BMH, Yung RWH. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. CHEST. 2004;126:845–50. doi: 10.1378/chest.126.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wong HH, Fung TS, Fang S, Huang M, Le MT, Liu DX. Accessory proteins 8b and 8ab of severe acute respiratory syndrome coronavirus suppress the interferon signaling pathway by mediating ubiquitin-dependent rapid degradation of interferon regulatory factor 3. Virology. 2018;515:165–75. doi: 10.1016/j.virol.2017.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keyaerts E, Vijgen L, Maes P, Neyts J, Ranst MV. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264–68. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lau EHY, Cowling BJ, Muller MP, Ho L-M, Tsang T, LoS V, Louie M, Leung GM. Effectiveness of ribavirin and corticosteroids for severe acute respiratory syndrome. Am J Med. 2009;122(12). doi: 10.1016/j.amjmed.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peiris JSM. Severe acute respiratory syndrome (SARS). J Clin Virol. 2003;28(3):245–47. doi: 10.1016/j.jcv.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hui DSC, Chan PKS. Severe acute respiratory syndrome and coronavirus. Infect Dis Clin North Am. 2010;24(3):619–38. doi: 10.1016/j.idc.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ding Y-Q, He L, Zhang Q, Huang Z, Che X, Hou J-L, Wang H, Shen H, Qiu L, Li Z, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622–30. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu G, Yan S. Potential targets for anti-SARS drugs in the structural proteins from SARS related to coronavirus. Peptides. 2004;25(6):901–08. doi: 10.1016/j.peptides.2004.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu J-H, Zhang D-M, Wang G-L, Guo Z-M, Zhang C-H, Tan B-Y, Ouyang L-P, Lin L, Liu Y-M, Chen W-Q, et al. Variation analysis of the severe acute respiratory syndrome coronavirus putative non-structural protein 2 gene and construction of three-dimensional model. Chin Med J. 2005;118:707–13. [PubMed] [Google Scholar]
- 111.Rabenau HF, Kampf G, Cinatl J, Doerr HW. Efficacy of various disinfectants against SARS coronavirus. J Hosp Infect. 2005;61(2):107–11. doi: 10.1016/j.jhin.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cao C, Chen W, Zheng S, Zhao J, Wang J, Cao W. Analysus of spatiotemporal characteristics of pandemic SARS spread in mainland China. Biomed Res Int. 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Demmler GJ, Ligon BL. Severe acute respiratory syndrome (SARS): a review of the history, epidemiology, prevention and concerns for the future. Semin Pediatr Infect Dis. 2003;14(3):240–44. doi: 10.1016/S1045-1870(03)00056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maxwell C, McGeer A, Tai KFY, Sermer MN. 225-management guidelines for obstetric patients and neonates born to mothers with suspected or probable severe acute respiratory syndrome (SARS). J Obstet Gynaecol Can. 2017;39(8):e130–e137. doi: 10.1016/j.jogc.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yount B, Curtis KM, Fritz EA, Hensley LE, Jahrling PB, Prentice E, Denison MR, Geisbert TW, Baric RS. Reverse genetics with a full-length infectious cDNA of severe acute respiratory syndrome coronavirus. PNAS. 2003;100(22):12995–3000. doi: 10.1073/pnas.1735582100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170(4):1136–47. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Christian MD, Poutanen SM, Loutfy MR, Muller MP, Low DE. Severe acute respiratory syndrome. Clin Infect Dis. 2004;38:1420–27. doi: 10.1086/420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hijawi B, Abdallat M, Sayaydeh A, Alqasrawi S, Haddadin A, Jaarour N, et al. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J. 2013;19(Suppl 1):S12–8. doi: 10.26719/2013.19.supp1.S12. [DOI] [PubMed] [Google Scholar]
- 119.Chan JFW, Lau SKP, To KKW, Cheng VCC, Woo PCY, Yuen KY. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28(2):465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Al-Tawfiq JA, Memish ZA. Drivers of MERS-CoV transmission: what do we know? Expert Rev Respir Med. 2016;10:331–38. doi: 10.1586/17476348.2016.1150784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim Y, Lee S, Chu C, Choe S, Hong S, Shin Y. The characteristics of middle eastern respiratory syndrome coronavirus transmission dynamics in South Korea. Osong Public Health Res Perspect. 2016;7:49–55. doi: 10.1016/j.phrp.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jung S-Y, Kang KW, Lee E-Y, Seo D-W, Kim H-L, Kim H, Kwon TW, Park H-L, Kim H, Lee S-M, et al. Heterologous prime-boost vaccination with adenoviral vector and protein nanoparticles induces both Th1 and Th2 responses against Middle East respiratory syndrome coronavirus. Vaccine. 2018;36:3468–76. doi: 10.1016/j.vaccine.2018.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ko J-H, Kim S-H, Lee NY, Kim Y-J, Cho SY, Kang C-I, Chung DR, Peck KR. Effects of environmental disinfection on the isolation of vancomycin-resistant Enterococcus after a hospital-associated outbreak of Middle East respiratory syndrome. Am J Infect Control. 2019;47:1516–18. doi: 10.1016/j.ajic.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.De, Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RA, Galiano M, Gorbalenya AE, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–92. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 126.Eifan SA, Nour I, Hanif A, Zamzam AMM, Aljohani SM. A pandemic risk assessment of middle east respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia. Saudi J Biol Sci. 2017;24:1631–38. doi: 10.1016/j.sjbs.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Adegboye O, Saffary T, Adegboye M, Elfaki F. Individual and network characteristics associated with hospital-acquired Middle East Respiratory Syndrome coronavirus. J Infect Public Health. 2019;12:343–49. doi: 10.1016/j.jiph.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.World Health Organization . Middle East respiratory syndrome coronavirus (MERS-CoV): Thailand. Disease outbreak news. World Health Organization; 2015. http://www.who.int/csr/don/20-june-2015-mers-thailand/en/ [Google Scholar]
