Abstract
Asthma and influenza are leading causes of worldwide morbidity and mortality. Although these two conditions can co-exist in the same patient, the immune parameters that impact disease outcomes are not fully elucidated. The importance of macrophages to both conditions suggested a role for CD14, a co-receptor for endotoxin, as a regulatory mechanism for innate immune responses during asthma and influenza co-morbidity. Herein, we hypothesized that parameters of influenza morbidity will be reduced in the absence of CD14. Age and gender matched wild-type (WT) and CD14 knock-out (KO) mice were subjected to our validated model of Aspergillusinduced model of asthma and/or influenza. Characteristics of disease pathogenesis were investigated using standard methods in weight loss, flow cytometry, airway resistance, histology, quantitative real-time PCR, and viral titer quantification. The absence of CD14 did not have an impact on morbidity as these mice were equally susceptible to disease with similar airway resistance. Peribronchovascular inflammation and goblet cell content were equivalent between WT and KO mice in asthma alone and asthma and influenza co-morbidity. Co-morbid KO mice had less lymphocytes and eosinophils in the airways although their lung viral burden was equivalent to WT. Inflammatory gene signatures were altered in co-morbid mice in each genotype. CD14 expression on macrophages is necessary for airway inflammation but not for viral pathogenesis in allergic hosts.
Keywords: Fungal asthma, Mouse, Inflammation
1. Introduction
Asthma is a common chronic, multifactorial disease of the airways known to precipitate from both environmental triggers and genetic predispositions. Allergic asthma is especially prevalent, and fungal antigens are the most ubiquitous trigger. Amongst the various known characteristics of asthma such as airway hyperresponsiveness (AHR) and increased goblet cell metaplasia associated with mucus production, inflammation is a key pathological feature characterized by recruitment of inflammatory cells such as granulocytes, macrophages and lymphocytes [1].
Pattern recognition receptors (PRRs) are an important component of antigen recognition by cells of the innate immune system. Toll-like receptors (TLRs) are a subgroup of PRRs that are key players in host defense to a plethora of pathogens including fungi and viruses. TLR-4, activated by lipopolysaccharide (LPS), is known to be primarily expressed on the cell surface and plays a significant role in maintaining inflammation in diseases like asthma [2]. Functioning as an accessory molecule to TLR-4, CD14 is an endotoxin receptor found either on the surface of macrophages or in soluble form in the serum [3], and appears to be critical in the induction of a TH1 response. LPS signaling through this pathway has been shown to increase levels of IL-12 and IFN-γ as well as a coinciding increase in levels of circulatory CD14 and a reduction of IgE levels in various asthma models thereby skewing the immune response from TH2 to TH1 [4–8]. CD14 mutations may increase the risk for severe asthma [9], while polymorphisms in CD14 are associated with decreased levels of serum IgE in children [5]. Mutations in TLR4 have also been previously shown to diminish airway hyperresponsiveness in humans [10,11]. Therefore, the debate of CD14’s function in the development and severity of asthma and other allergic diseases is ongoing with publications showing differing results in both children and adults.
Asthma is generally considered a risk factor for hospitalization during influenza infections. However, the 2009 pandemic (p)H1N1 influenza led to the unexpected finding that of all patients hospitalized, those with asthma had significantly lower morbidity and mortality rates [12–14]. This counterintuitive finding has led to several discoveries concerning the interaction of influenza with the asthmatic lung [15–17]. Independent immune responses in each disease condition are complex, and those involved in asthma and influenza co-morbidity are even more complicated and multifaceted [17]. Therefore, investigation of host-pathogen interactions in asthma and influenza co-morbidities is desirable from both an academic and a public health standpoint. Herein, we aimed to delineate the mechanistic function of macrophage CD14 in asthma-influenza co-morbidity. We demonstrate that CD14 plays a pro-inflammatory role leading to increased inflammation during influenza in allergic mice, without impacting structural changes such as goblet cell metaplasia.
2. Materials and methods
2.1. Ethics statement
All animal work was performed in strict accordance with protocols approved by the Institutional Animal Care and Use Committee at St. Jude Children’s Research Hospital.
2.2. Animal model
Gender-matched C57BL/6J and CD14tm1Frm/J mice (CD14 deficiency in macrophages) at six weeks of age were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in micro-isolator cages with free access to food and water with a 12 h light and dark cycle and allowed to acclimatize for one week.
2.3. Acute asthma and influenza model (AA + Flu)
Mice were subjected to the AA + Flu model as previously described [16]. A well characterized model of fungal asthma was used to induce the hallmarks of allergic asthma in mice [18,19]. Briefly, mice were globally sensitized by intraperitoneal and subcutaneous injections with Aspergillus fumigatus extract (Greer Labs, Lenoir, NC) adjuvanted in Imject® Alum (Pierce, Rockford, IL). Three local sensitizations were performed by intranasal delivery of A. fumigatus extract diluted in phosphate buffered saline (PBS). One week later, mice were exposed to A. fumigatus mature conidia through a nose-only route for 10 min, two weeks apart. For the AA + Flu model, mice were infected with 50 μLof 1000 tissue culture infectious dose 50 (TCID50) of pH1N1 (A/California/04/2009) one week after the second inhalation challenge. Naïve control mice were mock-treated with PBS. Mice subjected to the asthma model only were referred to as the ‘Asthma’ group while those only infected with virus were referred to as the ‘Flu’ group. Tissues were harvested at day 5 after the virus infection to capture the intersection phase between innate and adaptive immunity.
2.4. Airway hyperresponsiveness measurements
Mice were anesthetized with xylazine (7.5 mg/mL) and then injected with pentobarbital (2.5 mg/mL) prior to intubation with a metal cannula. Cannulated mice were attached to a computer controlled small animal ventilator (flexiVent FX1, SCIREQ, Quebec, Canada) and challenged with 0 and 25 mg/mL acetyl-β-methylcholine (Sigma, St. Louis, MO) using a nebulizer to measure parameters of airway physiologic responses. The mean for each dose was calculated for each mouse and the data represented as the mean and standard error for each group of mice as a fold increase over baseline.
