Abstract
Healthcare-associated infections are a major public health concern for both patients and medical personnel. This has taken on greater urgency during the current COVID-19 pandemic. Radiation Personal Protective Equipment (RPPE) may contribute to risks of microbial contamination. This possibility was tested in 61 personal or shared-use lead aprons and thyroid collars at Columbia Presbyterian Irving Medical Center. 50% tested positive for either bacterial or fungal contamination, mostly around the neckline of lead vests and thyroid collars. Repeated testing of garments some weeks to months later confirmed continued presence of microbial contamination. The possibility that hospital approved disinfection agents could degrade the radio-protective features of these garments was also examined. Samples of identical construction to garments in regular use were subjected to either daily or weekly wipes with hypochlorite or alcohol-based hospital approved cleaning agents for six months. A third group of samples were maintained in contact with the cleaning agents for six months. All samples were fluoroscoped four times during the study. None demonstrated any degradation in radio-protection. All samples were photographed monthly. Physical degradation of the outer plastic covering by concentrated hypochlorite, and limited mechanical damage around stitched seams of the samples cleaned daily with alcohol, was noted. Based on the high prevalence of microbial contamination, regular cleaning and disinfection protocols should be implemented. Regular cleaning with medical facility approved cleaning and disinfecting agents is likely to be effective at reducing the microbial load and unlikely to result in significant reduction in radioprotective properties of these garments.
Keywords: Healthcare-associated infection risk, Hospital environmental cleaning, Personal Protective Equipment (PPE), Infection Control, Fluoroscopy, Anti-microbial agents
INTRODUCTION
Healthcare associated infections are a major public health concern involving more than 2 million patients and more than 90,000 deaths each year in the USA (Burke 2003, Shafer et al. 2019). This concern has taken on greater urgency for both patients and caregivers during the current COVID-19 pandemic (WHO, 2020). Disposable bio-protective garments worn by medical personnel are one such source of contamination in hospitals. Another underappreciated source of exposure may be occupational Radiation Personal Protective Equipment (RPPE) worn by those at risk of ionizing radiation exposure. In medical settings, these usually include both a one or two piece apron and a thyroid collar (Klein et al. 2009; Smilowitz et al. 2013). While most users understand that these garments and devices are designed to protect the wearer from radiation exposure, many users are less aware that RPPE can be biologically contaminated during normal use by the wearers themselves, from other workers, by patients or from the environment.
These garments may be personal, belonging to and worn by a single individual, or shared among multiple users. In many facilities, garments are placed on exposed coat racks in hallways or procedure rooms. After use, they are typically simply placed back onto the original rack. Visible contamination can sometimes be noted as stains on the coverings, indicating infrequent cleaning. Prior studies have indicated a variety of microorganisms including S. aureus, coagulase-negative Staphylococci and gram-positive rods can be detected on leaded garment in general surgery (Jain et al. 2019) and orthopedic (Grogan et al. 2011) operating suites. This manuscript is the first to describe microorganism contamination of RPPE in an interventional medical setting.
In some cases, RPPE may be worn by personnel underneath a disposable surgical gown and may therefore be less likely to be contaminated. Significant variation in microbial protection afforded by disposable gowns is well documented (Lovitt et al. 1992) and contamination of underlying lead aprons can occur. In practice, not all users within the interventional suite routinely don disposable coverings over their RPPE.
Individuals can contaminate their own RPPE simply by wearing it. Most items (e.g., lead aprons and thyroid-collars) are reused multiple times over many months to years. While some individuals utilize their own personal RPPE exclusively, in many cases, multiple users share the same equipment. This shared use increases the risk of cross- contamination.
Historically, only limited attention was given to RPPE infection control and cleaning. Procedures ranged from removing visible soiling to periodically shipping the RPPE to an external vendor for decontamination. Enhancements, partially motivated by the COVID-19 pandemic, should include improved regular cleaning and decontamination, as well as institutionalized standard operating policies and procedures.
This paper addresses some elements of microbiological decontamination of RPPE in a hospital or other health-care environment. Minimizing the potential health risks associated with surface bio-contamination should be a priority that is performed on a regular basis. Many health-care facilities maintain written policies and procedures that include approved cleaning materials and frequencies. Other cleaning materials, including those recommended by the PPE manufacturers, need to be reviewed and approved by the facility’s infection control officers before use.
Radiological inspection of RPPE for deterioration of its protective value is a routine radiation safety function commonly done annually. Experience has shown that most items pass. Additional testing may be required when a more robust RPPE cleaning program is initiated and these may differ among different practice settings.
