Abstract
The Hedgehog (Hh) signaling pathway coordinates cell–cell communication in development and regeneration. Defects in this pathway underlie diseases ranging from birth defects to cancer. Hh signals are transmitted across the plasma membrane by two proteins, Patched 1 (PTCH1) and Smoothened (SMO). PTCH1, a transporter-like tumor-suppressor protein, binds to Hh ligands, but SMO, a G-protein-coupled-receptor family oncoprotein, transmits the Hh signal across the membrane. Recent structural, biochemical and cell-biological studies have converged at the surprising model that a specific pool of plasma membrane cholesterol, termed accessible cholesterol, functions as a second messenger that conveys the signal between PTCH1 and SMO. Beyond solving a central puzzle in Hh signaling, these studies are revealing new principles in membrane biology: how proteins respond to and remodel cholesterol accessibility in membranes and how the cholesterol composition of organelle membranes is used to regulate protein function.
The history of the Hedgehog (Hh) signaling pathway provides an instructive narrative on how basic research on embryo segmentation in Drosophila ultimately led to the development of anticancer drugs used in the clinic today. Ligands that initiate Hh signaling in target cells (such as Sonic Hedgehog or SHH in vertebrates) function as paracrine signals to control patterning and morphogenesis in most of our tissues during development. After embryogenesis, Hh signaling is used to coordinate reparative and regenerative responses in many tissues such as the brain, bladder, skin and bone. Given these myriad roles, even subtle defects in Hh signaling have been implicated in diseases ranging from birth defects to cancer. Drugs that inhibit Hh signaling are now used to treat patients with basal cell cancer and acute myeloid leukemia.
Research on the mechanisms of Hh signaling1 has often revealed new regulatory principles in cell biology. For instance, Hh signaling in vertebrates was unexpectedly shown to depend on primary cilia, antenna-like organelles found in most cells in our bodies that play many roles in development and physiology2. Cilia function as compartments in which signal propagation is linked to the dynamic trafficking of components at all levels of Hh signaling, from the receptor Patched 1 (PTCH1) to the GLI family of transcriptional effectors3. The Hh pathway has served as a valuable model system to understand ciliary trafficking and the organization of signaling pathways at cilia. This Perspective will focus on the initiating step in the vertebrate Hh pathway and how its study is providing fresh insights into the role of cholesterol in controlling membrane-based signal-transduction events.
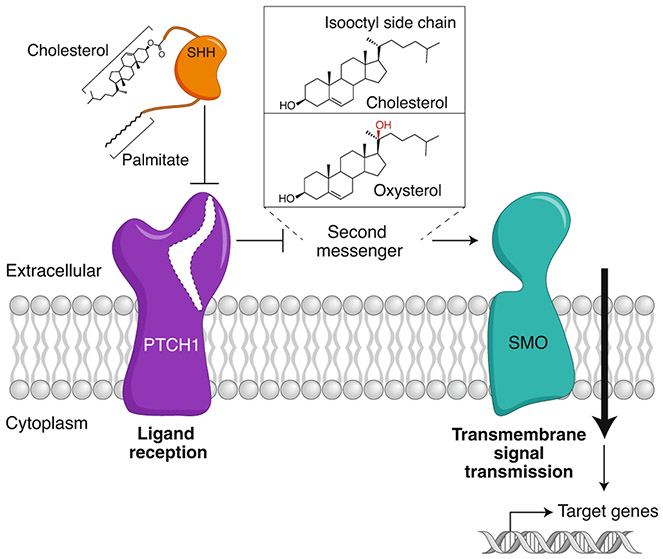
Ligands such as SHH are received at the extracellular side by PTCH1, a 12-pass transmembrane (TM) protein (Fig. 1). However, the Hh signal is transmitted across the plasma membrane by Smoothened (SMO), a 7-pass TM protein that belongs to the G-protein-coupled receptor (GPCR) superfamily4. PTCH1 inhibits SMO; inactivation of PTCH1 by direct binding of SHH allows SMO to adopt an active conformation and transmit the Hh signal to the cytoplasm. As PTCH1 and SMO do not physically interact, this arrangement requires the Hh signal to be transmitted from PTCH1 to SMO by a second messenger (Fig. 1). The puzzle of how PTCH1 inhibits SMO has remained unsolved for nearly 25 years. In this Perspective we describe research from diverse areas that has converged to suggest the new concept that the organization of cholesterol in cellular membranes is used as a second messenger to communicate the Hh signal between PTCH1 and SMO. PTCH1 uses its transporter-like function to diminish a biochemically distinct pool of membrane cholesterol, called accessible cholesterol, that activates SMO. PTCH1 inactivation by SHH leads to an increase in cholesterol accessibility, perhaps locally in the membrane of the primary cilium, thereby allowing SMO activation and transmission of the Hh signal to the cytoplasm. While this model is preliminary, and its predictions require further experimental testing, it serves as a useful guide for future research in Hh signaling and, more generally, in the role of accessible cholesterol in other membrane-dependent processes.
Fig. 1 ∣. Hedgehog signal transmission across the plasma membrane.
A double-negative mechanism initiates Hh signaling at the cell surface. PTCH1 inhibits SMO; SHH inhibits PTCH1, allowing SMO activation. Since ligand reception and transmembrane signaling are assigned to different proteins, a second messenger must communicate the signal between PTCH1 and SMO. Candidate second messengers include cholesterol and oxysterols (such as 20(S)-hydroxycholesterol shown here), both of which can bind and activate SMO. Interestingly, cholesterol is also covalently attached to SHH and plays a critical role in its biogenesis from a precursor protein.
The multiple roles of cholesterol in Hh signaling
Cholesterol is involved in both the biogenesis of ligands in producer cells and signal reception in target cells. Ligands that initiate Hh signaling are covalently attached to two lipids: a palmitoyl moiety at the N terminus and a cholesterol molecule at the C terminus5,6 (Fig. 1). Seemingly unrelated to its role in Hh ligand biogenesis, cholesterol is also required in target cells to receive Hh signals. Exposure to inhibitors of late steps in the cholesterol biosynthesis pathway during pregnancy can lead to holoprosencephaly, a birth defect also seen with genetic or pharmacological inhibition of Hh signaling7,8. Human syndromes caused by loss-of-function mutations in enzymes that catalyze late steps in cholesterol biosynthesis (like Smith-Lemli-Opitz Syndrome) are characterized by birth defects in tissues that are dependent on Hh signaling during development9,10. Indeed, Hh signaling in cultured cells is attenuated by loss-of-function mutations in these genes or by the direct depletion of cholesterol using methyl-ß-cyclodextrin (MβCD).
Side chain oxysterols bind and activate SMO
Though several exogenous SMO ligands have been identified over the years11,12, the identity of the endogenous ligand that mediates SMO activation has remained elusive. An important clue was provided by the discovery that side chain oxysterols are sufficient to activate Hh signaling in cultured cells and to induce the accumulation of SMO in primary cilia (just like SHH)13-15. Side chain oxysterols are oxygenated metabolites of cholesterol that contain hydroxyl or epoxy groups in the sterol’s isooctyl side chain projecting from the tetracyclic steroid nucleus (Fig. 1). Oxysterols were subsequently shown to directly bind and activate SMO16, leading to the proposal that the elusive endogenous ligand for SMO may be a sterol. Several cilia-enriched oxysterols have recently been shown to influence SMO activity17.
