Abstract
Background and Aims
Oat (Avena sativa) has human health benefits when consumed as a whole-grain food, attributed to the high content of (1,3;1,4)-β-d-glucan (mixed-linkage glucan [MLG]), but little is known about the synthase genes and synthesis mechanism of MLG polysaccharides in this species.
Methods
The concentration of oat MLGs under different light intensities was measured by a standard enzymatic approach and further verified by immunoelectron microscopy. The effect of light intensity on MLG synthase genes was examined by RT–qPCR and western blot analyses. The pattern of expression directed by the promoter of the oat MLG synthase gene was also investigated by histochemical β-glucuronidase (GUS) analysis.
Key Results
The oat orthologues of genes implicated in the synthesis of MLG in other cereals, including cellulose synthase-like (Csl) F, H and J gene families, were defined. Transcript profiling of these genes across oat tissues indicated that AsCslF6 transcripts dominated. Under high light intensities, the expression of AsCslF6, a major isoform of the MLG synthase genes, increased to >30 % of the dark growth control. The amount of MLG in oat rose from 0.07 to 1.06 % with increased light intensity. Histochemical tests showed that the AsCslF6 gene promoter preferentially directs GUS expression under high light intensity conditions.
Conclusions
Oat MLG synthesis is regulated by light. High light intensity upregulates the expression of the MLG synthase AsCslF6 gene, leading to an increase in the amount of MLG in oat leaves.
Keywords: Oat, β-glucan, cellulose synthase-like genes, enzymatic assay, transcript profiling, promoter analysis, light-mediated regulation
INTRODUCTION
The (1,3;1,4)-β-d-glucans (mixed-linkage glucan [MLGs]) are linear polysaccharides consisting of monomeric β-d-glucopyranosyl residues linked through their C(O)3 and C(O)4 atoms. Despite some isolated cases, MLGs are distributed almost exclusively within the Poaceae, where they appear to be components of both primary and secondary walls (Fincher, 2009). Furthermore, MLGs are thought to have human health benefits. Reports have shown soluble fibres in some cereal grains such as barley (Hordeum vulgare L.) and oat (Avena sativa L.), which consist mainly of MLGs and are beneficial in the prevention and treatment of serious human health conditions, including obesity, colorectal cancer, high serum cholesterol and cardiovascular disease (Brennan and Cleary, 2005; Wood, 2007).
Although the structures, tissue distribution and physicochemical properties of the MLGs are well defined, there is relatively little information on the enzymes involved in their biosynthesis. This is partly due to the complexity of the synthesis of MLGs, which have limited functional mutants. Comparative genomic technologies present an alternative approach to identifying the components of the MLG synthetic machinery. By comparing the detected barley MLG quantitative trait locus (QTL) with the genome sequence of rice, Burton et al. (2006) determined cellulose synthase-like (Csl) gene family members as candidate genes for MLG biosynthesis. Gain-of-function experiments on Arabidopsis, a dicot devoid of MLG, showed that MLG was produced and deposited in the cell walls by immunocytochemical and enzymatic methods when expressing rice OsCslF genes. This suggested that OsCslF genes are essential for MLG biosynthesis (Burton et al., 2006). Functional analysis of the HvCslF homologue of OsCslF in barley also confirmed the role of the CslF gene in MLG biosynthesis (Burton et al., 2011). Using similar approaches, other Csl family members that mediate MLG synthesis, including CslH and CslJ, were also identified recently (Doblin et al., 2009; Little et al., 2018). To date, no other candidate Csl family genes potentially interacting with the MLG synthesis pathway have been identified.
Barley is most commonly used in the study of MLG biosynthesis genes, partly due to its high levels of MLG compared with other crops. Furthermore, there is an interest in MLG in the barley processing industry (malting, brewing and animal feed) since high levels of MLG are undesirable. This interest led to the early development of QTL maps and molecular markers that contribute to levels of MLG in barley. Oat also contains high levels of MLG (3–7 %) and is distinct among the cereals due to its multifunctional characteristics and nutritional profile (Welch and Lloyd, 1989). Unlike barley, oats and oat by-products are considered functional foods in the human diet and have been linked to the health claims attributed to MLG (Butt et al., 2008). Oat research has often focused on MLGs since they are considered an important characteristic of oats. Several QTL studies of grain MLG content using both biparental and association mapping populations have been carried out in oat (Kianian et al., 2000; Newell et al., 2012). However, the mechanism of MLG biosynthesis in cultivated oat and the associated genes remain largely unknown, mainly due to oat’s ploidy and large genome. In this study, we defined MLG biosynthesis genes in oat and characterized their regulatory mechanisms.
MATERIALS AND METHODS
Plant material and growth conditions
Oat (Avena sativa ‘Xiayoumai’) plants were grown in a greenhouse under a day/night temperature regime of 28/15 °C. For gene isolation and gene tissue-specific expression analysis, tissue samples were collected from five oat plants and frozen immediately in liquid nitrogen. The pollination time of oat spikelets was deduced from the grain size, and grains taken ~15 d after pollination were selected for gene expression analysis. Other oat tissues, including stem, leaf and root, were sampled simultaneously from these plants. All experiments were carried out at least three times. For light treatments, high light (continuous white LED lighting system, 300 μmol m−2 s−1), low light (continuous white LED lighting system, 80 μmol m−2 s−1) and complete darkness were applied to intact plants incubated in a growth chamber for 2 d when the second leaf was fully expanded. To examine the expression level of MLG synthesis genes entirely in response to light treatment, whole plants were harvested and ground to powder for gene expression analysis. Experiments were always started in the middle of the light period, and were carried out at least three times. Segments were cut from the middle part of fully expanded second leaves for MLG detection.