This paper reports findings in a large university interventional fluoroscopy and surgery center. Concern about the need for more frequent cleaning of RPPE was driven by an initial pilot study that demonstrated consistent bacterial and fungal contamination on gowns and thyroid collars routinely used in the cath lab and in operating rooms. Based on these limited findings, cath lab senior personnel advised RPPE users to decontaminate their garment before donning in the morning using the same hospital supplied wipes used to decontaminate surfaces such as table-tops. After this recommendation, some users expressed concern that repeated cleaning and disinfection using these agents might deteriorate the radioprotective properties of the RPPE and/or otherwise damage the fabric outer covering. A prospective experiment was designed to address these concerns.
Materials and Methods
Surface contamination pilot study
A variety of shared and personal protective lead garments used by interventional cardiology medical personnel at New York Presbyterian Hospital, Columbia University Irving Medical Center in New York City were tested for the presence of culturable bacterial or fungal microorganisms. All garments were uniquely identified by their manufacturer’s or hospital assigned ID number. Medical personnel included physicians, nurses, technologists, and imaging staff. Sixty-one different garments were tested. In some cases, the same garment was retested up to three times over the course of several weeks.
RPPE lead aprons and thyroid collars used in the cath lab and operating room were tested for bio-contamination by physically wiping the items with culture swabs in a consistent pattern across the central chest area, inside and outside lining at the armpit area, and inside and outside the collar neckline. “S” shape swabbing patterns were confined to an approximately 5 × 5 cm region in each sampling area. BD CultureSwab™ MaxV collection and transport systems (Becton, Dickinson & Co, Franklin Lakes, NJ) were used to collect these samples as, according to the manufacturer, non-animal proteins imbedded into the swabs themselves “improve microorganism viability during transport” and “improve the recovery of fastidious organisms”. These tests were done on multiple occasions from 2017–2019. At each sampling, five control swabs for each type of agar were exposed within the cath lab to the same environment and then inserted back into the transport system.
Three types of 100 × 15 mm agar culture plates were utilized: Blood, Sabouraud, and MacConkey (ThermoFisher Scientific, Waltham, MA). Blood Agar is a general purpose media that is well suited for the growth of more fastidious bacteria such as streptococci. MacConkey Agar is specific for cultures of gram-negative bacteria. Sabouraud Agar is commonly used for fungal cultures as its low pH and gentamicin aminoglycoside antibiotic content inhibit bacterial growth. When the transport system was returned to the laboratory, the swabs were inoculated onto the agar plates in consistent identical “Z” swipes and the plates incubated at 37° C in a humidified environment for up to 72 hours. Plates were examined daily and, if present, colonies counted and each colony’s description recorded. In some case, plates were imaged using a cell phone. Some bacterial samples were re-cultured and then gram stained using a Remel™ Gram Stain Kit (Remel, Lenexa, KS). Specimens were observed under 100X magnification using oil immersion and shape and color noted.
Lead apron cleaning damage testing
Small samples of RPPE were obtained from one anonymous manufacturer. These consisted of one type of the manufacturer’s lead apron material with covering and binding identical to the full size items. Lead radiographic numbers were used to uniquely identify each sample throughout the study.
Cleaning Materials:
The two types of disposable disinfectant wipes were available and approved for use in the cath lab at the start of the study. Two-thirds of the way through this study, the hospital’s infection control policy changed and the more chemically aggressive sodium hypochlorite based wipe was replaced in the clinic by one containing hydrogen peroxide as the active ingredient. To maintain consistency during the study, instead of switching to the peroxide based wipe, full strength commercial laundry bleach was substituted and applied using surgical gauze pads (C2). This substitution was also expected to accelerate any damage caused by the earlier use of approved sodium hypochlorite wipes at the beginning of the study. Table 1 reports the cleaning agents used in this study.
Table 1.
Cleaning agents used in this study
| Label | Active Ingredient(s) | Trade Name | Form |
|---|---|---|---|
| A | 0.25% n-Alkyl (68% C12, 32% C14) dimethyl ethylbenzyl ammonium chlorides. 0.25% n-Alkyl (60% C14, 30% C16, 5% C12, 5% C18) dimethyl benzyl ammonium chlorides. 55% Isopropyl Alcohol. |
Super Sani-Cloth®
Germicidal Disposable Wipes |
Wipes from a dispensing container |
| C1 | 0.55% Sodium Hypochlorite 0.55% | Clorox Healthcare® Bleach Germicidal Wipes |
Wipes from a dispensing container |
| C2 | 6% Sodium Hypochlorite (Undiluted household bleach) |
Clorox®
Bleach |
From bottle, applied using gauze squares |
PPE Samples:
Fourteen samples were supplied by the manufacturer. Seven had an additional plastic layer over the fabric front face. The other seven had a simple fabric front face. The back of all samples was fabric. Samples were edge bound and stitched in the same manner as full-size lead aprons. Additional binding was stitched to the front of each sample. Twelve samples were exposed to disinfecting wipes. The remaining two samples served as controls.
