ABSTRACT
We conducted a phase I dose-escalation trial of radiation with ipilimumab in patients with melanoma with ≥2 metastatic lesions. Here, we report the final full clinical analysis. Patients received RT (6 or 8 Gy x 2 or 3 doses) to a single lesion followed by 4 cycles of ipilimumab. The primary endpoint was maximum tolerated dose of RT, and secondary endpoint was response at non-radiated sites. Twenty-two patients with treatment-naïve (n = 11) or treatment-refractory (n = 11) Stage IV melanoma were enrolled. There were 31 treatment-related adverse events (AEs), of which 16 were deemed immune-related. Eleven patients had grade 3 AEs (no grade 4/5). There were no dose-limiting toxicities related to the radiation/ipilimumab combination. Five of 22 patients (22.7%, 95% CI 7.8–45.4%) had partial response as best response and three (13.6%) had stable disease. Median overall survival was 10.7 months (95% CI, 4.9 months to not-estimable) and median progression-free survival 3.6 months (95% CI, 2.9 months to 7.8 months). Seven patients were still alive at the time of last follow-up (median follow-up 89.2 months), most of whom received pembrolizumab after progression. Radiotherapy followed by ipilimumab was well tolerated and yielded a response rate that compares favorably to the objective response rate with ipilimumab alone. Furthermore, 32% of patients are long-term survivors, most of whom received pembrolizumab. Based on these results, the recommended dose that was used in subsequent Phase 2 trials was 8 Gy x 3 doses.
Clinical Trial Registration: NCT01497808 (www.clinicaltrials.gov)
KEYWORDS: CTLA-4, ipilimumab, radiation, abscopal, hypofractionated radiation
Introduction
In 2010, Hodi et al. published a landmark phase 3 trial showing that ipilimumab, an anti-CTLA-4 antibody, improves survival in patients with metastatic melanoma,1 which launched the era of immune checkpoint blockade. In the same year, the University of Pennsylvania started a trial in which patients with metastatic melanoma were treated with the anti-CTLA-4 antibody tremelimumab and CP-870,893, an agonistic CD40 monoclonal antibody.2 One patient in particular was noted to be an exceptional responder. This patient developed rapid clinical deterioration and tumor progression before the end of the first cycle of immunotherapy and was removed from study. Subsequently painful rib metastases developed, for which the patient received a course of palliative radiation one month after completing immunotherapy. Surprisingly, even without any further therapy, a CT scan 4 months after radiation showed resolution of his widespread disease. This patient had a complete response (CR) and remains with no evidence of disease (NED) at 6+ years.2 The surprising response of this patient following radiation after progressing on immunotherapy suggested that perhaps the radiation potentiated the effects of immunotherapy.
There were also pre-clinical data at the time suggesting that the combination of anti-CTLA-4 antibody and radiation could be effective as anti-cancer therapy.3,4 Demaria et al. showed that the combination of local radiotherapy and anti-CTLA-4 antibody reduces the incidence of lung metastases in a model in which 4T1 mouse mammary carcinoma cells were grown as subcutaneous tumors while neither treatment by itself had much effect.3 Using a model in which tumors (either TSA breast carcinoma or MCA38 colon cancer) were grown in the bilateral flanks of mice, these investigators demonstrated that the combination of anti-CTLA-4 therapy and fractionated radiation to a single site leads to tumor control at the non-irradiated (abscopal) site.
Based on the combination of these pre-clinical data and our anecdotal patient, in 2011 we initiated a phase I trial in which patients with metastatic melanoma received 2 or 3 days of hypofractionated radiation therapy (HFRT) in combination with an anti-CTLA-4 antibody. In this study, a single metastatic lesion was irradiated in patients with at least two metastatic lesions, with the hope that there would be a measurable shrinkage of tumors outside of the radiation field (i.e. abscopal effect). Our primary objective was to assess whether the combination would be safe, namely to determine dose-limiting toxicities (DLT) and the maximally tolerated dose of radiation in combination with ipilimumab. Our secondary objective was to assess radiologic response of abscopal tumors.
The preliminary results of this trial have been published, 5 and in this paper we report the final results of this trial.
Materials and methods
Study design
The primary objective was to determine the maximum tolerated dose (MTD) of hypofractionated radiotherapy to a solitary metastatic focus (‘index lesion’) when followed by ipilimumab, in metastatic melanoma patients. The study and amendments were approved by the University of Pennsylvania institutional review board, and patients provided written informed consent prior to study enrollment. This trial is registered on https://clinicaltrials.gov/(NCT01497808), first posted on 12/23/2011.
