Abstract
Despite the ability of combination antiretroviral therapy to dramatically suppress viremia, the brain continues to be a reservoir of HIV-1 low-level replication. Adding further complexity to this is the comorbidity of drug abuse with HIV-1 associated neurocognitive disorders and neuroHIV. Among several abused drugs, the use of opiates is highly prevalent in HIV-1 infected individuals, both as an abused drug as well as for pain management. Opioids and their receptors have attained notable attention owing to their ability to modulate immune functions, in turn, impacting disease progression. Various cell culture, animal and human studies have implicated the role of opioids and their receptors in modulating viral replication and virus-mediated pathology both positively and negatively. Further, the combinatorial effects of HIV-1/HIV-1 proteins and morphine have demonstrated activation of inflammatory signaling in the host system. Herein, we summarized the current knowledge on the role of opioids on peripheral immunopathogenesis, viral immunopathogenesis, epigenetic profiles of the host and viral genome, neuropathogenesis of SIV/SHIV-infected non-human primates, blood-brain-barrier, HIV-1 viral latency, and viral rebound. Overall, this review provides recent insights into the role of opioids in HIV-1 immunopathogenesis.
Keywords: morphine, HIV-1, immunopathogenesis, blood-brain barrier, immunosuppression
Graphical Abstract
Introduction
HIV-1 prevalence continues to be a severe worldwide public health problem, with nearly 33 million people infected with HIV-1. Also, since the incidence of drug abuse among HIV-infected people is rapidly growing, there is an increased risk of the infected drug abusing people to develop HIV-associated neurocognitive disorders (HAND) compared to non-drug users infected with HIV-1. The Centers for Disease Control and Prevention describe HIV-1 infection and drug abuse as two intertwined epidemics, resulting in not only compromised combination antiretroviral therapy (cART) adherence but also to exacerbated pathogenesis of HAND. Among the several abused drugs, the use of opiates is highly prevalent in HIV-infected individuals, both as a recreational drug as well as for pain management (Atluri 2016; Basu et al. 2007; Hauser et al. 2012; Krashin et al. 2012; Lum and Tulsky 2006; Merlin et al. 2016; Noel et al. 2008). The interplay of HIV-1 and opiates thus raises concerns regarding the combinatorial effects of both on the progression of HAND. In recent years, chronic low-level inflammation likely mediated by residual viral proteins, abused drugs as well as long term cART usage (as these individuals continue to live longer), has been implicated as a significant underlying factor in the progression and pathology of HAND. Opioids and their receptors have attained notable attention owing to their ability to modulate immune functions, in turn, impacting disease progression. Various cell culture, animal and human studies have implicated the role of opioids and their receptors in modulating viral replication and virus-induced pathology both positively and negatively (Banerjee et al. 2011; Carr et al. 1996; Tahamtan et al. 2016; Welters 2003). Additionally, combinatorial effects of HIV-1/HIV-1 proteins and morphine have demonstrated activation of inflammatory signaling involving the generation of reactive oxygen species (ROS), calcium release, endoplasmic reticulum (ER) stress, dysregulated autophagy as well as activation of pattern recognition receptor signaling (Chivero et al. 2017; El-Hage et al. 2008; Lapierre et al. 2018; Mahajan et al. 2017; Rodriguez et al. 2017; Youn et al. 2017). In addition, emerging literature also suggests that opioids impact cognitive functioning and outcomes in HIV-infected individuals, thereby underscoring their contribution to the pathogenesis of HAND (Altice et al. 2011; Byrd et al. 2011; Chen et al. 2020; Dutta and Roy 2012; Fiellin et al. 2011; Hauser et al. 2012; Pirastu et al. 2006). The present review provides insights into the role of opioids in HIV-1 immunopathogenesis.
Role of morphine in the peripheral immunopathogenesis
In addition to their pain management potential, opioids can also modulate both the innate and acquired immune responses. Opioids have been traditionally known to be immunosuppressive; however, several recent reports have demonstrated immune activation by opioids involving multiple signaling pathways and mechanisms. In the following section, we focus on the role of morphine in peripheral immunopathogenesis.
Effects of morphine on T-cells
Morphine is a well-known, clinically used potent analgesic for alleviating pain. Despite its utility for pain management, several studies have demonstrated the immunosuppressive potential of opioids, including morphine and its derivatives, that could result in comorbid side-effects when used for pain management, especially in people with a compromised immune system such as those with cancer or infected with HIV-1. Opioid-mediated immunosuppression contributes to increased prevalence of diseases since the increased prevalence of opportunistic infections such as tuberculosis, HIV-1 infection, and pneumonia have been observed in chronic opioid abusers (Roy et al. 2006; Sacerdote 2006). Acute exposure of morphine has also been shown to play a role in selective differentiation of T-cells into T helper (Th)-2 cells via μ-opioid receptor signaling, both in vitro and in vivo (Han et al. 2020; Li et al. 2012). Several studies have demonstrated that chronic morphine exposure inhibits transcription of interleukin (IL)-2 in T-lymphocytes by inhibiting the transcription of the c-fos gene (Roy et al. 1997) and by modulating the cyclic adenosine monophosphate (cAMP) response element-binding transcription factors (Wang et al. 2007b). Similarly, in human naïve T cells, suppression of IL-2 was observed via the upregulation of cAMP, through modulation of IL-2 transactivators such as AP1, NFAT, and NF-κB (Borner et al. 2009). However, contradictory reports have demonstrated morphine-mediated downregulated expression of IL4 mRNA and protein (Borner et al. 2013). Morphine exposure was also shown to increase IL4 promoter activity in Jurkat T cells. Chronic exposure of morphine increases the binding of transcription factor NFAT to a DNA response element, resulting in the activation of T-cells with the concomitant production of cytokines (Roy et al. 2001; Wang et al. 2002). In another study, it was demonstrated that chronic morphine administration in rhesus macaques resulted in increased T-cell numbers expressing characteristic gut-homing cell surface markers (CD161 and CCR6) and, this was coupled with increased susceptibility to HIV-1 infection (Roy et al. 2011). Several reports have demonstrated that morphine-mediated induction of cAMP production leads to inhibition of cytokine expression in human Th1 cells (Borner et al. 2009), resulting in the production of IL-4. This involves multiple mechanisms such as interferon (IFN)-γ (Roy et al. 2001; Wang et al. 2002), GATA-3/T-bet switch (Roy et al. 2011), and Fas/Fas ligand-dependent activation-induced cell death of the Th1 cells (Greeneltch et al. 2005).
Effects of morphine on B-cells
Although limited studies have reported the effects of morphine on B-cells, some interesting studies have shown that administration of morphine pellet for 5 days resulted in a reduction of antibody-producing cells in the spleens of mice (Bhargava et al. 1994; Bussiere et al. 1992; Lefkowitz and Chiang 1975). It has also been demonstrated that these effects of morphine in B-cells were reversible in cells administered macrophage-derived proinflammatory cytokines such as IL-1β, 1L-6, or IFN-γ (Bussiere et al. 1993). Interestingly, it has been observed that morphine mediated decrease in the functioning of macrophages and polymorphonuclear leukocytes regulates activation and proliferation of B-cells (Rojavin et al. 1993; Weber et al. 1987). Overall, these studies indicate that morphine exerts immunosuppressive effects on B-cells.
Effects of morphine on natural killer cells
Similar to T and B cells, morphine is also shown to suppress the activity of natural killer (NK) cells (Saurer et al. 2006a). For example, a single administration of morphine into the periaqueductal grey region of the rat brain was shown to inhibit NK cell activity in spleen cells ex vivo. Injection of morphine into the rat hypothalamus, arcuate nucleus, medial amygdala, medial thalamus, and dorsal hippocampus, however, elicited no effect on NK cell activity, indicating thereby the unique effect of morphine injection in the periaqueductal grey region in modulating morphine-induced immune responses (Weber and Pert 1989). Lower doses of morphine, on the other hand, had the opposite effect. NK cells from chronic morphine users, however, failed to demonstrate any immunosuppression compared with opioid-naïve subjects (Tabellini et al. 2014). In healthy individuals, on the other hand, chronic morphine exposure has been shown to suppress NK cell activity (Yeager et al. 1995). In animal models of the pig, small acute doses of morphine prevented neoplastic transformations and viral infections by stimulating NK cell activity, and this was reversed by naltrexone (Borman et al. 2009). In macaques, however, chronic morphine administration exerted minimal alterations in the number of NK cells (Brown et al. 2012).
Effects of morphine on macrophages
It has also been reported that acute morphine impairs Toll-like receptor (TLR)-9-mediated NF-κB signaling, collectively leading to diminished bacterial clearance in mice during the early stages of Streptococcus pneumoniae infection (Wang et al. 2008). There have also been reports suggesting that chronic morphine administration in mice resulted in a shift in macrophage phenotype from an M1 to an M2 type, involving the cyclooxygenase-2-dependent mechanism (Godai et al. 2014). Acute morphine has also been shown to induce macrophage apoptosis by inhibiting inducible nitric oxide synthase and by promoting p53 as well as Bcl-2-associated X proteins, and the inhibitor for IL-converting enzyme-1 blocked these effects (Singhal et al. 1998). Chronic exposure of human blood monocyte-derived macrophages infected with HIV-1 to morphine (10 nM for 24 h) was shown to inhibit the expression of IFNs and IFN-inducible genes significantly with concomitant induction of negative regulators of the IFN signaling pathway (Wang et al. 2012). In a model of carcinoma, exposure of morphine up to 48 h was shown to modulate tumor angiogenesis by regulating macrophage protease production as well as M2 polarization in the tumor microenvironment (Khabbazi et al. 2015).
Effects of morphine on neutrophils
Neutrophils are one of the most critical phagocytic cells of the innate immune system. Studies have shown that opiates inhibit the bactericidal function of neutrophils while also reducing the ability of neutrophils to produce superoxide in response to the Escherichia coli metabolite, N-formyl methionyl leucyl phenylalanine (FMLP), resulting in reduced bacterial clearance (Sharp et al. 1985; Simpkins et al. 1986). Several reports have demonstrated the suppressive effects of morphine on recruitment and activation of neutrophils during an innate immune response. For example, exposure of human blood neutrophils to exogenous morphine (acute) resulted in the inhibition of IL-8 production (Glattard et al. 2010), no change in IL-8 receptor expression (Yossuck et al. 2008) as well as IL-8-induced chemotaxis (Grimm et al. 1998). Chronic opioid administration in murine models has also been shown to inhibit neutrophil migration (Clark et al. 2007) and decrease S. pneumoniae mediated production of IL-23/IL-17 in macrophages, resulting in delayed/reduced neutrophil migration, culminating, in turn, into the onset of severe lung infection and systemic infection (Ma et al. 2010). In a murine wound healing model, chronic morphine exposure led to reduced recruitment of neutrophils and macrophages at the wound sites likely due to reduced expression of monocyte chemotactic protein-1 (Martin et al. 2010). Cancer patients chronically taking morphine for pain management demonstrated a significant increase in total white blood cell count, neutrophils, and platelets (Karki et al. 2018). In an animal model of Acinetobacter baumannii infection, however, chronic morphine was shown to decrease the total number of neutrophils, with a decrease in the total number of macrophages, thereby potentiating infection (Breslow et al. 2011). Morphine thus modulates the bactericidal ability of neutrophils via alteration of white blood cell counts, neutrophil migration, and activity.