- 129.Cha MJ, Chung MJ, Kim K, Lee KS, Kim TJ, Kim TS. Clinical implication of radiographic scores in acute Middle East respiratory syndrome coronavirus pneumonia: report from a single tertiary-referral center of South Korea. Eur J Radiol. 2018;107:196–202. doi: 10.1016/j.ejrad.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yavarian J, Shafiei Jandaghi NZ, Naseri M, Hemmati P, Dadras M, Gouya MM, Azad TM. Influenza virus but not MERS coronavirus circulation in Iran, 2013–2016: comparison between pilgrims and general population. Travel Med Infect Dis. 2018;21:51–55. doi: 10.1016/j.tmaid.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Al-Tawfiq JA, Memish ZA. Emerging respiratory viral infections: MERS-CoV and influenza. Lancet Respir Med. 2014;2(1):23–25. doi: 10.1016/S2213-2600(13)70255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Almaghrabi RS, Omrani AS. Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Br J Hosp Med (Lond). 2017;78(1):23–26. doi: 10.12968/hmed.2017.78.1.23. [DOI] [PubMed] [Google Scholar]
- 133.Mailles A, Blanckaert K, Chaud P, van der Werf S, Lina B, Caro V, et al. First cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infections in France, investigations and implications for the prevention of human-to-human transmission, France, May 2013. Euro Surveill. 2013;18(24). [PubMed] [Google Scholar]
- 134.Choudhry H, Bakhrebah MA, Abdulaal WH, Zamzami MA, Baothman OA, Hassan MA, Zeyadi M, Helmi N, Alzahrani FA, Ali A, et al. Middle East respiratory syndrome: pathogenesis and therapeutic developments. Future Virol. 2019;14(4):237–46. doi: 10.2217/fvl-2018-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, Alabdullatid ZN, Assad M, Almulhim A, Makhdoom H, et al. Hospital outbreak or Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407–16. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Arabi YM, Arifi AA, Kalkhy HH, Najm H, Aldawwod AS, Ghabashi A, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–97. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 137.Al-Tawfiq JA, Zumla A, Memish ZA. Travel implications of emerging coronaviruses: SARS and MERS-CoV. Travel Med Infect Dis. 2014;12:422–28. doi: 10.1016/j.tmaid.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Noorwali AA, Turkistani AM, Asiri SI, Trabulsi FA, Alwafi OM, Alzahrani SH, et al. Descriptive epidemiology and characteristics of confirmed cases of Middle East respiratory syndrome coronavirus infection in the Makkah Region of Saudi Arabia. March to June 2014. Ann Saudi Med. 2015;35:203–09. doi: 10.5144/0256-4947.2015.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shalhoub S, Farahat F, Al-Jiffri A, Simhairi R, Shamma O, Siddiqi N, et al. IFN-α2a or IFN-β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. Antimicrob Chemother. 2015;70:2129–32. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Eng J Med. 2013;369:884–86. doi: 10.1056/NEJMc1308698. [DOI] [PubMed] [Google Scholar]
- 141.Memish ZA, Al-Tawfiq JA. Middle East respiratory syndrome coronavirus infection control: the missing piece? Am J Infect Control. 2014;42. doi: 10.1016/j.ajic.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Assiri A, Abedi GR, Saeed AAB, Abdalla MA, al-Masry M. Multifacility outbreak of Middle East respiratory syndrome in Tarif. Saudi Arabia Emerg Infect Dis. 2016;22:32–40. doi: 10.3201/eid2201.151370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hui DS, Perlman S, Zumla A. Spread of MERS to South Korea and China. Lancet Respir Med. 2015;3:509–10. doi: 10.1016/S2213-2600(15)00238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.De Wit E, Munster VJ. MERS-CoV: the intermediate host identified? Lancet Infect Dis. 2013;13:827–28. doi: 10.1016/S1473-3099(13)70193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Corman VM, Olschlager S, Wendtner C-M, Drexler JF, Hess M, Drosten C. Performance and clinical validation of the RealStar MERS-CoV kit for detection of Middle East respiratory syndrome coronavirus RNA. J Clin Virol. 2014;60:168–71. doi: 10.1016/j.jcv.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Munster VJ, Adney DR, van Doremalen N, Brown VR, Miazgowicz KL, Milne-Price S, Bushmaker T, Rosenke R, Scott D, Hawkinson A, et al. Replication and shedding of MERS-CoV in Jamaican fruit bats (Artibeus jamaicensis). Sci Rep. 2016;6:21878. doi: 10.1038/srep21878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meyerholz DK, Lambertz AM, McCray Jr PB. Dipeptidyl peptidase 4 distribution in the human respiratory tract. Am J Pathol. 2016;186(1):78–86. doi: 10.1016/j.ajpath.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.World Health Organization . Middle East respiratory syndrome coronavirus [MERS-CoV]; 2016. (http://www,who.int/emergencies/mers-cov/en/)
- 149.Alagaili AN, Briese T, Amor NMS, Mohammed OB, Lipkin WI. Waterpipe smoking as a public health risk: potential risk for transmission of MERS-CoV. Saudi J Biol Sci. 2019;26:938–41. doi: 10.1016/j.sjbs.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Poissy J, Goffard A, Parmentier-Decruq E, Favory R, Kauv M, Kipnis E, Mathieu D, Guery B. The MERS-CoV Biology Group. 2014. Kinetics and pattern of viral excretion in biological specimens of two MERS-CoV cases. J Clin Virol. 2014;61:275–78. doi: 10.1016/j.jcv.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Faridi U. Middle East respiratory syndrome coronavirus (MERS-CoV): impact on Saudi Arabia, 2015. Saudi J Biol Sci. 2018;25:1402–05. doi: 10.1016/j.sjbs.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Amer H, Alqahtani AS, Alaklobi F, Altayeb J, Memish ZA. Healthcare worker exposure to Middle East respiratory syndrome coronavirus (MERS-CoV): revision of screening strategies urgently needed. Int J Infect Dis. 2018;71:113–16. doi: 10.1016/j.ijid.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Park SY, Lee JS, Son JS, Ko JH, Peck KR, Jung Y, Woo HJ, Joo YS, Eom JS, Shi H. Post-exposure prophylaxis for Middle East respiratory syndrome in healthcare workers. J Hosp Infect. 2019;101:42–46. doi: 10.1016/j.jhin.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chowell G, Blumberg S, Simonsen L, Miller MA, Viboud C. Synthesizing data and models for the spread of MERS-CoV, 2013; key role of index cases and hospital transmission. Epidemics. 2014;9:40–51. doi: 10.1016/j.epidem.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cowling BJ, Park M, Fang VJ, Wu P, Leung GM, Wu JT. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Eurosurveillance. 2015;20(25). doi: 10.2807/1560-7917.ES2015.20.25.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kucharski AJ, Althaus CL. The role of superspreading in Middle East respiratory syndrome coronavirus (MERS-CoV) transmission. Eurosurveillance. 2015;20(25). doi: 10.2807/1560-7917.ES2015.20.25.21167. [DOI] [PubMed] [Google Scholar]