When the trial was designed, the optimal dose and fractionation to induce an abscopal response was unknown. Pre-clinical studies suggested that 8 Gy x 3 fractions might be more effective than single fraction or lower dose/fraction regimens, 4 however there were few clinical data at the time to guide us in terms of the safety of this fractionation combined with immunotherapy in humans. Therefore, the trial design allowed for dose escalation up to this target dose (Figure 1). Patients were stratified by site of index lesion: stratum 1: bone or lung and stratum 2: liver or subcutaneous/nodal. The reason for the lower dose for liver or subcutaneous/nodal lesions was because of concerns regarding toxicity at these sites. Dose-limiting toxicity (DLT) was defined as any treatment-related Grade 4 or higher immune-related toxicity or Grade 3 or higher nonimmune-related toxicity. The maximum tolerated radiation fraction (MTD) was defined separately for each stratum. The plan was to treat patients in cohorts of six patients. The study began with Dose Level (DL) 1:2 fractions of radiation (either 6 Gy or 8 Gy depending on stratum). Dose Level (DL) 2 escalated to 3 fractions (6 or 8 Gy depending on the stratum). MTD was defined as the fraction dose level at which 0–1 out of 6 patients experience DLT and at least 2 patients treated at the next higher dose level experience DLT. The plan was to enroll a maximum of 12 patients for each stratum (total 24 patients). Blood was drawn for correlative immunologic analysis, and results were previously reported.5
Figure 1.
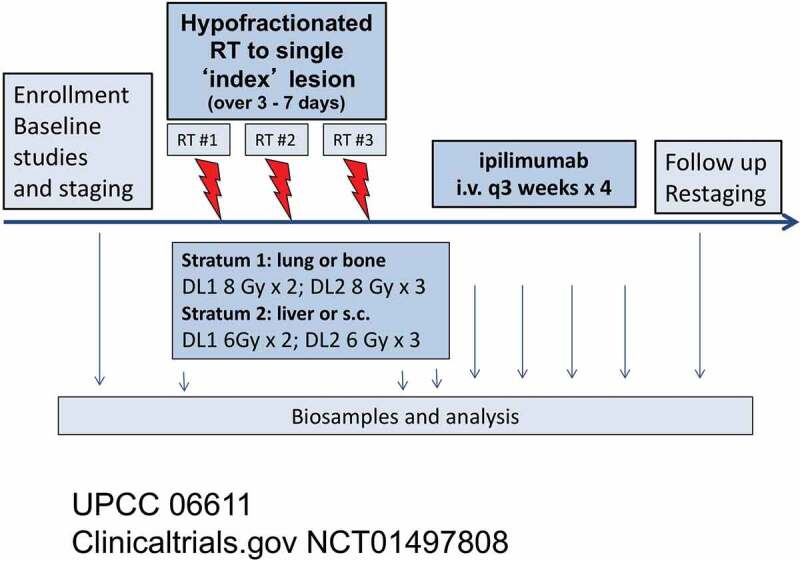
Trial schema
Twenty-two stage IV patients with metastatic melanoma were stratified by treatment site of a single index metastasis, which is the irradiated tumor. Two dosing levels for hypofractionated radiation (HFRT) are in each stratum. After enrollment, 2 or 3 fractions of HFRT were given over 3–7 days (red arrows). Ipilimumab (3 mg/kg) was initiated within 7 days of the final HFRT fraction, every 3 weeks x 4 (blue arrows). s.c – subcutaneous
Patient eligibility
Patients with an Eastern Cooperative Oncology Group performance status of 0 or 1 with adequate renal, hepatic and hematologic function to permit ipilimumab therapy were eligible for enrollment if they were 18 years or older with histologically proven metastatic melanoma. They were required to have an index lesion ≥1 cm that was amenable to radiotherapy, as well as at least one other metastatic lesion that was not irradiated but could be followed for response using Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 criteria. Prior radiotherapy was allowed as long as it did not preclude radiotherapy to the index lesion. Patients were allowed to have had prior systemic therapy (but no anti-CTLA4 or anti-PD-1 therapy) but could not have been treated with 14 days of starting radiation and must have been deemed by the PI to have recovered from side effects from this therapy. Notable exclusions included history of CNS metastases and long-term use of systemic corticosteroids.
Radiation therapy
Many patients were treated with stereotactic body radiation therapy (SBRT) although this was not required if the treating physician was comfortable using a non-SBRT plan. For SBRT, radiation details such as number of beams and planning target volume (PTV) coverage were specified in the protocol. Patients received 2 or 3 fractions of radiotherapy over a span of 3–7 days. The choice of lesion to irradiate was left to the discretion of the treating oncologist. Generally, considerations included the tumor volume, location relative to critical radiosensitive organs (e.g. bowel), and the desire to minimize dose to a non-target lesion in order to observe an abscopal response.
Study medication
Commercially available ipilimumab at 3 mg/kg intravenously every 3 weeks (± 2 days) was to be initiated within 7 days after the final radiation fraction. A total of 4 doses of ipilimumab was planned.
Response assessment
A secondary study objective was to measure disease response which was done by performing PET CT or CT scanning at baseline, 1–2 months after last ipilimumab treatment, and then every several months after that. Assessment of efficacy was based on the tumor response defined by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.6 Disappearance of all known lesions was scored as complete response (CR). A reduction of at least 30% in the sum of the diameters of target lesions was defined as partial response (PR). Progressive disease (PD) was defined as ≥20% increase in the sum of diameters of target lesions from nadir (including baseline if it was the smallest sum). Responses that did not meet the definitions of PR or PD were scored as stable disease (SD). PD was also defined as unequivocal progression of existing non-target lesions that merited discontinuation of therapy. For evaluation by RECIST, irradiated lesions were not included as part of measurable disease.