Effects of morphine on dendritic cells
Dendritic cells (DCs) play an essential role in the adaptive immune responses and are involved in antigen presentation to T-cells (Banchereau and Steinman 1998). Chronic morphine exposure has been shown to inhibit the production of IL-23 in Streptococcus pneumoniae-infected murine DCs involving the MyD88-IRAK1/4-dependent TLR2 and Nod2 signaling pathways. Morphine (chronic)-mediated inhibition of IL-23 production in S. pneumoniae-infected mice macrophages and dendritic cells were shown to involve MyD88-IRAK1/4-dependent TLR2 and Nod2 signaling pathways (Wang et al. 2011a). In a macaque model of morphine-dependence, morphine was shown to inhibit the expansion of myeloid DCs (Cornwell et al. 2016). Chronic administration of morphine has also been shown to suppress the maturation of mice bone marrow-derived DCs, antigen-presenting abilities, and the ability to activate antigen-specific CD8+T cells (Chang et al. 2016). In morphine administered mice, there was evidence of immunosuppression in bone-marrow monocyte (BMM)-derived DCs, which exhibited increased secretion of the anti-inflammatory cytokine, IL-10. Reciprocally, these cells also showed reduced expression of the proinflammatory cytokines IL-6 and tumor necrosis factor (TNF)-α. Furthermore, this study also showed that morphine exposure resulted in inhibition of DC-mediated anti-tumor immunity (Chang et al. 2016). In another report, intestinal sections from sepsis patients on opioids demonstrated increased expression of IL-17A. It can thus be envisioned that neutralization of IL-17A could be developed as an effective therapeutic strategy to manage gram-positive sepsis in patients on an opioid regimen (Meng et al. 2015a).
Effects of morphine on mast cells
Morphine exposure has been shown to induce selective immunosuppression in the peritoneal cavity in the mast cell-deficient as well as mast cell reconstituted mice (C57BL6/J) following chronic morphine exposure (Madera-Salcedo et al. 2011). In this study, morphine administration also inhibited the release of TNF-α following LPS challenge, an effect that was speculated to be mediated by its direct effect on resident peritoneal mast cells via negative crosstalk between opioid receptor and TLR4 signaling pathways (Madera-Salcedo et al. 2011). Furthermore, it was also shown that depending on the strains (CBA and Swiss) of mice, low dose morphine (5 mg/kg of body weight) attenuated pain in both the strains. In comparison, a high dose (20 mg/kg of body weight) inhibited the intraperitoneal accumulation of murine exudative leukocytes in Swiss mice but not in CBA mice (Plytycz and Natorska 2002; Stankiewicz et al. 2004). The difference in the outcomes between the mice strains was attributed to the release of histamine by the peritoneal mast cells (Stankiewicz et al. 2004). Mast cells have also been shown to play a role in morphine-mediated gut permeability and by facilitating pathogens to cross the gut barrier. Mucosal exposure of ilia to the microbial product, FMLP was associated with increased permeability to dextran 4400, which was ablated by acute morphine exposure, thereby underscoring the effects of morphine on mast cell-mediated mucosal permeability (Harari et al. 2006). In bone marrow-derived mast cells, it has been shown that activation of both μ and δ opioid receptors following morphine exposure suppressed TLR4-mediated release of TNF following LPS stimulation (Madera-Salcedo et al. 2013). In a tumor model, chronic morphine exposure has been shown to result in increased tumor angiogenesis, peri-tumoural lymphangiogenesis, mast cell activation, and increased generation of cytokines and Substance P (Nguyen et al. 2014).
Morphine withdrawal-induced immune suppression
Morphine withdrawal in mice has been associated with splenic plaque-forming cells and suppressed splenic cytokine production in macrophages (Rahim et al. 2002). There are other reports indicating that abrupt morphine withdrawal results in a range of changes including decreased splenic cytotoxic T cell activity, in vitro (Kelschenbach et al. 2008), B cell proliferation and IL-2 production in mice (Bhargava et al. 1994), suppressed splenic T cell proliferation, decreased IFN-γ production, increased serum TNFα levels in rats (West et al. 1999), activation of a population of suppressor macrophages and B cells, as well as decreased IFN-γ suppressed macrophage function in mice (Rahim et al. 2005). Morphine withdrawal in mice following 3 days of morphine injection was shown to result in decreased expression of IFN-γ - the Th1 signature cytokine, and increased expression of the Th2 cytokine, IL-4. Additionally, it was also observed that morphine withdrawal in mice induced Th2 differentiation involving the transcription factors Stat-6 and GATA-3. It was also shown that following withdrawal, the expression of the Th1-polarizing cytokine IL-12 was significantly downregulated, thereby supporting the notion that morphine induces Th2 differentiation which, in turn, generates an innate immune response, which, in turn, direct subsequent adaptive Th1/Th2 responses in these mice (Kelschenbach et al. 2005). Other studies have shown that morphine withdrawal leads to reduced binding of NF-κB, AP-1, and C/EBP to their consensus binding sequences in the IL-12 p40 promoter, thereby downregulating the expression of IL-12p40. This effect was shown to be abrogated in μ-opioid receptor knockout mice, thus underscoring the role of opioid receptor pathway in this process (Das et al. 2011). These studies thus demonstrate that morphine is immunosuppressive in the periphery, irrespective of whether the exposure is acute or chronic. Further studies are warranted to tease out specific unique effects of acute vs. chronic exposure of morphine.
Morphine impacts viral neuropathogenesis via modulation of the immune responses
Drug abuse is a significant comorbidity of HIV-1 infection and often leads to increased disease severity and complications (Norman and Kumar 2006). It has been well-documented that chronic opioid use directly correlates with HIV-1 infection and disease progression (Chang and Connaghan 2012; Nath 2002; Roy et al. 2011). Non-human primates have been the gold standard model in the field for studying HIV/SIV infection and the comorbidity of opioid abuse. In an earlier study, it has been reported that morphine exposure failed to exhibit any significant effect on viremia, CSF viral titers, or survival in SIV- infected Indian rhesus macaques (Marcario et al. 2008). Additionally, in this study, it was also shown that in the presence of morphine, there were increased white matter tract lesions in the brain compared with those in the SIV-infected animals administered saline, which primarily developed gray matter encephalitis. Morphine, on the other hand, had a significant effect on the immunopathogenesis of SIV infection, as evidenced by inhibition of ELISPOT responses against the virus (Marcario et al. 2008). Several reports suggest that abused drugs including opioids in combination with HIV-1 synergize to enhance viral load, immunodeficiency, neuronal dysfunction and neuronal degeneration (Nath et al. 2000; Nath et al. 2002; Wang et al. 2005). Acute morphine exposure also inhibited the non-cytotoxic, anti-HIV-1 activity of CD8+ T cells in HIV-1 latently infected cells (promonocytic (U1) and T (J1.1 and 1G5) cell lines), suggesting that morphine suppresses the host immune system, in turn, leading to enhanced HIV-1 infection and pathogenesis (Wang et al. 2005). In chronic morphine-dependent, SIV-infected rhesus macaques, it was demonstrated that morphine exposure potentiated both the plasma and CSF viral loads compared with the infected, saline-administered animals. Furthermore, morphine-dependent, SIV infected macaques also shown a trend of increased influx of infected monocyte/macrophages and increased virus build-up in the brain compared with infected animals administered saline (Bokhari et al. 2011). Using a mathematical model that incorporates experimentally observed effects of morphine on HIV-1 co-receptor expression, Vaidhya et al. (2016) reported that morphine enhances virus replication rate, maintaining a higher steady-state viral load and also inducing a significant drop in CD4 counts (Vaidya et al. 2016). Morphine exposure was also shown to potentiate viral load in plasma as well as in CSF of male Indian rhesus macaques infected with a mixture of SHIV(KU), SHIV(89.6)P, and SIV/17E-Fr (Kumar et al. 2006). This study also suggested that morphine enhanced viral migration through the BBB, and induced cellular and humoral immune responses in the infected macaques (Kumar et al. 2006). Other research findings have also demonstrated that morphine dependence increased rapid disease progression in the non-human primate model of AIDS, and this was accompanied by decreased virus evolution in SHIV/SIV-infected rhesus macaques (Rivera-Amill et al. 2010; Tirado and Kumar 2006).
Morphine impacts viral pathogenesis via modulation of host factors
Administration of opioids and activation of their receptors has been reported to alter immune functions while also upregulating the expression of HIV-1 entry co-receptors such as the chemokine receptors CCR5 and CXCR4, that promote HIV-1 replication and impact disease progression (Steele et al. 2003). There have been reports on the diminution of virus-specific neutralizing antibodies critical for enhancing viral clearance in SIV-infected, morphine-dependent macaques. In this study, morphine exposure was shown to increase the severity of viral infection, decrease viral clearance, increase the viral load, and increase the loss of CD4+ T cells (Mutua et al. 2019). It has also been demonstrated that HIV-1 Tat alone or in combination with morphine significantly accelerated glial activation in the striatum of wild type mice compared to C-C motif chemokine ligand 2 (CCL2) knockout mice (El-Hage et al. 2006a). These results thus suggested that CCR2 contributed significantly to the activation of macrophages and glia, an effect observed in the brains of HIV-1 infected individuals with and without a history of drug abuse. In another study, Meng et al. (2015) explored the role of opioids in the gastrointestinal tract and found that administration of opioids in mice resulted in changes in gastrointestinal motility, barriers functions, and gut bacterial homeostasis leading, in turn, to increased bacterial translocation and activation of the immune system, ultimately accelerating HIV-1 associated complications of the gastrointestinal tract (Meng et al. 2015b). The molecular mechanism(s) underlying morphine-mediated modulation of host factor(s) and the associated immune responses, however, needs to be explored further to understand better the effects of morphine on HIV-1 infection, pathogenesis, and development of HAND. Research in this area is warranted to gain further insights into the effects of drugs of abuse on host immune responses in the context of HIV-1 infection.
Morphine alters viral pathogenesis via modulating host cell signaling
Binding of opioids to their cognate receptors, localized on several peripheral as well as brain cells, results in the activation of various intracellular signaling pathways. Opiates such as morphine promote HIV-1 pathogenesis via a decrease in BBB integrity, increasing glial cell activation, and promoting neuronal damage (Dalvi et al. 2016; Dutta and Roy 2012; Gonek et al. 2018; Masvekar et al. 2014; Rodriguez et al. 2017). Below we describe specific cell signaling pathways mediated by opioids and/or HIV-1/HIV-1 proteins in distinct cell types. Although several studies are extant in the field, more work is warranted to assess the specific pharmacological targets of cellular signaling and to find potent pharmacological interventions that could modulate these signaling targets.