- 157.Al-Jasser FS, Nouh RM, Youssef RM. Epidemiology and predictors of survival of MERS-CoV infections in Riyadh region, 2014–2015. J Infect Public Health. 2019;12:171–77. doi: 10.1016/j.jiph.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Poletto C, Gomes MF, Pastore Y, Piontti A, Rossi L, Bioglio L, Chao DL, Longini IM, Halloran ME, Colizza A, et al. Assessing the impact of travel restrictions on international spread of the 2014 West African Ebola epidemic. Euro Surveill. 2014;19(42). doi: 10.2807/1560-7917.ES2014.19.42.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bogoch II, Creatore MI, Cetron MS, Brownstein JS, Pesik N, Miniota J, Tam T, Hu W, Nicolucci A, Ahmed S, et al. Assessment of the potential for international dissemination of Ebola virus via commercial air travel during the 2014 west African outbreak. Lancet. 2015;385(9962):29–35. doi: 10.1016/S0140-6736(14)61828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang N, Shi X, Jiang L, Zhang S, Wang D, Tong P, et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23(8):986–93. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zumla A, Chan JFW, Azhar EI, Hui DSC, Yuen KY. Coronaviruses-drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–47. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wernery U, Lau SKP, Woo PCY. Middle East respiratory syndrome (MERS) coronavirus and dromedaries. Vet J. 2017;220:75–79. doi: 10.1016/j.tvjl.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Haverkamp A-K, Bosch BJ, Spitzbarth I, Lehmbecker A, Te N, Bensaid A, Segales J, Baumgartner W. Detection of MERS-CoV antigen on formalin-fixed paraffin-embedded nasal tissue of alpacas by immunohistochemistry using human monoclonal antibodies directed against different epitopes of the spike protein. Vet Immunol Immunopathol. 2019;218:109939. doi: 10.1016/j.vetimm.2019.109939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lu G, Hu Y, Wang Q, Qi J, Gao F, Li Y, Zhang Y, Zhang W, Yuan Y, Bao J, et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–31. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Inn K-S, Kim Y, Aigerim A, Park U, Hwang E-S, Choi M-S, Kim Y-S, Cho N-H. Reduction of soluble dipeptidyl peptidase 4 levels in plasma of patients infected with Middle East respiratory syndrome coronavirus. Virology. 2018;518:324–27. doi: 10.1016/j.virol.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li W, Hulswit RJG, Widjaja I, Raj VS, McBride R, Peng W, Widagdo W, Tortotici MA, van Dieren B, Lang Y, et al. Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc Natl Acad Sci U S A. 2017;114:E8508–E8517. doi: 10.1073/pnas.1712592114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Du L, Yang Y, Zhou Y, Lu L, Li F, Jiang S. MERS-CoV spike protein: a key target for antivirals. Expert Opin Ther Targets. 2017;21:131–43. doi: 10.1080/14728222.2017.1271415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang Q, Wong G, Lu G, Yan J, Gao GF. MERS-CoV spike protein: targets for vaccines and therapeutics. Antiviral Res. 2016;133:165–77. doi: 10.1016/j.antiviral.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Du LY, He YX, Zhou YS, Liu SW, Zheng BJ, Jiang SB. The spike protein of SARS-CoV- a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226–36. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang N, Jiang S, Du L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert Rev Vaccines. 2014;13:761–74. doi: 10.1586/14760584.2014.912134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Papaneri AB, Johnson RF, Wada J, Bollinger L, Jahrling PB, Kuhn JH. Middle East respiratory syndrome: obstacles and prospects for vaccine development. Expert Rev Vaccines. 2015;14:949–62. doi: 10.1586/14760584.2015.1036033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Raj VS, Mou H, Smits SL, Dekkers DHW, Muller MA, Dijkman R, Muth D, Demmers JAA, Zaki A, Fouchier RAM, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–54. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pallesen J, Wang N, Corbett KS, Warpp D, Kirchdoerfer RN, Turner HL, Cottrell CA, Becker MM, Wang L, Shi W, et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci U S A. 2017;114:E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Meyer B, Muller MA, Corman VM, Reusken CB, Ritz D, Godeke GJ, Lattwein E, Kallies S, Siemens A, van Beek J, et al. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis. 2014;20(4):552–59. doi: 10.3201/eid2004.131746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Muller MA, Meyer B, Corman VM, Al-Masri M, Turkestani A, Ritz D, Sieberg A, Aldabbagh S, Bosch BJ, Lattwein E, et al. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015;15(5):559–64. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Song F, Fux R, Provacia LB, Volz A, Eickmann M, Becker S, et al. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccine virus Ankara efficiently induces virus-neutralizing antibodies. J Virol. 2013;87:11950–54. doi: 10.1128/JVI.01672-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim E, Okada K, Kenniston T, Raj VS, AlHajri MN, Farag EA, et al. Immunogenicity of an adenoviral-based Middle East respiratory syndrome coronavirus vaccine in BALB/c mice. Vaccine. 2014;32:5975–82. doi: 10.1016/j.vaccine.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guo X, Deng Y, Chen H, Lan J, Wang W, Zou X, et al. Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology. 2015;145:476–84. doi: 10.1111/imm.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Volz A, Kupke A, Song F, Jany S, Fux R, Shams-Eldin H, et al. Protective efficacy of recombinant modified vaccinia virus Ankara delivering Middle East respiratory syndrome coronavirus spike glycoprotein. J Virol. 2015;89(16):8651–56. doi: 10.1128/JVI.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Alharbi NK, Padron-Regalado E, Thompson CP, Kupke A, Wells D, Sloan MA, et al. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralizing antibodies and cellular immune responses in mice. Vaccine. 2017;35:3780–88. doi: 10.1016/j.vaccine.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ma C, Li Y, Wang L, Zhao G, Tao X, Tseng CT, et al. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: implication for designing novel mucosal MERS vaccines. Vaccine. 2014;32(18):2100–08. doi: 10.1016/j.vaccine.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tang J, Zhang N, Tao X, Zhao G, Guo Y, Tseng CT, et al. Optimization of antigen dose for a receptor-binding domain-based subunit vaccine against MERS coronavirus. Hum Vaccin Immunother. 