Abstract
This article comments on:
Xiaoxue Zeng, Gang Chen, Lei Wang, Akemi Tagiri, Shinji Kikuchi, Hidenori Sassa and Takao Komatsuda, The unique disarticulation layer formed in the rachis of Aegilops longissima probably results from the spatial co-expression of Btr1 and Btr2, Annals of Botany, Volume 127, Issue 3, 16 February 2021, Pages 297–304, https://doi.org/10.1093/aob/mcaa147
Keywords: Rachis, Aegilops longissima, seed dispersal
Some species of goatgrass (the genus Aegilops) drop their seeds in a peculiar way, with the inflorescence stalk (rachis) splitting in two about halfway up, yielding two otherwise unbroken units. In this issue of Annals of Botany, Zeng et al. (2020a) show that the genes controlling this process in Aegilops longissima may be similar to those already known in wild species of wheat (Triticum and Aegilops spp.) and barley (Hordeum spp.).
Plants have myriad ways to disperse their seeds, whether to enclose them in edible packages (fruits), fling them into the wind, or simply drop them onto the ground for passing insects to carry off. In all cases, the seeds or their enclosing structures fall off the plant in a precisely controlled way, with a specific break point known as an abscission zone. Think of it as the perforated line seen on some cardboard packaging where it is labelled ‘Tear along dotted line’. The abscission zone forms in a predictable place in a given plant and can often be seen in cross-sections as a set of cells that are distinctive in their size or response to specific stains (Yu and Kellogg, 2018).
In domesticated cereals, the breakage process is known as shattering. The earliest step in plant domestication was often unconscious selection against shattering. As our ancestors collected edible seeds, they would have chosen the ones still attached to the plant rather than those that had fallen in the dirt. When they then began to plant some of the seeds for a new crop, they were selecting for plants that held on to their seeds slightly longer than the wild ones. Thus all of our major cereal crops are mutants in which the abscission zone fails to form and to function.
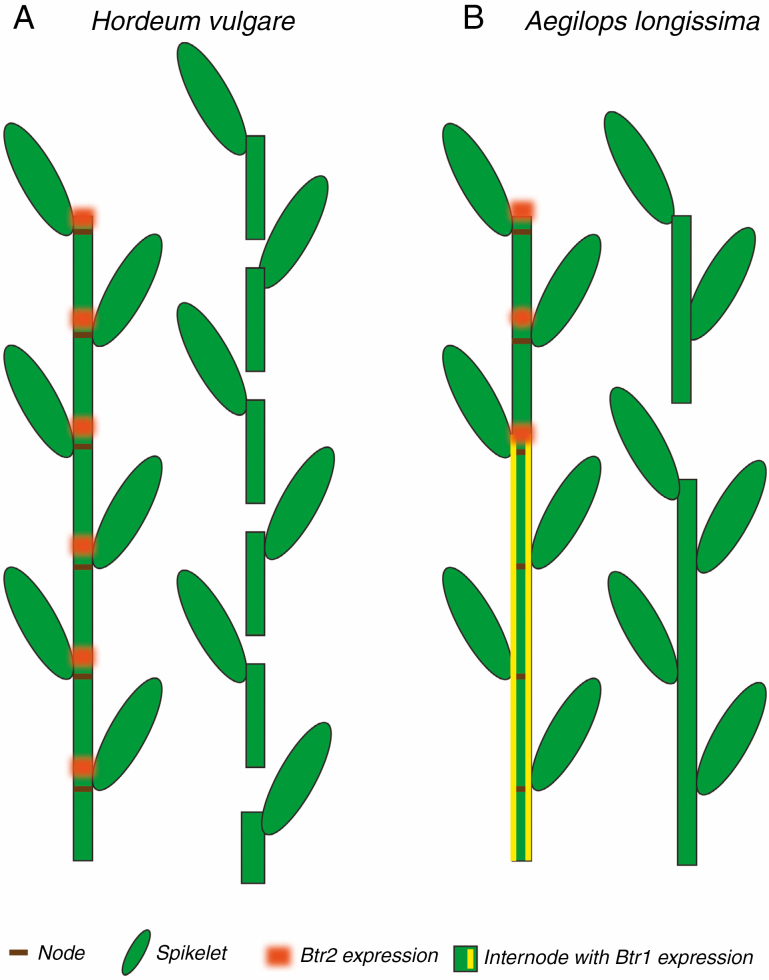
The domestication process in general, and loss of shattering in particular, has been studied extensively in wheat and barley, both members of the grass tribe Triticeae. In wild wheats and barley species, the inflorescence stalk, or rachis, breaks between the floral units (spikelets) (Fig. 1A). Breakage is controlled by two loci, Non-brittle rachis1 (Btr1) and Non-brittle rachis2 (Btr2). In barley (Hordeum vulgare), a mutation in either locus prevents shattering (i.e. disables the abscission zone) (Pourkheirandish et al., 2015). Btr2 is expressed just above the node (Fig. 1A, orange bands), suggesting it is intimately involved in forming the abscission zone (Pourkheirandish et al., 2015). Likewise, a mutation in Btr1 in Einkorn wheat (Triticum monococcum ssp. monococcum) creates a non-shattering inflorescence (Pourkheirandish et al., 2017). Bread wheat (Triticum aestivum) is a product of two hybridization events, resulting in a species made of three distinct genomes (an allohexaploid), one of which came from a species similar to Triticum urartu; the other two came from species of goatgrass. Each genome brings its own copy of Btr1 and Btr2, and mutations in Btr1 are required in both the A and B genomes to confer a non-shattering rachis (Zhao et al., 2019).
Fig. 1.