While exogenously added oxysterols can clearly activate SMO and Hh signaling, mice lacking oxysterol biosynthetic enzymes (even in combination) do not show developmental defects associated with reduced Hh signaling18. However, these studies are confounded by potential transplacental transfer of sterols from the mother during development, by redundant biosynthesis pathways and by synthesis of some oxysterols by non-enzymatic reactions. To address these issues, we conducted a loss-of-function CRISPR screen in cultured cells targeting all lipid-related genes (including all annotated enzymes assigned to sterol and steroid synthesis pathways)19. The screen was purposefully conducted under conditions designed to block cholesterol uptake from the media, thereby forcing cells to depend on their endogenous sterol synthesis pathways. No known oxysterol synthesis enzymes were found to be positive regulators of Hh signaling. By contrast, multiple enzymes at both early and late steps in the post-squalene pathway for cholesterol biosynthesis were identified as positive regulators. This focused screen implicates cholesterol itself, rather than a precursor or product sterol, as a requirement for Hh signaling in target cells.
The second piece of evidence that implicates cholesterol (rather than an oxysterol) is the observation that depletion of sphingomyelin (SM) potentiates Hh signaling in cultured cells. SM is known to form complexes with cholesterol, thereby sequestering cholesterol from other proteins (further discussed below)20-23. Depletion of SM would liberate cholesterol from SM–cholesterol complexes to promote SMO signaling. Importantly, oxysterols do not form such complexes with SM, and therefore SM depletion would not be expected to increase oxysterol access to SMO23. Taken together, data from structural studies, human genetics, CRISPR screens and manipulations of SM levels in cells all point to cholesterol as the endogenous sterol that mediates SMO activation. Oxysterols may play a role in modulating SMO activity and Hh signaling in specific oncogenic or metabolic contexts17.
Activation of SMO by cholesterol
Cholesterol is required for the proper function of many membrane proteins, including GPCRs. One striking example is the oxytocin receptor, whose affinity for its peptide ligand is reduced by ~80-fold when membrane cholesterol is depleted by MβCD24. However, although cholesterol can promote receptor stability or a specific receptor conformation, it is not sufficient on its own to activate signaling, which still requires an agonist. By contrast, two groups independently made the surprising observation that cholesterol can serve as a bona fide agonist for SMO25-27. A crystal structure of SMO unexpectedly revealed a cholesterol molecule bound to a hydrophobic groove in the extracellular cysteine-rich domain (CRD)25 (Fig. 2a,b). This same CRD groove also binds to oxysterols28-30. Cholesterol delivery to cells using MβCD as a carrier was sufficient to activate Hh signaling even in the absence of SHH26,27. Structure-guided mutations in residues that mediate hydrogen bonding with cholesterol’s 3β-hydroxyl group or interactions with its tetracyclic steroid nucleus resulted in diminished signaling responses to SHH in both cultured cells25-27 and mouse embryos31, showing that the interaction of cholesterol with the CRD was required for endogenous signaling. Interestingly, high-potency SMO inhibitors, including the cancer drug Vismodegib, that bind at the extracellular end of the transmembrane domain (TMD)11 (hereafter called the TMD1 site, Fig. 2a) induce a conformational change that prevents cholesterol from binding to CRD25,32 (Fig. 2c). A report has also suggested that cholesterol can be covalently linked to the CRD by an ester linkage to an aspartate residue31.
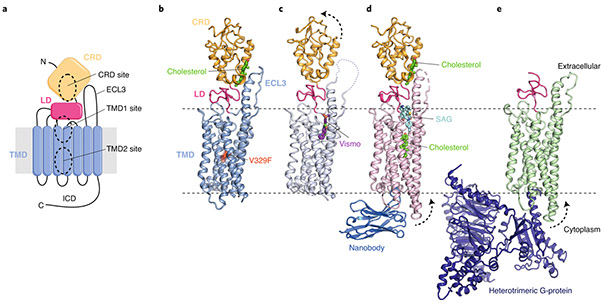
Fig. 2 ∣. Multiple sterol binding sites in SMO.
a, Schematic of SMO showing the extracellular cysteine-rich domain (CRD), linker domain (LD), transmembrane domain (TMD), third extracellular loop (ECL3) and intracellular domain (ICD). The three structurally and functionally characterized small-molecule binding sites (two in the TMD and one in the CRD) are highlighted. N and C refer to N- and C-termini, respectively. b–e, Cartoon representation of the closed SMO–cholesterol complex (b; PDB ID 5L7D), the SMO–Vismodegib (Vismo) complex in an inhibited conformation (c; PDB ID 5L7I), the SMO–cholesterol–nanobody-SAG (SMO agonist) complex in an activated conformation (d; PDB ID 6O3C) and the SMO–G-protein complex (e, PDB ID 6OT0). Dotted arrows indicate movement compared to that of the closed SMO–cholesterol complex (shown in b). Small molecules are labeled and depicted in stick representation. The V329F mutation that locks SMO in a closed conformation is highlighted as a red sphere in b.
The first structure of cholesterol-bound SMO used a mutation (valine 329 to phenylalanine, Fig. 2b) in the TMD to stabilize the protein in an inactive state and improve protein expression25. A putative active-state structure of SMO with its native valine 329 was later solved in complex with two synthetic agonists: a small-molecule SMO agonist (SAG) bound to the TMD1 site and a nanobody bound to intracellular loops33 (Fig. 2d). This structure confirmed the presence of a cholesterol bound to the CRD, but it also revealed a second cholesterol molecule bound at the center of the TMD (hereafter called the TMD2 site, Fig. 2a) at a position just below (and abutting) the SAG-binding site (Fig. 2d). Two putative active-state structures of SMO have been reported in the absence of SAG. One structure confirmed sterol binding to the CRD and suggested a tunnel through the center of the TMD (which encompasses the TMD1 and TMD2 sites) but did not find a bound sterol in the TMD2 site34. A second structure of SMO in complex with a heterotrimeric G-protein obtained by cryo-EM suggested the presence of a ligand in the SMO TMD (Fig. 2e), though the low resolution makes both the identity and the position of this ligand uncertain35. All of these putative active-state structures of SMO suggest that sterol binding to SMO can lead to the outward movement of TM5 and TM6 helices on the cytoplasmic face (dotted arrows in Fig. 2d,e), a conformational change associated with activation in other GPCRs33-35.
The question of which of the three ligand-binding sites in SMO (Fig. 2a) is regulated by PTCH1 during the course of endogenous signaling remains uncertain. Mutations in the TMD1 site do not impair SMO regulation by PTCH1 (ref. 36). However, mutations in both the CRD and the TMD2 sites impair signaling by SHH, so either (or both) may accommodate a sterol agonist regulated by PTCH1 (refs. 25-27,33). Point mutations in the CRD site also prevent SMO signaling during development, mimicking the effect of a complete SMO knockout31. Finally, experiments using a cholesterol analog containing an azide group on its isooctyl side chain showed that PTCH1 activity negatively regulates sterol access to the CRD31. The most compelling evidence that the TMD2 binding site is regulated by PTCH1 comes from analysis of a truncation mutant of SMO entirely lacking the CRD (ΔCRD-SMO). This mutant has high constitutive signaling activity because CRD interactions with the TMD stabilize the inactive state of SMO25. However, a small but reproducible SHH response is still observed in Smo−/− cells expressing ΔCRD-SMO, and the overexpression of PTCH1 beyond physiological levels can suppress its constitutive signaling activity25,29,30,37. Sterol binding to both the CRD and TMD2 sites may be required for full SMO activation, allowing SMO to respond in a switch-like fashion to changes in cholesterol abundance. Ordered binding is also a possibility, with one site occupied at low SHH concentration and both at high SHH concentrations. Finally, one of the sterol-binding sites may be constitutively occupied to promote SMO stability or trafficking, and the second one may be regulated by PTCH1. Despite the uncertainty surrounding the roles of the various sterol-binding sites, the key conclusion from both functional and structural studies is that SMO can be activated by both cholesterol and oxysterols.