The 12 exposed samples were divided into six groups of two. One plastic front and one fabric front sample was included in each group. Over the course of six months, two groups were wiped with the disinfectant every day the investigator was in the hospital (mirroring clinical practice), two groups were wiped weekly, and two groups were in continuous contact with wet disinfectant wipes material for the duration of the study. One pair in each set was treated with wipe A, the other pair with wipe C1 (later C2). All samples were wiped on both sides.
Visual inspection and photography:
Each sample was visually inspected whenever it was handled. All samples were photographed monthly. Additional close-up photographs were obtained of areas of concern.
X-ray Inspection:
All samples were qualitatively inspected for defective radiation shielding using fluoroscopy at the beginning, mid-points, and end of the study. An interventional fluoroscopic C-arm was used in a clinical ADRC mode.
Contrast sensitivity in the images was simultaneously visually assessed using a locally constructed lead step wedge, with 21 steps ranging from 0.0 to 0.8 mm of lead. All the steps near the samples’ nominal lead equivalence of 0.35 mm were seen during the inspections. Some thin steps are white clipped because the ADRC set X-ray factors based on transmission through the RPPE samples placed in the center of the imaging field.
Results
Microbial contamination:
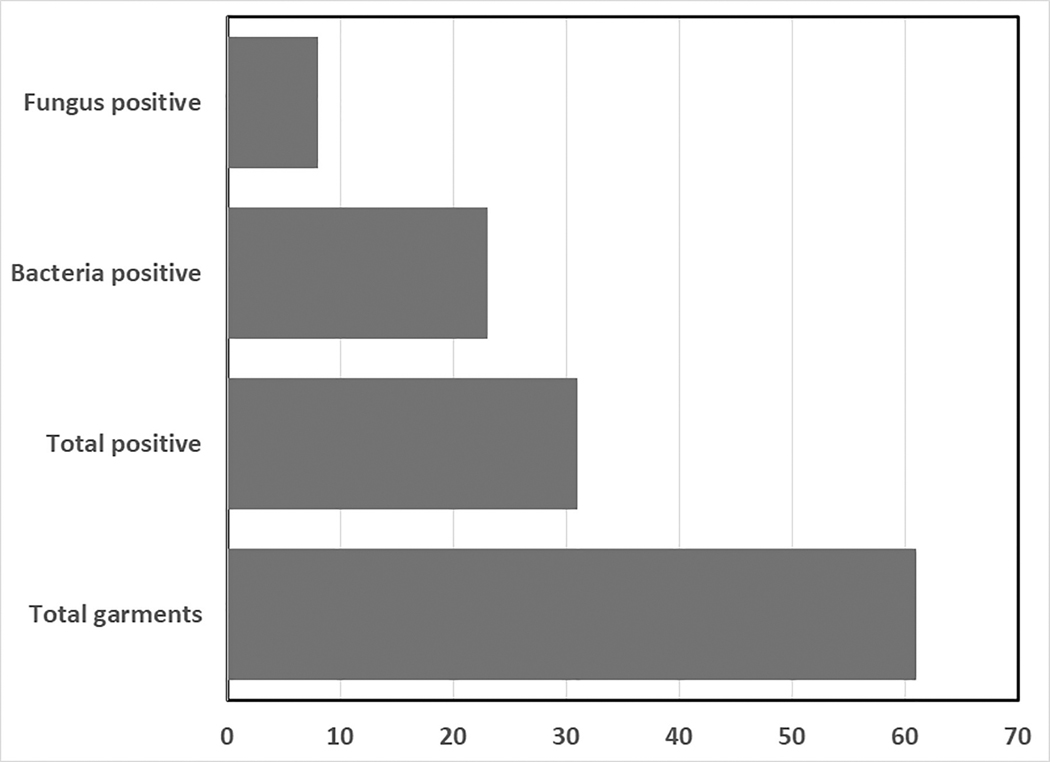
Table 2 depicts the findings from testing for microbial contamination on lead vests and thyroid collars located in a high volume, multi-procedure room interventional cardiology unit. Of 34 vests and 27 collars tested (61 total garments), 54 were personal garments and 7 were shared communal garments. Vests and collars are located on storage hooks located in multiple locations both within operating rooms and in outside hallways. Overall, 31/61 garments (50.8%) tested positive for microbial contamination while 30/61 (49.2%) tested negative. Bacteria was isolated on 23 (37.7%) of the garments while 8 (13.1%) demonstrated fungal growth (Fig.1). One vest and one collar (from two separate individuals) tested positive for both bacteria and fungi. Both garments were hung on hooks in the same exposed hallway outside a procedure room. Five garments tested positive for bacteria in more than one location.