2.5. Tissue harvest and processing
Following AHR measurements, mice were subjected to cervical dislocation as a secondary measure of euthanasia. Bronchoalveolar lavage (BAL) was performed with 1 mL of 1 × PBS and repeated with a total of 2 mL. Cardiac, middle and bottom half of proximal lung lobes were harvested for RNA and the distal and the remainder of the proximal lung lobes for viral titration. Pooled blood in the thoracic cavity was collected and stored in ice for 1 h until processing. Blood samples were centrifuged at 12,000 × g for 10 min and serum was transferred to new tubes and stored at −80 °C.
2.6. Flow cytometric analyses of BAL cells
BAL contents were centrifuged at 1500 × g for 10 min, and BAL fluid was removed and stored at −80 °C. BAL cells were suspended in staining media (PBS/5% PBS) containing human gamma globulin and incubated on ice for 30 min. Cells were washed and stained with antibodies that were fluorescently labeled for 30 min on ice. Streptavidin-BV605 was used to probe Mac-3-biotin. The samples which were harvested from AA + Flu and Flu-control mice were additionally stained with PB1-tetramer-PE (kindly provided by Dr. Paul Thomas, St. Jude Children’s Research Hospital). All cells were fixed with 1 × stabilizing fixative (BD Biosciences, San Jose, CA). Data were acquired using LSR Fortessa Cytometer (BD). The list of fluorochromes which were purchased from BD Biosciences which were diluted 1:50 unless indicated otherwise are as follows: (clones are indicated in parentheses) CD8α-FITC (53–6.7), CD19-PerCP/Cy5.5 (1D3), Ly6G-V450 (1A8), CD193 (CCR3)-Alexa Fluor 647 (83103), CD4-Alexa Fluor 700 (RM4–5), CD3ε-PE/Cy7 (145–2C11), CD107b (Mac-3)-Biotin (M3/84, BioLegend, 1:500). Controls included unstained cells, cells stained with single colors and matched isotypes. Data were analyzed using FlowJo v10.3 (Flowjo, LLC, Ashland, OR).
2.7. Quantitative real time PCR
Trizol (Invitrogen, Carlsbad, CA), and chloroform extraction method was used to extract RNA from lungs per standard protocol. Extracted RNA was quantified and DNase treated accordingly, and 1 μg of RNA was converted to cDNA using iSCRIPT™ (BioRad, Hercules, CA). Diluted cDNA was used to quantify gene expression for Hprt, Cd14, Tlr4, Retnla, Muc5ac, and Muc5b, with Quantitect primers and SYBR® Green master mix (Qiagen, Hilden, Germany) using an ABI 7500 (Applied Biosystems, Foster City, CA). Hprt was used as the housekeeping gene and all calculations were done using the 2−ΔΔCt method using naïve levels as the baseline. Data are represented as the mean (of all technical replicates in each biological replicate) and standard error of the mean.
2.8. Quantification of antibodies
Isotypes of antibodies in the serum were measured by ELISA kits following manufacturer’s guidelines. Samples were diluted 1:5000 for IgA and IgG1 (Bethyl Labs, Montgomery, TX), 1:500 for IgE and used neat for IgG2a (BD Biosciences).
2.9. Quantification of viral burden
From each mouse in the study, the weights of distal and half of the proximal lobes were recorded prior to homogenization in 1 mL of PI cocktail (Roche, Basel, Switzerland), and tissue-free supernatants were aliquotted and stored at −80 °C. Serially diluted (1:10) lung homogenates were added to 96 well plates with confluent MDCK cells (ATCC, Manassas, VA), and incubated for 1 h. Cells were washed with PBS, and infection media containing 1 μg/mL TPCK-trypsin (Worthington Biochemicals, Lakewood, NJ) was added to the plates and incubated for 72 h. Agglutinated chicken red blood cells were used to calculate the viral titer using the Reed-Muench method. The viral burden of each homogenate was then divided by the weight of the original sample, to determine the amount of virus per gram of lung tissue.
2.10. Histological analyses
Lobes of the left lung were harvested and formalin fixed ex vivo, sectioned at 4 μm, and affixed to glass slides and stained with periodic acid Schiff’s (PAS) stain to quantify goblet cells (GCs) along 100 μm of basement membrane in 10 large airways visualized using 200 × objective of a Nikon Eclipse 50i light microscope. The percentage of GCs in the airway epithelial layer was calculated for each mouse and averaged for the group.
2.11. Statistical analyses
Mice in both genotypes were separated into groups of five for each treatment (Naïve, Flu-control, Asthma-control, and AA + Flu). The mean and standard deviation (SD) were calculated for each treatment in each group and analyzed by two-way ANOVA followed by Tukey’s or Sidak’s multiple comparisons tests as appropriate using GraphPad Prism software v6.01 (La Jolla, CA) as noted in the Figure legends.
3. Results
We have previously shown that total populations of airway macrophages do not change during asthma and influenza co-morbidity [16]. However, the impact of CD14 on macrophages in immune responses during asthma and influenza co-morbidity has not been determined. Herein, using a mouse that lacks CD14 expression on macrophages, we investigated its function in two common lung diseases. In this study, our data indicate that while CD14 does not play a significant role in the major airway remodeling seen in allergic asthma, it does appear to play a pro-inflammatory role in allergic airways during virus infection. The absence of CD14 also altered the antibody and cytokine environment.
3.1. Absence of CD14 does not affect weight loss and minimally impacts lung viral burden
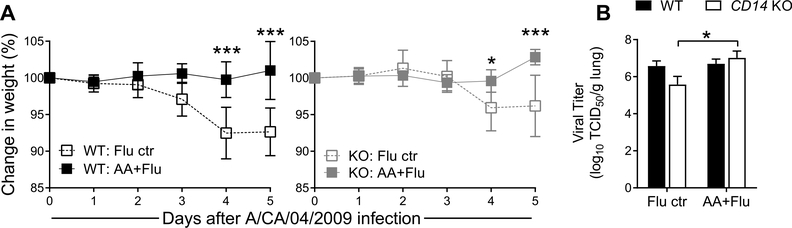
Weight loss is a hallmark of influenza morbidity in mice. Allergic mice infected with pH1N1 did not lose weight in either genotype while non-allergic virus-infected mice of both genotypes had significant weight loss (Fig. 1A). Overall, CD14 deficient mice lost less weight after pH1N1 infection compared to WT mice by day 5 (Fig. 1A). While preexisting allergic airways inflammation did not impact the lung viral burden in WT mice, a significant increase was observed in the KO AA + Flu group as compared to the KO mice infected with pH1N1 alone (Fig. 1B).