Inflorescences of (A) Hordeum vulgare and (B) Aegilops longissima. In H. vulgare, Btr2 is expressed in a zone just above the nodes. At maturity, the rachis breaks up to produce spikelets attached to the node and the internode below. In A. longissima, Btr2 is also expressed just above the nodes but only in the distal nodes of the inflorescence, mostly above node 4. Btr1 is expressed in the cortical cells in the proximal four nodes. The rachis breaks only around node 4, where expression of the two Btr genes overlaps. Hordeum has three spikelets per node but only the central fertile one is illustrated here.
In A. longissima, the inflorescence appears to be partly non-shattering and partly shattering. Only one or two of the middle nodes of the rachis fall apart so the most proximal (bottom-most) spikelets remain attached, and the distal ones fall off as a unit (Fig. 1B). Zeng et al. (2020a) examined expression of the Btr loci from A. longissima, and found that Btr1 was expressed in the cortex of the rachis in the lowermost four nodes and internodes (Fig. 1B, yellow lines), whereas Btr2 was expressed in areas just above the nodes starting around node 4 (Fig. 1B, orange bands). Only where the expression domain of the two genes overlapped did the rachis break up. The morphology of the cells at the break point is similar to that in barley, in that the cell walls are thinner than those above and below the break point and form a smooth layer after abscission occurs.
Because shattering and seed dispersal are near-universal among plants, one might assume the mechanisms and genetic controls discovered in Triticeae are similar and largely conserved, at least among the cereal crops. However, the position of the abscission zone differs among species and among major clades of grasses (Doust et al., 2014). Even within the single tribe Triticeae, abscission occurs just above the node in Hordeum and many Aegilops species (Fig. 1), but just below the node in other species (e.g. Aegilops tauschii, the donor of the D genome of wheat; Zeng et al., 2020b). Still other species, including most of the perennials, have a tough rachis and breakage occurs between the flowers. In addition, the break point above the node in Triticeae is histologically undifferentiated, whereas abscission zones in other species, such as rice, comprised a set of isodiametric cells that are noticeably smaller than the cells around them (Yu et al., 2020a). Differences in cell size and cell wall composition across the breakage zone are common in grasses in general, but far from universal (Yu et al., 2020a). For example, studies in rice imply that differential lignification is central to shattering, but the observations on A. longissima show that differential lignification is not required (Zeng et al., 2020a).
Not only does the position and histology of the abscission zone vary among grass species, but few if any ‘shattering genes’ are shared. A recent RNA-seq study of three disparate species of grasses (Setaria, Oryza, Brachypodium) specifically sought to identify a shared gene expression module that would be common to abscission in all of them (Yu et al., 2020b). Unexpectedly, no conserved expression module was found; only a couple of genes (an MYB transcription factor and a gene with a lysine decarboxylase domain) were expressed in the abscission zone of all three species. Both the study of Yu et al. (2020b) and other genetic literature point to genes that are truly restricted to particular lineages. The Btr loci occur only in Triticeae (Zeng et al., 2020b), where they appear to be derived via duplication from the paralogous genes Btr1-like and Btr2-like. The latter two loci are present in all grasses investigated, but there is no evidence that Btr1-like and Btr2-like have anything to do with abscission (Zeng et al., 2020b).