Table 2.
Information about garments sampled
| Total number of garments | Total number of microbial positive garments | Number of bacteria positive garments | Number of fungus positive garments | |
|---|---|---|---|---|
| All personal | 54 | 28 | 20 | 8 |
| All communal | 7 | 3 | 3 | 0 |
| Thyroid collars | 27 | 17 | 13 | 4 |
| Vests | 34 | 14 | 10 | 4 |
| Totals | 61 | 31 | 23 | 8 |
Figure 1).
Prevalence of bacterial and fungal contamination on lead protective garments in an interventional cardiology unit
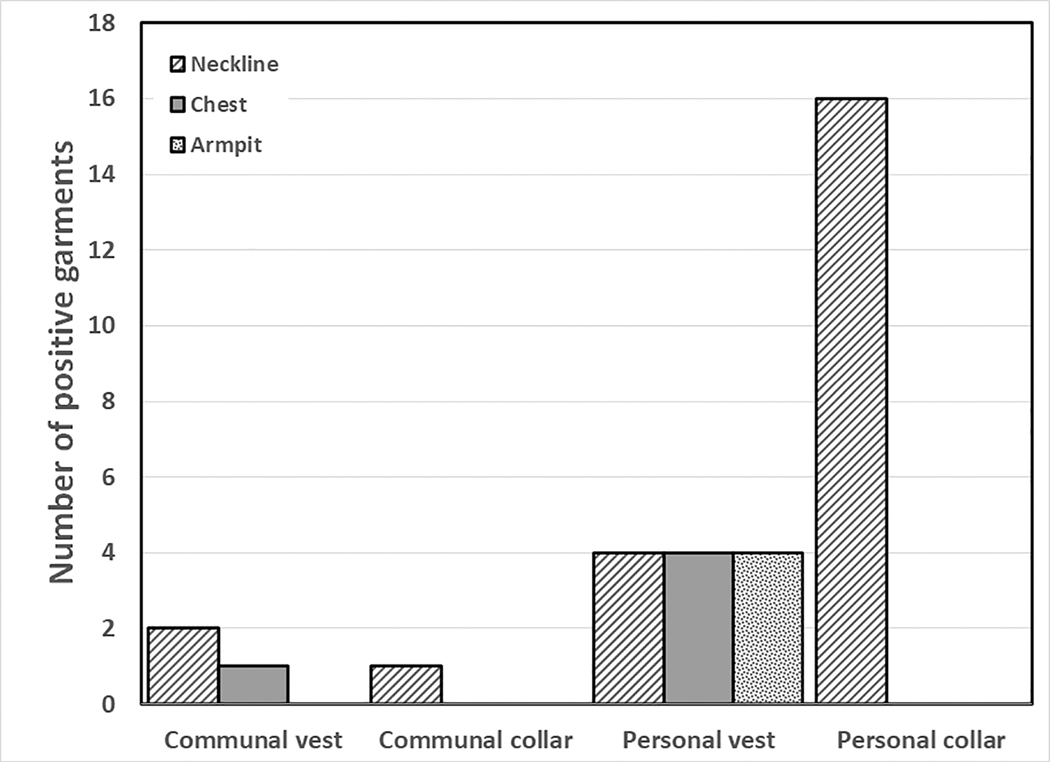
The greatest prevalence of microbial contamination was found around the neckline of thyroid collars (17/27; 63.0%) followed by the neck region of lead vests (6/34; 17.6%) and the chest region of lead vests (5/34; 14.7%) (Fig. 2). Surprisingly, the armpit region of lead vests was positive in only 4/34 lead vests (11.8%) There were too few communal garments sampled to draw statistical conclusions about differences in prevalence of microbial contamination on communal vs personal garments. Interestingly, one communal vest tested positive in three different locations with three different microorganisms.
Figure 2).
Prevalence of microbial contamination on chest, armpit or neckline areas of lead vests and neckline of lead collars
To further classify organisms, positive bacterial samples underwent gram staining. Of the 23 bacteria positive samples, 21 were gram-positive and cocci shaped. Two were gram-negative. Gram positive bacteria are more likely to survive for long periods on dry surfaces than gram-negative bacteria (Kramer, 2014). On the other hand, gram-positive bacteria are generally more susceptible to disinfection and killing by cleaning agents and antibiotics (McDonnell, 1999).
Cleaning and inspection frequencies:
There was an overall total of 99 daily wipes, 26 weekly wipes, 7 sets of photographs, and 4 fluoroscopic inspections performed during this six-month study. The sodium hypochlorite wipes (C1) were withdrawn from routine hospital use seven weeks before the end of the study. Laundry bleach (C2 – 6% sodium hypochlorite) was used for the remaining period.