Toxicity assessment
An adverse event was categorized as treatment-related if it was felt to be definitely, probably, or possibly related to ipilimumab or radiation which was observed during treatment or within 30 days of the final ipilimumab injection. Dose-limiting toxicity was defined as any treatment related Grade 4 or higher immune-related toxicity or Grade 3 or higher nonimmune-related toxicity which was observed during treatment or within 30 days of the final ipilimumab injection and which was probably or definitely related to treatment. All toxicities were graded by NCI CTCAE Version 4.0.
Statistical analysis
Plans for data analysis included toxicity grading and tabulation by stratum and fractionation cohort. Descriptive statistics were used to summarize patient characteristics. A comprehensive tabulation of tumor and treatment characteristics and clinical outcomes for patients was displayed by stratum and fractionation cohort. Event rates and exact 95% confidence intervals were calculated. Progression-free survival (PFS) was calculated from date of first fraction of radiation to date of progressive disease, death, or censored at last clinical follow-up that documented progression-free status. Overall survival (OS) was calculated from the date of first fraction of radiation to date of death due to any cause or censored at last patient contact. PFS and OS curves were estimated using the Kaplan-Meier method. A waterfall plot of clinical response and Kaplan-Meier curves of PFS and OS were produced in R (https://www.R-project.org).
Results
Patient characteristics
Twenty-two patients were enrolled between January 2012 and August 2014. Table 1 shows the distribution of patient characteristics by stratum and dose. All patients had metastatic melanoma: 2 M1a, 5 M1b, and 15 M1c by AJCC 7th Edition. Eleven patients had no prior systemic therapy before starting the protocol (Supplemental Table 1). Two patients had received vemurafenib, two had temozolomide and carboplatin/paclitaxel, two had a BRAF/MEK inhibitor, one had dacarbazine and vemurafenib, one had a BRAF/MEK inhibitor and vemurafenib, one had temozolomide, one had tremelimumab and CD40 agonist, and one had temozolomide, carboplatin/paclitaxel, and mTOR inhibitor and hydroxychloroquine. Two of the 22 patients had received prior local irradiation (one to the axilla and one to the lower back).
Table 1.
Demographics
| Stratum |
1 |
2 |
||
|---|---|---|---|---|
| Dose: | Dose Level 1 8 Gy x 2 (n = 6) |
Dose Level 2 8 Gy x 3 (n = 4) |
Dose Level 1 6 Gy x 2 (n = 6) |
Dose Level 2 6 Gy x 3 (n = 6) |
| Site of index (irradiated) lesion | Lung | lung | liver/ subcutaneous |
liver/ subcutaneous |
| Prior Therapy* | ||||
| Treatment-naïve | 2 (33%) | 3 (75%) | 3 (50%) | 3 (50%) |
| Treatment-refractory | 4 (67%) | 1 (25%) | 3 (50%) | 3 (50%) |
| Stage | ||||
| M1a | 0 | 0 | 0 | 2 (33%) |
| M1b | 2 (33%) | 2 (50%) | 0 | 2 (33%) |
| M1c | 4 (67%) | 2 (50%) | 6 (100%) | 2 (33%) |
| LDH | ||||
| Elevated | 4 (67%) | 1 (25%) | 3 (50%) | 2 (50%) |
| Not elevated | 2 (33%) | 3 (75%) | 3 (50%) | 2 (50%) |
| Gender | ||||
| Male | 5 (83%) | 2 (50) | 5 (83) | 5 (83) |
| Female | 1 (17%) | 2 (50) | 1 (17%) | 1 (17%) |
| Patient age in years, n (%) | ||||
| 18–44 | 0 | 0 | 0 | 0 |
| 45–64 | 3 (50) | 3 (75) | 2 (33) | 0 |
| ≥65 | 3 (50) | 1 (25%) | 4 (67) | 6 (100) |
| Patients with ECOG PS, n (%) | ||||
| 0 | 4 (67) | 3 (75) | 3 (50) | 2 (33) |
| 1 | 2 (33) | 1 (25%) | 3 (50) | 4 (67) |
*Prior Therapies Detailed in Supplemental Table 1.
Treatment
For stratum 1, there were six patients treated at DL1 and 4 at DL2 (Table 1). For stratum 2, there were six patients treated at DL1 and 6 at DL2. In 2014 the anti-PD1 antibody pembrolizumab was approved by the FDA for the treatment of metastatic melanoma, and this became the preferred immune checkpoint agent; hence, we closed this trial, after having enrolled 22 out of a planned 24 patients. Accrual was completed in 3 out of the 4 dose levels. Overall treatment was very well-tolerated. Out of 22 patients (#7, 10, 11, 12, 13, 19, 2; Supplemental Table 1), 7 failed to complete 4 cycles of ipilimumab: 5 were taken off study because of disease progression and 2 due to toxicity. In addition to these seven patients, one patient was taken off study due to their preference (#2) and another patient (#6) due to disease progression, both of whom had completed four cycles of ipilimumab. All patients completed their planned radiation course. Time from last radiation dose to first ipilimumab dose ranged from 3 to 8 days.