2015;11:1244–50. doi: 10.1080/21645515.2015.1021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Coleman CM, Venkataraman T, Liu YV, Glenn GM, Smith GE, Flyer DC, et al. MERS-CoV spike nanoparticles protect mice from MERS-CoV infection. Vaccine. 2017;35(12):1586–89. doi: 10.1016/j.vaccine.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang L, Shi W, Joyce MG, Modjarrad K, Zhang Y, Leung K, et al. Evaluation of candidate vaccine approaches for MERS-CoV. Nat Commun. 2015;6:7712. doi: 10.1038/ncomms8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Muthumani K, Falzarano D, Reuschel EL, Tingey C, Flingai S, Villarreal DO, et al. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med. 2015;7(301):301ra132. doi: 10.1126/scitranslmed.aac7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Al-Tawfiq JA, Pear TM. Middle East respiratory syndrome coronavirus in healthcare settings. Curr Opin Infect Dis. 2015;28:392–96. doi: 10.1097/QCO.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 187.Ahmadzadeh J, Mobaraki K, Mousavi SJ, Aghazadeh-Attari J, Mirza-Aghazadeh-Attari M, Mohebbi I. The risk factors associated with MERS-CoV patient fatality: a global survey. Diagn Microbiol Infect Dis. 2020;96:114876. doi: 10.1016/j.diagmicrobio.2019.114876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nah K, Otsuki S, Chowell G, Nishiura H. Predicting the international spread of Middle East respiratory syndrome (MERS). BMC Infect Dis. 2016;16:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang Y, Sun J, Zhu A, Zhao J, Zhao J. Current understanding of middle east respiratory syndrome coronavirus infection in human and animal models. J Thorac Dis. 2018;10:S2260–S2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Al-Omari A, Rabaan AA, Salih S, Al-Tawfiq JA, Memish ZA. MERS coronavirus outbreak: implications for emerging viral infections. Diagn Microbiol Infect Dis. 2019;93:265–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Masters PS. Coronavirus genomic RNA packaging. Virology. 2019;537:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kasem S, Qasim I, Al-Hufofi A, Hashim O, Alkarar A, Abu-Obeida A, Gaafer A, Elfadil A, Zaki A, Al-Romaihi A, et al. Cross-sectional study of MERS-CoV-specific RNA and antibodies in animals that have had contact with MERS patients in Saudi Arabia. J Infect Public Health. 2018;11:331–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dighe A, Jombart T, Van Kerkhove MD, Ferguson N. A systematic review of MERS-CoV seroprevalence and RNA prevalence in dromedary camels: implications for animal vaccination. Epidemics. 2019;29:100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Al-Tawfiq JA, Rabaan AA, Hinedi K. Influenza is more common than Middle East respiratory syndrome coronavirus (MERS-CoV) among hospitalized adult Saudi patients. Travel Med Infect Dis. 2017;20:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Alfaraj SH, Al-Tawfiq JA, Assiri AY, Alzahrani NA, Alanazi AA, Memish ZA. Clinical predictors of mortality of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: a cohort study. Travel Med Infect Dis. 2019;29:48–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Corman VM, Ithete NL, Richards LR, Schoeman MC, Presier W, Drosten C, Drexler JF. Rooting the phylogenetic tree o Middle East respiratory syndrome coronavirus by characterization of a conspecific virus from and African bat. J Virol. 2014;88:11297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yoon JH, Lee JY, Lee J, Shin YS, Jeon S, Kim DE, Min JS, Song JH, Kim S, Kwon S, et al. Synthesis and biological evaluation of 3-acyl-2-phenylamino-1,4-dihydroquinolin-4(1H)-one derivatives as potential MERS-CoV inhibitors. Bioorg Med Chem Lett. 2019;29:126727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Qiu H, Sun S, Xiao H, Feng J, Guo Y, Tai WY, Du L, Zhao G, Zhou Y. Single-dose treatment with a humanized neutralizing antibody affords full protection of a human transgenic mouse model from lethal Middle East respiratory syndrome (MERS)-coronavirus infection. Antiviral Res. 2016;132:141–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Park HY, Lee EJ, Ryu YA, Kim Y, Kim H, Lee H, et al. Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea, May to June 2015. Eurosurveillance. 2015;20(June25):21169. [DOI] [PubMed] [Google Scholar]
- 200.Ahmed AE. Diagnostic delays 537 symptomatic cases of Middle East respiratory syndrome coronavirus infection in Saudi Arabia. Int J Infect Dis. 2017;62:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ahmed AE. Diagnostic delays in Middle East respiratory syndrome coronavirus patients and health systems. J Infect Public Health. 2019;12:767–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lee A, Cho J. The impact of city epidemics on rural labor market: the Korean Middle East Respiratory Syndrome case. Japan World Econ. 2017;43:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tang B, Bragazzi NL, Li Q, Tang S, Xiao Y, Wu J. An updated estimating of the risk of transmission of the novel coronavirus (2019-nCov). Infect Dis Model. 2020;5:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Alqahtani AS, Rashid H, Basyouni MH, Alhawassi TM, BinDhim NF. Public response to MERS-CoV in the Middle East: iPhone survey in six countries. J Infect Public Health. 2017;10:534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Almutairi KM, Al Helih EM, Moussa M, Boshaigah AE, Saleh Alajilan A, Vinluan JM, et al. Awareness, attitude, and practices related to coronavirus pandemic among public in Saudi Arabia. Farm Community Health. 2015;38(4):332–40. [DOI] [PubMed] [Google Scholar]