Macrophages/monocytes
In the context of HIV-1 infection, opioid exposure induces the expression of both cytokines and chemokines, in turn, increasing the trafficking of myeloid cells into the brain and thus contributing high viral load in CNS compartment (Dave 2012; Guo et al. 2002; Hollenbach et al. 2014; Suzuki et al. 2002; Wang et al. 2012). In monocyte-derived macrophages (MDMs), morphine (10 μM for 24 h) has been shown to potentiate HIV-1 viral replication at 5-, 10-, and 15-days post-infection by increasing the expression of galectin-1 and increasing the stabilization of HIV-1 onto MDMs (Reynolds et al. 2012). Intriguingly, concomitant incubation of morphine (10 μM for 24 h) with recombinant galectin-1 (2 μM for 30 min) prior to infection had a synergistic effect on the levels of p24 antigen in MDMs at 5-, 10- and 15-days post-infection. In another study, it was demonstrated that in HIV-infected human MDMs acute (0.1 μM for 24 h) as well as chronic (21 days) morphine exposure induced inflammatory responses with increased release of cytokines such as IL6 and MCP-2, without significantly affecting viral replication (Dave 2012). Several studies have shown that exposure of macrophages and microglia to morphine upregulates the expression of CCR5, an HIV-1 co-receptor, leading to potentiation of HIV-1 infection/replication and thus to enhanced neuroinflammation (El-Hage et al. 2013; Guo et al. 2002; Kim et al. 2018; Li et al. 2003). Another study also demonstrated that exposure of human blood MDMs to morphine (10 nM for 24 h) suppressed the critical regulators of IFN-signaling pathway, including IFN-inducible genes, cytokine signaling protein, protein inhibitors of activated STAT while also suppressing the expression of interferons (Wang et al. 2012). This, in turn, promoted HIV-1 Bal/SIV Delta (B670) infection and replication. In another study, it has been reported that exposure of neonatal MDMs to morphine (10 nM for 24 h) resulted in increased HIV-1 replication involving the upregulation of CCR5 receptor and inhibition of β-chemokine expression. Morphine thus can act as a contributing factor for perinatal HIV-1 transmission and infection (Li et al. 2003).
Microglia and Astrocytes
Several lines of evidence have demonstrated increased glial activation and enhanced neuroinflammation in a chronically-infected cohort of HIV+ patients with a history of opiate abuse (Anderson et al. 2003; Kim et al. 2018; Liu et al. 2016). Ample evidence also suggests that in glial cells, morphine-mediated potentiation of the neurodegenerative effects of HIV-1 Tat protein involves activation of μ-opioid receptors (Kim et al. 2018; Zou et al. 2011). A study by Turchan-Cholewo et al. (2009) reported that both morphine (500 nM for 24 h) and HIV-1 Tat (50 ng/mL for 24 h) synergistically increase the production of ROS and oxidative stress in N9 murine microglial cells, leading to increased release of proinflammatory cytokines such as TNFα, IL6, and the chemokine, MCP-1 by these cells (Turchan-Cholewo et al. 2009). Another study by the same group has also shown that in N9 murine microglial cells, morphine (500 nM for 24 h) exposure decreased the surface expression of μ-opioid receptors (MOR) expression in microglia but not in astrocyte. Exposure of cells to HIV-1 Tat (50 ng/ml for 24 h), however, did not affect the expression levels of MOR. Interestingly, combined exposure of morphine and HIV-1 Tat increased both the surface expression of MOR as well as their intracellular levels. Furthermore, HIV-1 Tat in the presence of morphine resulted in increased receptor synthesis but did not interfere with the internalization and/or trafficking of MORs (Turchan-Cholewo et al. 2008). In addition to its synergy with HIV-1 Tat, opiates have also been shown to cooperate with HIV-1 glycoprotein (gp) 120 to induce the generation of oxidative stress in human microglial cells, resulting in the dysregulation of the cell cycle in these cells (Samikkannu et al. 2015). There have been reports demonstrating the role of TLRs in morphine-mediated potentiation of HIV-1 neuropathogenesis, which was found to be associated with increased expression of proinflammatory cytokines such as IL-6 and TNF-α (Dutta et al. 2012; Wang et al. 2011a). In another report, it has been documented that morphine impaired the autophagy pathway via a beclin1-independent mechanism, resulting in enhanced HIV-1 replication and inflammatory responses in human primary microglial cells (El-Hage et al. 2015).
In mouse primary astrocytes, morphine (500 nM for 4 and 12 h) has been shown to time-dependently exacerbate HIV-1 Tat (100 nM for 4 and 12 h)-mediated induction of proinflammatory cyto/chemokines such as, TNFα, IL6, MCP-1, and RANTES via convergent effects on intracellular calcium and NFκB trafficking, thereby implicating a cooperative synergistic effect of opioids and HIV-1 proteins, which, in turn, could contribute to accelerated neuropathogenesis of HIV-1 (El-Hage et al. 2008; El-Hage et al. 2005). In human U373 astrocytoma cells, morphine exposure has also been shown to exacerbate the neurotoxic effects of HIV-1 gp120 via modulation of the expression of chemokines such as CCR3 and CCR5 (Mahajan et al. 2005a; Mahajan et al. 2005b). It has been well-recognized that the interaction of HIV-1 Tat (100 nM) and morphine (500 nM for 16 h) increases the neurodegenerative effects through μ-opioid receptor signaling in an in vitro co-culture model of neurons and glia as well as in doxycycline-inducible HIV-1 Tat transgenic mice administered twice-daily injections of morphine intraperitoneally (10 mg/kg) for 5 days (Zou et al. 2011). Reports on opiates exacerbating neuroAIDS have implicated increased astrocytic expression of μ-opioid receptor signaling, activation of which has been shown to trigger the production of chemokines including MCP-1 (a known chemoattractant for monocytes), resulting in increased neuroinflammation via promoting microglial activation and recruitment of macrophages in the CNS (El-Hage et al. 2006b). It has also been demonstrated that in human primary astrocytes, morphine (500 nM) exposure led to induction of the ER stress as well as defective autophagy, in turn leading to astrocytosis and neuroinflammation via the μ-opioid receptor, in vitro. These findings were also validated in the frontal cortex, occipital cortex, cerebellum, and basal ganglia region of chronic morphine (21 days with escalating doses from 6 to 12 mg/kg body weight/day) administered rhesus macaques (Sil et al. 2018).
Neurons
Although HIV-1 does not infect the neurons directly, soluble neurotoxic viral proteins such as HIV-1 Tat and gp120 and/or the host proinflammatory cytokines, chemokines, excitotoxins and proteases released from infected cells, can lead to neuronal damage, thereby contributing to the development and progression of HAND (Dutta and Roy 2012; Kovalevich and Langford 2012; Ru and Tang 2017). Morphine has been shown to impact the integrity and function of neurons. In an earlier report, it has been demonstrated that in exposure of mouse primary hippocampal neurons to morphine (10 μM for 24 h) induced synaptic alterations involving ER stress and autophagy pathways, findings analogous to the administration of morphine at an initial dose of 10 mg/kg followed by ramping the dose by 5 mg/kg/d for six consecutive days in mice. The detrimental effects of morphine were observed to be reversed by platelet-derived growth factor (PDGF)-BB, a key factor implicated in neuroprotection (Cai et al. 2016). Interestingly, morphine (500 nM for 3 days) also potentiated the neurotoxic effects of supernatants from HIV-1SF162-infected differentiated-U937 cells in neurons and glia in mixed co-cultures, thereby affecting the ability of neurons to recover from sub-lethal damage of HIV-1 (Masvekar et al. 2014). In another study, neuronal cultures exposed to supernatant fluids from HIV-1SF162-infected THP-1 cells in the presence of morphine (500 nM for 3 days) demonstrated neuronal injury that could be prevented by GSK3β inhibitors, such as valproate, SB415286 or GSK-3β inhibitor XXVI (Masvekar et al. 2015). It has also been reported that morphine (100 nM for 24 h) potentiates HIV-1 Tat (100 ng/mL) induced apoptosis in human neuronal cells, and this involves activation of the c-Jun N-terminal kinase (JNK)/extracellular signal-regulated kinase-1/2 (ERK1/2) pathway (Malik et al. 2011). Furthermore, pretreatment of human neuronal cells with a growth factor, PDGF-BB, was shown to prevent neuronal toxicity mediated by morphine and HIV-1 Tat (Malik et al. 2011). In another report, the exposure of mouse primary striatal neurons with a combination of HIV-1 Tat (50 nM) and morphine (500 nM for 10 min) resulted in enhanced synaptodendritic injury involving an increased influx of sodium and calcium as well as mitochondrial instability (Fitting et al. 2014). Interestingly, we have also reported that exposure of rat primary astrocytes, as well as A172 human astrocytoma cell line to morphine (10 μM for 24 h) and HIV-1 Tat (200 ng/mL), increased the release of exosomes enriched with miR-29b, which, in turn, could be taken up the neurons, resulting in increased neuronal apoptosis involving downregulation of the miR-29b target, PDGF-B. These findings were also validated in vivo in morphine-dependent SIV-infected macaques (Hu et al. 2012).
Morphine alters epigenetic profiles of the host and viral genome:
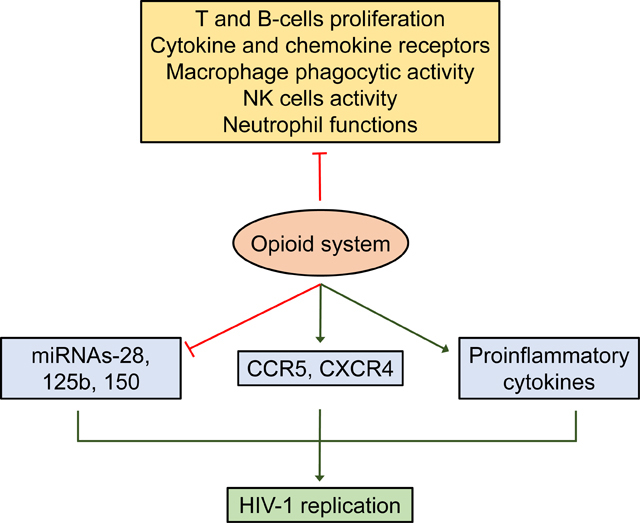
In the context of HIV-1, abused drugs such as opioids can induce epigenetic modifications on the viral promotor as well as the host chromatin, thereby promoting HIV-1 viral infection, replication, and disease progression (Shirazi et al. 2013). Several studies have demonstrated the effect of drug of abuse-mediated epigenetic modifications such as histone modifications, DNA methylation, and non-coding RNAs in maintaining tight regulation of HIV-1 integration and latency while also enhancing HIV-1 infection and replication (Bushman et al. 2005; Chandel et al. 2015; Desplats et al. 2014; Kauder et al. 2009; Tyagi et al. 2016; Tyagi et al. 2010; Wang et al. 2007a). It is well-known that the HIV-1 genome integrates into the host cell genome in the intronic regions. The specific integration of the HIV-1 genome is known to be facilitated either by host cellular components such as non-coding RNAs, and DNA methyltransferases or by viral factors including cellular gene analogs, and nucleosome proteins that define the overall chromatin structure in the long terminal repeat (LTR) regions (Maricato et al. 2015; Vandegraaff et al. 2006; Verdin 1991; Verdin et al. 1993; Wang et al. 2009; Wang et al. 2011b). Epigenetic regulation of the nucleosome (Nuc-0 and Nuc-1) proteins play a significant role in the integration and replication of the HIV-1 genome in the host (Jordan et al. 2001; Verdin et al. 1993). In addition to the nucleosome regulation, changes in the miRNA expression have also been reported to affect HIV-1 replication and pathogenesis (Tahamtan et al. 2016). Furthermore, in monocytes, activation of the opioid system has been shown to inhibit the expression of miRNAs, including miRNA-28, 125b, 150, and 382, that target 3′-UTR of HIV-1 transcripts, thus increasing the susceptibility of these cells to HIV-1 infection (Wang et al. 2011b). The molecular mechanism(s) underlying morphine-mediated epigenetic modifications and their role in HIV-1 infection, integration, and replication, as well as exacerbation of HIV-1 mediated neuropathogenesis, have not been studied in detail. Further research in these realms will pave a way to bridge a major gap in the fields of opiates abuse and HIV-1/AIDS biology and will set a stage for the future development of new therapeutic approaches aimed at targeting epigenetic players underlying HIV-1 neuropathogenesis.