Fig. 1. CD14 is not necessary for influenza (Flu) virus-mediated morbidity or the regulation of virus load.
Virus infection resulted in weight loss in wild-type (WT) and knock-out (KO) mice in the absence of allergy with no differences between genotypes (A). Viral load in the lungs were similar between WT and KO mice after infection although allergic KO mice had significantly more virus in the lungs compared to non-allergic KO mice (B). Data are represented as the mean and SD of n = 5 mice per group per genotype and analyzed with two-way ANOVA with Sidak’s (A) or Tukey’s (B) multiple comparisons test to compare data between groups. Significance marked by *p < 0.05and ***p < 0.001. TCID50 = Tissue Culture Infectious Dose 50.
3.2. Airway resistance was unaffected in allergic mice devoid of CD14
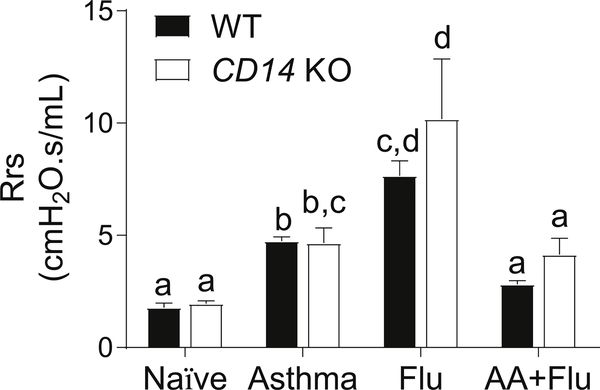
Generally, AHR is associated with lung inflammation and is an indicator of lung function [12]. We measured AHR by assessing the airway resistance after exposure to a non-specific spasmogen. Allergen exposure and virus infection caused a significant increase in AHR in both genotypes (Fig. 2). However, the co-occurrence of asthma and influenza led to a decrease in AHR in both genotypes (Fig. 2).
Fig. 2. The absence of CD14 on macrophages did not impact the resistance of the respiratory system (Rrs).
Allergen and virus exposure caused a significant increase in airway resistance in mice, but the co-pathogenesis of asthma and influenza resulted in no change. Data are represented as the mean and SEM of n = 5 mice per group per genotype and analyzed with two-way ANOVA with Tukey’s multiple comparisons test. Data are significant (p < 0.05) when letters above bars are dissimilar.
3.3. CD14 contributes to inflammation in response to asthma and influenza
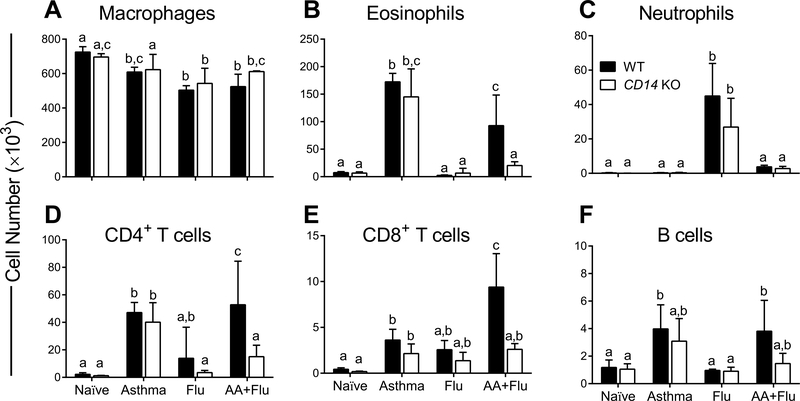
Airways inflammation is a hallmark of allergic asthma while tissue inflammation marks influenza. We used flow cytometry to delineate the leukocyte populations in the BAL compartment. Total macrophage numbers in the airways decreased from baseline in WT and KO mice during the course of all three disease conditions. There was no statistical difference in macrophage numbers between the two genotypes in any group (Fig. 3A). Similarly, all cell types investigated (except neutrophils, which were reduced in the KO mice with influenza) were equivalent between WT and KO mice in the Asthma and Flu groups (Fig. 3). Neutrophils were not recruited into the airways in either genotype in the presence of allergen stimulation (Fig. 3). Eosinophils and T lymphocytes were reduced in KO mice with co-morbidity (Fig. 3). B cells were increased in the airways of WT mice in response to allergen, but not in KO mice (Fig. 3). Together these data imply that CD14 may affect the dynamics of airway inflammation in asthma and influenza co-morbidity.
Fig. 3. Specific leukocytes were reduced in the airways of CD14 null mice during co-morbidity.
While disease states led to a reduction in the number of macrophages in wild-type (WT) and knowckout (KO) mice in general, flow cytometric analyses revealed no differences between WT and KO mice (A). Eosinophils were prominent after allergen exposure but were significantly reduced in the KO mice with asthma and influenza (B), while neutrophils were dominant in both genotypes after virus infection in the absence of asthma (C). Lymphocytes (D, E, and F) followed similar trends wherein KO mice had less infiltration of these cells in co-morbidity. Data are represented as the mean and SD of n = 5 mice per group per genotype and analyzed with two-way ANOVA with Tukey’s multiple comparisons test. Data are significant (p < 0.05) when letters above bars are dissimilar.