PTCH1 as a sterol transporter
More than 20 years ago, PTCH1 was noted to have sequence similarity to Niemann–Pick C1 (NPC1), a lysosomal membrane protein that transports cholesterol from the lumen of the lysosome to the limiting lysosomal membrane for delivery to other cellular destinations38,39 (Fig. 3a). Both PTCH1 and NPC1 contain a sterol-sensing domain (SSD), a module composed of five TM helices, which is found in several other proteins that handle or sense cholesterol40 (Fig. 3a). PTCH1 and NPC1 are distantly related to the resistance-nodulation-division (RND)-family of pumps, which uses transmembrane proton gradients to efflux toxic hydrophobic molecules out of Gram-negative bacteria41.
Fig. 3 ∣. Structural similarities between PTCH1 and the cholesterol transporter NPC1.
a, Both PTCH1 (top) and NPC1 (bottom) contain two large extracellular domains (ECD1 and ECD2 in PTCH1 and the middle (MLD) and C-terminal (CTD) luminal domains in NPC1) and a 12-pass transmembrane domain (TMD) that includes an evolutionarily conserved sterol-sensing domain (SSD). NPC1 contains an additional N-terminal transmembrane helix (1′) and an extracellular domain (NTD) involved in cholesterol transfer (see b). b,c, Structures of the NPC1–NPC2 complex (b; PDB ID 6W5V) and human PTCH1 (c; PDB ID 6RVD). Yellow surfaces show potential tunnels (calculated with CAVER99) connecting the extracellular/luminal and TMD cholesterol-binding sites. Blue arrows indicate possible transport routes for cholesterol. d,e, Inhibited-state structures of NPC1 (PDB ID 6UOX) and PTCH1 (PDB ID 6RVD). NPC1 is inhibited by itraconazole, which occupies the TMD cholesterol-binding site (d). Binding of SHH to PTCH1 results in a 2:1 PTCH1:SHH complex (e). SHH interacts with PTCH1 mol1 via a high-affinity protein–protein interface involving the SHH-metal binding sites (green and black spheres represent calcium and zinc ions, respectively) and by inserting its N- and C-terminal palmitate and cholesterol modifications into PTCH1 mol2. f,h, Close-up views of the TMD cholesterol-binding sites show that the palmitoyl moiety of SHH overlaps with the cholesterol- and itraconazole-binding sites, suggesting a shared binding site critical for cholesterol transport in both PTCH1 and NPC1.
Structures of PTCH1 and NPC1 suggest models for how they may transport cholesterol42-53. Both PTCH1 and NPC1 structures identified a tunnel through the protein that may serve as a conduit for sterol transport (Fig. 3b,c). Sterol-like densities have been identified at various positions throughout the tunnel in both proteins and may represent transport intermediates. The transport path is better defined for NPC1, in which the directionality of cholesterol transport from the lysosomal lumen to the limiting membrane is established. Cholesterol is delivered to NPC1 by NPC2, a soluble protein that binds cholesterol liberated from lipoprotein particles in the lysosome lumen. NPC2 transfers cholesterol to the N-terminal domain (NTD, Fig. 3a) of NPC1 (unique to NPC1 and not present in PTCH1), which then transfers it to a tunnel that runs through the protein to the outer leaflet of the lysosome membrane48,54-57 (Fig. 3b). Cholesterol molecules are also observed along the analogous tunnel in PTCH1 (Fig. 3c). For both PTCH1 and NPC1, the functional importance of the sterol tunnel is suggested by structures captured in inactive states43,44,51-53 (Fig. 3d-h). Unlike NPC1, neither the directionality of sterol transport nor the identity of the cholesterol donor or acceptor for PTCH1 is known. PTCH1 could transport cholesterol from the outer leaflet of the plasma membrane to a membrane or protein acceptor or receive a cholesterol molecule from a donor (like SMO) and transport it to the membrane1.
Taken together, these studies support a shared biochemical function of PTCH1 and NPC1 and suggest that PTCH1 transports a sterol to regulate SMO. Interestingly, the first functional evidence of cholesterol transport by PTCH1 pre-dated the recent flurry of PTCH1 structures by several years. A report in 2011 showed that PTCH1 could bind cholesterol and efflux a fluorescent cholesterol analog (BODIPY–cholesterol) from cells58. More recently, transport activity of PTCH1 has been inferred from experiments showing that its activity can reduce the abundance or accessibility of cholesterol in both the inner45 and outer19 leaflets of the plasma membrane.
The concept of accessible cholesterol in membranes
To get a semi-quantitative measure of the availability of cholesterol to SMO in the plasma membrane, consider that the cross-sectional footprint of SMO in a lipid bilayer would be an ellipse with major and minor axes lengths of 36.4 and 34 Å, respectively. Using an estimate of 50 Å2 for the average area of a lipid molecule in the plasma membrane59, simple geometric considerations predict that ~28 lipid molecules would fit in a single layer around SMO (very similar to the numbers measured for Rhodopsin in rod outer segments60). If cholesterol comprises ~40% of lipids in the vertebrate plasma membrane, the annular lipid shell around SMO in each leaflet would contain ~10 molecules of cholesterol (assuming uniform lipid distribution). How then can SMO activity in cells ever be turned off if its TMD is awash in such an abundance of cholesterol?
A potential solution to this conundrum emerges from the large body of data showing that plasma membrane cholesterol is organized into accessible (minor) and inaccessible (major) pools. This view has its origins in Leathes’ observation from nearly a century ago that the average molecular area of phospholipids in membranes is reduced when cholesterol is present61. This phenomenon, called the cholesterol ‘condensing’ effect, subsequently led to the proposal that cholesterol and phospholipids form complexes with specific stoichiometries20,62. A thermodynamic model of such ‘condensed complexes’ has been developed, which accounts for the area condensation effects, as well as phase behavior, NMR spectra and chemical potentials of membranes containing cholesterol and phospholipids20,63,64. Phospholipids vary in their ability to form condensed complexes with cholesterol, with the highest propensity shown by sphingolipids, followed by glycerophospholipids with saturated acyl chains21,65-67.
An important consequence of condensed complex formation is the reduction of cholesterol’s chemical activity, a thermodynamic quantity related to its chemical potential68. In a membrane composed of cholesterol and phospholipids, the chemical activity of cholesterol generally increases with increasing cholesterol concentration (dashed line, Fig. 4a). However, at lower concentrations complex formation can suppress cholesterol’s chemical activity below that expected in the absence of lipid–lipid interactions (solid red line, Fig. 4a). At higher concentrations, when phospholipids become limiting, the chemical activity of cholesterol rises, sometimes very sharply (Fig. 4a). The switch point between these two regimes is referred to as the ‘equivalence point’ (marked in Fig. 4a), as there is neither an excess of cholesterol or of phospholipids at this point. The chemical activity determines the rates of loss of cholesterol from the membrane to soluble acceptors, the extent of binding of cholesterol to proteins at the membrane interface, or the ability of cholesterol to participate in membrane reactions such as SMO activation. As all of these scenarios reflect access of cholesterol to proteins, we hereafter use ‘accessible cholesterol’ as a descriptive term to refer to the pool of cholesterol that has sharply higher chemical activity.