Isolation of genomic and cDNA clones of MLG synthesis genes in oat
Total RNA was extracted using TRIzol reagent (Invitrogen) and was treated with RNase-free DNase I (Promega) to remove genomic DNA according to the manufacturer’s instructions. Then, cDNA synthesis was conducted using SuperScript III reverse transcriptase for RT–PCR (Invitrogen) using purified RNA. The oat MLG synthesis genes were cloned by a comparative genomics approach. Firstly, the fragments of MLG biosynthesis genes of Csl families were amplified with primers designed to match conserved regions of identified MLG biosynthesis genes using cDNA prepared from various tissues as template. Subsequently, 3′ or 5′ ends of the cDNA were obtained by PCR using a SMART RACE kit according to the manufacturer’s (Clontech) instructions. Full-length cDNAs were identified by PCR amplification with gene-specific primers designed to the 5′ and 3′ untranslated regions. The fragments and cDNAs were cloned into the pGEM-T Easy vector (Promega) and sequenced on an ABI 3730xl analyser (Applied Biosystems).
The full-length DNA sequences of MLG synthesis genes, including putative promoter regions, were obtained using the Universal Genome Walker Kit (Clontech) according to the manufacturer’s instructions. Genomic DNA was isolated from young leaves using the DNeasy Plant DNA Kit (Qiagen). Then, the genomic DNA was digested with DraI, StuI, PvuII and EcoRV to make a blunt end. Genome walker adapters were ligated to the digests and the ligated products were then used as template to amplify promoter regions of MLG synthesis genes. The gene-specific primers were designed according to the cDNA sequence isolated as described above and the nested PCR products were cloned and sequenced. Finally, the full-length DNAs were identified by PCR amplification and sequencing. PCR primers used to isolate the full-length cDNA and corresponding genomic loci are listed in Supplementary Data Table S1.
Structure and conserved domain analysis of the isolated oat MLG synthesis genes
For gene structure analysis, the number and position of exons and introns were determined by comparing the coding sequences with their corresponding genomic DNA sequences, and maps of these gene structures were generated using the Gene Structure Display Server (Guo et al., 2007). By comparing the coding sequences with their genomic DNA sequences, regions ~2000 bp upstream of the start codon were extracted from the genomic DNA sequences and were designated as promoter sequences. These sequences were used to query the PLACE database (Higo et al., 1999), and the putative cis-acting regulatory DNA elements of the promoter sequences were identified. Functional motifs or domains of CSL protein sequences were analysed using PROSITE and the Conserved Domain database (Sigrist et al., 2002; Lu et al., 2020). MEME (http://meme-suite.org/) (Bailey et al., 2006) was used to identify motifs in candidate sequences. Transmembrane helices were predicted using the website http://www.cbs.dtu.dk/services/TMHMM-2.0/.
The phylogenetic analysis of the isolated genes was carried out using identified MLG synthesis genes from other gramineous crops, including rice, barley, sorghum and Brachypodium. These gene sequences were downloaded from public databases such as Phytozome, GenBank and GRAMENE (Ermawar et al., 2015). Phylogenetic trees were constructed with the MEGA 7.0 software using the neighbour-joining method and the bootstrap test was replicated 1000 times (Kumar et al., 2016).
Expression pattern of the oat MLG synthesis genes in different tissues and conditions
The identification of expression patterns of oat MLG synthesis genes in different tissues was carried out by RT–qPCR according to the method described by Burton (Burton et al., 2008). Firstly, oat vegetative tissues and developing grains were frozen in liquid nitrogen immediately after harvest and total RNA was isolated and digested with RNase-free DNase I. Then, 1 μg of purified total RNA was reverse-transcribed into cDNA using SuperScript III reverse transcriptase and diluted to 200 μL in sterile water as the RT–qPCR template. Reactions were performed on an ABI 7500 Real-Time PCR System (Applied Biosystems) using a Platinum SYBR Green qPCR SuperMix-UDG kit (Thermo Fisher). Three replicates per sample were carried out for RT–qPCR validation and the housekeeping genes EF1α and PP2A were selected as internal controls. The cycling conditions were as follows: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Primers used in these RT–qPCR experiments are listed in Supplementary Data Table S2. Considering the high similarity of these genes, each set of PCR primers was designed so that their sequences would match perfectly with the target gene but differ in at least three nucleotides from others. The specificity of amplification was verified at the end of each PCR run using 7500-system SDS Dissociation Curve Analysis Software, and melting curve analysis indicated that all the primers generated a single peak.
Characterization of the promoters of MLG synthesis genes
The effect of light on MLG synthesis gene expression was also studied by characterization of the gene promoter. Firstly, promoter regions of AsCslF4 (1734 bp) and AsCslF6 (2068 bp) were amplified from oat genomic DNA and ligated to the pCAMBIA1391z binary vector to form promoter–β-glucuronidase (GUS) gene fusions. The resulting recombinant plasmids were introduced into Agrobacterium tumefaciens strain EHA105 and transformed into Arabidopsis (ecotype Col-0) (Clough and Bent, 1998). Histochemical staining and quantitative analyses of GUS activity were conducted as described by Jefferson (1987). For histochemical staining, T2 generation transgenic Arabidopsis plants subjected to either continuous darkness or under appropriate light conditions were incubated in GUS reaction buffer (RTU4032, Real-Times, China) at 37 °C overnight, then washed twice with ethanol to remove chlorophyll. As the T2 generation may have null lines, transgenic Arabidopsis plants were screened by PCR and only positive strains of transgenic plants in the rosette stage were selected for promoter analysis. At least three independent lines were tested in triplicate for each assay.
