Abstract
Stress may contribute to progression of coronary heart disease (CHD) through inflammation, especially among women. Thus, we sought to examine whether increased inflammatory response to stress among patients with CHD is associated with a greater risk of cardiovascular events and whether this risk is higher in women. We examined inflammatory biomarkers known to increase with mental stress (speech task), including interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and matrix metallopeptidase-9 (MMP-9) among 562 patients with stable CHD. Inflammatory response, the difference between post-stress and resting values, was examined as a predictor of major adverse cardiovascular events (MACE) using subdistribution hazards models for competing risks adjusting for demographics, cardiovascular risk factors, and medications. MACE was defined as a composite endpoint of cardiovascular death, myocardial infarction, unstable angina with revascularization, and heart failure. All biomarkers were standardized. The mean age was 63 years (range 34-79) and 24% were women. During a median follow-up of 3 years, 71 patients experienced MACE. Overall, there was no significant association between inflammatory response to stress and risk of MACE, but there were sex-based interactions for IL-6 (p = 0.001) and MCP-1 (p = 0.01). The risk of MACE increased 56% (HR: 1.56; 95% CI: 1.21, 2.01; p = 0.001) and 30% (HR: 1.30; 95% CI: 1.09, 1.55; p = 0.004) for each standard deviation increase in IL-6 and MCP-1 response to mental stress for women, respectively, while there was no association among men. Increased inflammation in response to stress is associated with future adverse cardiovascular outcomes among women with CHD.
Keywords: Inflammation, inflammatory response, mental stress, cardiovascular events, women
1. Introduction
Inflammation plays a key role in the pathogenesis and exacerbation of coronary heart disease and cardiovascular events. Several inflammatory biomarkers, including interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and matrix metallopeptidase-9 (MMP-9), together with acute phase C-reactive protein (CRP), have been shown to predict cardiovascular events in healthy individuals as well as individuals with underlying coronary heart disease (CHD) (Blankenberg, Rupprecht et al., 2003; Daniels, 2017; de Lemos, Morrow et al., 2007; Fanola, Morrow et al., 2017; Held, White et al., 2017; Ridker, 2014; Ridker, Hennekens et al., 2000; Yabluchanskiy, Ma et al., 2013).
Increases in inflammatory biomarkers in response to exogenous stressful challenges, including psychological stress, may contribute to cardiovascular events, even though empirical data are limited (Ackerman, Martino et al., 1998; Endrighi, Steptoe et al., 2016; Goebel, Mills et al., 2000; Goldman-Mellor, Brydon et al., 2010; Hackett, Hamer et al., 2012; Kop, Gottdiener et al., 2002; Lu, Zhao et al., 2013; Maes, Song et al., 1998; Miller, Cohen et al., 2002). The sympathetic nervous system activates the inflammatory cascade that may adversely affects the cardiovascular system (Kivimaki & Steptoe, 2018; McEwen, 1998). An increase in pro-inflammatory cytokines in response to stress can be especially deleterious in patients with a previous diagnosis of CHD, because it can contribute to acute atherosclerotic plaque destabilization (Kivimaki & Steptoe, 2018).
A growing number of studies have shown elevations of inflammatory cytokines among patients with CHD in response to acute mental stress, especially IL-6, suggesting that inflammation may be one potential pathway by which stress contributes to progression of coronary atherosclerosis and to the triggering of acute coronary syndromes (Hammadah, Sullivan et al., 2018; Libby, Tabas et al., 2014; Marsland, Walsh et al., 2017; Steptoe, Hamer et al., 2007). However, no previous study has specifically examined whether inflammatory response to stress is associated with greater risk of cardiovascular events among patients with CHD.
Understanding sex differences in inflammatory response to stress among patients with CHD may help elucidate potential mechanisms that may increase women’s susceptibility to adverse cardiovascular outcomes. Women with CHD have less obstructive coronary artery disease (Almuwaqqat, Sullivan et al., 2019; Smilowitz, Sampson et al., 2011), yet greater risk of myocardial ischemia compared with similarly aged men (Bairey Merz, Shaw et al., 2006; Garcia, Mulvagh et al., 2016; Shaw, Bairey Merz et al., 2006; Sullivan, Hammadah et al., 2018a). There is growing recognition that emotional stress and physiological sex differences in response to stress are important in women’s cardiovascular vulnerability (Samad, Boyle et al., 2014; Vaccarino, Shah et al., 2014; Vaccarino, Wilmot et al., 2016). Several studies have suggested that women with CHD are at higher risk of adverse cardiovascular outcomes than men in the presence of psychosocial exposures (Pimple, Lima et al., 2019; Shah, Ghasemzadeh et al., 2014).
We previously showed that younger women with CHD have higher concentrations of IL-6 before and after mental stress, as well as a higher IL-6 response to stress, compared to similarly aged men (Sullivan, Hammadah et al., 2018b). Thus, circulating levels of IL-6 in response to stress may be an important pathway through which women with CHD are susceptible to increased risk of cardiovascular events. However, whether the inflammatory response to stress is related to the risk of cardiovascular outcomes among individuals with CHD, and women in particular, has not been previously examined.
The objectives of the current study are to: 1) examine whether a stress-induced rise in inflammatory biomarkers known to increase with acute psychological stress, a panel including IL-6, MCP-1, and MMP-9 (Hammadah, Sullivan et al., 2018), is associated with major adverse cardiovascular events, or MACE among patients with underlying CHD; and 2) examine whether these associations are moderated by sex such that women have greater risk of MACE than men per unit change in inflammatory biomarkers. We hypothesized that higher concentrations of inflammatory biomarkers in response to stress would be associated with greater risk of cardiovascular events among patients with CHD and that this risk would be larger among women.
2. Material and Methods
2.1. Study Design and Participants
The data that support the findings of this study are available from the corresponding author on reasonable request. Between June 2011 and August 2014, we enrolled 695 patients with stable CHD in the Mental Stress Ischemia Mechanisms and Prognosis Study (MIPS), a prospective cohort study designed to investigate mechanisms and prognosis of mental stress-induced ischemia. Patients from Emory University-affiliated hospitals and clinics with documented CHD were eligible for the study if they were between 30–79 years of age. Criteria for documented CHD included at least one of the following: 1) abnormal coronary angiography or intravascular ultrasound demonstrating atherosclerosis with at least luminal irregularities; 2) previous percutaneous or surgical coronary revascularization; 3) documented myocardial infarction; or 4) positive exercise or pharmacological nuclear stress test or electrocardiographic exercise stress test. Although patients in MIPS could have angiographic (but not necessarily incident CAD by history), 73% of patients in MIPS did have a prior history of MI, stroke, heart failure, or angina. Patients were excluded from the study if they were pregnant; if they were hospitalized in the previous week for unstable angina, decompensated heart failure, or myocardial infarction; if they had severe psychiatric conditions such as schizophrenia or a history of alcohol or substance abuse; or if they had active malignancy, end stage renal disease, or other severe medical problems expected to shorten life expectancy to less than 5 years.