3.4. Absence of CD14 on macrophages does not alter goblet cell metaplasia
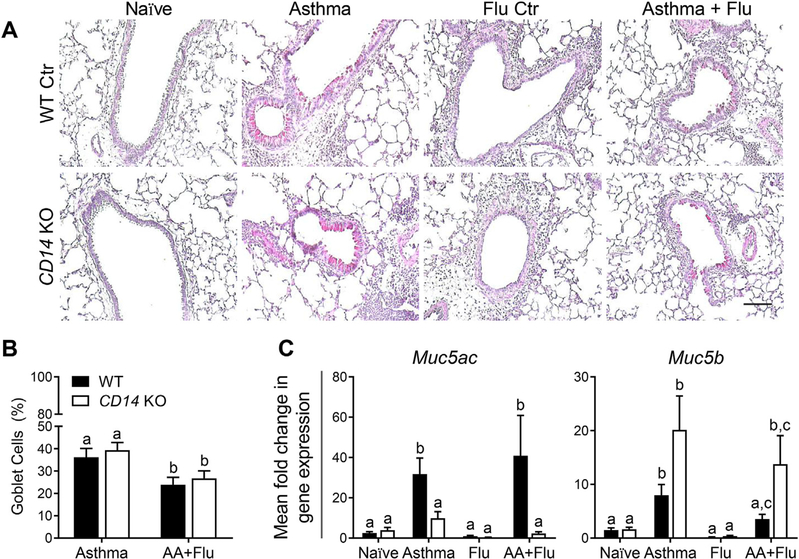
The upregulation of mucus producing GCs in the airway lining is a hallmark of asthma. Since the role of macrophage CD14 has not been investigated as a regulator of GC metaplasia, we enumerated GCs and relevant Muc genes in our model. The deletion of CD14 on macrophages did not result in a difference in airway GCs (visualized by PAS stain, Fig. 4A) in any treatment group compared to WT counterparts (Fig. 4B). Similar to our previous findings in pH1N1-infected WT mice [16], we did not observe GCs in the airways of pH1N1-infected KO mice (Fig. 4). Mucus composition in the airways is tightly regulated in airways diseases [13]. Muc5AC is considered to be a marker of GC metaplasia and has been implicated in the induction of AHR [20]. We measured Muc5ac and Muc5b levels in the lungs to determine if macrophage CD14 signaling plays a role in regulating mucin composition. Virus infection alone did not induce expression of either gene in WT or KO mice, while both genes were upregulated in WT mice exposed to allergen (Fig. 4C). Co-morbid WT mice had increased expression of Muc5ac but not Muc5b, which followed the opposite trend in the KO mice with asthma and influenza (Fig. 4C).
Fig. 4. The absence of CD14 did not affect goblet cells but impacted mucin genes.
Periodic acid Schiff’s stained lung sections (A) were used to enumerate goblet cells in the airway lining (B). Numbers of GCs were similar between wild-type (WT) and knockout (KO) mice with co-morbidity inducing a reduction compared to asthma. Mucin genes, Muc5ac and Muc5b were differentially regulated in the absence of CD14 (C). Data are represented as the mean and SD of n = 5 mice per group per genotype and were analyzed by two-way ANOVA with Tukey’s multiple comparisons test. Data are significant (p < 0.05) when letters above bars are dissimilar. Scale bar = 100 μm and applies to all images.
3.5. CD14 on macrophages may play a role in regulating humoral immune responses
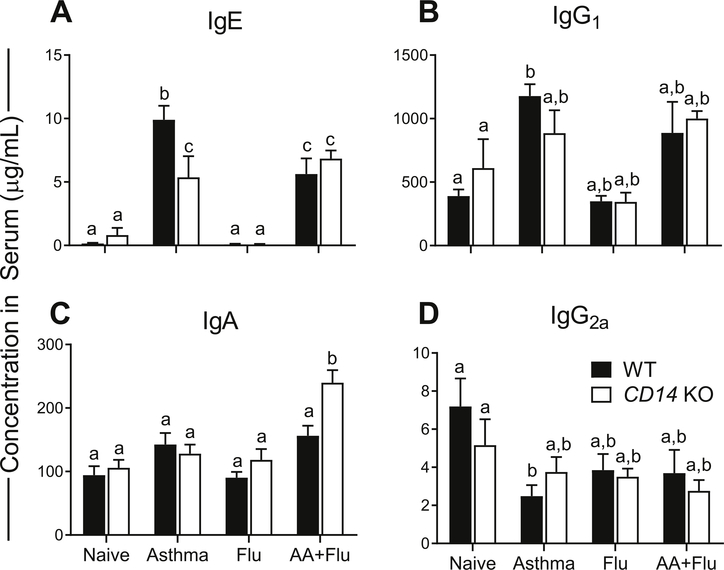
Antibody responses are important for antigen neutralization as well as activation of innate immune cells [14]. As expected, IgE levels were low in naïve and pH1N1 infected mice, but, elevated in allergen-exposed mice of both genotypes (Fig. 5A). Interestingly, allergic KO mice had significantly lower levels of IgE compared to WT controls (Fig. 5A). IgG1 plays a role in hypersensitivity and prevents anaphylaxis by limiting antigens from binding to IgE [21]. While IgG1 levels were increased in allergic mice, the increased levels only reached statistical significance in WT asthma control mice (Fig. 5B). IgA is a component of secreted innate immune barrier defenses and important for antigen neutralization at the mucosal surface [22]. Co-morbidity led to a significant increase in serum IgA levels in KO mice (Fig. 5C). IgG2a is capable of binding to extracellular pathogens and may combat virions during their relatively short extracellular phase [23]. This antibody isotype was significantly decreased in the WT asthma group but remained comparable to baseline levels in all other treatment groups in both WT and KO mice (Fig. 5D).
Fig. 5. CD14 has a minimal impact on humoral responses.
Serum antibody types were quantified by ELISA and found to be generally similar between wild-type (WT) and knockout (KO) mice. Immunoglobulin (Ig) E, was significantly reduced in KO allergic mice (A). IgG1 was not altered in KO mice during disease, but allergic WT mice had a significant increase (B). While the level of serum IgA remained at baseline in asthma and influenza, a significant increase occurred in KO mice during co-morbidity (C). Serum IgG2a was downregulated in WT allergic mice but remained at basal levels in other treatment groups in both genotypes (D). Data are represented as the mean and SD of n = 5 mice per group per genotype and analyzed by two-way ANOVA with Tukey’s multiple comparisons test. Data are significant (p < 0.05) when letters above bars are dissimilar.