Western blot analysis
Expression of AsCslF4 and AsCslF6 proteins was determined by western blot analysis. Oat samples were ground in liquid nitrogen using a pestle and mortar. Precooled RIPA protein extraction buffer was added to the ground powder, the preparation was incubated on ice for 20 min, and the mixture was centrifuged at 20 000 g for 20 min. The supernatant was collected and the protein concentration was measured by the BCA method. For western blot analysis, samples were loaded on different gels and transferred to nitrocellulose membranes separately. Forty micrograms of protein was loaded into wells and separated by 8 % SDS–PAGE. After electrophoresis, gels were blotted onto nitrocellulose and membranes were blocked in 3 % bovine serum albumin (BSA)–TBST for 30 min before incubation with rabbit anti-AsCslF4/6 antibody (1:1000 dilution). Membranes were washed five times in TBST, then incubated in goat anti-rabbit IgG HRP-conjugated antibody diluted 1:1000 in TBS containing 5 % w/v non-fat milk powder. Membranes were washed five times in TBST, and the signal was detected using a Pierce ECL Western Blotting Substrate kit according to the manufacturer’s instructions.
Determination of MLG concentration
The MLG concentration was determined using the mixed-linkage β-Glucan Assay Kit (Megazyme, Ireland) with small modifications. Samples were first oven-dried at 105 °C for 2 h followed by 80 °C for 24 h. Dry leaves were ground with Cyclotec (Foss, Sweden) and passed through a 0.5-mm sieve to get a homogeneous powder. To eliminate the influence of monosaccharides and chlorophyll, samples were washed with 75 % (v/v) ethanol for 10 min at 95 °C before analysis. To avoid sampling errors, three or more independent biological replicates of each treatment were performed. Data were analysed using SPSS and presented as the means and standard deviations of three replicates. The significance of differences was tested by Student’s t-test.
Immunoelectron microscopy
The effect of light intensity on MLG content in oat was further verified by transmission electron microscopy immunocytochemistry as described previously by Burton et al. (2011) and Wilson et al. (2006). To eliminate the possible influence of dark-induced accumulation of (1,3;1,4)-β-d-glucanase, which would result in MLG hydrolysis, all oat plants were dark-treated for 24 h then transferred to different light conditions. Dissected oat leaves were fixed by vacuum infiltration with 0.25 % glutaraldehyde, 4 % paraformaldehyde and 4 % sucrose in phosphate-buffered saline (PBS) and incubated at 4 °C overnight. After rinsing and dehydration in a graded ethanol series, samples were embedded in LR White resin over several days. Resin-embedded leaf sections were cut to ~70 nm thickness and collected on Pioloform-coated gold grids. Three slides were made, each containing biological replicates. Sections were rehydrated with PBS, incubated with 0.05 m glycine to inactivate residual aldehyde groups, and blocked with 1 % (w/v) BSA in PBS (blocking buffer) for 20 min. Sections were incubated with monoclonal anti-(1,3;1,4)-β-d-glucan antibody (Biosupplies, Australia) at 1:50 dilution for 60 min. After washing three times with blocking buffer, sections were incubated in a 1:20 dilution of rabbit-anti-mouse secondary antibody conjugated to 18 nm gold particles (Jackson ImmunoResearch, USA) for 60 min. Grids were washed three times with blocking buffer and rinsed several times with distilled water. Sections were examined using an HT7700 transmission electron microscope (Hitachi, Japan) at 80 kV.
RESULTS
Isolation of genomic and cDNA clones of MLG synthesis genes
Candidate MLG synthesis genes in oat were isolated through homology-based cloning and confirmed by sequence alignment. The nomenclature of the isolated genes was based on the orthologues from identified MLG synthesis genes in other cereals. As a result, genes orthologous to CslF3, CslF4, CslF8, CslF9, CslH and CslJ were identified in oat except for CslF6, which has been identified previously (Chawade et al., 2010; Jobling, 2015). The lengths of these isolated cDNAs for AsCslF3, AsCslF4, AsCslF8, AsCslF9, AsCslH and AsCslJ were 2716, 2885, 2772, 2825, 2534 and 2641 bp, respectively. The open reading frames (ORFs) of these cDNAs were predicted to encode polypeptides from 748 to 901 amino acid residues within the range defined by other species (Supplementary Data Table 3). The genome loci corresponding to these isolated genes were obtained by PCR amplification from genomic DNA up to 2000 bp upstream of the gene start codon site, using gene-specific primers. Once the sequences were assembled, PCR was used to generate near-full-length genomic sequences corresponding to each of these genes. The complete sequences for these genes were submitted to the public databases and assigned the following accession numbers: AsCslF3, MG543996; AsCslF4, MG543997; AsCslF6, MG543998; AsCslF8, MG543999; AsCslF9, MG544000; AsCslH, HQ128579; and AsCslJ, MK905204. Furthermore, we isolated the AsCslF6 genomic locus sequence, whose cDNA sequence has been found in GenBank previously.