Of the 695 patients with CHD in the MIPS dataset, 80 patients had missing plasma samples during the baseline visit and 133 had missing samples post mental stress because of technical difficulties in sample drawing or processing, or patent refusal. Thus, 562 patients with plasma samples at both baseline and post-stress were included in this analysis. The Institutional Review Board at Emory University approved the MIPS research protocol. Written informed consent was obtained from all patients enrolled in the study. More detailed information on the MIPS objectives and study design has been previously described (Hammadah, Al Mheid et al., 2016; Vaccarino, Wilmot et al., 2016).
2.2. Measurements
2.2.1. Major Adverse Cardiovascular Events (MACE)
Patients were prospectively followed for major adverse cardiovascular outcomes (MACE), including death, myocardial infarction, coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting surgery), unstable angina, and hospitalization for heart failure. Follow-up data were collected by clinic visits at 1 and 2 years and by phone calls at 3 years as well as medical record review (including outside medical records) and querying the Social Security Death Index. All events were adjudicated by study cardiologists (MH, AJS, and AQ) who were blinded to other study data. The main endpoint was a composite of events including cardiovascular death, myocardial infarction, unstable angina with revascularization, or hospitalization for heart failure.
2.2.2. Mental Stress Testing Procedure
We used a mental stress testing procedure in the laboratory following an established experimental approach in CHD patients to elicit sympathetic nervous system responses to emotional challenges (Ramachandruni, Fillingim et al., 2006; Steptoe & Vogele, 1991; Strike & Steptoe, 2003; Vaccarino, 2016). The mental stress protocol we used has been validated and widely used in patients with CHD (Goldberg, Becker et al., 1996; Kim, Bartholomew et al., 2003; Ramachandruni, Fillingim et al., 2006; Sheps, McMahon et al., 2002), and is highly reproducible and predictive of mental stress induced myocardial-ischemia and of hemodynamic and vascular responses to stress in our laboratory (Hammadah, Alkhoder et al., 2017; Sullivan, Hammadah et al., 2018a). Patients were tested using a standardized public speaking task after a 30-minute rest period, in a temperature controlled, quiet, and dimly lit room, as previously described (Goldberg, Becker et al., 1996; Kim, Bartholomew et al., 2003; Sullivan, Hammadah et al., 2018a; Vaccarino, Shah et al., 2014; Vaccarino, Wilmot et al., 2016). Briefly, patients were asked to imagine a real-life stressful situation, in which a close relative had been mistreated in a nursing home and asked to make up a realistic story around this scenario. Patients were given two minutes to prepare a statement and then three minutes to present it in front of a video camera and an audience wearing white coats. Participants were told that their speech would be evaluated by the laboratory staff for content, quality, and duration. Cardiovascular medications, including beta-blockers, calcium-channel blockers, long-acting nitrates, and other anti-ischemic medications, as well as xanthine derivatives and caffeine-containing products were withheld for approximately 24 hours prior to stress testing.
2.2.3. Inflammatory Biomarkers
Inflammatory biomarkers were measured from venous blood samples using indwelling catheters collected at rest and 90-minutes post mental stress testing, including IL-6, MCP-1, and MMP-9. The panel of biomarkers chosen for this cohort and the plasma collection time points were guided by published reviews and meta-analyses (Marsland, Walsh et al., 2017; Steptoe, Hamer et al., 2007) and also by our pilot data that showed that IL-6, MCP-1, and MMP-9 were most robust for changes with mental stress compared with other inflammatory biomarkers tested (Hammadah, Sullivan et al., 2018). Venous blood was collected in ice-cooled citrate tubes and immediately centrifuged at 4°C; obtained plasma was frozen at −70°C until further processing. We employed the MesoScale system (Meso Scale Diagnostics Rockville, Maryland) using the SECTOR Imager 2400 to measure IL-6, MCP-1, and MMP-9 according to the protocols supplied by the manufacturer. The Mesoscale multiplex assay system uses electrochemiluminescence for high sensitivity and broad dynamic range. All biomarkers were in the range of detection. The inter-assay coefficient of variations for midpoint standards were 5.78% for IL-6, 4.99% for MCP-1, and 9.38% for MMP-9. The intra-assay coefficients of variation were 3.29% for IL-6, 3.45% for MCP-1, and 5.95% for MMP-9.
2.2.4. Other Measurements
Demographic information was obtained using standardized questionnaires during the baseline enrollment visits. Previous medical history (diabetes, hypertension, previous myocardial infarction) and medication use (e.g. aspirin, beta blockers) were obtained by study nurses or physicians through medical history, clinical examinations and by reviewing medical records. Depressive symptoms were assessed with the Beck Depression Inventory-II (Beck, Steer et al., 1996), a reliable and valid self-report measure that has been widely used in cardiac as well as non-cardiac patients. Lifetime history of major depression, current major depression, and lifetime/current posttraumatic stress disorder were assessed using the Structured Clinical Interview for DSM-IV (SCID) (First, Spitzer et al., 1995) by a trained research nurse under the supervision of the study psychiatrist (JDB). We also administered the Cohen’s Perceived Stress Scale, a 10-item survey of general stress validated in multiethnic populations (Cohen, Kamarck et al., 1983). Height and weight were objectively measured during the clinical exam and used to calculate body mass index (BMI, kg/m2). Angiographic data were obtained from the most recent coronary angiogram and severity of coronary artery disease was measured using the Gensini Score (Gensini, 1983).
2.3. Statistical Analyses
For descriptive purposes, participants’ characteristics were stratified by sex, and differences tested using chi-squared tests for categorical variables and analysis of variance for continuous variables. Inflammatory response to mental stress was calculated as the difference between inflammatory concentrations 90 minutes post-mental stress and resting values. The relative risk of MACE was estimated using Fine and Gray’s subdistribution hazards models for competing risks after checking that the proportional hazards assumption was met. Circulating inflammatory concentrations at baseline and post-stress were checked using the Schoenfeld and deviance residuals to identify any potential outliers and their influence on the results. All analyses were conducted before and after adjusting for possible confounding factors considered a priori and included in sequential models after adjusting for baseline inflammatory values (model 1). First, we added demographic variables including age, gender, race, and education (model 2), followed by the addition of lifestyle and clinical risk factors known to affect inflammation, including current smoking, depression, BMI, diabetes, and hypertension (model 3). A subsequent model (model 4) adjusted for medication use and adverse cardiovascular risk profile including beta-blockers, anti-depressants, and previous congestive heart failure. A final model (model 5), adjusted for Gensini score, a measure of angiographic coronary artery disease severity. To determine whether inflammatory response to stress and MACE differed by sex, we included biomarker-by-sex interactions in the hazards model. We then estimated linear combinations of the regression coefficients for sex using a similar modeling strategy as for the main effects. The significance level for main effects was set at p < 0.05; while the statistical significance of interaction effects was set at p < 0.10. In supplemental analyses, we estimated relative risks for MACE using baseline and 90-minutes values of inflammatory biomarkers as predictors, rather than their difference. To better contrast results of inflammatory biomarkers with different units of measure, we standardized all biomarkers in regression analyses. Thus, all hazard ratios can be interpreted as the hazards for MACE per 1 standard deviation increase in the inflammatory variable. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
3. Results
3.1. Descriptive Characteristics
Of the 562 patients in the analytic sample, the mean age was 63.0 years (range: 34–79) and 24% were women. Excluded participants were more likely to be women, African American, have a lower income as well as current major depression, lifetime history of PTSD, hypertension, and heart failure (Supplemental Table 1). Compared to men, women were more likely to be African American, to have a lower income, and to have a greater burden of psychosocial stressors including depression, posttraumatic stress, and perceived stress, and on anti-depressants (Table 1). Men were more likely to have dyslipidemia, a higher Gensini Score (a measure of angiographic coronary artery disease severity), and on ACE inhibitors. Descriptive inflammatory profiles for women and men are presented in Table 2. In general, women had higher inflammatory concentrations of IL-6 and MCP-1 at baseline and 90-minutes after mental stress, while men had higher concentrations of MMP-9 after stress.