3.6. CD14 on macrophages functions as a regulator of innate immune factors in the lungs during disease
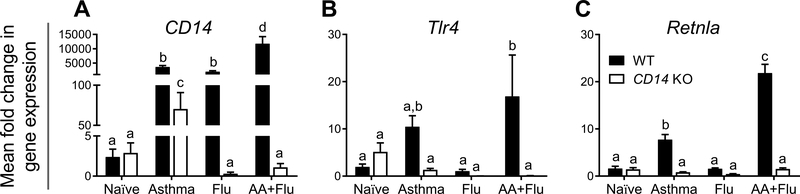
CD14 signaling on macrophages was deemed to play a role in the pathogenesis of asthma and influenza in our model. Since CD14 can be expressed on other myeloid cells and secreted, we measured CD14 expression in the lungs of mice in each treatment group and determined that this gene was markedly upregulated during disease in the WT animals (Fig. 6A). While CD14 expression was elevated in the KO mice in the asthma group, influenza resulted in a significant reduction in the KO mice compared to the mice of the WT control group (Fig. 6A). Interestingly, gene expression for TLR4 which acts as a PRR for A. fumigatus cell wall components [24], was significantly lower in the KO allergic asthma mice infected with pH1N1 compared to the WT mice (Fig. 6B). Resistin-like molecule-α is a marker of M2-polarized macrophages and has previously been shown to be important in allergen and parasite induced TH2 responses [25]. Our assessment of Retnla gene expression in the lungs showed that it was upregulated in WT mice exposed to allergen, but not in the KO mice (Fig. 6C).
Fig. 6. Mediators of innate immunity were downregulated in the absence of CD14.
The expression of CD14 in the lungs was significantly elevated in wild-type (WT) mice after each disease state, but not in the knockout (KO) mice (A). Tlr4 was significantly increased in the co-morbid WT mice but this gene was reduced in the KO (B). A marker of TH2 immunity, Retnla was increased in asthma and co-morbid conditions in the WT mice but not in the KO (C). Data are represented as the mean and SEM of n = 5 mice per group per genotype and analyzed by two-way ANOVA with Tukey’s multiple comparisons test. Data are significant (p < 0.05) when letters above bars are dissimilar.
4. Discussion
Allergic asthma is a multifaceted chronic inflammatory disorder that affects approximately 300 million people and estimated to result in approximately 250,000 deaths worldwide per annum [26]. While asthma can be either triggered by environmental factors or genetic predisposition [27], its etiology remains unclear. Asthma exacerbations can occur upon exposure to a respiratory virus and asthmatics were amongst the most hospitalized during the 2009 Influenza pandemic [28]. While various leukocytes play a role in asthma and influenza pathogenesis, macrophages are known to play a significant role in both conditions. Since A. fumigatus and pH1N1 activate the TLR4 pathway [29,30], we investigated the contribution of CD14 on macrophages in the pathogenesis of asthma and influenza.
Influenza virus infections result in a decrease in alveolar macrophage populations providing a window of opportunity for secondary bacterial infections [31], and different strains of H1N1 influenza viruses impact alveolar macrophage populations with differing kinetics [32]. Asthma has also been shown to impact macrophage activity by altering these cells into what has been classically called M2 macrophages [33] marked by high expression of Retnla/Fizz1 [34]. Exposure to an allergic model in mice alters peripheral blood monocyte gene activity, possibly by promoting TH2 responses [35]. In spite of these well-characterized functions of macrophages in asthma and influenza, their function during the co-pathogenesis of these two diseases remains poorly investigated and served as the impetus for our work described here.
In allergic asthma, exposure to an allergen results in a TH2 response which is characterized by goblet cell metaplasia with associated mucus hypersecretion. If CD14 signaling does induce a TH1 skew, we hypothesized that the removal of CD14 would result in increased GC metaplasia and mucus secretion. This hypothesis was not supported by our data wherein the number of GCs was unaffected by the deletion of CD14 on macrophages, although the inverse correlation between Muc5ac and Muc5b in KO mice compared to WT controls suggests an unexpected role for this receptor as a regulator of mucin composition in the airways during allergic disease. Previous studies have demonstrated that cross-talk between alveolar macrophages and epithelial cells lining the airways including mucus-producing cells [36]. Our data suggest that while macrophages may not promote GC metaplasia, they may guide the composition of mucin and that this role may be regulated by CD14. The dichotomy between Muc5ac and Muc5b expression in the KO mice suggest that alternative pathways of regulation occur for these two dominant mucins that play a role in GC metaplasia, and that this regulatory network may directly/indirectly rely on CD14 signaling on macrophages. Interestingly, alveolar macrophages in the BAL of patients with chronic obstructive pulmonary disease express MUC5B mRNA and stored apo-MUC5B within vacuoles and granules [37]. Since Muc5b is critical for mucociliary clearance and airway defense against infectious agents [38], Muc5b interaction with virus may serve a dual role in host defense. Human macrophages induce MUC5B through GC hyperplasia of bronchial epithelial cells [36], and if similar mechanisms exist in mice, our data suggest that CD14 expression on macrophages is inhibitory to this pathway (Fig. 7). It would be of interest to differentially stain for Muc5AC and Muc5B in KO mice in comparison to WT controls in asthma and co-morbidity to identify the cells that produce these proteins for further analysis into the mechanisms by which mucins are regulated by CD14 signaling.
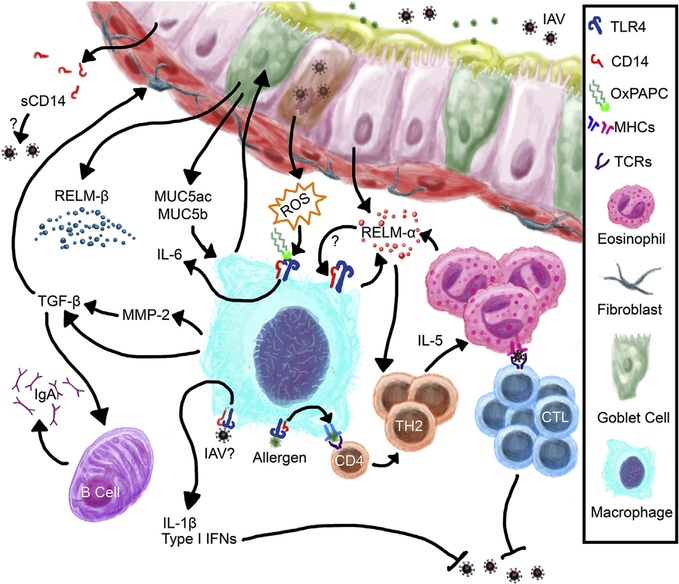
Fig. 7. Schematic representation of the possible functions of macrophage CD14 in the co-pathogenesis of asthma and influenza.