Fig. 4 ∣. Cholesterol accessibility in cellular membranes.
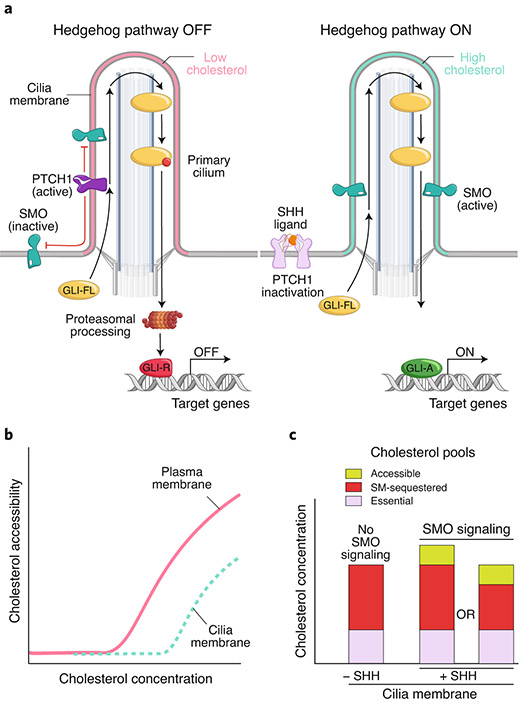
a, Calculated chemical activity (related to the chemical potential) of cholesterol as a function of membrane cholesterol content (expressed as the mole fraction of cholesterol relative to total lipids). Shown are chemical activities for binary membrane mixtures of cholesterol and phospholipid assuming no interactions (blue curve) or a complex containing one cholesterol and two phospholipid molecules (pink curve, derived from a regular solution free energy calculation100). At concentrations below the equivalence point, cholesterol is sequestered by phospholipids. Above the equivalence point, cholesterol exceeds the sequestering capacity of phospholipids, and its accessibility sharply rises. b, Three pools of cholesterol in plasma membranes, along with toxin-based probes that can be used to detect and manipulate the sphingomyelin (SM)-sequestered (OlyA) and accessible (ALOD4 or PFO) pools. c, Schematic showing the three cholesterol pools in unperturbed plasma membranes or after cholesterol loading or SM depletion.
Sharp rises in cholesterol accessibility have been detected in model membranes through measurements of cholesterol extraction by cyclodextrins59,68, oxidation by cholesterol oxidase65,69 and binding to cholesterol-dependent cytolysins such as perfringolysin O (PFO) or anthrolysin O (ALO)70,71. In all of these measurements, the increase in cholesterol accessibility with increasing cholesterol concentration was sigmoidal, rather than linear, with little change until a threshold concentration was reached (similar to the solid line in Fig. 4a). Once the cholesterol content of the membrane exceeded this threshold, cholesterol accessibility increased dramatically. These same three methods have shown that cholesterol accessibility in the plasma membranes of cultured cells also rises sharply at threshold concentrations72-74. Sphingomyelin (SM), a lipid confined to the outer leaflet of the plasma membrane, plays a particularly important role in determining cholesterol accessibility due to its high affinity for cholesterol. Depletion of SM is commonly used to increase cholesterol accessibility at the plasma membrane75.
More recently, studies with PFO*, a mutant version of PFO that does not lyse cells at 4 °C, have revealed that plasma membrane cholesterol is present in at least three pools: an accessible pool, a SM-sequestered pool and a third pool that is sequestered by other membrane factors and is essential for membrane integrity75 (Fig. 4b). Investigation of these plasma membrane cholesterol pools has been greatly aided by the development of a domain of ALO designated as ALOD4, which senses accessible cholesterol and is non-lytic even at 37 °C71,76, and the discovery of a non-lytic fungal toxin, Ostreolysin A (OlyA), which selectively binds to SM–cholesterol complexes (but not to accessible cholesterol or free SM)23,77 (Fig. 4b). Expansion of accessible cholesterol levels by cholesterol addition or SM depletion (Fig. 4c), as defined by PFO* or ALOD4 binding, leads to its rapid translocation from the plasma membrane to the endoplasmic reticulum (ER)75,76. In the ER, accessible cholesterol binds to Scap, a cholesterol sensor protein78. This binding terminates the activation of lipogenic transcription factors called sterol regulatory element binding proteins (SREBPs), leading to reduced cholesterol synthesis and uptake and thus restoring cholesterol homeostasis. By restricting signaling to just the accessible pool, cells are able to maintain optimal cholesterol levels in plasma membranes while avoiding cholesterol overaccumulation.
The work summarized above provides a framework for how the extent of cholesterol binding to SMO could be driven not by its total concentration in plasma membranes, but rather by a smaller pool of accessible cholesterol that is free of sequestration by SM and other membrane factors.
Regulation of Hedgehog signaling by accessible cholesterol
Increasing cholesterol accessibility in the plasma membrane by depleting SM does not change the total cholesterol levels, yet it suppresses cholesterol biosynthesis and uptake75,79,80. Conversely, sequestering accessible cholesterol in the outer leaflet of the plasma membrane with ALOD4 (again without changing total membrane cholesterol) induces cholesterol biosynthesis and uptake to restore accessible cholesterol to its homeostatic setpoint76. In striking similarity to the Scap-SREBP system, SM depletion potentiates Hh signaling whereas ALOD4 dampens Hh signaling (Fig. 5)19. As these perturbations do not change the total cholesterol content of cells, we conclude that Hh signaling is also sensitive to the accessible pool of cholesterol (Fig. 5b). Importantly, in some cell lines, SM depletion is sufficient to activate Hh signaling even in the absence of SHH, further highlighting the direct role of accessible cholesterol in signaling19.
Fig. 5 ∣. Changes in accessible cholesterol influence Hh signaling in target cells.
a, Accessible cholesterol can be reduced (left) in the plasma membrane by trapping it in the outer leaflet with ALOD4 (Fig. 4b) or by removing it with methyl-β-cyclodextrin (MβCD). Conversely, accessible cholesterol can be increased (right) by depleting sphingomyelin (SM) or by delivering cholesterol to the outer leaflet using MβCD–cholesterol complexes. Due to rapid flip-flop of cholesterol between the leaflets, changes in the outer leaflet are also transmitted to the inner leaflet. b, Changes in total cholesterol, the accessible pool of cholesterol and Hedgehog signaling strength after each of the manipulations shown immediately above in a. c, Conceptual cholesterol activity vs. concentration curves (see Fig. 4a for description) depicting how the manipulations shown in a change total and accessible cholesterol in the plasma membrane. The starting set-point in both panels is point A. ALOD4 (left panel) traps cholesterol (and shifts the curve to the right) without changing total cholesterol abundance (A→B), while cholesterol removal by MβCD reduces total and accessible cholesterol (A→C). Right panel shows two manipulations that increase cholesterol accessibility either by shifting the curve to the left (SM depletion, A→B) or by increasing total cholesterol (A→C).