Table 1.
Descriptive Characteristics for Patients by Sex, MIPS (n = 562).
| Women | Men | p-value* | |
|---|---|---|---|
| Total, n (%) | 133 (23.7) | 429 (76.3) | |
| Demographics | |||
| Age, years, mean (SD) | 62.7 (9.3) | 63.0 (8.9) | 0.72 |
| Age > 50 years, n (%) | 119 (89.5) | 392 (91.4) | 0.50 |
| African American, n (%) | 49 (36.8) | 95 (22.1) | 0.001 |
| Income < $20,000/yr. | 29 (21.8) | 49 (11.6) | 0.003 |
| Education High School or Less | 37 (27.8) | 104 (24.3) | 0.41 |
| Psychosocial Risk Factors | |||
| Lifetime History Major Depression, n (%) | 48 (37.2) | 94 (22.4) | 0.001 |
| Current Major Depression, n (%) | 13 (10.2) | 25 (6.0) | 0.11 |
| Beck Depression Inventory, mean, (SD) | 10.7 (8.7) | 7.6 (8.0) | 0.0002 |
| Lifetime History of PTSD, n (%) | 4 (3.2) | 25 (6.0) | 0.22 |
| Current PTSD, n (%) | 1 (25.0) | 18 (72.0) | 0.10 |
| PTSD Symptom Checklist, mean (SD) | 29.2 (11.3) | 25.5 (10.2) | 0.001 |
| Perceived Stress Scale, n (%) | 14.8 (8.2) | 11.5 (7.3) | <.0001 |
| Subjective Units of Distress, mean (SD) † | 12.0 (25.4) | 10.1 (16.6) | 0.43 |
| Cardiovascular Risk Factors | |||
| BMI, kg/m2, mean (SD) | 30.4 (6.5) | 29.5 (4.8) | 0.15 |
| Current Smoker, n (%) | 24 (18.1) | 55 (12.9) | 0.14 |
| Diabetes, n (%) | 47 (35.3) | 126 (29.4) | 0.19 |
| Hypertension, n (%) | 101 (75.9) | 319 (74.4) | 0.71 |
| Dyslipidemia, n (%) | 97 (72.9) | 358 (83.5) | 0.01 |
| Medication Use | |||
| Aspirin, n (%) | 112 (84.2) | 370 (86.5) | 0.52 |
| Beta Blocker, n (%) | 106 (79.7) | 308 (72.0) | 0.08 |
| ACE Inhibitors, n (%) | 41 (31.1) | 209 (49.0) | 0.0003 |
| Anti-Depressant, n (%) | 46 (34.6) | 89 (20.8) | 0.001 |
| Statins, n (%) | 107 (81.1) | 370 (86.7) | 0.11 |
| Clinical Characteristics | |||
| Previous MI, n (%) | 52 (39.1) | 152 (35.4) | 0.44 |
| Heart Failure, n (%) | 27 (20.3) | 93 (21.9) | 0.70 |
| Revascularization, n (%) | 102 (76.7) | 330 (76.9) | 0.96 |
| Gensini Score, median (IQR) | 17.0 (4.5, 50.0) | 28.0 (10.0, 65.3) | 0.001 |
Abbreviations: BMI: Body mass index; MI: Myocardial infarction; MIPS: Mental Stress Ischemia Mechanisms And Prognosis Study; PTSD: Post-traumatic stress disorder; SD: Standard deviation.
Statistical tests: Categorical variables: Chi-square; continuous variables: Student’s t test or Wilcoxon-Mann Whitley U Test when appropriate.
Difference between posttest and pretest values. A positive value indicates higher distress with mental stress.
Table 2.
Descriptive Inflammatory Profiles at Rest and 90-Minutes after Mental Stress by Sex. Values Reported are Geometric Mean Concentrations of IL-6, MCP-1, and MMP-9 (n=562).
| Women | Men | ||||
|---|---|---|---|---|---|
| N | Geometric Mean (95% CI) | N | Geometric Mean (95% CI) | p-value | |
| IL-6, pg/mL | |||||
| Rest | 133 | 1.60 (1.43, 1.78) | 429 | 1.40 (1.31, 1.48) | 0.03 |
| 90-Minutes | 133 | 2.03 (1.81, 2.27) | 429 | 1.75 (1.64, 1.87) | 0.03 |
| MCP-1, pg/mL | |||||
| Rest | 133 | 138.0 (131.8, 144.4) | 429 | 116.98 (114.0, 120.0) | <.0001 |
| 90-Minutes | 133 | 143.2 (136.5, 150.3) | 429 | 123.6 (120.3, 127.0) | <.0001 |
| MMP-9, ng/mL | |||||
| Rest | 133 | 58.2 (52.3, 64.8) | 429 | 64.4 (60.7, 68.4) | 0.10 |
| 90-Minutes | 133 | 60.4 (54.2, 67.2) | 429 | 71.9 (67.8, 76.4) | 0.01 |
Abbreviations: IL-6; interleukin-6; MCP-1: monocyte chemoattractant protein-1; MMP-9: matrix metallopeptidase 9.
Patients were followed for a median of 3 years and 71 (12.7 %) experienced MACE during follow-up (Table 3). Women and men had a similar incidence of MACE (12.8% vs. 12.7%). Of patients who experienced MACE, 35 (6.3%) events were hospitalizations for unstable angina with revascularization followed by nonfatal myocardial infarction and congestive heart failure hospitalizations.
Table 3.
Numbers* and Percentages of Patients who Developed Major Adverse Cardiovascular Events (MACE) by Sex.
| Total Population | Women | Men | p-value† | |
|---|---|---|---|---|
| Total Cardiovascular Events, n (%) | 71 (12.7) | 17 (12.8) | 54 (12.7) | 0.98 |
| Cardiovascular death, n (%) | 14 (2.5) | 2 (1.5) | 12 (2.8) | 0.40 |
| Myocardial infarction, n (%) | 22 (3.9) | 7 (5.3) | 15 (3.5) | 0.37 |
| Heart failure, n (%) | 19 (3.4) | 2 (1.5) | 17 (4.0) | 0.27 |
| Unstable angina with revascularization, n (%) | 35 (6.3) | 10 (7.5) | 25 (5.9) | 0.50 |
Individual events do not sum up to total events because of overall (same individuals having multiple events during follow-up).
Chi-square test or Fisher’s exact test when appropriate.