- 207.Kharma MY, Alalwani MS, Amer MF, Tarakji B, Aws G. Assessing of the awareness level of dental students toward Middle East Respiratory Syndrome-coronavirus. J Int Soc Prev Community Dent. 2015;5:163–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Al-Mohrej OA, Al-Shirian SD, Al-Otaibi SK, Tamim HM, Masuadi EM, Fakhoury HM. Is the Saudi public aware of Middle East respiratory syndrome? J Infect Public Health. 2016;9:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bawazir A, Al-Mazroo E, Jradi H, Ahmed A, Badir M. MERS-CoV infection: mind the public knowledge gap. J Infect Public Health. 2018;11:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Al-Tawfiq JA. Middle East Respiratory Syndrome-coronavirus infection: an overview. J Infect Public Health. 2013;6:319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hashem AM, Al-Amri SS, Al-Subhi TL, Siddiq LA, Hassan AM, Alawi MM, Alhabbab RY, Hindawi SI, Mohammed OB, Amor NS, et al. Development and validation of different indirect ELISAs for MERS-CoV serological testing. J Immunol Methods. 2019;466:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Al-Tawfiq JA, Auwaerter PG. Healthcare-associated infections: the hallmark of Middle East respiratory syndrome coronavirus with review of the literature. J Hosp Infect. 2019;101:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kim Y. Nurses, experiences of care for patients with Middle East respiratory syndrome-coronavirus in South Korea. Am J Infect Control. 2018;46:781–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Al-Tawfiq JA, Abdrabalnabi R, Taher A, Mathew S, Rahman KA. Infection control influence of Middle East respiratory syndrome coronavirus: a hospital-based analysis. Am J Infect Control. 2019;47:431–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Douglas MG, Kocher JF, Scobey T, Baric RS, Cockrell AS. Adaptive evolution influences the infectious dose of MERS-CoV necessary to achieve severe respiratory disease. Virology. 2018;517:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nikiforuk AM, Leung A, Cook BWM, Court DA, Kobasa D, Theriault SS. Rapid one-step construction of a Middle East Respiratory Syndrome (MERS-CoV) infectious clone system by homologous recombination. J Virol Methods. 2016;236:178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ebihara H, Groseth A, Neumann G, Kawaoka Y, Feldmann H. The role of reverse genetics system in studying viral hemorrhagic fevers. Thromb Haemost. 2005;94:240–53. [DOI] [PubMed] [Google Scholar]
- 218.Letko M, Miazgowicz K, McMinn R, Seifert SN, Sola I, Enjuanes L, Carmody A, van Doremalen N, Munster V. Adaptive evolution of MERS-CoV to species variation in DPP4. Cell Rep. 2018;24:1730–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang S, Zhou P, Wang P, Li Y, Jiang L, Jia W, Wang H, Fan A, Wang D, Shi X, et al. Structural definition of a unique neutralization epitope on the receptor-binding domain of MERS-CoV spike glycoprotein. Cell Rep. 2018;24:441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mustafa S, Balkhy H, Gabere MN. Current treatment options and the role of peptides as potential therapeutic components for Middle East Respiratory Syndrome (MERS): a review. J Infect Public Health. 2018;11:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Baharoon S, Memish ZA. MERS-CoV as an emerging respiratory illness: a review of prevention methods. Travel Med Infect Dis. 2019;32:101520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Widagdo W, Okba NMA, Raj VS, Haagmans BL. MERS-coronavirus: from discovery to intervention. One Health. 2017;3:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu H, Wang -L-L, Zhao S-J, Kwak-Kim J, Mor G, Liao A-H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Reprod Immunol. 2020;139:103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Banik GR, Khandaker G, Rashid H. Middle East Respiratory Syndrome Coronavirus “MERS-CoV”: current knowledge gaps. Paediatr Respir Rev. 2015;16:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cunha CB, Opal SM. Middle East respiratory syndrome (MERS). Virulence. 2014;5:650–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hui DS, Azhar E, Kim Y-J, Memish ZA, Oh M-D, Zumla A. Middle East respiratory syndrome coronavirus: risj factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18:e217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Arabi Y, Balkhy H, Hajeer AH, et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rabaan AA, Alahmed SH, Bazzi AM, Alhani HM. A review of candidate therapies for Middle East respiratory syndrome from a molecular perspective. J Med Microbiol. 2017;66:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sheahan TP, Sims AC, Leist SR, Schafer A, Won J, Brown AJ, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Agostini ML, Pruijssers AJ, Chappell JD, et al. Small-molecule antiviral beta-d-n4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J Virol. 2019;93:e01348–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Behzadi MA, Leyva-Grado VH. Overview of current therapeutics and novel candidates against influenza, respiratory syncytial virus, and Middle East respiratory syndrome coronavirus infections. Front Microiol. 2019;10:1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Beigel JH, Nam HH, Adams PL, et al. Advances in respiratory virus therapeutics- a meeting report from the 6th ISIRV antiviral group conference. Antiviral Res. 2019;167:45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Momattin H, Al-Ali AY, Al-Tawfiq JA. A systematic review of therapeutic agents for the treatment of the Middle East respiratory syndrome coronavirus (MERS-CoV). Travel Med Infect Dis. 2019;30:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhou Y, Yang Y, Huang J, Jiang S, Du L. Advances in MERS-CoV vaccines and therapeutics based on the receptor-binding domain. Viruses. 2019;11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Memish ZA, Perlman S, van Kerkove MD, Zumla A. Middle East respiratory syndrome. Lancet. 2020;395:1063–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Perlman S, Vijay R. Middle East respiratory syndrome vaccines. Middle East respiratory syndrome vaccines. Int J Infect Dis. 2016;47:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dyall J, Cross R, Kindrachuk J, Johnson RF, Olinger GG, Hensley LE, Frieman MB, Jahrling PB. Middle East respiratory syndrome and severe acute respiratory syndrome: current therapeutic options and potential targets for novel therapies. Drugs. 2017;77:1935–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tai DYH. Pharmacologic treatment of SARS: current knowledge and recommendations. Ann Acad Med. 2007;36:438–43. [PubMed] [Google Scholar]