Effects of morphine on the neuropathogenesis of SIV/SHIV-infected non-human primates:
Opioids modulate the immune system either directly via opioid receptors located on immune cells or indirectly via opioid receptors in the CNS. Chronic morphine exposure affects both arms of the immune system. Activation of μ-opioid receptors on macrophages results in inhibition of phagocytosis (Szabo et al. 1993; Tomei and Renaud 1997) and increased nitric oxide production (Fecho et al. 1994); while on CD4+ T cells it leads to inhibition of Th1 cytokines, IL-2 and IFN-γ, and potentiation of Th2 cytokines, IL-4 and IL-5, ultimately biasing the cells towards a Th2 pathway (Roy et al. 2005; Roy et al. 2004). Morphine can also upregulate the expression of the CCR5 receptor on neutrophils as well as monocytes/macrophages (Miyagi et al. 2000; Suzuki et al. 2002). While the effects of morphine on the immune system have been reported extensively (Miyagi et al. 2000; Roy et al. 2005; Roy et al. 2004; Suzuki et al. 2002), the effects of morphine on the interactions between the virus (either HIV-1 or SIV infection) and the immune system and the progression of the disease are still controversial. It has been shown that morphine downregulates the splenic NK cell activity in rats administered a single high dose of morphine, that crosses the BBB. Interestingly, N-methylmorphine, which does not cross the BBB, had no effect on peripheral NK cell activity, thus indicating that the immunosuppressive effect of morphine (Shavit et al. 1986; Shavit et al. 1987). In the rat splenic cells, morphine exposure mediated suppression of NK cell activity involved opioid receptors in the periaqueductal gray matter of the mesencephalon, but not in the other brain regions (Weber and Pert 1989). Morphine-mediated suppression of NK cell activity is mediated via the α-adrenoreceptor stimulated circuits (Carr et al. 1994), nicotinic receptors (Mellon and Bayer 2001), innervation of the sympathetic nervous system (Felten and Olschowka 1987), neurotransmitter Y (Saurer et al. 2006b), and Fas signaling (Yin et al. 1999). Morphine can thus directly inhibit NK cell activity irrespective of its acute or chronic exposure.
Morphine is a well-known immunosuppressant; however, there is limited information available on the effects of morphine on the pathogenesis of HIV/SIV infection. Numerous in vitro and in vivo studies that investigated the effects of opioids in the context of HIV-1 infection with conflicting findings reported on its effect on disease pathogenesis (Bokhari et al. 2011; Chuang et al. 1993; Donahoe 2004; Kumar et al. 2006; Kumar et al. 2004; Marcario et al. 2008; Suzuki et al. 2002). Several epidemiological studies have also demonstrated contrasting findings on the effects of morphine on HIV-1 infection; with some reports demonstrating protective effects of opiates on HIV-1 pathogenesis (Spijkerman et al. 1996) while others are suggesting deleterious effects (Bouwman et al. 1998) and some with no effect at all (Thorpe et al. 2004). It must be recognized that these studies are challenging owing to the high variability of drug usage patterns, length of use, and multidrug usage, as well as differences in nutritional status and access to medical care among individuals (Kapadia et al. 2005). Furthermore, most studies rely on self-reported data, which may not be accurate. Animal models, therefore, provide a valuable approach to study the effects of opiates on viral pathogenesis in a more controlled environment. Many researchers have reported the effects of morphine on the pathogenesis of SIV infected rhesus macaques, and these findings include: (a) increased virus replication in non-human primates on long-term morphine dependence (Bokhari et al. 2011; Chuang et al. 1993; Kumar et al. 2004; Miyagi et al. 2000); (b) increased circulating SIV-infected T cells in morphine-administrated macaques (Cornwell et al. 2016); (c) increased plasma and CSF viral load in SIV-infected rhesus macaques dependent on morphine (Bokhari et al. 2011); (d) increased virus build-up in the brains that is accompanied with the increased influx of infected monocyte/macrophages in the brains of SIV-infected rhesus macaques that were morphine-dependent (Bokhari et al. 2011). There are also reports demonstrating that morphine administration inhibited the SIV/SHIV neuropathogenesis (Bouwman et al. 1998; Marcario et al. 2008). Controlled studies with constant morphine administration have shown that morphine has a trend of potentiating virus replication and neuropathogenesis, leading to increased mortality in a subset of macaques compared with the virus alone animals (Bokhari et al. 2011).
Effects of morphine on the blood-brain barrier (BBB)
BBB is a tightly regulated barrier (comprising of endothelial cells, pericytes, and astrocytes) separating the peripheral circulation from the CNS, and that maintains homeostasis within the CNS (Hawkins and Davis 2005). In vitro exposure of morphine (up to 10 μM for 3 days) to human umbilical arterial endothelial cells stimulated cell proliferation via mitogen-activated protein kinase (Leo et al. 2009). On the other hand, morphine and its metabolite morphine-3-glucuronide via TLR4-dependent inflammatory signaling were shown to activate cultured CNS endothelial cells lining the BBB in drug-reward areas of the brain, leading to modulatory effects in the reward regions of the brain, ultimately contributing to opioid-associated reward and addiction (Grace et al. 2014). In another in vitro study using human pericytes, it was shown that clinically relevant doses of morphine were shown to increase the secretion of PDGF-BB from human umblical vein endothelial cells, thereby activating PDGF-β and mitogen-activated protein kinase/extracellular signal-regulated kinase phosphorylation (Luk et al. 2012). Complementary to these in vitro findings, clinically relevant escalating dose of morphine for 7 weeks promoted tumor angiogenesis by increasing the desmin- and PDGFRβ-positive cells in the tumor vasculature of mice, suggestive of increased proliferation and/or recruitment of vessel-associated pericytes (Luk et al. 2012). The effects of morphine on astrocytes have been discussed in the previous section. Studies on gene and protein patterns of transporters and metabolizing enzymes have provided information regarding drug entry and metabolism in human endothelial cells (Dauchy et al. 2008; Shawahna et al. 2011; Uchida et al. 2011). It has been suggested that the transporter protein in endothelial cells, P-glycoprotein, contributes to the development of opioid tolerance by active brain-to-blood efflux at the BBB (Mercer and Coop 2011). Studies have shown that in rats, inhibition of BBB transporter, such as multidrug resistance-associated proteins, by probenecid, resulted in reduced clearance via brain efflux of morphine (Tunblad et al. 2003). It is of great interest in the effect of opioids on the differential expression of BBB proteins that can supply a possible correlation between the BBB disruption and the onset of opioid tolerance. Accumulating evidence suggests that increased breach of the BBB during opioid withdrawal (Baba et al. 1988; Chaves et al. 2017; Sharma and Ali 2006; Sharma et al. 2010). It has also been reported that opiates in combination with HIV-1 Tat protein downregulate the expression of tight junction proteins such as ZO-1 and Occludins in primary brain microvascular endothelial cells, thereby compromising BBB integrity and exacerbating HIV-1 neuropathogenesis (Mahajan et al. 2008). Detailed investigations are warranted for the future development of novel therapeutics aimed at abrogating BBB damage in opiate abusing HIV-1 patients.
Effects of morphine on HIV-1 viral latency and viral rebound
The reactivation of latent HIV-1 infection by opioids is one of the mechanisms that could affect HIV-1 disease pathogenesis in HIV-1 infected drug abusers. Few studies have examined the reactivation of HIV-1 in the context of opioids. In a study, Prottengeier et al. (2004) found that opioids such as morphine or heroin reactivated HIV-1 in vitro in latently-infected ACH-2 T lymphoblasts at drug concentrations that were higher than those used for analgesic regimens or those found in the plasma of intravenous drug users (Prottengeier et al. 2014). In vivo studies examining the reactivation of HIV-1 in animal models of HIV/SIV infection with morphine administration are scant.
Conclusions and future perspectives
The literature reviewed here highlights that morphine-mediated suppression of innate and adaptive immunity in the periphery plays a crucial role in the pathogenesis of not only HIV-1 infection but other viral and bacterial infections as well. Several cellular processes such as phagocytosis, autophagy, production of bioactive molecules (chemokines and cytokines), and immune activation are modulated by morphine in several cell types, including T cells, B cells, NK cells, mast cells, dendritic cells, neutrophils, and myeloid cells. In the context of HIV-1 infections, morphine appears to potentiate disease pathogenesis involving modulation of innate immune responses such as the interferon system, among others. In the CNS, however, most literature we reviewed suggests the neuroinflammatory effects of morphine involving alterations in the trafficking of immune cells into the brain, increased production of inflammatory mediators, modulation of the expression of tight junctional proteins and disruption of the BBB integrity. In particular, the neuroinflammatory effects of morphine emanating from microglia and astrocytes have been documented to contribute to the ensuing neurotoxicity. Intriguingly, the interactions of morphine and the immune system in the context of concurrent pathogenic infections is a very complex issue. Collectively, most of the studies we reviewed point to the role of morphine being an immunosuppressant in the periphery while being neuroinflammatory in the CNS in both rodent and non-human primate models. Seemingly this paradox is not well understood, and studies aimed at understanding this dichotomy are warranted, especially in human subjects. This complexity is partly because, in most studies, acute or chronic exposure to morphine fails to exhibit a uniformity of time points which, in turn, could translate into differential effects on inflammation and viral replication, thus making it difficult to fully understand the effects of morphine on HIV-1 infection and disease progression. Well-controlled studies (time points and drug concentrations) in higher animal models are warranted to enhance the comparison of acute versus chronic effects of morphine on HIV-1 infection and disease progression. In addition, research aimed at identifying the molecular mechanism(s) underlying opioids-mediated epigenetic modifications and their role in HIV-1 infection, integration, and replication, as well as exacerbation of HIV-1-mediated neuropathogenesis are needed to shed more light on the complex interactions of morphine and HIV-1 viral infections. A better understating of these areas could provide insights into the role of morphine in the pathogenesis of comorbid viral infections and pave the way for future therapeutics.