3.2. Inflammatory Response and Risk of MACE
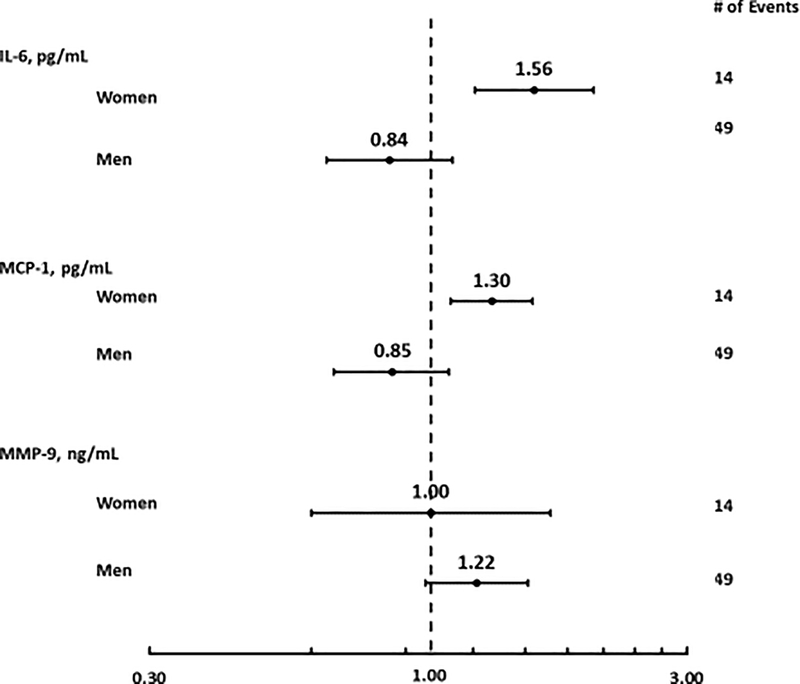
There were no significant associations between inflammatory response to stress and risk of MACE in the overall sample (Supplemental Table 2). However, significant sex differences were detected for IL-6 (p = 0.001 for IL-6*sex interaction) and MCP-1 (p = 0.01 for MCP-1*sex interaction) after adjusting for resting biomarker value, demographics, cardiovascular risk factors, medication use, and CHD severity (Table 4). The risk of MACE increased 56% (HR: 1.56; 95% CI: 1.21, 2.01; p = 0.001) and 30% (HR: 1.30; 95% 1.09. 1.55; p = 0.004) for each standard deviation increase in IL-6 and MCP-1 response to mental stress, respectively, while the trends were opposite among men (Table 4; Figure 1). Compared to women, men tended to have a higher risk of MACE with a greater MMP-9 response to stress; however, p-values for these HRs and interactions were marginally significant and were attenuated in fully adjusted models. Full results of models with covariates are shown in Supplemental Tables 3.
Table 4.
Unadjusted and Adjusted Fine & Gray’s Proportional Sub-Distribution Hazard Ratios and 95% Confidence Intervals for Major Adverse Cardiovascular Events (MACE) and Inflammatory Response (Post-stress – Resting Values) to Stress by Sex.
| Women | Men | ||||
|---|---|---|---|---|---|
| HR (95% CI) Per SD Biomarker Increase with Stress | p-value | HR (95 % CI) Per SD Biomarker Increase with Stress | p-value | p-value for interaction* | |
| IL-6, pg/mL | |||||
| Model 1 | 1.25 (0.90, 1.74) | 0.19 | 0.83 (0.67, 1.02) | 0.08 | 0.045 |
| Model 2 | 1.46 (1.04, 2.04) | 0.03 | 0.79 (0.64, 0.99) | 0.04 | 0.005 |
| Model 3 | 1.49 (1.11, 2.02) | 0.01 | 0.81 (0.64, 1.02) | 0.07 | 0.002 |
| Model 4 | 1.60 (1.19, 2.14) | 0.002 | 0.81 (0.62, 1.05) | 0.12 | 0.001 |
| Model 5 | 1.56 (1.21, 2.01) | 0.001 | 0.84 (0.64, 1.10) | 0.19 | 0.001 |
| MCP-1, pg/mL | |||||
| Model 1 | 1.50 (1.25, 1.80) | <.0001 | 0.81 (0.66, 1.00) | 0.05 | <.0001 |
| Model 2 | 1.54 (1.26, 1.88) | <.0001 | 0.78 (0.62, 0.98) | 0.03 | <.0001 |
| Model 3 | 1.50 (1.27, 1.78) | <.0001 | 0.77 (0.60, 0.97) | 0.03 | <.0001 |
| Model 4 | 1.33 (1.16, 1.53) | <.0001 | 0.80 (0.62, 1.03) | 0.08 | 0.001 |
| Model 5 | 1.30 (1.09, 1.55) | 0.004 | 0.85 (0.66, 1.08) | 0.18 | 0.01 |
| MMP-9, ng/mL | |||||
| Model 1 | 0.69 (0.40, 1.20) | 0.19 | 1.18 (0.98, 1.43) | 0.08 | 0.08 |
| Model 2 | 0.74 (0.44, 1.25) | 0.26 | 1.20 (0.99, 1.46) | 0.06 | 0.10 |
| Model 3 | 0.71 (0.40, 1.25) | 0.24 | 1.20 (0.97, 1.47) | 0.09 | 0.10 |
| Model 4 | 0.82 (0.47, 1.44) | 0.49 | 1.26 (1.02, 1.56) | 0.03 | 0.17 |
| Model 5 | 1.00 (0.60, 1.67) | 0.99 | 1.22 (0.98, 1.52) | 0.08 | 0.49 |
Abbreviations: CL: confidence limits; IL-6: interleukin-6; MCP-1: monocyte chemoattractant protein-1; MMP-9: matrix metallopeptidase 9.
p-value of sex-by-biomarker interaction.
Model 1 adjusted for biomarker, sex, biomarker*sex interaction, and resting value.
Model 2 adjusted for model 1 covariates + age, African American race, and education.
Model 3 adjusted for model 2 covariates + BDI, BMI, smoking status, diabetes, hypertension, and dyslipidemia.
Model 4 adjusted for model 3 covariates + previous heart failure, beta blockers and anti-depressants.
Model 5 adjusted for model 4 covariates + Gensini score.
Figure 1. Forest Plot of Fine & Gray’s Proportional Sub-Distribution Hazard Ratios for each standard deviation increase in IL-6, MCP-1, and MMP-9 Response to Stress and Major Adverse Cardiovascular Events.
Estimates are from fully adjusted model (model 5).
3.3. Inflammatory Concentrations at Rest and 90 Minutes Post-Stress with Risk of MACE
In the overall sample, there were no statistically significant associations between any inflammatory biomarker (IL-6, MCP-1, or MMP-9) at rest or 90-minues post-stress and risk of MACE (Supplemental Table 4). However, a significant interaction by sex was noted for MCP-1 at baseline (p = 0.06) such that each standard deviation increase was associated with a 32% increase in the risk of MACE among men only (HR: 1.32; 95% CI: 1.05, 1.67) in the fully adjusted model (Supplemental Table 5). Also, a significant interaction by sex was noted for post-stress values of IL-6 (p = 0.04) such that each standard deviation increase post-stress IL-6 levels was marginally associated with a 22% increase in risk for MACE among women (HR: 1.22; 95% CI: 0.99, 1.51; p=0.06), but not among men (HR: 0.82; 95% CI: 0.59, 1.14; p = 0.24) after adjusting for demographics, cardiovascular risk factors, and medication use (Supplemental Table 5). However, results were attenuated after subsequently adjusting for CHD severity.