- 239.Bal A, Destras G, Gaymard A, Bouscambert-Duchamp M, Valette M, Escuret V, Frobert E, Billaud G, Trouillet-Assant S, Cheynet V, et al. Molecular characterization of SARS-CoV-2 in the first COVID-19 cluster in France reveals an amino-acid deletion in nsp2 (Asp268Del). Clin Microbiol Infect. 2020. http://doi.10.1016/j.cmi.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Abduljali JM, Abduljali BM. Epidemiology, genome and clinical features of the pandemic SARS-CoV-2: a recent view. New Microbes New Infect. 2020. http://doi.10.1016/j.nmni.2020.100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Benvenuto D, Giovanetti M, Vassallo L, Angeletti S, Ciccozzi M. Application of the ARIMA model on the COVID-2019 epidemic dataset. Data Brief. 2020;29:105340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hsih W-H, Cheng M-Y, Ho M-W, Chou C-H, Lin P-C, Chi C-Y, Liao W-C, Chen C-Y, Leong L-Y, Tien N, et al. Featuring COVID-19 cases via screening symptomatic patients with epidemiologic link during flu season in a medical center of central Taiwan. J Microbiol Immunol Infect. 2020. http://doi.10.1016/j.jmii.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lai -C-C, Liu YH, Wang C-Y, Wang Y-H, Hsueh S-C, Yen M-Y, Ko W-C, Hsueh P-R. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronaviurs 2(SARS-CoV-2): fact and myths. J Microbiol Immunol Infect. 2020. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Robson B. Computers and viral disease. Preliminary bioinformatics studies on the design of a synthetic vaccine and a preventive peptidomimetic antagonist against the SARS-CoV-2 (2019-nCoV, COVID-19) coronavirus. Comput Biol Med. 2020;119:103670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Roda WC, Varughese MB, Han D, Li MY. Why is it difficult to accurately predict the COVID-19 epidemic? Infect Dis Model. 2020;5:271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Li C, Yang Y, Ren L. Genetic evolution analysis of 2019 novel coronavirus and coronavirus from other species. Infect Genet Evol. 2020;82:104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020;525:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cheng S-C, Chang Y-C, Chiang Y-LF, Chien Y-C, Cheng M, Yang C-H, Huang C-H, Hsu Y-N. First case of coronavirus disease 2019 (COVID-19) pneumonia in Taiwan. J Formos Med Assoc. 2020;119:747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Yu Jun IS, Anderson DE, Zheng Kang AE, Wang L-F, Rao P, Young BE, Lye DC, Agrawal R. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020. doi: 10.1016/j.ophtha.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gralinski LE, Menachery VD. Return of the Coronavirus: 2019-nCoV. Viruses. 2020;12:135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lupia T, Scabini S, Pinna SM, Di Perri G, De Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cyranoski D. Mystery deepens over animal source of coronavirus. Nature. 2020;579:18–19. doi: 10.1038/d41586-020-00548-w. [DOI] [PubMed] [Google Scholar]
- 255.Ji W, Wang W, Zhao X, Zai J, Li X. Cross species transmission of the newly identified coronavirus 2019-nCoV-Ji-2020. J Med Virol. 2020;92:433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clinica Chimica Acta. 2020;505:190–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lu D, Wang H, Yu R, Yang H, Zhao Y. Integrated infection control strategy to minimize nosocomial infection of coronavirus disease 2019 among ENT healthcare workers. J Hosp Infect. 2020. doi: 10.1016/j.jhin.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan. Plos One. 2020;15(3):e0230548. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sun K, Chen J, Viboud C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population-level observational study. Lancet Digital Health. 2020;2:e201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kang S, Peng W, Zhu Y, Lu S, Zhou M, Lin W, Wu W, Huang S, Jiang L, Luo X, et al. Recent progress in understanding 2019 novel coronavirus associated with human respiratory disease: detection, mechanism and treatment. Int J Antimicrob Agents. 2020. doi: 10.1016/j.ijantimicag.2020.105950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Tseng C-T, Sbrana E, Iwata-Yoshikawa N, Newman PC, Garron T, Atmar RL, Peters CJ, Couch RB. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with SARS virus. PLoS ONE. 2012;7:e35421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yuan Y, Cao D, Zhang Y, Ma J, Qi J, Wang Q, et al. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat Commun. 2017;8:15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furinlike cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yu F, Du L, Ojcius DM, Pan C, Jiang S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 2020;22:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Matsuyama S, Nao N, Shirato K, Kawase M, Saito S, Takayama I, Nagata N, Sekizuka T, Katoh H, Kato F, et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A. 2020. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians needs to know. Am J Obstet Gynecol. 2020. doi: 10.1016/j,ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, Xing F, Liu J, Yip CC-Y, Poon RW-S, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Khan S, Nabi G, Han G, Siddique R, Lian S, Shi H, Bashir N, Ali A, Adnan Shereen M. Novel coronavirus: how things are in Wuhan. Clin Microbiol Infect. 2020. doi: 10.1016/j.cmi.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Liu K-C, Xu P, Lv W-F, Qiu X-H, Yao J-L, Gu J-F, Wei W. CT manifestations of coronavirus disease-2019: a retrospective analysis of 73 cases by disease severity. Eur J Radiol. 2020;126:108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Han Q, Lin Q, Jin S, You L. Coronavirus 2019-nCoV: a brief perspective from the front line. J Infect. 2020;80:373–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sigrist CJA, Bridge A, Le Mercier P. A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Res. 2020;177:104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for the virus origins and receptor binding. Lancet. 2020;395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Luan J, Lu Y, Jin X, Zhang L. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem Biophys Res Commun. 2020. doi: 10.1016/j.bbrc.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wall AC, Park Y-J, Tortoici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, Chen H, Wang D, Liu N, Liu D, et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80:401–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.El-Zowalaty ME, Jarhult JD. From SARS to COVID-19: a previously unknown SARS-related coronavirus (SARS-CoV-2) of pandemic potential infecting humans- Call for a One Health approach. One Health. 2020;9:100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Jiang X, Deng L, Zhu Y, Ji H, Tao L, Liu L, Yang D, Ji W. Psychological crisis intervention during the outbreak period of new coronavirus pneumonia from experience in Shanghai. Psychiatry Res. 2020;286:112903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Du H-Z, Hou X-Y, Miao Y-H, Huang D-S, Liu D-H. Traditional Chinese medicine: an effective treatment for 2019 novel coronavirus pneumonia (NCP). Chin J Nat Med. 2020;18:206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Long C, Xu H, Shen Q, Zhang X, Fan B, Wang C, Zeng B, Li Z, Li X, Li H. Diagnosis of the coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126:108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Rello J, Tejada S, Userovici C, Arvaniti K, Pugin J, Waterer G. Coronavirus Disease 2019 (COVID-19): a critical care perspective beyond China. Anaesth Crit Care Pain Med. 2020. doi: 10.1016/j.accpm.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Rubin EJ, Baden LR, Morrissey S, Campion EW. Campion, Medical Journals and the 2019-nCoV Outbreak. N Engl J Med. 2020. [DOI] [PubMed] [Google Scholar]