CD14 on macrophages may play a central role in mediating inflammation during asthma and influenza through direct interaction with viral and allergen antigens via CD14/TLR4 pathways or through indirect activation through mediators in the microenvironment that become available during viral pathogenesis and allergen sensitization.
Severe asthma with fungal sensitization, as mimicked by our animal model system, has a mixed cytokine response although TH2 responses dominate. Influenza, on the other hand, is a disease with a TH1 bias, thereby making these two conditions immunologically distinct. CD14 signaling has been considered to polarize macrophages toward M1 cells that support TH1 immunity [39]. While we did not characterize macrophage markers to determine the dominant phenotypes, total macrophage populations did not change during the co-pathogenesis of asthma and influenza, nor did the markers of allergy decrease. Our findings that KO mice had similar expression profiles of cytokines in the airways (data not shown) also suggest that CD14 does not play a dominant role in regulating cytokine production during influenza contrary to a previous report [40], although differences in viral strain and dose may have accounted for this. While the canonical allergy prone antibody, IgE, was not affected by the loss of CD14 on macrophages, IgA was significantly elevated in the KO allergic mice infected with pH1N1 indicating a regulatory role in B cell activation. The reason for increased IgA in the KO AA + Flu group is unclear; it may be possible that CD14 signaling on macrophages decreases their production of mediators that promote B cell class switching to produce IgA, a regulatory mechanism that was lost in the KO mice. Further studies are necessary to identify whether IgA production by B cells is directly regulated by CD14 signaling on macrophages or if this regulatory pathway is indirect. Since the engagement of IgG and IgA antibodies to their respective receptors on macrophages [41], as well as TLR4 signaling [42], promote phagocytic capacity of these cells, it is interesting that the CD14 KO mice (which also showed a marked reduction in Tlr4) did not show an increase in the viral burden.
The absence of CD14 expression on macrophages resulted in a marked reduction in specific leukocyte populations in the AA + Flu mice suggesting a crucial role for this receptor as a regulator of airway inflammation during co-pathogenesis. A deficiency in macrophage phagocytosis through CD14/TLR pathway [42] may have caused a reduction in antigen presentation by these cells thereby leading to a reduction in T cell recruitment and sustenance in the airways. Furthermore, if CD14 binds to influenza A virus similar to its known functions as a receptor for respiratory syncytial virus [43,44], the absence of this receptor may reduce macrophage processing of viral antigens for presentation to CD8+ T cells (Fig. 7). Epithelial cell injury and the generation of reactive oxygen species (ROS) during influenza pathogenesis are important mediators of inflammation and leukocyte activation which serve as a positive feedback loop to augment ROS production in the lungs [45]. Oxidized 1-palmitoyl-2-arachidonoyl-phosphatidylcholine (oxPAPC) generated during lung injury induces pro-inflammatory cascades by binding to TLR4 [46,47]. While the role played by CD14 in this pathway has not been investigated, oxPAPCs have been shown to compete with CD14 to bind LPS, thereby reducing the impact of TLR4 signaling [48]. Based on our data, we propose that macrophages in CD14 null mice have reduced inflammatory potential via the ROS-mediated pathway thereby suggesting a role for this receptor as a mediator of host defense during asthma and influenza co-pathogenesis (Fig. 7).
Macrophages are pleiotropic cells that play a crucial role in tissue repair and homeostasis. As such, they produce a number of mediators that alter the matrix and promote wound healing. Removal of recruited inflammatory cells is a vital component of regaining tissue homeostasis. In this capacity, macrophage-derived matrix metalloproteinase (MMP)-2[49] may promote leukocyte egress into the airway lumen [50] and alter the extracellular matrix [51]. Eosinophil and T cell egression into the airway lumen is regulated by MMP-2 [52], and it may be possible that the reduction in these two cell types in the BAL of co-morbid KO mice were due to a reduction in CD14-mediated MMP-2 by macrophages, a mechanism that warrants further investigation. Furthermore, MMP-2-mediated activation of transforming growth factor (TGF)-β [53] may play a role in protecting the allergic airways from viral-induced tissue damage as well promoting IgA production by local B cells [54] (Fig. 7) as noted previously in our asthma and influenza model [55].
The cytokine resistin-like molecule (RELM)-α, is found in high levels in the allergic environment of the lungs during asthma [56] and a marker of M2-polarized macrophages [34]. While elucidation of functions of RELM-α in asthma and influenza are still ongoing in our laboratory, we recently identified that related molecule, RELM-β, plays an anti-fibrogenic role in fungal asthma [57]. Since CD14 null mice had a marked reduction in Retnla in the lungs, it is possible that macrophage CD14 is necessary for the induction of Retnla in disease. Even though functions of RELM-α have been elucidated in various disease states and organs [58,59], a specific receptor to which it binds to has not been identified to date. The marked reduction in Retnla in the CD14 null mice and its close resemblance to the pattern of Tlr4 expression suggests a link between these molecules leading to the tantalizing possibility that CD14/TLR4 may be a receptor for RELM-α in the lungs through a positive feedback loop (Fig. 7). Similarly, if the reduction in Tlr4 gene expression in these KO mice translates to a reduction in TLR4 receptor, all differences noted in these mice may have been a result of a TLR4 signaling deficiency.
5. Conclusion
Influenza viruses pose a significant threat to public health and patients with chronic lung diseases are at increased risk of infection. Our novel model system of asthma and influenza [16] recapitulates a subset of asthmatics that were resistant to severe influenza morbidity during the 2009 Swine Flu pandemic. While there may be many reasons for this unanticipated relationship [17], we identified a role for eosinophils as putative antigen presenting cells that led to enhanced cellular immune responses in the allergic hosts [15]. In this study, we explored the possibility that CD14 expression on macrophages was necessary for enhanced immune protection against pH1N1 infection in allergic hosts. Our data suggest that macrophage CD14 may perform a pro-inflammatory function in the airways during influenza in hosts with allergic asthma. While we only investigated a single timepoint at the intersection of innate and adaptive immune responses, it would be necessary to investigate the entire spectrum of viral disease and explore subpopulations of macrophages to determine the exact role played by this receptor in the co-pathogenesis of asthma and influenza.