Structure of the isolated oat MLG synthesis genes
The introns of the MLG synthesis genes were identified by comparison of the nucleotide sequences between the isolated cDNA and corresponding genomic loci (Fig. 1). The introns, which are flanked by consensus intron processing motifs, ranged in size from 79 to >3476 bp. Comparison with published MLG synthesis genes in other cereals, such as rice and barley, shows that the numbers and locations of these introns are relatively conserved among species (Burton et al., 2008).
Fig. 1.
Structure of the isolated AsCsl genes. Exons and introns are shown as boxes and lines, respectively. Numbers above boxes show the length of exons, while those below show the length of introns (in base pairs).
Phylogenetic analyses of the isolated oat CslF, CslH and CslJ sequences were performed with the deduced amino acid sequence using MEGA software, and they displayed high similarity with those of other species. An unrooted, radial phylogenetic tree of the isolated oat CslF, CslH and CslJ sequences and the corresponding genes of other species indicated the orthologous pairs (Supplementary Data Fig. S1). The resulting phylogenetic tree showed a clear division into different clades according to orthologous genes and highlights the orthologous relationships of these Csl oat genes.
Analysis of the predicted protein structures of these isolated genes showed that all predicted proteins have the characteristic glycosyltransferase motif D, D, D, QxxRW and therefore belong to the GT2 family of glycosyltransferases. Further analysis of the protein structures in other cereals such as rice, barley, sorghum and Brachypodium showed that the catalytic motifs of MLG synthesis proteins are highly conserved. The CslF3 enzyme has a QIVRW motif and the CslF6 enzyme has a QVLRW motif, while CslF8 and CslF9 share a QILRW motif. However, the CslF4 enzyme is different; in rice it has a QILRW motif, in sorghum it has a QLLRW motif, while in oat, Brachypodium and barley it has a QVLRW motif. In most cases, changes in amino acid residues around the motif are conserved (Supplementary Data Fig. S2). TMHMM analysis showed that the isolated AsCsl proteins possess transmembrane helices and are probably membrane proteins. For all AsCslF family members, eight transmembrane helices were predicted, two near the N-terminus and six near the C-terminus. For AsCslH six transmembrane helices were predicted and four near the C-terminus; for AsCslJ, seven transmembrane helices were predicted. These structural differences mean that they may play different roles in MLG synthesis.
Expression pattern of the isolated oat MLG synthesis genes in different tissues
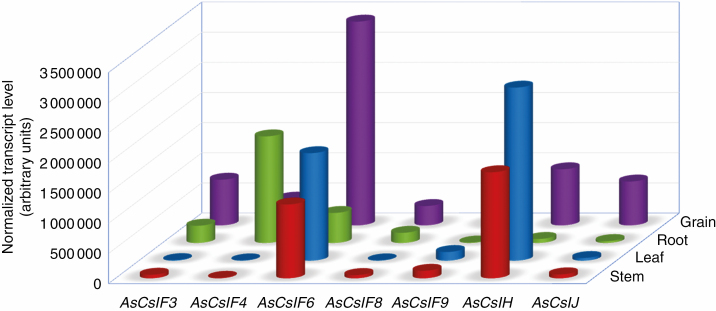
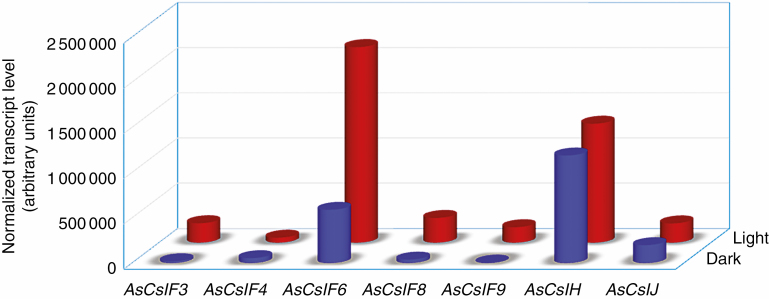
The abundance of the isolated oat AsCsl family genes in various tissues was determined by RT–qPCR in roots, stems, leaves and developing grains (~15 d after pollination) of ‘Xiayoumai’, an oat landrace with high glucan content (>6 %). The RT–qPCR results showed that the isolated AsCsl genes exhibited diverse expression levels in all four detected samples, indicating different roles of these genes for MLG synthesis in different tissues (Fig. 2). Among them, AsCslF6 had the highest transcript levels in all tested tissues, similar to previously reported CslF6 expression profiles in some other species (Burton et al., 2008; Ermawar et al., 2015). Unlike in other crops, such as sorghum, the AsCslH gene was highly expressed in most tissues examined. Comparison of gene expression levels among different tissues showed that AsCslF6 and AsCslH have higher seed specificity. Other AsCslF family gene transcripts were also abundant in grains and at relative low levels in roots except for AsCslF4, which was expressed at a higher level in roots than other tissues. Analysis of the promoter sequence prompted us to examine the possible effect of light on the expression profile of isolated Csl genes. As shown in Fig. 3, all individual Csl genes had different expression levels under dark and light conditions. Among them, AsCslF6 and AsCslF8 consistently exhibited about 3- to 8-fold higher expression level in the light than in the dark, while AsCslF4 and AsCslH showed minimal expression changes between light and dark. Remarkably, AsCslF3 and AsCslF9 exhibited >10-fold higher expression level under light conditions compared with the dark.