Acknowledgments
Funding information: This work is supported by NIH grants - DA040397, DA041751, MH062261, DA043164, DA044586, and Nebraska Center for Substance Abuse Research.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors declare that they have no conflict of interest.
References:
- Altice FL et al. (2011) HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study J Acquir Immune Defic Syndr 56 Suppl 1:S22–32 doi: 10.1097/QAI.0b013e318209751e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CE et al. (2003) Relationship of Nef-positive and GFAP-reactive astrocytes to drug use in early and late HIV infection Neuropathol Appl Neurobiol 29:378–388 doi: 10.1046/j.1365-2990.2003.00475.x [DOI] [PubMed] [Google Scholar]
- Atluri VS (2016) Editorial: HIV and Illicit Drugs of Abuse Front Microbiol 7:221 doi: 10.3389/fmicb.2016.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Oishi R, Saeki K (1988) Enhancement of blood-brain barrier permeability to sodium fluorescein by stimulation of mu opioid receptors in mice Naunyn Schmiedebergs Arch Pharmacol 337:423–428 doi: 10.1007/bf00169534 [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity Nature 392:245–252 doi: 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- Banerjee A, Strazza M, Wigdahl B, Pirrone V, Meucci O, Nonnemacher MR (2011) Role of muopioids as cofactors in human immunodeficiency virus type 1 disease progression and neuropathogenesis J Neurovirol 17:291–302 doi: 10.1007/s13365-011-0037-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Bruce RD, Barry DT, Altice FL (2007) Pharmacological pain control for human immunodeficiency virus-infected adults with a history of drug dependence J Subst Abuse Treat 32:399–409 doi: 10.1016/j.jsat.2006.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava HN, Thomas PT, Thorat S, House RV (1994) Effects of morphine tolerance and abstinence on cellular immune function Brain Res 642:1–10 doi: 10.1016/0006-8993(94)90899-0 [DOI] [PubMed] [Google Scholar]
- Bokhari SM et al. (2011) Morphine potentiates neuropathogenesis of SIV infection in rhesus macaques J Neuroimmune Pharmacol 6:626–639 doi: 10.1007/s11481-011-9272-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman A, Ciepielewski Z, Wrona D, Stojek W, Glac W, Leszkowicz E, Tokarski J (2009) Small doses of morphine can enhance NK cell cytotoxicity in pigs Int Immunopharmacol 9:277–283 doi: 10.1016/j.intimp.2008.11.006 [DOI] [PubMed] [Google Scholar]
- Borner C, Lanciotti S, Koch T, Hollt V, Kraus J (2013) mu opioid receptor agonist-selective regulation of interleukin-4 in T lymphocytes J Neuroimmunol 263:35–42 doi: 10.1016/j.jneuroim.2013.07.012 [DOI] [PubMed] [Google Scholar]
- Borner C et al. (2009) Mechanisms of opioid-mediated inhibition of human T cell receptor signaling J Immunol 183:882–889 doi: 10.4049/jimmunol.0802763 [DOI] [PubMed] [Google Scholar]
- Bouwman FH et al. (1998) Variable progression of HIV-associated dementia Neurology 50:1814–1820 doi: 10.1212/wnl.50.6.1814 [DOI] [PubMed] [Google Scholar]
- Breslow JM, Monroy MA, Daly JM, Meissler JJ, Gaughan J, Adler MW, Eisenstein TK (2011) Morphine, but not trauma, sensitizes to systemic Acinetobacter baumannii infection J Neuroimmune Pharmacol 6:551–565 doi: 10.1007/s11481-011-9303-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JN et al. (2012) Morphine produces immunosuppressive effects in nonhuman primates at the proteomic and cellular levels Mol Cell Proteomics 11:605–618 doi: 10.1074/mcp.M111.016121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman F, Lewinski M, Ciuffi A, Barr S, Leipzig J, Hannenhalli S, Hoffmann C (2005) Genome-wide analysis of retroviral DNA integration Nat Rev Microbiol 3:848–858 doi: 10.1038/nrmicro1263 [DOI] [PubMed] [Google Scholar]
- Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK (1992) Differential effects of morphine and naltrexone on the antibody response in various mouse strains Immunopharmacol Immunotoxicol 14:657–673 doi: 10.3109/08923979209005416 [DOI] [PubMed] [Google Scholar]
- Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK (1993) Cytokine reversal of morphine-induced suppression of the antibody response J Pharmacol Exp Ther 264:591–597 [PubMed] [Google Scholar]
- Byrd DA et al. (2011) Neurocognitive impact of substance use in HIV infection J Acquir Immune Defic Syndr 58:154–162 doi: 10.1097/QAI.0b013e318229ba41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y et al. (2016) Regulation of morphine-induced synaptic alterations: Role of oxidative stress, ER stress, and autophagy J Cell Biol 215:245–258 doi: 10.1083/jcb.201605065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Mayo S, Gebhardt BM, Porter J (1994) Central alpha-adrenergic involvement in morphine-mediated suppression of splenic natural killer activity J Neuroimmunol 53:53–63 doi: 10.1016/0165-5728(94)90064-7 [DOI] [PubMed] [Google Scholar]
- Carr DJ, Rogers TJ, Weber RJ (1996) The relevance of opioids and opioid receptors on immunocompetence and immune homeostasis Proc Soc Exp Biol Med 213:248–257 doi: 10.3181/00379727-213-44056 [DOI] [PubMed] [Google Scholar]
- Chandel N, Malhotra A, Singhal PC (2015) Vitamin D receptor and epigenetics in HIV infection and drug abuse Front Microbiol 6:788 doi: 10.3389/fmicb.2015.00788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC et al. (2016) Anti-CD40 antibody and toll-like receptor 3 ligand restore dendritic cell-mediated anti-tumor immunity suppressed by morphine Am J Cancer Res 6:157–172 [PMC free article] [PubMed] [Google Scholar]
- Chang SL, Connaghan KP (2012) Behavioral and molecular evidence for a feedback interaction between morphine and HIV-1 viral proteins J Neuroimmune Pharmacol 7:332–340 doi: 10.1007/s11481-011-9324-1 [DOI] [PubMed] [Google Scholar]
- Chaves C, Remiao F, Cisternino S, Decleves X (2017) Opioids and the Blood-Brain Barrier: A Dynamic Interaction with Consequences on Drug Disposition in Brain Curr Neuropharmacol 15:1156–1173 doi: 10.2174/1570159X15666170504095823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Phan T, Lin A, Sardo L, Mele AR, Nonnemacher MR, Klase Z (2020) Morphine exposure exacerbates HIV-1 Tat driven changes to neuroinflammatory factors in cultured astrocytes PLoS One 15:e0230563 doi: 10.1371/journal.pone.0230563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivero ET, Guo ML, Periyasamy P, Liao K, Callen SE, Buch S (2017) HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation J Neurosci 37:3599–3609 doi: 10.1523/JNEUROSCI.3045-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang LF, Killam KF Jr., Chuang RY (1993) Increased replication of simian immunodeficiency virus in CEM x174 cells by morphine sulfate Biochem Biophys Res Commun 195:1165–1173 doi: 10.1006/bbrc.1993.2167 [DOI] [PubMed] [Google Scholar]
- Clark JD, Shi X, Li X, Qiao Y, Liang D, Angst MS, Yeomans DC (2007) Morphine reduces local cytokine expression and neutrophil infiltration after incision Mol Pain 3:28 doi: 10.1186/1744-8069-3-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell WD, Wagner W, Lewis MG, Fan X, Rappaport J, Rogers TJ (2016) Effect of chronic morphine administration on circulating dendritic cells in SIV-infected rhesus macaques J Neuroimmunol 295-296:30–40 doi: 10.1016/j.jneuroim.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvi P et al. (2016) Enhanced autophagy in pulmonary endothelial cells on exposure to HIV-Tat and morphine: Role in HIV-related pulmonary arterial hypertension Autophagy 12:2420–2438 doi: 10.1080/15548627.2016.1238551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Kelschenbach J, Charboneau R, Barke RA, Roy S (2011) Morphine withdrawal stress modulates lipopolysaccharide-induced interleukin 12 p40 (IL-12p40) expression by activating extracellular signal-regulated kinase 1/2, which is further potentiated by glucocorticoids J Biol Chem 286:29806–29817 doi: 10.1074/jbc.M111.271460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchy S et al. (2008) ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier J Neurochem 107:1518–1528 doi: 10.1111/j.1471-4159.2008.05720.x [DOI] [PubMed] [Google Scholar]
- Dave RS (2012) Morphine affects HIV-induced inflammatory response without influencing viral replication in human monocyte-derived macrophages FEMS Immunol Med Microbiol 64:228–236 doi: 10.1111/j.1574-695X.2011.00894.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P et al. (2014) Epigenetic alterations in the brain associated with HIV-1 infection and methamphetamine dependence PLoS One 9:e102555 doi: 10.1371/journal.pone.0102555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahoe RM (2004) Multiple ways that drug abuse might influence AIDS progression: clues from a monkey model J Neuroimmunol 147:28–32 doi: 10.1016/j.jneuroim.2003.10.011 [DOI] [PubMed] [Google Scholar]
- Dutta R et al. (2012) Morphine modulation of toll-like receptors in microglial cells potentiates neuropathogenesis in a HIV-1 model of coinfection with pneumococcal pneumoniae J Neurosci 32:9917–9930 doi: 10.1523/JNEUROSCI.0870-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, Roy S (2012) Mechanism(s) involved in opioid drug abuse modulation of HAND Curr HIV Res 10:469–477 doi: 10.2174/157016212802138805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Bruce-Keller AJ, Yakovleva T, Bazov I, Bakalkin G, Knapp PE, Hauser KF (2008) Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca(2+)](i), NF-kappaB trafficking and transcription PLoS One 3:e4093 doi: 10.1371/journal.pone.0004093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Dever SM, Podhaizer EM, Arnatt CK, Zhang Y, Hauser KF (2013) A novel bivalent HIV-1 entry inhibitor reveals fundamental differences in CCR5-mu-opioid receptor interactions between human astroglia and microglia AIDS 27:2181–2190 doi: 10.1097/QAD.0b013e3283639804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF (2005) Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat Glia 50:91–106 doi: 10.1002/glia.20148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Rodriguez M, Dever SM, Masvekar RR, Gewirtz DA, Shacka JJ (2015) HIV-1 and morphine regulation of autophagy in microglia: limited interactions in the context of HIV-1 infection and opioid abuse J Virol 89:1024–1035 doi: 10.1128/JVI.02022-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Wu G, Ambati J, Bruce-Keller AJ, Knapp PE, Hauser KF (2006a) CCR2 mediates increases in glial activation caused by exposure to HIV-1 Tat and opiates J Neuroimmunol 178:9–16 doi: 10.1016/j.jneuroim.2006.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N et al. (2006b) HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines Glia 53:132–146 doi: 10.1002/glia.20262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecho K, Maslonek KA, Coussons-Read ME, Dykstra LA, Lysle DT (1994) Macrophage-derived nitric oxide is involved in the depressed concanavalin A responsiveness of splenic lymphocytes from rats administered morphine in vivo J Immunol 152:5845–5852 [PubMed] [Google Scholar]