4. Discussion
Among patients with pre-existing stable CHD, there were significant sex differences in the association between IL-6 and MCP-1 response to stress with risk of MACE such that there was a higher risk among women and no significant association found among men or in the overall sample. Specifically, we found that among women with stable CHD, but not men, each standard deviation increase in IL-6 in response to stress was associated with a 56% greater risk of adverse cardiovascular events. We also found that each standard deviation increase in MCP-1 in response to stress was associated with a 30% increase in risk of MACE among women only. These associations were robust after adjusting for sociodemographic characteristics, CHD risk factors, and medication use.
We were especially interested in potential sex differences in the relationship between inflammatory reactivity to stress and risk of cardiovascular events. In a previous study, we found that younger women with CHD had significantly higher concentrations of IL-6 before and after mental stress, as well as a higher response to stress, compared to similarly aged men with CHD (Sullivan, Hammadah et al., 2018b), which provided the premise for the current study.
IL-6 is an upstream inflammatory cytokine that is essential to the initiation of the atherosclerotic process and the downstream production of CRP (Held, White et al., 2017). This interleukin is a significant predictor of a plethora of adverse health outcomes including increased ambulatory blood pressure (Brydon & Steptoe, 2005), increased arterial stiffness (Mahmud & Feely, 2005), and increased risk for acute coronary syndromes (Blake & Ridker, 2003). Prior research has also shown that higher values of IL-6 were associated with the risk of clinical outcomes in stable CHD patients and after acute coronary syndromes, including recurrent coronary events and all-cause mortality (Fanola, Morrow et al., 2017; Held, White et al., 2017), and may be a more robust risk biomarker than CRP (Held, White et al., 2017). These previous findings suggest that IL-6 reflects a pathophysiological process for adverse cardiovascular outcomes rather than being a mere risk biomarker (Daniels, 2017). In this current study, we show that IL-6 is an important pathway through which women with CHD are susceptible to increased risk of adverse cardiovascular outcomes.
Similarly, an increase in MCP-1 response to stress was associated with adverse cardiovascular events among women and not men in our study. MCP-1 is a chemokine that increases with ischemia and reperfusion injury in many tissues and is also increased after ischemic stroke (Losy & Zaremba, 2001; Niu & Kolattukudy, 2009). MCP-1 is responsible for the recruitment of monocytes to sites of inflammation and plays a critical role at multiple stages in atherosclerosis and acute coronary syndromes, including the initiation of the fatty streak, promotion of plaque instability resulting in ischemic episodes, and remodeling after myocardial infarction (de Lemos, Morrow et al., 2003; Niu & Kolattukudy, 2009). After a myocardial infarction, monocytes are rapidly recruited to the infarct zone, where they promote wound healing and turnover of the extracellular matrix, and also contribute to adverse processes such as reperfusion injury, ventricular remodeling, and heart failure (Aukrust, Ueland et al., 1998; Birdsall, Green et al., 1997; de Lemos, Morrow et al., 2003; Parissis, Adamopoulos et al., 2002). Interestingly, our sample of women had less obstructive coronary artery disease (less atherosclerotic plaque) than men, but more ischemia induced by mental stress as previously reported (Vaccarino, Wilmot et al., 2016). Thus, an increase in MCP-1 with stress may occur in part in response to ischemia and may signal heightened risk but also directly contribute to a greater incidence of cardiovascular events among women.
We did not see similar trends with MMP-9. Men had higher levels of circulating levels of MMP-9 at baseline and post-stress than women, and the MMP-9 response to stress was marginally associated with an increased risk of MACE among men only. However, there were no statistically significant sex differences in fully adjusted models indicating that the associations among women and men were not statistically different. MMP-9 is a potential biomarker for cardiac remodeling and development of heart failure (Halade, Jin et al., 2013). In our cohort men had significantly greater coronary artery disease severity, as measured by the Gensini Score, which may have caused more cardiac remodeling among men. It is therefore possible that severity of coronary artery disease may have driven higher MMP-9 levels in men compared with women.
Understanding the potential mechanisms for these sex differences would help elucidate potential risk pathways among women, but much has yet to be clarified. Research suggests that women are more vulnerable to psychosocial stress, having greater molecular and biological effects that may be detrimental to cardiovascular health, especially women with CHD (Bangasser & Valentino, 2012; Vaccarino & Bremner, 2017). Previous studies, including ours, have shown that women have increased cardiovascular disease vulnerability to psychological stress (Pimple, Lima et al., 2019). In particular, women have increased inflammatory reactivity (Sullivan, Hammadah et al., 2018b), and platelet activation (Samad, Boyle et al., 2014), lower heart-rate variability (Ohira, Diez Roux et al., 2008), and are more likely to develop mental stress induced myocardial ischemia (Vaccarino, Shah et al., 2014; Vaccarino, Wilmot et al., 2016). Another potential mechanism is sex differences in glucocorticoid receptor sensitivity which may affect pro-inflammatory cytokine production after stress (Sullivan, Hammadah et al., 2018b). Previous research has shown sexual dimorphism in the immune response of humans and differences in immune responses between different female reproductive phases due to hormonal variations (Bouman, Heineman et al., 2005). Recent epidemiological research has shown that sex differences in circulating biomarkers of cardiovascular disease are most pronounced in premenopausal women compared with men, with attenuated sex differences among postmenopausal women (Lau, Paniagua et al., 2019). Research also suggests that estrogens may enhance cytokine production (Da Silva, 1999), thereby stimulating secretion of pro-inflammatory cytokines among women. However, in our previous work, we showed that a higher inflammatory response to stress was distinctive for young women with CHD, but not among female community controls matched for age, arguing against an estrogen effect (Sullivan, Hammadah et al., 2018b). Otherwise, similar patterns would have been seen in the community controls. There are also conflicting results about the effects of female sex hormones and the menopausal transition on inflammation, particularly IL-6 (Bouman, Heineman et al., 2005). Thus, sex hormones as mechanisms for sex differences in inflammatory responses to mental stress deserve further exploration. The possibility of “reverse-causation” must also be acknowledged, such that higher baseline coronary artery disease severity can lead to higher inflammation and to future CHD events. However, women in our sample had a significantly lower angiographic severity of coronary disease than men. It is also important to mention that women and men did not have statistically different emotional responses to mental stress as measured by the Subjective Units of Distress Scale (SUDS), calculated as the difference between post-test and pre-test values. In exploratory analysis, we added the SUDS score to our models and results were materially unchanged. Thus, our analysis suggests that our results were not attributable to differences in perceived emotional response to stress.
Several inflammatory biomarkers, including IL-6, MCP-1, and MMP-9 have been associated with the risk of future cardiovascular events in healthy individuals and patients with acute coronary syndrome (de Lemos, Morrow et al., 2003; Fanola, Morrow et al., 2017; Held, White et al., 2017; Ridker, Hennekens et al., 2000; Tunon, Blanco-Colio et al., 2014). However, in our study, we did not find significant associations between baseline (resting) concentrations of IL-6, MCP-1, or MMP-9, and greater risk of cardiovascular events in the overall sample. However, we found that MCP-1 at baseline was associated with greater risk of cardiovascular events among men. These inconsistencies may be attributable to differences across studies related to sample characteristics, length of follow-up, and ascertainment of cardiovascular events. In contrast, we found that higher concentrations of IL-6 and MCP-1 in response to stress were associated with a greater risk of adverse cardiovascular events among women only.