Visual inspection:
Figure 3 illustrates all twelve active RPPE samples at the start of the study. Additional sets of photographs were acquired monthly. Figure 4 illustrates the visual condition of the four continuously immersed samples at the conclusion of the six month study. The samples were removed from contact with their respective cleaning agents approximately one week before photography. These photographs were taken as the samples were being positioned for their final fluoroscopic inspections. Distortions are attributable to drying.
Figure 3).
Initial appearance of all 12 test samples. Lead numbers added for visual and radiographic identification.
Figure 4).
Final appearance Wipes A (left) and C (right) after 6 months of continuous contact with the cleaning agent.
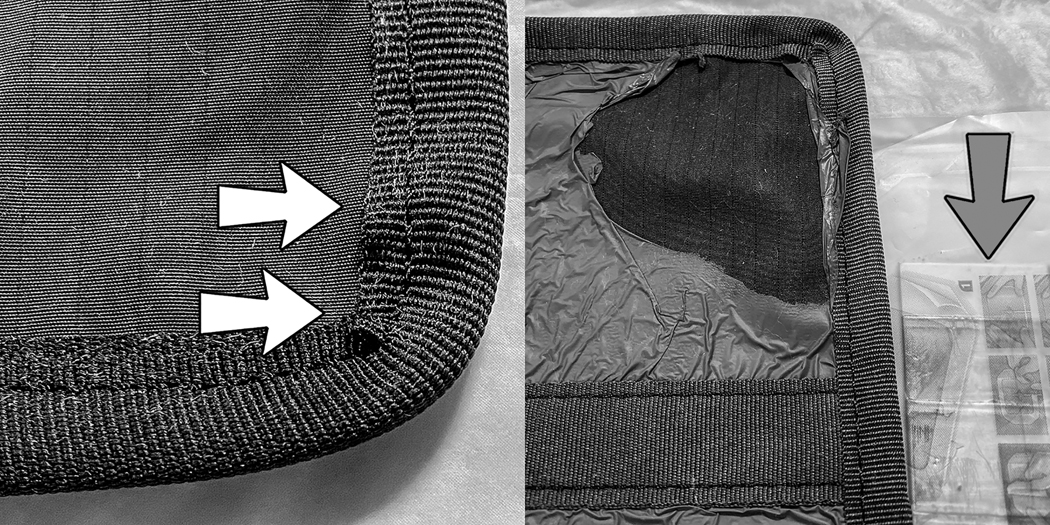
No major changes or discolorations of the fabric were noted on the six samples wiped with agent-A. Fully wetting the cloth surfaces using agent A required subjectively additional effort compared to wiping the agent-A plastic surfaces and all agent C1, C2 surfaces. The additional wiping effort affected the smoothness of the binding at the fabric-binding interface (Fig. 5, left). This resulted in small pockets under the binding that might trap foreign matter. This effect was more pronounced in the daily samples than the weekly samples and was not seen in the continuous samples. This indicates that the changes were mechanically induced by the cleaning process.
Figure 5).
Damage
Left: Wipe A (daily) Note pockets in binding (arrows)
Right: Wipe C (continuous) Note portion of lead reference wedge (arrow)
Fabrics and bindings wiped with C1 and C2 were bleached to a varying degree. The plastic covers on the samples were not affected by C1 but were damaged by C2. Some visible damage to the plastic was seen on the C2 daily sample. The plastic overlayer was severely damaged by continuous contact with C, as shown in Figure 5, right. This is attributed to the use of C2 (full strength laundry bleach) for the last seven weeks of the study. There was no visible mechanical damage to the cloth surfaces (including those exposed by damaged plastic overcovers) or the bindings. Both C1 and C2 wet the surfaces with minimal rubbing. This is probably why the bindings were relatively undistorted.
X-ray:
All fifteen samples were fluoroscopically inspected at the beginning, two intermediate points, and the end of the experiment. A locally fabricated lead foil reference wedge (Fig. 6), partially seen in Fig. 5, was used to confirm the contrast sensitivity of the inspection process.
FIGURE 6).
Lead reference wedge used to visually check fluoro inspection sensitivity. Indicated values (0.00 – 0.80 ) are mm of commercially pure lead.
The fluoroscopic images of every sample were smooth and homogenous at all four testing occasions. This finding suggests that any penetration of the sample by cleaning agents did not damage the radio-protective layer underneath. Typically, fluoroscopic inspections are utilized to detect mechanical damage to this layer. Historically, some protective layers have deteriorated in a short time for a variety of chemical and mechanical reasons (Lambert 2001; Oyar 2012; Matsuda 2016) The current investigation is limited in that the specimens were largely protected from mechanical damage; therefore, interactions between mechanical and chemical effects on the shielding layer were not examined.