- Felten SY, Olschowka J (1987) Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp J Neurosci Res 18:37–48 doi: 10.1002/jnr.490180108 [DOI] [PubMed] [Google Scholar]
- Fiellin DA et al. (2011) Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone J Acquir Immune Defic Syndr 56 Suppl 1:S33–38 doi: 10.1097/QAI.0b013e3182097537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Knapp PE, Zou S, Marks WD, Bowers MS, Akbarali HI, Hauser KF (2014) Interactive HIV-1 Tat and morphine-induced synaptodendritic injury is triggered through focal disruptions in Na(+) influx, mitochondrial instability, and Ca(2)(+) overload J Neurosci 34:12850–12864 doi: 10.1523/JNEUROSCI.5351-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glattard E et al. (2010) Endogenous morphine levels are increased in sepsis: a partial implication of neutrophils PLoS One 5:e8791 doi: 10.1371/journal.pone.0008791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godai K et al. (2014) Peripheral administration of morphine attenuates postincisional pain by regulating macrophage polarization through COX-2-dependent pathway Mol Pain 10:36 doi: 10.1186/1744-8069-10-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonek M et al. (2018) CCR5 mediates HIV-1 Tat-induced neuroinflammation and influences morphine tolerance, dependence, and reward Brain Behav Immun 69:124–138 doi: 10.1016/j.bbi.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM et al. (2014) Activation of adult rat CNS endothelial cells by opioid-induced toll-like receptor 4 (TLR4) signaling induces proinflammatory, biochemical, morphological, and behavioral sequelae Neuroscience 280:299–317 doi: 10.1016/j.neuroscience.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeneltch KM, Kelly-Welch AE, Shi Y, Keegan AD (2005) Chronic morphine treatment promotes specific Th2 cytokine production by murine T cells in vitro via a Fas/Fas ligand-dependent mechanism J Immunol 175:4999–5005 doi: 10.4049/jimmunol.175.8.4999 [DOI] [PubMed] [Google Scholar]
- Grimm MC, Ben-Baruch A, Taub DD, Howard OM, Wang JM, Oppenheim JJ (1998) Opiate inhibition of chemokine-induced chemotaxis Ann N Y Acad Sci 840:9–20 doi: 10.1111/j.1749-6632.1998.tb09544.x [DOI] [PubMed] [Google Scholar]
- Guo CJ, Li Y, Tian S, Wang X, Douglas SD, Ho WZ (2002) Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor J Investig Med 50:435–442 doi: 10.1136/jim-50-06-03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C et al. (2020) Morphine induces the differentiation of T helper cells to Th2 effector cells via the PKC-theta-GATA3 pathway Int Immunopharmacol 80:106133 doi: 10.1016/j.intimp.2019.106133 [DOI] [PubMed] [Google Scholar]
- Harari Y, Weisbrodt NW, Moody FG (2006) The effect of morphine on mast cell-mediated mucosal permeability Surgery 139:54–60 doi: 10.1016/j.surg.2005.07.009 [DOI] [PubMed] [Google Scholar]
- Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE (2012) Opiate drug use and the pathophysiology of neuroAIDS Curr HIV Res 10:435–452 doi: 10.2174/157016212802138779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease Pharmacol Rev 57:173–185 doi: 10.1124/pr.57.2.4 [DOI] [PubMed] [Google Scholar]
- Hollenbach R et al. (2014) Effect of morphine and SIV on dendritic cell trafficking into the central nervous system of rhesus macaques J Neurovirol 20:175–183 doi: 10.1007/s13365-013-0182-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G et al. (2012) Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction Cell Death Dis 3:e381 doi: 10.1038/cddis.2012.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan A, Defechereux P, Verdin E (2001) The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation EMBO J 20:1726–1738 doi: 10.1093/emboj/20.7.1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia F, Vlahov D, Donahoe RM, Friedland G (2005) The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations Clin Infect Dis 41:1027–1034 doi: 10.1086/433175 [DOI] [PubMed] [Google Scholar]
- Karki S, Timilsina S, Sharma S (2018) Does Morphine Influence Blood Count in Palliative Patients? : A Longitudinal Study from an Oncology Center in Nepal Journal of Pain Management & Medicine 04 doi: 10.35248/2684-1320.18.4.134 [DOI] [Google Scholar]
- Kauder SE, Bosque A, Lindqvist A, Planelles V, Verdin E (2009) Epigenetic regulation of HIV-1 latency by cytosine methylation PLoS Pathog 5:e1000495 doi: 10.1371/journal.ppat.1000495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelschenbach J, Barke RA, Roy S (2005) Morphine withdrawal contributes to Th cell differentiation by biasing cells toward the Th2 lineage J Immunol 175:2655–2665 doi: 10.4049/jimmunol.175.4.2655 [DOI] [PubMed] [Google Scholar]
- Kelschenbach J, Ninkovic J, Wang J, Krishnan A, Charboneau R, Barke RA, Roy S (2008) Morphine withdrawal inhibits IL-12 induction in a macrophage cell line through a mechanism that involves cAMP J Immunol 180:3670–3679 doi: 10.4049/jimmunol.180.6.3670 [DOI] [PubMed] [Google Scholar]
- Khabbazi S, Goumon Y, Parat MO (2015) Morphine Modulates Interleukin-4- or Breast Cancer Cell-induced Pro-metastatic Activation of Macrophages Sci Rep 5:11389 doi: 10.1038/srep11389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Hahn YK, Podhaizer EM, McLane VD, Zou S, Hauser KF, Knapp PE (2018) A central role for glial CCR5 in directing the neuropathological interactions of HIV-1 Tat and opiates J Neuroinflammation 15:285 doi: 10.1186/s12974-018-1320-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevich J, Langford D (2012) Neuronal toxicity in HIV CNS disease Future Virol 7:687–698 doi: 10.2217/fvl.12.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashin DL, Merrill JO, Trescot AM (2012) Opioids in the management of HIV-related pain Pain Physician 15:ES157–168 [PubMed] [Google Scholar]
- Kumar R et al. (2006) Chronic morphine exposure causes pronounced virus replication in cerebral compartment and accelerated onset of AIDS in SIV/SHIV-infected Indian rhesus macaques Virology 354:192–206 doi: 10.1016/j.virol.2006.06.020 [DOI] [PubMed] [Google Scholar]
- Kumar R et al. (2004) Modulation by morphine of viral set point in rhesus macaques infected with simian immunodeficiency virus and simian-human immunodeficiency virus J Virol 78:11425–11428 doi: 10.1128/JVI.78.20.11425-11428.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre J, Rodriguez M, Ojha CR, El-Hage N (2018) Critical Role of Beclin1 in HIV Tat and Morphine-Induced Inflammation and Calcium Release in Glial Cells from Autophagy Deficient Mouse J Neuroimmune Pharmacol 13:355–370 doi: 10.1007/s11481-018-9788-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz SS, Chiang CY (1975) Effects of certain abused drug s on hemolysin forming cells Life Sci 17:1763–1767 doi: 10.1016/0024-3205(75)90458-0 [DOI] [PubMed] [Google Scholar]
- Leo S, Nuydens R, Meert TF (2009) Opioid-induced proliferation of vascular endothelial cells J Pain Res 2:59–66 doi: 10.2147/jpr.s4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BH et al. (2012) Stat6 activity-related Th2 cytokine profile and tumor growth advantage of human colorectal cancer cells in vitro and in vivo Cell Signal 24:718–725 doi: 10.1016/j.cellsig.2011.11.005 [DOI] [PubMed] [Google Scholar]
- Li Y et al. (2003) Morphine enhances HIV infection of neonatal macrophages Pediatr Res 54:282–288 doi: 10.1203/01.PDR.0000074973.83826.4C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Liu X, Tang SJ (2016) Interactions of Opioids and HIV Infection in the Pathogenesis of Chronic Pain Front Microbiol 7:103 doi: 10.3389/fmicb.2016.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk K et al. (2012) Influence of morphine on pericyte-endothelial interaction: implications for antiangiogenic therapy J Oncol 2012:458385 doi: 10.1155/2012/458385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum PJ, Tulsky JP (2006) The medical management of opioid dependence in HIV primary care settings Curr HIV/AIDS Rep 3:195–204 doi: 10.1007/s11904-006-0016-z [DOI] [PubMed] [Google Scholar]
- Ma J, Wang J, Wan J, Charboneau R, Chang Y, Barke RA, Roy S (2010) Morphine disrupts interleukin-23 (IL-23)/IL-17-mediated pulmonary mucosal host defense against Streptococcus pneumoniae infection Infect Immun 78:830–837 doi: 10.1128/IAI.00914-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madera-Salcedo IK, Cruz SL, Gonzalez-Espinosa C (2011) Morphine decreases early peritoneal innate immunity responses in Swiss-Webster and C57BL6/J mice through the inhibition of mast cell TNF-alpha release J Neuroimmunol 232:101–107 doi: 10.1016/j.jneuroim.2010.10.017 [DOI] [PubMed] [Google Scholar]
- Madera-Salcedo IK, Cruz SL, Gonzalez-Espinosa C (2013) Morphine prevents lipopolysaccharide-induced TNF secretion in mast cells blocking IkappaB kinase activation and SNAP-23 phosphorylation: correlation with the formation of a beta-arrestin/TRAF6 complex J Immunol 191:3400–3409 doi: 10.4049/jimmunol.1202658 [DOI] [PubMed] [Google Scholar]
- Mahajan SD et al. (2017) Immunomodulatory Role of Complement Proteins in the Neuropathology Associated with Opiate Abuse and HIV-1 Co-Morbidity Immunol Invest 46:816–832 doi: 10.1080/08820139.2017.1371891 [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Reynolds JL, Nair BB, Fernandez SF, Schwartz SA, Nair MP (2005a) Morphine exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in U373 astrocytoma cells Curr HIV Res 3:277–288 doi: 10.2174/1570162054368048 [DOI] [PubMed] [Google Scholar]