Toxicity analysis
Supplemental Table 2 shows a comprehensive list of adverse toxicities by dose level and Table 2 shows a summary of these toxicities. There were no grade 4 or 5 toxicities. There were 31 adverse events (AEs) deemed to be treatment-related and 104 non-treatment-related events. There were 15 grade 3 toxicities: 3 treatment-related (1 case each of fatigue, colitis, and infusion-related anaphylaxis with hypotension and edema) and 12 non-treatment-related, generally related to disease progression (4 cases of anemia and one case each of weight loss, fatigue, cholecystitis, dyspnea, pneumothorax, wound infection and gastric hemorrhage due to stomach metastases and leukocytosis). Of the three treatment-related grade 3 toxicities, one was in Stratum 2, DL1 and two were in Stratum 1, DL1. Overall, 11 out of 22 patients (50%) developed a grade 3 AE, and in three of the 22 (14%) the grade 3 toxicity was felt to be treatment-related. Importantly, there were no DLTs felt to be related specifically to the combination of radiation and ipilimumab.
Table 2.
Summary of Adverse Events
| Any grade | Grades 1–2 | Grade 3 | |
|---|---|---|---|
| Any adverse event | 135 | 120 | 15 |
| Any treatment-related adverse event | 31 | 28 | 3 |
| Specific treatment-related adverse event (immune) | |||
| Anaphylaxis (with hypotension and angioedema) | 1 | 0 | 1 |
| Arthralgia (flare of rheumatoid arthritis) | 1 | 1 | 0 |
| Colitis* | 2 | 1 | 1 |
| Pruritus | 7 | 7 | 0 |
| Rash | 4 | 4 | 0 |
| Uveitis | 1 | 1 | 0 |
| Specific treatment-related adverse event (nonimmune) | |||
| Abdominal Pain | 1 | 1 | 0 |
| Anorexia | 2 | 2 | 0 |
| Chills | 1 | 1 | 0 |
| Dermatitis (radiation) | 1 | 1 | 0 |
| Diaphoresis | 1 | 1 | 0 |
| Diarrhea* | 1 | 1 | 0 |
| Fatigue | 3 | 2 | 1 |
| Headache | 1 | 1 | 0 |
| Nausea | 4 | 4 | 0 |
| Non-treatment related adverse events | 104 | 92 | 12 |
There were no grade 4 toxicities.
* Diarrhea was defined as a disorder characterized by frequent and watery bowel movements; colitis was defined as a disorder characterized by inflammation of the colon.
Of the treatment-related adverse events, 16 were classified as immune-related: 7 cases of pruritus, 4 cases of rash, two cases of colitis, one case of uveitis, and one case of arthralgia in a patient with rheumatoid arthritis whose disease flared on ipilimumab (Table 2). Overall, 11 patients out of 22 (50%) had an immune AE, but the vast majority were grade 1 or 2, with only two patients having a grade 3 immune AE (9.1%). There were no cases of pneumonitis or hepatitis, in spite of the fact that radiation was given to the lung in 10 patients and to the liver in 3. The patient with Grade 3 immune-related colitis was radiated to the right lateral liver and received no dose to the bowel. There was Grade 3 fatigue in one patient, who had rapid disease progression after treatment, and was shortly thereafter diagnosed with innumerable brain metastases. Given the small number of events, it is difficult to meaningfully assess the influence of various risk factors on treatment-related AEs.
Tumor response
To evaluate secondary efficacy endpoints, response was assessed using RECIST criteria.6 In examining out-of-field response using an aggregate diameter of nonirradiated RECIST target metastases, the best response was partial response (PR) in five patients (22.7%, 95% CI 7.8–45.4%), stable disease (SD) in three (13.6%) and progressive disease (PD) in 14 patients (63.4%), as shown in the waterfall plot in Figure 2. Twelve patients had radiographic progression, while two patients had clinical progression without imaging (denoted ** in Figure 2). RECIST criteria are not reliable in assessing response in irradiated lesions, especially in the lung, because post-radiation changes often occur and make adequate assessment of tumor response challenging. However, we did assess the metabolic response of the index (irradiated) lesion in the 12 patients who had follow-up PET scanning. None had progressive disease by PET scanning: 4 had complete metabolic response (CMR), 5 had partial metabolic response (PMR), and 3 had stable disease.
Figure 2.