Discussion
The findings of this study confirms our initial hypothesis that microbial contamination of radioprotective garments is a frequent occurrence. Significant and persistent levels of microbial contamination was found in more than 50% of the garments tested. We initially hypothesized that the neckline and armpit areas would be the locations most likely to be contaminated. Surprisingly, contamination in the armpit area was infrequent. In contrast, however, more than 37% of the garments exhibited microbial contamination around the neckline area. Neckline microbial contamination was much more prevalent in thyroid collars (63.0%) then in lead vests (17.6%). We hypothesize that contamination in the neckline area arises from biological material originating in the users’ face, neck, or hair regions and that use of thyroid collars partially shields the neckline region of the underlying vest from contamination. We suspect hair and scalp are the most likely source of microorganism contamination. In support of this hypothesis, Summers (1965) reported that hair and scalp from both patients and surgical staff harbor greater numbers of staphylococci than from the nostril region (Summers, 1965) and a recent report finds that the scalp contains a great variety of both bacterial and fungal microorganisms (Wang, 2015). Curiously, no significant gender based differences in rates of contamination were noted. This may, in part, be due to the use of protective head coverings worn by most users.
We also hypothesized that communal garments are more likely to be positive than personal, unshared equipment due to greater potential exposure to a variety of microorganisms. We were unable to rigorously test this hypothesis due to the limited numbers of communal garments analyzed but did observe that one communal garment exhibited contamination by three different microogranisms in three different locations. We did not, however, analyze the spectrum of microbial contamination to determine if there were more varied species in communal garments as compared to personal items.
The finding that gram-positive bacteria were the predominate type of micro-organism detected on leaded garments is not surprising given their relatively greater ability to survive for long times on dry surfaces. On the other hand, the relatively greater sensitivity of gram-positive bacteria to disinfecting agents suggests that periodic cleaning of RPPE is likely to be effective at reducing microbial contamination.
These findings are similar to those reported by other investigators in different medical settings. Studies have indicated the presence of S. aureus, coagulase-negative Staphylococci and gram-positive rods in various surgical operating units including orthopedics (Elsayed et al. 2005; Grogan et al. 2011; Jain et al. 2019), general surgery, cardiovascular thoracic surgery, radiology (Boyle 2010), urology and otolaryngology (La Fauci et al. 2016). The majority of studies were reported in orthopedic surgery settings, perhaps because of a perceived greater risk of microbial contamination from these types of operations. In these studies, rates of contamination ranged from 25% to over 80%. For example, Feirabend (2015) reported contamination approaching 80% on thyroid shields used in an orthopedic operating arena, consistent with our finding of greater than 63% contamination of thyroid collars in interventional cardiology. Contamination rates were reduced by over 70% when periodic cleaning regimens were introduced. Similar findings were reported by Boyle (2010) in a diagnostic imaging department, who found that regular cleaning greatly reduced the prevalence of contamination. Consistent with the present study, Boyle reported greater prevalence of contamination in the shoulder region as compared to the chest area. Curiously, one study in a dental clinic reported microbial contamination in almost all surfaces they termed “high-touch”, except for the lead aprons (Rahmatulla et al. 1996). The investigators had no explanation for this unusual finding but noted, like other studies, that regular use of disinfectant solution greatly reduced rates of surface contamination. These prior studies in orthopedics and other surgical environments where lead aprons are extensively used by medical personnel motived the present investigation in an interventional cardiology environment. To our knowledge, there have been no other similar studies reported to date.
Numerous earlier studies in other medical settings reported significant reduction in microbial flora after various disinfection procedures, We therefore considered its use in interventional cardiology to reduce microorganism load on radioprotective garments. We also recognized that shared communal garments might raise additional health concerns. Decontamination using hospital approved disinfectant wipes was considered to be a practical, simple, and efficient process to provide for patient and health care worker safety. We therefore further felt it prudent to advise each individual user decontaminate his/her own RPPE every day before donning, including those individuals who share communal RPPE.
A variety of approved cleaning materials are available for disinfecting RPPE in most interventional facilities. The materials list differs between facilities and may differ between departments within a single facility. RPPE manufacturers’ lists may not have any agents in common with those in use in a facility. Furthermore, RPPE materials and construction differ both within and between manufacturers. As noted in the Methods, only one material and three specific disinfection methods were utilized in the present manuscript. Therefore, while believe we have proved proof of principle, our findings may not be generalizable to all garments and disinfection protocols. It is suggested that each individual Radiation Safety Officer (RSO) verify the compatibility of each facility’s approved biological decontamination agent(s) and process(es) with the specific RPPEs utilized within each facility.