- 285.Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JI-P, Gutierrez RA, Gwee SXW, Chua PEY, Yang Q, et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Lin Q, Zhao S, Gao D, Lou Y, Yang S, Musa SS, Wang MH, Cai Y, Wang W, Yang L, et al. A conceptual model for the coronavirus disease 2019 (COVID-19) outbreak in Wuhan, China with individual reaction and governmental action. Int J Infect Dis. 2020;93:211–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Prem K, Liu Y, Russell TW, Kucharski AJ, Eggo RM, Davies N, et al. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modeling study. Lancet Public Health. 2020. doi: 10.1016/S2468-2667(20)30073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Sun Z, Thilakavathy K, Kumar SS, He G, Liu SV. Potential factors influencing repated SARS outbreaks in China. Int J Environ Res Public Health. 2020;17:1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kobayashi T, Jung S-M, Linton NM, Kinoshita R, Hayashi K, Miyama T, Anzai A, Yang Y, Yuan B, Akhmetzhanov AR, et al. Communicating the risk of death from novel coronavirus disease (COVID-19). J Clin Med. 2020;9:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Grubaugh ND, Petrone ME, Holmes EC. We should not worry when a virus mutates during disease outbreaks. Nat Microbiol. 2020. doi: 10.1038/s41564-020-0690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zhang Y-Z, Holmes EC. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Zhao S, Ling K, Yan H, Zhong L, Peng X, Yao S, Huang J, Chen X. Anesthetic management of patients with COVID 19, infections during emergency procedures. J Cardiothorac Vasc Anesth. 2020;34:1125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clinica Chimica Acta. 2020;506:145–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lv D-F, Ying Q-M, Weng Y-S, Shen C-B, Chu J-G, Kong J-P, Sun D-H, Gao X, Weng X-B, Chen X-Q. Dynamic change process of target genes by RT-PCR testing of SARS-CoV-2 during the course of a Coronavirus Disease 2019 patient. Clinica Chimica Acta. 2020;506:172–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Xie C, Jiang L, Huang G, Pu H, Gong B, Lin H, Ma S, Chen X, Long B, Si G, et al. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int J Infect Dis. 2020;93:264–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Guo H, Zhou Y, Liu X, Tan J. The impact of the COVID-19 epidemic on the utilization of emergency dental services. J Dent Sci. 2020. doi: 10.1016/j.jds.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9:eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wang K, Kang S, Tian R, Zhang X, Zhang X, Wang Y. Imaging manifestations and diagnostic value of chest CT of coronavirus disease 2019 (COVID-19) in the Xiaogan area. Clin Radiol. 2020. doi: 10.1016/j.crad.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Shen M, Zhou Y, Ye J, Al-maskri AAA, Kang Y, Zeng S, Cai S. Recent advances and perspectives of nucleic acid detection for coronavirus. J Pharm Anal. 2020. doi: 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Ghinai I, McPherson TD, Hunter JC, Kirking HL, Christiansen D, Joshi K, Rubin R, Morales-Estrada S, Black SR, Pacilli M, et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Bai L, Yang D, Wang X, Tong L, Zhu X, Zhong N, Bai C, et al. Chinese experts, consensus on the Internet of Things-aided diagnosis and treatment of coronavirus disease 2019 (COVID-19). Clin eHealth. 2020;3:7–15. [Google Scholar]
- 306.Li L, Yang Z, Dang Z, Meng C, Huang J, Meng H, Wang D, Chen G, Zhang J, Peng H, et al. Propagation analysis and prediction of the COVID-19. Infect Dis Model. 2020;5:282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Skariyachan S, Challapilli SB, Packirisamy S, Kumargowda ST, Sridhar VS. Recent aspects on the pathogenesis mechanism, animal models and novel therapeutic interventions for Middle East respiratory syndrome coronavirus infections. Front Microbiol. 2019;10:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zhao J, Li K, Wohlford-Lenane C, et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci USA. 2014;111:4970–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Agrawal AS, Tao X, Algaissi A, et al. Immunization with inactivated Middle East respiratory syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Human Vaccine Immunother. 2016;12:2351–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Spruth M, et al. A double-inactivated whole virus candidate SARS coronavirus vaccine stimulate neutralizing and protective anibody responses. Vaccine. 2006;24:652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Bolles M, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon. J Virol. 2011;85:12201–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Hilgenfeld R, Peiris M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antivir Res. 2013;100:286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zuma A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Liu R, Wang J, Shao Y, Wang X, Zhang H, Shuai L, Ge J, Wen Z, Bu Z. A recombinant VSV-vectored MERS-CoV vaccine induces neutralizing antibody and T cell responses in rhesus monkeys after single dose immunization. Antiviral Res. 2018;150:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Nyon MP, Du L, Tseng C-TK, Seid CA, Pollet J, Naceanceno KS, Agrawal A, Algaissi A, Peng B-H, Tai W, et al. Engineering a stable CHO cell line for the expression of a MERS-coronavirus vaccine antigen. Vaccine. 2018;36:1853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Ma C, Wang L, Tao X, Zhang N, Yang Y, Tseng C-TK, Li F, Zhou Y, Jiang S, Du L. Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments- The importance of immunofocusing in subunit vaccine design. Vaccine. 2014;32:6170–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ma C, Li Y, Wang L, Zhao G, Tao X, Tseng C-TK, Zhou Y, Du L, Jiang S. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: implication of designing novel mucosal MERS vaccines. Vaccine. 2014;32:2100–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Wang C, Zheng X, Gai W, Wong G, Wang H, Jin H, Feng N, Zhao Y, Zhang W, Li N, et al. Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice. Antivir Res. 2017;140:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Okada M, Okuno Y, Hashimoto S, Kita T, Kanamaru N, et al. Development of vaccines and passive immunotherapy against SARS corona virus using SCID-PBL/hu mouse models. Vaccine. 2007;25:3038–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Huang J, Ma R, Wu C-Y. Immunization with SARS-CoV S DNA vaccine generates memory CD4+ and CD8+ T cell immune responses. Vaccine. 2006;24:4905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.He Y, Zhou Y, Siddiqui P, Jiang S. Inactivated SARS-CoV vaccine elicits high titers of spike protein-specific antibodies that block receptor binding and virus entry. Biochem Biophys Res Commun. 2004;325:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Kuate S, Cinatl J, Doerr HW, Uberla K. Exosomal vaccines containing the S protin of the SARS coronavirus induce high levels of neutralizing antibodies. Virology. 2007;362:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wang X, Xu W, Tong D, Ni J, Gao H, Wang Y, Chu Y, Li P, Yang X, Xiong S. A chimeric multi-epitope DNA vaccine elicited specific antibody response against sevre acute respiratory syndrome-associated coronavirus which attenuated the virulence of SARS-CoV in vitro. Immunol Lett. 2008;119:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Xiong S, Wang Y-F, Zhang M-Y, Liu X-J, Zhang C-H, Liu -S-S, Qian C-W, Li J-X, Lu J-H, Wan Z-Y, et al. Immunogenicity of SARS inactivated vaccine in BALB/c mice. Immunol Lett. 2004;95:139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhou Z, Post P, Chubet R, Holtz K, McPherson C, Petric M, Cox M. A recombinant baculovirus-expressed S glycoprotein vaccine elicits high titers of SARS-associated coronavirus (SARS-CoV) neutralizing antibodies in mice. Vaccine. 2006;24:3624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Zakhartchouk AN, Liu Q, Petric M, Babiuk LA. Augmentation of immune responses to SARS coronavirus by a combination of DNA and whole killed virus vaccines. Vaccine. 2005;23:4385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Kapadia SU, Simon ID, Rose JK. SARS vaccine based on a replication-defective recombinant vesicular stomatitis virus is more potent than one based on a replication-competent vector. Virology. 2008;376:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Herst CV, Burkholz S, Sidney J, Sette A, Harris PE, et al. An effective CTL peptide vaccine for Eolca Zaire based on the survivors, CD8+ targeting of a particular nucleocapsid protein epitope with potential implications for COVID-19 vaccine design. Vaccine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2010;19:102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ji H, Yan Y, Ding B, Guo W, Brunswick M, Niethammer A, SooHoo W, Smith R, Nahama A, Zhang Y. Novel decoy cellular vaccine strategy utilizing transgenic antigen-expressin cells as immune presenter and adjuvant in vaccine prototype against SARS-CoV-2 virus. Med Drug Discov. 2020;5:100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Amanat F, Krammer R. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Taylor A, et al. Fc receptors in antibody-dependent enhancement of viral infection. Immunol Rev. 2015;268:340–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Smatti MK, et al. Viral-induced enhanced disease illness. Front Microb. 2018;9:2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wan Y, et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol. 2020;94:e02015–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Qin C, et al. Dysregulation of the immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ricke D, Malone R. Medical countermeasures analysis of 2019-nCoV and vaccine risks for antibody-dependent enhancement (ADE). 2020.
- 337.Quinlan BD, et al. The SARS-CoV-2 receptor-binding elicits a potent neutralizing response without antibody-dependent enhancement. Immunity. 2020. [Google Scholar]
- 338.Zhang B, et al. Immune phenotyping based on neutrophil-to-lymphocyte ration and lgC predicts severity and outcome for patients with COVID-19. medRxiv 2020. [DOI] [PMC free article] [PubMed]
- 339.Zhao J, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Du L, et al. Receptor-binding domain of SARS-CoV spike protein induces long-term protective immunity in an animal model. Vaccine. 2007;25:2832–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Chen WH, et al. Optimization of the production process and characterization of the yeast-expressed SARS-CoV recombinant receptor-binding domain (RBD219-N1), a SARS vaccine candidate. J Pharm Sci. 2017;106:1961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Yip MS, et al. Antibody-dependent enhancement of SARS coronavirus infection and its role in the pathogenesis of SARS. Hong Kong Med J. 2016;22:25–31. [PubMed] [Google Scholar]
- 344.Wang SF, et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Liu L, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4:e123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Tan W, et al. Viral kinetics and antibody responses in pateitns with COVID-10. Preprint at medRxiv 10.1101/2020.03.24.20042382 2020. [DOI]
- 347.Jiang H-W, et al. Global profiling of SARS-CoV-2 specific lgG/lgM responses of convalescents using proteome microarray. Preprint at medRxiv 10.1101/2020.03.20.20039495 2020. [DOI]
- 348.Wu F, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. Preprint at medRxiv Doi: 10.1101/2020.03.30.20047365 2020. [DOI]
- 349.Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nature. 2020;20:339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Wang J, Zand MS. The potential for antibody-dependent enhancement of SARS-CoV-2 infection: translational implications for vaccine development. J Clin Transl Sci. 2020;1–4. doi: 10.1017/cts.2020.39.32257403 [DOI] [Google Scholar]