How might PTCH1 reduce accessible cholesterol to control the activation of SMO? The simplest possibility is that PTCH1, using its transporter-like activity, depletes accessible cholesterol to levels below those required for SMO activation, similar to what is observed when cholesterol is depleted from plasma membranes by MβCD (Fig. 5). A prediction for this model is that the accessible cholesterol set point of a cell, determined approximately by the SM-to-cholesterol ratio, will determine the sensitivity of cells to SHH. This model explains the observation that the potency of SHH is enhanced by either cholesterol loading of cells or SM depletion, both of which will increase the cholesterol-to-SM ratio and hence the abundance of accessible cholesterol19,26,27 (Fig. 5). This will raise the transport burden on PTCH1, and consequently fewer molecules of PTCH1 would need to be inactivated by SHH to allow accessible cholesterol to rise above the threshold required to activate SMO. If PTCH1 is unable to keep up with the additional load of accessible cholesterol, the result is constitutive, SHH-independent signaling19.
Regulation of Hh signaling by ciliary cholesterol
The common use of accessible cholesterol to regulate both Hh signaling and cholesterol homeostasis raises a problem: how can the outputs of both pathways be independently regulated. For other shared second messengers, such as cAMP, this problem is solved by spatial segregation. Different pathways change second messenger levels in different cellular compartments or locations, which in turn also contain the cognate downstream signaling targets. An analogous solution to this problem for the Hh pathway is suggested based on the observation that PTCH1 regulates SMO at primary cilia14 (Fig. 6a). PTCH1 is concentrated in a punctate pattern along the membrane of the primary cilium and in a membrane invagination around the base of primary cilia known as the ciliary pocket14. SHH induces changes in the localization of PTCH1 and SMO: PTCH1 is inactivated and leaves the cilium, whereas SMO accumulates in the ciliary membrane14,81. SMO activation and accumulation in cilia are both required to transmit the Hh signal to the cytoplasm, likely because the downstream signaling machinery is localized in this organelle (Fig. 6a).
Fig. 6 ∣. Hh signal transmission by ligand-controlled changes in cholesterol accessibility of the ciliary membrane.
a, PTCH1 inhibits SMO (left) by reducing accessible cholesterol in the ciliary membrane (pink). Without SMO activity, full-length GLI proteins (GLI-FL) are proteolytically processed into transcriptional repressors (GLI-R). When PTCH1 is inactivated by SHH (right), accessible cholesterol levels in the ciliary membrane rise (blue), allowing SMO accumulation and activation and ultimately the formation of GLI activators (GLI-A). b, Conceptual cholesterol activity vs. concentration curves (see Fig. 4a) for the plasma membrane and ciliary membrane. The curve for the ciliary membrane is shifted to the right due to its higher sphingomyelin content. c, Schematic showing changes in cholesterol pools in the ciliary membrane in response to PTCH1 inactivation by SHH. Accessible cholesterol in the ciliary membrane rises in response to SHH, driving SMO activation. Two models for how inactivation of PTCH1 by SHH expands the ciliary accessible cholesterol pool are by increasing the total cholesterol in cilia or by converting some of the SM-sequestered cholesterol to an accessible form.
Primary cilia have distinct protein and lipid compositions compared to the bulk plasma membrane82. A barrier at the cilia base combined with elaborate trafficking systems maintain this distinct composition and also allows it to be dynamically altered to regulate the activity of cilia-localized proteins. Single-molecule imaging showed that the mobility and distribution of PTCH1 and SMO in the ciliary membrane can be altered by SHH or by cholesterol depletion with MβCD83. Membranes around flagella (analogs of cilia) found in single-cell protists like Paramecium and Trypanosoma brucei are enriched in sphingolipids and show a more ordered organization similar to those seen in membrane domains with condensed cholesterol–sphingolipid complexes84-87. A mutant of OlyA (OlyA_E69A) that binds to both cholesterol-complexed and cholesterol-free forms of SM revealed that primary cilia in mammalian cultured cells also have higher levels of total SM compared to the plasma membrane19,23.
If the total amount of cholesterol in ciliary and plasma membranes is the same, elevated SM levels in cilia would reduce the abundance of accessible cholesterol in cilia compared to the plasma membrane (Fig. 6b). Two observations support the scarcity of accessible cholesterol in the ciliary membrane. First, the membrane of the cilium is more resistant to permeabilization by cholesterol-binding detergents or toxins compared to the plasma membrane88. Second, probes that bind to accessible cholesterol on the plasma membrane fail to stain the ciliary membrane in cultured cells19. Most importantly, inactivation of PTCH1 by SHH leads to an increase in accessible cholesterol at primary cilia19 (Fig. 6c). This last observation provides the critical link that connects the biochemical and structural evidence implicating cholesterol in PTCH1–SMO regulation to the cell biological data identifying primary cilia as the subcellular compartment where PTCH1 regulates SMO.
Models for the function of PTCH1 at primary cilia
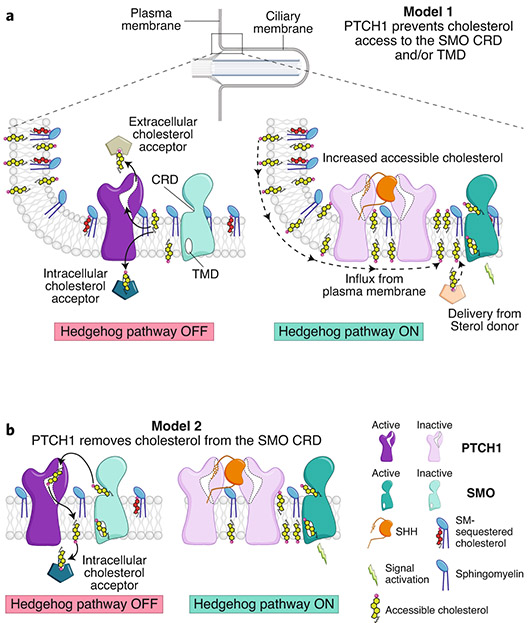
At equilibrium, the chemical activity or accessibility of cholesterol would be expected to be the same throughout the plasma membrane. Thus, despite the observation that SM levels are higher at primary cilia, an active transport mechanism would be required to maintain a difference in cholesterol accessibility between the ciliary and bulk plasma membranes. This concept suggests a ‘pump-leak’ model for PTCH1 inhibition of SMO at cilia19 (Fig. 7a). According to this model, PTCH1 uses its energy-driven transporter function to keep accessible cholesterol levels in the ciliary membrane below the threshold required for SMO activation. Excess cholesterol above this threshold is transported by PTCH1 to either an intracellular or an extracellular acceptor. The pumping action of PTCH1 would be opposed by the continual leak of cholesterol back into the ciliary membrane down its activity gradient. Consequently, when PTCH1 is inactivated by SHH, levels of accessible ciliary cholesterol would rise and activate SMO (Fig. 7a).
Fig. 7 ∣. Two models for the regulation of SMO by PTCH1 at primary cilia.
a, A cartoon representation of cilia is shown at the top, with a rectangle showing a region at the cilia base known as the ‘ciliary pocket’, magnified below. In model 1, PTCH1 uses its transporter function (left) to remove cholesterol from the ciliary membrane, transferring it to an acceptor. Because of the higher abundance of SM in the ciliary membrane, accessible cholesterol drops below the threshold required to activate SMO. Inactivation of PTCH1 (right) allows influx of cholesterol back into the ciliary membrane, driving SMO activity. b, An alternative model in which PTCH1 directly inactivates SMO by accepting cholesterol from its CRD, inspired by how cholesterol bound to the NTD of NPC1 is transferred to the CTD (see Fig. 3b).