- Mahajan SD et al. (2008) Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability J Clin Immunol 28:528–541 doi: 10.1007/s10875-008-9208-1 [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Aalinkeel R, Chawda RP, Sykes DE, Nair MP (2005b) Morphine modulates chemokine gene regulation in normal human astrocytes Clin Immunol 115:323–332 doi: 10.1016/j.clim.2005.02.004 [DOI] [PubMed] [Google Scholar]
- Malik S, Khalique H, Buch S, Seth P (2011) A growth factor attenuates HIV-1 Tat and morphine induced damage to human neurons: implication in HIV/AIDS-drug abuse cases PLoS One 6:e18116 doi: 10.1371/journal.pone.0018116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcario JK et al. (2008) Effect of morphine on the neuropathogenesis of SIVmac infection in Indian Rhesus Macaques J Neuroimmune Pharmacol 3:12–25 doi: 10.1007/s11481-007-9085-z [DOI] [PubMed] [Google Scholar]
- Maricato JT et al. (2015) Epigenetic modulations in activated cells early after HIV-1 infection and their possible functional consequences PLoS One 10:e0119234 doi: 10.1371/journal.pone.0119234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Koodie L, Krishnan AG, Charboneau R, Barke RA, Roy S (2010) Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site Am J Pathol 176:786–799 doi: 10.2353/ajpath.2010.090457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masvekar RR, El-Hage N, Hauser KF, Knapp PE (2014) Morphine enhances HIV-1SF162-mediated neuron death and delays recovery of injured neurites PLoS One 9:e100196 doi: 10.1371/journal.pone.0100196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masvekar RR, El-Hage N, Hauser KF, Knapp PE (2015) GSK3beta-activation is a point of convergence for HIV-1 and opiate-mediated interactive neurotoxicity Mol Cell Neurosci 65:11–20 doi: 10.1016/j.mcn.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon RD, Bayer BM (2001) Reversal of acute effects of high dose morphine on lymphocyte activity by chlorisondamine Drug Alcohol Depend 62:141–147 doi: 10.1016/s0376-8716(00)00184-8 [DOI] [PubMed] [Google Scholar]
- Meng J, Banerjee S, Li D, Sindberg GM, Wang F, Ma J, Roy S (2015a) Opioid Exacerbation of Gram-positive sepsis, induced by Gut Microbial Modulation, is Rescued by IL-17A Neutralization Sci Rep 5:10918 doi: 10.1038/srep10918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Sindberg GM, Roy S (2015b) Disruption of gut homeostasis by opioids accelerates HIV disease progression Front Microbiol 6:643 doi: 10.3389/fmicb.2015.00643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer SL, Coop A (2011) Opioid analgesics and P-glycoprotein efflux transporters: a potential systems-level contribution to analgesic tolerance Curr Top Med Chem 11:1157–1164 doi: 10.2174/156802611795371288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin JS, Bulls HW, Vucovich LA, Edelman EJ, Starrels JL (2016) Pharmacologic and non-pharmacologic treatments for chronic pain in individuals with HIV: a systematic review AIDS Care 28:1506–1515 doi: 10.1080/09540121.2016.1191612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi T, Chuang LF, Doi RH, Carlos MP, Torres JV, Chuang RY (2000) Morphine induces gene expression of CCR5 in human CEMx174 lymphocytes J Biol Chem 275:31305–31310 doi: 10.1074/jbc.M001269200 [DOI] [PubMed] [Google Scholar]
- Mutua JM, Perelson AS, Kumar A, Vaidya NK (2019) Modeling the Effects of Morphine-Altered Virus Specific Antibody Responses on HIV/SIV Dynamics Sci Rep 9:5423 doi: 10.1038/s41598-019-41751-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A (2002) Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia J Infect Dis 186 Suppl 2:S193–198 doi: 10.1086/344528 [DOI] [PubMed] [Google Scholar]
- Nath A et al. (2000) Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia J Psychopharmacol 14:222–227 doi: 10.1177/026988110001400305 [DOI] [PubMed] [Google Scholar]
- Nath A et al. (2002) Molecular basis for interactions of HIV and drugs of abuse J Acquir Immune Defic Syndr 31 Suppl 2:S62–69 doi: 10.1097/00126334-200210012-00006 [DOI] [PubMed] [Google Scholar]
- Nguyen J et al. (2014) Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer Br J Anaesth 113 Suppl 1:i4–13 doi: 10.1093/bja/aeu090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel RJ Jr., Rivera-Amill V, Buch S, Kumar A (2008) Opiates, immune system, acquired immunodeficiency syndrome, and nonhuman primate model J Neurovirol 14:279–285 doi: 10.1080/13550280802078209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman LR, Kumar A (2006) Neuropscyhological Complications of HIV Disease and Substances of Abuse Am J Infect Dis 2:67–73 doi: 10.3844/ajidsp.2006.67.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirastu R, Fais R, Messina M, Bini V, Spiga S, Falconieri D, Diana M (2006) Impaired decision-making in opiate-dependent subjects: effect of pharmacological therapies Drug Alcohol Depend 83:163–168 doi: 10.1016/j.drugalcdep.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Plytycz B, Natorska J (2002) Morphine attenuates pain and prevents inflammation in experimental peritonitis Trends Immunol 23:345–346 doi: 10.1016/s1471-4906(02)02257-3 [DOI] [PubMed] [Google Scholar]
- Prottengeier J, Koutsilieri E, Scheller C (2014) The effects of opioids on HIV reactivation in latently-infected T-lymphoblasts AIDS Res Ther 11:17 doi: 10.1186/1742-6405-11-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim RT, Adler MW, Meissler JJ Jr., Cowan A, Rogers TJ, Geller EB, Eisenstein TK (2002) Abrupt or precipitated withdrawal from morphine induces immunosuppression J Neuroimmunol 127:88–95 doi: 10.1016/s0165-5728(02)00103-0 [DOI] [PubMed] [Google Scholar]
- Rahim RT, Meissler JJ Jr., Adler MW, Eisenstein TK (2005) Splenic macrophages and B cells mediate immunosuppression following abrupt withdrawal from morphine J Leukoc Biol 78:1185–1191 doi: 10.1189/jlb.0304123 [DOI] [PubMed] [Google Scholar]
- Reynolds JL et al. (2012) Morphine and galectin-1 modulate HIV-1 infection of human monocyte-derived macrophages J Immunol 188:3757–3765 doi: 10.4049/jimmunol.1102276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Amill V, Silverstein PS, Noel RJ Jr., Kumar S, Kumar A (2010) Morphine and rapid disease progression in nonhuman primate model of AIDS: inverse correlation between disease progression and virus evolution J Neuroimmune Pharmacol 5:122–132 doi: 10.1007/s11481-009-9184-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M et al. (2017) Importance of Autophagy in Mediating Human Immunodeficiency Virus (HIV) and Morphine-Induced Metabolic Dysfunction and Inflammation in Human Astrocytes Viruses 9 doi: 10.3390/v9080201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojavin M, Szabo I, Bussiere JL, Rogers TJ, Adler MW, Eisenstein TK (1993) Morphine treatment in vitro or in vivo decreases phagocytic functions of murine macrophages Life Sci 53:997–1006 doi: 10.1016/0024-3205(93)90122-j [DOI] [PubMed] [Google Scholar]
- Roy S et al. (2001) Morphine directs T cells toward T(H2) differentiation Surgery 130:304–309 doi: 10.1067/msy.2001.116033 [DOI] [PubMed] [Google Scholar]
- Roy S, Chapin RB, Cain KJ, Charboneau RG, Ramakrishnan S, Barke RA (1997) Morphine inhibits transcriptional activation of IL-2 in mouse thymocytes Cell Immunol 179:1–9 doi: 10.1006/cimm.1997.1147 [DOI] [PubMed] [Google Scholar]
- Roy S et al. (2011) Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections J Neuroimmune Pharmacol 6:442–465 doi: 10.1007/s11481-011-9292-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Wang J, Charboneau R, Loh HH, Barke RA (2005) Morphine induces CD4+ T cell IL-4 expression through an adenylyl cyclase mechanism independent of the protein kinase A pathway J Immunol 175:6361–6367 doi: 10.4049/jimmunol.175.10.6361 [DOI] [PubMed] [Google Scholar]
- Roy S, Wang J, Gupta S, Charboneau R, Loh HH, Barke RA (2004) Chronic morphine treatment differentiates T helper cells to Th2 effector cells by modulating transcription factors GATA 3 and T-bet J Neuroimmunol 147:78–81 doi: 10.1016/j.jneuroim.2003.10.016 [DOI] [PubMed] [Google Scholar]
- Roy S, Wang J, Kelschenbach J, Koodie L, Martin J (2006) Modulation of immune function by morphine: implications for susceptibility to infection J Neuroimmune Pharmacol 1:77–89 doi: 10.1007/s11481-005-9009-8 [DOI] [PubMed] [Google Scholar]
- Ru W, Tang SJ (2017) HIV-associated synaptic degeneration Mol Brain 10:40 doi: 10.1186/s13041-017-0321-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacerdote P (2006) Opioids and the immune system Palliat Med 20 Suppl 1:s9–15 [PubMed] [Google Scholar]
- Samikkannu T, Ranjith D, Rao KV, Atluri VS, Pimentel E, El-Hage N, Nair MP (2015) HIV-1 gp120 and morphine induced oxidative stress: role in cell cycle regulation Front Microbiol 6:614 doi: 10.3389/fmicb.2015.00614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurer TB, Carrigan KA, Ijames SG, Lysle DT (2006a) Suppression of natural killer cell activity by morphine is mediated by the nucleus accumbens shell J Neuroimmunol 173:3–11 doi: 10.1016/j.jneuroim.2005.11.009 [DOI] [PubMed] [Google Scholar]
- Saurer TB, Ijames SG, Lysle DT (2006b) Neuropeptide Y Y1 receptors mediate morphine-induced reductions of natural killer cell activity J Neuroimmunol 177:18–26 doi: 10.1016/j.jneuroim.2006.05.002 [DOI] [PubMed] [Google Scholar]
- Sharma HS, Ali SF (2006) Alterations in blood-brain barrier function by morphine and methamphetamine Ann N Y Acad Sci 1074:198–224 doi: 10.1196/annals.1369.020 [DOI] [PubMed] [Google Scholar]
- Sharma HS, Sjoquist PO, Ali SF (2010) Alterations in blood-brain barrier function and brain pathology by morphine in the rat. Neuroprotective effects of antioxidant H-290/51 Acta Neurochir Suppl 106:61–66 doi: 10.1007/978-3-211-98811-4_10 [DOI] [PubMed] [Google Scholar]
- Sharp BM, Keane WF, Suh HJ, Gekker G, Tsukayama D, Peterson PK (1985) Opioid peptides rapidly stimulate superoxide production by human polymorphonuclear leukocytes and macrophages Endocrinology 117:793–795 doi: 10.1210/endo-117-2-793 [DOI] [PubMed] [Google Scholar]