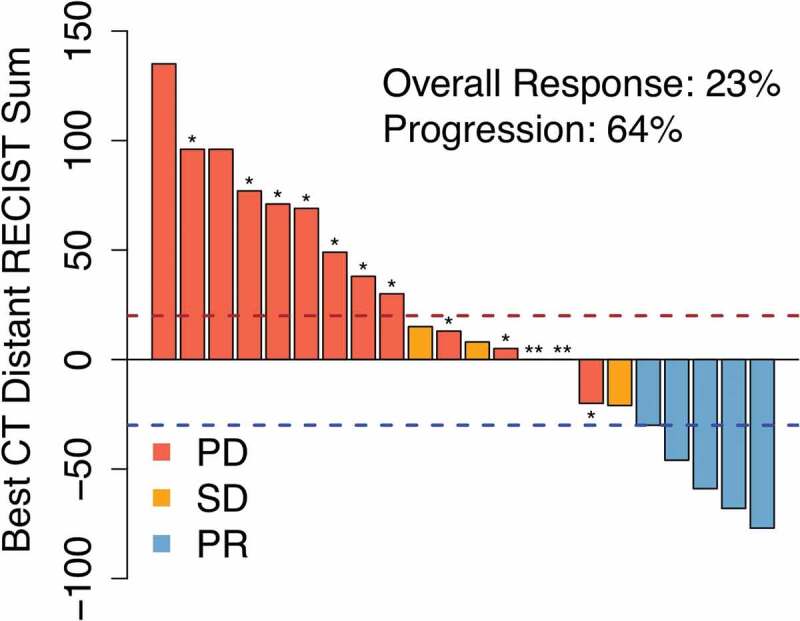
Radiation + ipilimumab is associated with regression of unirradiated tumors in some patients. Waterfall plot of clinical response in unirradiated tumors after radiation treatment (RT) to a single index lesion with ipilimumab. Dashed lines are thresholds for progressive disease (PR; red) and partial response (PR; blue). * Patients with new lesions. ** Clinical progression without imaging
Survival analysis
The length of follow-up ranged from 2.7 to 106.8 months all patients. The median length of follow-up for seven living patients was 89.2 months (range 24.9–106.8) (Supplemental Table 1). The median OS for all patients was 10.7 months (95% CI, 4.9 months to upper bound not estimable) and the median PFS was 3.6 months (95% CI, 2.9 months to 7.8 months) as shown in Figure 3. Almost all patients (21 out of 22) eventually had progression of disease. Twelve patients (57%) received pembrolizumab at relapse. Six of the 12 patients (50%) who received salvage pembrolizumab had favorable responses with long-term survival. Three of these patients also received some form of local therapy at relapse, including SBRT to a new lung lesion and resection of a brain metastasis in one patient, resection and gamma knife for brain metastases in a second patient, and mastectomy for progression in the breast in a third patient.
Figure 3.
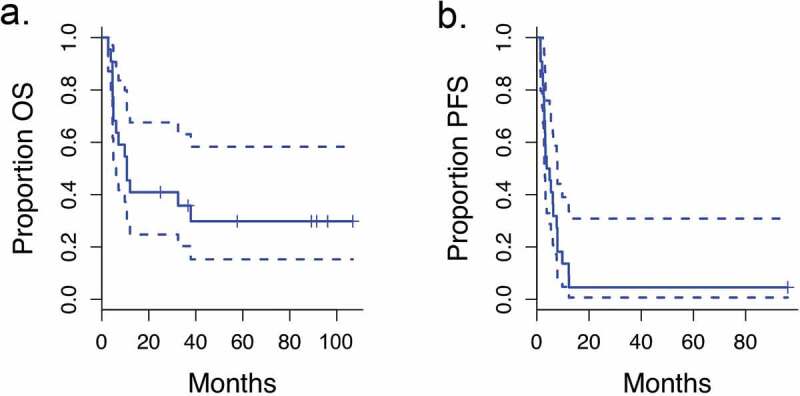
Survival curves
Progression-free survival (PFS) and overall survival (OS) for all patients (dashed line: 95% CI).
Seven patients treated on this trial were still alive at the time of last follow-up (Table 3). Three of the seven had PR as best distant response following radiation + ipilimumab (patient #10, 16, 22 in Table 3). Two of the seven had SD as best response to radiation + ipilimumab (#1, 20). The other two patients who are still alive (#8, 17) had progressive disease as best distant response after radiation + ipilimumab. Of the seven surviving patients, only one required no therapy following initial therapy on the trial. One was placed on a B-Raf inhibitor, and the remaining five were treated with pembrolizumab.
Table 3.