RPPE is often shared between individuals. This may be intentional in some cases where communal RPPE is provided for multiple users in a specific area. It may be unintentional in the sense that an individual may “borrow” an item assigned to another user. Although our findings do not indicate that shared RPPE is any more liable to be contaminated than single user items, we did not perform detailed microbial testing to investigate whether the type and variety of microorganisms differed between single user and shared RPPE.
Prevention of cross-contamination is among the responsibilities of every infection control department. Part of this task is the development of cleaning policies and procedures, including approval of specific decontamination agents for use in each facility. This can be a problem when RPPE manufacturers suggest specific cleaning materials for use with their products. Facility infection control policies may not permit the use of certain agents or these disinfectants may not be available in every region or facility. Even when such manufacturer specified agents are allowed, the use of any agent not supplied by the facility in large quantities poses operational issues: It can be difficult to get a non-standard agent into the hands of each RPPE user in sufficient quantities and in a timely manner. On the other hand, facility approved surface wipes tend to be readily available in most locations where RPPE might be used.
This study investigated the possibility of RPPE damage from repeated use of two disinfectant surface wipes approved for use in our cath lab in one representative example at the start of a half-year trial. Wipe-A was the most common. Wipe-C1 was available but much more caustic to individuals’ hands and, furthermore, corrosive to metal. As noted in the Methods section, hospital infection control withdrew this agent from routine use and reserved it for only limited specific indications. Because introducing a new chemical (H2O2) for the last seven weeks of the study was inadvisable, concentrated Clorox™ (Wipe C2) was substituted. The benefit of this substitution is that it accelerated the effects of hypochlorite on our samples.
In this study, damage to the RPPE samples appears to be superficial. No fluoroscopically detectable damage was noted in the radioprotective layers of the samples. In contrast, biological contamination was determined using real-world actual RPPE utilized in clinical practice. The biological effectiveness of germicidal wipes was not tested with the actual RPPE. One can speculate, however, that damage to the covering and binding might promote increased contamination in regions of high potential risk such as the thyroid collar and neckline area of lead aprons.
RPPE used in a clinical setting is subject to additional mechanical damage from repeated folding, hanging from hooks, potential tears and other physical trauma. Indeed, a main reason for routinely inspecting RPPE is to detect such damage. One can also speculate that mechanical damage to RPPE covering layers might expose the radioprotective layer to additional chemical deterioration from the disinfecting agents as well as possible entry of microorganisms into this layer.
Limitations of this study include testing of only two specific decontamination agents, evaluation of RPPE samples from only one manufacturer, a relatively short half-year testing interval to detect damage, and the aforementioned lack of wipe testing on actual garments in clinical use. Nevertheless, we feel the findings are useful for demonstrating the need for routine cleaning and disinfection of RPPE and a consistent protocol for testing the integrity of the underlying shielding.
Conclusions
The prevalence of microbial contamination of Radiation Personal Protective Equipment (RPPE) is greater than 50% during normal use in interventional fluoroscopy in an interventional cardiology setting. This finding strongly suggests periodic cleaning and disinfection of these garments should become routine clinical practice. The contamination patterns and observed organisms suggest that the RPPE’s wearer themselves are the most common source of microbial contamination. To decrease the prevalence of microbial contamination on RPPE, routine standardized sanitation protocols should be developed and implemented. We also suggest that garments be kept in more private areas, away from hallways and open spaces to minimize the potential for environmental contamination. Although not specifically tested in this manuscript due to limited numbers of samples, we suggest that communal use of RPPE could pose greater risk for increased number and variety of microorganisms than those used by only one person. Lastly, we point out the challenges of balancing the use of chemicals that can cause degradation of radioprotective materials with the need for routine cleaning and disinfection. The relatively little damage to outer coverings and no degradation of radioprotection is a strong argument for the routine cleaning/disinfection of this vitally important part of radiation safety practice. These processes should help guide the RSO in evaluation of RPPE in each facility.