There are several strengths and limitations of our study. This is the first study to investigate inflammatory response to stress and future cardiovascular events. The MIPS study is one of the largest and most comprehensive studies of mental stress to date among stable CHD patients. The large and diverse sample, including a sizable number of women, along with a well characterized population with comprehensive medical and psychological measures, are important strengths. The experimental manipulation of the exposure (mental stress) allows a controlled assessment of the effects of the inflammatory response to stress. Also, cardiovascular events and causes of death were adjudicated by experienced cardiologists using an established protocol. There were less women than men with limited number of events in this group, which may have affected the study power for some analyses. However, we found statistically significant interactions with sex, which argue against a type II error. Our sample size, especially the relatively small number of younger women, also precluded the examination of sex by age interactions with outcomes. It is also important to note that there are numerous inflammatory biomarkers that could have been investigated. The panel of biomarkers chosen for this cohort were guided by previous research (Marsland, Walsh et al., 2017; Steptoe, Hamer et al., 2007) and our own pilot data showing that IL-6, MCP-1, and MMP-9 were most responsive to mental stress among a panel of biomarkers tested (Hammadah, Sullivan et al., 2018). Other important limitations include the observational nature of the study design, residual confounding, and lack of generalizability. Finally, we only examined inflammatory biomarkers at two time points. However, previous data indicate that the 90 minutes post-stress time point is optimal for the detection of change in most biomarkers of inflammation (Edwards, Burns et al., 2006; Marsland, Walsh et al., 2017; Mendham, Donges et al., 2011; Moldoveanu, Shephard et al., 2000; Rooks, Ibeanu et al., 2016; Steptoe, Willemsen et al., 2001).
4.1. Conclusions
We show for the first time that, among patients with stable CHD, there are important sex differences in the link between inflammatory response to psychological stress and risk of future cardiovascular events. Specifically, heightened IL-6 and MCP-1 responses to stress are associated with a pronounced increase in adverse events in women only. Higher inflammatory cytokine and chemokine concentrations in response to stress among women with CHD may be important pathways linking stress to cardiovascular outcomes in this group.
Supplementary Material
Highlights.
First to study inflammatory response to stress and adverse cardiovascular events.
We found clear sex differences.
Higher IL-6 and MCP-1 stress response was associated with cardiovascular events among women only.
Stress may increase cardiovascular events among women through inflammation.
Funding
This work was supported by the National Institutes of Health (grant numbers P01HL101398, P20HL113451, P01HL086773-06A1, R56HL126558, R01HL109413, R01HL109413-02S1, R01HL125246, UL1TR000454, KL2TR000455, K24HL077506, K24 MH076955, K23HL127251, T32HL130025, R61HL138657, P30DK111024, R01HL095479, RF1AG051633, R01AG042127, 2P01HL086773, U54AG062334, R01HL141205, P01HL086773, R01NS064162, R01HL89650, DP3DK094346, K12HD085850, K01HL149982, L30HL148912, UL1TR002378) and American Heart Association grant 15SFCRN23910003. The authors of this article are solely responsible for the content of this paper. The funding agency had no role in the design and conduct of this study, in the collection, analysis, interpretation of the data, or in the preparation, review or approval of this manuscript.
Abbreviations and Acronyms
- BDI
beck depression inventory
- BMI
body mass index
- CHD
coronary heart disease
- IL-6
interleukin-6
- MACE
major adverse cardiovascular events
- MCP-1
monocyte chemoattractant protein-1
- MIPS
Mental Stress Ischemia Mechanisms and Prognosis Study
- MMP-9
matrix metallopeptidas
Footnotes
Declarations of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ackerman KD, Martino M, Heyman R, Moyna NM, & Rabin BS, 1998. Stressor-induced alteration of cytokine production in multiple sclerosis patients and controls. Psychosom Med, 60(4), 484–491. [DOI] [PubMed] [Google Scholar]
- Almuwaqqat Z, Sullivan S, Hammadah M, Lima BB, Shah AJ, Abdelhadi N, Fang S, Wilmot K, Al Mheid I, Bremner JD, Garcia E, Nye JA, Elon L, Li L, O’Neal WT, Raggi P, Quyyumi AA, & Vaccarino V, 2019. Sex-Specific Association Between Coronary Artery Disease Severity and Myocardial Ischemia Induced by Mental Stress. Psychosom Med, 81(1), 57–66. doi: 10.1097/PSY.0000000000000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukrust P, Ueland T, Muller F, Andreassen AK, Nordoy I, Aas H, Kjekshus J, Simonsen S, Froland SS, & Gullestad L, 1998. Elevated circulating levels of C-C chemokines in patients with congestive heart failure. Circulation, 97(12), 1136–1143. [DOI] [PubMed] [Google Scholar]
- Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G, & Investigators, Wise, 2006. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol, 47(3 Suppl), S21–29. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, & Valentino RJ, 2012. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol, 32(5), 709–723. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck Aaron Temkin, Steer Robert A., & Brown Gregory K. (1996). Beck depression inventory : BDI-II : manual. San Antonio: The Psychological Corporation : Harcourt Brace & Company. [Google Scholar]
- Birdsall HH, Green DM, Trial J, Youker KA, Burns AR, MacKay CR, LaRosa GJ, Hawkins HK, Smith CW, Michael LH, Entman ML, & Rossen RD, 1997. Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation, 95(3), 684–692. [DOI] [PubMed] [Google Scholar]
- Blake GJ, & Ridker PM, 2003. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. J Am Coll Cardiol, 41(4 Suppl S), 37S–42S. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L, & AtheroGene Investigators, 2003. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation, 107(12), 1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- Bouman A, Heineman MJ, & Faas MM, 2005. Sex hormones and the immune response in humans. Hum Reprod Update, 11(4), 411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- Brydon L, & Steptoe A, 2005. Stress-induced increases in interleukin-6 and fibrinogen predict ambulatory blood pressure at 3-year follow-up. J Hypertens, 23(5), 1001–1007. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R, 1983. A global measure of perceived stress. J Health Soc Behav, 24(4), 385–396. [PubMed] [Google Scholar]
- Da Silva JA, 1999. Sex hormones and glucocorticoids: interactions with the immune system. Ann N Y Acad Sci, 876, 102–117; discussion 117–108. [DOI] [PubMed] [Google Scholar]
- Daniels LB, 2017. Pretenders and Contenders: Inflammation, C-Reactive Protein, and Interleukin-6. J Am Heart Assoc, 6(10). doi: 10.1161/JAHA.117.007490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lemos JA, Morrow DA, Blazing MA, Jarolim P, Wiviott SD, Sabatine MS, Califf RM, & Braunwald E, 2007. Serial measurement of monocyte chemoattractant protein-1 after acute coronary syndromes: results from the A to Z trial. J Am Coll Cardiol, 50(22), 2117–2124. doi: 10.1016/j.jacc.2007.06.057. [DOI] [PubMed] [Google Scholar]