Several alternate models for the function of PTCH1 should be considered1,89. One model is suggested by recent structural and functional studies of the NPC1 protein, which shows that the N-terminal domain of NPC1 (the NTD, Fig. 3a,b) transfers cholesterol to the tunnel through NPC1 for transport to the lysosomal membrane48,49. By analogy, the extracellular domain of PTCH1 could accept a cholesterol molecule from the SMO CRD and transport it to the membrane, which is a direct inactivation mechanism (Fig. 7b). Furthermore, PTCH1 could increase ciliary SM or promote SM–cholesterol interactions. Finally, PTCH1 could promote the expulsion of accessible cholesterol in ciliary exovesicles90,91, as such vesicles from macrophages are enriched in accessible cholesterol92.
Transbilayer cholesterol distribution in PTCH1-SMO regulation
The issue of cholesterol distribution between the two leaflets is relevant to SMO activation because there is uncertainty about whether cholesterol gains access to SMO from the inner or outer leaflet19,33,34,45 (Fig. 5a). In one study, PTCH1 was overexpressed throughout the plasma membrane to show that it selectively reduces the abundance of inner-leaflet cholesterol45. The authors proposed that inactivation of PTCH1 would lead to an increase in inner-leaflet cholesterol, which would then move through a gap between two TM helices to bind the TMD2 site34. However, the CRD sterol-binding site on SMO, perched >10 Å above the membrane, is most likely to receive cholesterol from the outer leaflet. Indeed, our work using fluorescent PFO* added to intact cells expressing endogenous levels of PTCH1 shows that SHH induces an increase in the accessibility of outer-leaflet cholesterol at the ciliary membrane19.
These seemingly divergent observations are both consistent with PTCH1 inactivation resulting in an overall increase in cholesterol accessibility that is manifested in both leaflets. While the steady state concentration of total cholesterol may be different between the leaflets93-95, the chemical activity of cholesterol is likely similar. This is because cholesterol (unlike phospholipids with charged head groups) can rapidly flip-flop between the two leaflets of the plasma membrane on a subsecond time scale. Maintaining an activity gradient would require an energetically prohibitive active-transport mechanism96. Thus, changes in cholesterol activity induced in one leaflet, caused by cholesterol loading or depletion, ALOD4 binding or SM depletion, are likely to be reflected in both leaflets due to this rapid transbilayer movement of cholesterol19 (Fig. 5a).
Evolution of the Hh pathway
The many links between Hh signaling and cholesterol (Fig. 1) are best explained by the model that Hh signaling evolved from an ancient pathway for sensing and pumping sterol-like molecules (like hopanoids) in unicellular organisms97,98. Such a pathway would have functioned to maintain optimal membrane hopanoid composition using a combination of a sensor and transporter. A SMO-like protein (the sensor) would be activated by an increase in membrane hopanoids and initiate a signaling cascade that upregulates the production of a PTCH1-like transporter, which would return membrane composition to homeostatic levels (and consequently lead to inactivation of the sensor). This regulatory connection explains the unique inhibitory interaction between PTCH1 and SMO, with PTCH1 being a transporter for the same sterol that activates SMO (Fig. 1). In addition, the major negative-feedback loop in the present-day Hh pathway directly follows the logic of this homeostatic pathway: SMO activation induces PTCH1 transcription, which feeds back to attenuate SMO activity.
Adaptation of this sensor–transporter module to paracrine cell–cell communication in multicellular organisms requires a ligand secreted by one cell that can regulate the transporter in a neighboring cell. Basler and colleagues have suggested that the simplest way to accomplish this is to covalently link the substrate for the transporter to a protein that will sterically block transporter activity97. Support for this insightful idea comes from recent structural studies showing that the cholesterol molecule covalently linked to SHH binds to PTCH1 and seems to occlude a conduit for sterol transport51,52 (Fig. 3d). The observation that cholesterol is both a substrate and (when linked to SHH) an inhibitor of PTCH1 provides an explanation for the unusual requirement of cholesterol in both ligand production and ligand reception.
Conclusions
The work summarized above points to an answer to the longstanding mystery of how PTCH1 inhibits SMO in Hh signaling: PTCH1 functions as a membrane remodeling machine to inhibit SMO by changing the cholesterol composition of the ciliary membrane (Fig. 7a). This model provides a unifying explanation for many seemingly unrelated clues that have emerged over the last 25 years around the PTCH1–SMO interaction, including the role of cholesterol and oxysterols in SMO activation, the role of primary cilia, and the homology of PTCH1 to transporter proteins. We emphasize that this model is still provisional, with many aspects based on circumstantial evidence, and will require further testing and refinement as new data emerge.
Key questions for the future include how PTCH1 regulates membrane cholesterol and how the lipid composition of the ciliary membrane is regulated. A more general question is how cholesterol accessibility is sensed and then regulated in cells, either in the plasma membrane or in specific membrane compartments like primary cilia. Answers to these questions could have broad implications for diverse transmembrane signaling processes in cells.
Acknowledgements
We acknowledge G. Pusapati for help with the figures and comments on the manuscript. C.S. was supported by grants from Cancer Research UK (C20724/A26752) and the European Research Council (647278), R.R. by grants from the National Institutes of Health (GM118082 and GM106078), and A.R. by grants from the NIH (HL20948), Welch Foundation (I-1793) and Leducq Foundation (19CVD04).
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kong JH, Siebold C & Rohatgi R Biochemical mechanisms of vertebrate hedgehog signaling. Development 146, dev166892 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huangfu D et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83–87 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Goetz SC, Ocbina PJR & Anderson KV The primary cilium as a Hedgehog signal transduction machine. Methods Cell Biol. 94, 199–222 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C et al. Structure of the human smoothened receptor bound to an antitumour agent. Nature 497, 338–343 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter JA, Young KE & Beachy PA Cholesterol modification of Hedgehog signaling proteins in animal development. Science 274, 255–259 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Pepinsky RB et al. Identification of a palmitic acid-modified form of human Sonic hedgehog. J. Biol. Chem 273, 14037–14045 (1998). [DOI] [PubMed] [Google Scholar]
- 7.Chiang C et al. Cyclopia and defective axial patterning in mice lacking Sonic Hedgehog gene function. Nature 383, 407–413 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Cooper MK, Porter JA, Young KE & Beachy PA Teratogen-mediated inhibition of target tissue response to Shh signaling. Science 280, 1603–1607 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Cooper MK et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat. Genet 33, 508–513 (2003).Cholesterol depletion using MβCD or mutations in genes encoding terminal cholesterol biosynthetic enzymes impair Hh signaling in target cells.
- 10.Blassberg R, Macrae JI, Briscoe J & Jacob J Reduced cholesterol levels impair Smoothened activation in Smith-Lemli-Opitz syndrome. Hum. Mol. Genet 25, 693–705 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharpe HJ, Wang W, Hannoush RN & de Sauvage FJ Regulation of the oncoprotein Smoothened by small molecules. Nat. Chem. Biol 11, 246–255 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Wang C et al. Structural basis for Smoothened receptor modulation and chemoresistance to anticancer drugs. Nat. Commun 5, 4355 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corcoran RB & Scott MP Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc. Natl Acad. Sci. USA 103, 8408–8413 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohatgi R, Milenkovic L & Scott MP Patched1 regulates hedgehog signaling at the primary cilium. Science 317, 372–376 (2007).PTCH1 is localized in and around primary cilia and inhibits SMO activity and accumulation in the ciliary membrane.
- 15.Dwyer JR et al. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J. Biol. Chem 282, 8959–8968 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Nachtergaele S et al. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat. Chem. Biol 8, 211–220 (2012).Side chain oxysterols induce Hh responses in cultured cells by binding and activating SMO.