Acknowledgments
FUNDING:
SB: None
NJK, MR: National Institute of Environmental Health Sciences (NIEHS) 1-R25-ES025505–03
REFERENCES
- Boyle H, Strudwick RM. Do lead rubber aprons pose an infection risk? Radiography 16: 297–303; 2010. DOI: 10.1016/j.radi.2010.03.002. [DOI] [Google Scholar]
- Burke JP. Infection control - a problem for patient safety. N Engl J Med 348: 651–656; 2003. DOI: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- Chauveaux D Preventing surgical-site infections: measures other than antibiotics. Orthop Traumatol Surg Res 101(1 Suppl):S77–83; 2015. DOI: 10.1016/j.otsr.2014.07.028. [DOI] [PubMed] [Google Scholar]
- Elsayed S, Lloyd J, Cosker TDA, Hussainn A, Kulkarni R. Bacterial contamination of the theatre lead apron: A cause for concern. J Trauma Injury Infection Crit Care 2005; Abstracts of the papers presented at the annual scientific meeting of the Trauma Association of Canada, April 6–9, 59:542; 2005. 10.1097/00005373-200508000-00148. [DOI] [Google Scholar]
- Feierabend S, Siegel G. Potential infection risk from thyroid radiation protection. J Orthop Trauma 29:18–20; 2015. DOI: 10.1097/BOT.0000000000000161. [DOI] [PubMed] [Google Scholar]
- Grogan BF, Cranston WC, Lopez DM, Furbee C, Murray CK, Hsu JR, Skeletal Trauma Research Consortium. Do protective lead garments harbor harmful bacteria? Orthopedics 34:e765–767; 2011. DOI: 10.3928/01477447-20110922-09. [DOI] [PubMed] [Google Scholar]
- Jain S, Rajfer RA, Melton-Kreft R, Nistico L, Miller MC, Stoodley P, Altman DT, Altman GT. Evaluation of bacterial presence on lead X-ray aprons utilised in the operating room via IBIS and standard culture methods. J Infect Prev 20:191–196; 2019. DOI: 10.1177/1757177419833163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein LW, Miller DL, Balter S, Laskey W, Haines D, Norbash A, Mauro MA, Goldstein JA. Occupational health hazards in the interventional laboratory: time for a safer environment. Radiology 250:538–544; 2009. DOI: 10.1148/radiol.2502082558. [DOI] [PubMed] [Google Scholar]
- Klein LW, Miller DL, Balter S, Laskey W, Naito N, Haines D, Ross A, Mauro MA, Goldstein JA. Occupational health hazards in the interventional laboratory: Time for a safer environment. Catheter Cardiovasc Interv January 4; 2018. DOI: 10.1002/ccd.21772. [DOI] [PubMed] [Google Scholar]
- Kramer A, Assadian O. Survival of microorganisms on inanimate surfaces In: Borkow G, ed. Use of biocidal surfaces for reduction of healthcare acquired infections. New York, NY: Springer; 2014:7–26. doi: 10.1007/978-3-319-08057-4_2. PMCID: PMC7123372. [DOI] [Google Scholar]
- La Fauci V, Riso R, Facciola A, Merlina V,Squeri R. Surveillance of microbiological contamination and correct use of protective lead garments. Ann Ig 28:360–366; 2016. DOI: 10.7416/ai.2016.2116. [DOI] [PubMed] [Google Scholar]
- Lambert K, McKeon T Inspection of lead aprons: criteria for rejection. Health Phys 80(5 Suppl): S67–69; 2001. DOI: 10.1097/00004032-200105001-00008. [DOI] [PubMed] [Google Scholar]
- Lovitt SA, Nichols RL, Smith JW, Muzik AC, Pearce PF. Isolation gowns: a false sense of security? Am J Infect Control 20:185–191; 1992. DOI: 10.1016/s0196-6553(05)80144-0. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Suzuki T. Evaluation of lead aprons and their maintenance and management at our hospital. J Anesth 30:518–521; 2016. DOI: 10.1007/s00540-016-2140-2. [DOI] [PubMed] [Google Scholar]
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–79; 1999. DOI: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyar O, Kişlalioğlu A. How protective are the lead aprons we use against ionizing radiation? Diagn Interv Radiol 18:147–152; 2012. DOI: 10.4261/1305-3825.DIR.4526-11.1. [DOI] [PubMed] [Google Scholar]
- Rahmatulla M, Almas K, al-Bagieh N. Cross infection in the high-touch areas of dental radiology clinics. Indian J Dent Res 7:97–102; 1996. [PubMed] [Google Scholar]
- Shafer CW, Allison JR, Hogue AL, Huntington MK. Infectious Disease: Health Care-Associated Infections. FP Essent 476:30–42; 2019. [PubMed] [Google Scholar]
- Smilowitz NR, Balter S, Weisz G. Occupational hazards of interventional cardiology. Cardiovasc Revasc Med 14: 223–228; 2013. DOI: 10.1016/j.carrev.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Summers MM, Lynch PF, Black T. Hair as a reservoir of staphylococci. J Clin Pathol 18:13–15; 1965. DOI: 10.1136/jcp.18.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Clavaud C, Bar-Hen A, Cui M, Gao J, Liu Y, Liu C, Shibagaki N, Guéniche A, Jourdain R, Lan K, Zhang C, Altmeyer R, Breton L. Characterization of the major bacterial-fungal populations colonizing dandruff scalps in Shanghai, China, shows microbial disequilibrium. Exp Dermatol 24:398–400; 2015. DOI: 10.1111/exd.12684. [DOI] [PubMed] [Google Scholar]