Fig. 2.
Expression levels of the isolated AsCsl genes in a variety of tissues presented as normalized copies per microlitre of cDNA. Normalized levels of oat cellulose synthase-like gene transcripts in a range of tissues were calculated using RT–qPCR data. These transcripts were detected in all tissues examined and very high levels of AsCslF6 and AsCslH mRNA were detected in some tissues. The RT–qPCR data for the AsCsl genes include at least three biological replicates per tissue.
Fig. 3.
Comparison of AsCsl family gene member expression levels under dark and light conditions. Whole plants were harvested for gene expression analysis.
AsCslF promoters can confer differential expression under different light conditions
Analysis of the promoter regions of isolated genes revealed that multiple potential transcription factor-binding motifs were present upstream of the start codon. In addition to many necessary characteristic elements, such as TATA and CAAT boxes, some phytohormone response elements, such as the auxin-responsive TGA element and AuxRR-core element, MeJA-responsive CGTCA-motif and salicylic acid-responsive TCA element, were detected in the promoter regions. However, analysis showed that the most abundant cis elements in these isolated promoters are light-responsive elements. For example, AsCslF6 contains as-2-box, Box IV, CATT-motif, GAG-motif, G-box, I-box, LAMP-element, TCT-motif and Sp1 motif cis-acting regulatory elements involved in light responsiveness. Moreover, some cis elements have more than one copy; for example, seven copies of the Sp1 motif were detected in the promoter region of CslF6, indicating that the expression of AsCslF6 may be regulated by light. Other promoters have similar structural features. Figure 4 shows the exact location of some characteristic elements of putative promoters except the common core promoter elements such as TATA and CAAT boxes.
Fig. 4.
Characteristic cis-acting elements in the putative promoter region of isolated AsCsl genes. Putative cis-acting elements were predicted by PLACE. Lines represent upstream gene sequences and boxes of different colours represent different elements. Numbers above and below the boxes indicate the positions of corresponding elements upstream of the start codon. To simplify, common core promoter elements such as TATA and CAAT boxes are not shown.
To further verify the RT–qPCR results and the bioinformatics predictions, promoters of AsCslF4 and AsCslF6 were isolated and fusion constructs (PAsCslF:GUS) were transformed into the Arabidopsis genome to investigate the activity of these promoters. GUS staining and a fluorometric assay showed that GUS gene expression occurred in both leaf and root tissues in transformants, but mainly in the aerial parts of plants, with much lower levels in roots. Figure 5 shows that although expression was low under weak light intensity and in darkness, PAsCslF6 exhibited light-inducible expression activity in GUS staining assays of PAsCslF6:GUS fusion transformants, while minimal change was detected in PAsCslF4:GUS fusion transformants. These results indicate that light can induce the expression of GUS genes in PAsCslF6:GUS fusion transformants, but has little effect on the AsCslF4 promoter. These findings confirmed the RT–qPCR results and the in silico prediction that the AsCslF6 promoter is regulated by light.
Fig. 5.
GUS activity in PAsCslF4:GUS and PAsCslF6:GUS transformants under different light intensities. The effects of light intensity on promoter activity of AsCslF4 and AsCslF6 were estimated by quantitative GUS activity assays. Transformants containing PAsCslF4:GUS or PAsCslF6:GUS were exposed to different light intensities for 48 h and GUS activity was measured. Levels of GUS activity at different light intensity are shown for seedlings grown in darkness (DK), low light intensity (LL) and high light intensity (HL).
Protein and glucan determination
To test whether expression of the AsCslF protein is regulated by light, polyclonal antibodies raised against AsCslF4 and AsCslF6 proteins were used to investigate expression changes under different light intensities by western blot analysis. Supplementary Data Fig. S3 shows that expression of the AsCslF6 protein increased with increasing light intensity, which confirmed the light-responsive characteristics of AsCslF6. Although changes in the expression of AsCslF4 protein were minimal, AsCslF6 was actually expressed at much higher levels than AsCslF4 under all detected conditions.
To investigate changes in MLG content in response to variation in illumination, the concentration of oat MLG under different light intensities was measured by a standard enzymatic approach. Figure 6D shows that the amount of MLG in oat leaves exhibited obvious differences under different light conditions. Compared with values for leaves under dark growth conditions, the detected MLG content was significantly higher under light growth conditions. To monitor the deposition of MLG under different light intensities and confirm the detected changes, the amount of MLG was further detected using MLG-specific antibody and transmission electron microscopy. Figure 6A–C shows that MLG deposition was barely detectable near the cell wall in dark-grown oat. Compared with dark-grown oat, more MLG was detected around the cell wall of oat grown under low light intensity. Under high light intensity the cell wall of oat was fully filled with MLG molecules.
Fig. 6.
Amount of MLG in leaves of oat grown under different light conditions. Transmission electron micrographs of leaf cross-sections from leaves of oat grown in darkness (A), low light intensity (B) and high light intensity (C), showing immunogold labelling with (1,3;1,4)-β-d-glucan-specific antibody. (D) Concentration of MLG in leaves of oat grown in different light conditions. Values are mean ± s.e. of triplicates. **P < 0.01 compared with dark-grown control (Student’s t-test). CW, cell wall; PM, plasma membrane; DK, grown in darkness; LL, grown in low light intensity; HL, grown in high light intensity.