- 17.Raleigh DR et al. Cilia-associated oxysterols activate Smoothened. Mol. Cell 72, 316–327.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Chen G, Head DL, Mangelsdorf DJ & Russell DW Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 5, 73–79 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinnebrew M et al. Cholesterol accessibility at the ciliary membrane controls hedgehog signaling. eLife 8, e50051 (2019).A CRISPR screen and sphingomyelin (SM) depletion showed that Hh signaling is activated selectively by the accessible pool of membrane cholesterol. Fluorescent probes described in ref. 23 showed that Hh ligands trigger an increase in accessible cholesterol in the ciliary membrane by inactivating PTCH1, allowing SMO activation.
- 20.McConnell HM & Radhakrishnan A Condensed complexes of cholesterol and phospholipids. Biochim. Biophys. Acta 1610, 159–173 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Demel RA, Jansen JW, van Dijck PW & van Deenen LL The preferential interaction of cholesterol with different classes of phospholipids. Biochim. Biophys. Acta 465, 1–10 (1977). [DOI] [PubMed] [Google Scholar]
- 22.Finean JB Phospholipid-cholesterol complex in the structure of myelin. Experientia 9, 17–19 (1953). [DOI] [PubMed] [Google Scholar]
- 23.Endapally S et al. Molecular discrimination between two conformations of sphingomyelin in plasma membranes. Cell 176, 1040–1053.e17 (2019).Fluorescently labeled probes derived from microbial and fungal toxins that can be used to distinguish between the accessible and sequestered pools of cholesterol were developed.
- 24.Klein U, Gimpl G & Fahrenholz F Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry 34, 13784–13793 (1995). [DOI] [PubMed] [Google Scholar]
- 25.Byrne EFX et al. Structural basis of Smoothened regulation by its extracellular domains. Nature 535, 517–522 (2016).A 3.2 Å multi-domain crystal structure of SMO unexpectedly revealed a cholesterol molecule bound in the cysteine-rich domain (CRD).Clinically used SMO inhibitors induce a conformational change that prevents cholesterol access to the CRD.
- 26.Luchetti G et al. Cholesterol activates the G-protein coupled receptor Smoothened to promote Hedgehog signaling. eLife 5, e20304 (2016).Refs. 26 and 27 showed that cholesterol can function as an instructive agonist for SMO and is both necessary and sufficient to activate Hh signaling.
- 27.Huang P et al. Cellular cholesterol directly activates Smoothened in Hedgehog signaling. Cell 166, 1176–1187.e14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nedelcu D, Liu J, Xu Y, Jao C & Salic A Oxysterol binding to the extracellular domain of Smoothened in Hedgehog signaling. Nat. Chem. Biol 9, 557–564 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers BR et al. Hedgehog pathway modulation by multiple lipid binding sites on the smoothened effector of signal response. Dev. Cell 26, 346–357 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nachtergaele S et al. Structure and function of the Smoothened extracellular domain in vertebrate Hedgehog signaling. eLife 2, e01340 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao X et al. Cholesterol modification of Smoothened is required for Hedgehog signaling. Mol. Cell 66, 154–162.e10 (2017).Mutations in the CRD cholesterol-binding site abolished SMO function in mouse embryos, and PTCH1 can attenuate cholesterol access to the SMO CRD.
- 32.Zhang X et al. Crystal structure of a multi-domain human smoothened receptor in complex with a super stabilizing ligand. Nat. Commun 8, 15383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deshpande I et al. Smoothened stimulation by membrane sterols drives Hedgehog pathway activity. Nature 571, 284–288 (2019).An active-state structure of SMO bound to two synthetic agonists revealed a second cholesterol-binding site in the middle of the TMD, in addition to the previously identified site in the CRD.
- 34.Huang P et al. Structural basis of Smoothened activation in Hedgehog signaling. Cell 175, 295–297 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi X et al. Cryo-EM structure of oxysterol-bound human Smoothened coupled to a heterotrimeric Gi. Nature 571, 279–283 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yauch RL et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science 326, 572–574 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers BR, Neahring L, Zhang Y, Roberts KJ & Beachy PA Rapid, direct activity assays for Smoothened reveal Hedgehog pathway regulation by membrane cholesterol and extracellular sodium. Proc. Natl Acad. Sci. USA 114, E11141–E11150 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loftus SK et al. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science 277, 232–235 (1997). [DOI] [PubMed] [Google Scholar]
- 39.Carstea ED et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science 277, 228–231 (1997). [DOI] [PubMed] [Google Scholar]
- 40.Davies JP & Ioannou YA Topological analysis of Niemann-Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J. Biol. Chem 275, 24367–24374 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Tseng TT et al. The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol. Biotechnol 1, 107–125 (1999). [PubMed] [Google Scholar]
- 42.Gong X et al. Structural basis for the recognition of Sonic Hedgehog by human Patched1. Science 361, eaas8935 (2018).Refs. 42-45 report liganded and unliganded cryo-EM structures of PTCH1, suggesting a tunnel through the protein that may function in sterol transport.Ref. 45 demonstrated that overexpression of PTCH1 reduces recruitment of a cholesterol-binding probe to the inner leaflet of the plasma membrane.
- 43.Qi X, Schmiege P, Coutavas E & Li X Two Patched molecules engage distinct sites on Hedgehog yielding a signaling-competent complex. Science 362, aas8843 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi X, Schmiege P, Coutavas E, Wang J & Li X Structures of human Patched and its complex with native palmitoylated sonic hedgehog. Nature 560, 128–132 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y et al. Structural basis for cholesterol transport-like activity of the Hedgehog receptor patched. Cell 175, 1352–1364.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X et al. Structure of human Niemann-Pick C1 protein. Proc. Natl Acad. Sci. USA 113, 8212–8217 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong X et al. Structural insights into the Niemann-Pick C1 (NPC1)-mediated cholesterol transfer and Ebola infection. Cell 165, 1467–1478 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian H et al. Structural basis of low-pH-dependent lysosomal cholesterol egress by NPC1 and NPC2. Cell 182, 98–111.e18 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Winkler MBL et al. Structural insight into eukaryotic sterol transport through Niemann-Pick type C proteins. Cell 179, 485–497.e18 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Qi C, Di Minin G, Vercellino I, Wutz A & Korkhov VM Structural basis of sterol recognition by human hedgehog receptor PTCH1. Sci. Adv 5, eaaw6490 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudolf AF et al. The morphogen Sonic hedgehog inhibits its receptor Patched by a pincer grasp mechanism. Nat. Chem. Biol 15, 975–982 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian H et al. Inhibition of tetrameric Patched1 by Sonic Hedgehog through an asymmetric paradigm. Nat. Commun 10, 2320 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long T et al. Structural basis for itraconazole-mediated NPC1 inhibition. Nat. Commun 11, 152 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Infante RE et al. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J. Biol. Chem 283, 1052–1063 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Infante RE et al. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J. Biol. Chem 283, 1064–1075 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Infante RE et al. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc. Natl Acad. Sci. USA 105, 15287–15292 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwon HJ et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 137, 1213–1224 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bidet M et al. The hedgehog receptor patched is involved in cholesterol transport. PLoS One 6, e23834 (2011).PTCH1 can promote efflux of a fluorescent cholesterol analog (BODIPY-cholesterol) from cells.