Acknowledgements
We thank Brandi Livingston at the Animal Resource Center at St. Jude Children’s Research Hospital for animal care. AES would like to thank Dr. Jonathan McCullers at St. Jude Children’s Research Hospital for funding this project at inception. This project was funded through the American Lebanese Syrian Associated Charities (ALSAC) at the St. Jude Children’s Research Hospital (to J. A. McCullers, MD) and through the Department of Pediatrics at the University of Tennessee Health Science Center (to Samarasinghe).
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.micpath.2018.12.008.
References
- [1].Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma, Lancet 391 (2018) 783–800. [DOI] [PubMed] [Google Scholar]
- [2].Shao H, Wang C, Zhu W, Huang X, Guo Z, Zhang H, et al. , Different regulation of Toll-like receptor 4 expression on blood CD14(+) monocytes by simvastatin in patients with sepsis and severe sepsis, Int. J. Clin. Exp. Med. 8 (2015) 13830–13835. [PMC free article] [PubMed] [Google Scholar]
- [3].Martinez FD, CD14, endotoxin, and asthma risk: actions and interactions, Proc. Am. Thorac. Soc. 4 (2007) 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Keane-Myers A, Wysocka M, Trinchieri G, Wills-Karp M, Resistance to antigen-induced airway hyperresponsiveness requires endogenous production of IL-12, J. Immunol. 161 (1998) 919–926. [PubMed] [Google Scholar]
- [5].Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD, A Polymorphism* in the 5’ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E, Am. J. Respir. Cell Mol. Biol. 20 (1999) 976–983. [DOI] [PubMed] [Google Scholar]
- [6].Hubacek JA, Rothe G, Piťha J, Skodova Z, Stanek V, Poledne R, et al. , C(-260) – > T polymorphism in the promoter of the CD14 monocyte receptor gene as a risk factor for myocardial infarction, Circulation 99 (1999) 3218–3220. [DOI] [PubMed] [Google Scholar]
- [7].Koppelman GH, Reijmerink NE, Colin Stine O, Howard TD, Whittaker PA, Meyers DA, et al. , Association of a promoter polymorphism of the CD14 gene and atopy, Am. J. Respir. Crit. Care Med. 163 (2001) 965–969. [DOI] [PubMed] [Google Scholar]
- [8].Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. , Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene, Science 282 (1998) 2085–2088. [DOI] [PubMed] [Google Scholar]
- [9].Mauro Baldini A polymorphism* in the 5’ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E, Am. J. Respir. Cell Mol. Biol. 20 (1999) 976–983. [DOI] [PubMed] [Google Scholar]
- [10].Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, et al. , TLR4 mutations are associated with endotoxin hyporesponsiveness in humans, Nat. Genet. 25 (2000) 187–191. [DOI] [PubMed] [Google Scholar]
- [11].Dabbagh K, Dahl ME, Stepick-Biek P, Lewis DB, Toll-like receptor 4 is required for optimal development of Th2 immune responses: role of dendritic cells, J. Immunol. 168 (2002) 4524–4530. [DOI] [PubMed] [Google Scholar]
- [12].Tomlinson KL, Davies GC, Sutton DJ, Palframan RT, Neutralisation of inter-leukin-13 in mice prevents airway pathology caused by chronic exposure to house dust mite, PLoS One (2010) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM, Airway mucus: from production to secretion, Am. J. Respir. Cell Mol. Biol. 34 (2006) 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, et al. , Programming the magnitude and persistence of antibody responses with innate immunity, Nature 470 (2011) 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Samarasinghe AE, Melo RC, Duan S, LeMessurier KS, Liedmann S, Surman SL, et al. , Eosinophils promote antiviral immunity in mice infected with influenza a virus, J. Immunol. 198 (2017) 3214–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Samarasinghe AE, Woolard SN, Boyd KL, Hoselton SA, Schuh JM, McCullers JA, The immune profile associated with acute allergic asthma accelerates clearance of influenza virus, Immunol. Cell Biol. 92 (2014) 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Veerapandian R, Snyder JD, Samarasinghe AE, Influenza in asthmatics: for better or for worse? Front. Immunol. 9 (2018) 1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hoselton SA, Samarasinghe AE, Seydel JM, Schuh JM, An inhalation model of airway allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice, Med. Mycol. 48 (2010) 1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Samarasinghe AE, Hoselton SA, Schuh JM, The absence of the VPAC(2) receptor does not protect mice from Aspergillus induced allergic asthma, Peptides 31 (2010) 1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN, et al. , The polymeric mucin Muc5ac is required for allergic airway hyperreactivity, Nat. Commun. 6 (2015) 6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Oshiba A, Hamelmann E, Takeda K, Bradley KL, Loader JE, Larsen GL, et al. , Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig) E and IgG1 in mice, J. Clin. Invest. 97 (1996) 1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].WJ M, KM A, IgA function – variations on a theme, Immunology 113 (2004) 175–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Coutelier JP, van der Logt JT, Heessen FW, Warnier G, Van Snick J, IgG2a restriction of murine antibodies elicited by viral infections, J. Exp. Med. 165 (1987) 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chai LY, Kullberg BJ, Vonk AG, Warris A, Cambi A, Latge JP, et al. , Modulation of Toll-like receptor 2 (TLR2) and TLR4 responses by Aspergillus fumigatus, Infect. Immun. 77 (2009) 2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Munitz A, Cole ET, Karo-Atar D, Finkelman FD, Rothenberg ME, Resistin-like molecule-alpha regulates IL-13-induced chemokine production but not allergen-induced airway responses, Am. J. Respir. Cell Mol. Biol. 46 (2012) 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].D’Amato G, Vitale C, Molino A, Stanziola A, Sanduzzi A, Vatrella A, et al. , Asthma-related deaths, Multidiscip. Respir. Med. 11 (2016) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald JM, et al. , Global strategy for asthma management and prevention: GINA executive summary, Eur. Respir. J. 31 (2008) 143–178. [DOI] [PubMed] [Google Scholar]
- [28].Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. , Hospitalized patients with 2009 H1N1 influenza in the United States, N. Engl. J. Med. 2009 (361) (April-June 2009) 1935–1944. [DOI] [PubMed] [Google Scholar]