Patients alive at last follow-up
| Study subject # | Radiation site | Stratum | Dose | Best distant response (RECIST) | Time to progression from RT (months) | Therapy at progression | Status at last f/u | Time from RT to last f/u or death (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | Lung | 1 | 8 Gy x 2 | SD | 9.8 | SBRT to new lung lesion; resection of brain met; pembro | Alive; SD on pembro | 97.4 |
| 8 | Lung | 1 | 8 Gy x 2 | PD | 5.4 | pembro | Alive; SD on pembro | 36.7 |
| 10 | Liver | 2 | 6 Gy x 2 | PR | N/A | N/A | NED | 88.7 |
| 16 | Lung | 1 | 8 Gy x 3 | PR | 12.2 | Gamma Knife and resection of brain mets; then pembro | Stable disease on pembro | 82.6 |
| 17 | Axillary nodal mass | 2 | 6 Gy x 3 | PD | 3.3 | pembro; then progressed in breast; mastectomy; placed on B-raf inhibitors | Alive; SD on B-Raf inhibitors | 82.2 |
| 20 | Lung | 1 | 8 Gy x 3 | SD | 7.6 | pembro | SD on pembro | 24.9 |
| 22 | Lung | 1 | 8 Gy x 3 | PR | 12.3 | pembro | Stable disease on pembro | 57.6 |
Discussion
We started this prospective study based on our own anecdotal experience and pre-clinical reports suggesting that radiation could potentiate the effect of anti-CTLA therapy. Shortly after the trial started, a case report was published that appeared to validate the concept.7 This report described a patient with metastatic melanoma who had progressive disease on ipilimumab and was then treated palliatively with radiation therapy to a paraspinal mass (9.5 Gy x 3 fractions). The patient then showed regression of both the paraspinal tumor and a non-irradiated metastatic lesion.7 This anecdotal case report spurred efforts to combine radiotherapy with immune checkpoint blockade to stimulate an abscopal response.8,9 The final results of our initial trial demonstrate that the combination of radiation and ipilimumab was well tolerated, yielded a 23% response rate of unirradiated lesions, and there are several long-term survivors.
Regarding our primary endpoint, we found no DLTs related to the combination of radiation and ipilimumab, even at the highest dose level, 24 Gy (8 Gy x 3 fractions). We saw no obvious increase in toxicity as a result of adding radiation to ipilimumab. Specifically, there were no grade 4 toxicities. Grade 3 toxicity occurred in 50% of patients, but the rate of treatment-related grade 3 toxicity was 14%, which is identical to the treatment-related grade 3–4 toxicity rate seen in another prospective trial testing the combination of ipilimumab and radiation from Hiniker et al. .10 These rates are not higher than those observed in large randomized trials using ipilimumab at the same dose (3 mg/kg). In the ipilimumab-only arm in the trial reported by Hodi et al. the overall rate of grade 3–4 toxicity was 45.8%, the drug-related grade 3–4 toxicity was 22.9%, and the immune-related grade 3–4 adverse toxicity rate was 14.5% .1 In other large randomized trials including KEYNOTE-006, the drug-related grade 3–5 toxicity rate in the ipilimumab arm has ranged from 20% to 24% .11,12 Grade 3 immune-related toxicity occurred in 9.1% of patients on our trial. We found no evidence that immune-related adverse effects increased in an organ when radiotherapy was delivered near that organ. Specifically, we found no cases of pneumonitis or hepatitis in patients who received lung or liver irradiation, respectively. This lack of increased immune-related toxicity when radiation is added to ipilimumab is consistent with what others have found in retrospective series.13–16
Our secondary endpoint in this study was to determine the effect of radiation and ipilimumab on the radiologic response of unirradiated tumors, which we found to be 23%. By way of comparison, the ipilimumab-only arms in multiple large prospective studies have reported an objective response rate of approximately 11%.1,11,12,17 Hence, our observed response rate of 23%, although based on a very small number of patients, could represent an improvement over that expected with ipilimumab alone. Several retrospective studies have suggested this as well.14–16,18 In general, patients reported in these prior studies received conventional radiation to one or more lesions for the purpose of palliation. In one such study, the rates of overall responses (including irradiated lesions) were 37.1% and 19.4% (p = .11) respectively in patients who received ipilimumab/radiation versus ipilimumab alone15 and in another study 37.8% and 17.9%.4 The latter study also examined abscopal effects and found that in 4 out of 19 patients (21%) who received ipilimumab and radiation, there was a response in metastatic sites distant from locally treated sites. Both of these studies also found improved overall survival with the addition of radiation to ipilimumab. However, these retrospective studies are difficult to interpret because there is tremendous variation in the dose and timing of radiation with respect to ipilimumab administration. Furthermore, there may be a bias in which patients are referred for palliative radiation.