DISCUSSION
Environmental conditions can exert significant effects on the MLG content. Several lines of evidence showed that the amount of MLG was affected by many environmental factors, such as water, pH, light and temperature. In rice, submergence of seedlings decreased the MLG content in the cell (Kimpara et al., 2008). In barley, the MLG concentration of naturally growing seedlings was found to be significantly decreased when transferred into continuous darkness, and these changes were attributed to the increased activity of MLG hydrolase (Roulin et al., 2002). In general, the amount of cell wall polysaccharides is determined by both synthesis and degradation. Although darkness was proved to induce expression of MLG hydrolase genes, the impact of light intensity on MLG synthesis has not been studied so far. Previous studies found that degradation of MLG in dark-incubated leaves can be completely reversed by illumination (Roulin and Feller, 2001). This finding provides an opportunity to study the changes in MLG synthase activity under different light conditions and its regulation. In the present study, to eliminate the influence of hydrolase on MLG content, incubation in darkness and subsequent re-exposure to light were employed to study the effect of different light intensities on MLG content in oat seedlings. The results of enzymatic assay as well as immunoelectron microscopy showed greater accumulation of MLG at higher light intensity.
To determine whether the greater accumulation of MLG involves an increase in the synthesis of the synthase enzyme, the expression levels of MLG synthase genes under different light conditions were measured by RT–qPCR. Investigation of Csl gene expression showed that transcription levels of most CslF family members were much higher under high light intensity. Further immunoblotting analysis of two typical differentially transcribed AsCslF gene family members, AsCslF4 and AsCslF6, showed that expression of the AsCslF6 protein increased with increasing light intensity, which confirmed the RT–qPCR results. Although changes in the expression of AsCslF4 protein were minimal, considering that expression of the AsCslF6 gene was more than an order of magnitude higher than that of other gene family members, there is reason to believe that the accumulation of MLG is accompanied by an increased production of MLG synthase, such as AsCslF6 protein, in response to higher light intensity.
In wheat, studies on glucanase and carbohydrate levels under different light conditions showed that both enzyme activity and protein levels rapidly and markedly changed with the carbohydrate levels (Wälti et al., 2002). Considering that insufficient light supply and other adverse environmental factors usually lead to decreases in photosynthetic products, the decrease in MLG content was considered to be a feedback of carbohydrate depletion (Wälti et al., 2002). However, our study on the expression of the MLG synthase gene showed that it can be actively regulated by light intensity. Analysis of the promoter sequence provides valuable information on gene regulation. In this study, analysis of the isolated Csl gene promoter sequence revealed many potential transcription factor-binding motifs in the Csl gene loci, and most are light-responsive cis elements. Further investigation identified numerous light-responsive cis elements, and these regulatory element sequences are not only reproducible but are also mostly close together. Previous studies showed that pairwise combinations of the light-responsive elements, but not the individual elements alone, can confer light-inducible expression of the reporter gene (Puente et al., 1996). This finding suggests that these promoters are light-regulated promoters and that Csl genes may also be regulated by light. Investigation of Csl gene expression under different light conditions by RT–qPCR showed not only that the expression of AsCslF6 was regulated by light, but also that the expression level of AsCslF6 was significantly higher than that of other genes, indicating that AsCslF6 is the dominant gene responsible for the synthesis of the majority of MLG in oat. Comparing with other MLG synthase genes, AsCslF4 was the least affected gene in response to light treatment according to the qPCR data. To verify these findings, the promoters of AsCslF4 and AsCslF6 were cloned and ligated to a GUS-containing expression vector for promoter analysis. Results of histochemical staining for GUS clearly demonstrated the light-inducible activity of the AsCslF6 promoter and the light insensitivity of the AsCslF4 promoter, which also verified the RT–qPCR results. These observations suggest that the change in MLG synthase activity can be directly regulated by light intensity and is not merely a result of glucose substrate-induced regulation. When light is insufficient, oat seedlings will actively reduce the synthesis of MLG by reducing the expression of the MLG synthase gene. Although both AsCslF4 and AsCslF6 had light-regulatory elements in their promoters, RT–qPCR and GUS assay results showed that only the AsCslF6 promoter was significantly regulated by light. These findings indicate that although bioinformatics analysis suggests that both promoters are rich in light-regulatory elements, the results of this analysis can only be regarded as predictions, and specific functions of these promoters need to be verified by further experiments. These findings also provide an opportunity to explore the regulation mechanisms of MLG synthesis. Using some biochemical methods for the analysis of DNA–protein interactions, such as the yeast one-hybrid and electrophoretic mobility shift assays, it is possible to validate the transcriptional activation and signal transduction involved in the regulation of MLG synthesis.
Although plant biologists have identified some genes that encode MLG synthesis, the regulation mechanisms of MLG synthesis are largely unknown. MLG synthase can produce a wide range of ratios of cellotriose units to cellotetraose units in vitro, while the distribution of the units in vivo is tightly regulated within a specific grass species (Wood et al., 1994). This difference indicates that the mechanism of MLG synthesis in vivo is different from that in vitro. There are some kinds of regulatory factors in oat that can modulate glucose synthesis, but these factors are not yet known. In maize, treatments that induce a large decrease in substrate UDP-glucose concentration did not result in marked changes in cellodextrin oligomeric ratios or in molecular size of the newly synthesized MLG (Buckeridge et al., 1999). These data indicate that in vivo synthesis of MLG is regulated by factors independent of cytosolic carbohydrate concentration. Our finding that MLG synthesis can be regulated by light provide another possible clue in the comprehensive study of the mechanism of MLG synthesis in vivo. Gibeaut and Carpita (1993) found that MLG synthase activity can be stimulated by addition of small amounts of ATP or by steepening of the pH gradient across the Golgi membranes, which are also correlated with light. As cool-season crops, oats are to a high degree affected by adverse environmental factors, and the ability for rapid accumulation and hydrolysis of MLG in response to light may impart a more flexible architecture able to respond rapidly to initiate wall extension during growth under varied conditions.