- [29].Mambula SS, Sau K, Henneke P, Golenbock DT, Levitz SM, Toll-like receptor (TLR) signaling in response to Aspergillus fumigatus, J. Biol. Chem. 277 (2002) 39320–39326. [DOI] [PubMed] [Google Scholar]
- [30].Nhu QM, Shirey K, Teijaro JR, Farber DL, Netzel-Arnett S, Antalis TM, et al. , Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo, Mucosal Immunol. 3 (2010) 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ghoneim HE, Thomas PG, McCullers JA, Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections, J. Immunol. 191 (2013) 1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].LeMessurier KS, Lin Y, McCullers JA, Samarasinghe AE, Antimicrobial peptides alter early immune response to influenza A virus infection in C57BL/6 mice, Antivir. Res. 133 (2016) 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gordon S, Alternative activation of macrophages, Nat. Rev. Immunol. 3 (2003) 23. [DOI] [PubMed] [Google Scholar]
- [34].Murray PJ, Macrophage polarization, Annu. Rev. Physiol. 79 (2017) 541–566. [DOI] [PubMed] [Google Scholar]
- [35].DG P, HS A, SA R, SA E, V-DE E, DS T, et al. , Gene expression profiling and network analysis of peripheral blood monocytes in a chronic model of allergic asthma, Microbiol. Immunol. 54 (2010) 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Silva MA, Bercik P, Macrophages are related to goblet cell hyperplasia and induce MUC5B but not MUC5AC in human bronchus epithelial cells, Lab. Invest.; J. Tech. Methods Pathol. 92 (2012) 937–948. [DOI] [PubMed] [Google Scholar]
- [37].Sepper R, Prikk K, Metsis M, Sergejeva S, Pugatsjova N, Bragina O, et al. , Mucin5B expression by lung alveolar macrophages is increased in long-term smokers, J. Leukoc. Biol. 92 (2012) 319–324. [DOI] [PubMed] [Google Scholar]
- [38].Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. , Muc5b is required for airway defence, Nature 505 (2014) 412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tundup S, Srivastava L, Nagy T, Harn D, CD14 influences host immune responses and alternative activation of macrophages during Schistosoma mansoni infection, Infect. Immun. 82 (2014) 3240–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pauligk C, Nain M, Reiling N, Gemsa D, Kaufmann A, CD14 is required for influenza A virus-induced cytokine and chemokine production, Immunobiology 209 (2004) 3–10. [DOI] [PubMed] [Google Scholar]
- [41].Janssen WJ, Stefanski AL, Bochner BS, Evans CM, Control of lung defence by mucins and macrophages: ancient defence mechanisms with modern functions, Eur. Respir. J. 48 (2016) 1201–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Blander JM, Medzhitov R, Regulation of phagosome maturation by signals from toll-like receptors, Science 304 (2004) 1014–1018. [DOI] [PubMed] [Google Scholar]
- [43].Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, et al. , Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus, Nat. Immunol. 1 (2000) 398. [DOI] [PubMed] [Google Scholar]
- [44].Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR, Murine retroviruses activate B cells via interaction with toll-like receptor 4, Proc. Natl. Acad. Sci. 99 (2002) 2281–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Snelgrove RJ, Edwards L, Rae AJ, Hussell T, An absence of reactive oxygen species improves the resolution of lung influenza infection, Eur. J. Immunol. 36 (2006) 1364–1373. [DOI] [PubMed] [Google Scholar]
- [46].Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, et al. , Identification of oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury, Cell 133 (2008) 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shirey KA, Lai W, Scott AJ, Lipsky M, Mistry P, Pletneva LM, et al. , The TLR4 antagonist Eritoran protects mice from lethal influenza infection, Nature 497 (2013) 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Erridge C, Kennedy S, Spickett CM, Webb DJ, Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition, J. Biol. Chem. 283 (2008) 24748–24759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, et al. , Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture, Circulation 92 (1995) 1565–1569. [PubMed] [Google Scholar]
- [50].Corry DB, Rishi K, Kanellis J, Kiss A, Song Lz LZ, Xu J, et al. , Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency, Nat. Immunol. 3 (2002) 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ulrich G, Franz-Xaver G-R, Thomas C, Gillian M, DA JP, Wolfram B, The C-terminal (haemopexin-like) domain structure of human gelatinase A (MMP2): structural implications for its function, FEBS (Fed. Eur. Biochem. Soc.) Lett. 378 (1996) 126–130. [DOI] [PubMed] [Google Scholar]
- [52].Corry DB, Kiss A, Song LZ, Song L, Xu J, Lee SH, et al. , Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines, Faseb J.: Offic. Publ. Fed. Am. Soc. Exp. Biol. 18 (2004) 995–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gomes LR, Terra LF, Wailemann RA, Labriola L, Sogayar MC, TGF-beta1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells, BMC Canc. 12 (2012) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cazac BB, Roes J, TGF-β receptor controls B cell responsiveness and induction of IgA in Vivo, Immunity 13 (2000) 443–451. [DOI] [PubMed] [Google Scholar]
- [55].Doorley LA, LeMessurier KS, Iverson AR, Palipane M, Samarasinghe AE, Humoral immune responses during asthma and influenza co-morbidity in mice, Immunobiology 222 (2017) 1064–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, et al. , FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family, EMBO J. 19 (2000) 4046–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].LeMessurier KS, Palipane M, Tiwary M, Gavin B, Samarasinghe AE, Chronic features of allergic asthma are enhanced in the absence of resistin-like molecule-beta, Sci. Rep. 8 (2018) 7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Munitz A, Cole ET, Karo-Atar D, Finkelman FD, Rothenberg ME, Resistin-like molecule–α regulates IL-13–induced chemokine production but not allergen-induced airway responses, Am. J. Respir. Cell Mol. Biol. 46 (2012) 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Munitz A, Waddell A, Seidu L, Cole ET, Ahrens R, Hogan SP, et al. , Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis, J. Allergy Clin. Immunol. 122 (2008) 1200–1207 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]