The prospective trial from Hiniker et al. found an abscopal radiologic response similar to ours; out of 22 patients treated with ipilimumab and radiation, three had a CR of unirradiated lesions and three had a PR (overall response rate 6/22 = 27.2%).10 However, in the only other prospective trial of ipilimumab and non-CNS radiation for melanoma that has been published of which we are aware, only one patient out of 13 had a response (7.7%) .19 There are some key differences between our study and these two other trials. In our study, radiation was given prior to the first dose of ipilimumab whereas in the other studies it was given after either the first dose10 or the second dose .19 The timing of RT relative to ipilimumab administration might make a difference, as one pre-clinical study showed that anti-CTLA4 therapy was most effective in a mouse model when given prior to radiation therapy, in part due to regulatory T-cell depletion, 20 whereas in our trial, the first dose of ipilimumab was delayed until after radiation was completed. The dose of radiation was in our study was lower than in the other two studies. We used 6 or 8 Gy for 2 or 3 doses whereas Sundahl et al. used 8, 10, or 12 Gy for 3 doses19 and in Hiniker et al. most patients received 30–45 Gy, in 5–15 fractions .10 In fact the optimal timing of radiation with respect to immune checkpoint block or even the radiation dose to elicit the best response remains unclear.21
We had some radiologic responders in our study, but the more important question is whether this treatment regimen impacted long-term outcomes. Unfortunately, most patients progressed after initial treatment with radiation/ipilimumab (Figure 2). However, we do have seven survivors out of 22 patients (31.8%), with a median follow-up of 82.2 months. One patient is alive at 88.7 months without requiring any further therapy. One patient had local therapy after progression and is on a B-raf inhibitor; the remaining six patients were placed on pembrolizumab (± local therapy) after progression. Whether this long-term OS rate of 31.8% is better than what would be expected with upfront ipilimumab alone is unclear. In a systematic retrospective analysis of 1,034 patients with advanced melanoma included in a European Expanded Access Program (EAP) (EURO-VOYAGE) the interim analysis showed that the 4-year OS rate was only 8%.22 However, in a pooled analysis that included patients with metastatic melanoma from 12 studies of ipilimumab (n = 1,861) as well as patients enrolled on an U.S. EAP (n = 2,985) the 3-year OS rate was 21%.23 5-year OS rates were 31% and 26%, respectively, in the ipilimumab arms in the KEYNOTE-00612 and CheckMate-67 trials.24
Since the majority of long-term survivors in our study received pembrolizumab after initial progression, this raises the question of whether SBRT + ipilimumab potentiates the response to salvage PD-1 inhibition relative to upfront ipilimumab alone. On our trial, 6 of 12 patients (50%) who received salvage pembrolizumab had favorable responses with long-term survival, compared to the 6-month progression-free survival of 34% reported on KEYNOTE-00225 and an objective response rate of 32% on the CheckMate 037 study using nivolumab after progression on anti-CTLA-4 therapy.26 The importance of our results is that we provide long-term follow-up for the first prospective trial testing the combination of radiation with ipilimumab, showing that this treatment can be given safely. Our off-target objective response rate of 23% with the combination is very similar to what others have seen in patients who have received radiation and ipilimumab.10,14 It might be higher than the radiologic response rate seen in large studies using ipilimumab alone, 11,12,17 which would give some support to the hypothesis that radiation can potentiate immunotherapy. A number of studies in mouse models have also suggested this.3,4,20,27 This hypothesis is also supported by clinical studies that find that the combination of radiation and ipilimumab in non-small cell lung cancer, which generally does not respond to anti-CTLA-4 therapy, leads to responses in a subset of patients.28,29
While it is disappointing that our response rate was not higher, in our initial report, we showed in mouse models that resistance to radiation and anti-CTLA-4 therapy was due to upregulation of PD-L1 on melanoma cells and was associated with T-cell exhaustion.5 Using samples from patients on the clinical trial reported in this paper, we showed that patients whose tumors exhibited high PD-L1 expression did not respond to radiation plus anti-CTLA-4, demonstrated persistent T-cell exhaustion, and rapidly progressed. Furthermore, in that study we showed that mice bearing melanoma tumors did better when treated with the triple therapy of radiation, anti-CTLA4 therapy and anti-PD-1 therapy. Of note, many of the patients in our trial who did progress responded very well to pembrolizumab, and many are alive years later.
The major limitation of this study is the small number of patients, which makes it difficult to draw firm conclusions about the toxicity of this approach, or the oncologic outcomes. Furthermore, the treatment after progression was heterogeneous.
As ipilimumab monotherapy is no longer first-line treatment for patients with advanced melanoma, but rather anti-PD1 therapy, we have subsequently conducted a trial combining hypofractionated radiation and pembrolizumab, for which results from the safety phase have been published.30 Furthermore, we are currently testing the combination of nivolumab and ipilimumab with and without radiation in a randomized study (NCT03646617).
Supplementary Material
Funding Statement
This work was supported by NIH P01 CA210944 (R.H.V., A.M, A.J.M, R.M) and Melanoma Research Alliance Pilot Award (R.R.).
Disclosure of Potential Conflicts of Interest
A.M. has received research funding from Merck.
A.J.M. has received research funding from Merck. He has also received honoraria and travel support from Merck, AstraZeneca, Pfizer, H3 Biomedicine, and Takeda. A.J.M. is an inventor on patents related to the IFN pathway and CAR T cells.
R.H.V. has received consulting fees or honoraria from Celgene, Celldex, Janssen, Lilly, Medimmune, and Verastem; and research funding from Apexigen, Fibrogen, Inovio, Janssen, and Lilly. He is an inventor on a licensed patent relating to cancer cellular immunotherapy and receives royalties from Children’s Hospital Boston for a licensed research-only monoclonal antibody.
T.M. has received honoraria from Merck and Bristol Myers Squibb.
R.R. has received honoraria from Astra-Zeneca.