Field trials also showed that environmental conditions had a great impact on oat MLG content, yet the mechanisms remain largely unknown. The effects of climate on oat MLG content have been described by several authors. Saastamoinen (1995) found that oat MLG content was dependent on the growing period temperature, while high precipitation decreased MLG content. Similar results were observed previously (Redaelli et al., 2013). In contrast, some studies found no correlation between growth temperature or total precipitation and MLG content in oats (Lim et al., 1992; Andersson and Börjesdotter, 2011). These studies tried to correlate specific weather conditions, in particular temperature and water availability during the growth season, to the accumulation of MLG but no significant correlations were found. Our finding that MLG synthase Csl genes are regulated by light well explains this apparent contradiction. High precipitation usually results in less sunlight; therefore it is possible that the association of low MLG content with high precipitation may be due to the lack of sunlight. Likewise, more sunlight or higher light intensity leads to higher temperatures, which is also associated with high MLG content. Thus, light is the primary cause of changes in MLG content in oat. Furthermore, this may also explain why the MLG level in grains grown in Spain (hot and higher light intensity) was higher than in Scotland (cooler and lower light intensity) (Swanston et al., 1997). Coles et al. (1991) showed that a correlation between MLG synthesis and transpiration occurs during periods of high transpiration rate. This also relates to higher light intensity, which induces a higher respiration rate. In this study, we demonstrate the role of light in the synthesis of oat MLG. Considering that MLG content determines much of the value of the oat crop to the producer, our findings will provide new insights and speculations to produce better-quality oats. High solar radiation and cooler summer weather without excessive rains during grain filling will generate the best oat yields with high-quality grain.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: list of primers used to isolate AsCsl genes. Table S2: oligonucleotide primers used in RT–qPCR experiments. Table S3: identity among the isolated AsCsl genes and deduced proteins Figure S1: phylogenetic relationships of the isolated oat Csl genes among plant species. Figure S2: part of the glycosyltransferase GT2 motif with surrounding amino acid residues. Figure S3: influence of light on the expression of AsCslF4 and AsCslF6 proteins.
ACCESSION NUMBERS
Sequence data from this article can be found in the EMBL/GenBank data libraries under the following accession numbers: AsCslF3 (MG543996), AsCslF4 (MG543997), AsCslF6 (MG543998), AsCslF8 (MG543999), AsCslF9 (MG544000), AsCslH (Q128579) and AsCslJ (MK905204).
FUNDING
This work was supported by grants from the Chinese Agricultural Research System (CARS-07-A1), the Chinese Ministry of Agriculture, the Agricultural Science and Technology Innovation Program of CAAS and the National Natural Science Foundation of China (grant number 30800699). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LITERATURE CITED
- Andersson AAM, Börjesdotter D. 2011. Effects of environment and variety on content and molecular weight of β-glucan in oats. Journal of Cereal Science 54: 122–128. [Google Scholar]
- Bailey TL, Williams N, Misleh C, Li WW. 2006. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Research 34: 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan CS, Cleary LJ. 2005. The potential use of cereal (1,3;1,4)-β-d-glucans as functional food ingredients. Journal of Cereal Science 42: 1–13. [Google Scholar]
- Buckeridge MS, Vergara CE, Carpita NC. 1999. The mechanism of synthesis of a mixed-linkage (1→3),(1→4)β-d-glucan in maize. Evidence for multiple sites of glucosyl transfer in the synthase complex. Plant Physiology 120: 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Wilson SM, Hrmova M, et al. 2006. Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)-beta-d-glucans. Science 311: 1940–1942. [DOI] [PubMed] [Google Scholar]
- Burton RA, Jobling SA, Harvey AJ, et al. 2008. The genetics and transcriptional profiles of the cellulose synthase-like HvCslF gene family in barley. Plant Physiology 146: 1821–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Collins HM, Kibble NA, et al. 2011. Over-expression of specific HvCslF cellulose synthase-like genes in transgenic barley increases the levels of cell wall (1,3;1,4)-β-d-glucans and alters their fine structure. Plant Biotechnology Journal 9: 117–135. [DOI] [PubMed] [Google Scholar]
- Butt MS, Tahir-Nadeem M, Khan MK, Shabir R, Butt MS. 2008. Oat: unique among the cereals. European Journal of Nutrition 47: 68–79. [DOI] [PubMed] [Google Scholar]
- Chawade A, Sikora P, Bräutigam M, et al. 2010. Development and characterization of an oat TILLING-population and identification of mutations in lignin and β-glucan biosynthesis genes. BMC Plant Biology 10: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant Journal 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Coles GD, Jamieson PD, Haslemore RM. 1991. Effect of moisture stress on malting quality in Triumph barley. Journal of Cereal Science 14: 161–177. [Google Scholar]