- Shavit Y et al. (1986) Involvement of brain opiate receptors in the immune-suppressive effect of morphine Proc Natl Acad Sci U S A 83:7114–7117 doi: 10.1073/pnas.83.18.7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavit Y et al. (1987) Effects of a single administration of morphine or footshock stress on natural killer cell cytotoxicity Brain Behav Immun 1:318–328 doi: 10.1016/0889-1591(87)90034-1 [DOI] [PubMed] [Google Scholar]
- Shawahna R et al. (2011) Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels Mol Pharm 8:1332–1341 doi: 10.1021/mp200129p [DOI] [PubMed] [Google Scholar]
- Shirazi J, Shah S, Sagar D, Nonnemacher MR, Wigdahl B, Khan ZK, Jain P (2013) Epigenetics, drugs of abuse, and the retroviral promoter J Neuroimmune Pharmacol 8:1181–1196 doi: 10.1007/s11481-013-9508-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil S, Periyasamy P, Guo ML, Callen S, Buch S (2018) Morphine-Mediated Brain Region-Specific Astrocytosis Involves the ER Stress-Autophagy Axis Mol Neurobiol 55:6713–6733 doi: 10.1007/s12035-018-0878-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins CO, Alailima ST, Tate EA, Johnson M (1986) The effect of enkephalins and prostaglandins on O-2 release by neutrophils J Surg Res 41:645–652 doi: 10.1016/0022-4804(86)90090-9 [DOI] [PubMed] [Google Scholar]
- Singhal PC, Sharma P, Kapasi AA, Reddy K, Franki N, Gibbons N (1998) Morphine enhances macrophage apoptosis J Immunol 160:1886–1893 [PubMed] [Google Scholar]
- Spijkerman IJ et al. (1996) Differences in progression to AIDS between injection drug users and homosexual men with documented dates of seroconversion Epidemiology 7:571–577 doi: 10.1097/00001648-199611000-00002 [DOI] [PubMed] [Google Scholar]
- Stankiewicz E, Wypasek E, Plytycz B (2004) Mast cells are responsible for the lack of anti-inflammatory effects of morphine in CBA mice Mediators Inflamm 13:365–368 doi: 10.1080/09629350400008778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ (2003) Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication Virology 309:99–107 doi: 10.1016/s0042-6822(03)00015-1 [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang AJ, Chuang LF, Doi RH, Chuang RY (2002) Morphine promotes simian acquired immunodeficiency syndrome virus replication in monkey peripheral mononuclear cells: induction of CC chemokine receptor 5 expression for virus entry J Infect Dis 185:1826–1829 doi: 10.1086/340816 [DOI] [PubMed] [Google Scholar]
- Szabo I, Rojavin M, Bussiere JL, Eisenstein TK, Adler MW, Rogers TJ (1993) Suppression of peritoneal macrophage phagocytosis of Candida albicans by opioids J Pharmacol Exp Ther 267:703–706 [PubMed] [Google Scholar]
- Tabellini G et al. (2014) Effects of opioid therapy on human natural killer cells Int Immunopharmacol 18:169–174 doi: 10.1016/j.intimp.2013.11.015 [DOI] [PubMed] [Google Scholar]
- Tahamtan A, Tavakoli-Yaraki M, Mokhtari-Azad T, Teymoori-Rad M, Bont L, Shokri F, Salimi V (2016) Opioids and Viral Infections: A Double-Edged Sword Front Microbiol 7:970 doi: 10.3389/fmicb.2016.00970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe LE et al. (2004) Effect of hard-drug use on CD4 cell percentage, HIV RNA level, and progression to AIDS-defining class C events among HIV-infected women J Acquir Immune Defic Syndr 37:1423–1430 doi: 10.1097/01.qai.0000127354.78706.5d [DOI] [PubMed] [Google Scholar]
- Tirado G, Kumar A (2006) Evolution of SIV envelope in morphine-dependent rhesus macaques with rapid disease progression AIDS Res Hum Retroviruses 22:114–119 doi: 10.1089/aid.2006.22.114 [DOI] [PubMed] [Google Scholar]
- Tomei EZ, Renaud FL (1997) Effect of morphine on Fc-mediated phagocytosis by murine macrophages in vitro J Neuroimmunol 74:111–116 doi: 10.1016/s0165-5728(96)00213-5 [DOI] [PubMed] [Google Scholar]
- Tunblad K, Jonsson EN, Hammarlund-Udenaes M (2003) Morphine blood-brain barrier transport is influenced by probenecid co-administration Pharm Res 20:618–623 doi: 10.1023/a:1023250900462 [DOI] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga FO, Ding Q, Keller JN, Hauser KF, Knapp PE, Bruce-Keller AJ (2008) Cell-specific actions of HIV-Tat and morphine on opioid receptor expression in glia J Neurosci Res 86:2100–2110 doi: 10.1002/jnr.21653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga FO, Gupta S, Keller JN, Knapp PE, Hauser KF, Bruce-Keller AJ (2009) Morphine and HIV-Tat increase microglial-free radical production and oxidative stress: possible role in cytokine regulation J Neurochem 108:202–215 doi: 10.1111/j.1471-4159.2008.05756.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi M, Bukrinsky M, Simon GL (2016) Mechanisms of HIV Transcriptional Regulation by Drugs of Abuse Curr HIV Res 14:442–454 doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi M, Pearson RJ, Karn J (2010) Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction J Virol 84:6425–6437 doi: 10.1128/JVI.01519-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T (2011) Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors J Neurochem 117:333–345 doi: 10.1111/j.1471-4159.2011.07208.x [DOI] [PubMed] [Google Scholar]
- Vaidya NK, Ribeiro RM, Perelson AS, Kumar A (2016) Modeling the Effects of Morphine on Simian Immunodeficiency Virus Dynamics PLoS Comput Biol 12:e1005127 doi: 10.1371/journal.pcbi.1005127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegraaff N, Devroe E, Turlure F, Silver PA, Engelman A (2006) Biochemical and genetic analyses of integrase-interacting proteins lens epithelium-derived growth factor (LEDGF)/p75 and hepatoma-derived growth factor related protein 2 (HRP2) in preintegration complex function and HIV-1 replication Virology 346:415–426 doi: 10.1016/j.virol.2005.11.022 [DOI] [PubMed] [Google Scholar]
- Verdin E (1991) DNase I-hypersensitive sites are associated with both long terminal repeats and with the intragenic enhancer of integrated human immunodeficiency virus type 1 J Virol 65:6790–6799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E, Paras P Jr., Van Lint C (1993) Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation EMBO J 12:3249–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GP, Ciuffi A, Leipzig J, Berry CC, Bushman FD (2007a) HIV integration site selection: analysis by massively parallel pyrosequencing reveals association with epigenetic modifications Genome Res 17:1186–1194 doi: 10.1101/gr.6286907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Schwendener R, Roy S (2008) Morphine induces defects in early response of alveolar macrophages to Streptococcus pneumoniae by modulating TLR9-NF-kappa B signaling J Immunol 180:3594–3600 doi: 10.4049/jimmunol.180.5.3594 [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Roy S (2007b) Transcriptional and epigenetic regulation of interleukin-2 gene in activated T cells by morphine J Biol Chem 282:7164–7171 doi: 10.1074/jbc.M604367200 [DOI] [PubMed] [Google Scholar]
- Wang J, Charboneau R, Balasubramanian S, Barke RA, Loh HH, Roy S (2002) The immunosuppressive effects of chronic morphine treatment are partially dependent on corticosterone and mediated by the mu-opioid receptor J Leukoc Biol 71:782–790 [PubMed] [Google Scholar]
- Wang J, Ma J, Charboneau R, Barke R, Roy S (2011a) Morphine inhibits murine dendritic cell IL-23 production by modulating Toll-like receptor 2 and Nod2 signaling J Biol Chem 286:10225–10232 doi: 10.1074/jbc.M110.188680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tan N, Douglas SD, Zhang T, Wang YJ, Ho WZ (2005) Morphine inhibits CD8+ T cell-mediated, noncytolytic, anti-HIV activity in latently infected immune cells J Leukoc Biol 78:772–776 doi: 10.1189/jlb.0305167 [DOI] [PubMed] [Google Scholar]
- Wang X, Ye L, Hou W, Zhou Y, Wang YJ, Metzger DS, Ho WZ (2009) Cellular microRNA expression correlates with susceptibility of monocytes/macrophages to HIV-1 infection Blood 113:671–674 doi: 10.1182/blood-2008-09-175000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ye L, Zhou Y, Liu MQ, Zhou DJ, Ho WZ (2011b) Inhibition of anti-HIV microRNA expression: a mechanism for opioid-mediated enhancement of HIV infection of monocytes Am J Pathol 178:41–47 doi: 10.1016/j.ajpath.2010.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang X, Ye L, Li J, Song L, Fulambarkar N, Ho W (2012) Morphine suppresses IFN signaling pathway and enhances AIDS virus infection PLoS One 7:e31167 doi: 10.1371/journal.pone.0031167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RJ, Ikejiri B, Rice KC, Pert A, Hagan AA (1987) Opiate receptor mediated regulation of the immune response in vivo NIDA Res Monogr 76:341–348 [PubMed] [Google Scholar]
- Weber RJ, Pert A (1989) The periaqueductal gray matter mediates opiate-induced immunosuppression Science 245:188–190 doi: 10.1126/science.2749256 [DOI] [PubMed] [Google Scholar]
- Welters ID (2003) Is immunomodulation by opioid drugs of clinical relevance? Curr Opin Anaesthesiol 16:509–513 doi: 10.1097/00001503-200310000-00011 [DOI] [PubMed] [Google Scholar]
- West JP, Dykstra LA, Lysle DT (1999) Immunomodulatory effects of morphine withdrawal in the rat are time dependent and reversible by clonidine Psychopharmacology (Berl) 146:320–327 doi: 10.1007/s002130051123 [DOI] [PubMed] [Google Scholar]
- Yeager MP, Colacchio TA, Yu CT, Hildebrandt L, Howell AL, Weiss J, Guyre PM (1995) Morphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteers Anesthesiology 83:500–508 doi: 10.1097/00000542-199509000-00008 [DOI] [PubMed] [Google Scholar]
- Yin D, Mufson RA, Wang R, Shi Y (1999) Fas-mediated cell death promoted by opioids Nature 397:218 doi: 10.1038/16612 [DOI] [PubMed] [Google Scholar]
- Yossuck P, Nightengale BJ, Fortney JE, Gibson LF (2008) Effect of morphine sulfate on neonatal neutrophil chemotaxis Clin J Pain 24:76–82 doi: 10.1097/AJP.0b013e3181582c76 [DOI] [PubMed] [Google Scholar]
- Youn GS, Cho H, Kim D, Choi SY, Park J (2017) Crosstalk between HDAC6 and Nox2-based NADPH oxidase mediates HIV-1 Tat-induced pro-inflammatory responses in astrocytes Redox Biol 12:978–986 doi: 10.1016/j.redox.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Fitting S, Hahn YK, Welch SP, El-Hage N, Hauser KF, Knapp PE (2011) Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at mu-opioid receptor-expressing glia Brain 134:3616–3631 doi: 10.1093/brain/awr281 [DOI] [PMC free article] [PubMed] [Google Scholar]