- de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, & Braunwald E, 2003. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation, 107(5), 690–695. [DOI] [PubMed] [Google Scholar]
- Edwards KM, Burns VE, Ring C, & Carroll D, 2006. Sex differences in the interleukin-6 response to acute psychological stress. Biol Psychol, 71(3), 236–239. doi: 10.1016/j.biopsycho.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Endrighi R, Steptoe A, & Hamer M, 2016. The effect of experimentally induced sedentariness on mood and psychobiological responses to mental stress. Br J Psychiatry, 208(3), 245–251. doi: 10.1192/bjp.bp.114.150755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanola CL, Morrow DA, Cannon CP, Jarolim P, Lukas MA, Bode C, Hochman JS, Goodrich EL, Braunwald E, & O’Donoghue ML, 2017. Interleukin-6 and the Risk of Adverse Outcomes in Patients After an Acute Coronary Syndrome: Observations From the SOLID-TIMI 52 (Stabilization of Plaque Using Darapladib-Thrombolysis in Myocardial Infarction 52) Trial. J Am Heart Assoc, 6(10). doi: 10.1161/JAHA.117.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, & Gibbon M, 1995. Structured Clinical Interview for DSM IV-Patient Edition (SCID-P) Washington. DC: American Psychiatric Press Inc. [Google Scholar]
- Garcia M, Mulvagh SL, Merz CN, Buring JE, & Manson JE, 2016. Cardiovascular Disease in Women: Clinical Perspectives. Circ Res, 118(8), 1273–1293. doi: 10.1161/CIRCRESAHA.116.307547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensini GG, 1983. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol, 51(3), 606. [DOI] [PubMed] [Google Scholar]
- Goebel MU, Mills PJ, Irwin MR, & Ziegler MG, 2000. Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosom Med, 62(4), 591–598. [DOI] [PubMed] [Google Scholar]
- Goldberg AD, Becker LC, Bonsall R, Cohen JD, Ketterer MW, Kaufman PG, Krantz DS, Light KC, McMahon RP, Noreuil T, Pepine CJ, Raczynski J, Stone PH, Strother D, Taylor H, & Sheps DS, 1996. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI). Circulation, 94(10), 2402–2409. [DOI] [PubMed] [Google Scholar]
- Goldman-Mellor S, Brydon L, & Steptoe A, 2010. Psychological distress and circulating inflammatory markers in healthy young adults. Psychological Medicine, 40(12), 2079–2087. doi: 10.1017/S0033291710000267. [DOI] [PubMed] [Google Scholar]
- Hackett RA, Hamer M, Endrighi R, Brydon L, & Steptoe A, 2012. Loneliness and stress-related inflammatory and neuroendocrine responses in older men and women. Psychoneuroendocrinology, 37(11), 1801–1809. doi: 10.1016/j.psyneuen.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Halade Ganesh V, Jin Yu-Fang, & Lindsey Merry L, 2013. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacology & therapeutics, 139(1), 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V, & Quyyumi AA, 2016. The Mental Stress Ischemia Prognosis Study (MIPS): Objectives, Study Design, and Prevalence of Inducible Ischemia. Psychosom Med. doi: 10.1097/PSY.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, Chou D, Obideen M, O’Neal WT, Sullivan S, Tahhan AS, Kelli HM, Ramadan R, Pimple P, Sandesara P, Shah AJ, Ward L, Ko YA, Sun Y, Uphoff I, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Sheps DS, Raggi P, Vaccarino V, & Quyyumi AA, 2017. Hemodynamic, catecholamine, vasomotor and vascular responses: Determinants of myocardial ischemia during mental stress. Int J Cardiol doi: 10.1016/j.ijcard.2017.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M, Sullivan S, Pearce B, Al Mheid I, Wilmot K, Ramadan R, Tahhan AS, O’Neal WT, Obideen M, Alkhoder A, Abdelhadi N, Mohamed Kelli H, Ghafeer MM, Pimple P, Sandesara P, Shah AJ, Hosny KM, Ward L, Ko YA, Sun YV, Weng L, Kutner M, Bremner JD, Sheps DS, Esteves F, Raggi P, Vaccarino V, & Quyyumi AA, 2018. Inflammatory response to mental stress and mental stress induced myocardial ischemia. Brain Behav Immun, 68, 90–97. doi: 10.1016/j.bbi.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, Koenig W, Siegbahn A, Steg PG, Soffer J, Weaver WD, Ostlund O, Wallentin L, & Investigators, Stability, 2017. Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: Experiences From the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J Am Heart Assoc, 6(10). doi: 10.1161/JAHA.116.005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CK, Bartholomew BA, Mastin ST, Taasan VC, Carson KM, & Sheps DS, 2003. Detection and reproducibility of mental stress-induced myocardial ischemia with Tc-99m sestamibi SPECT in normal and coronary artery disease populations. J Nucl Cardiol, 10(1), 56–62. doi: 10.1067/mnc.2003.26. [DOI] [PubMed] [Google Scholar]
- Kivimaki M, & Steptoe A, 2018. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol, 15(4), 215–229. doi: 10.1038/nrcardio.2017.189. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J, Newman A, Hirsch C, & Tracy RP, 2002. Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol, 89(4), 419–424. doi:S0002914901022640 [pii]. [DOI] [PubMed] [Google Scholar]
- Lau ES, Paniagua SM, Guseh JS, Bhambhani V, Zanni MV, Courchesne P, Lyass A, Larson MG, Levy D, & Ho JE, 2019. Sex Differences in Circulating Biomarkers of Cardiovascular Disease. J Am Coll Cardiol, 74(12), 1543–1553. doi: 10.1016/j.jacc.2019.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Tabas I, Fredman G, & Fisher EA, 2014. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res, 114(12), 1867–1879. doi: 10.1161/CIRCRESAHA.114.302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losy J, & Zaremba J, 2001. Monocyte chemoattractant protein-1 is increased in the cerebrospinal fluid of patients with ischemic stroke. Stroke, 32(11), 2695–2696. [DOI] [PubMed] [Google Scholar]
- Lu XT, Zhao YX, Zhang Y, & Jiang F, 2013. Psychological stress, vascular inflammation, and atherogenesis: potential roles of circulating cytokines. J Cardiovasc Pharmacol, 62(1), 6–12. doi: 10.1097/FJC.0b013e3182858fac. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, & Smith RS, 1998. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine, 10(4), 313–318. doi:S1043466697902908 [pii]. [DOI] [PubMed] [Google Scholar]
- Mahmud A, & Feely J, 2005. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension, 46(5), 1118–1122. doi: 10.1161/01.HYP.0000185463.27209.b0. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, & John-Henderson NA, 2017. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav Immun, 64, 208–219. doi: 10.1016/j.bbi.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 1998. Protective and damaging effects of stress mediators. N Engl J Med, 338(3), 171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Mendham AE, Donges CE, Liberts EA, & Duffield R, 2011. Effects of mode and intensity on the acute exercise-induced IL-6 and CRP responses in a sedentary, overweight population. Eur J Appl Physiol, 111(6), 1035–1045. doi: 10.1007/s00421-010-1724-z. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, & Ritchey AK, 2002. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol, 21(6), 531–541. [DOI] [PubMed] [Google Scholar]