- 59.Litz JP, Thakkar N, Portet T & Keller SL Depletion with cyclodextrin reveals two populations of cholesterol in model lipid membranes. Biophys. J 110, 635–645 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watts A, Volotovski ID & Marsh D Rhodopsin-lipid associations in bovine rod outer segment membranes. Identification of immobilized lipid by spin-labels. Biochemistry 18, 5006–5013 (1979). [DOI] [PubMed] [Google Scholar]
- 61.Leathes JB Condensing effect of cholesterol on monolayers. Lancet 208, 853–856 (1925). [Google Scholar]
- 62.Finegold LX Cholesterol in Membrane Models. (CRC Press, 1992). [Google Scholar]
- 63.McConnell H & Radhakrishnan A Theory of the deuterium NMR of sterol-phospholipid membranes. Proc. Natl Acad. Sci. USA 103, 1184–1189 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Radhakrishnan A & McConnell H Condensed complexes in vesicles containing cholesterol and phospholipids. Proc. Natl Acad. Sci. USA 102, 12662–12666 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lange Y, Tabei SMA, Ye J & Steck TL Stability and stoichiometry of bilayer phospholipid-cholesterol complexes: relationship to cellular sterol distribution and homeostasis. Biochemistry 52, 6950–6959 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keller SL, Radhakrishnan A & McConnell HM Saturated phospholipids with high melting temperatures form complexes with cholesterol in monolayers. J. Phys. Chem. B 104, 7522–7527 (2000). [Google Scholar]
- 67.Lönnfors M, Doux JPF, Killian JA, Nyholm TKM & Slotte JP Sterols have higher affinity for sphingomyelin than for phosphatidylcholine bilayers even at equal acyl-chain order. Biophys. J 100, 2633–2641 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Radhakrishnan A & McConnell HM Chemical activity of cholesterol in membranes. Biochemistry 39, 8119–8124 (2000). [DOI] [PubMed] [Google Scholar]
- 69.Ahn K-W & Sampson NS Cholesterol oxidase senses subtle changes in lipid bilayer structure. Biochemistry 43, 827–836 (2004). [DOI] [PubMed] [Google Scholar]
- 70.Flanagan JJ, Tweten RK, Johnson AE & Heuck AP Cholesterol exposure at the membrane surface is necessary and sufficient to trigger perfringolysin O binding. Biochemistry 48, 3977–3987 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gay A, Rye D & Radhakrishnan A Switch-like responses of two cholesterol sensors do not require protein oligomerization in membranes. Biophys. J 108, 1459–1469 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haynes MP, Phillips MC & Rothblat GH Efflux of cholesterol from different cellular pools. Biochemistry 39, 4508–4517 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Lange Y, Ye J & Steck TL How cholesterol homeostasis is regulated by plasma membrane cholesterol in excess of phospholipids. Proc. Natl Acad. Sci. USA 101, 11664–11667 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Das A, Goldstein JL, Anderson DD, Brown MS & Radhakrishnan A Use of mutant 125I-perfringolysin O to probe transport and organization of cholesterol in membranes of animal cells. Proc. Natl Acad. Sci. USA 110, 10580–10585 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Das A, Brown MS, Anderson DD, Goldstein JL & Radhakrishnan A Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. eLife 3, e02882 (2014).Sphingomyelin regulates the partitioning of cholesterol between the accessible and sequestered pools.
- 76.Infante RE & Radhakrishnan A Continuous transport of a small fraction of plasma membrane cholesterol to endoplasmic reticulum regulates total cellular cholesterol. eLife 6, e25466 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skočaj M et al. Tracking cholesterol/sphingomyelin-rich membrane domains with the ostreolysin A-mCherry protein. PLoS One 9, e92783 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldstein JL & Brown MS A century of cholesterol and coronaries: from plaques to genes to statins. Cell 161, 161–172 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Slotte JP & Bierman EL Depletion of plasma-membrane sphingomyelin rapidly alters the distribution of cholesterol between plasma membranes and intracellular cholesterol pools in cultured fibroblasts. Biochem. J 250, 653–658 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scheek S, Brown MS & Goldstein JL Sphingomyelin depletion in cultured cells blocks proteolysis of sterol regulatory element binding proteins at site 1. Proc. Natl Acad. Sci. USA 94, 11179–11183 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corbit KC et al. Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018–1021 (2005).Hh ligands promote the accumulation of SMO in primary cilia, the likely subcellular location from which it signals to the cytoplasm.
- 82.Nachury MV & Mick DU Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Mol. Cell Biol 20, 389–405 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weiss LE, Milenkovic L, Yoon J, Stearns T & Moerner WE Motional dynamics of single Patched1 molecules in cilia are controlled by Hedgehog and cholesterol. Proc. Natl Acad. Sci. USA 116, 5550–5557 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaiser F, Huebecker M & Wachten D Sphingolipids controlling ciliary and microvillar function. FEBS Lett. 10.1002/1873-3468.13816 (2020). [DOI] [PubMed] [Google Scholar]
- 85.Serricchio M et al. Flagellar membranes are rich in raft-forming phospholipids. Biol. Open 4, 1143–1153 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tyler KM et al. Flagellar membrane localization via association with lipid rafts. J. Cell Sci 122, 859–866 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaneshiro ES, Matesic DF & Jayasimhulu K Characterizations of six ethanolamine sphingophospholipids from Paramecium cells and cilia. J. Lipid Res 25, 369–377 (1984). [PubMed] [Google Scholar]
- 88.Breslow DK, Koslover EF, Seydel F, Spakowitz AJ & Nachury MV An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J. Cell Biol 203, 129–147 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kowatsch C, Woolley RE, Kinnebrew M, Rohatgi R & Siebold C Structures of vertebrate Patched and Smoothened reveal intimate links between cholesterol and Hedgehog signalling. Curr. Opin. Struct. Biol 57, 204–214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nager AR et al. An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell 168, 252–263.e14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Phua SC et al. Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell 168, 264–279.e15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He C et al. Macrophages release plasma membrane-derived particles rich in accessible cholesterol. Proc. Natl Acad. Sci. USA 115, E8499–E8508 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mondal M, Mesmin B, Mukherjee S & Maxfield FR Sterols are mainly in the cytoplasmic leaflet of the plasma membrane and the endocytic recycling compartment in CHO cells. Mol. Biol. Cell 20, 581–588 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Courtney KC et al. C24 sphingolipids govern the transbilayer asymmetry of cholesterol and lateral organization of model and live-cell plasma membranes. Cell Reports 24, 1037–1049 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Liu S-L et al. Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol. Nat. Chem. Biol 13, 268–274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Steck TL & Lange Y Transverse distribution of plasma membrane bilayer cholesterol: Picking sides. Traffic 19, 750–760 (2018). [DOI] [PubMed] [Google Scholar]
- 97.Hausmann G, von Mering C & Basler K The hedgehog signaling pathway: where did it come from? PLoS Biol. 7, e1000146 (2009).A hypothesis that Hh signaling may have evolved from an ancient pathway for sensing and transporting hopanoids.
- 98.Bazan JF & de Sauvage FJ Structural ties between cholesterol transport and morphogen signaling. Cell 138, 1055–1056 (2009). [DOI] [PubMed] [Google Scholar]
- 99.Chovancova E et al. CAVER 3.0: a tool for the analysis of transport pathways in dynamic protein structures. PLOS Comput. Biol 8, e1002708 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sokolov A & Radhakrishnan A Accessibility of cholesterol in endoplasmic reticulum membranes and activation of SREBP-2 switch abruptly at a common cholesterol threshold. J. Biol. Chem 285, 29480–29490 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]