The lack of similarity in morphology, histology and controls of abscission differ in every species examined compromises our ability to infer anything about unstudied species. That is, if studying disarticulation in rice does not illuminate the process in wheat and barley and vice versa (and it indeed does not), how much insight can be offered from any one species? The paper by Zeng et al. (2020a) fortunately offers some hope in this regard. By showing that the Btr loci may control shattering in A. longissima, we can infer that the rachis may break up in the same way and under the same genetic control in many of the annual species of Triticeae, most of which have a shattering rachis that breaks just above the nodes. This result may appear simply confirmatory, but in fact it is important in establishing the level of generality. In the future, it will be intriguing to know if the Btr loci are present and functional in the perennial Triticeae (e.g. Leymus, Elymus, Pseudoroegneria, Psathyrostachys, etc.) in which the rachis is generally tough.
One emerging possibility is that most of the proteins that regulate shattering are general patterning genes that govern plant development and morphology, and are not specific to the process of abscission at all. Possibly the disparate genes that regulate abscission in different crops are the very genes that make the crops morphologically distinct. For example, the Btr proteins could be involved in controlling rachis architecture and nodal anatomy. That their mutation leads to loss of shattering could be incidental. Only with further studies of their cellular and developmental function will this hypothesis be tested.
Finally, a missing link in the literature on abscission is the underlying mechanism behind breakage. Data on abscission of floral organs in tomato suggest an active process, whereby a signalling module is activated, enzymes are exported from the cell and the cell wall breaks down (Reichardt et al., 2020). However, there is no evidence for or against such a mechanism in the cereals. Many ‘domestication’ genes are transcription factors, but it is unclear what downstream processes they regulate. The function of the Btr loci is completely unknown even though their developmental role is now confirmed in multiple Triticeae species. Molecular genetic analysis of the Btr1/2 loci and biochemical study of their products will be needed to understand their precise function in abscission regulation.
LITERATURE CITED
- Doust AN, Mauro-Herrera M, Francis AD, Shand LC. 2014. Morphological diversity and genetic regulation of inflorescence abscission zones in grasses. American Journal of Botany 101: 1759–1769. [DOI] [PubMed] [Google Scholar]
- Pourkheirandish M, Hensel G, Kilian B, et al. 2015. Evolution of the grain dispersal system in barley. Cell 162: 527–539. [DOI] [PubMed] [Google Scholar]
- Pourkheirandish M, Dai F, Sakuma S, et al. 2017. On the origin of the non-brittle rachis trait of domesticated Einkorn wheat. Frontiers in Plant Science 8: 2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt S, Piepho HP, Stintzi A, Schaller A. 2020. Peptide signaling for drought-induced tomato flower drop. Science 367: 1482–1485. [DOI] [PubMed] [Google Scholar]
- Yu Y, Kellogg EA. 2018. Inflorescence abscission zones in grasses: diversity and genetic regulation. Annual Plant Reviews 1: 1–35. [Google Scholar]
- Yu Y, Leyva P, Tavares RL, Kellogg EA. 2020a The anatomy of abscission zones is diverse among grass species. American Journal of Botany 107: 549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Hu H, Doust AN, Kellogg EA. 2020b Divergent gene expression networks underlie morphological diversity of abscission zones in grasses. New Phytologist 225: 1799–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Chen G, Wang L, et al. 2020. a The unique disarticulation layer formed in the rachis of Aegilops longissima likely results from the spatial co-expression of Btr1 and Btr2. Annals of Botany 127: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Mishina K, Jia J, et al. 2020. b The brittle rachis trait in species belonging to the Triticeae and its controlling genes Btr1 and Btr2. Frontiers in Plant Science 11: 1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xie P, Guan P, et al. 2019. Btr1-A induces grain shattering and affects spike morphology and yield-related traits in wheat. Plant & Cell Physiology 60: 1342–1353. [DOI] [PubMed] [Google Scholar]