R.M, J.N.L., D.P., A.P.M., L.S., and R.A. have no relevant conflicts of interest to report.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–8. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajor DL, Mick R, Riese MJ, Huang AC, Sullivan B, Richman LP, Torigian DA, George SM, Stelekati E, Chen F, et al. Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. Oncoimmunology. 2018;7:e1468956. doi: 10.1080/2162402X.2018.1468956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 4.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S.. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo LY, O’Hara MH, Mitchell TC, Vonderheide RH, Wherry EJ, Minn AJ, Maity A. Combining radiation with immunotherapy: the University of Pennsylvania experience. Semin Radiat Oncol. 2020;30:173–180. doi: 10.1016/j.semradonc.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39:644–655. doi: 10.1016/j.it.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiniker SM, Reddy SA, Maecker HT, Subrahmanyam PB, Rosenberg-Hasson Y, Swetter SM, Saha S, Shura L, Knox SJ. A prospective clinical trial combining radiation therapy with systemic immunotherapy in metastatic melanoma. Int J Radiat Oncol Biol Phys. 2016;96(3):578–588. doi: 10.1016/j.ijrobp.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239–1251. doi: 10.1016/S1470-2045(19)30388-2. [DOI] [PubMed] [Google Scholar]
- 13.Barker CA, Postow MA, Khan SA, Beal K, Parhar PK, Yamada Y, Lee NY, Wolchok JD. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res. 2013;1:92–98. doi: 10.1158/2326-6066.CIR-13-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theurich S, Rothschild SI, Hoffmann M, Fabri M, Sommer A, Garcia-Marquez M, Thelen M, Schill C, Merki R, Schmid T, et al. Local tumor treatment in combination with systemic ipilimumab immunotherapy prolongs overall survival in patients with advanced malignant melanoma. Cancer Immunol Res. 2016;4:744–754. [DOI] [PubMed] [Google Scholar]
- 15.Koller KM, Mackley HB, Liu J, Wagner H, Talamo G, Schell TD, Pameijer C, Neves RI, Anderson B, Kokolus KM, et al. Improved survival and complete response rates in patients with advanced melanoma treated with concurrent ipilimumab and radiotherapy versus ipilimumab alone. Cancer Biol Ther. 2017;18:36–42. doi: 10.1080/15384047.2016.1264543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin R, Olson A, Singh B, Thomas S, Wolf S, Bhavsar NA, Hanks BA, Salama JK, Salama AKS. Safety and efficacy of radiation therapy in advanced melanoma patients treated with ipilimumab. Int J Radiat Oncol Biol Phys. 2016;96:72–77. doi: 10.1016/j.ijrobp.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 18.Chandra RA, Wilhite TJ, Balboni TA, Alexander BM, Spektor A, Ott PA, Ng AK, Hodi FS, Schoenfeld JD. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology. 2015;4:e1046028. doi: 10.1080/2162402X.2015.1046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundahl N, De Wolf K, Kruse V, Meireson A, Reynders D, Goetghebeur E, Van Gele M, Speeckaert R, Hennart B, Brochez L, et al. Phase 1 dose escalation trial of ipilimumab and stereotactic body radiation therapy in metastatic melanoma. Int J Radiat Oncol Biol Phys. 2018;100:906–915. doi: 10.1016/j.ijrobp.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, Messenheimer DJ, Fox B, Newell P, Bahjat KS, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One. 2016;11:e0157164. doi: 10.1371/journal.pone.0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandhi SJ, Minn AJ, Vonderheide RH, Wherry EJ, Hahn SM, Maity A. Awakening the immune system with radiation: optimal dose and fractionation. Cancer Lett. 2015;368:185–190. doi: 10.1016/j.canlet.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Rogiers A, Boekhout A, Schwarze JK, Awada G, Blank CU, Neyns B. Long-term survival, quality of life, and psychosocial outcomes in advanced melanoma patients treated with immune checkpoint inhibitors. J Oncol. 2019;2019:5269062. doi: 10.1155/2019/5269062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen -T-T, Berman DM, Wolchok JD, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob -J-J, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al . Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 25.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Lao CD, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 27.Marciscano AE, Ghasemzadeh A, Nirschl TR, Theodros D, Kochel CM, Francica BJ, Muroyama Y, Anders RA, Sharabi AB, Velarde E, et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin Cancer Res. 2018;24:5058–5071. doi: 10.1158/1078-0432.CCR-17-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, Vanpouille-Box C, Friedman K, Ferrari de Andrade L, Wucherpfennig KW, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24:1845–1851. doi: 10.1038/s41591-018-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, Basu S, Curran MA, Cabanillas ME, Subbiah V, et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res. 2017;23:1388–1396. doi: 10.1158/1078-0432.CCR-16-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maity A, Mick R, Huang AC, George SM, Farwell MD, Lukens JN, Berman AT, Mitchell TC, Bauml J, Schuchter LM, et al. A phase I trial of pembrolizumab with hypofractionated radiotherapy in patients with metastatic solid tumours. Br J Cancer. 2018;119:1200–1207. doi: 10.1038/s41416-018-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