- Moldoveanu AI, Shephard RJ, & Shek PN, 2000. Exercise elevates plasma levels but not gene expression of IL-1beta, IL-6, and TNF-alpha in blood mononuclear cells. J Appl Physiol (1985), 89(4), 1499–1504. [DOI] [PubMed] [Google Scholar]
- Niu J, & Kolattukudy PE, 2009. Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin Sci (Lond), 117(3), 95–109. doi: 10.1042/CS20080581. [DOI] [PubMed] [Google Scholar]
- Ohira T, Diez Roux AV, Prineas RJ, Kizilbash MA, Carnethon MR, & Folsom AR, 2008. Associations of psychosocial factors with heart rate and its short-term variability: multi-ethnic study of atherosclerosis. Psychosom Med, 70(2), 141–146. doi: 10.1097/PSY.0b013e318160686a. [DOI] [PubMed] [Google Scholar]
- Parissis JT, Adamopoulos S, Venetsanou KF, Mentzikof DG, Karas SM, & Kremastinos DT, 2002. Serum profiles of C-C chemokines in acute myocardial infarction: possible implication in postinfarction left ventricular remodeling. J Interferon Cytokine Res, 22(2), 223–229. doi: 10.1089/107999002753536194. [DOI] [PubMed] [Google Scholar]
- Pimple P, Lima BB, Hammadah M, Wilmot K, Ramadan R, Levantsevych O, Sullivan S, Kim JH, Kaseer B, Shah AJ, Ward L, Raggi P, Bremner JD, Hanfelt J, Lewis T, Quyyumi AA, & Vaccarino V, 2019. Psychological Distress and Subsequent Cardiovascular Events in Individuals With Coronary Artery Disease. J Am Heart Assoc, 8(9), e011866. doi: 10.1161/JAHA.118.011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandruni S, Fillingim RB, McGorray SP, Schmalfuss CM, Cooper GR, Schofield RS, & Sheps DS, 2006. Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. J Am Coll Cardiol, 47(5), 987–991. doi: 10.1016/j.jacc.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Ridker PM, 2014. Inflammation, C-reactive protein, and cardiovascular disease: moving past the marker versus mediator debate. Circ Res, 114(4), 594–595. doi: 10.1161/CIRCRESAHA.114.303215. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, & Rifai N, 2000. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med, 342(12), 836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Rooks CR, Ibeanu I, Shah A, Pimple P, Murrah N, Shallenberger L, Pace T, Douglas Bremner J, Raggi P, & Vaccarino V, 2016. Young women post-MI have higher plasma concentrations of interleukin-6 before and after stress testing. Brain Behav Immun, 51, 92–98. doi: 10.1016/j.bbi.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad Z, Boyle S, Ersboll M, Vora AN, Zhang Y, Becker RC, Williams R, Kuhn C, Ortel TL, Rogers JG, O’Connor CM, Velazquez EJ, Jiang W, & Investigators, Remit, 2014. Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: insights from the REMIT study. J Am Coll Cardiol, 64(16), 1669–1678. doi: 10.1016/j.jacc.2014.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AJ, Ghasemzadeh N, Zaragoza-Macias E, Patel R, Eapen DJ, Neeland IJ, Pimple PM, Zafari AM, Quyyumi AA, & Vaccarino V, 2014. Sex and age differences in the association of depression with obstructive coronary artery disease and adverse cardiovascular events. J Am Heart Assoc, 3(3), e000741. doi: 10.1161/JAHA.113.000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G, & Investigators, Wise, 2006. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol, 47(3 Suppl), S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, Sheffield D, Goldberg AD, Ketterer MW, Pepine CJ, Raczynski JM, Light K, Krantz DS, Stone PH, Knatterud GL, & Kaufmann PG, 2002. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation, 105(15), 1780–1784. [DOI] [PubMed] [Google Scholar]
- Smilowitz NR, Sampson BA, Abrecht CR, Siegfried JS, Hochman JS, & Reynolds HR, 2011. Women have less severe and extensive coronary atherosclerosis in fatal cases of ischemic heart disease: an autopsy study. Am Heart J, 161(4), 681–688. doi: 10.1016/j.ahj.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, & Chida Y, 2007. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun, 21(7), 901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Steptoe A, & Vogele C, 1991. Methodology of mental stress testing in cardiovascular research. Circulation, 83(4 Suppl), II14–24. [PubMed] [Google Scholar]
- Steptoe A, Willemsen G, Owen N, Flower L, & Mohamed-Ali V, 2001. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin Sci (Lond), 101(2), 185–192. [PubMed] [Google Scholar]
- Strike PC, & Steptoe A, 2003. Systematic review of mental stress-induced myocardial ischaemia. Eur Heart J, 24(8), 690–703. [DOI] [PubMed] [Google Scholar]
- Sullivan S, Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, Isakadze N, Shah A, Levantsevych O, Pimple PM, Kutner M, Ward L, Garcia EV, Nye J, Mehta PK, Lewis TT, Bremner JD, Raggi P, Quyyumi AA, & Vaccarino V, 2018a. Sex Differences in Hemodynamic and Microvascular Mechanisms of Myocardial Ischemia Induced by Mental Stress. Arterioscler Thromb Vasc Biol, 38(2), 473–480. doi: 10.1161/atvbaha.117.309535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S, Hammadah M, Wilmot K, Ramadan R, Pearce BD, Shah A, Kaseer B, Gafeer MM, Lima BB, Kim JH, Ward L, Ko YA, Lewis TT, Hankus A, Elon L, Li L, Bremner JD, Raggi P, Quyyumi A, & Vaccarino V, 2018b. Young Women With Coronary Artery Disease Exhibit Higher Concentrations of Interleukin-6 at Baseline and in Response to Mental Stress. J Am Heart Assoc, 7(23), e010329. doi: 10.1161/JAHA.118.010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunon J, Blanco-Colio L, Cristobal C, Tarin N, Higueras J, Huelmos A, Alonso J, Egido J, Asensio D, Lorenzo O, Mahillo-Fernandez I, Rodriguez-Artalejo F, Farre J, Martin-Ventura JL, & Lopez-Bescos L, 2014. Usefulness of a combination of monocyte chemoattractant protein-1, galectin-3, and N-terminal probrain natriuretic peptide to predict cardiovascular events in patients with coronary artery disease. Am J Cardiol, 113(3), 434–440. doi: 10.1016/j.amjcard.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, & Bremner JD, 2017. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev, 74(Pt B), 297–309. doi: 10.1016/j.neubiorev.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Shah AJ, Rooks C, Ibeanu I, Nye JA, Pimple P, Salerno A, D’Marco L, Karohl C, Bremner JD, & Raggi P, 2014. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med, 76(3), 171–180. doi: 10.1097/PSY.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Wilmot K, Al Mheid I, Ramadan R, Pimple P, Shah AJ, Garcia EV, Nye J, Ward L, Hammadah M, Kutner M, Long Q, Bremner JD, Esteves F, Raggi P, & Quyyumi AA, 2016. Sex Differences in Mental Stress-Induced Myocardial Ischemia in Patients With Coronary Heart Disease. J Am Heart Assoc, 5(9). doi: 10.1161/JAHA.116.003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino Viola. (2016). Mental Stress-Induced Myocardial Ischemia Cardiovascular Diseases and Depression (pp. 105–121): Springer. [Google Scholar]
- Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, & Lindsey ML, 2013. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology (Bethesda), 28(6), 391–403. doi: 10.1152/physiol.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.