- Doblin MS, Pettolino FA, Wilson SM, et al. 2009. A barley cellulose synthase-like CSLH gene mediates (1,3;1,4)-β-d-glucan synthesis in transgenic Arabidopsis. Proceedings of the National Academy of Sciences of the USA 106: 5996–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermawar RA, Collins HM, Byrt CS, et al. 2015. Distribution, structure and biosynthetic gene families of (1,3;1,4)-β-glucan in Sorghum bicolor. Journal of Integrative Plant Biology 57: 429–445. [DOI] [PubMed] [Google Scholar]
- Fincher GB 2009. Exploring the evolution of (1,3;1,4)-β-d-glucans in plant cell walls: comparative genomics can help! Current Opinion in Plant Biology 12: 140–147. [DOI] [PubMed] [Google Scholar]
- Gibeaut DM, Carpita NC. 1993. Synthesis of (1→3),(1→4)-β-d-glucan in the Golgi apparatus of maize coleoptiles. Proceedings of the National Academy of Sciences of the USA 90: 3850–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo AY, Zhu QH, Chen X, Luo JC. 2007. GSDS: a gene structure display server. Yi Chuan 29: 1023–1026. [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. 1999. Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Research 27: 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA 1987. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Molecular Biology Reporter 5: 387–405. [Google Scholar]
- Jobling SA 2015. Membrane pore architecture of the CslF6 protein controls (1-3,1-4)-β-glucan structure. Science Advances 1: e1500069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianian SF, Phillips RL, Rines HW, Fulcher RG, Webster FH, Stuthman DD. 2000. Quantitative trait loci influencing beta-glucan content in oat (Avena sativa, 2n = 6x = 42). Theoretical and Applied Genetics 101: 1039–1048. [Google Scholar]
- Kimpara T, Aohara T, Soga K, et al. 2008. β-1,3:1,4-Glucan synthase activity in rice seedlings under water. Annals of Botany 102: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HS, White PJ, Fry KJ. 1992. Genotypic effects on β-glucan content of oat lines grown in two consecutive years. Cereal Chemistry 69: 262–265. [Google Scholar]
- Little A, Schwerdt JG, Shirley NJ, et al. 2018. Revised phylogeny of the cellulose synthase gene superfamily: insights into cell wall evolution. Plant Physiology 177: 1124–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SN, Wang JY, Chitsaz F, et al. 2020. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Research 48: D265–D268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell MA, Asoro FG, Scott MP, White PJ, Beavis WD, Jannink, JL. 2012. Genome-wide association study for oat (Avena sativa L.) beta-glucan concentration using germplasm of worldwide origin. Theoretical and Applied Genetics 125: 1687–1696. [DOI] [PubMed] [Google Scholar]
- Puente P, Wei N, Deng XW. 1996. Combinatorial interplay of promoter elements constitutes the minimal determinants for light and developmental control of gene expression in Arabidopsis. EMBO Journal 15: 3732–3743. [PMC free article] [PubMed] [Google Scholar]
- Redaelli R, Del Frate V, Bellato S, et al. 2013. Genetic and environmental variability of total and soluble β-glucan in European oat genotypes. Journal of Cereal Science 57: 193–199. [Google Scholar]
- Roulin S, Feller U. 2001. Reversible accumulation of (1-3,1-4)-β-glucan endohydrolase in wheat leaves under sugar depletion. Journal of Experimental Botany 52: 2323–2332. [DOI] [PubMed] [Google Scholar]
- Roulin S, Buchala AJ, Fincher GB. 2002. Induction of (1→3,1→4)-β-d-glucan hydrolases in leaves of dark-incubated barley seedlings. Planta 215: 51–59. [DOI] [PubMed] [Google Scholar]
- Saastamoinen M 1995. Effects of environmental factors on the β-glucan content of two oat varieties. Acta Agriculturae Scandinavica Section B 45: 181–187. [Google Scholar]
- Sigrist CJA, Cerutti L, Hulo N, et al. 2002. PROSITE: a documented database using patterns and profiles as motif descriptors. Briefings in Bioinformatics 3: 265–274. [DOI] [PubMed] [Google Scholar]
- Swanston JS, Ellis RP, Perez Vendrell A, Voltas J, Molina Cano JL. 1997. Patterns of barley grain development in Spain and Scotland and their implications for malting quality. Cereal Chemistry 74: 456–461. [Google Scholar]
- Wälti M, Roulin S, Feller U. 2002. Effects of pH, light and temperature on (1-3,1-4)-β-glucanase stability in wheat leaves. Plant Physiology and Biochemistry 40: 363–371. [Google Scholar]
- Welch WR, Lloyd JD. 1989. Kernel (1-3),(1-4)-β-d-glucan content of oat genotypes. Journal of Cereal Science 9: 35–40. [Google Scholar]
- Wilson SM, Burton RA, Doblin MS, et al. 2006. Temporal and spatial appearance of wall polysaccharides during cellularization of barley (Hordeum vulgare) endosperm. Planta 224: 655–667. [DOI] [PubMed] [Google Scholar]
- Wood PJ 2007. Cereal β-glucans in diet and health. Journal of Cereal Science 46: 230–238. [Google Scholar]
- Wood PJ, Weisz J, Blackwell BA. 1994. Structural studies of (1→3),(1→4)-β-d-glucans by 13C-nuclear magnetic resonance spectroscopy and by rapid analysis of cellulose-like regions using high-performance anion-exchange chromatography of oligosaccharides released by lichenase. Cereal Chemistry 71: 301–307. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.